Abstract
Background
Vaginitis due to Trichomonas vaginalis is one of the most common of sexually transmitted diseases. Trichomoniasis affects women during pregnancy as well but it is not clearly established whether it causes preterm birth and other pregnancy complications.
Objectives
The objective of this review was to assess the effects of various treatments for trichomoniasis during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (14 January 2011).
Selection criteria
Randomized trials comparing anti‐trichomonas agents during pregnancy. Trials including symptomatic or asymptomatic women with trichomoniasis were eligible.
Data collection and analysis
Two review authors assessed eligibility and trial quality.
Main results
We included two trials with 842 pregnant women. In both trials around 90% of women were cleared of trichomonas in the vagina after treatment. In the US trial, women with asymptomatic trichomoniasis between 16 and 23 weeks were treated with metronidazole on two occasions at least two weeks apart. The trial was stopped before reaching its target recruitment because metronidazole was not effective in reducing preterm birth and there was a likelihood of harm (risk ratio 1.78; 95% confidence interval 1.19 to 2.66). The South African trial recruited women later in pregnancy and did not have the design and power to address adverse clinical outcomes. We excluded two recent studies, identified for the current update, because they did not address the primary question.
Authors' conclusions
Metronidazole, given as a single dose, is likely to provide parasitological cure for trichomoniasis, but it is not known whether this treatment will have any effect on pregnancy outcomes. The cure rate could probably be higher if more partners used the treatment.
Plain language summary
Interventions for trichomoniasis in pregnancy
Metronidazole is effective against a trichomoniasis infection during pregnancy, but may increase the risk of preterm and low birthweight babies.
Trichomoniasis is a very common sexually transmitted infection. Symptoms include vaginal itching and discharge. It is not clear if pregnant women with trichomoniasis are more likely to give birth preterm, or have other pregnancy complications. The review of two trials, involving 842 women, found that the drug metronidazole is effective against trichomoniasis when taken by women and their partners during pregnancy, but it may harm the baby due to early birth. One of the trials was stopped early because women taking metronidazole were more likely to give birth preterm and have low birthweight babies. Further research into trichomoniasis treatments for pregnant women is needed.
Background
Vaginitis due to Trichomonas vaginalis is probably the most common sexually transmitted disease. According to WHO the total number of new cases of the four main STIs in 2005 was estimated to be 448 million, broken down as, 101 million new cases of Chlamydia, 88 million new cases of Neisseria gonorrhoeae, 11 million new cases of Syphilis, and 248 million new cases of Trichomonas vaginalis. And at any point in 2005 there were 153 million cases with Trichomonas vaginalis (WHO 2011). Infection is characterized by green‐yellow frothy vaginal discharge, dyspareunia, irritation of the vulva and urethra causing vulvovaginal soreness, itching and dysuria. The diagnosis is usually made on clinical findings and identification of the parasite in wetmount smear. Wetmount smear is a cheap and quick method whereby motile protozoa are identified under the light microscope. More sensitive techniques such as culture, immunofluorescence and enzyme immunoassay are also available although they are more costly, time consuming and therefore not used very often in busy clinics, especially in developing countries (Lossick 1991).
It is not clear whether Trichomonas vaginalis infection during pregnancy has any effect on adverse pregnancy outcomes. Concern has been raised about the possibility of increasing the transmission of HIV infection because of impairment of the vaginal mucosal barrier. It may, however, be difficult to single out any micro‐organism with regard to these adverse effects, as many types of vaginitis are polymicrobial. Metronidazole has been the main agent used in the treatment of trichomoniasis since the 1960s. It is generally advised to withhold metronidazole treatment during pregnancy until after the first trimester (Lossick 1991; Murphy 1994). In early pregnancy other agents such as clotrimazole have been recommended as a local application. Tinidazole, ornidazole and nimorazole are other nitroimidazoles which are also effective against trichomonas. The Cochrane review comparing the effectiveness of various treatment options for trichomoniasis in nonpregnant women found metronidazole and other nitroimidazole group drugs effective in treating the infection (Forna 2003). There were no major differences between different nitroimidazoles.
Trichomoniasis during pregnancy is a common occurrence in both developing and developed countries, treated by various healthcare professionals. The possible effects of trichomoniasis on pregnancy, the effectiveness of different preparations and different routes are potential areas of controversy. It is therefore important to document the evidence from randomized trials regarding the effectiveness and safety of various treatment protocols.
Objectives
To determine the effectiveness of various drug treatments for trichomoniasis in pregnant women.
Methods
Criteria for considering studies for this review
Types of studies
Any randomized trial in which an attempt is made to compare different forms of treatment for trichomoniasis during pregnancy.
Types of participants
Pregnant women with:
symptomatic trichomoniasis, diagnosed by either wetmount smear or any other laboratory test in addition to clinical findings;
asymptomatic trichomoniasis, with a laboratory diagnosis.
Types of interventions
Any treatment versus no treatment.
Comparison of two different agents.
Comparison of different doses of the same agent.
Systemic versus local treatment.
Single dose (including one day) versus longer (five to 10 day) treatment.
Types of outcome measures
Primary outcomes
Adverse pregnancy outcomes such as preterm birth, low birthweight and intrauterine infection.
Parasitological cure confirmed by repeat testing after treatment.
Symptomatic relief (clearance of discharge, soreness, itching).
Side effects and complications of treatment.
Recurrence of infection.
Satisfaction with treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (14 January 2011).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1
For this update we intended to use the current recommended method to assess the methodological quality using The Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2008). However, the two trials did not meet the inclusion criteria for the review.
Selection of studies
Two review authors (M Azhar (MA), AM Gulmezoglu (AMG)) independently assessed for inclusion all the potential studies that were identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, MA and AMG extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2008) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered the studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results.
We assessed the methods as:
low risk, high risk or unclear risk of bias for participants;
low risk, high risk or unclear risk of bias for personnel;
low risk, high risk or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we re‐included missing data in the analyses which was undertaken. We assessed methods as:
low risk of bias;
high risk bias;
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2008). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardized mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
The review focuses on individual randomized trials.
Dealing with missing data
For included studies, we noted the levels of attrition. As there was just one trial for analysis of most outcomes, we have not carried out sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses, and analyzed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if T² was greater than zero and either I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis where there was only one study per analysis and also for combining data where it was reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Results
Description of studies
We included two trials that differed in several ways. Ross 1983 was conducted in South Africa and included both asymptomatic and symptomatic women, whereas Klebanoff 2001 included only asymptomatic women attending antenatal clinics in 15 centres across the USA.
The diagnosis was made by wetmount smear in the South African trial and by culture in the US trial. The US trial was set to investigate whether treatment of asymptomatic trichomoniasis could prevent preterm birth and the enrolment took place between 16 and 23 weeks, whereas in the South African trial enrolment was much later in pregnancy. The dose of metronidazole used in the US trial was double (2 g 48 hours apart) the dose used in the South African trial and was repeated after two weeks.
In the South African trial, 225 out of the 376 (60%) antenatal women tested were positive. In the US trial only 7.6% (2377/31,157) of women were trichomonas positive.
Metronidazole was given to women for their partners to take in both studies (Klebanoff 2001; Ross 1983).
Risk of bias in included studies
In Klebanoff 2001, adequate sequence generation was generated by a computer while in the Ross 1983 study, sequence was not generated and the method of alternate allocation was used which is prone to selection bias.
Allocation
Klebanoff 2001 does not provide any information regarding the allocation concealment and actual manner with which allocation was made. In the South African trial, women were randomly assigned to the intervention and control group and no further information is available to judge if allocation concealment was done (Ross 1983).
Blinding
In the Ross 1983 trial, the technicians assessing the parasite did not have any knowledge of the source of specimen. The Klebanoff 2001 trial was double blind with the use of identical placebos.
Incomplete outcome data
The details relevant to attrition and exclusions were detailed in Klebanoff 2001 and Ross 1983.
Selective reporting
The trials seem to be free of selective reporting (Klebanoff 2001; Ross 1983).
Other potential sources of bias
The choice of alternate allocation in Ross 1983 make it prone to selection bias.
Effects of interventions
Both trials, Ross 1983 and Klebanoff 2001, showed high rates of parasitological cure (around 90%) following treatment. The risks of preterm birth (risk ratio (RR) 1.78; 95% confidence interval (CI) 1.19 to 2.66) and low birthweight (RR 1.38; 95% CI 0.92 to 2.06) were increased in the metronidazole group in the US study (Klebanoff 2001).
In the South African trial (Ross 1983) there were no differences in mean birthweight, gestational age and the incidence of low birthweight (12% in treated versus 11% in control) between the two groups.
Discussion
Trichomoniasis is a troublesome infection which causes significant discomfort and is associated with adverse pregnancy outcomes (Cotch 1997; French 1999). The literature on metronidazole treatment during pregnancy and preterm birth is not conclusive. Hauth 1995 used metronidazole and erythromycin and Morales 1994 used metronidazole alone in women with bacterial vaginosis and high risk of preterm birth; in both studies, preterm birth rates were reduced. McDonald 1997 on the other hand did not find a clinically or statistically significant difference in preterm birth rate with metronidazole in women with bacterial vaginosis. The trials excluded from the review where metronidazole was given in combination with the other antibiotics for treatment of trichomoniasis for sexually transmitted infection control and prevention of HIV (Kigozi 2003) and for reduction of perinatal HIV transmission due to chorioamnionitis (Stringer 2010) did not yield significant differences in terms of preterm birth and low birthweight.
Summary of main results
Metronidazole is effective in clearing the trichomonas infection in the vagina. The increased tendency towards preterm birth as well as low birthweight in the intervention arm as compared to the control arm (Klebanoff 2001) is difficult to explain and it is unknown whether it is real or not.
Overall completeness and applicability of evidence
The statistical assessment of evidence suggests that treatment of trichomoniasis with metronidazole does not reduce the incidence of preterm birth and delivery of low birthweight babies. Therefore treatment may be encouraged keeping in view the effectiveness of metronidazole in parasitological cure of trichomoniasis (Ross 1983) for mainly women who are symptomatic.
Quality of the evidence
Two of the trials included in the review show reasonable rates of parasitological cure (Klebanoff 2001; Ross 1983) and this could probably be improved if more emphasis is put on partner treatment. Despite the association of Trichomonas vaginalis with preterm birth and low birthweight, the US trial results suggest that a protective effect on preterm birth and low birthweight is unlikely. Surprisingly, there was an increase in preterm births in women receiving metronidazole (Klebanoff 2001). The trial was stopped because it was highly unlikely that the treatment would be effective if all women would have been recruited. However, the question of whether the drug actually increases the preterm birth rate remains unanswered. Recruitment was stopped in one of the trials after 617 women were randomized (32% of total planned sample size) (Klebanoff 2001). The authors discuss the possible reasons for an increase in preterm birth rate including the possibility of toxic substances being released from destroyed Trichomonas and an unpredicted change in the vaginal flora triggered by the high‐dose metronidazole treatment. Whether inclusion of symptomatic women would have changed the result is unknown. With the limited evidence currently available, metronidazole treatment of asymptomatic trichomoniasis infection does not seem to reduce preterm birth or improve low birthweight while it does provide parasitological cure for trichomoniasis infection. From a methodological point of view the results of prematurely stopped trials should be interpreted cautiously as they can be misleading.
Authors' conclusions
Implications for practice.
This review found no evidence to support the use of metronidazole in pregnant asymptomatic women with trichomonas vaginalis. It is not clear why metronidazole should cause adverse pregnancy outcomes when it is effective in clearing the infection. Given that Trichomonas vaginalis is a sexually transmitted infection with unpleasant symptoms and associated with adverse outcomes, including facilitating HIV transmission (Sorvillo 2001), it would seem prudent to treat symptomatic women during pregnancy.
Implications for research.
Metronidazole, or nitro‐imidazoles in general, are the first choice agents against Trichomonas vaginalis. There are no other readily available medications to replace this class of drugs for the treatment of trichomonas infections. Metronidazole when given in combination with other antibiotics did not show decline in the rate of preterm birth or low birthweight. There are two research questions that need to be answered:
whether the treatment of pregnant women with symptoms (trichomonas vaginitis) is effective in reducing preterm birth;
whether the adverse effect of increased preterm birth in treated asymptomatic women with trichomonas observed in one, prematurely stopped trial is real.
What's new
| Date | Event | Description |
|---|---|---|
| 14 January 2011 | New citation required but conclusions have not changed | New author updated the review. |
| 14 January 2011 | New search has been performed | Search updated. The two reports identified by a new search did not meet the inclusion criteria for this review (Kigozi 2003; Stringer 2010). Both studies were the sub‐analysis of trials designed for other interventions including control of sexually transmitted diseases for prevention of HIV (Kigozi 2003) and for use of a combination of antibiotics for reduction in perinatal HIV transmission due to chorioamnionitis. Therefore both the studies were excluded as their basis and randomization was not based on trichomoniasis and its treatment during pregnancy. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | Amended | Search updated. One report added to Studies awaiting classification (Kigozi 2003). |
| 24 July 2009 | Amended | Contact details updated. |
| 11 September 2008 | Amended | Converted to new review format. |
| 15 January 2004 | New search has been performed | Search rerun but no new trials identified. |
| 24 May 2002 | New citation required and conclusions have changed | Substantive amendment |
| 13 March 2002 | New search has been performed | Review updated with one new trial. |
Acknowledgements
Dr S Ross for providing additional information about the trial and Dr Zulfiqar A Bhutta of Aga Khan University, Pakistan, for his support.
The World Health Organization retains copyright and all other rights in the manuscript of this Review as submitted for publication, including any revisions or updates to the manuscript which WHO may make from time to time.
Appendices
Appendix 1. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Klebanoff 2001; Ross 1983.
Trials under consideration were evaluated for methodological quality and appropriateness for inclusion, without consideration of their results.
Methodological quality was assessed in terms of adequacy of allocation concealment as described in Clarke 2000.
Included trial data were processed as described in Clarke 2000.
Data and analyses
Comparison 1. Metronidazole versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (< 37 weeks) | 1 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.19, 2.66] |
| 2 Low birthweight (< 2500 g) | 1 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.92, 2.06] |
| 3 No parasitological cure | 2 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.08, 0.17] |
| 4 Birthweight (kg) | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.24, 0.04] |
| 5 Gestational age (weeks) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.69, 0.09] |
1.1. Analysis.
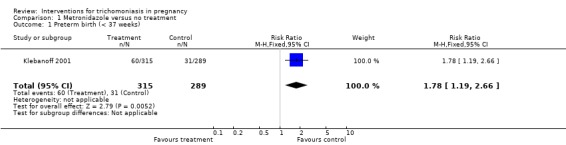
Comparison 1 Metronidazole versus no treatment, Outcome 1 Preterm birth (< 37 weeks).
1.2. Analysis.
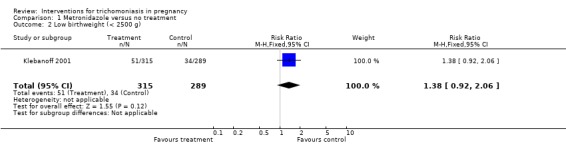
Comparison 1 Metronidazole versus no treatment, Outcome 2 Low birthweight (< 2500 g).
1.3. Analysis.
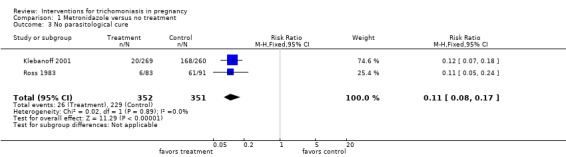
Comparison 1 Metronidazole versus no treatment, Outcome 3 No parasitological cure.
1.4. Analysis.
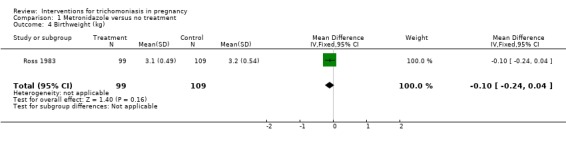
Comparison 1 Metronidazole versus no treatment, Outcome 4 Birthweight (kg).
1.5. Analysis.
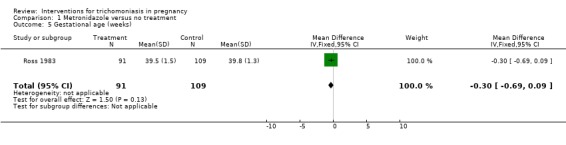
Comparison 1 Metronidazole versus no treatment, Outcome 5 Gestational age (weeks).
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Ross 1983.
| Methods | Randomization was by alternate allocation. Placebos were not used. Technicians doing the parasitological assessments had no knowledge of the source of the specimens. | |
| Participants | 376 women attending a midwife operated antenatal clinic. Women were enrolled in 2 groups. The first group included those who booked before 34 weeks; the second group were initially uninfected but then found to be infected at 38 weeks. In each group women were randomly allocated to treatment (110 women) and no treatment (115 women) groups. 151 women were found not to be infected. This latter group was followed until delivery but not included in either group. | |
| Interventions | Benzoylmetronidazole 50 ml (2 g metronidazole equivalent) oral, single dose vs no treatment. An extra dose of the medication was given to the women to take to their partners and they were asked to refrain from coitus until the follow‐up visit. Untreated symptomatic patients received symptomatic relief but details of the agent used for symptomatic treatment are not given. | |
| Outcomes | Perinatal outcome (mean birthweight, low birthweight). Gestational age at delivery. Parasitological follow‐up at 1 and 4 weeks. | |
| Notes | Trichomoniasis was diagnosed by saline wetmount technique. Loss to follow‐up: 8/110 in metronidazole group lost at first control and a further 14 could not have outcome assessments because of delivery or loss to follow‐up. In the no treatment group 5/115 were lost to follow‐up and a further 19 attended too irregularly to assess parasitological diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients found to be infected were randomly divided into two groups." |
| Allocation concealment (selection bias) | High risk | None. Alternate allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: probably done (technicians doing the parasitological assessments had no knowledge of the source of the specimens). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Probably done. |
| Selective reporting (reporting bias) | Unclear risk | Unknown because protocol is not accessible. |
| Other bias | Low risk | The study seems to be free of other types of bias. |
Klebanoff 2001.
| Methods | Double‐blind randomized controlled trial using identical placebos. Randomization sequence was generated by computer. The method of allocation concealment and random allocation are not mentioned. | |
| Participants | 617 asymptomatic women in 15 centres in the USA. Women were screened from 8 weeks until 23 weeks and enrolled between 16 to 23 weeks. Exclusion criteria: increased vaginal discharge with symptoms, allergy to metronidazole, current ethanol abuse, antibiotic therapy within the previous 14 days, an intention to continue antenatal care or plan delivery outside catchment area, language barrier precluding informed consent, planned antibiotic therapy before delivery, current or planned cervical cerclage, preterm labor before screening, current or planned tocolytic therapy, fetal death, major congenital abnormality, multiple gestation, medical illness. | |
| Interventions | Metronidazole 2 g (250 mg capsules x 8) on randomization + 2 g after 48 hours repeated after 2 weeks versus placebo (lactose) capsules. The first treatment was before 23 weeks and the second between 24 to 29 weeks but at least 14 days after the initial treatment. | |
| Outcomes | Preterm birth (< 37 completed weeks) was the primary outcome. | |
| Notes | 2 women were randomized without having trichomoniasis. Compliance was around 80% in both groups. 11.8% of all women were lost to follow‐up. The authors report that the proportion of lost to follow‐up was not significantly different between the 2 groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The urn method of randomization, with stratification according to clinical center, was used to create the computer‐generated randomization sequence". |
| Allocation concealment (selection bias) | Unclear risk | Likely but not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "After specimens were obtained, the women were randomly assigned in a double‐blind manner to receive eight capsules containing either 250 mg of generic metronidazole each or a lactose placebo". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In this study, 544 of the 617 women (88.2 percent) returned for the follow‐up visit and provided information on side effects. The reasons for failure to return were loss of contact (47 women), delivery before the scheduled visit (16 women), refusal to continue in the study (8 women), and other reasons (2 women); there was no significant difference between the groups in the proportion of women who did not return for a follow‐up visit". |
| Selective reporting (reporting bias) | Unclear risk | The protocol is not accessible. |
| Other bias | Low risk | |
vs: versus
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Robinson 1965 | This trial was excluded because it was not clear whether the comparisons had been made between randomized groups. The authors extended a series of metronidazole treated pregnant and nonpregnant women to include a group of women randomly allocated to a treatment and placebo. Consequently, there is an imbalance in the sample sizes of 2 groups and it is not possible to identify randomized groups in the tables presented. |
| Roos 1978 | There was no randomized comparison. |
| Kigozi 2003 | This study is excluded as it is a secondary analysis of a randomized trial designed for evaluation of interventions for STD control for prevention of HIV. In this trial the intervention was a community‐based trial of presumptive treatment of several antimicrobials including metronidazole as antitrichomoniasis agent. Therefore, to receive the treatment (or no treatment) the women were not required to have trichomoniasis in pregnancy. Also, the authors have acknowledged that some women in the intervention arm were likely to have received (presumptive) treatment before pregnancy. |
| Stringer 2010 | The trial is a secondary analysis of a randomized trial where the main comparison is the use of several antenatal and intrapartum antibiotics for reduction of chorioamnionitis‐related perinatal HIV transmission against no use of antibiotics. |
HIV: human immunodeficiency virus
STD: sexually transmitted disease
Contributions of authors
Both authors contributed equally to the development of this update.
Sources of support
Internal sources
National Perinatal Epidemiology Unit, Oxford, UK.
UK Cochrane Centre, NHS R&D Programme, Oxford, UK.
HRP ‐ UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
External sources
Nuffield Provincial Hospitals Trust, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Klebanoff 2001 {published data only}
- Carey JC, Klebanoff M for the NICHD MFMU Network. Metronidazole treatment increased the risk of preterm birth in asymptomatic women with trichomonas. American Journal of Obstetrics and Gynecology 2000;182(1):Ss13. [Google Scholar]
- Klebanoff M, Carey C, Hauth J, Hillier S, Nugent R, Thom E, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic trichomonas vaginalis infection. New England Journal of Medicine 2001;345(7):487‐93. [DOI] [PubMed] [Google Scholar]
Ross 1983 {published data only}
- Ross SM, Middelkoop A. Trichomonas infection in pregnancy ‐ does it affect perinatal outcome?. South African Medical Journal 1983;63:566‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Kigozi 2003 {published data only}
- Kigozi GG, Brahmbhatt H, Wabwire‐Mangen F, Wawer MJ, Serwadda D, Sewankambo N, et al. Treatment of trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. American Journal of Obstetrics and Gynecology 2003;189:1398‐400. [DOI] [PubMed] [Google Scholar]
Robinson 1965 {published data only}
- Robinson SC, Gopi M. Trichomonas vaginalis. V. Further observations on metronidazole (Flagyl) (including infant follow‐up). American Journal of Obstetrics and Gynecology 1965;93:502‐5. [PubMed] [Google Scholar]
Roos 1978 {published data only}
- Roos RF. Trichomoniasis treated with a single dose of benzoylmetronidazole. South African Medical Journal 1978;54:869‐70. [PubMed] [Google Scholar]
Stringer 2010 {published data only}
- Stringer E, Read JS, Hoffman I, Valentine M, Aboud S, Goldenberg RL. Treatment of trichomoniasis in pregnancy in sub‐Saharan Africa does not appear to be associated with low birth weight or preterm birth. South African Medical Journal 2010;100(1):58‐64. [PMC free article] [PubMed] [Google Scholar]
Additional references
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers’ Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Cotch 1997
- Cotch MF, Pastorek JG 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sexually Transmitted Diseases 1997;24(6):353‐60. [DOI] [PubMed] [Google Scholar]
Forna 2003
- Forna F, Gülmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000218] [DOI] [PMC free article] [PubMed] [Google Scholar]
French 1999
- French JI, McGregor JA, Draper D, Parker R, McFee J. Gestational bleeding, bacterial vaginosis, and common reproductive tract infections: risk for preterm birth and benefit of treatment. Obstetrics and Gynecology 1999;93:715‐24. [DOI] [PubMed] [Google Scholar]
Hauth 1995
- Hauth JC, Goldenberg RL, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. New England Journal of Medicine 1995;333:1732‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Lossick 1991
- Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. American Journal of Obstetrics and Gynecology 1991;165:1217‐22. [DOI] [PubMed] [Google Scholar]
McDonald 1997
- McDonald HM, O'Loughlin JA, Vigneswaran R, Jolley PT, Harvey JA, Bof A, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(12):1391‐7. [DOI] [PubMed] [Google Scholar]
Morales 1994
- Morales WJ, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo‐controlled, double‐blind study. American Journal of Obstetrics and Gynecology 1994;171:345‐9. [DOI] [PubMed] [Google Scholar]
Murphy 1994
- Murphy PA, Jones E. Use of oral metronidazole in pregnancy. Journal of Nurse Midwifery 1994;39:214‐20. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sorvillo 2001
- Sorvillo F, Smith L, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and African‐Americans. Emerging Infectious Diseases 2001;7:927‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Prevalence and incidence of selected sexually transmitted infections. Chlamydia, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis. Geneva: WHO, in press. [Google Scholar]


