Summary
Altered states of embodiment are fundamental to the scientific understanding of bodily self consciousness. The feeling of disembodiment during everyday activities is common to clinical conditions; however, the direct study of disembodiment in experimental setups is rare compared to the extensive investigation of illusory embodiment of an external object. Using mixed reality to modulate embodiment through temporally mismatching sensory signals from the own body, we assessed how such mismatches affect phenomenal and physiological aspects of embodiment and measured perceptual thresholds for these across multimodal signals. The results of a principal component analysis suggest that multimodal mismatches generally induce disembodiment by increasing the sense of disownership and deafference and decreasing embodiment; however, this was not generally reflected in physiological changes. Although visual delay decreased embodiment both during active movement and passive touch, the effect was stronger for the former. We discuss the relevance of these findings for understanding bodily self plasticity.
Subject Areas: Sensory Neuroscience, Cognitive Neuroscience, Applied Sensory Psychophysics
Graphical Abstract
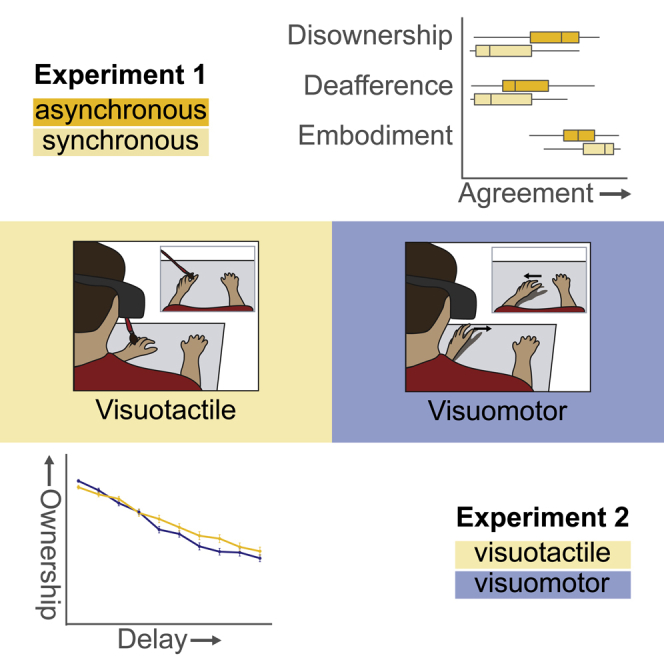
Highlights
-
•
Temporal multimodal conflict from own body induces disembodiment
-
•
Main components of disembodiment are disownership, deafference, and reduced embodiment
-
•
Subjective reports are not reflected by implicit measures
-
•
Disembodiment increases faster with visuomotor than with visuotactile mismatch
Sensory Neuroscience; Cognitive Neuroscience; Applied Sensory Psychophysics
Introduction
Over the past two decades, experimental evidence has shown that the sense of body of healthy subjects is remarkably plastic and built upon a constant prediction, weighting, and integration of multimodal signals (e.g. Blanke, 2012, Ehrsson, 2012, Kilteni et al., 2015). Protocols involving multimodal stimulation suggest that a majority of healthy individuals embody foreign or virtual limbs or full bodies when bodily sensations (e.g. body movements or touch) are visually displayed in synchrony to matching sensations on the hidden body (e.g. Botvinick and Cohen, 1998, Tsakiris et al., 2006, Lenggenhager et al., 2007, Slater et al., 2010). Such illusory embodiment is usually manifested by the senses of body ownership and agency (Kalckert and Ehrsson, 2012, Tsakiris et al., 2006) as well as self location (Longo et al., 2008) and has been evidenced using a variety of experimental setups using both explicit (e.g. questionnaire) and implicit (e.g. proprioceptive drift or physiological response) measures (Blanke et al., 2015).
This line of research predominately investigated the influence of multimodal coherence on illusory embodiment of an external or supernumerary bodily object; far more elusive, however, is how breaking multimodal information about the own body might reduce embodiment or even induce a feeling of disembodiment (Gentile et al., 2013, Graham et al., 2015; Hoover and Harris, 2012; Kannape et al., 2019, Newport and Preston, 2011, Otsuru et al., 2014, Newport and Gilpin, 2011, Longo and Haggard, 2009, Osumi et al., 2018). This is surprising, as disorders of bodily self awareness in clinical populations predominantly manifest in a loss of embodiment, as a break of (own) body ownership and one's sense of agency (Aglioti et al., 1996, Brugger and Lenggenhager, 2014, Otsuru et al., 2014, Vallar and Ronchi, 2009). For example, in the case of somatoparaphrenia, resulting from a brain lesion, patients lack the feeling of ownership for the contralesional arm, often attributing that arm to someone else (Aglioti et al., 1996, Brugger and Lenggenhager, 2014, Vallar and Ronchi, 2009) or even showing aggression toward it (Loetscher et al., 2006). Similarly, individuals suffering from body integrity dysphoria feel strong alienation from one or several body parts often combined with a desire for amputation (Blom et al., 2012, Brugger and Lenggenhager, 2014, Lenggenhager et al., 2015). Such a feeling of disembodiment might also extend to the full body, both in neurological (Smit et al., 2018) as well as in psychiatric disorders, as during depersonalization (Davidson, 1966, Sierra et al., 2005) or borderline personality disorder (Löffler et al., 2019).
Important theoretical differences between ownership of an external body, reduced ownership for one's own body, and body disownership have been proposed (de Vignemont, 2011), and the degree of alteration in embodiment of the own body in illusory limb or full-body ownership paradigms remains elusive. Although some authors suggest decreased ownership for the real body based on questionnaire (Longo et al., 2008, Moseley et al., 2008, Lane et al., 2017) or even immunological data (Barnsley et al., 2011), others found disownership of one's own body to be rare and rather weak in rubber-hand-illusion-like setups (Folegatti et al., 2009). Data from individuals with clinically caused alterations leading to loss of own-body ownership generally suggest enhanced illusory ownership for an external body, pointing to different mechanisms between embodiment and disembodiment in patients suffering from schizophrenia (Thakkar et al., 2011; see Shaqiri et al., 2018 for alternative findings during full body illusions), body integrity dysphoria (Lenggenhager et al., 2015), or somatoparaphrenia (Smit et al., 2018, vanStralen et al., 2013, White and Aimola Davies, 2017). This is further evidenced by a voxel-based lesion symptom mapping study that found a partial dissociation between brain areas involved in own-limb disembodiment as compared with supernumerary embodiment (Martinaud et al., 2017).
Here we directly manipulated embodiment of one's biological hand using a controlled multisensory conflict, without the use of a proxy/rubber hand. Previous studies suggest a feeling of disownership and numbness during delayed and therefore conflicting visual feedback of a tactile or motor event in a mixed reality setup using an infrared camera feed (Kannape et al., 2019), a mixed reality setup using a prerecorded video (Gentile et al., 2013), or in the MIRAGE setup where participants enter their hand in a box where visual aspects (spatial or temporal) of the hand are altered (e.g. Newport and Preston, 2010, Newport and Preston, 2011). We adapted such setups to be more realistic and ecologically valid using a wide field of view webcam mounted on a head-mounted display (HMD), providing an online, naturally colored, view of the video feed. This setup provides a direct view on the own full body in its current environment as seen from a first-person perspective, roughly corresponding to the direct view of the own body. Our setup was created to induce a strong prior assumption of actually viewing one's own body and surroundings. We then manipulated the delay of the video feed digitally, thus controlling the latency of visual as compared to other bodily signals (i.e. tactile, motor, or potentially others). We used this setup in two different experiments to evaluate the relative influence of multimodal mismatches about one's own body on the sense of embodiment and its physiological correlates. Importantly, although previous studies investigated the effect of either visuotactile incongruency or visuomotor incongruency on the sense of embodiment (e.g. Tsakiris et al., 2010, Kalckert and Ehrsson, 2012, Kalckert and Ehrsson, 2014), the systematic comparison of these and their contribution to disembodiment remains scarce. Yet, differential roles of motor and somatosensory signals in the sense of body have been suggested (Asai, 2015, Tsakiris et al., 2006, Tsakiris et al., 2010), and the role of actively (moving) in comparison to passively perceiving bodily signals to the bodily self has been extensively discussed (Grechuta et al., 2019, Pia et al., 2019). We thus compared the relative contribution of breaking visuomotor versus breaking visuotactile signals to disembodiment.
In Experiment 1 (Figure 1A), we manipulated visuotactile coherence, which is classically used to induce altered embodiment in rubber-hand-illusion-like paradigms. For two stimulation durations (1 and 3 min) the participant's hand was stroked with a paintbrush while the visual feedback, was either delayed (∼disembodiment illusion condition) or not (∼control condition). Alterations in embodiment, ownership, sensations of deafferentation, and related phenomenal sensations were measured using questionnaires adapted from Botvinick and Cohen, 1998, Kannape et al., 2019, Lenggenhager et al., 2007, and Longo et al. (2008). To further understand the phenomenal qualities, we used a psychometric approach by performing a principal component analysis (PCA) on the questionnaire data of a larger sample. Furthermore, previously suggested implicit correlates of embodiment, namely skin temperature (see Moseley et al., 2008, but see also de Haan et al., 2017 for a critical view) and skin conductance responses (SCR) to threat (see Armel and Ramachandran, 2003) were assessed. Heart rate variability (HRV) measures were added, as homeostatic processes have suggested to be altered in conditions of alteration in body ownership (Barnsley et al., 2011). A measure of interoceptive accuracy has been included, as poor accuracy has previously shown to be related to higher susceptibility to illusory ownership and thus a more plastic bodily self (Monti et al., 2019, Tsakiris et al., 2011; but for exceptions see Crucianelli et al., 2018 and David et al., 2014). We hypothesized that the sensory conflict between tactile and delayed visual feedback would result in a reduced sense of embodiment and enhanced sense of disembodiment, which would be reflected in both explicit (subjective) and implicit (physiological) measures, especially in participants with a weak interoceptive accuracy.
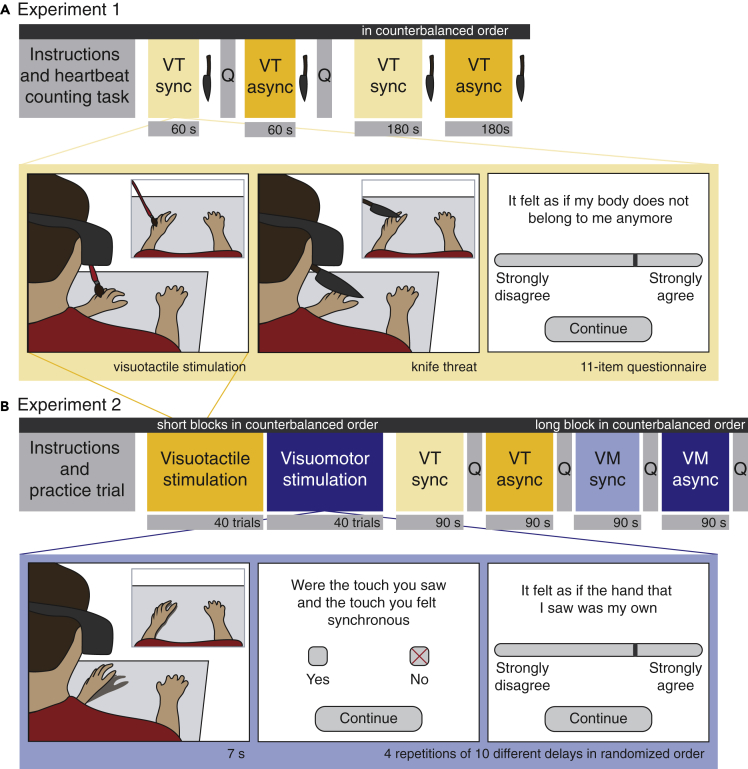
Figure 1.
Experimental Procedure
Experimental setup in (A) Experiment 1 and (B) Experiment 2. In both experiments participants were sitting down with both hands placed on a table. In Experiment 1, the visuotactile stimulation was either synchronous (VT sync) or asynchronous (VT async). Each stimulation was followed by a knife threat and in the 60 s blocks also by the embodiment questionnaire (Q). The 60 s blocks were always presented first, followed by the 180 s blocks. The order of VT sync and VT async was counterbalanced across participants. In Experiment 2 the visuotactile stimulation was similar to that of Experiment 1. Here, visuomotor or visuotactile stimulation were presented for 7 s during which the participant's hand was stroked two times. After each 7 s trial two questions appeared on the HMD. This was repeated 40 times for each modality, with four repetitions of 10 possible delay steps. Then, a long block followed with synchronous visuotactile (VT sync) and visuomotor (VM sync) as well as asynchronous visuotactile (VT async) and visuomotor (VM async) stimulation presented in counterbalanced order.
In Experiment 2 (Figure 1B), we investigated the temporal thresholds for detecting synchrony for visuomotor as compared to visuotactile delays and how different delays relate to the feeling of disembodiment. Participants were exposed to 40 trials in each condition with differing delays across a range of 139–733 ms, and after each trial synchrony perception and the feeling of ownership were assessed. These were followed by a block of longer stimulation of 90 s in both a visuomotor and visuotactile condition where the visual feedback was either delayed (∼disembodiment illusion condition) or not (∼control condition). Systematic empirical comparisons of these multimodal couplings remain rather scarce, with some studies suggesting similarly strong bodily illusions for visuomotor and visuotactile synchrony (Kalckert and Ehrsson, 2012, Kalckert and Ehrsson, 2014), others suggesting that visuomotor synchrony may be more important for illusory embodiment than visuotactile synchrony (Kokkinara and Slater, 2014; Roel Lesur et al., 2018), and some suggesting the relative importance of active movements versus passive touch for an integrated and global sense of body (Burin et al., 2015, Tsakiris et al., 2010, Tsakiris et al., 2006). Moreover, there is evidence suggesting that the temporal window of multisensory integration of peripheral signals is narrowed when followed by efferent signals as compared to only afferent signals (Zierul et al., 2019); however, these findings have not explicitly been linked to the sense of body.
Results
Results of Experiment 1
Principal Component Analysis Reveals Three Main Components of Disembodiment
A PCA was used to investigate the structure of participants' experience and to quantify the complex experience during this illusion. The PCA was conducted on the questionnaire data after synchronous or asynchronous visuotactile stimulation. Data from Experiment 1 (n = 30), Experiment 2 (n = 32), and additional data from an unpublished experiment of 15 participants were used for the PCA. After running a primary PCA to determine the number of components, a secondary PCA with three components was used for the analysis. The three components together explained 68% of the variance in the questionnaire data (see Table 1 for component loadings after varimax rotation and explained variance of each component). The first component was termed disownership and comprised items that refer to the experience of not belonging of the body, alienation, and perceiving the body as an image rather than an actual body (q4, q6, and q9). The second component was termed deafference (Longo et al., 2008) and included items related to the feeling of numbness, vividness, and disappearing of the body (q10, q8, and q7). The final component, embodiment, consisted of items related to the experience of own body ownership, agency, and looking at one's own body (q1, q11, and q5).
Table 1.
Factor Loadings from the PCA on Nine Items of the Questionnaire in the Asynchronous Visuotactile Condition
| Varimax Rotated Factor Loadings |
Commonalities | ||||
|---|---|---|---|---|---|
| Sometimes I Felt … | Component 1 Disownership |
Component 2 Deafference |
Component 3 Embodiment |
||
| q4 | Alienation from my body | .81 | .21 | .05 | .70 |
| q6 | As if my body does not belong to me anymore | .78 | .18 | .29 | .73 |
| q9 | The seen body as an image rather than as my actual body | .65 | .24 | -.04 | .50 |
| q10 | As if my body was numb | .12 | .81 | -.03 | .68 |
| q8 | As though the experience of my hand was less vivid than normal | .23 | .81 | -.14 | .73 |
| q7 | As though my body had disappeared | .25 | .78 | .30 | .75 |
| q1 | As if the body I saw was my own | .02 | .00 | .89 | .79 |
| q11 | As if I could move the seen body | .10 | .05 | .81 | .67 |
| q5 | As if I was looking at another person's body | .53 | -.05 | .57 | .61 |
| Eigenvalues | 2.11 | 2.06 | 1.98 | ||
| % Of variance | 23 | 23 | 22 | ||
Note. Factor loadings >.50 are in boldface.
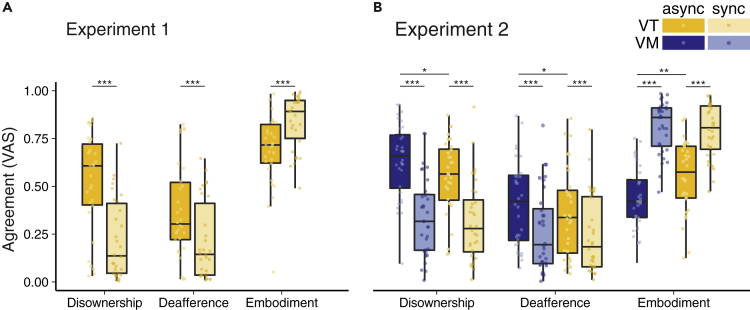
Responses to questionnaire items are in line with our hypotheses (Figure 2, see Table S2 for descriptive statistics and results of the comparisons for all individual items). Participants reported a relative increase of disownership, deafference (though generally low [<0.5] in both conditions), and a relative reduction of embodiment (though generally still high [>0.5] in both conditions) after asynchronous visuotactile stimulation compared with synchronous stimulation. As expected, responses to the control item (q3) did not differ between conditions, and the manipulation check item (q2) differed between the synchronous and asynchronous condition, which confirmed that participants were able to perceive the manipulation.
Figure 2.
Questionnaire Responses Clustered by the PCA Factors Questionnaire data, medians, and interquartile ranges are displayed.
The three components of the questionnaire differed significantly between the synchronous (sync) and asynchronous (async) visuotactile (VT) stimulation in Experiment 1 (A). In Experiment 2 (B) there were significant differences between the synchronous and asynchronous stimulation for both the visuotactile, and visuomotor (VM) stimulation, as well as between visuotactile and visuomotor stimulation in the asynchronous, but not the synchronous, condition. *p < .05, ** p < 0.01, ***p < 0.001.
Skin Conductance Response to Threat Is Not Altered
Previous studies demonstrated that SCRs to threats increased after synchronous stroking in rubber-hand–illusion-like paradigms (e.g. Armel and Ramachandran, 2003, Petkova and Ehrsson, 2008): a study showed reduced SCR to a threat after multisensory mismatching stimulation (Gentile et al., 2013), another a reduction of SCR after stimulating illusory dissapearance of the hand (Newport and Gilpin, 2011), and a similar pattern after asynchronous multimodal stimulation (Newport and Preston, 2010, Newport and Preston, 2011). As disownership was higher in the asynchronous condition, we hypothesized that SCRs would be reduced as compared to the synchronous condition. Even though we found an increase in skin conductance after threat, we did not observe significant differences between the synchronous and asynchronous condition in neither the short, (synchronous: Mdn = 1.13, IQR = 0.90–1.38; asynchronous: Mdn = 0.94, IQR = 0.71–1.16; Z = −1.42, p = .16) nor the long block (synchronous: Mdn = 1.01, IQR = 0.60–1.19; asynchronous Mdn = 0.94, IQR = 0.58–1.10; Z = −0.48, p = .63). This indicates that there was no evidence for a difference in the sympathetic activation in response to a threatening stimulus to the hand after asynchronous as compared to synchronous stimulation, even though participants subjectively experienced less embodiment and increased disownership over their own hand according to the questionnaire.
Skin Temperature Is Not Altered
Comparisons between synchronous and asynchronous conditions in the short block did not reveal any significant differences between conditions in temperature change for the neck (p = .76), right hand (p = .38), or left hand (p = .27). However, in the long block, there was a significantly smaller increase in skin temperature of the left hand across the trial in the asynchronous (Mdn = 0.038, IQR = −0.009–0.143) than synchronous condition (Mdn = 0.078, IQR = 0.012–0.158; Z = −2.09, p = .04, r = −.30). We further aimed to disentangle this small but significant effect for the left hand, by assessing differences between the conditions in each of the three minutes separately, but these analyses did not show any significant differences for any of these time periods (all ps> .48). There were no significant differences for the neck (p = .37) or the right hand (p = .72).
Heartrate Variability Is Not Altered
HRV, as quantified by the RMSSD, did not differ between the synchronous (Mdn = 30.62, IQR = 21.22–54.37) and the asynchronous condition (Mdn = 32.84, IQR = 24.61–45.30; Z = −0.25).
No Relation of Illusion Strength and Interoceptive Accuracy
Overall mean interoceptive accuracy was 0.62 ± 0.17, which is comparable to other studies (e.g. Garfinkel et al., 2015). We performed a median split on interoceptive accuracy scores to assess the differences in previously reported significant effects of synchrony between participants with high (Mdn = 0.77, IQR = 0.67–0.85) and low (Mdn = 0.46, IQR = 0.43–0.52) interoceptive accuracy. There was no significant difference between participants with high and low accuracy in the subjective strength of the illusion (difference between category average in synchronous and asynchronous) for the disownership (Z = −0.48, p = .63), deafference (Z = −0.33, p = .74), and embodiment (Z = −0.63, p = .53) category.
Summarized Results of Experiment 1
In this first experiment we showed that asynchronously shown stroking of one's own real hand using a video-based virtual reality setup leads, as predicted, to consistent and significant changes in the subjective sense of the bodily self as indicated by the responses to the questionnaire. According to the principal component analysis the response to this questionnaire can be clustered in three main components, namely disownership, deafference, and embodiment. During asynchronous as compared with synchronous stroking embodiment for one's own body is reduced, whereas the sense of disowernship and deafference is enhanced. In contrast to our prediction based on rubber-hand-illusion-like setups, despite these manipulations we did not evidence any physiological changes. There was no evidence for changes in the electrodermal response to threat, and the temperature measure only showed a mild trend toward a lesser increase in hand temperature in the asynchronous condition. Furthermore, we did not find the predicted relation between the individual strength of interoception and the subjective measures of the illusion.
Results of Experiment 2
Questionnaire Ratings Reveal Subjective Changes after Asynchronous Stimulation with a Stronger Effect of Visuomotor Than Visuotactile Signals
To assess the subjective experience of participants after 90 s of visuotactile or visuomotor stimulation, differences between responses to questionnaire items in the asynchronous and synchronous conditions were assessed (see Figure 3; and Tables S4 and S5 for descriptive statistics and results for each individual item). The results confirmed our hypothesis that asynchronous visuotactile and visuomotor stimulation induced a feeling of disownership for the seen body and followed a same pattern as in Experiment 1. There was a significant main effect of condition for the three illusion-related factors that were determined in the PCA (see section Principal Component Analysis Reveals Three Main Components of Disembodiment). Interestingly, the reduction of embodiment and increase in deafference and disownership were stronger in the asynchronous visuomotor than visuotactile condition. There were no significant differences between conditions for the control item (q3), and the differences in q2 confirmed that participants were able to perceive the manipulation.
Figure 3.
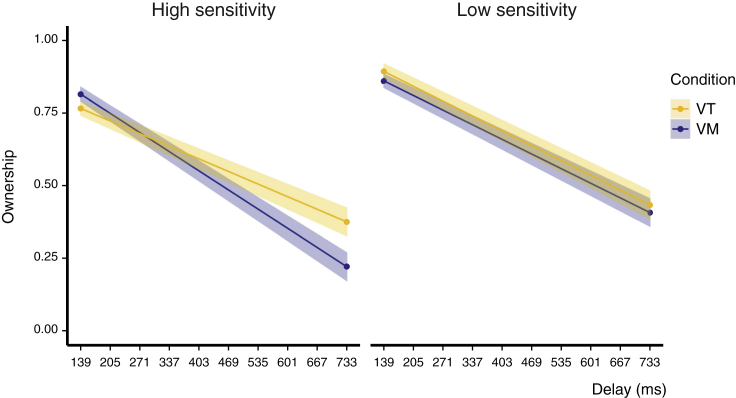
Predicted Ownership by Delay and Sensitivity to Delay
The three-way interaction of delay, modality (visuotactile [VT] and visuomotor [VM]), and sensitivity is displayed. Lines show predicted values from the model, where sensitivity was set to M − 1 SD for high sensitivity and M + 1 SD for low sensitivity for visualization purposes.
Synchrony Judgements Did Not Differ between Modalities
To assess whether sensitivity to delay was affected by modality of stimulation, we compared the PSE in the visuomotor (M = 0.338, SE = 0.015) and visuotactile condition (M = 0.327, SE = 0.014). There was no significant difference between the two conditions (Z =−.55, p = .58). Sensitivity was also not correlated with relative changes in any of the questionnaire components between the synchronous and asynchronous stimulation in both the visuotactile (all ps> .59) and visuomotor (all ps> .44) conditions. Crucially, responses to only 2.3% of all trials in the visuotactile and 0.8% in the visuomotor condition stated that the 0 ms delay was asynchronous, thus indicating that stimulation with the intrinsic delay was generally perceived as synchronous.
VAS Body Ownership Ratings Drop with Increasing Delay
To assess the influence of delay, modality, and delay perception on ownership, we fitted mixed models in a stepwise procedure. First, we fitted a model that included fixed effects for delay and condition and their interaction (see Table S6 for model coefficients). To explore whether sensitivity to delay for the different modalities, as quantified by the PSE, explained additional variance, we added the main effect and the two- and three-way interactions with delay and condition in a second model. The model fit of the PSE-model was better than that of the initial model (BIC model 1 = −1676.7, BIC PSE-model = −1709.5), and the PSE-model explained 32% of the variance in VAS ownership ratings (pseudo R2 = .32). Adding age as a predictor did not improve the model fit (BIC age-model: −1705.2) and was thus removed from the model. There was a significant three-way interaction of all predictors (delay × modality × PSE; b = 2.19, 95% CI: 1.15, 3.23, t(2213.7) = 4.13, p < .001; see Table S7 for all model coefficients). Overall, VAS ratings of ownership decreased with increasing delay. A stronger decrease in ownership was present especially in the visuomotor condition for participants with high sensitivity for delay. For lower sensitivity to delay there was no strong difference in the decrease of ownership between the visuotactile and visuomotor conditions (see Figure 3).
Summarized Results of Experiment 2
The results from the long stimulation in Experiment 2 show that visuomotor asynchrony when actively moving the hand in the same setup as in Experiment 1 also induces a decrease in embodiment coupled with an increase in disownership and sense of deafference. These changes were significantly stronger during visuomotor than during visuotactile mismatch. In line with this, the results from the short time exposure to various delays show that although increasing delay attenuates embodiment in both modalities, in participants with high delay sensitivity visuomotor delays affected embodiment already at smaller delays. Together these results might suggest a stronger contribution of visuomotor as compared to visuotactile synchrony in maintaining embodiment of the own hand or/and a heightened sensitivity to mismatch during active body movements as compared with passive touch. It should be noted, however, that the visuomotor task included a tactile component when participants put their hand on the table after each movement trajectory, in which case it may be the trimodal interaction that affects disembodiment more strongly.
Discussion
In two separate experiments and a PCA for a larger sample, we set out to assess how mismatching multimodal signals about one's own body alter the sense of embodiment in healthy participants. For this, the participant's hand was passively stroked or actively moved while their own body was seen from a first-person perspective on an HMD in a realistic video-based environment. The visual signals were either delayed (asynchronous; experimental condition) or presented simultaneously (synchronous; control condition; although including the system delay of ∼139 ms) compared to the bodily signals (i.e. tactile or motor related). We used a (dis)embodiment questionnaire as well as physiological measures that have previously reported to correlate with body ownership (Experiment 1) and a series of synchrony and embodiment judgements across different visuotactile and visuomotor delays (Experiment 2). The two studies revealed three main findings. First, both visuotactile and visuomotor mismatches led to increased disembodiment, which predominantly involved the feelings of disownership, deafference, and embodiment (PCA results Experiment 1 and Experiment 2). Second, visuomotor delay when actively moving the hand led to a stronger feeling of disembodiment than visuotactile delay during passive touch. In participants with high delay sensitivity this was also evidenced by a steeper decay of body ownership with increased delay for visuomotor signals (Exp 2). Third, implicit measures of body ownership such as SCR and skin temperature showed overall no evidence of being modulated by the illusion, except for a small difference in the hand temperature that should be taken with caution because it was only significant for the whole duration of the long-stimulation block and not for shorter periods within that block (Experiment 1).
Multimodal Temporal Mismatches from the Own Hand Alter the Bodily Self
Subjective changes in embodiment were measured with a questionnaire given to the participants after a stroking period. In line with previous studies (e.g. Gentile et al., 2013, Kannape et al., 2019) asynchronous stimulation generally reduced the feeling of embodiment, suggesting that synchronous multisensory inputs are crucial not only to induce embodiment over a fake body (e.g. Botvinick and Cohen, 1998) but also to maintain the sense of embodying one's own body. In a PCA based on the asynchronous visuotactile stroking, we identified three main factors of the subjective disembodiment experience. These are disownership corresponding to the experience of not belonging of the body, alienation, and perceiving the body as an image rather than as an actual body; deafference, which, in accordance to Longo et al. (2008) includes numbness and vividness, plus in our case disappearance of the own body; and embodiment, consisting of the experience of body ownership, agency, and the feeling of looking at one's own body. Our results show that both visuotactile and visuomotor mismatches lead to increased disownership, deafference, and decreased embodiment respectively, when compared to synchronous stimulation.
In the case of synchronous stimulation, only two main factors were identified in the PCA, namely embodiment and disownership, together accounting for 71% of the variance (see Table S3). These results exclude the deafference component found for asynchronous stimulation. Although this might be expected because our bodily experience is not generally accompanied by a sense of deafference, it should be noted that asynchronous signals led not only to a disruption of the components found for synchronous signals but also to a new phenomenal component (see Longo et al., 2008 for similar results using a rubber hand illusion). This suggests that disembodiment does not only vary along the dimensions of embodiment and disownership, but also includes a sense of deafference.
As mentioned in the Introduction, the direct study of disembodiment in contrast to embodiment of a fake limb is not trivial, as important conceptual (e.g. de Vignemont, 2011, Folegatti et al., 2009) and neuroanatomical (Martinaud et al., 2017, Zeller et al., 2011) differences between these two mechanisms have been suggested. Furthermore, there is only indirect, sparse, and non-conclusive evidence of embodiment of a fake limb altering disembodiment (de Vignemont, 2011, Folegatti et al., 2009). Thus the currently most common way to study disembodiment, namely in RHI-like paradigms (Barnsley et al., 2011, Longo et al., 2008, Moseley et al., 2008), is problematic, as it (1) does not necessarily apply to some disturbances in body ownership and (2) may not actually induce the phenomena of interest. In this sense, the direct stimulation of disembodiment by altering own-body related signals may be important and more ecologically valid than using fake limbs. Several experimental setups for stimulating disembodiment on the own body exist, using mirror-based (e.g. McCabe et al., 2005), MIRAGE (e.g. Newport et al., 2010, Newport and Gilpin, 2011, Newport and Preston, 2011), infrared camera feed (Kannape et al., 2019), or pre-recorded setups (Gentile et al., 2013), yet we add to this palette the capacity to show the full body and the natural environment from a first-person perspective and alter it in real time (see Stanton et al., 2018 for a similar setup for manipulating non-temporal aspects of the body).
The Effect of Visuomotor as Compared with Visuotactile Mismatch on the Phenomenology of Disembodiment
Our questionnaire data from Experiment 2 replicated and extended the findings of Experiment 1 by showing that both asynchronous visuotactile signals as well as asynchronous visuomotor signals lead to increased disembodiment. Moreover, prolonged asynchronous visuomotor signals had a stronger effect on disembodiment compared with that of visuotactile signals. Although previous studies using foreign bodies or body parts have suggested that the tolerance for asynchronous visuotactile versus visuomotor stimulation during embodiment might differ (Kalckert and Ehrsson, 2012; Kokkinara and Slater, 2014; Roel Lesur et al., 2018; Tsakiris et al., 2006) and the specific contribution of actively moving on the bodily self has been intensively discussed (Grechuta et al., 2019, Pia et al., 2019), we here compared the relative contribution of these couplings by directly manipulating signals explicitly related to the own body. Although it is known that in the clinical population both alterations in the sensory and motor systems might correlate with feelings of disembodiment, our results suggest that there may be a stronger contribution of the latter to disembodiment. On these lines, for example the rubber hand illusion has been related to activity in the premotor cortex (Ehrsson et al., 2005, Ehrsson et al., 2004); and in clinical cases Burin et al. (2015) found that participants with left upper-limb hemiplegia experienced a greater rubber hand illusion in their affected hand when compared with both their unaffected hand and a control group, arguing that the reduction of efferent signals in these participants contributed to weakening their own body ownership, resulting in a more plastic sense of body. Our results further extend these findings showing that in healthy participants, breaking visuomotor synchrony facilitates the sense of disembodiment.
The data from the short trials of different delay steps might provide a more sensitive measure of the relation between small multimodal mismatches and its subjective interpretation and disembodiment. As hypothesized, the results generally showed better asynchrony detection and a decreased sense of ownership over one's own body with increased delay. This finding was true for both the tactile and the motor modality, and there were no significant differences in terms of perceived delay between multimodal couplings. This is surprising as previous literature suggested a greater delay sensitivity depending on the strength of efferent signals (Hoover and Harris, 2012; Lau et al., 2004, Winter et al., 2008; the latter however without a statistically significant difference). A possible reason for this difference to previous literature is that our protocol might not have had a high enough temporal resolution to assess small differences in synchrony judgments, as previous literature has found it varying between 22 ms (Hoover and Harris, 2012) and 29 ms (Winter et al., 2008). Moreover, theoretical models would suggest that with the presence of efferent signals, there would be a stronger expectation of afferent signals (Wolpert, 1997), thus affecting the perception of the afferent stimuli.
High sensitivity to delay, however, predicted overall lower ownership ratings and especially in the visuomotor condition a faster decay. Although previous studies have shown that greater temporal binding windows (TBW) of multisensory integration increase susceptibility to illusory embodiment of a rubber hand (Costantini et al., 2016), our results show that participants with high delay sensitivity have an overall stronger tendency to lose body ownership with increased delay between visuotactile or visuomotor signals than participants with lower sensitivity. A recent study found that the binding of incongruent multisensory signals in the ventriloquist effect (an effect where the location of an auditory stimulus is mapped to that of a visual stimulus: Pick et al., 1969, Talsma et al., 2010) drops after active movements (Zierul et al., 2019), i.e., incongruent signals are more easily bound when no efferent signals are involved. Zierul et al. (2019) expected, following a predictive coding account, that action would modulate the predictions and therefore bind incongruent stimuli more with action than without; however, their results showed the contrary. The authors thus hypothesize that action did form a stronger prediction, yet resulting mismatches were more salient and therefore multisensory incongruences were more evident. In our results, a similar explanation could be applied, i.e. expectations based on the motor-prediction were broken, whereas for the only visuotactile signals these expectations were not present. Moreover, in the visuomotor task, there is, next to matching between the motor command and the seen visual consequence, an additional mismatch of proprioceptive and visual signals that is not present during purely visuotactile tasks. This might explain the steeper decay of body ownership with increased delay. In this sense, the matching of motor predictions with their sensory consequences is important not only for the sense of agency but also for the maintenance of a healthy sense of ownership (perhaps even more than the temporal coherence of somatosensory signals).
Importantly, low sensitivity to delay did not differently influence ownership sensation in the visuomotor and visuotactile tasks but rather generally predicted higher ownership. This could suggest that the effect might be mediated by stronger visual dependence: participants with stronger visual dependence would not be so sensitive to incongruencies to other senses because they rely stronger on vision as compared to other senses (Witkin and Asch, 1948). Indeed visual dependence has shown to be correlated with susceptibility to various multisensory illusions (David et al., 2014, Rothacher et al., 2018). A stronger dependence on visual signals could thus explain why there was no difference in the decay of ownership for visuomotor and visuotactile tasks for participants with low delay sensitivity; however, we did not objectively assess such dependence.
No Evidence for Physiological Changes
The generally strong effect in the subjective measures of the illusion was not mirrored in the chosen implicit measures (skin temperature, SCR, HRV), where no evidence for, or only weak effects, were found. Only the temperature measure tentatively suggests a condition-specific effect by revealing a significantly smaller increase of temperature for asynchronous compared with synchronous stroking. This is in line with literature suggesting that a decrease in body temperature links to own-body disembodiment during illusory embodiment of a fake body (Moseley et al., 2008, Salomon et al., 2013; but see also de Haan et al., 2017) or in neurological damage (Moseley et al., 2008; but see also Lenggenhager et al., 2015). As in previous literature, such relatively lower temperature was in our data specifically found for the stimulated hand (Macauda et al., 2015) and only after longer stimulation (cp. Macauda et al., 2015, Moseley et al., 2008, both reporting a drop in temperature only after more than a minute of stimulation), which might be related to the adaptation time homeostatic processes might need. However, when comparing temperature for different time periods of the long stimulation block, we found no significant differences between time periods. Thus, these results should be taken with caution. Moreover, an increasing amount of literature doubts a meaningful relationship between body ownership and body temperature (de Haan et al., 2017).
SCR is an indicator of physiological reactions to threat (Armel and Ramachandran, 2003, Ehrsson, 2007). Previous studies have linked embodiment of an external body part to an SCR when such body part is threatened (Armel and Ramachandran, 2003, Ehrsson, 2007), a study has found a weaker SCR with decreased embodiment of the own body in a setup similar to ours (Gentile et al., 2013), and other studies using the MIRAGE illusion box for stimulating the hand found a significantly weaker SCR after illusory disappearance of the hand (Newport and Gilpin, 2011) and asynchronous multimodal stimulation (Newport and Preston, 2010, Newport and Preston, 2011). Following this, we hypothesized to find a weaker response in the asynchronous compared with the synchronous stimulation condition. However, such an effect was not evident in our data, with both conditions showing a response to threat. On the other hand, given that HRV has been suggested to be a measure of homeostatic processes (Berntson et al., 1997), we expected to find lower HRV during asynchronous stimulation due to a homeostatic disturbance, which was however not evidenced in our analysis.
So far, we can only speculate on the reasons for this lack of significant results in the chosen threat-related implicit measures. Although generally the relationship between explicit and implicit measures of embodiment manipulations has been questioned (de Haan et al., 2017, Rohde et al., 2011, Rohde et al., 2013), and in the case of HRV a recent study found no differences after altering embodiment in a full-body illusion (Park et al., 2016), it may be that the ecological congruency of the seen environment and body might have impeded an effect on our implicit measures. That is, in our setup participants are actually seeing their own hand and surroundings, with a higher degree of ecological plausibility compared with previous setups (e.g. Gentile et al., 2013). From an ecological point of view, it makes sense that participants would more readily extend the physiological reaction (protective space) to an external object than diminishing it. Additionally, although there is a significant increase of subjective disembodiment following the asynchronous stimulation, the degree of the embodiment component is still relatively high (>0.5 on the scale), which may account for the sustained physiological response. Alternatively, it may be that even if there is an increase of disembodiment of one's own body during asynchronous stimulation, it might be too fragile and that body perception may be immediately restored when attention is shifted away from the asynchronous stroking, regardless of limb-related multisensory synchrony. Along these lines it has been proposed a low degree of ownership does not necessarily result in disownership but that attention to the lack of ownership may (de Vignemont, 2011).
Lastly, although not directly linked to physiological changes but to conscious monitoring of physiological changes, high interoceptive accuracy has previously been related to lower malleability of the bodily self in the context of the rubber hand illusion paradigm (Monti et al., 2019, Tsakiris et al., 2011). We thus expected interoceptive accuracy as measured by a heartbeat counting task to predict the degree of disembodiment after asynchronous stimulation. Yet, interoceptive accuracy did not predict the strength of disembodiment in the current study. Our findings are in line with recent studies showing no relation between interoception and suggestibility to bodily illusions (Crucianelli et al., 2018, David et al., 2014).
General Considerations, Challenges, and Outlook
Setups involving the own body to manipulate embodiment in contrast to requiring supernumerary body parts (such as the rubber hand illusion) may be more directly related to the loss of ownership described in certain psychiatric and neurological conditions and may thus be important for understanding such disorders. The use of an HMD for showing and manipulating the full body viewed from a first-person perspective, and not exclusively a limb, may offer additional advantages. The protocol used in Experiment 2 allows for a sensitive assessment of the contribution of various multimodal mismatches to the loss of body ownership and can be expanded to measure the temporal thresholds in relation to body ownership for other multimodal couplings. In contrast to illusory supernumerary ownership, which has described to occur after 11 s in visuotactile rubber hand setups (Ehrsson et al., 2004), 22.8 s in active visuomotor (Kalckert and Ehrsson, 2017), 36 s in a visuotactile virtual hand setups (Perez-Marcos et al., 2012), and 20 s in MIRAGE mixed reality setups (e.g. Newport et al., 2010, Newport and Preston, 2011, Preston and Newport, 2011), our Experiment 2 shows that even after short periods of stimulation (7 s), it is possible to manipulate the sense of one's own body consistently and reliably cp. also (Kannape et al., 2019). Such a procedure can be sensitive for comparing individual differences as well as between different populations. Future studies comparing populations and multisensory mismatches are encouraged to shed light on the concept of bodily self plasticity. On these lines, a direct comparison between the rubber hand or a virtual hand illusion and our setup would offer important insights.
Although the plasticity of the bodily self has traditionally been measured as the susceptibility to illusory supernumerary embodiment, there is currently no consensus on whether higher delay sensitivity in terms of own-body embodiment is a result of a more or less plastic bodily self or vice-versa. Motivating the question of whether there is a “general body plasticity” or promptness to own-body disembodiment and to supernumerary embodiment may be separate components of such plasticity. Costantini et al. (2016) found that a small TBW leads to lower susceptibility to illusory embodiment of a rubber hand, whereas we found that small TBWs lead to higher susceptibility to own-body disembodiment. This may seem paradoxical, because the same condition (small TBW) leads to both lower susceptibility to supernumerary embodiment and higher to own-body disembodiment. Such a contrast may suggest the need of separate components of bodily self plasticity, say one for supernumerary embodiment and one for own-body disembodiment. This would follow neuroanatomical findings in patients with disorders of embodiment (Martinaud et al., 2017, Zeller et al., 2011). This differentiation could help to explain the different results in implicit measures between previous literature and our study. However, it could also be that more proneness to a disembodiment illusion is actually a result of a less plastic bodily self, thus a weaker susceptibility to supernumerary embodiment. In this scenario, participants with a highly plastic sense of body would still maintain embodiment even during stronger multimodal mismatches, adapting their bodily sense to the ongoing mismatching signals. Although our data are inconclusive regarding this point, we propose that this is an important debate in the field of bodily self consciousness, which in our view has not received enough attention. We hope to encourage future experimental inquiries that disentangle these questions; studies directly comparing our protocol with the rubber hand illusion may offer additional insights.
With the increasing availability of mixed reality technologies, and in particular with the growing availability of augmented reality, understanding how our sense of body may change through our interactions with a mediated view of reality and the temporal mismatches that this may entail is of great importance. Again, the study of bodily self consciousness would benefit of studying more on how seeing one's own body, instead of fake or virtual bodies, through digital visual manipulations affects embodiment. This is thus not only important at a theoretical and clinical level but may imply relevant practical knowledge for a near future where mixed reality technologies may be ubiquitous and thus constantly manipulate our sense of body. The study of disembodiment is relevant for various clinical conditions and has been studied rather indirectly in the general population. Our disembodiment protocol may be important for the scientific study of bodily self consciousness, both to induce a sense of disembodiment and as an assessment tool. In particular, it may be a useful method to measure the degree and sensory weighting of bodily self plasticity in the general as well as clinical populations. The results of the two experiments presented here extend the previous literature showing that mismatching multisensory signals contribute to increased disembodiment of one's own body as expressed by the phenomenal dimensions of disownership, deafference, and embodiment. Moreover, they provide evidence for the differential contribution of sensorimotor signals compared to somatosensory in maintaining our sense of body. Lastly, we promote a debate regarding the concept of bodily self plasticity, proposing either that it has two independent dimensions for supernumerary embodiment and for disembodiment respectively or that strong susceptibility to disembodiment is a reflection of low bodily self plasticity.
Limitations of the Study
Readers should note that in our visuomotor task, participants were instructed to start and end every movement trajectory with their hand on the table during the visuomotor task; therefore the procedure also involved touch. Along these lines, the presence of an acoustic metronome may be an additional source of multisensory binding. Future studies should aim at constraining to the modalities in question. The experimenter was not blinded to the condition when performing the threats in Experiment 1, and neither speed nor kinematics were controlled for. Lastly, our short trials of Experiment 2 do not allow us to disentangle whether the explicit judgment of synchrony may affect the participants' subsequent response regarding body ownership. Future studies should address this point.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
M.R.L., M.L.W., and B.L. were supported by the Swiss National Science Foundation (grant number: PP00P1_170511).
Author Contributions
M.R.L., M.L.W., and B.L. contributed to the experimental design and the writing of the manuscript. M.R.L., M.L.W., and C.S. collected the data. M.L.W. and C.S. performed the statistical analysis. O.A.K. contributed with a thorough revision of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100901.
Contributor Information
Marte Roel Lesur, Email: marteroel@gmail.com.
Bigna Lenggenhager, Email: bigna.lenggenhager@gmail.com.
Supplemental Information
References
- Aglioti S., Smania N., Manfredi M., Berlucchi G. Disownership of left hand and objects related to it in a patient with right brain damage. NeuroReport. 1996;8:293. doi: 10.1097/00001756-199612200-00058. [DOI] [PubMed] [Google Scholar]
- Armel K.C., Ramachandran V.S. Projecting sensations to external objects: evidence from skin conductance response. Proc. R. Soc. B: Biol. Sci. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T. Feedback control of one’s own action: self-other sensory attribution in motor control. Conscious.Cogn. 2015;38:118–129. doi: 10.1016/j.concog.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Barnsley N., McAuley J.H., Mohan R., Dey A., Thomas P., Moseley G.L. The rubber hand illusion increases histamine reactivity in the real arm. Curr.Biol. 2011;21:R945–R946. doi: 10.1016/j.cub.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Bigger J.T., Jr., Eckberg D.L., Grossman P., Kaufmann P.G., Malik M., Nagaraja H.N., Porges S.W., Saul J.P., Stone P.H., Van Der Molen M.W. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 2012;13:556–571. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- Blanke O., Slater M., Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88:145–166. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Blom R.M., Hennekam R.C., Denys D. Body integrity identity disorder. PLoSOne. 2012;7:e34702. doi: 10.1371/journal.pone.0034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Cohen J. Rubber hands “feel” touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Brugger P., Lenggenhager B. The bodily self and its disorders: neurological, psychological and social aspects. Curr.Opin.Neurol. 2014;27:644. doi: 10.1097/WCO.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Burin D., Livelli A., Garbarini F., Fossataro C., Folegatti A., Gindri P., Pia L. Are movements necessary for the sense of body ownership? Evidence from the rubber hand illusion in pure hemiplegic patients. PLoSOne. 2015;10:e0117155. doi: 10.1371/journal.pone.0117155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M., Robinson J., Migliorati D., Donno B., Ferri F., Northoff G. Temporal limits on rubber hand illusion reflect individuals’ temporal resolution in multisensory perception. Cognition. 2016;157:39–48. doi: 10.1016/j.cognition.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Krahé C., Jenkinson P.M., Fotopoulou A.K. Interoceptive ingredients of body ownership: affective touch and cardiac awareness in the rubber hand illusion. Cortex. 2018;104:180–192. doi: 10.1016/j.cortex.2017.04.018. [DOI] [PubMed] [Google Scholar]
- David N., Fiori F., Aglioti S.M. Susceptibility to the rubber hand illusion does not tell the whole body-awareness story. Cogn.AffectiveBehav.Neurosci. 2014;14:297–306. doi: 10.3758/s13415-013-0190-6. [DOI] [PubMed] [Google Scholar]
- Davidson P.W. Depersonalization phenomena in 214 adult psychiatric in-patients. Psychiatr. Q. 1966;40:702–722. [Google Scholar]
- de Haan A.M., van Stralen H.E., Smit M., Keizer A., Van der Stigchel S., Dijkerman H.C. No consistent cooling of the real hand in the rubber hand illusion. ActaPsychol. 2017;179:68–77. doi: 10.1016/j.actpsy.2017.07.003. [DOI] [PubMed] [Google Scholar]
- de Vignemont F. Embodiment, ownership and disownership. Conscious.Cogn. 2011;20:82–93. doi: 10.1016/j.concog.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Ehrsson H.H. The experimental induction of out-of-body experiences. Science. 2007;317:1048. doi: 10.1126/science.1142175. [DOI] [PubMed] [Google Scholar]
- Ehrsson H.H. The concept of body ownership and its relation to multisensory integration. In: Stein B.E., editor. The New Handbook of Multisensory Processes. MIT Press; 2012. pp. 775–792. [Google Scholar]
- Ehrsson H.H., Holmes N.P., Passingham R.E. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J. Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson H.H., Spence C., Passingham R.E. That’smy hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Folegatti A., Vignemont F., dePavani F., Rossetti Y., Farnè A. Losing one’s hand: visual-proprioceptive conflict affects touch perception. PLoSOne. 2009;4:e6920. doi: 10.1371/journal.pone.0006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S.N., Seth A.K., Barrett A.B., Suzuki K., Critchley H.D. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Gentile G., Guterstam A., Brozzoli C., Ehrsson H.H. Disintegration of multisensory signals from the real hand reduces default limb self-attribution: an fMRI study. J. Neurosci. 2013;33:13350–13366. doi: 10.1523/JNEUROSCI.1363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.T., Martin-Iverson M.T., Holmes N.P., Waters F.A. The projected hand illusion: component structure in a community sample and association with demographics, cognition, and psychotic-like experiences. Attention Percept. Psychophys. 2015;77:207–219. doi: 10.3758/s13414-014-0748-6. [DOI] [PubMed] [Google Scholar]
- Grechuta K., Ulysse L., Rubio Ballester B., Verschure P.F.M.J. Self beyond the body: action-driven and task-relevant purely distal cues modulate performance and body ownership. Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover A.E.N., Harris L.R. Detecting delay in visual feedback of an action as a monitor of self recognition. Exp. Brain Res. 2012;222:389–397. doi: 10.1007/s00221-012-3224-3. [DOI] [PubMed] [Google Scholar]
- Kalckert A., Ehrsson H.H. Moving a rubber hand that feels like your own: a dissociation of ownership and agency. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckert A., Ehrsson H.H. The moving rubber hand illusion revisited: comparing movements and visuotactile stimulation to induce illusory ownership. Conscious.Cogn. 2014;26:117–132. doi: 10.1016/j.concog.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Kalckert A., Ehrsson H.H. Theonset time of the ownership sensation in the moving rubber hand illusion. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannape O.A., Smith E.J.T., Moseley P., Roy M.P., Lenggenhager B. Experimentally induced limb-disownership in mixed reality. Neuropsychologia. 2019;124:161–170. doi: 10.1016/j.neuropsychologia.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Kilteni K., Maselli A., Kording K.P., Slater M. Over my fake body: body ownership illusions for studying the multisensory basis of own-body perception. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinara E., Slater M. Measuring the effects through time of the influence of visuomotor and visuotactile synchronous stimulation on a virtual body ownership illusion. Perception. 2014;43:43–58. doi: 10.1068/p7545. [DOI] [PubMed] [Google Scholar]
- Lane T., Yeh S.-L., Tseng P., Chang A.-Y. Timing disownership experiences in the rubber hand illusion. Cogn. Res. Princ.Implic. 2017;2:4. doi: 10.1186/s41235-016-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H.C., Rogers R.D., Haggard P., Passingham R.E. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B., Hilti L., Brugger P. Disturbed body integrity and the “rubber foot illusion.”. Neuropsychology. 2015;29:205–211. doi: 10.1037/neu0000143. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B., Tadi T., Metzinger T., Blanke O. Video ergo sum: manipulating bodily self-consciousness. Science. 2007;317:1096–1099. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Loetscher T., Regard M., Brugger P. Misoplegia: a review of the literature and a case without hemiplegia. J. Neurol. Neurosurg. Psychiatry. 2006;77:1099–1100. doi: 10.1136/jnnp.2005.087163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler A., Kleindienst N., Cackowski S., Schmidinger I., Bekrater-Bodmann R. Reductions in whole-body ownership in borderline personality disorder – a phenomenological manifestation of dissociation. J. Trauma Dissociation. 2019:1–14. doi: 10.1080/15299732.2019.1678213. [DOI] [PubMed] [Google Scholar]
- Longo M.R., Haggard P. Sense of agency primes manual motor responses. Perception. 2009;38:69–78. doi: 10.1068/p6045. [DOI] [PubMed] [Google Scholar]
- Longo M.R., Schüür F., Kammers M.P.M., Tsakiris M., Haggard P. What is embodiment? A psychometric approach. Cognition. 2008;107:978–998. doi: 10.1016/j.cognition.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Macauda G., Bertolini G., Palla A., Straumann D., Brugger P., Lenggenhager B. Binding body and self in visuo-vestibular conflicts. Eur. J. Neurosci. 2015;41:810–817. doi: 10.1111/ejn.12809. [DOI] [PubMed] [Google Scholar]
- Martinaud O., Besharati S., Jenkinson P.M., Fotopoulou A. Ownership illusions in patients with body delusions: different neural profiles of visual capture and disownership. Cortex. 2017;87:174–185. doi: 10.1016/j.cortex.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C.S., Haigh R.C., Halligan P.W., Blake D.R. Simulating sensory–motor incongruence in healthy volunteers: implications for a cortical model of pain. Rheumatology. 2005;44:509–516. doi: 10.1093/rheumatology/keh529. [DOI] [PubMed] [Google Scholar]
- Monti A., Porciello G., Tieri G., Aglioti S.M. “Embreathment” illusion reveals hierarchical influence of respiratory, visual and spatial signals on corporeal awareness. 2019. [DOI]
- Moseley G.L., Olthof N., Venema A., Don S., Wijers M., Gallace A., Spence C. Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proc. Natl. Acad. Sci. USA. 2008;105:13169–13173. doi: 10.1073/pnas.0803768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport R., Pearce R., Preston C. Fake hands in action: embodiment and control of supernumerary limbs. Exp. Brain Res. 2010;204:385–395. doi: 10.1007/s00221-009-2104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport R., Gilpin H.R. Multisensory disintegration and the disappearing hand trick. Curr.Biol. 2011;21:R804–R805. doi: 10.1016/j.cub.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Newport R., Preston C. Pulling the finger off disrupts agency, embodiment and peripersonal space. Perception. 2010;39:1296–1298. doi: 10.1068/p6742. [DOI] [PubMed] [Google Scholar]
- Newport R., Preston C. Disownership and disembodiment of the real limb without visuoproprioceptive mismatch. Cogn.Neurosci. 2011;2:179–185. doi: 10.1080/17588928.2011.565120. [DOI] [PubMed] [Google Scholar]
- Osumi M., Nobusako S., Zama T., Taniguchi M., Shimada S., Morioka S. Sensorimotor incongruence alters limb perception and movement. Hum. Mov. Sci. 2018;57:251–257. doi: 10.1016/j.humov.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Otsuru N., Hashizume A., Nakamura D., Endo Y., Inui K., Kakigi R., Yuge L. Sensory incongruence leading to hand disownership modulates somatosensory cortical processing. Cortex. 2014;58:1–8. doi: 10.1016/j.cortex.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Park H.-D., Bernasconi F., Bello-Ruiz J., Pfeiffer C., Salomon R., Blanke O. Transient modulations of neural responses to heartbeats covary with bodily self-consciousness. J. Neurosci. 2016;36:8453–8460. doi: 10.1523/JNEUROSCI.0311-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Marcos D., Sanchez-Vives M.V., Slater M. Is my hand connected to my body? The impact of body continuity and arm alignment on the virtual hand illusion. Cogn.Neurodyn. 2012;6:295–305. doi: 10.1007/s11571-011-9178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova V.I., Ehrsson H.H. If I were you: perceptual illusion of body swapping. PLoSOne. 2008;3:e3832. doi: 10.1371/journal.pone.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pia L., Garbarini F., Kalckert A., Wong H.Y. Editorial: owning a body + moving a body = me? Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick H.L., Warren D.H., Hay J.C. Sensory conflict in judgments of spatial direction. Percept.Psychophys. 1969;6:203–205. [Google Scholar]
- Preston C., Newport R. Differential effects of perceived hand location on the disruption of embodiment by apparent physical encroachment of the limb. Cogn.Neurosci. 2011;2:163–170. doi: 10.1080/17588928.2011.582944. [DOI] [PubMed] [Google Scholar]
- Roel Lesur M., Gaebler M., Bertrand P., Lenggenhager B. The plasticity of the bodily self: head movements in bodily illusions and their relation to gallagher’s body image and body schema. Constructivist Foundations. 2018;14:94–105. [Google Scholar]
- Rohde M., Luca M.D., Ernst M.O. The rubber hand illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoSOne. 2011;6:e21659. doi: 10.1371/journal.pone.0021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M., Wold A., Karnath H.-O., Ernst M.O. Thehuman touch: skin temperature during the rubber hand illusion in manual and automated stroking procedures. PLoSOne. 2013;8:e80688. doi: 10.1371/journal.pone.0080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothacher Y., Nguyen A., Lenggenhager B., Kunz A., Brugger P. Visual capture of gait during redirected walking. Sci. Rep. 2018;8:17974. doi: 10.1038/s41598-018-36035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Lim M., Pfeiffer C., Gassert R., Blanke O. Full body illusion is associated with widespread skin temperature reduction. Front. Behav.Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaqiri A., Roinishvili M., Kaliuzhna M., Favrod O., Chkonia E., Herzog M.H., Blanke O., Salomon R. Rethinking body ownership in schizophrenia: experimental and meta-analytical approaches show no evidence for deficits. Schizophr. Bull. 2018;44:643–652. doi: 10.1093/schbul/sbx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M., Baker D., Medford N., David A.S. Unpacking the depersonalization syndrome: an exploratory factor analysis on the Cambridge Depersonalization Scale. Psychol. Med. 2005;35:1523–1532. doi: 10.1017/S0033291705005325. [DOI] [PubMed] [Google Scholar]
- Slater M., Spanlang B., Sanchez-Vives M.V., Blanke O. First person experience of body transfer in virtual reality. PLoSOne. 2010;5:e10564. doi: 10.1371/journal.pone.0010564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M., van Stralen H.E., van den Munckhof B., Snijders T.J., Dijkerman H.C. The man who lost his body: suboptimal multisensory integration yields body awareness problems after a right temporoparietal brain tumour. J. Neuropsychol. 2018 doi: 10.1111/jnp.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T.R., Gilpin H.R., Edwards L., Moseley G.L., Newport R. Illusory resizing of the painful knee is analgesic in symptomatic knee osteoarthritis. PeerJ. 2018;6 doi: 10.7717/peerj.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D., Senkowski D., Soto-Faraco S., Woldorff M.G. The multifaceted interplay between attention and multisensory integration. Trends Cogn. Sci. 2010;14:400–410. doi: 10.1016/j.tics.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Nichols H.S., McIntosh L.G., Park S. Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoSOne. 2011;6:e27089. doi: 10.1371/journal.pone.0027089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M., Jiménez A.T., Costantini M. Just a heartbeat away from one’s body: interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. Lond. B: Biol. Sci. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M., Longo M.R., Haggard P. Having a body versus moving your body: neural signatures of agency and body-ownership. Neuropsychologia. 2010;48:2740–2749. doi: 10.1016/j.neuropsychologia.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Prabhu G., Haggard P. Having a body versus moving your body: how agency structures body-ownership. Conscious.Cogn. 2006;15:423–432. doi: 10.1016/j.concog.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Vallar G., Ronchi R. Somatoparaphrenia: a body delusion. A review of the neuropsychological literature. Exp. Brain Res. 2009;192:533–551. doi: 10.1007/s00221-008-1562-y. [DOI] [PubMed] [Google Scholar]
- van Stralen H.E., van Zandvoort M.J.E., Kappelle L.J., Dijkerman H.C. The rubber hand illusion in a patient with hand disownership. Perception. 2013;42:991–993. doi: 10.1068/p7583. [DOI] [PubMed] [Google Scholar]
- White R.C., AimolaDavies A.M. Asynchrony in the rubber hand paradigm: unexpected illusions following stroke. Cortex. 2017;93:224–226. doi: 10.1016/j.cortex.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Winter R., Harrar V., Gozdzik M., Harris L.R. The relative timing of active and passive touch. Brain Res. 2008;1242:54–58. doi: 10.1016/j.brainres.2008.06.090. [DOI] [PubMed] [Google Scholar]
- Witkin H.A., Asch S.E. Studies in space orientation. IV. Further experiments on perception of the upright with displaced visual fields. J. Exp. Psychol. 1948;38:762–782. doi: 10.1037/h0053671. [DOI] [PubMed] [Google Scholar]
- Wolpert D.M. Computational approaches to motor control. Trends Cogn. Sci. 1997;1:209–216. doi: 10.1016/S1364-6613(97)01070-X. [DOI] [PubMed] [Google Scholar]
- Zeller D., Gross C., Bartsch A., Johansen-Berg H., Classen J. Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: a lesion study. J. Neurosci. 2011;31:4852–4857. doi: 10.1523/JNEUROSCI.5154-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierul B., Tong J., Bruns P., Röder B. Reduced multisensory integration of self-initiated stimuli. Cognition. 2019;182:349–359. doi: 10.1016/j.cognition.2018.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.