Abstract
Background
The key role of Vitamin D is to maintain an adequate calcium and phosphorus metabolism. Vitamin D plays an antagonistic role with the parathyroid hormone. 25 OH Vitamin D is the major circulating form and the best indicator to monitor Vitamin D levels.
Methods
A cross-sectional study was conducted in 1339 individuals ≥18 years old. The main objective was to establish the nutritional status of Vitamin D and its association with PTH and ionized calcium levels. Other objectives were to compare the levels of 25 OH Vitamin D based on sun exposure habits, and to identify the minimum cut-off point for the levels of 25 OH Vitamin D that could give rise to a concomitant increase in PTH and ionized calcium levels.
Results
14.2% of participants presented Vitamin D deficiency, and 28.8% presented insufficiency; ≥89% of the participants with deficiency or insufficiency were exposed to sunlight <30 minutes per week. A value of 25 OH Vitamin D >30 ng/mL was associated with a more stable and “flat” PTH value. The median of 25 OH Vit-D associated with hypercalcemia was <10 ng/mL.
Conclusion
In Colombia, low 25 OH Vitamin D values are highly prevalent; this may be accounted for by poor sun-exposure habits and frequent use of sunscreen. Just as in other similar trials, the lower the levels of 25 OH Vit-D, the higher the effect on PTH and ionized calcium elevation.
Keywords: Biological sciences, Chemistry, Environmental science, Food science, Health sciences, Vitamin D, PTH, 25 OH vitamin D, Ionized calcium, Plateau
Biological sciences; Chemistry; Environmental science; Food science; Health sciences; Vitamin D; PTH; 25 OH vitamin D; Ionized calcium; Plateau.
1. Introduction
The nutritional status of Vitamin D (Vit-D) derives from two sources: diet (10%) and endogenous production (90%). Endogenous production of Vit-D is induced by the exposure to ultraviolet B rays (UVB), generated through the photolytic conversion of 7-dehydrocholesterol to pre-Vit-D3, which via a thermal isomerization process produces Vit-D3 [1, 2, 3]. Vit-D hydroxylation initially occurs in the liver (converting into 25 OH Vit-D), and subsequently –particularly in the kidney and mediated by 1-α hydroxylase-converts into 1,25 (OH)2D (or active Vit-D) [4, 5, 6]. This latter form of Vit-D has “calcemic” actions such as the regulation of calcemia and phosphatemia, as well as “non-calcemic” actions, such as cell differentiation and some anti-proliferative actions [7, 8]. 1,25 (OH)2D induces biological cellular effects through genomic and non-genomic mechanisms; the former are initiated with the interaction between 1,25 (OH)2D and its receptor (VDR), leading to gene transcription changes, while the latter intervene in the opening of ionic channels or in the formation of second messengers [9, 10]. The key role of Vit-D is to maintain an adequate calcium and phosphorus metabolism, and hence bone homeostasis. Hypovitaminosis D has been associated with countless bone and metabolic conditions, including rickets, osteomalacia, osteoporosis, increased falls risk, autoimmunity, cancer, diabetes mellitus, cardiovascular disease, etc. [11, 12]. Vit-D plays an antagonistic role with the parathyroid hormone (PTH), and hence “hypovitaminosis D” stimulates PTH secretion, inducing the removal of calcium from the bone. 25 OH Vit-D is the major circulating form and the best indicator to monitor Vit-D levels [13, 14]. In 2011, the Institute of Medicine concluded that the adequate level of 25 OH Vit-D for optimum bone health was ≥20 ng/mL. This same year, the Endocrine Society defined the levels of Vit-D (25 OH Vit-D) in the population, indicating that levels >30 ng/mL are “optimal”, 20–30 ng/mL are “insufficient”, and <20 ng/mL are “deficient” [15, 16]. Current estimates indicate that around 15% of the world population is Vit-D deficient or insufficient and some of the factors involved are insufficient sun exposure, poor Vit-D diets, use of sunscreens, old age, dark skin, inter alia [17, 18]. The main objective of this study was to establish the nutritional status of Vit-D (deficiency, insufficiency, and optimum values) and its association with PTH and ionized calcium levels in a population of the southeastern region in Colombia. Other objectives were: to do a socio-demographic characterization of the population, to compare the levels of 25 OH Vit-D based on socio-demographic characteristics and sun exposure habits; to identify any changes in PTH values and ionized calcium for different levels of 25 OH Vit-D; and to identify the minimum cut-off point for the levels of 25 OH Vit-D that could give rise to a concomitant increase in PTH and ionized calcium levels.
2. Methods
An analytical cross-sectional study was conducted. The study population is comprised of individuals from an urban area in Popayán-Colombia, who visited a medium-complexity internal medicine and endocrinology outpatient clinic between May 2016 and February 2018. The inclusion criteria were: individuals ≥18 years old that presented with pathologies other than bone metabolism, and with creatinine and creatinine clearance measurements taken over the past 6 months. The exclusion criteria were: pregnant women and individuals with recent intake (last 6 months) of calcium, Vit-D, magnesium, or phosphorus supplements; individuals who were in active osteoporosis treatment, subjects using steroids, anticonvulsant drugs, thiazide diuretics, anticoagulants, HIV-therapy; history of parathyroid surgery, bariatric surgery, hyperthyroidism, hypercalcemia, or hypocalcemia undergoing specific therapy, creatinine clearance ≤40 mL/min, or creatinine >1.3 mg/dL in males and >1.1 mg/dL in females, alcohol and/or tobacco use, kidney transplant, dialysis (or in a pre-dialysis condition), and individuals diagnosed with liver cirrhosis or liver failure. The final sample was selected using non-probabilistic sequential sampling. Patients that met the inclusion criteria were invited to participate in the study; upon their acceptance, patients were asked to sign the informed consent and a survey was administered requiring the following information: age (in years); sex (male-female); socio-economic level (SEL) – in Colombia, the SEL is classified according to the National Department of Statistics (2015), as follows: SELs 1, 2 and 3 are low-income levels comprising the populations with limited resources who are recipients of subsidies for public utilities. People in the SEL 4 receive no subsidies but are exempt from any public utilities surcharges; however, SELs 5 and 6 are the high-income levels and comprise the population with higher economic resources that are required to make additional contributions to the public utilities rates. Base on the above, 3 categories of SELs were established: Low (levels 1, 2, and 3); medium (level 4); and high (level ≥5)]; sun exposure habits based on exposure of any skin area (unprotected by clothing or footwear); time of sun exposure per week (<30 min or ≥30 min); use of sunscreen [at least 3 times per week (yes/no), on the areas not protected by clothing and footwear, regardless of the brand and sun protection factor –SPF-]. Body weight, height, and body mass index –BMI- [(kg/m2) classified as <18.5: low weight; between 18.5 and 24.9: normal; between 25 and 29.9: overweight; and ≥30: obese] were measured in all of the subjects. Then, fasting serum levels of 25 OH Vit-D, ionized calcium and PTH were also measured. The levels of 25 OH Vit-D (ng/mL) were measured using ELFA (Enzyme Linked Fluorescent Assay); and the results were quantitatively analyzed and classified based on The Endocrine Society (2011) guidelines. PTH (intact molecule) levels were measured through a solid phase chemiluminescent sequential immunoassay, considering 11–67 pg/mL as a normal value. Ionized calcium levels were measured through an electrolyte analyzer, based on ion-selective electrode measurements, with normal values ranging from 1.13-1.51 mmol/L [19, 20, 21].
2.1. Ethics committee approval
All personal data were confidential and managed exclusively by the principal investigator, according to the legal standards on confidentiality of medical records and adhering to the rules of the Institutional Review Committee of Human Ethics (reference number: ID 5075). Universidad del Cauca, Popayán-Cauca-Colombia. Written informed consents were obtained from all participants.
2.2. Statistical analysis
The univariate analysis of qualitative variables was conducted using frequencies and percentages; the quantitative variables (considering that a non-normal distribution was identified in every case) were analyzed with nonparametric statistics [median, range, interquartile range (IQR)] using Shapiro Wilk. In the bivariate analysis, 25 OH Vit-D presented a non-normal distribution, and the Mann Withney U test was used to compare and contrast the 25 OH Vit-D values, against a number of variables including gender, sun exposure and use of sunscreen. The Kruskall Wallis test was used to compare the levels of 25 OH Vit-D in accordance with variables such as SEL, and BMI. Additionally, Spearman's correlation coefficient was used to assess the relationship with quantitative variables (age, ionized calcium, PTH); this analysis considered the following scale: 0–0.25: poor or null correlation; 0.26–0.50: weak correlation; 0.51–0.75: moderate to strong correlation; 0.76–1.00: strong to perfect correlation. In order to identify any changes in the ionized calcium levels and PTH for the different levels of 25 OH Vit-D, summary measurements were presented at each level. These analyses were accompanied by tendency lines for each variable. ROC curves were used to identify the minimum cut-off point for the 25 OH Vit-D levels that resulted in a concomitant elevation of PTH or ionized calcium levels. A 95% confidence interval was considered for all the analyses and the allowable α error was 0.05. Consequently, a p < 0.05 value was considered statistically significant. The statistical analysis was conducted using SPSS Statistics V21.0.
3. Results
Out of 1457 individuals that met the criteria to participate in the study, 1339 participants were included (of the remaining 118 subjects, 49 refused to participate and 69 failed to properly complete the survey information, or the laboratory parameters).
3.1. Socio-demographic characteristics, sun exposure habits, and distribution of 25 OH Vit-D, PTH, and ionized calcium levels
Among the 1339 participants, 85.7% were females; 50% of the participants were between 44 and 68 years old (IQR:24); 63.7% claimed they had less than 30 min of sun exposure per week, and 57% used sunscreen <3 times per week. 56.8% of the participants were overweight, and less than 15% had some level of obesity. The median 25 OH Vit-D was 32.3 ng/mL (IQR: 23.20). Moreover, 14.2% of the population presented Vit-D deficiency, 28.8% presented insufficiency, and 57% had optimum levels. The median PTH was 44.3 pg/mL, and 50% of the subjects exhibited levels between 29.4-73.9 pg/mL (IQR: 44.50). The ionized calcium median was 1.37 mmol/L, and 50% had values between 1.33-1.41 mmol/L (IQR: 0.08) (Table 1).
Table 1.
Socio-demographic and clinical baseline characteristics, sun exposure habits, and distribution of 25 OH Vit-D levels, ionized calcium, and PTH in the study population.
| Sex | N (%) |
|---|---|
| Females | 1148 (85.7%) |
| Males | 191 (14.3%) |
| Median age (years) | IQR |
| 57 | 24 |
| BMI (kg/m2) | N (%) |
| Low weight | 18 (1.34%) |
| Normal weight | 364 (27.18%) |
| Overweight | 761 (56.83%) |
| Obesity | 196 (14.64%) |
| Sun exposure | N (%) |
| <30 min/week | 853 (63.7%) |
| ≥30 min/week | 486 (36.3%) |
| Use of sunscreen | N (%) |
| <3 times/week | 763 (57%) |
| ≥3 times/week | 576 (43%) |
| Distribution of 25 OH Vit-D levels | N (%) |
| Optimum | 764 (57.1%) |
| Insufficient | 386 (28.8%) |
| Deficient | 189 (14.1%) |
| 25 OH Vit-D Median (ng/mL) | IQR |
| 32.3 | 23.2 |
| PTH Median (pg/mL) | IQR |
| 44.3 | 44.5 |
| Ionized calcium Median (mmol/L) | IQR |
| 1.37 | 0.08 |
| Socio-Economic Level (SEL) Classification and distribution | N (%) |
| Low | 112 (8.36%) |
| Middle | 933 (69.68%) |
| High | 294 (21.96%) |
Abbreviations: SEL: Socio-economic level; BMI: body mass index; IQR: interquartile range; PTH: parathyroid hormone; Vit-D: vitamin D.
3.2. Levels of 25 OH Vit-D, based on socio-demographic characteristics and sun exposure habits
When comparing the levels of 25 OH Vit-D with the qualitative variables (using Mann-Withney U test) the levels of 25 OH Vit-D were found to be higher in women that received >30 min of sun exposure per week, and who claimed to use sunscreen <3 times/week (p = 0.000). No statistically significant differences were found (using the Kruskall Wallis test) between the levels of 25 OH Vit-D and the SEL (p = 0.482) (Table 2).
Table 2.
Levels of 25 OH Vit-D according to socio-demographic variables, sun exposure habits, and use of sunscreen.
| Variable | Levels of 25 OH Vit-D (ng/mL) |
||||
|---|---|---|---|---|---|
| Median | Min. | Max. | IQR | P value | |
| Gender | |||||
| Male | 30.50 | 5.12 | 97.10 | 17.40 | 0.014∗ |
| Female | 32.65 | 1.36 | 213 | 24.67 | |
| SEL | |||||
| Low | 31.3 | 8.80 | 97.80 | 22.33 | 0.482 |
| Middle | 32.60 | 1.36 | 134 | 23.80 | |
| High | 31.90 | 8.90 | 213 | 21.83 | |
| Sun exposure | |||||
| <30 min | 27 | 1.36 | 213 | 11.64 | ≤0.001∗ |
| ≥30 min | 51.90 | 8.23 | 134 | 23.15 | |
| Sunscreen use | |||||
| ≥3 times/week | 23.30 | 1.36 | 84.60 | 8.58 | ≤0.001∗ |
| <3 times/week | 43.50 | 8.80 | 213 | 25 | |
Abbreviations: Min: minimum, Max: maximum, SEL: socio-economic level, IQR: interquartile range, Vit-D: vitamin D.
Statistically significant findings.
3.3. Distribution of the levels of 25 OH Vit-D, based on socio-demographic characteristics, sun exposure habits, PTH and ionized calcium
The highest proportion of individuals with 25 OH Vit-D levels defined as “deficient” (<20 ng/mL) or insufficient (20–29.9 ng/mL)” were within the 51-70-year-old age range, belonged to the middle SEL, and were overweight. ≥89% of the participants with deficiency or insufficiency were exposed to sunlight <30 min per week, and >85% used some kind of sunscreen. Among the group of subjects that were deficient, 83% exhibited PTH values > 67 pg/mL; among the subjects who were insufficient, 33% had PTH values > 67 pg/mL. Similarly, among the participants with deficiency or insufficiency, the proportion of subjects with ionized calcium levels >1.51 mmol/L was 37.6% and 9.7%, respectively. The assessment using the Spearman's correlation coefficient showed an inverse and significant correlation (though scarce) between the levels of 25 OH Vit-D and age (R: 0.073; p = 0.007). An inverse and scarce (non-significant) correlation was also found between the levels of 25 OH Vit-D and BMI (-0.025; p = 0.367) (Table 3).
Table 3.
Levels of 25 OH Vit-D according to socio-demographic characteristics, habits, and ionized calcium and PTH values.
| Variable | 25 OH Vit-D (ng/mL) |
Total (n = 1339) | |||
|---|---|---|---|---|---|
| <20 (n = 189) | 20-29 (n = 386) | ≥30 (n = 764) | |||
| Sex | Male | 26 (13.8%) | 67 (17.4%) | 98 (12.8%) | 191 (14.3%) |
| Female | 163 (86.2%) | 319 (82.6%) | 666 (87.2%) | 1148 (85.7%) | |
| Age (years) | 18–30 | 20 (10.6%) | 47 (12.2%) | 73 (9.6%) | 140 (10.5%) |
| 31–50 | 44 (23.3%) | 110 (28.5%) | 184 (24.1%) | 338 (25.2%) | |
| 51–70 | 85 (45.0%) | 163 (42.2%) | 361 (47.3%) | 609 (45.5%) | |
| >70 | 40 (21.2%) | 66 (17.1%) | 146 (19.1%) | 252 (18.8%) | |
| SEL | Low | 15 (7.94%) | 35 (9.07%) | 62 (8.12%) | 112 (8.36%) |
| Middle | 125 (66.14%) | 266 (68.91%) | 542 (70.94%) | 933 (69.68%) | |
| High | 49 (25.93%) | 85 (22.02%) | 160 (20.94%) | 294 (21.96%) | |
| BMI (kg/m2) | Low weight | 6 (3.2%) | 3 (0.8%) | 9 (1.2%) | 18 (1.3%) |
| Normal weight | 42 (22.2%) | 108 (28.0%) | 214 (28.0%) | 364 (27.2%) | |
| Overweight | 113 (59.8%) | 217 (56.2%) | 431 (56.4%) | 761 (56.8%) | |
| Obesity | 28 (14.8%) | 58 (15.0%) | 110 (14.4%) | 196 (14.6%) | |
| Sun exposure (minutes/week) | <30 | 169 (89.4%) | 363 (94.0%) | 321 (42.0%) | 853 (63.7%) |
| ≥30 | 20 (10.6%) | 23 (6.0%) | 443 (58.0%) | 486 (36.3%) | |
| Sunscreen use | <3 times/week | 25 (13.23%) | 56 (14.51%) | 682 (89.27%) | 763 (56.98%) |
| ≥3 times/week | 164 (86.77%) | 330 (85.49%) | 82 (10.73%) | 576 (43.02%) | |
| Ionized calcium (mmol/L) | <1.13 | 2 (1.1%) | 12 (0.3%) | 34 (4.5%) | 48 (3.6%) |
| 1.13 a 1.51 | 116 (61.4%) | 361 (15.9%) | 716 (93.7%) | 1193 (89.1%) | |
| >1.51 | 71 (37.6%) | 13 (9.7%) | 14 (1.8%) | 98 (7.3%) | |
| PTH (pg/mL) | <11 | 1 (0.5%) | 2 (0.5%) | 14 (1.8%) | 17 (1.3%) |
| 11 a 67 | 31 (16.4%) | 257 (66.6%) | 643 (84.2%) | 931 (69.5%) | |
| >67 | 157 (83.1%) | 127 (32.9%) | 107 (14.0%) | 391 (29.2%) | |
Abbreviations: SEL: socio-economic level; BMI: body mass index; PTH: parathyroid hormone; Vit-D: vitamin D.
3.4. Correlation between the levels of 25 OH Vit-D, PTH and ionized calcium
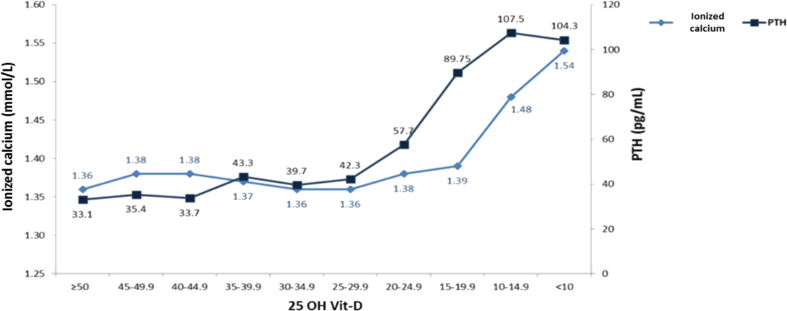
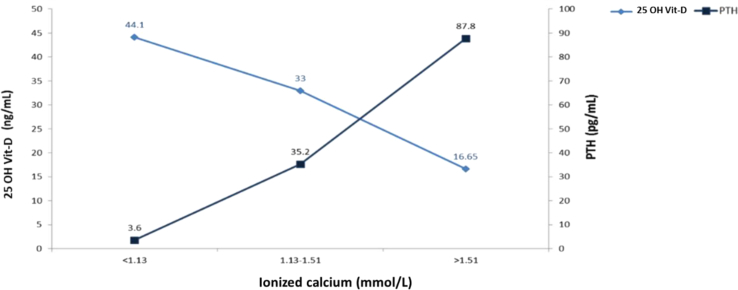
A low level of 25 OH Vit-D generated an increase in the PTH and ionized calcium values. The largest increase in the median PTH (from 57.7 to 89.75 pg/mL) occurred when changing 25 OH Vit-D from a range of 20–24.99 ng/mL to 15–19.99 ng/mL. In contrast, the highest increase in the ionized calcium median (from 1.39 to 1.48 mmol/L) occurred when changing 25 OH Vit-D from a range of 15–19.99 ng/mL to a range of 10–14.99 ng/mL. A median of 25 OH Vit-D of 33 ng/mL was associated with a PTH value of 35.2 pg/mL, and with an ionized calcium value of 1.13–1.15 mmol/L. Finally, a median of 25 OH Vit-D of 16.65 ng/mL was associated with PTH and ionized calcium values of 87.8 pg/mL and >1.51 mmol/L, respectively (Figures 1 and 2).
Figure 1.
Ionized calcium values and PTH, according to 25 OH Vit-D levels.
Figure 2.
Levels of 25 OH Vit and PTH, according to ionized calcium values.
3.5. Level of 25 OH Vit-D that better discriminates the normal PTH values
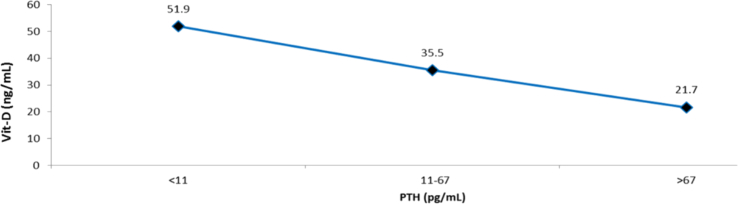
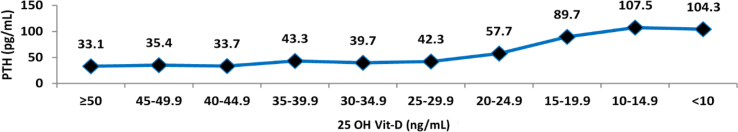
The median value of 25 OH Vit-D that was associated with a normal range PTH value (11–67 pg/mL) was 35.5 ng/mL. In contrast, a level of <20 ng/mL was associated with PTH values > 67 pg/mL, while a value of >30 ng/mL was associated with a more stable and “flat” PTH value (Figures 3 and 4). When evaluating the correlation between the levels of 25 OH Vit-D and PTH (Spearman's coefficient), a weak and significant correlation was found (-0.453; p = 0.000); furthermore, a 25 OH Vit-D range between 25-29.99 ng/mL was found to generate a significant change in the PTH median from 39.70 ng/mL to 42.30 pg/mL.
Figure 3.
Median of 25 OH Vit-D and PTH values.
Figure 4.
Relationship between the levels of 25 OH Vit-D and PTH values.
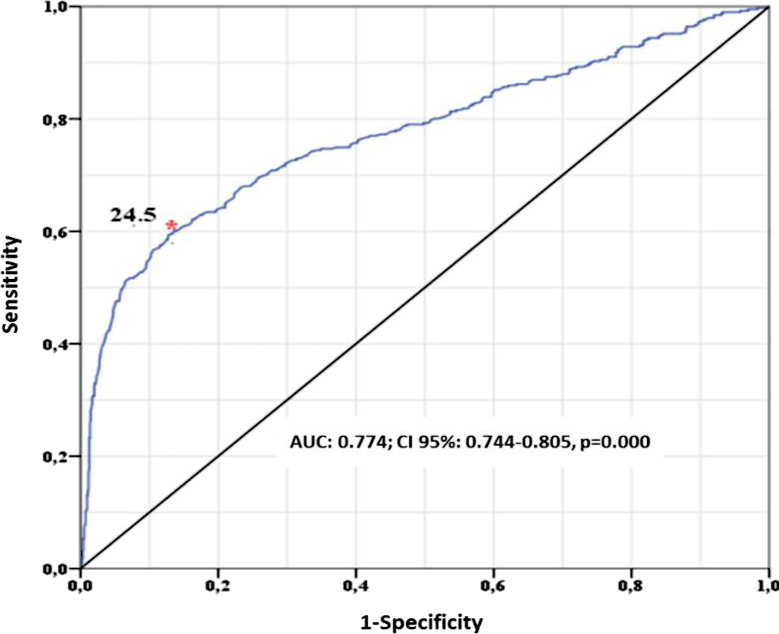
Additionally, a complementary analysis was conducted using ROC curves, classifying the PTH levels into 2 groups; the first one with values of ≤67 pg/mL, and the second one comprised subjects with values > 67 pg/mL. The 25 OH Vit-D value that best discriminated this difference was 24.5 ng/mL (Figure 5).
Figure 5.
Level of 25 OH Vit-D and critical PTH value.
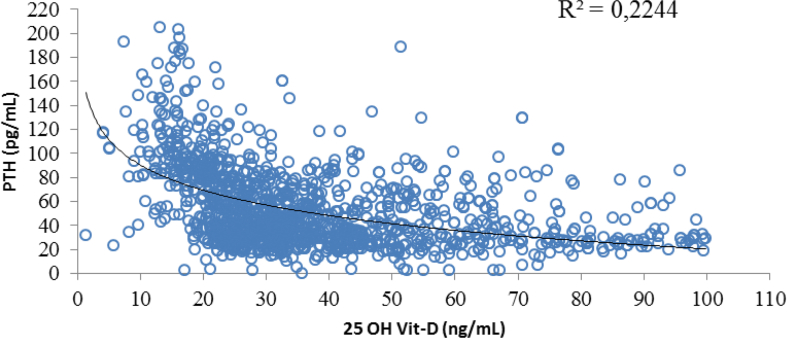
The regression analysis excluded 10 atypical extreme data (those that were ≥3 fold away from the IQR); 2 related to the 25 OH Vit-D variable, and 8 related to PTH. Upon removing these data, a non-normal distribution persisted, generating a result of 0.908 (p: 0.00) and of 0.888 (p: 0.00) for 25 OH Vit-D and PTH, respectively, with the Shapiro Wilk test. Our evaluation of the correlation using the Spearman's, test identified an inverse scarce and statistically significant correlation (-0.48, p: 0.000). A complementary analysis was conducted to explore the relationship between the levels of 25 OH Vit-D and PTH values, through a non-lineal (logarithmic) regression. Similarly, the ANOVA analysis found that the logarithmic model was significant to explain the dependent variable based on the independent variable, and verified that both B0 (159.433, p: 0.000) and B1 (-30.130, p: 0.000) were statistically different from 0. When checking the validity of the model adjustment, a determination coefficient (R2) of 0.22 was identified (Figure 6). The residual assumptions were checked to validate the model's predictive capacity, but the findings indicated that the residuals did not follow a normal distribution (P: 0.000), the residuals were not independent [checked through the run test - (p: 0.000) and the assumption of homoscedasticity was not met, since when increasing the adjustment, the variability increased]. All of this is accounted for by the weak correlation between the 2 variables. Notwithstanding the fact that the model showed a low predictive ability, the results obtained therefrom were used, which resulted in Y = a+b*lnX, so that in the logarithmic regression equation the following values were identified: Y = 159.433 + (-30.130 (lnX)); a = 159.433, b = -30.130, and Y = 67.01. Consequently, the 25 OH Vit-D value that originated a PTH value of >67 pg/mL was 21.49 ng/mL.
Figure 6.
Correlation between levels of 25 OH Vit-D and PTH values.
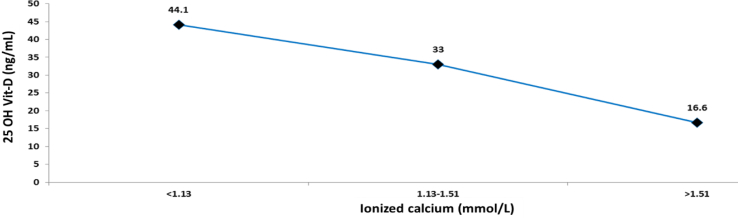
3.6. Correlation between the levels of 25 OH Vit-D and ionized calcium
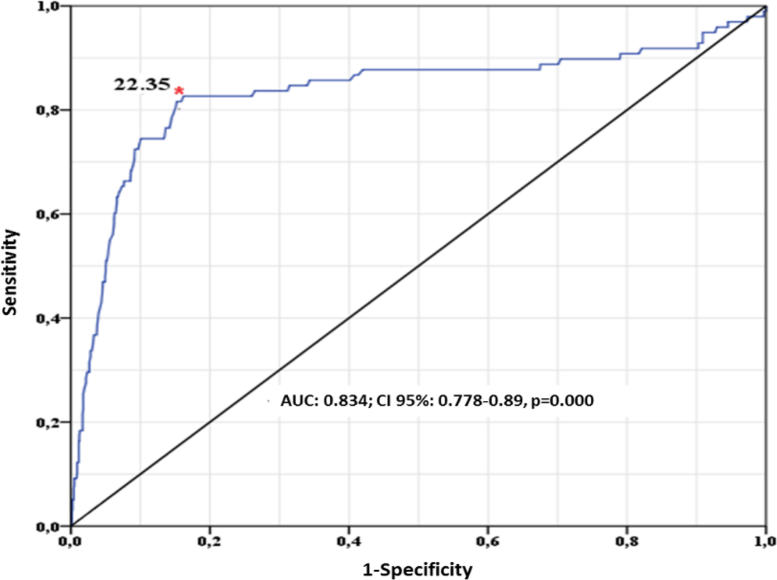
A median of 25 OH Vit-D of 33 ng/mL was associated with normal ionized calcium values (1.13–1.51 mmol/L); whilst a median of <10 ng/mL was associated with ionized calcium values > 1.51 mmol/L (Figure 7). When assessing the correlation between 25 OH Vit-D and ionized calcium (using Spearman's coefficient), an inverse and significant correlation was identified (-2.258; p = 0.000). A complementary ROC curves-based analysis was conducted, classifying individuals into 2 groups: the first group was comprised of those individuals with ionized calcium levels ≤1.51 mmol/L; and the second group was comprised of individuals with levels of >1.51 mmol/L. The level of 25 OH Vit-D that best discriminated the difference between these 2 groups was 22.35 ng/mL (Figure 8). Finally, a range of 25 OH Vit-D between 20-24.99 ng/mL led to a change in the ionized calcium median of 1.36–1.8 mmol/L (p = 0.000).
Figure 7.
Relationship between levels of 25 OH Vit-D and ionized calcium values.
Figure 8.
Levels of 25 OH Vit-D and critical ionized calcium value.
3.7. Correlation between PTH and ionized calcium
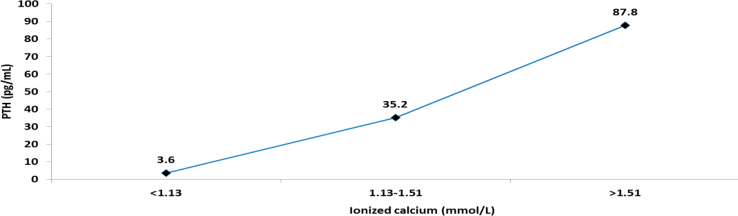
A PTH median of 35.2 pg/mL was associated with normal ionized calcium levels (1.13–1.51 mmol/L); in contrast, a PTH median of 87.8 pg/mL was associated with an increased ionized calcium level >1.51 mmol/L. Furthermore, a PTH median of 3.6 pg/mL was associated with an ionized calcium level of <1.13 mmol/L (Figure 9).
Figure 9.
Relationship between the PTH values and ionized calcium.
4. Discussion
Popayán is located in the southwestern region of Colombia, at an altitude of 1738 m above sea level at 2.43823 latitude and -76.6131592 longitude, in the northern hemisphere. The average sunshine per year is estimated at 4.5 h per day, with an annual average with no sunshine (in number of days) of 1.7. Hence, it is estimated that Popayán receives almost the same amount of sun radiation throughout the year [22, 23]. This study found that the prevalence of optimum 25 OH Vit-D levels was significantly higher among women with a sun exposure of >30 min per week, who claimed to use sunscreen <3 times per week. Forty three percent of the population studied exhibited at least 25 OH Vit-D insufficiency (28.8% presented with values of <30 ng/mL, and 14.2% with values < 20 ng/mL); these results differ from those in trials - for example in Europe, it has been estimated that 13% of the people have 25 OH Vit-D concentrations of <12 ng/mL throughout the year, regardless of age, ethnicity, and latitude. In those trials, when using a cut-off point for 25 OH Vit-D of <20 ng/mL, the prevalence of deficiency documented was 60–75% [during the extended winter season (November–March), and of 30–45% during the extended summer season (April–October)] [24, 25]. Moreover, the NHANES (National Health and Nutrition Examination Survey) data between 2001-2006, indicate that 33% of the US population exhibited levels of 25 OH Vit-D of <20 ng/mL, and 77% exhibited values of <30 ng/mL [26]. It is impossible to compare our study results against the above-mentioned percentages because there are no seasons in Colombia –there is just a rainy season and a dry season, and the solar radiation is almost constant throughout the year-. Moreover, the relationship between sun exposure and the different clinical/biological results, requires an accurate and reliable measurement of UV radiation (particularly UVB). There are multiple measurement approaches [questionnaires, ecology indicators (latitude, seasons, levels of intensity or duration as part of UV radiation, inter alia)]; however, no method is perfect, neither have any methods been validated for our environment. In this study, the approach to assess sun exposure was based on the universal recommendation that exposing 20% of the body surface for 15 min (between 10:00 am and 3:00 pm, at least 2 times/week), helps to maintain an adequate Vit-D level in the population [27]. In our population, over 90% of the participants with 25 OH Vit-D insufficiency of deficiency, had little sun exposure. Moreover, over 85% of the individuals who were insufficient or deficient, used some type of sunscreen ≥3 times/week (and 43% of all the participants used sunscreen with the same frequency); this information should also be emphasized, since it has been shown that the use of sunscreens reduces the absorption of UVB rays, and hence, reduces the production of Vit-D (an SPF 8 sunscreen may reduce the synthesis of Vit-D by 95% and an SPF 15 may reduce the synthesis of Vit-D by 98%) [27]. Additionally, the largest proportion of individuals with Vit-D insufficiency or deficiency, were in the age range between 51-70 years old, and were overweight. This finding is consistent with previous studies that have shown that age is a variable that effects the Vit-D status in the population (the older the individual, the lower the ability to synthetize Vit-D through the skin). Moreover, obesity has an inverse relationship with the levels of Vit-D (due to “sequestration” of Vit-D into the body fat, and because of decreased bioavailability) [28, 29]. However, we failed to find an association between BMI and 25 OH Vit-D levels, probably because of the small number of obese individuals among the population studied (<15%). We also found that a median of 35.5 ng/mL of Vit-D was associated with a normal range PTH value (11–67 pg/mL), and that a median of 25 OH Vit-D of 33 ng/mL was associated with a PTH value of 35.2 pg/mL (with ionized calcium of 1.13–1.51 mmol/L). These findings are very similar to those of other studies that have considered that the PTH “plateau” is reached at a 25 OH Vit-D level of 30 ng/mL [30, 31]. This is an indication of the fact that if the 25 OH Vit-D levels are >30 ng/mL, PTH reaches a value of around 35 pg/mL. In contrast, levels between 20 ng/mL and 30 ng/mL progressively activate PTH and further separate it from the plateau values; likewise, levels <20 ng/mL are associated with a steep PTH activation slope, and eventually result in hypercalcemia and hyperparathyroidism. Other studies have shown that values of 25 OH Vit-D <16 ng/mL are associated with a significant PTH elevation, and still others have shown a significant change in the PTH value with a level of 25 OH Vit-D of 31.56 ng/mL [32, 33, 34]. This has led to the conclusion that the PTH plateau is reached with a 25 OH Vit-D level ranging between 8-44 ng/mL –but mostly between 30-40 ng/mL- [35, 36]. Consequently, the plateau should be considered a “turning point”, which refers to the stimulation of PTH secretion when the levels of 25 OH Vit-D reach the limit to maintain the homeostasis of calcium. The variations in the cut-off point for 25 OH Vit-D among the various populations (resulting from the PTH plateau) may be at least partially accounted for by seasonal variations, demographic considerations, the way 25 OH Vit-D is measured, and ethnicity, inter alia. Notwithstanding the available evidence, the value of measuring the PTH concentration (to identify the optimum level of Vit-D) continues to be controversial and inconsistent; there is yet no threshold to define which is a “sufficient” Vit-D status [37, 38]. Notwithstanding this situation, measuring 25 OH Vit-D continues to be the best indicator of the Vit-D status of the population because it does not depend on PTH levels and it directly reflects the body's Vit-D stores [39, 40, 41]. We found an inverse, weak and significant correlation (probably due to the non-lineal nature of the association), among the levels of 25 OH Vit-D and PTH; and we were able to identify that the level of 25 OH Vit-D that originated a significant change in PTH values was 24.5 ng/mL. However, the level of 25 OH Vit-D that resulted in a PTH value above the upper normal value (>67 pg/mL) was 21.49 ng/mL (this value will determine the PTH plateau for our population, since it was the “turning point” for the maximum PTH suppression). We therefore consider that a level of 25 OH Vit-D of <25 ng/mL may be adequate to determine Vit-D fortification and/or supplementation. An additional finding was that low levels of 25 OH Vit-D resulted in significant changes in ionized calcium values; for instance, the level of 25 OH Vit-D that generated a significant change in the level of ionized calcium was 22.35 ng/mL, but the median of 25 OH Vit-D associated with an ionized calcium value above the upper normal level was <10 ng/mL. These results indicate –as expected-that measuring ionized calcium is useless for the early diagnosis of Vit-D insufficiency or deficiency (whilst PTH elevation seems to be an earlier predictor of such condition). Finally, a median of 25 OH Vit-D of 16.65 ng/mL was associated with a PTH and ionized calcium value of 87.8 pg/mL and >1.51 mmol/L, respectively, that determines the presence of hypercalcemic hyperparathyroidism. This study has some limitations, including the fact that the participants were followed at an intermediate complexity care center that cared for other various health conditions, different from metabolic bone disorders; therefore, this precludes the assumption that the results may be extrapolated to the rest of the population. Moreover, other aspects that could interfere with the values of 25 OH Vit-D were not considered (i.e., foods containing Vit-D, vegetarian-vegan lifestyles, physical activity, and skin pigmentation); additionally, the measurement of 25 OH Vit-D was done through immunoassay (as opposed to chromatography, which is a more consistent and accurate method). Having a history of osteomalacia, osteoporosis and/or fractures was not considered either, and hence we may not assume that the results obtained with regards to 25 OH Vit-D, PTH and ionized calcium values have any impact on the bone health of this population. Furthermore, only one measurement of 25 OH Vit-D, PTH and ionized calcium was taken from the participants and so these results reflect the Vit-D status of the population at one point in time, and not necessarily over time.
In contrast, the study has a number strengths such as the fact that the subjects did not receive any Vit-D, calcium or magnesium supplements (so the results may be considered independent from the use of those supplements); solar radiation in the geographical area is considered to be constant throughout the 12 months of the year (ruling out the potential “annual circadian rhythm” of 25 OH Vit-D which is typical of seasonal variations). Ionized calcium instead of total serum calcium was measured, so there was no need to correct for albumin; moreover, ionized calcium values are a better reflection of calcium metabolism. Both, creatinine and glomerular filtration rate were considered, and any individuals with health conditions and/or use of medications that could eventually alter the levels of 25 OH Vit-D were excluded.
In summary, the high prevalence of 25 OH Vit-D insufficiency and deficiency in this population is to a large extent the result of low sun exposure and of the frequent use of sunscreens (this does not preclude other confounding factors such as ethnicity, genetics, polymorphisms, skin pigmentation, physical activity, inter alia). A median of 25 OH Vit-D of 35.5 ng/mL was associated with a normal PTH range (11–67 pg/mL); whilst a range of 25 OH Vit-D between 20-24.99 ng/mL leads to a significant increase in PTH and a level of 25 OH Vit-D <10 ng/mL results in a significant increase in the level of ionized calcium. Finally, a level of 25 OH Vit-D of 16.65 ng/mL was associated with a significant and concomitant increase in PTH and ionized calcium.
Further studies in this geographical area should assess other aspects associated with the nutritional status of Vit-D, particularly among vulnerable population groups (children, adolescents, pregnant women, displaced populations, inter alia). Likewise, other sources of Vit-D, different from sun exposure, shall be assessed, as well as its impact on bone health, on various types of cancers, and on chronic and autoimmune diseases.
5. Conclusion
In Colombia, where the amount of solar radiation is constant throughout the year, low 25 OH Vit-D values are highly prevalent; this may be accounted for by poor sun-exposure habits and frequent use of sunscreen. The PTH plateau was reached with a level of 25 OH Vit-D of 21.49 ng/mL, and the value of 25 OH Vit-D that led to a significant change in PTH values was 24.5 ng/mL. The significant increase in the levels of ionized calcium was experienced by individuals with low levels of 25 OH Vit-D, and concomitant PTH elevation, suggesting that the level of ionized calcium is not a useful marker for early detection of Vit-D deficiency/insufficiency. This suggests that in similar populations, a level of 25 OH Vit-D <25 ng/mL could be considered as the cut-off point to start Vit-D supplementation.
Declarations
Author contribution statement
H. Vargas-Uricoechea: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Pinzón-Fernández and V. Agredo: Performed the experiments; Contributed reagents, materials, analysis tools or data.
A. Mera-Mamián: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11(3):E676. doi: 10.3390/nu11030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris H.A., Anderson P.H. Autocrine and paracrine actions of vitamin D. Clin. Biochem. Rev. 2010;31:129–138. [PMC free article] [PubMed] [Google Scholar]
- 3.Goltzman D., Mannstadt M., Marcocci C. Physiology of the calcium-parathyroid hormone-vitamin D Axis. Front. Horm. Res. 2018;50:1–13. doi: 10.1159/000486060. [DOI] [PubMed] [Google Scholar]
- 4.Mutchie T.R., Yu O.B., Di Milo E.S., Arnold L.A. Alternative binding sites at the vitamin D receptor and their ligands. Mol. Cell. Endocrinol. 2019;485:1–8. doi: 10.1016/j.mce.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawatsubashi S., Nishimura K., Mori J., Kouzmenko A., Kato S. The function of the vitamin D receptor and a possible role of enhancer RNA in epigenomic regulation of target genes: implications for bone metabolism. J. Bone Metab. 2019;26(1):3–12. doi: 10.11005/jbm.2019.26.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katayama Y. Vitamin D receptor: A critical regulator of inter-organ communication between skeletal and hematopoietic system. J. Steroid Biochem. Mol. Biol. 2019;190:281–283. doi: 10.1016/j.jsbmb.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S., Trummer C., Pandis M., Schwetz V., Aberer F., Grübler M., Verheyen N., Tomaschitz A., März W. Vitamin D: current guidelines and future outlook. Anticancer Res. 2018;38(2):1145–1151. doi: 10.21873/anticanres.12333. [DOI] [PubMed] [Google Scholar]
- 8.Caccamo D., Ricca S., Currò M., Ientile R. Health risks of hypovitaminosis D: a review of new molecular insights. Int. J. Mol. Sci. 2018;19(3):E892. doi: 10.3390/ijms19030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacquillet G., Unwin R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi) Pflügers Archiv. 2019;471(1):83–98. doi: 10.1007/s00424-018-2231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sai A.J., Walters R.W., Fang X., Gallagher J.C. Relationship between vitamin D, parathyroid hormone, and bone health. J. Clin. Encocrinol. Metab. 2011;96:E436–E446. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IOM (Institute of Medicine) The National Academies Press; Washington, DC: 2011. Dietary Reference Intakes for Calcium and Vitamin D; pp. 260–262. [PubMed] [Google Scholar]
- 12.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 13.Pfotenhauer K.M., Shubrook J.H. Vitamin D deficiency, its role in health and disease, and current supplementation recommendations. J. Am. Osteopath. Assoc. 2017;117(5):301–305. doi: 10.7556/jaoa.2017.055. [DOI] [PubMed] [Google Scholar]
- 14.van Schoor N., Lips P. Global overview of vitamin D status. Endocrinol Metab. Clin. N. Am. 2017;46(4):845–870. doi: 10.1016/j.ecl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Akoglu H. User's guide to correlation coefficients. Turk. J. Emerg. Med. 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlas de Radiación Solar, Ultravioleta y Ozono de Colombia. http://atlas.ideam.gov.co/visorAtlasRadiacion.html Available from:
- 17.Aoun A., Maalouf J., Fahed M., El Jabbour F. When and how to diagnose and treat vitamin D deficiency in adults: a practical and clinical update. J. Diet. Suppl. 2019:1–19. doi: 10.1080/19390211.2019.1577935. [DOI] [PubMed] [Google Scholar]
- 18.Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A., Pierroz D.D., Weber P., Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 19.Roth D.E., Abrams S.A., Aloia J., Bergeron G., Bourassa M.W., Brown K.H., Calvo M.S., Cashman K.D., Combs G., De-Regil L.M., Jefferds M.E. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018;1430(1):44–79. doi: 10.1111/nyas.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parva N.R., Tadepalli S., Singh P., Qian A., Joshi R., Kandala H., Nookala V.K., Cheriyath P. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012) Cureus. 2018;10(6) doi: 10.7759/cureus.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saraff V., Shaw N. Sunshine and vitamin D. Arch. Dis. Child. 2016;101(2):190–192. doi: 10.1136/archdischild-2014-307214. [DOI] [PubMed] [Google Scholar]
- 22.http://atlas.ideam.gov.co/visorAtlasRadiacion.html
- 23.UPME/IDEAM, Atlas de Radiación Solar de Colombia. 2005. [Google Scholar]
- 24.Cashman K.D., Dowling K.G., Škrabáková Z., Gonzalez-Gross M., Valtueña J., De Henauw S., Moreno L., Damsgaard C.T., Michaelsen K.F., Mølgaard C., Jorde R., Grimnes G. Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Food Safety Authority Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016;14(10):4547. [Google Scholar]
- 26.Ginde A.A., Liu M.C., Camargo C.A., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch. Intern. Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 28.Bahrami A., Sadeghnia H.R., Tabatabaeizadeh S.A., Bahrami-Taghanaki H., Behboodi N., Esmaeili H., Ferns G.A., Mobarhan M.G., Avan A. Genetic and epigenetic factors influencing vitamin D status. J. Cell. Physiol. 2018;233(5):4033–4043. doi: 10.1002/jcp.26216. [DOI] [PubMed] [Google Scholar]
- 29.Savastano S., Barrea L., Savanelli M.C., Nappi F., Di Somma C., Orio F., Colao A. Low vitamin D status and obesity: role of nutritionist. Rev. Endocr. Metab. Disord. 2017;18(2):215–225. doi: 10.1007/s11154-017-9410-7. [DOI] [PubMed] [Google Scholar]
- 30.Saliba W., Barnett O., Rennert H.S., Lavi I., Rennert G. The relationship between serum 25(OH)D and parathyroid hormone levels. Am. J. Med. 2011;124(12):1165–1170. doi: 10.1016/j.amjmed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Holick M.F. The D-batable parathyroid hormone plateau. Am. J. Med. 2011;124(12):1095–1096. doi: 10.1016/j.amjmed.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Li M., Lv F., Zhang Z., Deng W., Li Y., Deng Z., Jiang Y., Wang O., Xing X., Xu L., Xia W. Establishment of a normal reference value of parathyroid hormone in a large healthy Chinese population and evaluation of its relation to bone turnover and bone mineral density. Osteoporos. Int. 2016;27(5):1907–1916. doi: 10.1007/s00198-015-3475-5. [DOI] [PubMed] [Google Scholar]
- 33.Pludowski P., Holick M.F., Grant W.B., Konstantynowicz J., Mascarenhas M.R., Haq A., Povoroznyuk V., Balatska N., Barbosa A.P., Karonova T., Rudenka E., Misiorowski W., Zakharova I., Rudenka A., Łukaszkiewicz J., Marcinowska-Suchowierska E., Łaszcz N., Abramowicz P., Bhattoa H.P., Wimalawansa S.J. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018;175:125–135. doi: 10.1016/j.jsbmb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Pilz S., Zittermann A., Trummer C., Theiler-Schwetz V., Lerchbaum E., Keppel M.H., Grübler M.R., März W., Pandis M. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect. 2019;8(2):R27–R43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okazaki R., Ozono K., Fukumoto S., Inoue D., Yamauchi M., Minagawa M., Michigami T., Takeuchi Y., Matsumoto T., Sugimoto T. Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the research program of intractable diseases, Ministry of health, labour and welfare, Japan, the Japanese society for bone and mineral research and the Japan endocrine society [opinion] J. Bone Miner. Metabol. 2017;35(1):1–5. doi: 10.1007/s00774-016-0805-4. [DOI] [PubMed] [Google Scholar]
- 36.Priemel M., von Domarus C., Klatte T.O., Kessler S., Schlie J., Meier S., Proksch N., Pastor F., Netter C., Streichert T., Püschel K., Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 2010;25(2):305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 37.Ebeling P.R. Vitamin D and bone health: epidemiologic studies. BoneKEy Rep. 2014;3:511. doi: 10.1038/bonekey.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cesareo R., Attanasio R., Caputo M., Castello R., Chiodini I., Falchetti A., Guglielmi R., Papini E., Santonati A., Scillitani A., Toscano V., Triggiani V., Vescini F., Zini M. AME and Italian AACE chapter. Italian association of clinical endocrinologists (AME) and Italian chapter of the American association of clinical endocrinologists (AACE) position statement: clinical Management of vitamin D deficiency in adults. Nutrients. 2018;10(5):E546. doi: 10.3390/nu10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabenberg M., Scheidt-Nave C., Busch M.A., Thamm M., Rieckmann N., Durazo-Arvizu R.A., Dowling K.G., Škrabáková Z., Cashman K.D., Sempos C.T., Mensink G.B.M. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Publ. Health. 2018;18(1):845. doi: 10.1186/s12889-018-5769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scientific Advisory Committee on Nutrition Vitamin D and Health. 2016. https://www.gov.uk/government/publications/sacn-vitamind-and-health-report Available from: [Google Scholar]
- 41.Preiss D., Sattar N. Research digest: vitamin D supplementation. Lancet Diabetes Endocrinol. 2019;7(2):91. doi: 10.1016/S2213-8587(19)30007-5. [DOI] [PubMed] [Google Scholar]