Abstract
Although therapeutic hypothermia (TH) improves outcomes after neonatal encephalopathy (NE), the safety and efficacy of preemptive opioid sedation during cooling therapy is unclear. We performed a secondary analysis of the data from a large multicountry prospective observational study (Magnetic Resonance Biomarkers in Neonatal Encephalopathy [MARBLE]) to examine the association of preemptive morphine infusion during TH on brain injury and neurodevelopmental outcomes after NE. All recruited infants had 3.0 Tesla magnetic resonance imaging and spectroscopy at 1 week, and neurodevelopmental outcome assessments at 22 months. Of 223 babies recruited to the MARBLE study, the data on sedation were available from 169 babies with moderate (n = 150) or severe NE (n = 19). Although the baseline characteristics and admission status were similar, the babies who received morphine infusion (n = 141) were more hypotensive (49% vs. 25%, p = 0.02) and had a significantly longer hospital stay (12 days vs. 9 days, p = 0.009) than those who did not (n = 28). Basal ganglia/thalamic injury (score ≥1) and cortical injury (score ≥1) was seen in 34/141 (24%) and 37/141 (26%), respectively, of the morphine group and 4/28 (14%) and 3/28 (11%) of the nonmorphine group (p > 0.05). On regression modeling adjusted for potential confounders, preemptive morphine was not associated with mean (standard deviation [SD]) thalamic N-acetylaspartate (NAA) concentration (6.9 ± 0.9 vs. 6.5 ± 1.5; p = 0.97), and median (interquartile range) lactate/NAA peak area ratios (0.16 [0.12–0.21] vs. 0.13 [0.11–0.18]; p = 0.20) at 1 week, and mean (SD) Bayley-III composite motor (92 ± 23 vs. 94 ± 10; p = 0.98), language (89 ± 22 vs. 93 ± 8; p = 0.53), and cognitive scores (95 ± 21 vs. 99 ± 13; p = 0.56) at 22 months. Adverse neurodevelopmental outcome (adjusted for severity of encephalopathy) was seen in 26 (18%) of the morphine group, and none of the nonmorphine group (p = 0.11). Preemptive morphine sedation during TH does not offer any neuroprotective benefits and may be associated with increased hospital stay. Optimal sedation during induced hypothermia requires further evaluation in clinical trials.
Keywords: hypothermia, neonatal encephalopathy, sedation, MR spectroscopy
Background
Therapeutic hypothermia (TH) has become an established therapy for moderate-to-severe neonatal encephalopathy in high-income countries (National Institute for Health and Clinical Excellence, 2010), following evidence from several major cooling trials that a controlled and moderate reduction of core body temperature is associated with reduced brain injury and improved survival without long-term neurodisability (Gluckman et al., 2005; Shankaran et al., 2005; Azzopardi et al., 2009). However, the administration of opioid sedation during TH and its effect on patient outcomes remains controversial.
Although most cooling trials (Gluckman et al., 2005; Shankaran et al., 2005; Azzopardi et al., 2009), except one (Simbruner et al., 2010), did not mandate specified preemptive sedation, routine opioid sedation for reducing presumed discomfort during cooling has become a common practice worldwide. In a recent survey of tertiary cooling centers in the United Kingdom and United States, over 80% reported preemptive opioid sedation during cooling (Markati et al., 2018). Discomfort of cooling therapy and loss of hypothermic neuroprotection without sedation, were the most common reasons for opioid sedation (Markati et al., 2018).
Preclinical data on sedation during cooling are limited and conflicting. Thoresen and colleagues (Thoresen et al., 2001) conducted a study using a newborn piglet model for hypoxic injury, which demonstrated no significant reduction in neuropathology scores in piglets that were cooled for 24 hours compared with those that were not cooled. This was in contrast with earlier studies using the same model that had demonstrated significant neuroprotection. The authors hypothesized that this difference was due to the piglets in this study not receiving general anesthesia during the cooling period and thereby generating a stress response, which led to loss of neuroprotection. However, the clinical applicability of this study to human infants is unclear, in particular the differences in thermogenesis pathways, cooling duration, and target cooling temperature.
In contrast, Gunn and colleagues (1997) reported significant reductions in neuropathology scores in fetal lambs cooled for 72 hours compared with those that were not, without sedation or general anesthesia.
The secondary analysis of the National Institute of Child Health and Human Development (NICHD) whole-body cooling trial did not show any association of sedation with neurodevelopmental outcome in cooled babies (Natarajan et al., 2018). Worryingly, a combination of opioid sedation and anticonvulsants were associated with worse outcomes. Recent discontinuation of the Procedural Pain in Premature Infants trial due to the adverse effects of morphine has raised further concerns about opioid sedation in babies (Hartley et al., 2018).
This secondary analysis of the Magnetic Resonance Biomarkers in Neonatal Encephalopathy (MARBLE) study (Lally et al., 2018) compared the brain injury and neurodevelopmental outcomes of babies who received preemptive morphine sedation during TH with those who did not receive morphine. In a subgroup of babies, we examined the total cumulative dose of morphine during cooling therapy and its relationship with whole brain white matter fractional anisotropy.
Methods
MARBLE was a large, international, prospective, multicenter study involving 223 encephalopathic babies undergoing TH from eight centers in the United Kingdom and United States, between January 2013 and June 2016 (Lally et al., 2018). The North London Research Ethics Committee and clinical sites approved the study (11/H0717/6), and informed parental consent was obtained from parents or legal representatives of the infants. The secondary analysis reported in this study included only babies with moderate or severe encephalopathy as assessed by the NICHD neurological examination. During the cooling period, the use of preemptive morphine or other sedative agents was not mandated and if given, was done so at the discretion of the attending clinician.
All babies had magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) between 4 to 14 days after birth on a 3.0 Tesla scanner using carefully harmonized protocols. Briefly, this included three-dimensional T1-weighted imaging and two-dimensional axial T2-weighted imaging, thalamic single-voxel spectroscopy, and diffusion tensor imaging (Lally et al., 2018).
The MRS data were analyzed centrally by an MR physicist (P.J.L.; 7 years' experience in neonatal MRS) and the standard MRI data were reported, masked to clinical outcomes, by a consultant neonatologist (S.T.; 10 years' experience in neonatal MRI) using a ratified scoring system (Rutherford et al., 2010). We used an in-house MRS postprocessing software for quality assurance and then used LCModel (Provencher 1993) for analysis.
We examined the neurodevelopmental outcome between 20 to 24 months using Bayley Scales of Infant Development exam (Bayley-III, 3rd Edition), and detailed neurological examination. The neurodevelopmental assessments were carried out by trained clinicians blinded to the MRS data.
The primary outcome was death or neurodisability defined as either moderate [Bayley-III composite cognitive score 70–84 plus any of: Gross Motor Function Classification System (GMFCS; Palisano et al., 1997) 2, on-going seizures, or hearing impairment] or severe (Bayley-III composite cognitive score <70, significant hearing or visual impairments or a GMFCS level of 3–5). The secondary outcomes included metabolite peak area ratios and N-acetylaspartate (NAA) concentrations derived from thalamic proton MRS, and short-term clinical morbidity.
Initial analyses compared the characteristics of babies given and not given morphine. Categorical variables were compared between the groups using either the Chi-square test or Fisher's exact test. The unpaired t-test was used to compare continuous measures between groups, except for non-normally distributed variables, where the Mann–Whitney was preferred.
Subsequent analyses compared study outcomes between babies receiving and not receiving morphine. Continuous outcomes were analyzed using linear regression (with a log transformation if the values were positively skewed). Both unadjusted and adjusted analyses (accounting for possible confounders) were performed. Variables were included as possible confounders in the adjusted analyses if there was any suggestion that they varied between patients with and without morphine. As none of the babies in the nonmorphine group had an adverse outcome, the analysis was performed using exact logistic regression. In a subgroup of babies scanned on a single 3T Philips MR scanner, we examined the association of morphine dose with white matter fractional anisotropy using Tract-Based Spatial Statistics (TBSS; Smith et al., 2006), after adjusting for gestational age at the time of scanning, and the severity of encephalopathy at admission.
Results
Of 223 babies recruited to the MARBLE study, 183 had moderate or severe encephalopathy meeting the NICHD criteria for cooling therapy. Of these, 141 (76%) received morphine infusion for sedation, and 28 (15%) did not (Table 1). Data on sedation were not available in 14 (8%) babies.
Table 1.
Baseline Characteristics and Clinical Course
| Morphine sedation (n = 141) | No morphine sedation (n = 28) | p | |
|---|---|---|---|
| Birth weight g, mean (SD) | 3434 (569) | 3348 (522) | 0.4 |
| Gestation weeks, mean (SD) | 39.9 (1.5) | 39.7 (1.2) | 0.5 |
| Cord blood pH, mean (SD) | 6.9 (1.9) | 6.9 (1.9) | 0.6 |
| Cord blood base excess, mean (SD) | −14.7 (7.1) | −15.7 (6.2) | 0.5 |
| Apgar 10 minutes, median (IQR) | 5.3 (2.2) | 5.6 (2) | 0.61 |
| Encephalopathy stage at admission | |||
| Moderate, n (%) | 123 (87) | 27 (96) | 0.21 |
| Severe, n (%) | 18 (13) | 1 (4) | |
| Clinical course | |||
| PPHN, n (%) | 16 (12) | 0 (0) | 0.08 |
| Pulmonary bleed, n (%) | 3 (2) | 0 | 0.6 |
| Anticonvulsant use, n (%) | 79/128 (62) | 10/19 (53) | 0.45 |
| Hypotension, n (%) | 68 (49) | 7 (25) | 0.02 |
| Blood stream positive sepsis, n (%) | 10/132 (8) | 0/26 (0) | 0.37 |
| Hospital stay days, mean (SD) | 12 (7) | 9 (4) | 0.009 |
Hypotension, persistent mean blood pressure of <40 mmHg; IQR, interquartile range; PPHN, persistent pulmonary hypertension of the newborn (Severe hypoxemia disproportionate to the severity of lung disease and evidence of a right to left shunt); SD, standard deviation.
Although the baseline characteristics and admission status were similar, the babies who received morphine infusion were more hypotensive (49% vs. 25%, p = 0.02) and had a significantly longer hospital stay (12 days vs. 9 days, p = 0.009) (Table 1).
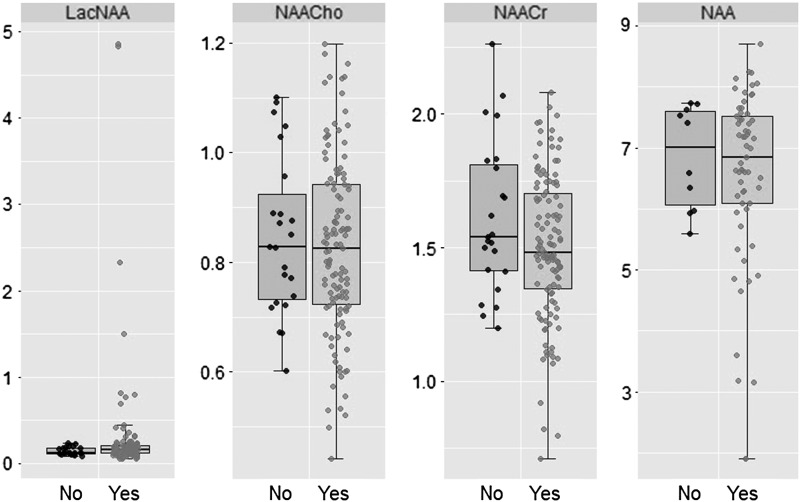
Basal ganglia/thalamic injury (score ≥1) and cortical injury (score ≥1) was seen in 34/141 (24%) and 37/141 (26%), respectively, of the morphine group and 4/28 (14%) and 3/28 (11%) of the nonmorphine group (p > 0.05). White matter injury (score ≥2) was seen in 46/141 (32%) of the morphine and 6/28 (21%) of the nonmorphine group (p > 0.05). Mean (standard deviation SD) thalamic NAA (6.5 ± 1.5 mmol/kg wet weight vs. 6.9 ± 0.9 mmol/kg wet weight) and median (interquartile range) lactate/NAA peak area ratio (0.16 [0.12–0.21] vs. 0.13 [0.11–0.18]) were also similar in both groups (p > 0.05) (Table 2; Fig. 1).
Table 2.
Association of Preemptive Morphine Sedation During Therapeutic Hypothermia with Magnetic Resonance Spectroscopy Biomarkers and Neurodevelopmental Outcomes
| Outcome | Sedation | n | Mean ± SD | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | p | Mean (95% CI) | p | ||||
| Bayley cognitive | No | 17 | 99.0 ± 12.5 | 0 | 0.43 | 0 | 0.71 |
| Yes | 115 | 94.8 ± 21.1 | −4.2 (−14.6 to 6.2) | −1.9 (−11.7 to 7.9) | |||
| Bayley language | No | 15 | 93.1 ± 8.0 | 0 | 0.44 | 0 | 0.68 |
| Yes | 111 | 88.6 ± 22.3 | −4.5 (−16.1 to 7.0) | −2.2 (−12.8 to 8.4) | |||
| Bayley motor | No | 17 | 93.6 ± 10.1 | 0 | 0.71 | 0 | 0.97 |
| Yes | 115 | 91.5 ± 22.5 | −2.0 (−13.0 to 8.9) | 0.2 (−9.9 to 10.2) | |||
| NAA | No | 10 | 6.85 ± 0.85 | 0 | 0.52 | 0 | 0.97 |
| Yes | 61 | 6.54 ± 1.49 | −0.31 (−1.28 to 0.65) | −0.02 (−0.91 to 0.95) | |||
| NAA/Cr | No | 23 | 1.61 ± 0.29 | 0 | 0.08 | 0 | 0.21 |
| Yes | 119 | 1.50 ± 0.27 | −0.11 (−0.23 to 0.01) | −0.08 (−0.21 to 0.05) | |||
| Outcome | Sedation | n | Median [IQR] | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|---|---|
| Ratio (95% CI) | p | Ratio (95% CI) | p | ||||
| Lac/NAA | No |
23 |
0.13 [0.11 to 0.18] |
1 |
0.09 |
1 |
0.20 |
| Yes | 119 | 0.16 [0.12 to 0.21] | 1.30 (0.96 to 1.76) | 1.22 (0.90 to 1.65) | |||
| Outcome | Sedation | n | n (%) | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||||
| Adverse neuro |
No |
17 |
0 (0) |
1 |
0.05 |
1 |
0.11 |
| Outcome | Yes | 125 | 26 (21) | 6.15 (0.99 to ∞) | 4.94 (0.72 to ∞) | ||
Adjusted for PPHN, hypotension, and encephalopathy severity score at admission.
CI, confidence interval; IQR, interquartile range; NAA, N-acetylaspartate; OR, odds ratio; PPHN, persistent pulmonary hypertension of the newborn.
FIG. 1.
Boxplot demonstrating no significant difference in magnetic resonance spectroscopy biomarker outcome measures between group receiving morphine and group not receiving morphine. NAA, N-acetylaspartate.
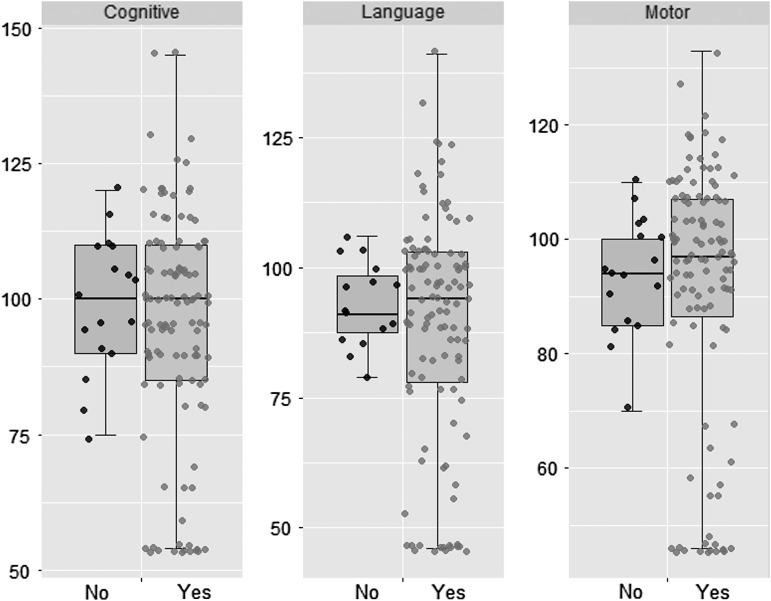
Neurodevelopmental outcomes at 22 months were available in 142 (83%) babies and the outcomes were compared between groups using exact logistic regression. Before adjusting for confounding variables, adverse outcomes (moderate/severe disability or death) occurred in 26 (18%) of the morphine group, and in none of the nonmorphine group (p = 0.05). These results were similar after exclusion of babies with severe encephalopathy. Further analyses adjusted for possible confounding variables. The adjusted results suggested some evidence that an adverse outcome remained higher in the morphine group, but the difference did not reach statistical significance (p = 0.11) (Table 2). Furthermore, the mean (SD) composite Bayley-III cognitive scores (95 ± 21 vs. 99 ± 13), language scores (89 ± 22 vs. 93 ± 8), and motor scores (92 ± 23 vs. 94 ± 10) were similar in the morphine and nonmorphine groups (Fig. 2).
FIG. 2.
Boxplot demonstrating no significant difference in neurodevelopmental outcome measures (Bayley III composite scores) between group receiving morphine and group not receiving morphine.
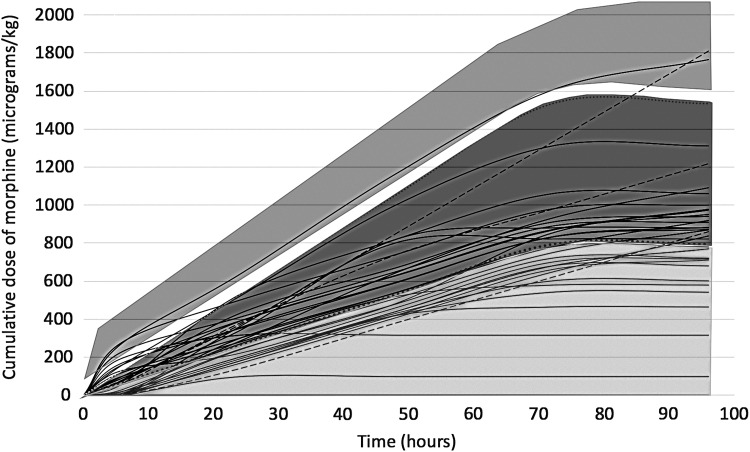
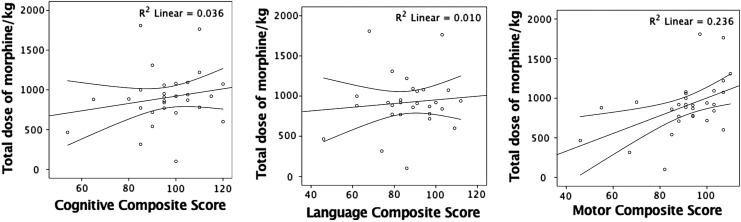
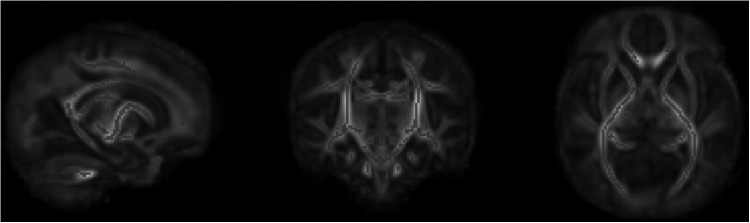
In the subgroup of 34 babies scanned on a single MR scanner, no relationship was observed between the morphine dose and white matter fractional anisotropy, adjusted for the severity for encephalopathy at admission. The total morphine dose given over the first 4 days after birth varied between 0 and 1.8 mg/kg, equating to a range of infusion rates between 0 to 25 μg/(kg·h) (Fig. 3). At 6 hours, 15 (44%) babies and at 24 hours, 20 (59%) babies were receiving a morphine infusion of 10–20 μg/(kg·h). Seven (20.6%) babies at 6 hours and 2 (6%) babies at 24 hours were receiving a morphine infusion rate >20 μg/(kg·h). No relationship was observed between the total morphine dose and any of the composite cognitive, language, or motor scores (Fig. 4), or white matter fractional anisotropy (Fig. 5).
FIG. 3.
Cumulative morphine dose during therapeutic hypothermia in babies with (dotted lines) and without (solid lines) persistent pulmonary hypertension. light gray shade on the bottom indicates cumulative dose at morphine infusion rate <10 μg/(kg·h), dark gray shade indicates infusion rates 10–20 μg/(kg·h) and medium gray shade on top indicates >20 μg/(kg·h) for the first 72 hours.
FIG. 4.
Relationship between total morphine dose and composite language, motor, and cognitive scores on Bayley III.
FIG. 5.
Linear regression of morphine dose with whole brain white matter fractional anisotropy using Tract-Based Spatial Statistics.
Discussion
In this secondary analysis of a large, prospective, multicenter observational study, preemptive morphine sedation during TH was associated with longer hospital stays, but not with brain injury on conventional MRI, proton MRS thalamic NAA and lactate/NAA peak area ratios, or neurodevelopment at 22 months. While data on morphine dosing were collected in only a subgroup of patients, it demonstrated that a large number (59%) of the infants were receiving relatively high doses (>10 μg/[kg·h]) at 24 hours. The total morphine dose was not associated with whole white matter fractional anisotropy on TBSS.
Pain management has long been a controversial aspect in neonatal medicine. In the 1950s and 1960s, a widespread belief was that neonates were incapable of perceiving pain (Merskey, 1970), leading to an unfortunate number of painful procedures, including surgeries, being performed without anesthesia or sedation until the 1980s (Lippmann et al., 1976). Gradually, it became clear that this belief was incorrect, and that neonates demonstrated clear stress responses during surgery through changes in their endocrinological and metabolic profiles (Anand et al., 1985).
This shift in our understanding of neonatal pain then led to the use of preemptive morphine and opioids routinely during ventilation of preterm neonates, with doses as high as 50–100 μg/(kg·h) in some studies (Quinn et al., 1992, 1993). However, the large NEOPAIN trial, in which ventilated preterm neonates were randomly assigned to either placebo or preemptive morphine infusions, there were significantly higher rates of severe intraventricular hemorrhage, death, and periventricular leukomalacia in the group who received open-label morphine compared with the placebo group (Anand et al., 2004). Thus, a later Cochrane review concluded there was insufficient evidence to recommend routine use of opioid sedation during ventilation in neonates (Bellù et al., 2008). Follow-up studies of those babies routinely sedated for ventilation have also demonstrated significant differences in morphometrics (i.e., smaller head circumference and body weight) and impaired behavioral/social development at school age in those that received preemptive morphine compared with those that did not (de Graaf et al., 2011; Ferguson et al., 2012).
A further difficult aspect to pain relief and pain management in neonates is the difficulty of reliably interpreting and classifying the background stress and discomfort during cooling. There are a variety of pain assessment tools available (Lawrence et al., 1993; Krechel and Bildner, 1995; Stevens et al., 2014), but they are generally limited to assessment of short-term painful stimuli (acute stress), such as a heel prick or venepuncture rather than for the assessment of more prolonged pain in neonates, that might be more analogous to the discomfort felt by cooled infants (Hall and Anand, 2014). The dissociation of cortical and behavioral pain responses in babies during high stress states add further complexity in quantifying nociception.
The question regarding preemptive morphine use in TH and its relationship with neuroprotection, as touched on above, stemmed from preclinical data (Thoresen et al., 2001). However, despite the widespread use of preemptive sedation during cooling and some supportive data on potential neuroprotective effects (Angeles et al., 2005), there have been concerns raised about opioid use. The effect of opioid use in normothermic term and preterm infants has previously been assessed both in animal models as well as in infants. Early studies showed that exposure to opioids in the postnatal period resulted in poor brain growth in rats (Seatriz and Hammer, 1993) and more recently, a study by Sabir and colleagues (2018) concluded that continuous infusions of high-dose fentanyl may lead to apoptosis in the internal granular cell layer of the cerebellum of healthy newborn pigs.
Given the growing concern for use of excessive opioids in normothermic, healthy newborns, there is a pressing need to assess their effects during TH. In adults, TH is used following cardiac arrest and, as standard, the patients often receive sedation and neuromuscular blockade. However, as discussed in systematic reviews, the exact medications used and the protocol in which they are deployed is widely variable (Chamorro et al., 2010). Adequate TH cannot be achieved in adults, children, or in newborn piglets without deep sedation and muscle relaxation to suppress shivering. However, newborn infants have nonshivering thermogenesis due to brown fat, and hence sedation and muscle relaxation should not be required to induce hypothermia.
Despite this, a recent survey of sedation practices of the U.K. cooling centers reported that (Markati et al., 2018) 88% used routine preemptive morphine during cooling therapy. Discomfort from cooling (81%) and presumed loss of neuroprotection without sedation (44%) were the commonest reasons given by clinicians for initiating preemptive morphine.
Renal and hepatic dysfunction is common following cardiac arrest in adults, and is similarly seen following hypoxic ischemic encephalopathy in infants (Shankaran et al., 2008). This could lead to prolonged or delayed clearance of opioids (Tortorici et al., 2007; Arpino and Greer, 2008). In adult studies it has also been shown that hypothermia itself leads to delayed breakdown and metabolism of drugs, thus ultimately changing the drugs' pharmacokinetics. Indeed, this effect has been borne out in a study showing slower rates of morphine clearance in babies undergoing TH compared with those treated with normothermia (Roka et al., 2008; Frymoyer et al., 2017). These studies describe possible toxicity in cooled infants at infusions greater than 10 μg/(kg·h).
Furthermore, sedative agents such as opioids are likely to interfere with accurate neurological assessments and may affect interpretation of amplitude-integrated electroencephalography readings. This, in turn, may cloud the overall clinical picture and delay prognostication (Natarajan et al., 2018). Sedation is also likely to prolong mechanical ventilation and cause hemodynamic compromise (Wassink et al., 2015).
Finally, a recent qualitative study (Craig et al., 2018) has also demonstrated that parents' perceptions of the use of opioids in TH are overall negative. Opioids were often given when health care professionals perceived the infant to be in discomfort, but many parents felt the assessment of the infant's discomfort was variable and differing from their own perception of their infant. Parents frequently associated morphine use with death or dying, following previous experiences of other loved ones in end-of-life care. Given the growing evidence that integrated family delivered care has positive effects on outcomes in babies treated on neonatal intensive care units (O'Brien et al., 2018), the perception and participation of parents is an important aspect to consider when treating with opioids.
Although our study was a large, multicenter study with carefully harmonized MR and outcome assessments, there are several limitations. First, this was not a randomized controlled trial, and it is possible that the babies who received morphine were sicker than those who did not. However, the results adjusted for the severity of encephalopathy suggested some evidence that an adverse outcome still remained higher in the morphine group, although any difference was not statistically significant (p = 0.11).
Second, we do not know the exact reason for preemptive sedation, and the morphine use was not standardized. It is likely that this was a clinical decision taken by the attending consultant based on the clinical needs, and a retrospective analysis may not have captured all the complex medical needs of the individual babies. Accurate assessments of the safety and efficacy of preemptive morphine can be assessed only in double-blind, placebo-controlled, randomized controlled trials.
Third, we collected the precise dose and duration of morphine only in a small subgroup of babies. We also did not have any data on pain or sedation scores of the babies, and hence are not able to judge if the opioid sedation was appropriate for individual babies. There was no requirement for explicit documentation of reasons behind starting morphine and this was left as a clinical decision to be made by the attending clinicians.
Finally, we did not have any data on the morphine blood levels. Renal dysfunction in conjunction with cooling therapy might have resulted in toxic morphine levels in some babies where the morphine infusion rates exceeded 10 μg/(kg·h) (Fig. 3), thus adversely affecting the outcome (Frymoyer et al., 2017).
Preemptive morphine sedation during TH is common clinical practice for preventing discomfort during cooling. Our data suggest that such a practice may prolong hospital stay without any additional neuroprotective benefit. Morphine levels should be routinely monitored in babies undergoing cooling therapy. Carefully designed clinical trials, including measurement of serum morphine levels, will help to elucidate the question of toxic accumulation of these drugs during TH. This will enable establishment of optimal sedation practices and supportive care during TH.
Acknowledgments
The authors thank Harvey Wilson, student, who assisted with statistical analysis. This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Center based at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Anand KJ, Brown MJ, Causon RC, Christofides ND, Bloom SR, Aynsley-Green A. Can the human neonate mount an endocrine and metabolic response to surgery?. J Pediatr Surg 1985;20:41–48 [DOI] [PubMed] [Google Scholar]
- Anand KJS, Whit Hall R, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA; NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363:1673–1682 [DOI] [PubMed] [Google Scholar]
- Angeles DM, Wycliffe N, Michelson D, Holshouser BA, Deming DD, Pearce WJ, Sowers LC, Ashwal S. Use of opioids in asphyxiated term neonates: effects on neuroimaging and clinical outcome. Pediatr Res 2005;57:873–878 [DOI] [PubMed] [Google Scholar]
- Arpino PA, Greer DM. Practical pharmacologic aspects of therapeutic hypothermia after cardiac arrest. Pharmacotherapy 2008;28:102–111 [DOI] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P; Toby Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–1358 [DOI] [PubMed] [Google Scholar]
- Bellù R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev 2008;Issue 1. Art. No.: CD004212. DOI: 10.1002/14651858.CD004212.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandín B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg 2010;110:1328–1335 [DOI] [PubMed] [Google Scholar]
- Craig AK, Gerwin R, Bainter J, Evans S, James C. Exploring parent expectations of neonatal therapeutic hypothermia. J Perinatol 2018;38:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf J, van Lingen RA, Simons SHP, Anand KJS, Duivenvoorden HJ, Weisglas-Kuperus N, Roofthooft DWE, Groot Jebbink LJM, Veenstra RR, Tibboel D, and van Dijk M. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain 2011;152:1391–1397 [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Ward WL, Paule MG, Whit Hall R, Anand KJS. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol 2012;34:47–55 [DOI] [PubMed] [Google Scholar]
- Frymoyer A, Bonifacio SL, Drover DR, Su F, Wustoff CJ, Van Meurs KP. Decreased morphine clearance in neonates with hypoxic ischemic encephalopathy receiving hypothermia. J Clin Pharmacol 2017;57:64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–670 [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997;99:248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RW, Anand KJS. Pain management in newborns. Clin Perinatol 2014;41:895–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley H, Moultrie F, Hoskin A, Green G, Monk V, Bell JL, King AR, Buckle M, Vaart M, Gursul D, Goksan S, Juszczak E, Norman JE, Rogers R, Patel C, Adams E, Slater R. Procedural Pain in Premature Infants (POPPI): a stopped blinded randomised placebo-controlled trial investigating the analgesic efficacy and safety of morphine. Lancet 2018;392:2595–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth 1995;5:53–61 [DOI] [PubMed] [Google Scholar]
- Lally PJ, Montaldo P, Oliveira V, Soe A, Swamy R, Bassett P, Mendoza J, Atreja G, Kariholu U, Pattnayak S, Sashikumar P, Harizaj H, Mitchell M, Ganesh V, Harigopal S, Dixon J, English P, Clarke P, Muthukumar P, Satodia P, Wayte S, Abernethy LJ, Yajamanyam K, Bainbridge A, Price D, Huertas A, Sharp DJ, Kalra V, Chawla S, Shankaran S, Thayyil S; MARBLE consortium. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol 2019;18:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw 1993;12:59–66 [PubMed] [Google Scholar]
- Lippmann M, Nelson RJ, Emmanouilides GC, Diskin J, Thibeault DW. Ligation of patent ductus arteriosus in premature infants. Br J Anaesth 1976;48:365–369 [DOI] [PubMed] [Google Scholar]
- Markati T, Montaldo P, Shankaran S, Thayyil S. Pre-emptive opioid sedation during therapeutic hypothermia for neonatal encephalopathy: a national survey. In: Presented at the Neonatal Society 2018 Autumn Meeting, London, 2018 [Google Scholar]
- Merske H. On the development of pain. Headache 1970;10:116–123 [DOI] [PubMed] [Google Scholar]
- Natarajan G, Shankaran S, Laptook A, McDonald SA, Pappas A, Hintz SR, Das A. Association between sedation-analgesia and neurodevelopment outcomes in neonatal hypoxic-ischemic encephalopathy. J Perinatol 2018;38:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury. 2010. Available at https://www.nice.org.uk/guidance/ipg347 (accessed February16, 2019)
- O'Brien K, Robson K, Bracht M, Cruz M, Lui K, Alvaro R, da Silva O, Monterrosa L, Narvey M, Ng E, Soraisham A, Ye XY, Mirea L, Tarnow-Mordi W, Lee SK; FICare Study Group, and FICare Parent Advisory Board. Effectiveness of Family Integrated Care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health 2018;2:245–254 [DOI] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–223 [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679 [DOI] [PubMed] [Google Scholar]
- Quinn MW, Otoo F, Rushforth JA, Dean HG, Puntis JWL, Wild J, Levene MI. Effect of morphine and pancuronium on the stress response in ventilated preterm infants. Early Hum Dev 1992;30:241–248 [DOI] [PubMed] [Google Scholar]
- Quinn MW, Wild J, Dean HG, Hartley R, Rushforth JA, Puntis JW, Levene MI. Randomised double-blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre-term babies. Lancet 1993;342:324–327 [DOI] [PubMed] [Google Scholar]
- Roka A, Melinda KT, Vasarhelyi B, Machay T, Azzopardi D, Szabo M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics 2008;121:e844–e849 [DOI] [PubMed] [Google Scholar]
- Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir H, Dingley J, Scull-Brown E, Chakkarapani E, Thoresen M. Fentanyl induces cerebellar internal granular cell layer apoptosis in healthy newborn pigs. Front Neurol 2018;9:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seatriz JV, Hammer RP. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull 1993;30:523–527 [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Health National Institute of Child, and Network Human Development Neonatal Research. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–1584 [DOI] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, Laptook AR, MacDonald SA, Ehrenkranz RA, Tyson JE, Walsh M, Goldberg RN, Higgins RD, Das A; NICHD Neonatal Research Network. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics 2008;122:e791–e798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 2010;126:e771–e778 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, Taddio A. The Premature Infant Pain Profile-Revised (PIPP-R). Clin J Pain 2014;30:238–243 [DOI] [PubMed] [Google Scholar]
- Thoresen M, Satas S, Løberg EM, Whitelaw A, Acolet D, Lindgren C, Penrice J, Robertson N, Haug E, Steen PA. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res 2001;50:405–411 [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 2007;35:2196–2204 [DOI] [PubMed] [Google Scholar]
- Wassink G, Lear CA, Gunn KC, Dean JM, Bennet L, Gunn AJ. Analgesics, sedatives, anticonvulsant drugs, and the cooled brain. Semin Fetal Neonatal Med 2015;20:109–114 [DOI] [PubMed] [Google Scholar]