Abstract
Cell replacement therapy is a promising treatment strategy for Parkinson's disease (PD); however, the poor survival rate of transplanted neurons is a critical barrier to functional recovery. In this study, we used self-assembling peptide nanofiber scaffolds (SAPNS) based on the peptide RADA16-I to support the in vitro maturation and in vivo post-transplantation survival of encapsulated human dopaminergic (DA) neurons derived from induced pluripotent stem cells. Neurons encapsulated within the SAPNS expressed mature neuronal and midbrain DA markers and demonstrated in vitro functional activity similar to neurons cultured in two dimensions. A microfluidic droplet generation method was used to encapsulate cells within monodisperse SAPNS microspheres, which were subsequently used to transplant adherent, functional networks of DA neurons into the striatum of a 6-hydroxydopamine-lesioned PD mouse model. SAPNS microspheres significantly increased the in vivo survival of encapsulated neurons compared with neurons transplanted in suspension, and they enabled significant recovery in motor function compared with control lesioned mice using approximately an order of magnitude fewer neurons than have been previously needed to demonstrate behavioral recovery. These results indicate that such biomaterial scaffolds can be used as neuronal transplantation vehicles to successfully improve the outcome of cell replacement therapies for PD.
Impact Statement
Transplantation of dopaminergic (DA) neurons holds potential as a treatment for Parkinson's disease (PD), but low survival rates of transplanted neurons is a barrier to successfully improving motor function. In this study, we used hydrogel scaffolds to transplant DA neurons into PD model mice. The hydrogel scaffolds enhanced survival of the transplanted neurons compared with neurons that were transplanted in a conventional manner, and they also improved recovery of motor function by using significantly fewer neurons than have typically been transplanted to see functional benefits. This cell transplantation technology has the capability to improve the outcome of neuron transplantation therapies.
Keywords: induced pluripotent stem cells, cell transplantation, cell encapsulation, hydrogel, neuron, biomaterials
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta region of the midbrain. Although current pharmacological treatments such as levodopa or dopamine agonists can offer symptomatic relief during the early stages of PD, these treatments lose efficacy over time and also fail to address the continuing death of neurons as the disease advances.1
Cell replacement therapy has been explored as a promising treatment strategy for PD and other neurodegenerative diseases, and initial clinical trials have been performed by using fetal ventral mesencephalon (VM) grafts.2 However, less than 20% of the DA neurons contained in VM grafts have been shown to survive after transplantation.3 There are also several ethical and logistical concerns regarding the use of fetal tissue for clinical therapeutic use. Human induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) have been used as a source of DA neurons, which have been transplanted into the brains of PD rodent models with in vivo cell survival rates as low as 1–5%.4–6 With such low survival rates, large numbers of cells must be transplanted to realize any functional benefit provided by the remaining viable cells. These challenges highlight the need for strategies to enhance the survival and function of transplanted DA neurons.
The majority of grafted cell death occurs within the first few days after transplantation, largely due to apoptosis.7–9 There are several detrimental conditions associated with the transplant procedure and post-transplantation in vivo environment that can trigger cell death, including hypoxia, withdrawal of trophic factors, and anoikis.10,11 During preparation for transplantation, cells are typically dissociated into a single cell suspension, which itself can induce apoptosis due to disruption of survival signals from contact with the extracellular matrix and neighboring cells.11,12 Additional cell death that occurs after neuronal transplantation can be attributed to immune rejection, trauma at the site of injection, which causes bleeding and tissue damage, or necrosis due to poor graft vascularization and lack of trophic support.13–15
Culturing cells within an injectable biomaterial scaffold precludes the need for cell dissociation before transplantation, maintaining cell identity as well as intact cell–cell and cell–biomaterial interactions. These scaffolds can potentially serve as a delivery platform to improve the survival of transplanted cells by providing structural support for functional, adherent networks of neurons. One such type of scaffold can be formed from the self-assembling peptide RADA16-I, which is fabricated from alternating sequences of the amino acids arginine (R), alanine (A), and aspartic acid (D).16 This synthetic peptide spontaneously assembles into nanofibrous hydrogel scaffolds when exposed to millimolar amounts of monovalent cations and is able to support the growth of a variety of cells, including neural stem cells and neurons.17–22
We have previously shown that RADA16-I-based self-assembling peptide nanofiber scaffolds (SAPNS) can be used to transplant reprogrammed human neurons into the mouse brain, resulting in a more than 100-fold increase in survival compared with neurons in suspension.19 The question of whether similar scaffolds can be used for the transplantation of subtype-specific neurons for the treatment of PD has not yet been addressed.
In this study, we report that the SAPNS can be configured as three-dimensional (3D) substrates for the culture and maturation of human DA neurons derived from iPSCs. A microfluidic droplet generation procedure was used to encapsulate DA neurons within monodisperse RADA16-I microspheres. These microspheres were used as vehicles to transplant adherent networks of DA neurons into the striatum of a unilateral 6-hydroxydopamine (6-OHDA)-lesioned mouse model of PD, facilitating long-term cell survival and support of motor function recovery.
Methods
Cell culture
All cell types were cultured in a Galaxy 170R incubator (Eppendorf) and maintained at 37°C in a humidified atmosphere with 5% CO2 and 5% O2. Human iPSCs (Supplementary Figure S1) were maintained in PeproGrow hESC medium (PeproTech) with 1:100 penicillin/streptomycin (ThermoFisher Scientific) and passaged by using Cell Passaging/Non-Enzymatic Detachment Buffer (PeproTech). For initial neurosphere formation, dissociated iPSCs were plated onto a non–tissue-culture-treated six-well dish (Eppendorf) in DOPA1 medium consisting of DOPA base medium (PeproGrow Neural Stem Cell [NSC] medium with 1 × insulin-containing NSC supplement [PeproTech], 200 μM L-ascorbic acid [Cat. No. 16457; Cayman], and 10 μg/mL gentamycin [15710064; ThermoFisher]) supplemented with 10 μM EC-23 (1044131; Biogems) and 10 μM Y-27632 (1293823; Biogems).
After 48 h, 0.5 μM Smoothened Agonist (SAG) was added to the medium and the concentration of EC-23 was lowered to 1 μM. Twenty-four hours later, neurospheres were plated onto a six-well tissue culture polystyrene (TCPS) dish (Eppendorf) (Supplementary Figure S2) coated with 5 μg/mL laminin (MilliporeSigma), in DOPA2 medium (base medium supplemented with 0.5 μM SAG [9128694; Biogems]), 5 μM Y-27632, and 10 ng/mL of the following growth factors (PeproTech): stromal cell-derived factor 1-alpha (SDF-1α); Pleiotrophin; hepatocyte growth factor (HGF); insulin-like growth factor-2 (IGF-2); vascular endothelial growth factor-D (VEGF-D); secreted frizzled-related protein 1 (SFRP-1), fibroblast growth factor-8 (FGF-8); and 20 ng/mL cerebral dopamine neurotrophic factor (CDNF). Cells were maintained in DOPA2 medium for at least 7 days or up to 35 passages for expansion of DA neural progenitors. For the initial three passages, neuronal type cells were removed from the TC dish by using a brief treatment with Detachment Buffer coupled with slow and gentle trituration using a P1000 type micropipette.
Thereafter, DOPA2 cells were passaged by incubation with Detachment Buffer for up to 5 min, removal of buffer before cell detachment, and gentle trituration in DOPA medium. For expansion, DOPA2 cells were plated at a density of 10K–40K cells per cm2, and for differentiation at a density of 50K–100K cells per cm2.
For final differentiation to mature DA neurons, cells were passaged onto a TCPS dish (Eppendorf) coated with 5 μg/mL laminin, into DOPA2 medium and 24 h later switched to DOPA3 medium for up to 30 days (base medium supplemented with the following growth factors [PeproTech]: 10 ng/mL brain-derived neurotrophic factor [BDNF] and glial cell line-derived neurotrophic factor [GDNF], 1 ng/mL transforming growth factor [TGF]-β3, 2.5 ng/mL Activin A, and 20 ng/mL CDNF; and the following small molecules: 250 μM dibutyryl cyclic AMP [dbcAMP, 1698950; Biogems], 10 μM N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-(S)-phenylglycine t-butyl ester [DAPT] [2088055; Biogems]).
Encapsulation of cells within bulk self-assembling peptide nanofiber scaffolds
The self-assembling peptide RADA16-I was kindly provided by 3-D Matrix, Inc. (Waltham, MA). Encapsulation of cells within bulk SAPNS was performed as previously described with minor modifications.19 To encapsulate cells within RADA16-I (diluted to 0.5% in 10% sucrose, final pH 3.0), DOPA2 cells were passaged with Detachment Buffer and washed in 9% sucrose before resuspending in fresh 9% sucrose (pH 6.5) at a concentration of 50 × 106 cells/mL. The cell suspension and RADA16-I peptide were mixed in a 1:1 ratio before transferring the mixture into a non-TCPS plate and adding DOPA2 culture medium to induce gelation, resulting in a final cell concentration of 25 × 106 cells/mL. After 48 h, the medium was changed to DOPA3 and the encapsulated cells were maintained at 37°C and 5% CO2/5% O2. The medium was changed every other day for the duration of the experiments.
SAPNS microsphere fabrication
Cells were encapsulated within RADA16-I microspheres by using a microfluidic droplet generation procedure as previously described with minor modifications.19 Fluorinated ethylene propylene tubing (0.01′′ inner diameter) and polyether ether ketone micro-T-junction connectors (0.02′′ inner diameter) (IDEX Health and Science) were connected to the X-junction in a hydrophobic glass droplet junction chip (190 μm etch depth) (Dolomite Microfluidics).
A horizontal 5-mL syringe, containing hexadecane with 1% Span 80 (MilliporeSigma), was connected to a syringe pump (flow rate: 0.15 mL/min) (ThermoFisher Scientific). A vertical 1-mL syringe, containing 0.5% RADA16-I+cell suspension in 9% sucrose (1:1), was connected to a second syringe pump (flow rate: 0.01 mL/min). The resulting microspheres, containing cells encapsulated at a final concentration of 25 × 106 cells/mL, were collected in a microcentrifuge tube containing DOPA2 medium with 0.25% Tween 20, centrifuged at 200 g for 30 s to pellet the microspheres, and washed once with DOPA2 medium before transferring the microspheres to a non-TCPS plate in fresh DOPA2 medium and culturing as described earlier.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated by using an RNeasy Plus Mini Kit (Qiagen) and reverse transcribed by using a High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific) according to the manufacturer's instructions. The cDNA was used with commercially available TaqMan gene expression assays (listed in Supplementary Table S2) on a PikoReal 96 RealTime PCR System (ThermoFisher Scientific). The cycling parameters were as follows: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Relative gene expression was calculated by using the ΔΔCT method after normalizing to GAPDH expression with reference to DOPA2 cells. Each data point represents three technical replicates from three independent biological samples.
Quantification of dopaminergic levels
Intracellular DA levels were measured after 22 days of differentiation by using high performance liquid chromatography (HPLC) with electrochemical detection (Waters 2695 Separations Module with Waters 2465 Electrochemical Detector, Milford, MA). Cells were lysed in 0.4 N perchloric acid with 0.1 mM Ethylenediaminetetraacetic acid (EDTA), and the homogenate was centrifuged at 13,200 g for 15 min at 4°C. The supernatant was removed and stored at −80°C until analysis. The remaining cell pellet was lysed with 0.5 M NaOH, and protein levels were quantified by using a MicroBCA assay (Pierce).
Samples were injected onto a Biophase ODS C-18 reverse-phase column (5 mm, 250 × 4.6 mm i.d.) at a constant flow rate of 1.0 mL/min with electrochemical detector settings of Vout = +0.002V, Range = 500 nA, Ic = 0.0nA, Ec = +0.70V. The mobile phase consisted of 0.1375 M sodium phosphate (dibasic), 0.0625 M citric acid, 5.0 mg EDTA, and 10% methanol. Quantitative analysis was performed by comparing sample peak heights with known external standards of DA and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and 5-hydroxytryptamine (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA).
Calcium imaging
Neurons were labeled with 3 μM Fluo-4 acetoxymethyl (AM) for 30 min at room temperature in an extracellular bath solution (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4) containing 0.02% Pluronic F-127 (MilliporeSigma). The cells were incubated in bath solution for an additional 30 min at room temperature before imaging. Time-lapse imaging was performed on a Zeiss LSM 780 confocal microscope at a resolution of 512 × 256 pixels and a time resolution of 16.5 Hz. High KCl buffer (100 mM) was added during imaging to induce depolarization. Image analysis was performed by using Fiji/ImageJ23 for ROI selection and quantification of change in fluorescence over time (ΔF/F0, where F0 = average fluorescence intensity over the first 200 frames). Cells were considered responsive if they displayed an increase in fluorescence at least three standard deviations above F0 (average fluorescence intensity over the first 200 frames) after addition of the high KCl buffer. Twenty fields of view and a minimum of 600 cells were analyzed for each experimental group.
In vivo transplantation
6-OHDA lesion
All animal experiments were carried out according to the Rutgers University Policy on Animal Welfare and were approved by the Institutional Animal Care and Use Committee (IACUC) at Rutgers University Robert Wood Johnson Medical School. NOD-SCID IL2Rγc null male mice (8–9 weeks old; Jackson Laboratory) were anesthetized with isoflurane (induction at 4% and maintained at 0.5–1% inhalation). Before surgery, mice received an intraperitoneal (i.p.) injection of desipramine (25 mg/kg) and pargyline (5 mg/kg) to increase the efficacy and selectivity of the 6-OHDA lesion.24–26
Mice were positioned in a stereotaxic frame (Kopf Instruments), and 6-OHDA hydrobromide (MilliporeSigma) (reconstituted in 0.9% saline +0.02% ascorbic acid) was injected into the left medial forebrain bundle by using a Neuros syringe (Hamilton) at the following coordinates (in mm): anteroposterior (AP): −1.2 (from bregma); mediolateral (ML): −1.1; dorsoventral (DV): −5.0 (from dura). A total mass of 4 μg 6-OHDA (free base) was injected over a period of 5 min (0.2 μL total volume). The needle was allowed to remain in place for an additional 6 min after injection before slowly retracting the syringe. Three weeks after the 6-OHDA lesion surgery, mice were assessed to determine the lesion severity. Mice displaying more than seven contralateral rotations/min after s.c. injection of apomorphine (see Behavioral testing) were selected for cell transplantation.
Cell transplantation
Cell transplantation was performed four weeks after the 6-OHDA lesion surgery. Cells were treated with 1 μg/mL Mitomycin C (MilliporeSigma) for 3 h on day 20 of differentiation to inactivate residual mitotic cells before transplantation,27 and they were harvested for transplantation on day 22 of differentiation.
DA neurons grown in two-dimensional (2D) were treated with Detachment Buffer, and SAPNS-encapsulated DA neurons were collected and resuspended in ice cold Hank's Balanced Salt Solution (Gibco). The dissociated DA neurons (12,000 cells; 5 μL volume) or SAPNS-encapsulated DA neurons (5 μL) were stereotaxically injected into the striatum by using a Hamilton 7000 Series Modified Microliter Syringe (with 24G needle) at the following coordinates (in mm): AP: 0.5 (from bregma); ML: −2.0; DV: −4.0 (2.5 μL of cell suspension), needle retracted to −3.0 to inject the remaining 2.5 μL. Control mice received injections of an equivalent number of dead cells (freeze-thawed) as previously described.28 The needle was allowed to remain in place for an additional 5 min after injection before slowly retracting the syringe.
Behavioral testing
See Supplementary Data.
Tissue processing and immunohistochemistry
See Supplementary Data.
Statistical analysis
Statistical analysis was performed by using GraphPad Prism 8 software (GraphPad). Data were analyzed by using one-way ANOVA followed by Tukey's post hoc multiple-comparisons test or unpaired two-tailed t-test for comparison of two groups, with p < 0.05 considered statistically significant. All data are reported as mean ± standard error.
Results
SAPNS support DA neuronal differentiation
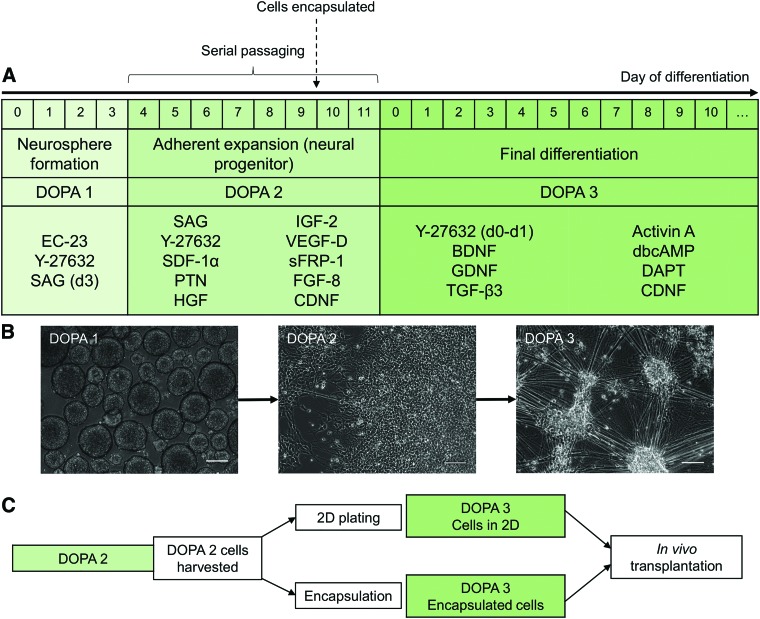
DA neurons were differentiated from iPSCs by using a modified protocol based on the stromal-derived inducing activity caused by growth factors secreted from PA6 stromal cells.29–33 Here, we developed a three-step approach for generating DA neurons, which comprised an initial neurosphere formation stage, an expansion stage where DA neural progenitors could be serially passaged up to 35 times and frozen for later use, and a final differentiation stage (Fig. 1). Initial neurosphere formation was induced with EC-23, a photostable retinoic acid analog that induces neural differentiation of pluripotent stem cells (PSCs), and the ROCK inhibitor Y-27632, which counteracts dissociation-induced apoptosis.34,35
FIG. 1.
Protocol for differentiation and encapsulation of DA neurons. (A) Protocol for expansion of DA neural progenitors and final differentiation of DA neurons. (B) Representative phase-contrast images of DOPA1, DOPA2, and DOPA3 cells. Scale bar: 100 μm. (C) Timeline for encapsulation within SAPNS. DA, dopaminergic; SAPNS, self-assembling peptide nanofiber scaffolds. Color images are available online.
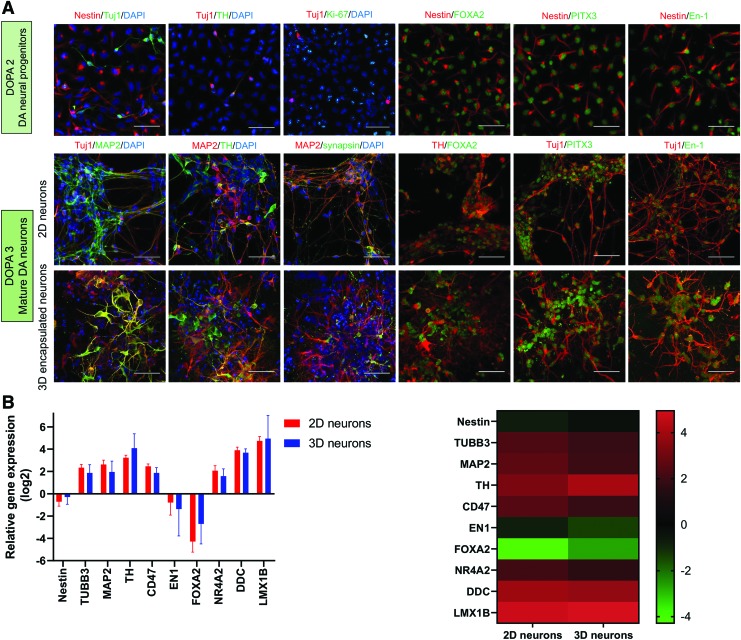
Growth factors and cytokines that induce DA neuronal differentiation (SDF-1α, pleiotrophin, HGF, IGF-2, VEGF-D, sFRP-1) were previously identified through gene expression analysis of PA6 cells and were included within the culture medium used to generate and expand DA neuronal progenitors.29,33 This medium was further supplemented with the Sonic hedgehog agonist SAG, which specifies the ventral pole of the neural tube, FGF-8, which has been observed to promote maturation of DA neurons, and CDNF, which has neuroprotective and regenerative effects on DA neurons.36–38 The resultant DA neural progenitors widely expressed the neural progenitor marker Nestin and midbrain DA markers FOXA2, PITX3, and En-1 (Fig. 2A; individual images shown in Supplementary Figures S3, S4, S5).
FIG. 2.
Characterization of DA neural progenitors and DA neurons. (A) Immunocytochemical staining of the following markers: Nestin: neural stem/progenitor, Tuj1 (β-III tubulin): neuron, MAP2: mature neuron, TH: DA neuron, Ki-67: proliferative cells, Synapsin: synaptic vesicle, FOXA2: midbrain DA neuron, PITX3: midbrain DA neuron, En-1 (Engrailed-1): midbrain DA neuron, DAPI: nucleus. Scale bar: 50 μm. (B) qRT-PCR analysis comparing gene expression in 2D and 3D (SAPNS-encapsulated) DA neurons at day 22 of differentiation. Data are presented as log2 of the fold change in expression relative to DOPA2 neural progenitors, shown in a bar graph (left) and heat map (right). Error bars represent standard deviation. p > 0.05, Student's unpaired t-test. 2D, two-dimensional; 3D, three-dimensional; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; TH, tyrosine hydroxylase. Color images are available online.
Final differentiation was initiated in medium supplemented with the growth factors BDNF, GDNF, TGF-β3, and CDNF, to support differentiation and survival of mature neurons, and Activin A, dbcAMP, and the Notch inhibitor DAPT, which further promote neuronal differentiation.39–42 Cells were encapsulated in bulk RADA16-I SAPNS 48 h before initiating final differentiation. Cellular phenotype was assessed at day 22 of differentiation (10 days after initiating final differentiation in DOPA3 medium) via immunocytochemistry and quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis. Cells in both 2D and 3D configurations expressed the mature neuronal markers MAP2 and synapsin, and the DA neuronal markers tyrosine hydroxylase (TH), FOXA2, PITX3, and En-1 (Fig. 2A). Quantification of immunocytochemical staining indicated high levels of MAP2 and TH expression in both 2D and 3D configurations that were not significantly different (MAP2: 94.1% ± 0.76% in 2D vs. 93.6% ± 1.53% in 3D, p = 0.7897; TH: 92.5% ± 1.73% in 2D vs. 92.3% ± 1.52% in 3D, p = 0.9624).
Quantitative RT-PCR (TaqMan) was used to analyze the gene expression of DA neurons cultured in 2D and 3D, relative to the DA neural progenitors before encapsulation (Fig. 2B). Analysis revealed upregulation of the neuronal markers MAP2 and TUBB3, along with DA neuron-specific markers such as TH, NR4A2, LMX1B, and DDC, and CD47, an integrin-associated protein that has been used for enrichment of hPSC-derived DA neural progenitors.43 Expression of the midbrain DA markers EN1 and FOXA2, the neural progenitor marker Nestin, and astrocytic marker GFAP was downregulated relative to the DA neural progenitors. There was no significant difference in expression of these markers in the DA neurons cultured in 2D or 3D relative to neural progenitors or through direct comparison (Supplementary Tables S3 and S4), indicating that DA neuronal differentiation was similar in both configurations.
SAPNS support in vitro functional maturation of DA neurons
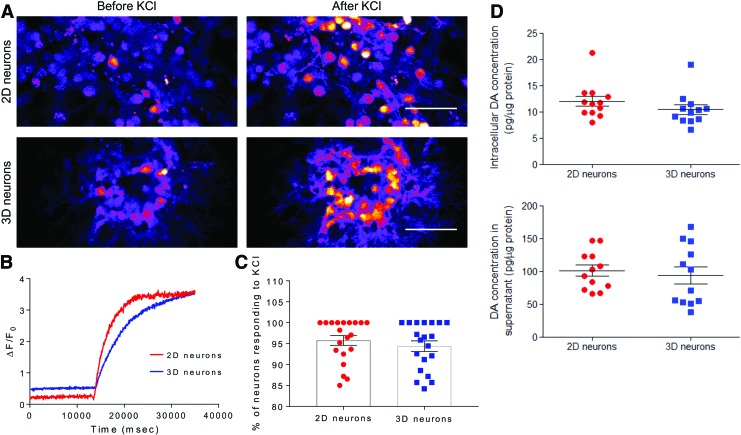
A population-wide analysis of the functional activity of DA neurons encapsulated within 3D SAPNS or cultured in 2D was performed by loading cells with the calcium indicator dye Fluo-4 AM and quantifying the change in somatic fluorescence in response to depolarization induced by the addition of a 100 mM KCl buffer. Addition of KCl caused a significant increase in somatic fluorescence compared with baseline levels in the DA neurons in both the 2D and 3D SAPNS, as shown in still images taken from time lapse videos and representative traces of the change in fluorescence intensity over time (Fig. 3A, B). The percentage of DA neurons encapsulated within the SAPNS that responded to the addition of KCl (94.4% ± 1.26%) was not significantly different from the percentage of responding DA neurons in 2D (95.8% ± 1.15%) (Fig. 3C).
FIG. 3.
SAPNS supports the functional maturation of encapsulated neurons. (A) Still images from time lapse videos during live cell calcium imaging before and after the addition of high KCl buffer. Scale bar: 50 μm. (B) Representative plot of the change in fluorescence compared with baseline fluorescence (ΔF/F0). (C) Quantification of the percentage of neurons in 2D and 3D responding to the addition of high KCl buffer (100 mM). Data are presented as mean ± SEM. p > 0.05, Student's unpaired t-test. (D) HPLC-ECD quantification of DA present intracellularly and in 24-h conditioned medium, normalized to the mass of protein present. Data are presented as mean ± SEM. p > 0.05, Student's unpaired t-test. HPLC-ECD, high performance liquid chromatography-electrochemical detection; SEM, standard error of the mean. Color images are available online.
Functional maturation was also assessed by quantifying intracellular DA content and the concentration of DA released into the culture medium supernatant. Using HPLC with electrochemical detection, DA was detected intracellularly within DA neurons grown on a 2D culture surface and within the 3D SAPNS (2D: 12.1 ± 0.98 pg DA/μg protein vs. 3D: 10.5 ± 0.90 pg DA/μg protein, p > 0.05) (Fig. 3D). DA released into the culture medium over a 24-h period was also detected in both 2D and 3D cultures (2D: 101.2 ± 8.43 pg DA/μg protein vs. 3D: 97.7 ± 12.4 pg DA/μg protein, p > 0.05). These data indicate that the DA neurons cultured within the SAPNS can maintain their identity and are capable of synthesizing and releasing DA.
Fabrication of monodisperse SAPNS microspheres
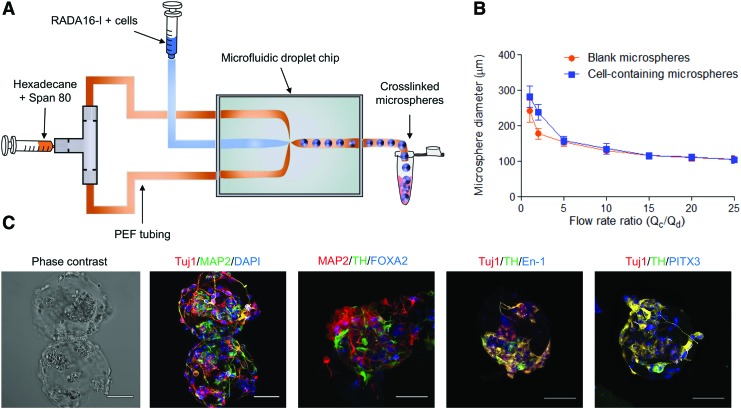
SAPNS microspheres were fabricated by using a microfluidic droplet generation protocol adapted from our previous work.19 A microfluidic X-junction was used to merge the flow of RADA16-I peptide mixed with DOPA2 cells and an immiscible organic phase of hexadecane containing 1% Span 80 as a water-in-oil droplet stabilizer (Fig. 4A) to produce microspheres containing cells encapsulated at a density of 25 × 106 cells/mL. There was an inverse correlation between the flow rate ratio [(flow rate of continuous organic phase (Qc)/flow rate of discontinuous phase of RADA16-I+cells (Qd))] and microsphere diameter, ranging in diameter from 241.5 ± 3.09 μm (flow rate ratio: 1) to 105.4 ± 9.82 μm (flow rate ratio: 25) for blank microspheres and from 282.6 ± 5.06 μm (flow rate ratio: 1) to 104.0 ± 1.03 μm (flow rate ratio: 25) for microspheres containing cells (Fig. 4B). Cells encapsulated and matured within the SAPNS microspheres also expressed the neuronal markers Tuj1 and MAP2, and the DA neuronal markers TH, En-1, PITX3, and FOXA2 and displayed neurite outgrowth similar to DA neurons cultured within the bulk SAPNS (Fig. 4C).
FIG. 4.
Fabrication of SAPNS microspheres using a microfluidic droplet generation method. (A) Schematic of the microsphere fabrication process. (B) Quantification of microsphere diameter versus flow rate ratio; mean ± SEM; (C) Immunocytochemical staining of cells encapsulated within SAPNS microspheres displaying the following markers: Tuj1 (β-III tubulin): neuron, MAP2: mature neuron, TH: DA neuron, FOXA2: midbrain DA neuron, PITX3: midbrain DA neuron, En-1 (Engrailed-1): midbrain DA neuron, DAPI: nucleus. Scale bar: 50 μm. Color images are available online.
SAPNS microspheres enhance survival of transplanted DA neurons in a Parkinson's disease mouse model
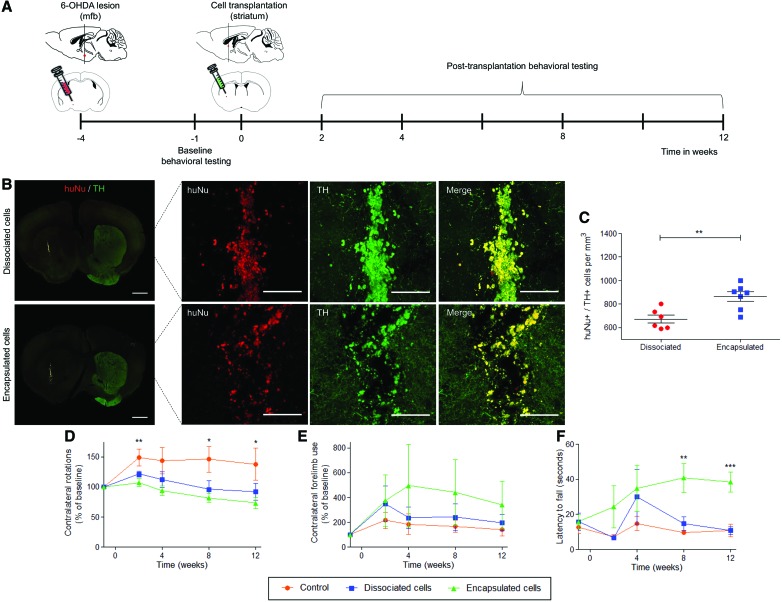
We next addressed whether SAPNS microspheres could serve as transplantation vehicles for encapsulated networks of DA neurons within the brains of PD model mice. Immunodeficient NOD-scid IL2Rgnull mice were unilaterally lesioned by injecting 6-OHDA into the medial forebrain bundle, which resulted in a significant loss of DA neurons in the ipsilateral substantia nigra and striatum (Supplementary Figure S6).44,45 Equivalent numbers of live cells in suspension or encapsulated within SAPNS microspheres were transplanted into the ipsilateral striatum four weeks after the initial lesion surgery (Fig. 5A). Cell numbers for transplantation were calculated by determining the average number of live cells present in each microsphere (50 ± 4 cells/microsphere) by counting Calcein AM-stained microspheres on the day before transplantation, and by counting the average number of microspheres within the injection volume (240 ± 13 microspheres in 5 μL) (Supplementary Figure S7).
FIG. 5.
In vivo survival of DA neurons transplanted within a 6-OHDA mouse model of PD. (A) Timeline for in vivo cell transplantation and behavioral testing. (B) Immunohistochemical staining of transplanted cells expressing human-specific nuclei (huNu) and TH within the mouse striatum. Scale bar for whole brain slice: 1 mm. Scale bar for inset: 100 μm. (C) Quantification of transplanted cells expressing TH and huNu within the mouse striatum. Data are presented as mean ± SEM. **p < 0.01, Student's unpaired t-test. (D) Change in apomorphine-induced contralateral rotations compared with baseline number of rotations. *p < 0.05, **p < 0.01. (E) Change in contralateral forelimb usage during the cylinder test compared with baseline contralateral forelimb usage. (F) Latency to fall during 25 rpm fixed speed rotarod. **p < 0.01, ***p < 0.001. Behavioral data are presented as mean ± SEM and were analyzed by one-way ANOVA with Tukey post hoc test at each time point. (D–F) Red: control (n = 6), Blue: dissociated cells (n = 6), Green: encapsulated cells (n = 7). 6-OHDA, 6-hydroxydopamine; ANOVA, analysis of variance; PD, Parkinson's disease. Color images are available online.
Survival of transplanted cells was quantified 13 weeks after transplantation, by counting cells expressing both TH and human-specific nuclei (huNu) within the graft (Fig. 5B). All animals survived after cell transplantation, until the designated end point of the study. Quantification revealed that SAPNS microspheres significantly increased the in vivo survival of encapsulated DA neurons (3016 ± 91; 25.1% survival) compared with neurons in suspension (2280 ± 105; 19% survival, p = 0.0002, Fig. 5C).
Transplantation of SAPNS-encapsulated DA neurons improves recovery of motor function in a PD mouse model
We also examined whether transplanting DA neurons within SAPNS microspheres could support motor recovery in the 6-OHDA-lesioned mice, as assessed by various behavioral tests before and at 2, 4, 8, and 12 weeks after cell transplantation. Rotational behavior was evaluated after the subcutaneous injection of apomorphine, a DA agonist, which preferentially stimulates the supersensitive DA receptors in the denervated hemisphere and, subsequently, causes contralateral rotations.46,47 At 8 and 12 weeks post-transplantation, mice that received transplants of SAPNS-encapsulated DA neurons showed a significant decrease in contralateral rotations compared with control mice (p < 0.05, Fig. 5D). This result indicated that the SAPNS-encapsulated DA neurons integrated well at the injection site and released DA within the denervated striatum ipsilateral to the 6-OHDA lesion, thereby reducing the DA receptor sensitization that causes apomorphine-induced rotations.
Forelimb asymmetry was assessed by counting the number of ipsilateral and contralateral forepaw touches when exploring the walls of a cylinder when rearing. Overall, 6-OHDA unilaterally lesioned mice display asymmetrical forelimb use, with a strong preference for using the unimpaired ipsilateral forepaw during weight-bearing movements. Mice that received transplants of encapsulated DA neurons showed an increase in contralateral forelimb use over 12 weeks; however, this difference was not statistically significant compared with the contralateral forelimb use in the control mice or mice receiving dissociated cell transplants (Fig. 5E).
Motor coordination was assessed by quantifying the latency to fall from a rotarod. At 8 and 12 weeks post-transplantation, mice that received transplants of SAPNS-encapsulated DA neurons were able to stay on the rotarod significantly longer than both the control mice and mice that received transplants of dissociated DA neurons (p < 0.01, 8 weeks; p < 0.001, 12 weeks), indicating that the SAPNS-encapsulated neurons released sufficient DA within the striatum to cause significant improvements in motor coordination (Fig. 5F). Overall, the effects on motor function indicate that the transplanted DA neurons were functional in vivo and transplantation using SAPNS microspheres supported and rescued several important indicators of motor function in the 6-OHDA lesioned mice.
Discussion
Successful grafting of mature neurons into the injured or neurodegenerative brain is challenged by a number of factors, including disruption of intact neuronal connections during cell dissociation, absence of structural support for the transplanted neurons, and a lack of cues to promote survival and integration within the injured CNS.11 Strategies to address these challenges are crucial to the success of future cell replacement therapies for PD. Here, we used microspheres of self-assembling peptide-based nanofibrous hydrogels (RADA16-I SAPNS) to transplant adherent networks of human DA neurons into the striatum of 6-OHDA lesioned mice, significantly improving post-transplantation survival and supporting recovery of motor function. The SAPNS microspheres provided a growth permissive environment and protected encapsulated DA neurons throughout various stages of the transplantation process that can trigger cell death: cell harvesting, the injection procedure, and the acute postinjection period.3
Hydrogel scaffolds have been previously used to improve the post-transplantation survival of neural progenitor cells; however, grafting immature precursors into the brain can lead to unchecked proliferation and teratoma formation in vivo.18,48–50 Further, neural progenitors and immature neurons are only appropriate for PD cell replacement therapies if they are able to fully mature into functional DA neurons after transplantation and integrate appropriately in vivo.51 Encapsulating embryonic VM cells within hydrogel scaffolds has also been shown to significantly improve their survival and function in vivo, yet this does not solve the problem of the serotonergic neurons contained within VM grafts that are known to cause graft-induced dyskinesias.52–54
Mature, postmitotic DA neurons derived from PSCs are fully lineage-committed, but harvesting these fragile cells for transplantation has been a challenge due to the direct physical trauma imposed on the elaborate neuronal networks that have formed during in vitro culture.55 We hypothesized that culturing and maturing DA neurons within the SAPNS hydrogels before transplantation offers a biocompatible 3D microenvironment that maintains the cell–cell and cell–material interactions that would otherwise be disrupted during dissociation. Allowing neurons to mature within the gels enabled us to transplant intact functional networks of DA neurons in vivo, thereby avoiding the cell dissociation that is prone to causing apoptosis in postmitotic neurons. Further, encapsulating cells within RADA16-I at the neural progenitor stage protects the more delicate mature DA neurons from direct exposure to the low pH of the ungelled peptide, which caused significant amounts of neuronal death. Thus, the hydrogel encapsulation approach offers a versatile technology to promote both differentiation of subtype-specific neurons and maturation of neuronal networks ready for transplantation.
Typically, the neuronal injection procedure itself is a stage of the transplantation process that can cause rapid cell death. Cells exposed to syringe needle flow experience linear shear and extensional flow forces that can damage the cell membrane, particularly when cells are suspended in a low viscosity solution. Extensional flow is caused by abrupt changes in geometry such as the tapering from the syringe to the needle, resulting in large increases in fluid flow velocity, which causes cells to stretch and deform, ultimately leading to cell death.56 Hydrogel carriers can protect encapsulated cells during injection via “plug flow,” which occurs when a thin layer of hydrogel near the needle wall acts as a lubricant, allowing the rest of the hydrogel to pass through the needle intact.56,57 Using SAPNS in the form of microspheres for transplantation protected encapsulated cells from the mechanical trauma of dissociation during the cell harvesting process and during injection, which, we propose, significantly improved survival of human DA neurons within the striatum of 6-OHDA lesioned mice.
Similar improvements in survival of mature DA neurons have been reported by using a functionalized hyaluronic acid-based hydrogel for transplantation, indicating that the strategy of using hydrogel scaffolds as transplantation vehicles is beneficial for improving the success of neuronal transplantation therapies.58 In our study, additional factors that may have contributed to improvements in post-transplantation survival are the use of CDNF within the culture medium and culturing cells in a biologically relevant low O2 environment. CDNF has been shown to be neuroprotective of DA neurons, and our low O2 culture environment (5% compared with the typical 21% atmospheric oxygen levels) may have had the effect of pre-acclimating the cells to the hypoxic graft environment.38
The increase in DA neuronal survival seen in mice receiving grafts of SAPNS-encapsulated neurons corresponded with parallel improvements in motor function of the lesioned mice. The apomorphine test is an indirect measurement of increases in striatal DA due to cell transplantation. Transplanted cells that release DA in the striatum decrease the supersensitivity of the DA receptors in the lesioned hemisphere, normalizing the response to apomorphine relative to the intact hemisphere.59 Although mice receiving transplants of SAPNS-encapsulated neurons showed significantly fewer contralateral rotations compared with the control mice, there was no significant difference in the rotation decrease compared with mice receiving neurons in suspension. It is likely that with regard to this behavioral test, differences in cell survival or DA release between these two experimental groups were not large enough to show a significant difference in the response to apomorphine relative to the intact hemisphere, particularly with the relatively low number of neurons transplanted.
Using the cylinder test, a measure of spontaneous forelimb usage/forelimb asymmetry, there was an increase in contralateral paw usage for mice receiving encapsulated neurons, though this increase was not significantly different. Grealish et al. found that the cylinder test was unable to distinguish between a severe, intermediate, or mild 6-OHDA lesion, so it is likely that this test was also not sensitive enough to detect any differences between the experimental groups used in our study.60 In comparison to the cylinder test, the rotarod has been found to be the best spontaneous motor test predictor of nigrostriatal cell loss and is a particularly sensitive test for motor coordination and balance.61 Using this test, we found significant increases in the latency to fall for mice receiving encapsulated neurons when compared with both mice receiving neurons in suspension and control mice.
These behavioral improvements mirror those described in a recent study by Moriarty et al., who used GDNF-loaded collagen hydrogels to transplant 400,000 encapsulated embryonic VM cells into the striatum of a PD rodent model.54 Direct comparisons of functional recovery cannot be made with this particular study since different behavioral tests were used. Adil et al. report the use of a hyaluronic acid-based hydrogel to achieve improvements in survival and functional recovery after transplanting 100,000 postmitotic DA neurons.62 Similar to our work, this group showed significantly reduced apomorphine-induced rotations for mice receiving injections of encapsulated neurons as early as 4–8 weeks post-transplantation, and improved contralateral forelimb function in the cylinder test at 4 weeks, although forelimb asymmetry was not significantly different from mice receiving dissociated neurons in subsequent time points analyzed.62
This behavioral function corresponds to a total of ∼1500 surviving cells in the dissociated cell cohort (1.5% survival) compared with ∼6100 surviving cells in the 3D gel cohort (6.1% survival), indicating that significant differences in motor function can, indeed, be observed with minor improvements in the survival of transplanted cells.62 Notably, the behavioral recovery we report here was achieved after transplanting merely 12,000 cells, which is markedly lower than the number of cells previously reported to achieve significant improvements in motor function compared with control rodents. Using the smallest possible number of cells for transplantation can decrease potential damage at the injection site and reduce the risk of tumorigenesis.
Previous studies have reported significant improvements in motor function after transplanting 100,000–400,000 mature DA neurons in vivo.4,63–65 Improvements in behavioral recovery that are seen in rodents receiving such large numbers of transplanted cells can be partially attributed to damaging striatal output neurons, which also has the effect of eliminating supersensitive DA receptors and thereby mitigating apomorphine-induced rotations.66 Improving the outcomes of cell transplantation therapies is likely to be a combinatorial approach, which can include the strategy of transplanting scaffold-encapsulated neurons and other effective treatments to maximize the survival of transplanted cells.
The studies reported earlier supplemented the hydrogels with key bioactive factors (Moriarty et al.: GDNF; Adil et al.: a combination of Ephrin-B2, HGF, and GDNF) to promote both survival and significant enhancements in functional recovery. Only the groups receiving transplants of neurons within these supplemented hydrogels demonstrated significantly improved motor function for the entire duration of the study.54,62 Although the chosen chemistry of the SAPNS used in our study lacks explicit bioactive domains, the RADA16-I peptide sequence can be readily modified with various neurotrophic or neuroprotective functional motifs to further improve survival, particularly within immunocompetent animals. Further, neurotrophic factors can also be included within the SAPNS for controlled release as described earlier, which could further improve survival and functional benefits.54,62
In conclusion, using SAPNS microspheres as delivery vehicles for human DA neurons in a PD mouse model significantly improved post-transplantation cell survival and supported rescue of motor function. This cell transplantation paradigm protected DA neurons at crucial stages during the transplantation process and the SAPNS provided a permissive environment for growth, retaining cells at the injection site and facilitating long-term survival, maturation, and function. Overall, the use of hydrogel-encapsulated human neurons shows significant potential as a basis for improving the success of stem cell transplantation therapies.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge 3-D Matrix for the gift of the RADA16-I self-assembling peptide (PuraMatrix).
Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported here was supported by NIH R21 NS095082, NIH T32EB005583, and NIH R01 AA023797.
Supplementary Material
References
- 1. DeMaagd G., and Philip A.. Part 2: introduction to the pharmacotherapy of Parkinson's disease, with a focus on the use of dopaminergic agents. Pharm Ther 40, 590, 2015 [PMC free article] [PubMed] [Google Scholar]
- 2. Lindvall O., and Björklund A.. Cell therapy in Parkinson's disease. NeuroRx 1, 382, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brundin P., Karlsson J., Emgård M., et al. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant 9, 179, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Kriks S., Shim J.W., Piao J., et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doi D., Samata B., Katsukawa M., et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Reports 2, 337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grealish S., Diguet E., Kirkeby A., et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell 15, 653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahalik T.J., Hahn W.E., Clayton G.H., and Owens G.P.. Programmed cell death in developing grafts of fetal substantia nigra. Exp Neurol 129, 27, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Barker R.A., Dunnett S.B., Faissner A., and Fawcett J.W.. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol 141, 79, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Zawada W.M., Zastrow D.J., Clarkson E.D., Adams F.S., Bell K.P., and Freed C.R.. Growth factors improve immediate survival of embryonic dopamine neurons after transplantation into rats. Brain Res 786, 96, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Marchionini D.M., Collier T.J., Camargo M., McGuire S., Pitzer M., and Sortwell C.E.. Interference with anoikis-induced cell death of dopamine neurons: implications for augmenting embryonic graft survival in a rat model of Parkinson's disease. J Comp Neurol 464, 172, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Marquardt L.M., and Heilshorn S.C.. Design of injectable materials to improve stem cell transplantation. Curr Stem Cell Reports 2, 207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schierle G.S., Hansson O., Leist M., Nicotera P., Widner H., and Brundin P.. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med 5, 97, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Emmett C., Jaques-Berg W., and Seeley P.. Microtransplantation of neural cells into adult rat brain. Neuroscience 38, 213, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Ideguchi M., Shinoyama M., Gomi M., Hayashi H., Hashimoto N., and Takahashi J.. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell–derived neural precursor cells. J Neurosci Res 86, 1936, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Emgård M., Hallin U., Karlsson J., Bahr B., Brundin P., and Blomgren K.. Both apoptosis and necrosis occur early after intracerebral grafting of ventral mesencephalic tissue: a role for protease activation. J Neurochem 86, 1223, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Zhang S., Holmes T.C., DiPersio C.M., Hynes R.O., Su X., and Rich A.. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 16, 1385, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Aligholi H., Rezayat S.M., Azari H., et al. Preparing neural stem/progenitor cells in PuraMatrix hydrogel for transplantation after brain injury in rats: a comparative methodological study. Brain Res 1642, 197, 2016 [DOI] [PubMed] [Google Scholar]
- 18. Cheng T.-Y., Chen M.-H., Chang W.-H., Huang M.-Y., and Wang T.-W.. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 34, 2005, 2013 [DOI] [PubMed] [Google Scholar]
- 19. Francis N.L., Bennett N.K., Halikere A., Pang Z.P., and Moghe P.V.. Self-assembling peptide nanofiber scaffolds for 3-D reprogramming and transplantation of human pluripotent stem cell-derived neurons. ACS Biomater Sci Eng 2, 1030, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmes T.C., de Lacalle S., Su X., Liu G., Rich A., and Zhang S.. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci 97, 6728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ylä-Outinen L., Joki T., Varjola M., Skottman H., and Narkilahti S.. Three-dimensional growth matrix for human embryonic stem cellsderived neuronal cells. J Tissue Eng Regen Med 8, 186, 2014 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z.X., Zheng Q.X., Wu Y.C., and Hao D.J.. Compatibility of neural stem cells with functionalized self-assembling peptide scaffold in vitro. Biotechnol Bioprocess Eng 15, 545, 2010 [Google Scholar]
- 23. Schindelin J., Arganda-Carreras I., Frise E., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiele S.L., Warre R., and Nash J.E.. Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson's disease. J Vis Exp 60, 3234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schallert T., Fleming S.M., Leasure J.L., Tillerson J.L., and Bland S.T.. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39, 777, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Henry B., Crossman A., and Brotchie J.M.. Characterization of a rodent model in which to investigate the molecular and cellular mechanisms underlying the pathophysiology of L-dopa-induced dyskinesia. Adv Neurol 78, 53, 1998 [PubMed] [Google Scholar]
- 27. Acquarone M., de Melo T.M., Meireles F., et al. Mitomycin-treated undifferentiated embryonic stem cells as a safe and effective therapeutic strategy in a mouse model of Parkinson's disease. Front Cell Neurosci 9, 97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang W.-Z., Liu T.-T., Cao J.-W., et al. Fear erasure facilitated by immature inhibitory neuron transplantation. Neuron 92, 1352, 2016 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz C.M., Tavakoli T., Jamias C., et al. Stromal factors SDF1α, sFRP1, and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J Neurosci Res 90, 1367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawasaki H., Mizuseki K., Nishikawa S., et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell–derived inducing activity. Neuron 28, 31, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Perrier A.L., Tabar V., Barberi T., et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A 101, 12543, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawasaki H., Suemori H., Mizuseki K., et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci 99, 1580, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vazin T., Becker K.G., Chen J., et al. A novel combination of factors, termed SPIE, which promotes dopaminergic neuron differentiation from human embryonic stem cells. PLoS One 4, e6606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clemens G., Flower K.R., Henderson A.P., et al. The action of all-trans-retinoic acid (ATRA) and synthetic retinoid analogues (EC19 and EC23) on human pluripotent stem cells differentiation investigated using single cell infrared microspectroscopy. Mol Biosyst 9, 677, 2013 [DOI] [PubMed] [Google Scholar]
- 35. Watanabe K., Ueno M., Kamiya D., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25, 681, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Lee K.J., and Jessell T.M.. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci 22, 261, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Lim M.-S., Lee S.Y., and Park C.-H.. FGF8 is essential for functionality of induced neural precursor cell-derived dopaminergic neurons. Int J Stem Cells 8, 228, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindholm P., Voutilainen M.H., Laurén J., et al. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448, 73, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Link A.S., Zheng F., and Alzheimer C.. Activin signaling in the pathogenesis and therapy of neuropsychiatric diseases. Front Mol Neurosci 9, 32, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mena M.A., Casarejos M.J., Bonin A., Ramos J.A., and De Yébenes J.G.. Effects of dibutyryl cyclic AMP and retinoic acid on the differentiation of dopamine neurons: prevention of cell death by dibutyryl cyclic AMP. J Neurochem 65, 2612, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Borghese L., Dolezalova D., Opitz T., et al. Inhibition of notch signaling in human embryonic stem cell–derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 28, 955, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Crawford T.Q., and Roelink H.. The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling. Dev Dyn 236, 886, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Lehnen D., Barral S., Cardoso T., et al. IAP-based cell sorting results in homogeneous transplantable dopaminergic precursor cells derived from human pluripotent stem cells. Stem Cell Reports 9, 1207, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Glajch K.E., Fleming S.M., Surmeier D.J., and Osten P.. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson's disease. Behav Brain Res 230, 309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boix J., Padel T., and Paul G.. A partial lesion model of Parkinson's disease in mice—characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav Brain Res 284, 196, 2015 [DOI] [PubMed] [Google Scholar]
- 46. Hudson J.L., van Horne C.G., Strömberg I., et al. Correlation of apomorphine-and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 626, 167, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand 82, 69, 1971 [DOI] [PubMed] [Google Scholar]
- 48. Roy N.S., Cleren C., Singh S.K., Yang L., Beal M.F., and Goldman S.A.. Functional engraftment of human ES cell–derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12, 1259, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Sonntag K.C., Pruszak J., Yoshizaki T., Van Arensbergen J., Sanchez-Pernaute R., and Isacson O.. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells 25, 411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ballios B.G., Cooke M.J., Donaldson L., et al. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell Reports 4, 1031, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grade S., and Götz M.. Neuronal replacement therapy: previous achievements and challenges ahead. NPJ Regen Med 2, 29, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Politis M., Wu K., Loane C., et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci Transl Med 2, 38ra46, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Wang T.Y., Bruggeman K.F., Kauhausen J.A., Rodriguez A.L., Nisbet D.R., and Parish C.L.. Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of Parkinson's disease. Biomaterials 74, 89, 2016 [DOI] [PubMed] [Google Scholar]
- 54. Moriarty N., Pandit A., and Dowd E.. Encapsulation of primary dopaminergic neurons in a GDNF-loaded collagen hydrogel increases their survival, re-innervation and function after intra-striatal transplantation. Sci Rep 7, 16033, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jönsson M.E., Ono Y., Björklund A., and Thompson L.H.. Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp Neurol 219, 341, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Aguado B.A., Mulyasasmita W., Su J., Lampe K.J., and Heilshorn S.C.. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 18, 806, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leslie S.K., Cohen D.J., Sedlaczek J., Pinsker E.J., Boyan B.D., and Schwartz Z.. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials 34, 8172, 2013 [DOI] [PubMed] [Google Scholar]
- 58. Adil M.M., Vazin T., Ananthanarayanan B., et al. Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials 136, 1, 2017 [DOI] [PubMed] [Google Scholar]
- 59. Boronat-García A., Guerra-Crespo M., and Drucker-Colín R.. Historical perspective of cell transplantation in Parkinson's disease. World J Transplant 7, 179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grealish S., Mattsson B., Draxler P., and Björklund A.. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson's disease. Eur J Neurosci 31, 2266, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Iancu R., Mohapel P., Brundin P., and Paul G.. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res 162, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Adil M.M., Rao A.T., Ramadoss G.N., et al. Dopaminergic neurons transplanted using cell-instructive biomaterials alleviate parkinsonism in rodents. Adv Funct Mater 28, 1804144, 2018 [Google Scholar]
- 63. Parish C.L., Castelo-Branco G., Rawal N., et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Investig 118, 149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wernig M., Zhao J.-P., Pruszak J., et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A 105, 5856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hargus G., Cooper O., Deleidi M., et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A 107, 15921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goldman S.A., Roy N.S., Beal M.F., and Cleren C.. Large stem cell grafts could lead to erroneous interpretations of behavioral results? Nat Med 13, 118, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.