Abstract
Objective: High-intensity laser therapy (HILT) combined with photobiomodulation therapy (PBMT) using a diode or CO2 laser was administered after extraction of the left first molar in rats. Effects on socket preservation (preservation of the alveolar bone and healing time after extraction) were evaluated histopathologically.
Background: Irradiation using a diode or CO2 laser has been shown to hasten wound healing, but the effects remain controversial.
Methods: Five-week-old male Wistar rats that underwent extraction of the left maxillary first molar were divided into three groups: diode laser irradiation (diode group), CO2 laser irradiation (CO2 group), and no laser irradiation (control group). HILT (27 J) was performed immediately after tooth extraction to enhance blood coagulation, followed by PBMT (0.7 J) 1 day later to enhance healing. Tissues, including the extraction socket, were removed en bloc 3, 5, 7, 10, and 21 days postextraction to determine the morphological characteristics of wound healing and the distribution of myofibroblasts involved in scar formation.
Results: In the diode and CO2 groups, new bone formation and cancellous bone maturation were observed at an early stage of wound healing. The number of myofibroblasts was significantly lower in the laser treatment groups than the control (p < 0.001), and both treatment groups had a significantly higher alveolar crest height (p < 0.01), with almost no concavity in the mucosa of the extraction wound.
Conclusions: Combined HILT and PBMT following tooth extraction hastened wound healing and preserved alveolar crest height, suggesting a role in socket preservation.
Keywords: socket preservation, tooth extraction, enhanced healing process, diode laser, carbon dioxide laser, myofibroblast, scar tissue
Introduction
Loss of alveolar bone height can often be caused by preexisting periodontal disease. For example, smoking exacerbates loss of attached gingiva, gingival recession, and loss of alveolar bone height due to vasoconstriction in periodontal tissue by nicotine.1–3 In addition to periodontal disease, there are various factors associated with loss of alveolar bone height such as tooth impaction or the presence of supernumerary tooth,4 oral injury, oral disease, and oral surgery for tumors of the jaw.5 Another such factor is tooth extraction. Although tooth extraction is one of the most frequent procedures in dental practice, including for pre-prosthetic preparation, it has a high likelihood of causing loss of the alveolar crest height.
Previous studies on changes in alveolar bone after tooth extraction have indicated that most of the decrease in alveolar bone height occurs within 3 months after extraction.6,7 In addition, Schropp et al. have reported an average decrease of 3–5 mm in the bone width of the alveolar ridge within 6 months after tooth extraction, of which 80% occurred within 3 months after extraction, and they found an average decrease of 50% within 12 months after extraction.8 Furthermore, Huebsch et al. have reported that the difference in the position of blood clot formation in the extraction socket affects the height of new bone formation.9
With the hemostatic methods typically used after tooth extraction (i.e., compression and suturing), blood is not retained in the extraction socket, leading to considerable outflow of blood and eventual loss of alveolar bone height. In this way, worsened condition of alveolar bone leads to difficulty in occlusion and long-term maintenance of prosthetic function. Thus, preserving alveolar crest height, together with rapid wound healing after tooth extraction, is essential. This is the concept behind socket preservation.
Currently, autologous bone grafting,10 using a bone filler or a collagen sponge,11 is performed for socket preservation. This procedure effectively preserves the blood clot in the extraction socket and offers coverage with osteoanagenesis-inducing substances. However, favorable outcomes are not necessarily achieved, probably due to infected granulation tissue and chronic residual inflammation in the socket, infection after bone or artificial bone grafting, and incomplete substitution of bone with synthetic material.12,13 Thus, thorough curettage of the socket, sufficient bleeding from the socket wall, and good preservation of the blood clot are essential. Laser irradiation to hasten wound healing and achieve reliable retention of blood in the extraction socket is reported to minimize alveolar bone resorption in the clinical setting.14,15
The U.S. Food and Drug Administration (FDA) has guidelines for the use of lasers in dentistry, wherein the efficacy of laser irradiation after tooth extraction, namely, “coagulation of extraction sites,” is mentioned.16 Two types of lasers are recommended: diode and carbon dioxide (CO2) lasers. Several clinical studies have evaluated laser irradiation after tooth extraction, but its utility remains unclear because objective evaluation was not possible due to differences in underlying disease and periodontal conditions.
We have been studying changes in the wound healing process induced by high-intensity laser therapy (HILT) that protects the wound surface through coagulation,17,18 and photobiomodulation therapy (PBMT) that activates tissue19–22 using a CO2 laser,23 and confirmed the presence of numerous osteoclasts with rapid and specific new bone formation early in the socket wound healing process. In contrast, other groups have reported accelerated wound healing in the extraction socket and suppressed scar formation in granulation tissue after postextraction PBMT using a tissue transmission-type laser.24–30 However, the laser irradiation conditions used in these studies did not represent conditions used in the clinical setting, precluding evaluation of clinical efficacy of postextraction laser irradiation.
In this study, we used diode and CO2 lasers for HILT combined with PBMT after tooth extraction in rats, in a manner that mimics the clinical procedure, and performed histopathological examination of the extraction sockets to examine the morphology of healing in alveolar bone and the proliferation of myofibroblasts involved in early scar formation.
Materials and Methods
Five-week-old male Wistar rats (weight, 130–150 g) were used. Three rats were housed in one cage with free access to food (CLEA Rodent Diet CE-2; CLEA Japan, Inc.) and tap water. The room was maintained at 24 ± 2°C and 50 ± 5% humidity with 12-h light/12-h dark cycles. Rats were divided into three groups depending on postextraction treatment: a group treated with no laser irradiation (control group); a group treated with a combination of HILT and PBMT using a diode laser (diode group); and a group treated with a combination of HILT and PBMT using a CO2 laser (CO2 group). The term “laser treatment groups” is used to describe the diode group and the CO2 group collectively. Observation time points were days 3, 5, 7, 10, and 21 postextraction. A total of 90 rats, 6 at each observation time point, were examined in this study.
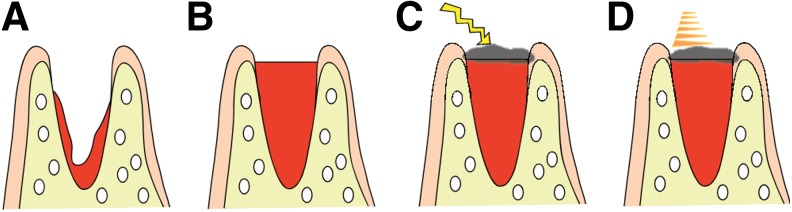
Rats were anesthetized by intraperitoneal administration of pentobarbital sodium and subjected to extraction of the left maxillary first molar using rat-use elevator and mosquito forceps. Extraction sockets were compressed with dry cotton balls to stop bleeding in the control group, while coagulation by HILT without compression hemostasis was performed in the laser treatment groups. The following day, the sockets were disinfected with the benzalkonium derivative Germitol® in all groups, and PBMT was performed in the laser treatment groups (Fig. 1).
FIG. 1.
Laser treatment protocol. (A) Extraction site immediately after tooth extraction. (B) Blood in the extraction socket was allowed to fill to the level of the surrounding gingiva. (C) The blood surface at the extraction socket was carbonized with high-intensity laser therapy, resulting in the formation of a carbonized layer. (D) The day after extraction, photobiomodulation therapy was performed to enhance healing.
Lasers used and detailed irradiation conditions are shown in Tables 1–3.
Table 1.
Laser Device Specifications
| Diode laser | CO2 laser | |
|---|---|---|
| Equipment model | iLase™ | PanalasCO5Σ |
| Manufacturer | Biolase Technology, Inc. | Panasonic Shikoku Electronics Cy., Ltd. |
| Geographical location | Irvine, CA | Osaka, Japan |
| Wavelength | 940 nm | 10,600 nm |
| Laser tip | Fiber tip with spot diameter of 0.4 mm | Taper 1A (transmittance 90%) (inner diameter of 1.5 mm) |
| Laser delivery system | Optical fiber | Articulated arm |
Table 2.
High-Intensity Laser Therapy Irradiation Conditions
| Diode laser | CO2 laser | |
|---|---|---|
| Power | 1.0 W | 1.0 W |
| Irradiation mode | Continuous wave | Continuous wave |
| Air | — | None |
| Irradiation time | 27 sec | 30 sec |
| Energy | 27 J | 27 J |
| Blood contact | Contact | Noncontact |
Table 3.
Photobiomodulation Therapy Irradiation Conditions
| Diode laser | CO2 laser | |
|---|---|---|
| Power | 0.3 W | 1.0 W |
| Irradiation mode | CP-1 mode | Σ modea |
| Air | — | None |
| Pulse width | 0.0001 sec | 0.0008 sec |
| Pulse interval | 0.0002 sec | 0.03 sec |
| Pulse frequency | 3333 Hz | 32.5 Hz |
| J/sec | 0.1 J/sec | 0.052 J/secb |
| Irradiation time | 7 sec | 15 sec |
| Energy | 0.7 J | 0.7 J |
| Blood contact | Slight contact | Slight contact |
Σ-mode uses an ultrashort pulse width to increase peak power during irradiation, thereby enabling photobiomodulation.
Calculated using the joule conversion table for PanalasCO5Σ.
Histopathological analysis
The extraction socket with the surrounding tissue was removed en bloc from each rat after a lethal dose of the anesthetic and fixed in 4% paraformaldehyde for 48 h. After 3-week decalcification in 10% ethylenediaminetetraacetic acid (EDTA) followed by dehydration using an alcohol gradient, tissue samples were embedded in paraffin. Consecutive sagittal sections (4 μm thick) were then prepared using a microtome and subjected to hematoxylin–eosin staining.
Morphometric analysis
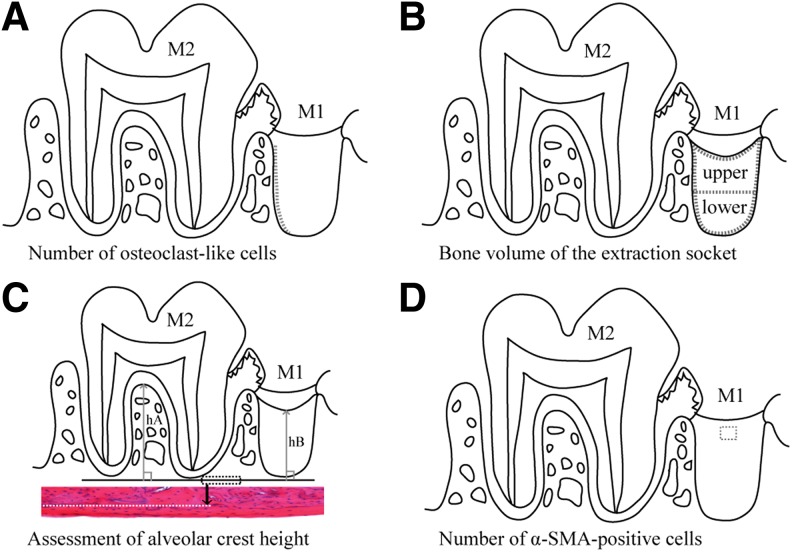
We counted the numbers of osteoclast-like cells in the extraction socket wall on day 3 postextraction, measured bone volume (BV) of extraction sockets on day 7 postextraction,25 and measured the height of the alveolar crest on day 21 postextraction,30,31 which is when the formation of trabeculae of alveolar bone was considered to be complete. The detailed measurement procedure is shown in Fig. 2A–C.
FIG. 2.
Morphological measurement methods. (A) Number of osteoclast-like cells. Length of the BS (dotted line) of the distal extraction socket wall was measured, and the number of the osteoclast-like cells on the surface was counted. The number of the cells per 1 mm on the surface of the extraction socket wall (N.Oc/BS) was then calculated on day 3 postextraction. (B) BV of the extraction socket. The extraction socket is divided into three parts (enclosed with dotted lines): the upper layer, the lower layer, and the whole (the upper+lower layers), and the ratio of the BV of new bone (cancellous bones) per TV in each area (BV/TV) was calculated on day 7 postextraction. (C) Assessment of alveolar crest height. Dotted rectangle shows the line of the inferior edge of the maxillary lamellar bone (reference line). H&E staining; original magnification × 100. The longest distance (hA) from the reference line perpendicular to the interradicular septum of the second molar (M2) and the shortest distance (hB) from the reference line to the surface layer of the new bone formed in the distal root socket of the extracted first molar (M1) were measured; hB/hA (mean ± standard deviation) was calculated as an index on day 21 postextraction. (D) Number of α-SMA-positive cells. α-SMA positive cells in a 150 × 150 μm square in the granulation tissue or the lamina propria in the surface layer of the socket (area within the dotted outline) of the first molar (M1) were counted on day 3 postextraction. BS, bone surface; BV, bone volume; H&E, hematoxylin–eosin; SMA, smooth muscle actin; TV, tissue volume.
Immunohistological observation and measurements
Proliferation of myoblasts involved in early scar formation on day 3 postextraction was examined using anti-human α-smooth muscle actin (SMA)-monoclonal antibody (Clone1A4 N1584; DAKO, Tokyo, Japan) as the primary antibody. Consecutive sagittal sections were incubated with a 1:50 dilution of primary antibody at room temperature, counterstained with Mayer's hematoxylin solution, and observed.
The detailed measurement procedure for counting the number of α-SMA-positive cells is shown in Fig. 2D. Images were scanned using a digital microscope (VZ-9000; Keyence Co., Ltd., Osaka, Japan) and analyzed using Scion-image software (Scion Corporation, Frederick, MD). A coauthor of this study performed blinded measurements. Results are shown as the mean ± standard deviation. Fisher's exact test (STATISTICA; StatSoft, Tulsa, OK) was used for statistical significance (p < 0.05).
The study protocol was in compliance with the Osaka Dental University Guidelines for Animal Experiments (approval no.: 18-01008).
Results
Histopathological and morphometric analyses
Day 3 postextraction
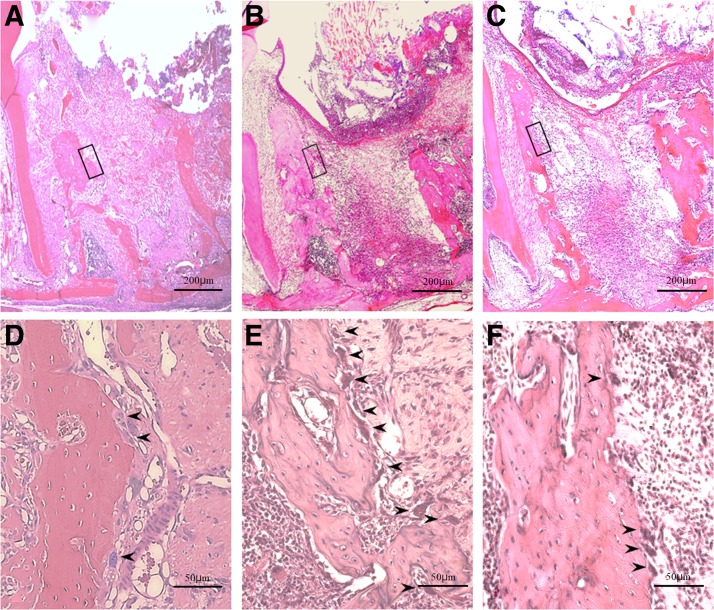
Sockets were almost completely filled with a blood clot in the control group, while the progression of organization from the socket walls was observed in the laser treatment groups (Fig. 4A–C). Organization from the socket fundus was also observed in the diode group. Magnified views of socket walls showed few osteoclast-like cells in the control group (8.737 ± 1.601) and the diode group (9.203 ± 1.336), but many osteoclast-like cells in the CO2 group (12.783 ± 2.901) (p < 0.01; Fig. 3A), and revealed findings indicative of active bone resorption in the CO2 group (Fig. 4D–F).
FIG. 4.
Histopathology on day 3 after tooth extraction. (A–C) Entire extraction socket, original magnification × 40. (D–F) Enlargement of areas indicated by the dotted line in (A–C), original magnification × 100. (A, D) Diode group; (B, E) CO2 group; (C, F) control group. Arrowheads (➤) indicate osteoclast-like cells. Progression of organization from socket surroundings is seen with blood clot only at the center in the laser treatment groups (A, B). In contrast, the socket is almost completely filled with a blood clot in the control group (C). Near absence of osteoclast-like cells with rapid organization in the diode group (D). Numerous osteoclast-like cells and active bone resorption in the CO2 group (E). Few osteoclast-like cells are seen in the control group (F).
FIG. 3.
Morphological measurement results. (A) Number of osteoclast-like cells. (B) BV in the upper layer of the extraction socket. (C) BV in the lower layer of the extraction socket. (D) Total BV of the extraction socket. (E) Assessment of alveolar crest height. (F) Number of α-SMA-positive cells (*p < 0.01; **p < 0.001).
Day 5 postextraction
New bone formation, which was not confirmed on day 3 postextraction, was seen in all three groups. New bone formation was apparent at only the socket fundus in the control group, but in the area from the socket fundus to the middle layer of the socket in the diode group, and at the socket fundus and the area from the middle layer to the shallow layers of the extraction socket in the CO2 group (Fig. 5A–C).
FIG. 5.
Histopathology on days 5 and 7 after tooth extraction. H&E staining; original magnification × 40. (A, D) Diode group; (B, E) CO2 group; (C, F) control group. Day 5 postextraction: new bone formation seen from the socket fundus in the diode group and the control group, with a lesser amount in the control group (A, C). In contrast, newly formed immature bone seen extending from the shallow to the middle layer of the extraction socket in the CO2 group (B). Day 7 postextraction: new bone formation seen from the fundus to the shallow layer of the extraction socket in the diode group and from the shallow to the middle layer with cross-linking pattern in the CO2 group. Denser cancellous bone is seen in the CO2 group than in the diode group (D, E). Cancellous bone formation in the extraction socket is immature, weak, and not continuous in the control group (F).
Day 7 postextraction
Simultaneous bone resorption by osteoclast-like cells and bone formation around the socket were seen in the control group, while few osteoclast-like cells were observed in the laser treatment groups. The amount of cancellous bone was lowest, and cancellous bone formation was immature in the control group. New bone formation was confirmed from the socket fundus to the shallow layer of the socket in the diode group and from the middle to the shallow layer with a cross-linking pattern in the CO2 group (Fig. 5D–F).
BV was significantly higher in the lower layer of the extraction socket in the diode group compared with the control group (0.414 ± 0.051 vs. 0.317 ± 0.054; p < 0.01), but was significantly higher in the upper layer of the extraction socket in the CO2 group (0.424 ± 0.056), which exhibited cross-linked bone formation, compared with the diode group (0.244 ± 0.041) and the control group (0.262 ± 0.051) (both p < 0.01). Furthermore, in the whole socket, BV showed a tendency to be lower in the control group (0.289 ± 0.050) than in both of the laser groups (diode group: 0.328 ± 0.024; CO2 group: 0.390 ± 0.050) (Fig. 3B–D).
Day 10 postextraction
Extraction sockets were filled with newly formed bone, and there were many cells in the bone marrow and in the area around cancellous bone in all three groups. However, the bone marrow area was wide, and the cancellous bone was immature in the control group (Fig. 6A–C).
FIG. 6.
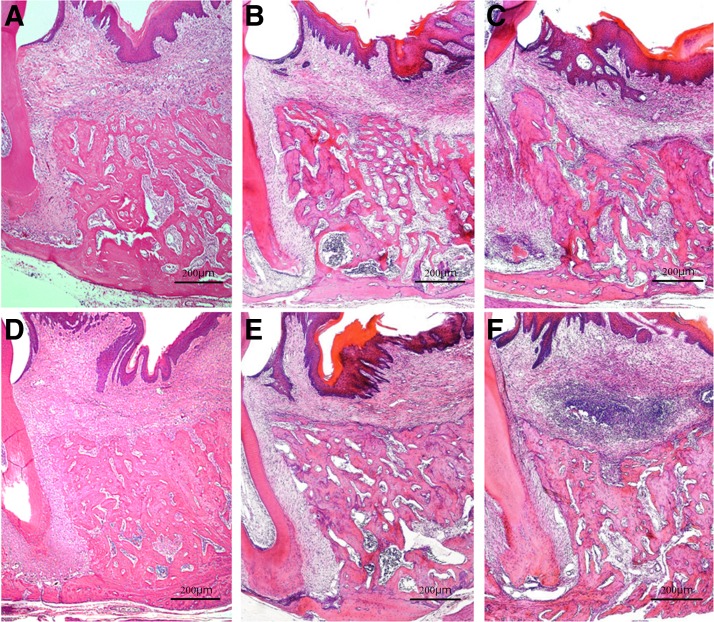
Histopathology on days 10 and 21 after tooth extraction. H&E staining; original magnification × 40. (A, D) Diode group; (B, E) CO2 group; (C, F) control group. Day 10 postextraction: extraction sockets are seen filled with newly formed bone. Numerous cells are observed in the bone marrow and the area around the cancellous bone in all groups (A–C). Day 21 postextraction: flat alveolar crest surface with almost no concavity seen in the laser treatment groups; cancellous bone is denser in the diode group than in the CO2 group (D, E). A dish-shaped concavity on the surface of the alveolar crest seen in the control group, and the less dense cancellous bone compared with that in the laser treatment groups (F).
Day 21 postextraction
Extraction sockets were filled with mature new bone with dense cancellous bone in all three groups. However, a concavity was noted at the center of the alveolar crest in the control group, while no concavity was seen in the laser treatment groups (Fig. 6D–F). The alveolar height index was significantly higher in the CO2 group (0.766 ± 0.039) and the diode group (0.798 ± 0.036) than in the control group (0.652 ± 0.079) (p < 0.01; Fig. 3E).
Regeneration of the mucosal epithelium in the extraction socket
We confirmed that a carbonized blood layer had formed on the extraction socket surface by applying HILT immediately after tooth extraction in the laser treatment groups. Regeneration of the mucosal epithelium was observed in all three groups at postextraction day 7. At postextraction day 21, there were no differences in histopathology findings of the mucosa among the three groups.
Immunohistological observation and measurement using α-SMA
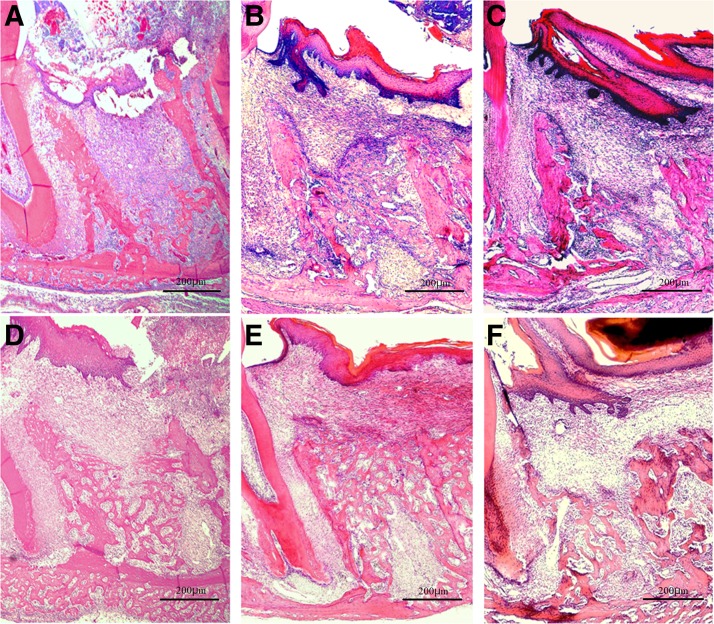
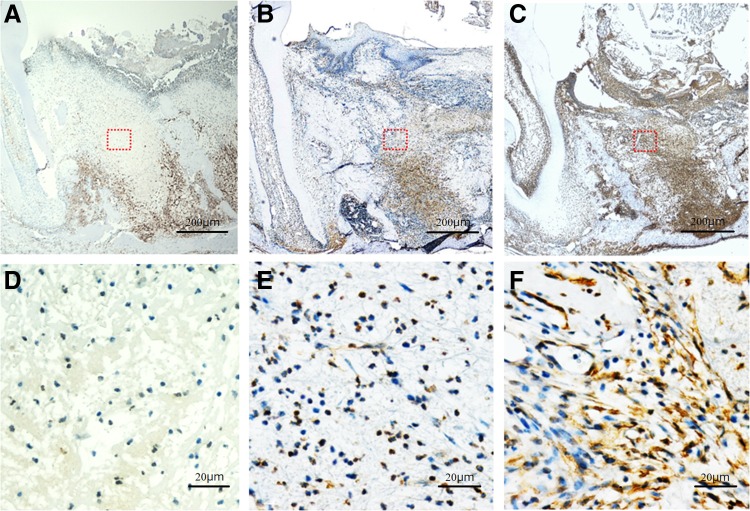
α-SMA-positive cells were distributed across the extraction socket and mucosal epithelia of the extraction wound in the control group and in the CO2 group, but the number of positive cells was significantly higher in the control group than in the CO2 group (29.389 ± 7.846 vs. 10.130 ± 5.019; p < 0.001). In contrast, in the diode group, few α-SMA-positive cells were seen across the surface layer and the deep layer of the mucosa of the extraction wound with a significantly lower positive cell count compared with the control group (3.361 ± 1.556 vs. 29.389 ± 7.846; p < 0.001) (Figs. 3F and 7).
FIG. 7.
Histopathology and anti-α-SMA immunostaining of the extraction socket on day 3 after tooth extraction. (A–C) Original magnification × 40. (D–F) Original magnification × 400. (A, D) Diode group; (B, E) CO2 group; (C, F) control group. α-SMA-positive cells are seen in the extraction socket, but are found around the extraction socket in the diode group (A, D). Numerous α-SMA-positive cells are seen across the extraction socket in the CO2 group and the control group, but are significantly fewer in the CO2 group than in the control group (B–F).
Discussion
The clinical efficacy of laser irradiation after tooth extraction was recently reported.14,15 Basic research showed that irradiation with a tissue transmission-type laser (typically a diode laser) resulted in considerable fibroblast proliferation and organization in the early stage of healing, enhanced new bone formation and cancellous bone maturation, and a significant increase in BV.24,28,29 In addition, several in vitro biochemical studies showed significantly increased expression of osteocalcin secreted by osteoblasts,25,26 as well as type I collagen.27 While in vitro studies showed increased alkaline phosphatase activity and type I collagen and osteopontin expression levels 72 h after CO2 laser irradiation of human osteoblast-like cells,32 TGF-β1, BMP-4, and BMP-7 expression levels were increased from 4 days after irradiation, followed by enhanced calcification.33
Meanwhile, results similar to those of extraction wound healing by PBMT have been reported. Mendes et al. applied hyaluronate sodium in the extraction sockets in rats. They found that active alveolar bone resorption was followed by marked new bone formation 7 days after extraction in the hyaluronate sodium-treated group; bone density was higher 21 days after extraction; and the boundary between existing alveolar bone and newly formed bone became unclear early.34 These results also indicate that PBMT enhances wound healing.
In this study, the HILT and PBMT conditions were set according to a study by Fukuoka et al.23 More precisely, the energy for HILT was calculated based on the time required for surface layer coagulation of the extraction socket in rats based on the output power and irradiation mode commonly used in clinical HILT. For PBMT, the rat gingiva was irradiated, and the output power, irradiation mode, and energy that did not cause irreversible changes were determined based on histopathological findings. The most significant point in this morphometrical analysis is that the two kinds of lasers differ in terms of characteristics recommended by the FDA.
Based on this difference between the lasers used, we measured the bone quantity of the extraction socket divided into three regions, namely, the upper part of the extraction socket which was expected to reflect the effect of the CO2 laser, the lower part of the socket which was expected to reflect the effect of the diode laser, and the entire socket. In addition, we selected day 7 postextraction for this measurement because the boundary between the extraction socket wall and newly formed bone became unclear with the healing of the socket, so conducting measurement became difficult on and after day 10 postextraction.
In the measurement of the height of alveolar bone, the rat maxillary first molar has five dental roots. Because damage to the interradicular septum during tooth extraction could cause measurement failure, our measurements were based on the relative evaluation of the height of the interradicular septum (hA) of the second molar and the surface layer of new bone (hB) in the extraction socket of the first molar (Fig. 2C). Fukuoka et al. and Noda et al. had almost the same opinions as ours about these measurement methods.23,25
Results of this study indicated an important role for carbonization of blood on the socket surface that resulted in the formation of artificial scab by HILT, in addition to the well-documented tissue activation by PBMT. This artificial scab enabled rapid physical closure of the extraction socket and clot retention high up in the socket with inhibition of mucosal epithelial invagination into the extraction socket, ultimately resulting in formation of new bone with good height (a space-making effect of the artificial scab in the extraction socket).
New bone formation in the extraction socket usually starts from the fundus, but a cross-linking pattern of new bone formation was observed in the shallow layer of the socket in the CO2 group. The penetration depth of CO2 laser light was shallow at 0.05 mm, and thus, the effect of photobioactive reactivity may be concentrated on the surface layer of the extraction socket. In contrast, the penetration depth of diode laser light is deep with an area of photobioactive reactivity, suggesting enhancement of new bone formation at the lower layer of the extraction socket.
The photobioactive reactivity caused by irradiation with these lasers was considered to promote the differentiation and proliferation of cells by stimulating undifferentiated mesenchymal stem cells, bone marrow stem cells, osteoblasts, osteoclasts, and bone lining cells, thereby activating bone remodeling.35,36 Subsequently, the stimulated osteoblasts produced collagen fibers and proteins associated with bone formation.37–39 In addition, the osteoblasts migrated on the fibers forming scaffolds for new bone formation. We therefore surmise that characteristic bone formation in the same area occurred in our study. These phenomena were similar to the finding that an artificial collagen sponge implanted in the extraction socket promotes the differentiation and proliferation of mesenchymal stem cells and provides a scaffold for bone formation.40,41
Recently, tissue activation by PBMT was shown to be strongly associated with mitochondrial adenosine tri-phosphate (ATP) production facilitated by cytochrome C oxidase, but the precise effects of the laser wavelengths used in the present study remain to be clarified.
Infection is a potential concern, but bacteria will be dispersed following HILT, and the carbonized blood on the surface of the extraction socket will prevent entry of bacteria and food debris. Accordingly, the risk of infection in the extraction socket below the carbonized blood is expected to be minimal.
In terms of expression of myofibroblasts in the extraction wound in this study, decreased myofibroblast counts were seen in only the surface layer of the extraction wound in the CO2 group, while few myofibroblasts were seen across the extraction wound in the diode group. This may also be explained by the difference in penetration depth between the two lasers. de Freitas et al. reported that the number of myofibroblasts proliferating in an incision wound on the back of the tongue was smaller when a CO2 laser was used than when a scalpel was used in rats.42 Pinheiro et al. also reported that the number of myofibroblasts in an incision wound on the back was increased when rats were irradiated with polarized light, but decreased with a diode laser.43 These are in good agreement with decreased myofibroblast number in the early wound healing stage shown in the present study.
The method examined in this study is promising for broad clinical application in humans, with potential use for nearly all patients who undergo tooth extraction. This method would be very effective for patients taking anticoagulants or antithrombotic agents or those prone to bleeding. In addition, for cases with limited blood supply from the extraction socket wall, it would be effective to soak artificial filling material (e.g., a collagen sponge) with blood, place it in the upper extraction socket, and char the surface layer. This is consistent with “coagulation of the extraction site” advocated by the FDA.
Conclusions
Combination of HILT and PBMT achieved good socket preservation, specifically, preservation of good alveolar crest height and enhanced wound healing in a rat model of tooth extraction. This method appears promising for clinical application in humans and warrants further investigation.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS-KAKENHI-Grant no. 15K11183).
References
- 1. Tatullo M, Gentile S, Paduano F, Santacroce L, Marrelli M. Crosstalk between oral and general health status in e-smokers. Medicine 2016;95:e5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Razali M, Palmer RM, Coward P, Wilson RF. A retrospective study of periodontal disease severity in smokers and non-smokers. Br Dent J 2005;198:495–498 [DOI] [PubMed] [Google Scholar]
- 3. Müller HP, Stadermann S, Heinecke A. Gingival recession in smokers and non-smokers with minimal periodontal disease. J Clin Periodontol 2002;29:129–136 [DOI] [PubMed] [Google Scholar]
- 4. Inchingolo F, Tatullo M, Abenavoli FM, et al. . Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: case report. Int J Med Sci 2010;7:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inchingolo F, Tatullo M, Abenavoli FM, et al. . Non-Hodgkin lymphoma affecting the tongue: unusual intra-oral location. Head Neck Oncol 2011;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent 1967;17:21–27 [DOI] [PubMed] [Google Scholar]
- 7. Lekovic V, Kenney EB, Weinlaender M, et al. . A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontal 1997;68:563–570 [DOI] [PubMed] [Google Scholar]
- 8. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent 2003;23:313–323 [PubMed] [Google Scholar]
- 9. Huebsch RF, Hansen LS. A histopathologic study of extraction wounds in dogs. Oral Surg Oral Med Oral Pathol 1969:28;187–196 [DOI] [PubMed] [Google Scholar]
- 10. Caplanis N, Lozada JL, Kan JY. Extraction defect assessment, classification, and management. J Calif Dent Assoc 2005;33:853–863 [PubMed] [Google Scholar]
- 11. Mannai C, Leake D, Pizzoferrato A, Ciapetti G, Sangiorgi C. Histologic evaluation of purified bovine tendon collagen sponge in tooth extraction sites in dogs. Oral Surg Oral Med Oral Pathol 1986;61:315–323 [DOI] [PubMed] [Google Scholar]
- 12. Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res 2002;13:103–111 [DOI] [PubMed] [Google Scholar]
- 13. Chan HL, Lin GH, Fu JH, Wang HL. Alterations in bone quality after socket preservation with grafting material: a systematic review. Int J Oral Maxillofac Implants 2013;28:710–720 [DOI] [PubMed] [Google Scholar]
- 14. Brawn PR, Kwong-Hing A. Histologic comparison of light emitting diode phototherapy-treated hydroxyapatite-grafted extraction sockets: a same-mouth case study. Implant Dent 2007;16:204–211 [DOI] [PubMed] [Google Scholar]
- 15. Cranska JP. Post-extraction laser hemostasis with immediate insertion of a bonded bridge. Dent Today 2008;27:108, 110 [PubMed] [Google Scholar]
- 16. Sulewski JG. Clearing the FDA hurdle, from initial device application through regulatory approval to the clinical operatory: an update on dental laser marketing clearances. J Laser Dent 2009;17:81–86 [Google Scholar]
- 17. Convissar RA. The top ten myths about CO2 lasers in dentistry. Dent Today 2009;28:68, 70,, 72–76 [PubMed] [Google Scholar]
- 18. Parker S. Lasers and soft tissue: ‘loose’ soft tissue surgery. Br Dent J 2007;202:185–191 [DOI] [PubMed] [Google Scholar]
- 19. Sharon-Buller A, Sela M. CO2-laser treatment of ulcerative lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:332–334 [DOI] [PubMed] [Google Scholar]
- 20. Saltmarche AE. Low level laser therapy for healing acute and chronic wounds-the extendicare experience. Int Wound J 2008;5:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jakse N, Payer M, Tangl S, Brgohold A, Kirmeier R, Lorenzoni M. Influence of low-level laser treatment on bone regeneration and osseointegration of dental implants following sinus augmentation. An experimental study on sheep. Clin Oral Implants Res 2007;18:517–524 [DOI] [PubMed] [Google Scholar]
- 22. Kuerová H, Dostálová T, Himmlova L, Bártobá J, Mazánek J. Low-level laser therapy after molar extraction. J Clin Laser Med Surg 2000;18:309–315 [PubMed] [Google Scholar]
- 23. Fukuoka H, Daigo Y, Enoki N, Taniguchi K, Sato H. Influence of carbon dioxide laser irradiation on the healing process of extraction sockets. Acta Odontol Scand 2011;69:33–40 [DOI] [PubMed] [Google Scholar]
- 24. Takeda Y. Irradiation effect of low-energy laser on alveolar bone after tooth extraction. Experimental study in rats. Int J Oral Maxillofac Surg 1988;17:388–391 [DOI] [PubMed] [Google Scholar]
- 25. Noda M, Aoki A, Mizutani K, et al. . High-frequency pulsed low-level diode laser therapy accelerates wound healing of tooth extraction socket: an in vivo study. Lasers Surg Med 2016; 48: 955–964 [DOI] [PubMed] [Google Scholar]
- 26. Mergoni G, Vescovi P, Sala R, et al. . The effect of laser therapy on the expression of osteocalcin and osteopontin after tooth extraction in rats treated with zoledronate and dexamethasone. Support Care Cancer 2016;24:807–813 [DOI] [PubMed] [Google Scholar]
- 27. Park JJ, Kang KL. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Laser Med Sci 2012;27:223–230 [DOI] [PubMed] [Google Scholar]
- 28. Korany NS, Mehannia SS, Hakam HM, El-Maghraby EMF. Evaluation of socket healing in irradiated rats after diode laser exposure (histological and morphometric studies). Arch Oral Biol 2012;57: 884–891 [DOI] [PubMed] [Google Scholar]
- 29. Hamad SA, Nail JS, Abdullah MA. Effect of diode laser on healing of tooth extraction socket: an experimental study in rabbits. J Maxillofac Oral Surg 2016;15:308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen 2007;15:866–874 [DOI] [PubMed] [Google Scholar]
- 31. Araùjo MG, Silva JC, Mendonca AF, Lindhe J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin Oral Implants Res 2015;26:407–412 [DOI] [PubMed] [Google Scholar]
- 32. Stein E, Koehn J, Sutter W, et al. . Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenshr 2008;120:112–117 [DOI] [PubMed] [Google Scholar]
- 33. Saracino S, Mozzati M, Martinasso G, Pol R, Canuto RA, Mizio G. Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Laser Surg Med 2009;41:298–304 [DOI] [PubMed] [Google Scholar]
- 34. Mendes RM, Silva GA, Lima MF, et al. . Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol 2008;53:1155–1162 [DOI] [PubMed] [Google Scholar]
- 35. Hirata S, Kitamura C, Fukushiama H, et al. . Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J Cell Biochem 2010;111:1445–1452 [DOI] [PubMed] [Google Scholar]
- 36. Fujimoto K, Kiyosaki T, Mitsui N, et al. . Low-intensity laser irradiation stimulates mineralization via increased BMPs in MT3T3-E1 cells. Laser Surg Med 2010;42:519–526 [DOI] [PubMed] [Google Scholar]
- 37. Nowak KC, McCormack M, Koch RJ. The effect of superpulsed carbon dioxide laser energy on keloid and normal dermal fibroblast secretion of growth factors: a serum-free study. Plast Reconstr Surg 2000;105:2039–2048 [DOI] [PubMed] [Google Scholar]
- 38. Ozawa Y, Shimizu N, Kariya G, Abiko Y. Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 1998;22:347–354 [DOI] [PubMed] [Google Scholar]
- 39. Khadra M, Kasem N, Haanaes HR, Ellingsen JE, Lyngstadaas SP. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;97:693–700 [DOI] [PubMed] [Google Scholar]
- 40. George J, Kuboki Y, Miyata T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng 2006;95:404–411 [DOI] [PubMed] [Google Scholar]
- 41. Hisanaga Y, Suzuki E, Aoki H, et al. . Effect of the combined use of enamel matrix derivative and atelocollagen sponge scaffold on osteoblastic differentiation of mouse induced pluripotent stem cells in vitro. J Periodontal Res 2018;53:240–249 [DOI] [PubMed] [Google Scholar]
- 42. de Freitas AC, Pinheiro AL, de Oliveira MG, Ramalho LM. Assessment of the behavior of myofibroblasts on scalpel and CO(2) laser wounds: an immunohistochemical study in rats. J Clin Laser Med Surg 2002;20:221–225 [DOI] [PubMed] [Google Scholar]
- 43. Pinheiro AL, Pozza DH, Oliveira MG, Weissmann R, Ramalho LM. Polarized light (400–2000 nm) and non-ablative laser (685 nm): a description of the wound healing process using immunohistochemical analysis. Photomed Laser Surg 2005;23:485–492 [DOI] [PubMed] [Google Scholar]