Abstract
Background
Induction of labour involves stimulating uterine contractions artificially to promote the onset of labour. There are several pharmacological, surgical and mechanical methods used to induce labour. Membrane sweeping is a mechanical technique whereby a clinician inserts one or two fingers into the cervix and using a continuous circular sweeping motion detaches the inferior pole of the membranes from the lower uterine segment. This produces hormones that encourage effacement and dilatation potentially promoting labour. This review is an update to a review first published in 2005.
Objectives
To assess the effects and safety of membrane sweeping for induction of labour in women at or near term (≥ 36 weeks' gestation).
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register (25 February 2019), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (25 February 2019), and reference lists of retrieved studies.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing membrane sweeping used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed on a predefined list of labour induction methods. Cluster‐randomised trials were eligible, but none were identified.
Data collection and analysis
Two review authors independently assessed studies for inclusion, risk of bias and extracted data. Data were checked for accuracy. Disagreements were resolved by discussion, or by including a third review author. The certainty of the evidence was assessed using the GRADE approach.
Main results
We included 44 studies (20 new to this update), reporting data for 6940 women and their infants. We used random‐effects throughout.
Overall, the risk of bias was assessed as low or unclear risk in most domains across studies. Evidence certainty, assessed using GRADE, was found to be generally low, mainly due to study design, inconsistency and imprecision. Six studies (n = 1284) compared membrane sweeping with more than one intervention and were thus included in more than one comparison.
No trials reported on the outcomes uterine hyperstimulation with/without fetal heart rate (FHR) change, uterine rupture or neonatal encephalopathy.
Forty studies (6548 participants) compared membrane sweeping with no treatment/sham
Women randomised to membrane sweeping may be more likely to experience:
· spontaneous onset of labour (average risk ratio (aRR) 1.21, 95% confidence interval (CI) 1.08 to 1.34, 17 studies, 3170 participants, low‐certainty evidence).
but less likely to experience:
· induction (aRR 0.73, 95% CI 0.56 to 0.94, 16 studies, 3224 participants, low‐certainty evidence);
There may be little to no difference between groups for:
· caesareans (aRR 0.94, 95% CI 0.85 to 1.04, 32 studies, 5499 participants, moderate‐certainty evidence);
· spontaneous vaginal birth (aRR 1.03, 95% CI 0.99 to 1.07, 26 studies, 4538 participants, moderate‐certainty evidence);
· maternal death or serious morbidity (aRR 0.83, 95% CI 0.57 to 1.20, 17 studies, 2749 participants, low‐certainty evidence);
· neonatal perinatal death or serious morbidity (aRR 0.83, 95% CI 0.59 to 1.17, 18 studies, 3696 participants, low‐certainty evidence).
Four studies reported data for 480 women comparing membrane sweeping with vaginal/intracervical prostaglandins
There may be little to no difference between groups for the outcomes:
· spontaneous onset of labour (aRR, 1.24, 95% CI 0.98 to 1.57, 3 studies, 339 participants, low‐certainty evidence);
· induction (aRR 0.90, 95% CI 0.56 to 1.45, 2 studies, 157 participants, low‐certainty evidence);
· caesarean (aRR 0.69, 95% CI 0.44 to 1.09, 3 studies, 339 participants, low‐certainty evidence);
· spontaneous vaginal birth (aRR 1.12, 95% CI 0.95 to 1.32, 2 studies, 252 participants, low‐certainty evidence);
· maternal death or serious morbidity (aRR 0.93, 95% CI 0.27 to 3.21, 1 study, 87 participants, low‐certainty evidence);
· neonatal perinatal death or serious morbidity (aRR 0.40, 95% CI 0.12 to 1.33, 2 studies, 269 participants, low‐certainty evidence).
One study, reported data for 104 women, comparing membrane sweeping with intravenous oxytocin +/‐ amniotomy
There may be little to no difference between groups for:
· spontaneous onset of labour (aRR 1.32, 95% CI 88 to 1.96, 1 study, 69 participants, low‐certainty evidence);
· induction (aRR 0.51, 95% CI 0.05 to 5.42, 1 study, 69 participants, low‐certainty evidence);
· caesarean (aRR 0.69, 95% CI 0.12 to 3.85, 1 study, 69 participants, low‐certainty evidence);
· maternal death or serious morbidity was reported on, but there were no events.
Two studies providing data for 160 women compared membrane sweeping with vaginal/oral misoprostol
There may be little to no difference between groups for:
· caesareans (RR 0.82, 95% CI 0.31 to 2.17, 1 study, 96 participants, low‐certainty evidence).
One study providing data for 355 women which compared once weekly membrane sweep with twice‐weekly membrane sweep and a sham procedure
There may be little to no difference between groups for:
· induction (RR 1.19, 95% CI 0.76 to 1.85, 1 study, 234 participants, low‐certainty);
· caesareans (RR 0.93, 95% CI 0.60 to 1.46, 1 study, 234 participants, low‐certainty evidence);
· spontaneous vaginal birth (RR 1.00, 95% CI 0.86 to 1.17, 1 study, 234 participants, moderate‐certainty evidence);
· maternal death or serious maternal morbidity (RR 0.78, 95% CI 0.30 to 2.02, 1 study, 234 participants, low‐certainty evidence);
· neonatal death or serious neonatal perinatal morbidity (RR 2.00, 95% CI 0.18 to 21.76, 1 study, 234 participants, low‐certainty evidence);
We found no studies that compared membrane sweeping with amniotomy only or mechanical methods.
Three studies, providing data for 675 women, reported that women indicated favourably on their experience of membrane sweeping with one study reporting that 88% (n = 312) of women questioned in the postnatal period would choose membrane sweeping in the next pregnancy.
Two studies reporting data for 290 women reported that membrane sweeping is more cost‐effective than using prostaglandins, although more research should be undertaken in this area.
Authors' conclusions
Membrane sweeping may be effective in achieving a spontaneous onset of labour, but the evidence for this was of low certainty. When compared to expectant management, it potentially reduces the incidence of formal induction of labour. Questions remain as to whether there is an optimal number of membrane sweeps and timings and gestation of these to facilitate induction of labour.
Keywords: Female; Humans; Pregnancy; Amnion; Amnion/physiology; Cervical Ripening; Labor, Induced; Labor, Induced/methods; Mechanical Phenomena; Pregnancy Outcome; Randomized Controlled Trials as Topic; Risk Factors; Term Birth; Term Birth/physiology
Plain language summary
Membrane sweeping for induction of labour
What is the question?
The aim of this Cochrane Review is to find out if membrane sweeping is a safe and effective way of inducing labour at or near term and if it is more effective than the formal methods of induction.
Why is this important?
Most commonly, formal induction of labour is offered to women when continuing with a pregnancy is considered probably more harmful for the mother or baby than the adverse effects of induction. The most common reason for formal induction of labour is post‐term pregnancy (pregnancies that continue past 42 weeks' gestation).
Membrane sweeping is a relatively simple, low‐cost procedure that seeks to reduce the use of formal induction of labour and it can be performed without the need for hospitalisation. It involves the clinician inserting one or two fingers into the lower part of the uterus (the cervix) and using a continuous circular sweeping motion to free the membrane from the lower uterus. Formal induction of labour involves artificially stimulating the uterus with drugs such as prostaglandins or oxytocin or by breaking the amniotic sack that holds the baby (breaking the waters).
What evidence did we find?
We searched for evidence on 25 February 2019. We included 44 randomised studies that reported findings for 6940 women from a wide range of countries including high‐, middle‐ and low‐income countries.
Studies compared membrane sweeping with no intervention or sham intervention, and also compared membrane sweeping with vaginal or intracervical prostaglandins, oral misoprostol, oxytocin and repeated membrane sweeping.
Of the seven studies that reported financial funding, two studies reported funding from pharmaceutical companies. Overall, the certainty of the evidence was found to be low.
Key results
Compared with no intervention or a sham sweep (40 studies involving 6548 women), allocated to membrane sweeping may be more likely to have spontaneous onset of labour, but we found no clear difference in unassisted vaginal births. Women may also be less likely to have formal induction of labour. We also found no clear differences between the groups for caesarean section, instrumental vaginal births or serious illness or death of the mother or baby.
Compared with vaginal or intracervical prostaglandins (four studies involving 480 women), we found no difference in any outcomes although data were limited.
We found insufficient data to draw any conclusions in the studies comparing membrane sweep with intravenous oxytocin, with or without breaking the waters, or with vaginal/oral misoprostol. Similarly for the comparison between different frequencies of membrane sweeping.
What does this mean?
Membrane sweeping appears to be effective in promoting labour but current evidence suggests this did not, overall, follow‐on to unassisted vaginal births. Membrane sweeping may reduce formal induction of labour. Only three studies reported on women’s satisfaction with membrane sweeping. Women reported feeling positive about membrane sweeping. While acknowledging that it may be uncomfortable, they felt the benefits outweighed the harms and most would recommend it to other women. Further research is needed to confirm our review findings and to identify the ideal time for membrane sweep and whether having more than one sweep would be beneficial. Further information on women’s views is also needed.
Summary of findings
Summary of findings for the main comparison. Amniotic membranes sweeping compared to no treatment/sham.
| Amniotic membrane sweeping compared to no treatment/sham for induction of labour | ||||||
| Patient or population: pregnant women carrying a live fetus at or near term (≥ 36 weeks' gestation). Setting: antenatal environments where amniotic membrane sweeping is likely to be used. Intervention: amniotic membrane sweeping Comparison: no treatment/sham | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment/sham | Risk with amniotic membranes sweeping | |||||
| Spontaneous onset of labour | Study population | RR 1.21 (1.08 to 1.34) | 3170 (17 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 598 per 1000 | 723 per 1000 (646 to 801) | |||||
| Induction of labour | Study population | RR 0.73 (0.56 to 0.94) | 3224 (16 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 313 per 1000 | 228 per 1000 (175 to 294) | |||||
| Caesarean section | Study population | RR 0.94 (0.85 to 1.04) | 5499 (32 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | ||
| 165 per 1000 | 155 per 1000 (140 to 171) | |||||
| Spontaneous vaginal birth | Study population | RR 1.03 (0.99 to 1.07) | 4538 (26 RCTs) | ⊕⊕⊕⊝ MODERATE 6 | ||
| 711 per 1000 | 733 per 1000 (704 to 761) | |||||
| Uterine hyperstimulation with/without fetal heart rate (FHR) changes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported on this outcome. |
| Maternal death or serious maternal morbidity | Study population | RR 0.83 (0.57 to 1.20) | 2749 (17 RCTs) | ⊕⊕⊝⊝ LOW 7 8 | ||
| 44 per 1000 | 36 per 1000 (25 to 53) | |||||
| Neonatal death or serious neonatal perinatal morbidity | Study population | RR 0.83 (0.59 to 1.17) | 3696 (18 RCTs) | ⊕⊕⊝⊝ LOW 9 10 | ||
| 36 per 1000 | 30 per 1000 (22 to 43) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious risk of bias due to evidence of design limitations in all trials. Three trials had unclear risk of bias for randomisation. Nine trials had unclear allocation concealment and one had a high risk of bias. No trial was blinded. Twelve trials had unclear risk of bias for blinding of outcome assessment and three were high risk of bias. One trial was at high risk of selective reporting bias.
2 We downgraded (1) level for serious risk of inconsistency due to evidence of statistical heterogeneity (Tau² = 0.03; Chi² = 59.79, df = 16 (P < 0.00001); I² = 73%)
3 We downgraded (1) level for serious risk of bias due to evidence of design limitations in all trials. Three trials had unclear risk of bias for randomisation. Ten trials had unclear allocation concealment. No trial was blinded. Ten trials had unclear risk of bias for blinding of outcome assessment and two were high risk of bias. Two trials were at high risk of attrition bias and two trials were at high risk of selective reporting bias. One trial was at high risk of selective reporting bias.
4 We downgraded (1) level for serious risk of inconsistency due to evidence of statistical heterogeneity (Tau² = 0.17; Chi² = 60.72, df = 15 (P < 0.00001); I² = 75%)
5 We downgraded (1) level for serious risk of bias due to evidence of design limitations in all trials. Seven trials had unclear risk of bias for randomisation with one trial at a high risk of bias. Nineteen trials had unclear allocation concealment and two had a high risk of bias. No trial was blinded. Twenty‐two trials had unclear risk of bias for blinding of outcome assessment and five were high risk of bias. One trial was at high risk of attrition bias and two trials were at high risk of selective reporting bias.
6 We downgraded (1) level for serious risk of bias due to evidence of design limitations in all trials. Five trials had unclear risk of bias for randomisation with one trial at a high risk of bias. Sixteen trials had unclear allocation concealment. No trial was blinded. Nineteen trials had unclear risk of bias for blinding of outcome assessment and three were high risk of bias. Two trials were at high risk of selective reporting bias.
7 We downgraded (1) level for serious risk of bias due to evidence of design limitations in all trials. Two trials had unclear risk of bias for randomisation with one trial at a high risk of bias. Twelve trials had unclear allocation concealment and one trial had a high risk of bias. No trial was blinded. Eleven trials had unclear risk of bias for blinding of outcome assessment and three were high risk of bias. Two trials were at high risk of attrition bias and two trials were at high risk of selective reporting bias.
8 We downgraded (1) level for serious risk of imprecision due to the total (cumulative) sample size of 2749 being less than the optimal information size (OIS) of 15342.
9 We downgraded (1) level for serious risk of bias due to evidence of design limitations in all trials. Two trials had unclear risk of bias for randomisation. Ten trials had unclear allocation concealment. No trial was blinded. Eleven trials had unclear risk of bias for blinding of outcome assessment and two were high risk of bias. Two trials had a high risk of attrition bias and two trials had a high risk of reporting bias
10 We downgraded (1) level for serious risk of imprecision due to the total (cumulative) sample size of 3696 being less than the optimal information size (OIS) of 18716.
Summary of findings 2. Amniotic membranes sweeping compared to vaginal/intracervical prostaglandins for induction of labour.
| Amniotic membrane sweeping compared to vaginal/intracervical prostaglandins for induction of labour | ||||||
| Patient or population: pregnant women carrying a live fetus at or near term (≥ 36 weeks' gestation). Setting: antenatal environments where amniotic membrane sweeping is likely to be used. Intervention: amniotic membrane sweeping Comparison: vaginal/intracervical prostaglandins | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with vaginal/intracervical prostaglandins | Risk with amniotic membrane sweeping | |||||
| Spontaneous onset of labour | Study population | RR 1.24 (0.98 to 1.57) | 339 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 521 per 1000 | 647 per 1000 (511 to 819) | |||||
| Induction of labour | Study population | RR 0.90 (0.56 to 1.45) | 157 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 319 per 1000 | 288 per 1000 (179 to 463) | |||||
| Caesarean section | Study population | RR 0.69 (0.44 to 1.09) | 339 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 5 | ||
| 221 per 1000 | 152 per 1000 (97 to 241) | |||||
| Spontaneous vaginal birth | Study population | RR 1.12 (0.95 to 1.32) | 252 (2 RCTs) | ⊕⊕⊝⊝ LOW 6 7 | ||
| 659 per 1000 | 738 per 1000 (626 to 870) | |||||
| Uterine hyperstimulation with/without fetal heart rate (FHR) changes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported on this outcome |
| Maternal death or serious maternal morbidity | Study population | RR 0.93 (0.27 to 3.21) | 87 (1 RCT) | ⊕⊕⊝⊝ LOW 8 9 | ||
| 108 per 1000 | 101 per 1000 (29 to 347) | |||||
| Neonatal death or serious neonatal perinatal morbidity | Study population | RR 0.40 (0.12 to 1.33) | 269 (2 RCTs) | ⊕⊕⊝⊝ LOW 10 11 | ||
| 70 per 1000 | 28 per 1000 (8 to 94) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for risk of serious bias due to evidence of design limitations in all trials. All trials had unclear risk of selection bias (allocation concealment) and detection bias (blinding of outcome assessment). All three trials have high risk of performance bias (blinding of participants and personnel). One trial was at high risk of other bias.
2 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 339 being less than the optimal information size (OIS) of 704.
3 We downgraded (1) level for risk of serious bias due to evidence of design limitations in all trials. All trials had unclear risk of selection bias (allocation concealment) and detection bias (blinding of outcome assessment). All trials have high risk of performance bias (blinding of participants and personnel). One trial was at high risk of other bias.
4 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 157 being less than the optimal information size (OIS) of 1572
5 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 339 being less than the optimal information size (OIS) of 2568
6 We downgraded (1) level for risk of serious bias due to evidence of design limitations in all trials. All trials had unclear risk of selection bias (allocation concealment) and detection bias (blinding of outcome assessment). All trials have high risk of performance bias (blinding of participants and personnel).
7 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 252 being less than the optimal information size (OIS) of 358
8 We downgraded (1) level for risk of serious bias due to evidence of design limitations. We found an unclear risk of selection bias (allocation concealment) and detection bias (blinding of outcome assessment). We found this trial to be of high risk of performance bias (blinding of participants and personnel) and other bias.
9 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 80 being less than the optimal information size (OIS) of 5908
10 We downgraded (1) level for risk of serious bias due to evidence of design limitations in all trials. All trials had unclear risk of selection bias (allocation concealment) and detection bias (blinding of outcome assessment). All trials have high risk of performance bias (blinding of participants and personnel). One trial was at high risk of other bias.
11 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 269 being less than the optimal information size (OIS) of 9496
Summary of findings 3. Amniotic membranes sweeping compared to intravenous oxytocin/amniotomy for induction of labour.
| Amniotic membrane sweeping compared to intravenous oxytocin +/‐ amniotomy for induction of labour | ||||||
| Patient or population: pregnant women carrying a live fetus at or near term (≥ 36 weeks' gestation). Setting: antenatal environments where amniotic membrane sweeping is likely to be used. Intervention: amniotic membrane sweeping Comparison: intravenous oxytocin +/‐ amniotomy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with intravenous oxytocin +/‐ amniotomy | Risk with amniotic membrane sweeping | |||||
| Spontaneous onset of labour | Study population | RR 1.32 (0.88 to 1.96) | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 514 per 1000 | 679 per 1000 (453 to 1000) | |||||
| Induction of labour | Study population | RR 0.51 (0.05 to 5.42) | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 57 per 1000 | 29 per 1000 (3 to 310) | |||||
| Caesarean section | Study population | RR 0.69 (0.12 to 3.85) | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 86 per 1000 | 59 per 1000 (10 to 330) | |||||
| Spontaneous vaginal birth ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported on. |
| Uterine hyperstimulation with/without fetal heart (FHR) rate changes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported on. |
| Maternal death or serious maternal morbidity | Study population | not estimable | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 1 5 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Neonatal death or serious neonatal perinatal morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported on. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for risk of serious bias due to evidence of design limitations in this trial. We found unclear risk of selection bias (random sequence generation and allocation concealment). We found high risk of performance bias. We found unclear risk of both detection bias and reporting bias.
2 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 69 being less than the optimal information size (OIS) of 718
3 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 69 being less than the optimal information size (OIS) of 11212
4 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 69 being less than the optimal information size (OIS) of 7642
5 We downgraded (1) level for risk of serious imprecision due to small sample size with no events recorded.
Summary of findings 4. Amniotic membranes sweeping compared to vaginal/oral misoprostol for induction of labour.
| Amniotic membrane sweeping compared to vaginal/oral misoprostol for induction of labour | ||||||
| Patient or population: pregnant women carrying a live fetus at or near term (≥ 36 weeks' gestation). Setting: antenatal environments where amniotic membrane sweeping is likely to be used. Intervention: amniotic membrane sweeping Comparison: vaginal/oral misoprostol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with vaginal/oral misoprostol | Risk with amniotic membrane sweeping | |||||
| Spontaneous onset of labour ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Induction of labour ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Caesarean section | Study population | RR 0.82 (0.31 to 2.17) | 96 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 160 per 1000 | 131 per 1000 (50 to 347) | |||||
| Spontaneous vaginal birth ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported |
| Uterine hyperstimulation with/without fetal heart rate (FHR) changes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported |
| Maternal death or serious maternal morbidity | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported |
| Neonatal death or serious neonatal perinatal morbidity ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for risk of serious bias due to evidence of design limitations in this trial. We found high risk of performance bias and an unclear risk of both detection bias and reporting bias.
2 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 96 being less than the optimal information size (OIS) of 3776
Summary of findings 5. One frequency of amniotic membranes sweeping compared to another frequency of amniotic membrane sweeping for induction of labour.
| One frequency of amniotic membrane sweeping compared to another frequency of amniotic membrane sweeping for induction of labour | ||||||
| Patient or population: pregnant women carrying a live fetus at or near term (≥ 36 weeks' gestation). Setting: antenatal environments where amniotic membrane sweeping is likely to be used. Intervention: 1 frequency of amniotic membrane sweeping Comparison: another frequency of amniotic membrane sweeping | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with another frequency of amniotic membrane sweeping | Risk with one frequency of amniotic membrane sweeping | |||||
| Spontaneous onset of labour ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Induction of labour | Study population | RR 1.19 (0.76 to 1.85) | 234 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 231 per 1000 | 275 per 1000 (175 to 427) | |||||
| Caesarean section | Study population | RR 0.93 (0.60 to 1.46) | 234 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 256 per 1000 | 238 per 1000 (154 to 374) | |||||
| Spontaneous vaginal birth | Study population | RR 1.00 (0.86 to 1.17) | 234 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 735 per 1000 | 735 per 1000 (632 to 860) | |||||
| Uterine hyperstimulation with/without fetal heart rate (FHR) changes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported |
| Maternal death or serious maternal morbidity | Study population | RR 0.78 (0.30 to 2.02) | 234 (1 RCT) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 77 per 1000 | 60 per 1000 (23 to 155) | |||||
| Neonatal death or serious neonatal perinatal morbidity | Study population | RR 2.00 (0.18 to 21.76) | 234 (1 RCT) | ⊕⊕⊝⊝ LOW 1 5 | ||
| 9 per 1000 | 17 per 1000 (2 to 186) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for risk of serious bias due to evidence of design limitations in this trial. We found unclear risk of selection bias (allocation concealment) and we found high risk of performance bias.
2 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 350 being less than the optimal information size (OIS) of 1414
3 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 350 being less than the optimal information size (OIS) of 2252
4 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 350 being less than the optimal information size (OIS) of 6182
5 We downgraded (1) level for risk of serious imprecision due to the total (cumulative) sample size of 350 being less than the optimal information size (OIS) of 83538
Background
This systematic review is an update of a Cochrane Review ‘Membrane sweeping for induction of labour’ first published on 24th January 2005 (Boulvain 2005). The previous review was one of a series of systematic reviews on methods of labour induction. This cohort of systematic reviews were utilised to compare and evaluate methods of labour induction at or near term. This current (2019) update is a stand‐alone review.
Description of the condition
Labour and childbirth are physiological processes and for the majority of women the onset of labour is spontaneous. However, some women will have an induction of labour. Induction of labour is the process of artificially stimulating uterine contractions to initiate the onset of labour. Approximately one in four pregnancies in high‐middle income settings will end with an induction of labour (Bakker 2013; World Health Organization 2011). Worldwide, the incidence of induction of labour varies with 28% of women in Australia, 26.8% in England, 21.8% in Canada and 25% in Ireland having their labours induced (Australian Institute of Health and Welfare 2016; Health Canada 2008; Health Service Executive 2016; National Childbirth Trust 2017). Obstetric statistics demonstrate a significant temporal increase in these rates, a trend set to continue (Alfirevic 2016).
Current international guidelines state that induction of labour, as with any intervention, carries risks and advise it be performed only when there are clear indications that continuing with the pregnancy is of greater risk to the mother or fetus than the risk of induction of labour (ACOG 2009; Middleton 2018; World Health Organization 2011). However, recent studies have reported that elective pharmacological induction of labour for post‐term pregnancy results in a lower risk of caesarean section than expectant management (Grobman 2018; Middleton 2018). Current medical indications for an induction of labour include preterm premature rupture of membrane (PPROM), intrauterine growth restriction, hypertensive disorders of pregnancy, intrauterine fetal death and post‐term pregnancies (SOGC 2013). Of these, induction of labour for pregnancy considered post‐term is the most common (NHS Digital 2014; Nippita 2015; Sue‐A‐Quan 1999).
A pregnancy is considered to have reached full term at 37 completed weeks' gestation, however, up to 10% of pregnancies will continue past 42 weeks’ gestation and are then considered “post‐term” (Middleton 2018; Olesen 2003).
Although the reasons why some pregnancies become post‐term are not understood fully, nulliparity, high body mass index and increased maternal age are all recognised risk factors (Roos 2010). Birth post 42 weeks’ gestation carries increased risk for the neonate including meconium aspiration, neonatal acidaemia, low Apgar scores, macrosomia and neonatal death (0.018% at day 287 versus 0.51% at day 301+) (ACOG 2014; Heimstad 2008). The incidence of maternal complications such as severe perineal injury (third‐ and fourth‐degree perineal lacerations) related to macrosomia (3.3% versus 2.6% at term), postpartum haemorrhage, chorioamnionitis and endomyometritis are seen to increase post‐term (Hedegaard 2014).
Labour may be induced using pharmacological, surgical and mechanical methods (Alfirevic 2016).
Pharmacological methods include the use of prostaglandins, such as dinoprostone administered either vaginally or intracervical, misoprostol administered orally, vaginally or intracervical, and oxytocin administered intravenously (Alfirevic 2014). Pharmacological methods of induction of labour are not suitable for all women (NICE 2008). Reduced levels of prostaglandins are indicated in women with a high parity and the use of prostaglandins are contraindicated in cases of women with a previous caesarean section (NICE 2008). Pharmacological induction of labour increases the risk of uterine rupture, hyperstimulation, prolonged labour and fetal and maternal compromise (World Health Organization 2011). The WHO recommend that women undergoing a pharmacological induction of labour should never be unattended, potentially increasing healthcare costs.
Surgically, labour may be induced using procedures including the deliberate rupturing of the amniotic membrane known as amniotomy (Caughey 2009). Amniotomy carries the risk of umbilical cord prolapse when the presenting part of the fetus is not engaged in the pelvis. It increases the risk of infection for mother and fetus and is contraindicated in HIV positive women (Bricker 2000).
Mechanical methods were among the first reported methods of induction of labour. When inducing labour, the favourability of the cervix, as assessed by the Bishops score, is the main indication of the likelihood of success (Bishop 1964). Mechanical methods of induction of labour are used to ripen and dilate the cervix encouraging the spontaneous onset of labour through manual manipulation of the cervix (de Vaan 2019). Mechanical methods include the use of an intracervical Foley catheter and membrane sweeping, also referred to as ‘stripping’ or ‘stretch and sweep’ of the membrane.
Description of the intervention
Membrane sweep is performed with consent during a vaginal examination. It involves the clinician inserting one or two fingers into the woman’s cervix and detaching the inferior pole of the membrane from the lower uterine segment in a circular motion (Boulvain 2008). Alternatively, the cervix may be massaged if the cervical os is closed. Membrane sweeping is a simple procedure and may be used independently or in combination with other means of induction and can be repeated multiple times.
How the intervention might work
Membrane sweeping is used to promote the normal physiological onset of labour by releasing localised prostaglandins F2α, phospholipase A2 and cytokines from the intrauterine tissues (Blackburn 2013). These hormones act on the cervix to augment cervical ripening potentially instigating uterine contractions. The stretching of the cervix may help to initiate the Ferguson reflex by releasing oxytocin, thereby increasing uterine activity (Blackburn 2013). The aim of this intervention is to soften and ripen the cervix, increasing cervical favourability and promoting uterine activity, to stimulate spontaneous uterine contractions potentially leading to the onset of labour and the avoidance of a formal induction of labour.
Why it is important to do this review
Twenty‐five per cent of all pregnancies in high‐middle income settings end in a formal induction of labour. Formal induction of labour is defined as the process of artificially stimulating the uterus to start labour through pharmacological or surgical methods (World Health Organization 2000). Membrane sweeping is an intervention that seeks to reduce the need for formal induction of labour. Post‐term pregnancy is by far the most common reason for formal induction of labour and membrane sweeping potentially offers a low‐risk, low‐cost method to reduce this. Membrane sweeping is a technically simple intervention that is routinely used. It has the advantage that it may be used independently or in combination with other means of induction and can be repeated multiple times. It can be performed by obstetricians or midwives in community or clinical settings (NICE 2008; Wong 2002). Guidelines supported by bodies including the National Institute for Health and Care Excellence (NICE 2008), the Society of Obstetricians and Gynaecologists of Canada (Public Health Canada 2008), the Department of Health, South Australia (South Australia DOH 2014) and the World Health Organization (World Health Organization 2011) state that women should be offered the option of membrane sweeping at or near term. The NICE guidelines state that a membrane sweep be offered to nulliparous women at term gestation and women who have had one or more infants at 41 weeks' gestation. In addition, it recommends that women be offered further membrane sweeps during subsequent antennal visits if labour does not commence (NICE 2008).
Questions remain on aspects of this intervention including the optimal frequency of membrane sweeping for induction of labour for differing parities and gestation, women’s satisfaction levels with this method and the use of cervical massage. Internationally, numerous guidelines have repeatedly identified the need for research to clarify these uncertainties (NICE 2008; Queensland DOH 2017). This systematic review will evaluate the available evidence to assess the effects of membrane sweeping for induction of labour in women with a live fetus at or near term (≥ 36 weeks' gestation) and address these uncertainties.
Objectives
The aim of this review is to assess the effects and safety of membrane sweeping for induction of labour in women at or near term (≥ 36 weeks' gestation).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised trials comparing membrane sweeping for labour induction with placebo/no treatment or other methods for labour induction. This review will include randomised controlled trials which cannot be blinded due to the nature of the intervention. Randomised controlled trials and quasi‐randomised trials found only as abstract trial reports were eligible for inclusion. Cluster‐randomised trials were eligible for inclusion in the analyses along with individually‐randomised trials.
Types of participants
Pregnant women carrying a live fetus at or near term (≥ 36 weeks' gestation).
Types of interventions
Amniotic membrane sweeping.
Comparisons
Amniotic membrane sweeping versus no treatment/sham treatment – all women
Amniotic membrane sweeping versus vaginal/intracervical prostaglandins – all women
Amniotic membrane sweeping versus intravenous oxytocin +/‐ amniotomy – all women
Amniotic membrane sweeping versus amniotomy only ‐ all women
Amniotic membrane sweeping versus vaginal/oral misoprostol – all women
Amniotic membrane sweeping versus mechanical methods (including extra‐amniotic Foley catheter) – all women
Amniotic membrane sweep versus differing frequencies of amniotic membrane sweeping – all women
For the purpose of this review, membrane sweeping is defined as the manual detachment of the inferior pole of the amniotic membrane from the lower uterine segment. This is performed with consent by a clinician digitally through a circular motion during a vaginal examination at or near term gestation. If the cervical os is closed massage of the cervix will be accepted.
Types of outcome measures
We examined the effect of membrane sweeping had on clinical measures of maternal and infant morbidity, mortality and maternal satisfaction.
Primary outcomes
Maternal
1. Spontaneous onset of labour
2. Induction of labour (defined as the process of artificially stimulating the uterus to start labour (World Health Organization 2000))
3. Caesarean section
4. Spontaneous vaginal birth
5. Uterine hyperstimulation with/without fetal heart rate (FHR) changes. Uterine hyperstimulation defined as uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonicity (a contraction lasting at least two minutes). These may or not be associated with changes in the FHR pattern (persistent decelerations, tachycardia or decreased short‐term variability) (Hofmeyer 2009)
6. Maternal death or serious maternal morbidity (i.e. uterine rupture, admission to intensive care unit, septicaemia)
Neonatal
7. Neonatal death or serious neonatal perinatal morbidity (i.e. neonatal sepsis, seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood)
The above seven outcomes were used in the 'Summary of findings' table.
Secondary outcomes
Maternal
8. Instrumental vaginal birth
9. Epidural analgesia
10. Postpartum haemorrhage (as defined by the trial authors)
11. Uterine rupture; all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery will be excluded (Hofmeyer 2009)
12. Augmentation of labour (defined as “the process of stimulating the uterus to increase the frequency, duration and intensity of contractions after the onset of spontaneous labour” (World Health Organization 2014)
Neonatal
13. Apgar score less than seven at five minutes
14. Neonatal encephalopathy
15. Perinatal death
Measures of satisfaction
16. Woman’s satisfaction
17. Cost
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (25 February 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (25 February 2019 using the search methods detailed in Appendix 1).
Searching other resources
We searched the reference lists of trial reports and reviews.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeBoulvain 2005.
For this update, the following methods were used for assessing the 58 reports that were identified as a result of the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (EF and DD) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details. Where contact was made, we have noted this in the Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias (where there is insufficient information to inform a judgement).
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias (where there is insufficient information to inform a judgement).
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we included missing data in the analyses we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias (where there is insufficient information to inform a judgement).
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias (where there is insufficient information to inform a judgement).
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the certainty of the evidence using the GRADE approach
For this update the certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes.
Maternal
Spontaneous onset of labour
Induction of labour (World Health Organization 2000)
Caesarean section
Spontaneous vaginal birth
Uterine hyperstimulation with/without FHR changes. Uterine hyperstimulation defined as uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonicity (a contraction lasting at least two minutes). These may or not be associated with changes in the FHR pattern (persistent decelerations, tachycardia or decreased short‐term variability) (Hofmeyer 2009)
Maternal death or serious maternal morbidity (i.e. uterine rupture, admission to intensive care unit, septicaemia)
Neonatal
Neonatal perinatal death or serious neonatal perinatal morbidity (i.e. neonatal sepsis, seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood)
GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
No continuous data were analysed in this review. In future updates, if appropriate, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials were eligible for inclusion in the analyses along with individually‐randomised trials. However, we did not identify any eligible cluster‐randomised studies.
Cross‐over trials
Trials with cross‐over designs were not eligible for inclusion.
Other unit of analysis issues
Studies with multiple arms
For studies with multiple treatment arms, we combined all relevant experimental intervention groups in the study (e.g. groups with different timings of membrane sweeping) into a single group and all comparable relevant control intervention groups into a single control group. We did not combine control groups with different types of interventions (e.g. different types of prostaglandins) in a single meta‐analysis; instead we analysed these separately.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data (> 20%) in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either theTau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we explored it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We anticipated clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials and therefore used a random‐effects meta‐analysis to produce an overall summary (we felt that an average treatment effect across trials was considered clinically meaningful). The random‐effects summary is treated as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. Had average treatment effects not been clinically meaningful, we would not have combined trials. Results are presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. Where the data allowed, we analysed the results by the following clinical categories of participants.
Primiparae, intact membrane versus multiparae, intact membrane.
All women, intact membrane, unfavourable cervix (defined as Bishop score ≤ 6) versus all women, intact membrane, favourable cervix (defined as Bishop score ≥ 6).
Subgroup analyses was restricted to primary outcomes.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We conducted a sensitivity analysis on trial quality and on missing data. We limited sensitivity analyses to primary outcomes.
Trial quality: we excluded all studies at high or unclear risk of bias for either sequence generation and/or allocation concealment, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).
Missing data: we excluded studies with high (> 20%) or unclear risk of attrition bias.
Results
Description of studies
Results of the search
See: Figure 1.
1.
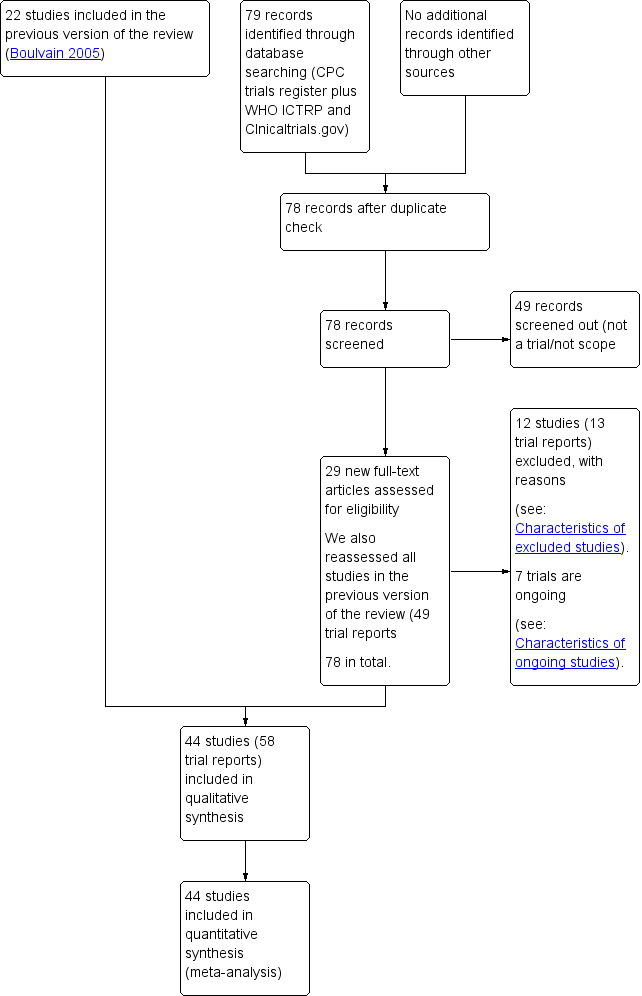
Study flow diagram.
For this update we assessed 29 new trial reports and reassessed the 49 reports in the previous version of the review. We included 44 trials (58 trial reports) and excluded 12 (13 trial reports). Of the five trials excluded in the previous version of this review, we judged two (Gemer 2001; McColgin 1993) as suitable for inclusion. Gemer 2001 was excluded previously for a high risk of allocation concealment (selection bias) 'The study was excluded based on an inadequate method of concealment of the allocation’.McColgin 1993 was excluded in the previous version of this review because ‘No clinical outcomes reported.’ Seven trials are ongoing.
Included studies
SeeCharacteristics of included studies.
Forty‐four studies associated with 58 reports are included. The included studies reported data for 6940 women. Seven studies did not offer any data for outcomes included in this review (Gemer 2001; Imsuwan 1999; McColgin 1993; Salmanian 2012; Weissberg 1977; Yaddehige 2015; Yasmeen 2014).
Design
Of the 44 included studies, all were randomised at the individual level.
Description of intervention
Thirty‐four studies (34/44) offered a detailed description of how they performed a membrane sweep (Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Goldenberg 1996; Gupta 1998; Hamdan 2009; Hill 2008a; Kashanian 2006; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yasmeen 2014; Yildirim 2010; Zamzami 2014).
Ten (10/44) studies did not offer any description of how they performed a membrane sweep (Adeniji 2013; Averill 1999; Gemer 2001; Imsuwan 1999; Janakiraman 2011; Magann 1998a; Magann 1998b; Netta 2002; Salmanian 2012; Yaddehige 2015). Three studies (3/44) reported using a standardised method of membrane sweeping within the trial (Kashanian 2006; Tannirandorn 1999; Wong 2002). Fourteen studies (14/44) (n = 2808) stated they performed cervical massage if the cervix was closed and was not favourable for a membrane sweep (Andersen 2013; Cammu 1998; Crane 1997; de Miranda 2006; Doany 1997; El‐Torkey 1992; Kashanian 2006; Magann 1998a; Putnam 2011; Ramya 2015; Wong 2002; Yasmeen 2014; Yildirim 2010; Zamzami 2014).
Sample sizes
Sample sizes of the included studies ranged from 50 (Gemer 2001) to 377 participants (de Miranda 2006).
Setting
The included studies were undertaken in hospital settings from a wide range of economic regions, as defined by The Word Bank 2018, including high income (25/44) (Allott 1993; Andersen 2013; Averill 1999; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; de Miranda 2006; Doany 1997; El‐Torkey 1992;Gemer 2001; Goldenberg 1996; Hill 2008a; Janakiraman 2011; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Putnam 2011; Salamalekis 2000; Weissberg 1977; Zamzami 2014), upper‐middle income (9/44) (Hamdan 2009; Imsuwan 1999; Kashanian 2006; Parlakgumus 2014; Salmanian 2012; Tannirandorn 1999; Wiriyasirivaj 1996; Wong 2002; Yildirim 2010) and low‐middle income (10/44) (Adeniji 2013; Afzal 2015; Alcoseba‐Lim 1992; Dare 2002; Gupta 1998; Ramya 2015; Saichandran 2015; Ugwu 2014; Yaddehige 2015; Yasmeen 2014) countries.
Five of the studies took place in military hospitals in the USA (5/44) (Hill 2008a; Magann 1998a; Magann 1998b; Magann 1999; Putnam 2011).
Seven studies reported study funding sources (7/44) (Alcoseba‐Lim 1992; Boulvain 1998; Magann 1998b; Magann 1999; McColgin 1993; Parlakgumus 2014; Wong 2002), of which two reported funding from pharmaceutical companies (2/44) (Alcoseba‐Lim 1992; Boulvain 1998) (see Characteristics of included studies).
Of the 44 included trials:
14 were conducted in the USA (Averill 1999; Berghella 1996; Doany 1997; Hill 2008a; Janakiraman 2011; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Putnam 2011; Weissberg 1977);
three in India (Gupta 1998; Ramya 2015; Saichandran 2015);
three in Thailand (Imsuwan 1999; Tannirandorn 1999; Wiriyasirivaj 1996);
three in Nigeria (Adeniji 2013; Dare 2002; Ugwu 2014);
two in the UK (Allott 1993; El‐Torkey 1992);
two in Canada (Boulvain 1998; Crane 1997);
two in Iran (Kashanian 2006; Salmanian 2012);
two in Turkey (Parlakgumus 2014; Yildirim 2010);
one in the Phillippines (Alcoseba‐Lim 1992);
one in Denmark (Andersen 2013);
one in Belgium (Cammu 1998);
two in Israel (Gemer 2001; Goldenberg 1996);
one in the Netherlands (de Miranda 2006);
one in Malaysia (Hamdan 2009);
one in Greece (Salamalekis 2000);
one in China (Wong 2002);
one in Sri Lanka (Yaddehige 2015);
two in Pakistan (Afzal 2015; Yasmeen 2014);
one in Saudi Arabia (Zamzami 2014).
Participants
Three studies (n = 482) only included nulliparous women (3/44) (Cammu 1998; Gupta 1998; Salamalekis 2000). Five studies (n = 817) included multiparous women only (5/44) (Afzal 2015; Hamdan 2009; Imsuwan 1999; Ramya 2015; Yasmeen 2014). Thirty‐five studies (n = 5567) included mixed parity (36/44) (Adeniji 2013; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Berghella 1996; Boulvain 1998; Crane 1997;Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Gemer 2001; Goldenberg 1996; Hill 2008a; Janakiraman 2011; Kashanian 2006; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Parlakgumus 2014; Putnam 2011; Saichandran 2015; Salmanian 2012; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yaddehige 2015; Yildirim 2010; Zamzami 2014). One study (n = 74) did not report on parity (1/44) (Averill 1999).
Three studies (n = 473) included only women with a history of a caesarean section (3/44) (Afzal 2015; Hamdan 2009; Ramya 2015). Twelve studies (n = 1600) excluded women with a history of caesarean section or a uterine scare (12/44) (Adeniji 2013; Alcoseba‐Lim 1992; Doany 1997; Kashanian 2006; Magann 1998a; Parlakgumus 2014; Saichandran 2015; Tannirandorn 1999; Ugwu 2014; Wiriyasirivaj 1996; Wong 2002; Yildirim 2010). Nine studies (n = 1740) included only women with an unfavourable cervix (9/44) (Adeniji 2013; Cammu 1998; Magann 1998a; Magann 1999; Putnam 2011; Ramya 2015; Salamalekis 2000; Yaddehige 2015; Yildirim 2010). Four studies (n = 574) excluded women with a closed cervix (4/44) (Allott 1993; Berghella 1996; Dare 2002; Gupta 1998).
Inclusion criteria for gestational age varied among studies. Three studies (n = 441) included women with pregnancies from 36 weeks’ gestation (3/44) (Alcoseba‐Lim 1992; Hamdan 2009; Netta 2002). Four (n = 398) included women with pregnancies from 37 weeks’ gestation (4/44) (Afzal 2015; Averill 1999; Janakiraman 2011; Weissberg 1977). Fourteen studies (n = 2395) included women pregnancies from 38 weeks’ gestation (14/44) (Berghella 1996; Boulvain 1998; Crane 1997; Dare 2002; Goldenberg 1996; Gupta 1998; Hill 2008a; McColgin 1990a; McColgin 1990b; McColgin 1993; Parlakgumus 2014; Wiriyasirivaj 1996; Yildirim 2010; Zamzami 2014). Six studies (n = 1050) included women pregnancies from 39 weeks’ gestation (6/44) (Cammu 1998; Kashanian 2006; Magann 1998a; Putnam 2011; Ramya 2015; Tannirandorn 1999). Ten studies (n = 1410) included women pregnancies from 40 weeks’ gestation (10/44) (Adeniji 2013; Allott 1993; de Miranda 2006; Saichandran 2015; Salamalekis 2000; Salmanian 2012; Ugwu 2014; Wong 2002; Yaddehige 2015; Yasmeen 2014). Six studies (n = 1196) included women pregnancies from 41 weeks’ gestation (6/44) (Andersen 2013; Doany 1997; El‐Torkey 1992; Imsuwan 1999; Magann 1998b; Magann 1999).
Two studies (n = 221) (2/44) (Janakiraman 2011; Netta 2002) examined membrane sweeping in women who were group B streptococcus positive. No additional maternal or fetal risk was noted with membrane sweeping. However, both studies were small and only abstracts were available to assess results.
The dates studies were conducted varied, with one study conducted over 40 years ago (Weissberg 1977). Twenty studies were conducted during the 1990s (Alcoseba‐Lim 1992; Allott 1993; Averill 1999; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Doany 1997; El‐Torkey 1992; Goldenberg 1996; Gupta 1998; Imsuwan 1999; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Tannirandorn 1999; Wiriyasirivaj 1996) and 23 studies conducted in the 21st century (Adeniji 2013; Afzal 2015; Andersen 2013; Dare 2002; de Miranda 2006; Gemer 2001; Hamdan 2009; Hill 2008a; Janakiraman 2011; Kashanian 2006; Netta 2002; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Salmanian 2012; Ugwu 2014; Wong 2002; Yaddehige 2015; Yasmeen 2014; Yildirim 2010; Zamzami 2014). Of these seven were conducted in the last five years (Afzal 2015; Parlakgumus 2014; Ramya 2015; Saichandran 2015; Yaddehige 2015; Yasmeen 2014; Zamzami 2014).
Interventions and Comparisons
Amniotic membrane sweeping versus no treatment/sham treatment
Of the 44 studies included, 40 (n = 6548) compared membrane sweeping with no treatment or sham treatment (40/44) (Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Averill 1999; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Goldenberg 1996; Gupta 1998; Hamdan 2009; Hill 2008a; Imsuwan 1999; Janakiraman 2011; Kashanian 2006; Magann 1998a; Magann 1998b; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yaddehige 2015; Yasmeen 2014Yildirim 2010; Zamzami 2014).
Amniotic membrane sweeping versus vaginal/intracervical prostaglandins
Four studies (n = 480) compared membrane sweeping with vaginal/intracervical prostaglandins (4/44) (Doany 1997; Gemer 2001; Magann 1998b; Magann 1999).
Amniotic membrane sweeping versus intravenous oxytocin +/‐ amniotomy
One study (n = 104) compared membrane sweeping with intravenous oxytocin +/‐ amniotomy (1/44) (Salamalekis 2000).
Amniotic membrane sweeping versus amniotomy only
No studies compared membrane sweeping with amniotomy only.
Amniotic membrane sweeping versus vaginal/oral misoprostol
Two studies n = 160) compared membrane sweeping with vaginal/oral misoprostol (2/44) (Adeniji 2013; Salmanian 2012).
Amniotic membrane sweeping versus mechanical methods
No study compared membrane sweeping with mechanical methods.
One frequency of amniotic membrane sweeping versus another frequency of amniotic membrane sweeping
One study (n = 355) compared differing frequencies of membrane sweeping (1/44) (Putnam 2011).
Six studies (n = 1284) compared membrane sweeping with more than one intervention (6/44) (Andersen 2013; Doany 1997; Magann 1998b; Putnam 2011; Salamalekis 2000; Yaddehige 2015). Seven studies provided no data (7/44) (Gemer 2001; Imsuwan 1999; McColgin 1993; Salmanian 2012; Weissberg 1977; Yaddehige 2015; Yasmeen 2014).
Outcomes
Maternal primary outcomes
Spontaneous onset of labour was reported in 18 studies (Andersen 2013; Cammu 1998; Crane 1997; de Miranda 2006; Doany 1997; El‐Torkey 1992; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Magann 1998a; Magann 1998b; Magann 1999; Ramya 2015; Saichandran 2015; Salamalekis 2000; Wong 2002; Yildirim 2010).
Induction of labour was reported in 16 studies (Allott 1993;Boulvain 1998; Cammu 1998; Crane 1997; de Miranda 2006; Doany 1997; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Magann 1998b; Parlakgumus 2014; Putnam 2011; Saichandran 2015; Salamalekis 2000; Wong 2002).
Caesarean section was reported in 34 studies (Adeniji 2013; Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Averill 1999; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Goldenberg 1996; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Kashanian 2006; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Tannirandorn 1999; Wiriyasirivaj 1996; Wong 2002; Yildirim 2010; Zamzami 2014).
Spontaneous vaginal birth was reported in 27 studies (Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; El‐Torkey 1992; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Tannirandorn 1999; Wiriyasirivaj 1996; Wong 2002; Zamzami 2014).
Maternal death or serious maternal morbidity was reported in 17 studies (Alcoseba‐Lim 1992; Dare 2002; Doany 1997; Goldenberg 1996; Gupta 1998; Hill 2008a; Janakiraman 2011; Kashanian 2006; McColgin 1990a; McColgin 1990b; Putnam 2011; Salamalekis 2000; Tannirandorn 1999; Ugwu 2014; Wiriyasirivaj 1996; Wong 2002; Yildirim 2010).
Uterine hyperstimulation was not reported on.
Neonatal primary outcomes
Neonatal death or serious neonatal perinatal morbidity was reported in 19 studies (Allott 1993; Andersen 2013; Boulvain 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Magann 1999; McColgin 1990b; Netta 2002; Putnam 2011; Saichandran 2015; Wong 2002; Yildirim 2010).
Maternal secondary outcomes
Instrumental vaginal birth was reported in 23 studies (Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Gupta 1998; Hamdan 2009; Magann 1998b; Magann 1999; McColgin 1990a; Putnam 2011; Ramya 2015; Tannirandorn 1999; Wiriyasirivaj 1996; Wong 2002, Zamzami 2014).
Epidural delivery was reported in nine studies (Allott 1993; Andersen 2013; Boulvain 1998; Cammu 1998; Crane 1997; de Miranda 2006; El‐Torkey 1992; Hamdan 2009; Wong 2002).
Postpartum haemorrhage was reported in five studies (Andersen 2013; Hamdan 2009; Tannirandorn 1999; Wiriyasirivaj 1996; Zamzami 2014).
Augmentation of labour was reported in 10 studies (Adeniji 2013; Andersen 2013; Cammu 1998; de Miranda 2006; Doany 1997; Goldenberg 1996; Magann 1998a; Ramya 2015; Saichandran 2015; Wiriyasirivaj 1996).
Uterine rupture was not reported on.
Neonatal secondary outcomes
Apgar score less than seven at five minutes was reported in 12 studies (Adeniji 2013; Andersen 2013; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; Doany 1997;Goldenberg 1996; Hamdan 2009; Magann 1998b; Magann 1999; Putnam 2011).
Neonatal encephalopathy was not reported on.
Woman’s satisfaction
Three studies providing data for (n = 675) women reported on maternal satisfaction (Adeniji 2013; Boulvain 1998; de Miranda 2006). One study compared membrane sweeping with oral misoprostol (Adeniji 2013). Boulvain 1998 compared membrane sweeping with a control group who underwent a vaginal examination for Bishop scoring only. de Miranda 2006 compared membrane sweeping to a control group where vaginal examination was not performed until the onset of labour
Cost
Two studies (n = 290) women reported on a cost analysis (Magann 1998b; Magann 1999). Both reported a cost per person (US dollars) and compared membrane sweeping with vaginal/intracervical prostaglandins.
Excluded studies
We excluded 12 studies, see Characteristics of excluded studies. Of these, 11 studies were excluded because the interventions compared did not meet our inclusion criteria (Al‐Harmi 2015; Bergsjo 1989; Day 2009; Foong 2000; Ifnan 2006; Kaul 2004; Laddad 2013; Park 2013; Park 2015; Shravage 2009; Tan 2006). One study did not demonstrate an adequate method of random sequence generation or allocation concealment (Swann 1958). Of the five trials excluded in the previous version of this review, we assessed two (Gemer 2001; McColgin 1993) as suitable for inclusion. Gemer 2001 was excluded previously for a high risk of allocation concealment (selection bias) 'The study was excluded based on an inadequate method of concealment of the allocation’.McColgin 1993 was excluded in the previous version of this review because ‘No clinical outcomes reported.’
Risk of bias in included studies
See Figure 2 for a summary of 'Risk of bias' assessments and Figure 3 for review authors’ judgements about each risk of bias item across all included studies.
2.
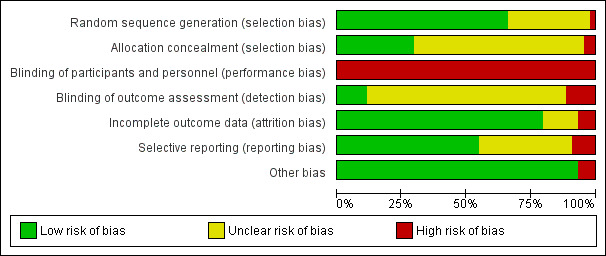
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
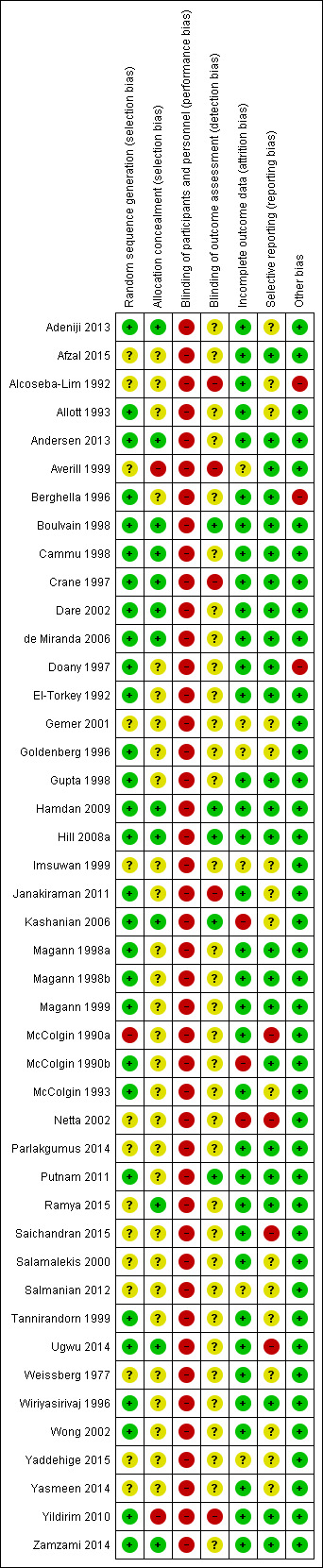
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Twenty‐nine studies were judged to be at a low risk for selection bias in random sequence generation (Adeniji 2013; Allott 1993; Andersen 2013; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992;Goldenberg 1996; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Kashanian 2006; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990b; McColgin 1993; Putnam 2011; Tannirandorn 1999; Ugwu 2014; Wiriyasirivaj 1996; Wong 2002; Yildirim 2010; Zamzami 2014). We judged studies to be at low risk for selection bias in random sequence generation if they had stated an appropriate randomisation method clearly, e.g. Adeniji 2013 stated that 'Computer‐generated random numbers were used for patient allocation'. Fourteen studies were judged to have unclear methods of random sequence generation primarily for lack of published methodological detail, e.g. Afzal 2015 states that trial participants 'were randomly allocated', with no further detail provided of the methods used given (Afzal 2015; Alcoseba‐Lim 1992; Averill 1999; Gemer 2001; Imsuwan 1999; Netta 2002; Parlakgumus 2014; Ramya 2015; Saichandran 2015; Salamalekis 2000; Salmanian 2012; Weissberg 1977; Yaddehige 2015; Yasmeen 2014). McColgin 1990a was judged to be of high risk for bias as it stated that women were 'prospectively assigned' to either receive a membrane sweep group or a control group'. See Characteristics of included studies.
Allocation concealment
Thirteen studies were judged to be of low risk of bias for allocation concealment (Adeniji 2013; Andersen 2013; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Hamdan 2009; Hill 2008a; Kashanian 2006; Ramya 2015; Ugwu 2014; Zamzami 2014). We found studies to be at low risk of bias for allocation concealment when a study reported fully the methodology used for allocation concealment, e.g. Andersen 2013 states “the allocations were contained in a series of opaque, sealed and consecutively numbered envelopes, kept in the delivery unit” “clerk opened the next envelope and informed the doctor of the woman’s allocation”. Twenty‐nine were judged to be of unclear risk of bias for allocation concealment due to insufficient reporting of methodological methods, e.g. Alcoseba‐Lim 1992 provided no evidence of the methods used to ensure allocation concealment (Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Berghella 1996; Doany 1997; El‐Torkey 1992; Gemer 2001; Goldenberg 1996; Gupta 1998; Imsuwan 1999; Janakiraman 2011; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Parlakgumus 2014; Putnam 2011; Saichandran 2015; Salamalekis 2000; Salmanian 2012; Tannirandorn 1999; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yaddehige 2015; Yasmeen 2014). Two studies (Averill 1999; Yildirim 2010) were judged to be high risk of bias for allocation concealment. Yildirim 2010 was found to be of high risk of bias for allocation concealment as the "investigator was not blinded to the allocation procedure” and "sealed opaque envelopes" were "withdrawn from the appropriate box and allocated to the woman" by the investigator. See Characteristics of included studies.
Blinding
Performance bias
All 44 studies in our review were judged to be of high risk for performance bias. Clinicians were not blinded to the intervention in any study and it is unclear (and unlikely in our view) in most studies if study participants were blinded post allocation. For some outcomes, e.g. “induction of labour”, knowledge of the allocation may have encouraged the clinician to modify the date for the procedure. See Characteristics of included studies.
Detection bias
Five studies were judged to be of low risk for detection bias (Boulvain 1998; Hamdan 2009; Hill 2008a; Kashanian 2006; Putnam 2011). We judged studies to be at low risk for detection bias if they had clearly stated an appropriate methodology to prevent detection bias, e.g. Hill 2008a states “All data were collected and all chart analysis was done by the primary author, who was also blinded to the group allocations. Unblinding did not occur until the time of data analysis.” Thirty‐four were judged to be of unclear risk of bias primarily due to a lack of methodological detail (Adeniji 2013; Afzal 2015; Allott 1993; Andersen 2013; Berghella 1996; Cammu 1998; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Gemer 2001; Goldenberg 1996; Gupta 1998; Imsuwan 1999; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Parlakgumus 2014; Ramya 2015; Saichandran 2015; Salamalekis 2000; Salmanian 2012; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002;Yaddehige 2015; Yasmeen 2014; Zamzami 2014). Five studies were judged to be of high risk of bias as the outcome assessors were aware of allocation, e.g. Janakiraman 2011 states that “No blinding was attempted” in the study (Alcoseba‐Lim 1992; Averill 1999; Crane 1997; Janakiraman 2011; Yildirim 2010). See Characteristics of included studies.
Incomplete outcome data
Thirty‐five studies were judged to be of low risk for attrition bias with minimal or no attrition noted (Adeniji 2013; Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1993; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yasmeen 2014; Yildirim 2010; Zamzami 2014).
Six studies were judged to be of unclear risk of bias as there was insufficient information to make an informed decision (Averill 1999; Gemer 2001; Goldenberg 1996; Imsuwan 1999; Salmanian 2012; Yaddehige 2015). Three studies were assessed as high risk of bias. Two were judged to be of high risk of bias due to high attrition rates, Netta 2002 (52%, 51/98) and Kashanian 2006 (33.5%, 51/152). McColgin 1990b was judged to be of high risk of bias as 29 of 209 women initially recruited were excluded. See Characteristics of included studies.
Selective reporting
Twenty‐four studies were judged as low risk for reporting bias (Afzal 2015; Andersen 2013; Averill 1999; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Gupta 1998; Hamdan 2009; Hill 2008a; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990b; Parlakgumus 2014; Putnam 2011; Ramya 2015; Wiriyasirivaj 1996; Yildirim 2010; Zamzami 2014). Sixteen were judged to be of unclear risk for reporting bias. Allott 1993 was judged as unclear risk of reporting bias as data were reported unclearly, with inconsistencies (see Characteristics of included studies) (Adeniji 2013; Alcoseba‐Lim 1992; Allott 1993; Gemer 2001; Goldenberg 1996; Imsuwan 1999; Janakiraman 2011; Kashanian 2006; McColgin 1993; Salamalekis 2000; Salmanian 2012; Tannirandorn 1999; Weissberg 1977; Wong 2002; Yaddehige 2015; Yasmeen 2014). Four studies were judged high risk for reporting bias. Two as primary outcomes were not reported (McColgin 1990a; Saichandran 2015). One study was deemed high risk as it only reported data on nulliparous women with a mixed parity trial (Netta 2002), and another as the study only reported outcomes for participants who did not exceed 41 + 3 weeks' gestation (Ugwu 2014). See Characteristics of included studies.
Other potential sources of bias
Forty‐one studies were judged to be at low risk for other sources of bias (Adeniji 2013; Afzal 2015; Allott 1993; Andersen 2013; Averill 1999; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; El‐Torkey 1992; Gemer 2001; Goldenberg 1996; Gupta 1998; Hamdan 2009; Hill 2008a; Imsuwan 1999; Janakiraman 2011; Kashanian 2006; Magann 1998a; Magann 1998b; Magann 1999; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Salmanian 2012; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yaddehige 2015; Yasmeen 2014; Yildirim 2010; Zamzami 2014). Three studies were assessed as high risk of bias, i.e. Alcoseba‐Lim 1992 for imbalance within groups in baseline Bishop score, Berghella 1996 for imbalance within groups in baseline parity and Doany 1997 for unbalanced group sizes. See Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
See: Table 1 for the main comparison: membrane sweeping compared with no treatment or a sham treatment.
Forty‐four studies associated with 58 publications were included. The included studies reported data for 6940 women. Six studies did not provide data for outcomes included in this review (Gemer 2001; Imsuwan 1999; McColgin 1993; Salmanian 2012; Yaddehige 2015; Yasmeen 2014).
Comparison 1: Amniotic membrane sweeping versus no treatment/sham
Forty studies reported data for 6548 women comparing membrane sweeping with no treatment or a sham treatment (Afzal 2015; Alcoseba‐Lim 1992; Allott 1993; Andersen 2013; Averill 1999; Berghella 1996; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Doany 1997; El‐Torkey 1992; Goldenberg 1996; Gupta 1998; Hamdan 2009; Hill 2008a; Imsuwan 1999; Janakiraman 2011; Kashanian 2006; Magann 1998a; Magann 1998b; McColgin 1990a; McColgin 1990b; McColgin 1993; Netta 2002; Parlakgumus 2014; Putnam 2011; Ramya 2015; Saichandran 2015; Salamalekis 2000; Tannirandorn 1999; Ugwu 2014; Weissberg 1977; Wiriyasirivaj 1996; Wong 2002; Yaddehige 2015; Yasmeen 2014; Yildirim 2010; Zamzami 2014).
Primary outcomes
1.1 Spontaneous onset of labour
Seventeen studies reported on spontaneous onset of labour within this comparison. Women in the membrane sweeping group may, on average, be more likely to experience spontaneous onset of labour compared to women in the control group (average risk ratio (RR), 1.21 95% confidence interval (CI) 1.08 to 1.34, 17 studies, 3170 participants, low‐certainty evidence Analysis 1.1). We found substantial heterogeneity (Tau² 0.03, I² = 73%, P < 0.00001) between the trials contributing data. While heterogeneity remains unexplained, we note the following differences in populations. Study size varied from n = 65 (El‐Torkey 1992) to n = 377 (de Miranda 2006). Three studies excluded multiparous women (Cammu 1998; Gupta 1998; Salamalekis 2000), and two excluded nulliparous women (Hamdan 2009; Ramya 2015). Five studies excluded women with a history of a uterine scar (Doany 1997; Magann 1998a; Saichandran 2015; Wong 2002; Yildirim 2010), and two studies included women with a history of a previous caesarean section or uterine scar (Hamdan 2009; Ramya 2015). Gestation at group allocation varied with a gestational difference of five weeks between Hamdan 2009 (> 36/40 weeks' gestation) and Doany 1997 (> 41/40 weeks' gestation). Five studies included only women with an unfavourable cervix (Cammu 1998; Magann 1998a; Magann 1998b; Ramya 2015; Salamalekis 2000) and one study included only women with a favourable cervix (Andersen 2013). Netta 2002 provided data for subgroup analysis of parity only. Ten of the 17 studies performed cervical massage if the cervix was closed on vaginal examination (Andersen 2013; Cammu 1998; Crane 1997; de Miranda 2006; Doany 1997; El‐Torkey 1992; Magann 1998a; Ramya 2015; Wong 2002; Yildirim 2010). Ten studies did not perform cervical massage or did not report this aspect of the intervention.
1.1. Analysis.
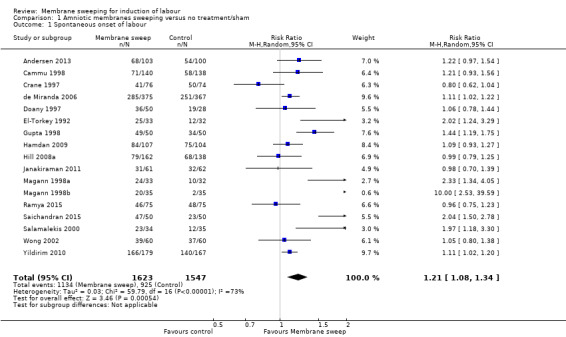
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 1 Spontaneous onset of labour.
As we identified substantial heterogeneity, we investigated it using a priori subgroup and sensitivity analyses.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Three studies reported data for primiparous women. Two studies reported data for multiparous women and 12 reported data for women of unknown parity. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² 5.92, P = 0.05, I² = 66.2%), suggesting that parity does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the multiparous and primiparous subgroups than to the unknown parity subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 8.1).
8.1. Analysis.
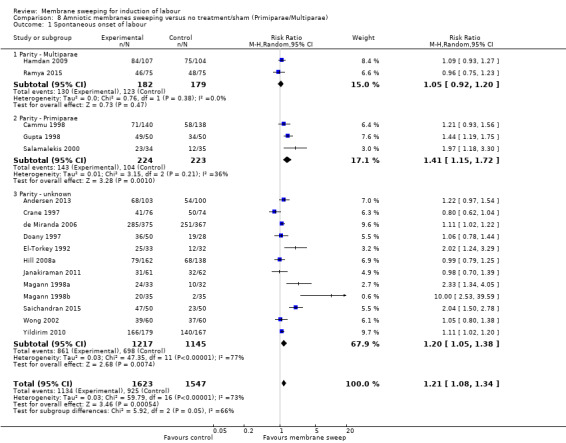
Comparison 8 Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae), Outcome 1 Spontaneous onset of labour.
Cervical status
No study reported data for a favourable cervix. Five studies reported data for an unfavourable cervix and 12 studies reported data for unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² 2.01, P = 0.16, I² = 50.4%), suggesting that cervical status does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the favourable and unfavourable subgroups than to the unknown cervical status subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 13.1).
13.1. Analysis.
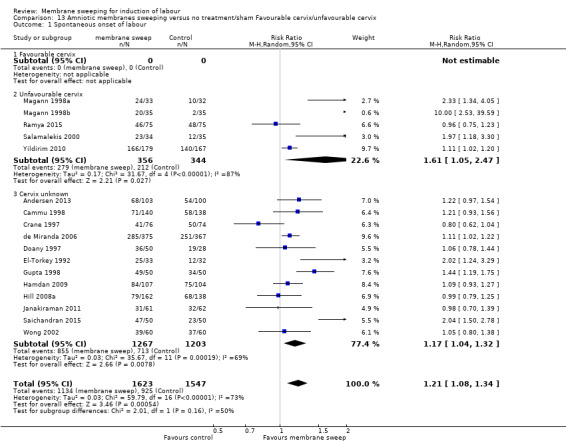
Comparison 13 Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix, Outcome 1 Spontaneous onset of labour.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
1.2. Induction of labour
Sixteen studies reported on induction of labour. When comparing membrane sweeping with no treatment or sham, women in the membrane sweeping group may, on average, be less likely to experience an induction of labour (average RR 0.73, 95% CI 0.56 to 0.94, 16 studies, 3224 participants, low‐certainty evidence; Analysis 1.2). There was substantial heterogeneity (Tau² 0.17, I² = 75%, P < 0.00001) between the trials contributing data. While heterogeneity remains unexplained, we note the following differences in populations. Study size varied from n = 69 (Salamalekis 2000) to n = 742 (de Miranda 2006). The inclusion criteria for Hamdan 2009 is multiparous women with a history of a previous caesarean section or uterine scar. Four studies did not include women with a history of uterine scar (Doany 1997; Parlakgumus 2014; Saichandran 2015; Wong 2002). Three studies excluded multiparous women (Cammu 1998; Gupta 1998; Salamalekis 2000). Twelve studies included women of mixed parity (Allott 1993; Boulvain 1998; Crane 1997; de Miranda 2006; Doany 1997; Hill 2008a; Janakiraman 2011; Magann 1998b; Parlakgumus 2014; Putnam 2011; Saichandran 2015; Wong 2002). Gestation at allocation varied, with a five‐week difference noted between Hamdan 2009 (> 36/40 weeks' gestation) and Doany 1997 (> 41/40 weeks' gestation). Three studies included participants with an unfavourable cervix (Bishop score < 6) at allocation (Cammu 1998; Putnam 2011; Salamalekis 2000). Two studies included participants with a favourable cervix (Bishop score > 6) at allocation 2/16 (Allott 1993; Gupta 1998). Seven studies performed cervical massage if the cervix was closed (Boulvain 1998; Cammu 1998; Crane 1997; de Miranda 2006; Doany 1997; Putnam 2011; Wong 2002). Nine studies did not state if cervical massage was used (Allott 1993; Gupta 1998; Hamdan 2009; Hill 2008a; Janakiraman 2011; Magann 1998b; Parlakgumus 2014; Saichandran 2015; Salamalekis 2000).
1.2. Analysis.
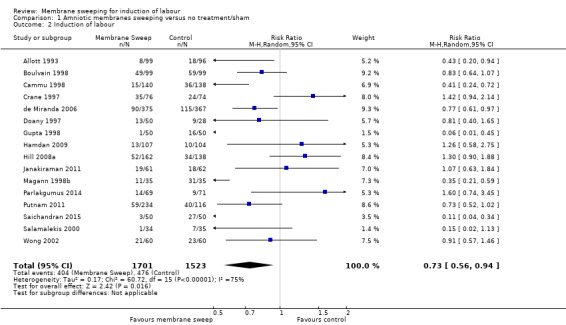
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 2 Induction of labour.
As we identified substantial heterogeneity, we investigated it using a priori subgroup and sensitivity analyses.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Five studies reported data for primiparous women. Two studies reported data for multiparous women and eleven studies reported data for women of unknown parity. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 3.24, P = 0.20, I² = 38.3%), suggesting that parity does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the multiparous and primiparous subgroups than to the unknown parity subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 8.2).
8.2. Analysis.
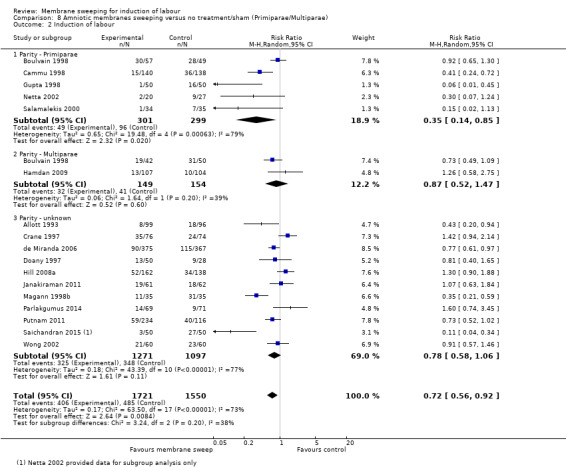
Comparison 8 Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae), Outcome 2 Induction of labour.
Cervical status
One study reported data for a favourable cervix. Four studies reported data for an unfavourable cervix and 13 studies reported data for unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 3.63, P = 0.16, I² = 44.9%), suggesting that cervical status does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the favourable and unfavourable subgroups than to the unknown cervical status subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 13.2).
13.2. Analysis.
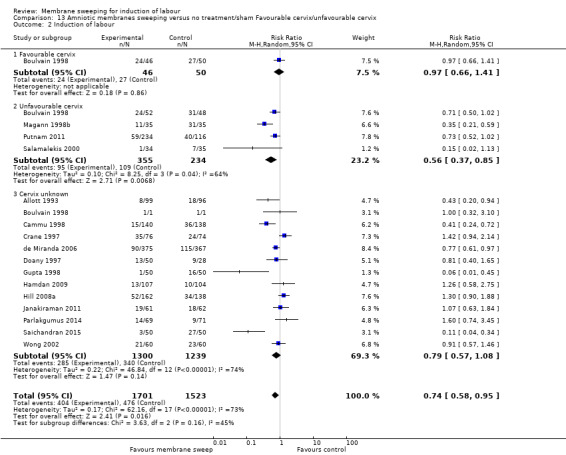
Comparison 13 Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix, Outcome 2 Induction of labour.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
1.3 Caesarean section
Caesarean section was reported in 32 studies. Compared to control/sham, membrane sweeping may, on average, have little to no effect on the risk of caesarean section (average RR 0.94, 95% CI 0.85 to 1.04, 32 studies, 5499 participants, moderate‐certainty evidence; Analysis 1.3). Heterogeneity was low (between the trials contributing data (Tau² 0.00, I² = 1%, P = 0.45).
1.3. Analysis.
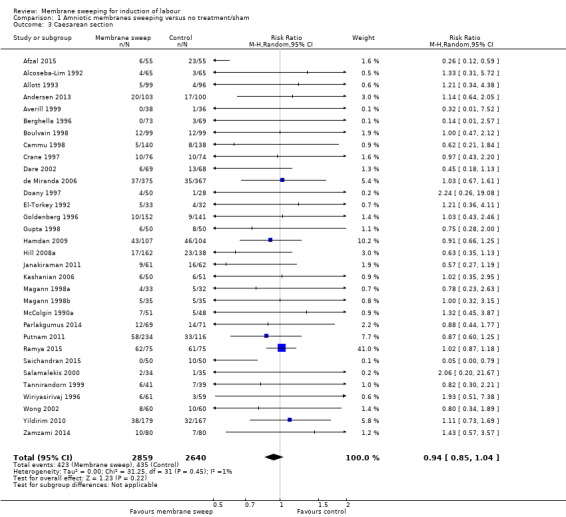
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 3 Caesarean section.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Four studies reported data for primiparous women. Four studies reported data for multiparous women and 25 studies reported data for women of unknown parity. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.65, P = 0.72, I² = 0%), suggesting that parity does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the multiparous and primiparous subgroups than to the unknown parity subgroup, meaning that the analysis may not be able to detect subgroup differences ().
Cervical status
One study reported data for a favourable cervix. Seven studies reported data for an unfavourable cervix and 24 studies reported data for women of unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 2.87, P = 0.24, I² = 30.2%), suggesting that cervical status does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the favourable and unfavourable subgroups than to the unknown cervical status subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 13.3).
13.3. Analysis.
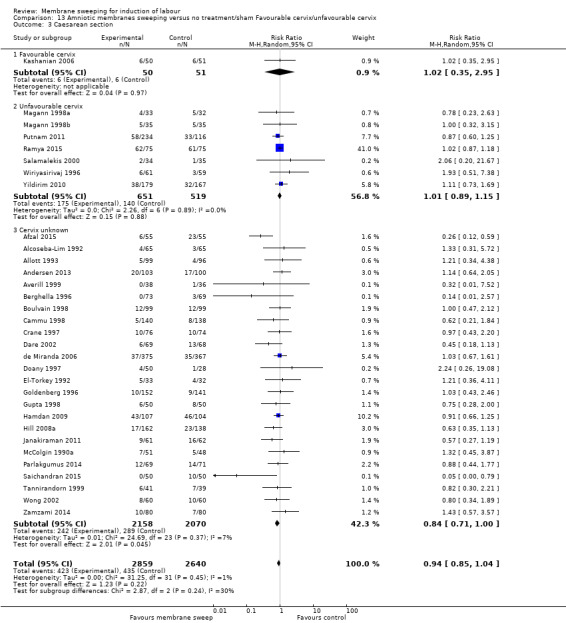
Comparison 13 Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix, Outcome 3 Caesarean section.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
1.4. Spontaneous vaginal birth
Spontaneous vaginal birth was reported in 26 studies. Compared to control/sham, membrane sweeping may have, on average, little to no effect on the risk of spontaneous vaginal birth (average RR 1.03, 95% CI 0.99 to 1.07, 26 studies, 4538 participants, moderate certainty evidence; Analysis 1.4). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 14%, P = 0.26).
1.4. Analysis.
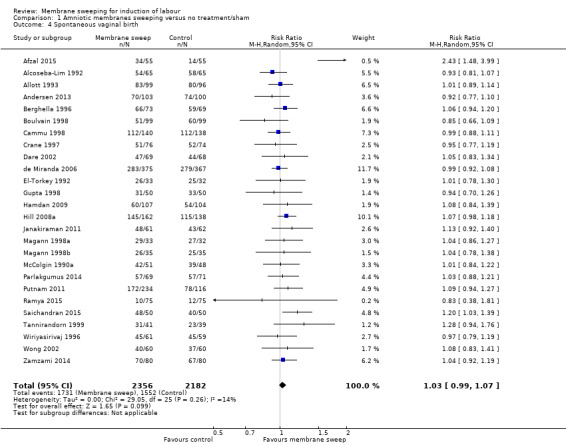
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 4 Spontaneous vaginal birth.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Three studies reported data for primiparous women. Four studies reported data for multiparous women and 20 studies reported data for women of unknown parity. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.62, P = 0.73, I² = 0%), suggesting that parity does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the multiparous and primiparous subgroups than to the unknown parity subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 8.4).
8.4. Analysis.
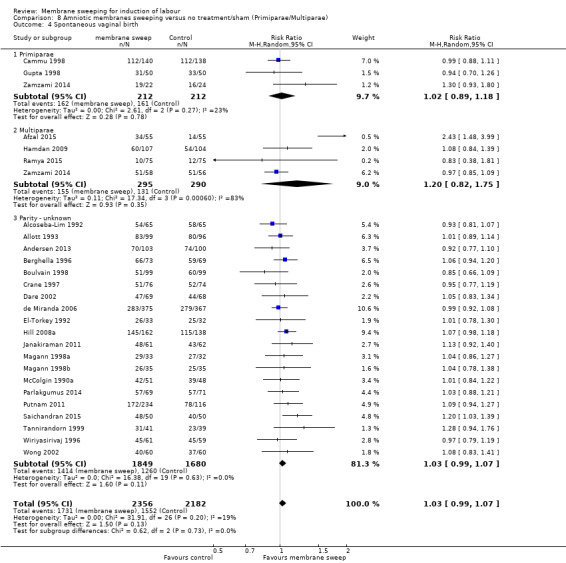
Comparison 8 Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae), Outcome 4 Spontaneous vaginal birth.
Cervical status
No studies reported data for a favourable cervix. Five studies reported data for an unfavourable cervix and 21 studies reported data for women of unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.04, P = 0.83, I² = 0%), suggesting that cervical status does not modify intervention effect. However, we note a smaller number of trials and participants contributed data to the favourable and unfavourable subgroups than to the unknown cervical status subgroup, meaning that the analysis may not be able to detect subgroup differences (Analysis 13.4).
13.4. Analysis.
Comparison 13 Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix, Outcome 4 Spontaneous vaginal birth.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
1.5. Uterine hyperstimulation with/without fetal heart rate (FHR) changes
No studies reported on uterine hyperstimulation with/without FHR changes.
1.6. Maternal death or serious maternal morbidity
Seventeen studies reported on maternal death or serious maternal morbidity. Compared to control/sham, membrane sweeping may have, on average, little to no effect on the risk of maternal death or serious maternal morbidity (average RR 0.83, 95% CI 0.57 to 1.20, 17 studies, 2749 participants, low‐certainty evidence; Analysis 1.5). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 0%, P = 0.84).
1.5. Analysis.
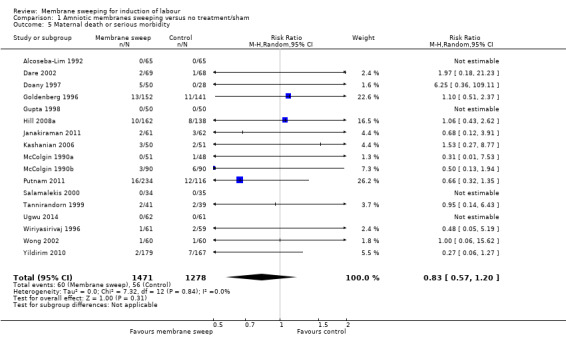
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 5 Maternal death or serious morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Two studies reported data for primiparous women, but no events were reported. No studies reported data for multiparous women and 15 studies reported data for women of unknown parity. Therefore, tests for subgroup interaction effects were not possible (Analysis 8.5).
8.5. Analysis.
Comparison 8 Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae), Outcome 5 Maternal death or serious morbidity.
Cervical status
No studies reported data for a favourable cervix. Four studies reported data for an unfavourable cervix and 13 studies reported data for women of unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 2.32, P = 0.13, I² = 56.9%), suggesting that cervical status does not modify intervention effect. However, we note no studies contributed data to the favourable subgroup and a smaller number of trials and participants contributed data to the unfavourable subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 13.5).
13.5. Analysis.
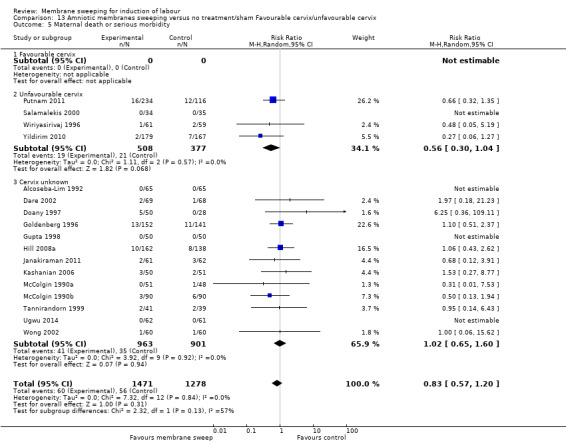
Comparison 13 Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix, Outcome 5 Maternal death or serious morbidity.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots.
We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
1.7 Neonatal death or serious neonatal perinatal morbidity
Eighteen studies reported on neonatal death or serious neonatal perinatal morbidity. Compared to control/sham, membrane sweeping may have, on average, little to no effect on the risk of neonatal perinatal death or serious neonatal perinatal morbidity (average RR 0.83, 95% CI 0.59 to 1.17, 18 studies, 3696 participants, low‐certainty evidence; Analysis 1.6). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 0%, P = 0.99).
1.6. Analysis.
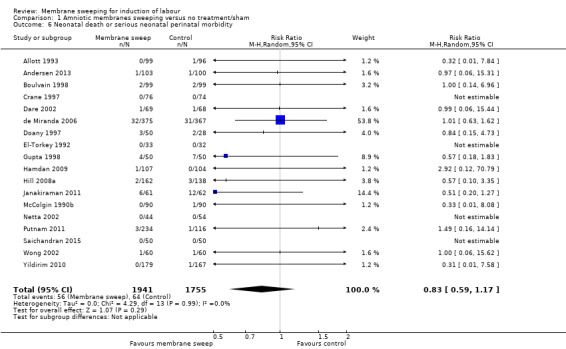
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 6 Neonatal death or serious neonatal perinatal morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
One study reported data for primiparous women. No studies reported data for multiparous women and 17 studies reported data for women of unknown parity. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.43, P = 0.51, I² = 0%), suggesting that parity does not modify intervention effect. However, we note no studies contributed data to the multiparous subgroup and only one contributed data to the primiparous subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 8.6).
8.6. Analysis.
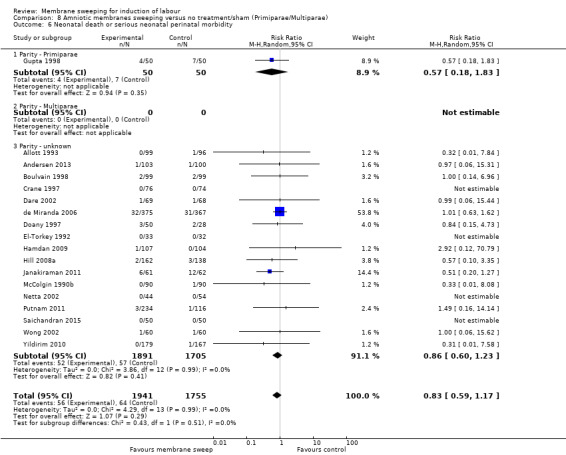
Comparison 8 Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae), Outcome 6 Neonatal death or serious neonatal perinatal morbidity.
Cervical status
No study reported data for a favourable cervix. One study reported data for an unfavourable cervix and 17 studies reported data for women of unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.37 P = 0.55, I² = 0%), suggesting that cervical status does not modify intervention effect. However, we note no studies contributed data to the favourable subgroup and only one contributed data to the unfavourable subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 13.6).
13.6. Analysis.
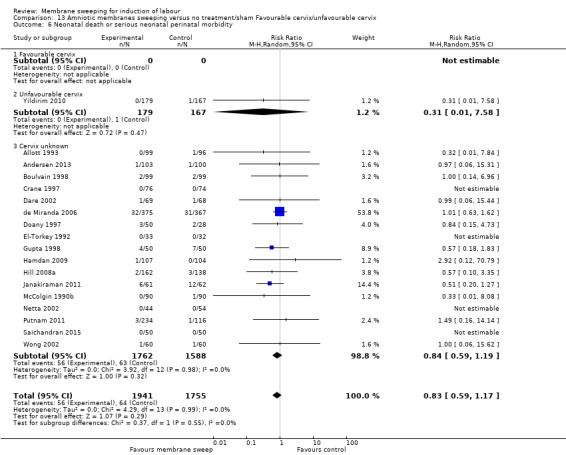
Comparison 13 Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix, Outcome 6 Neonatal death or serious neonatal perinatal morbidity.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots.
We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
Secondary outcomes
1.8 Instrumental vaginal birth
Twenty‐two studies reported on instrumental vaginal birth. Compared to control/sham, membrane sweeping may, on average, have little to no effect on the risk of an instrumental vaginal birth (average RR 1.06, 95% CI 0.91 to 1.25, 22 studies, 3888 participants, low‐certainty evidence; Analysis 1.7). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 0%, P = 0.67).
1.7. Analysis.
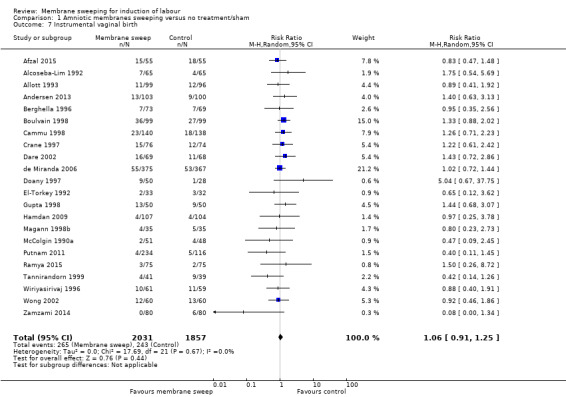
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 7 Instrumental vaginal birth.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots.
We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017).
1.9 Epidural analgesia
Nine studies reported on epidural analgesia. Compared to control/sham, membrane sweeping may, on average, have little to no effect on the risk of epidural analgesia (average RR 1.14, 95% CI 0.97 to 1.33, 9 studies, 2162 participants, low‐certainty evidence; Analysis 1.8. Heterogeneity was low between the trials contributing data (Tau² 0.02, I² = 29%, P = 0.18).
1.8. Analysis.
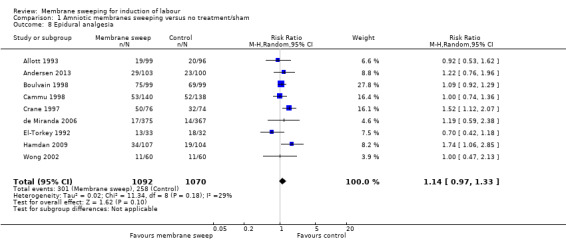
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 8 Epidural analgesia.
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots.
1.10 Postpartum haemorrhage
Five studies reported on postpartum haemorrhage. Compared to control/sham, membrane sweeping may, on average, have little to no effect on the risk of a postpartum haemorrhage (average RR 0.89, 95% CI 0.57 to 1.39, 5 studies, 760 participants, low‐certainty evidence; Analysis 1.9). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 0%, P = 0.95).
1.9. Analysis.
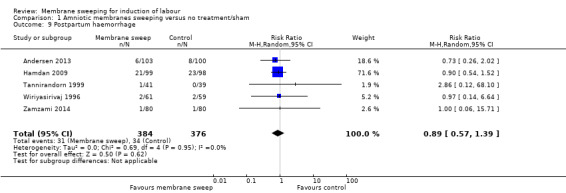
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 9 Postpartum haemorrhage.
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots.
1.11. Uterine rupture
No studies reported on the outcome uterine rupture.
1.12. Augmentation of labour
Nine studies reported on augmentation of labour. Compared to control/sham, membrane sweeping may, on average, have little to no effect on the risk of an augmentation of labour (average RR 0.92, 95% CI 0.72 to 1.17, 9 studies, 2011 participants, low‐certainty evidence; Analysis 1.10). Heterogeneity was high between the trials contributing data (Tau² 0.09, I² = 69%, P = 0.001). While heterogeneity remains unexplained, we note the following differences in populations. Study size varied from n = 23 (Magann 1998a) to n = 742 (de Miranda 2006). The inclusion criteria for Ramya 2015 is multiparous women with a history of a previous caesarean section or uterine scar. Three studies did not include women with a history of uterine scar (Doany 1997; Magann 1998a; Saichandran 2015). One study excluded multiparous women (Cammu 1998). One study excluded primiparous women (Ramya 2015). Gestation at group allocation varied, with a three‐week difference noted between Goldenberg 1996 (> 38/40) and Ramya 2015 (> 41/40). Three studies included participants with an unfavourable cervix (Bishop score < 6) at allocation (Cammu 1998; Magann 1998a; Ramya 2015). Six studies performed cervical massage if the cervix was closed (Andersen 2013; Cammu 1998; de Miranda 2006; Doany 1997; Magann 1998a; Ramya 2015).
1.10. Analysis.
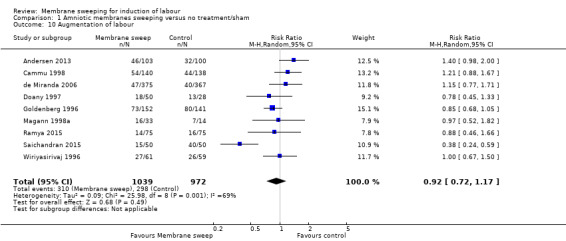
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 10 Augmentation of labour.
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots.
1.13 Apgar score less than seven at five minutes
Ten studies reported on Apgar score less than seven at five minutes. Compared to control/sham, membrane sweeping may, on average, have little to no effect on the risk of an Apgar score less than seven at five minutes (average RR 1.11, 95% CI 0.51 to 2.40, 10 studies, 1958 participants, low‐certainty evidence; Analysis 1.11). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 0%, P = 0.74).
1.11. Analysis.
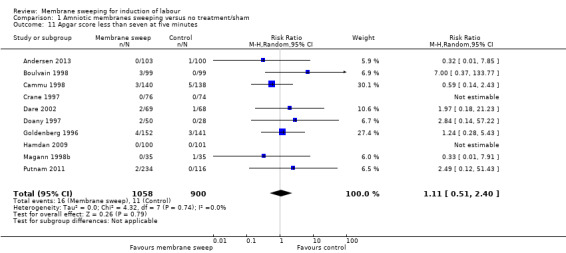
Comparison 1 Amniotic membranes sweeping versus no treatment/sham, Outcome 11 Apgar score less than seven at five minutes.
Assessment of reporting biases
As there were more than 10 studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots.
We assessed funnel plot asymmetry visually. As asymmetry was not suggested by a visual assessment, we did not perform exploratory analyses to investigate it further (Sterne 2017). Heterogeneity was low (I² = 0%) between the trials contributing data.
1.14 Neonatal encephalopathy
No studies reported on the outcome neonatal encephalopathy.
Sensitivity analyses
We conducted a sensitivity analysis excluding studies at high or unclear risk of bias for either sequence generation and/or allocation concealment, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).We also excluded studies with high (> 20%) or unclear risk of attrition bias. Twelve of the 40 trials were judged to be of low risk of bias and included in the sensitivity analysis (Adeniji 2013; Andersen 2013; Boulvain 1998; Cammu 1998; Crane 1997; Dare 2002; de Miranda 2006; Hamdan 2009; Hill 2008a; Kashanian 2006; Ugwu 2014; Zamzami 2014). On sensitivity analyses, all pre‐specified outcomes, with the exception of spontaneous onset of labour and induction of labour, were consistent with overall summary effect estimates. On sensitivity analysis, we found no difference between groups for the outcome spontaneous onset of labour (average RR 1.08, 95% CI 0.98 to 1.18, 6 studies, 1884 participants, low‐certainty evidence; Analysis 20.1). Heterogeneity was moderate between the trials contributing data (Tau² 0.00, I² = 37%, P = 0.16). We found no difference between groups for the outcome induction of labour (average RR 0.92, 95% CI 0.68 to 1.24, 6 studies, 1879 participants, low certainty evidence; Analysis 20.2). Heterogeneity was high between the trials contributing data (Tau² 0.10, I² = 74%, P = 0.002). See: Analysis 20.1; Analysis 20.2; Analysis 20.3; Analysis 20.4; Analysis 20.5; Analysis 20.6.
20.1. Analysis.
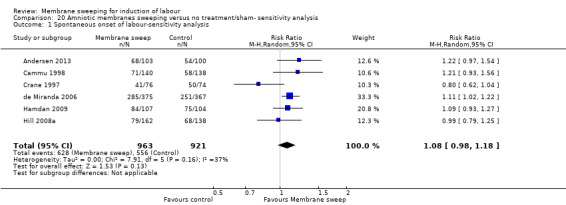
Comparison 20 Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis, Outcome 1 Spontaneous onset of labour‐sensitivity analysis.
20.2. Analysis.
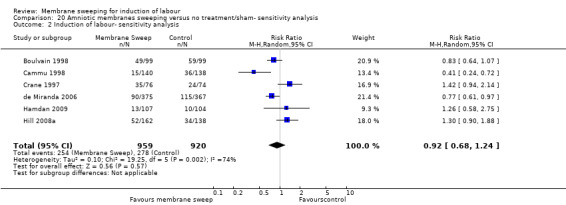
Comparison 20 Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis, Outcome 2 Induction of labour‐ sensitivity analysis.
20.3. Analysis.
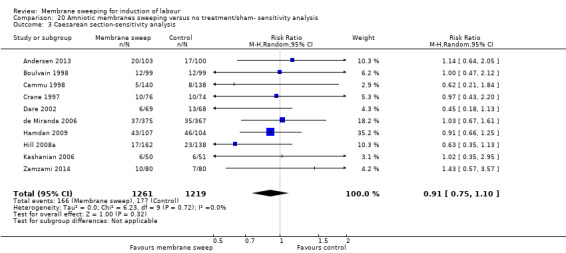
Comparison 20 Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis, Outcome 3 Caesarean section‐sensitivity analysis.
20.4. Analysis.
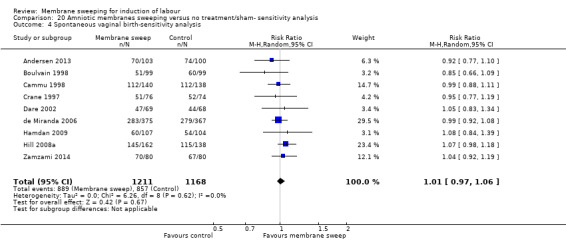
Comparison 20 Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis, Outcome 4 Spontaneous vaginal birth‐sensitivity analysis.
20.5. Analysis.
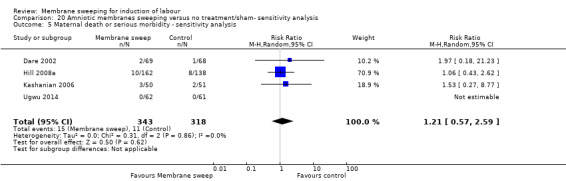
Comparison 20 Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis, Outcome 5 Maternal death or serious morbidity ‐ sensitivity analysis.
20.6. Analysis.
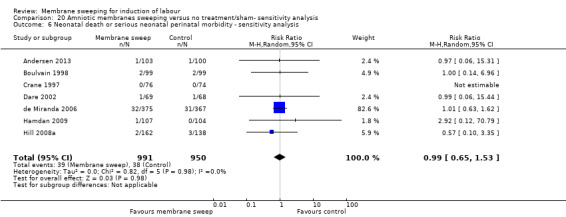
Comparison 20 Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis, Outcome 6 Neonatal death or serious neonatal perinatal morbidity ‐ sensitivity analysis.
Comparison 2: Amniotic membrane sweeping versus vaginal/intracervical prostaglandins
Four studies reported data for 480 women comparing membrane sweeping with vaginal/intracervical prostaglandins (Doany 1997; Gemer 2001; Magann 1998b; Magann 1999). Doany 1997 compared membrane sweeping with intravaginal PGE2 gel (4 mL at 0.5 mg/mL concentration), repeated at regular intervals until either the spontaneous onset of labour or 43 weeks and six days. Gemer 2001 compared membrane sweeping with intracervical prostaglandin E2 0.5 mg gel as a single time intervention. Magann 1998b compared daily membrane sweeping with daily intracervical prostaglandin E2 (PGE2) gel 0.5 mg. Magann 1999 compared daily membrane sweeping with daily placement of a dinoprostone vaginal suppository (Cervidil).
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots for any outcome.
Primary outcomes
2.1 Spontaneous onset of labour
Three studies reported on spontaneous onset of labour within this comparison (Doany 1997; Magann 1998b; Magann 1999). Compared to vaginal/intracervical prostaglandins, membrane sweeping may, on average, have little to no effect on the risk of a spontaneous onset (average RR 1.24, 95% CI 0.98 to 1.57, 3 studies, 339 participants, low‐certainty evidence; Analysis 2.1). There was moderate heterogeneity between the trials contributing data (Tau² 0.02, I² = 40%, P = 0.19).
2.1. Analysis.
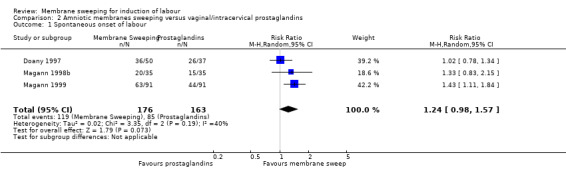
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 1 Spontaneous onset of labour.
While heterogeneity remains unexplained, we note the following differences in populations. Doany 1997 compared membrane sweeping with intravaginal PGE2 Gel (4 mL at 0.5 mg/mL concentration) repeated at regular intervals until either the spontaneous onset of labour or 43 weeks and six days. Magann 1998b compared daily membrane sweeping with daily intracervical prostaglandin E2 (PGE2) gel 0.5 mg. Magann 1999 compared daily membrane sweeping with daily placement of a dinoprostone vaginal suppository (Cervidil). Study size varied from n = 70 (Magann 1998b) to n = 182 (Magann 1999). Doany 1997 excluded women with a history of a previous caesarean section or uterine scar. Magann 1999 included women with an unfavourable cervix (Doany 1997;Magann 1998b) included women of mixed or unknown cervix status. Doany 1997 performed cervical massage if the cervix was closed on vaginal examination.
As we identified substantial heterogeneity, we investigated it using subgroup analyses.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
No studies reported on subgroup analysis by parity for the outcome spontaneous onset of labour.
Cervical status
No study reported data for a favourable cervix. Two studies reported data for an unfavourable cervix and one study reported data for unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 3.16, P = 0.08, I² = 68.4%), suggesting that cervical status does not modify intervention effect. However, we note no studies contributed data to the favourable subgroup and only two contributed data to the unfavourable subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 14.1).
14.1. Analysis.
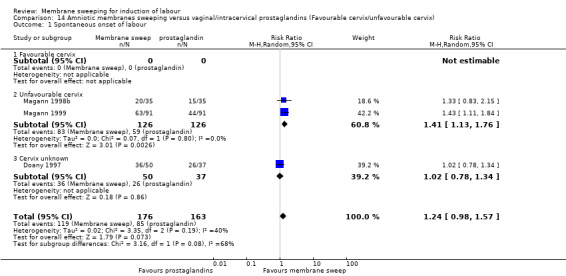
Comparison 14 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins (Favourable cervix/unfavourable cervix), Outcome 1 Spontaneous onset of labour.
2.2 Induction of labour
Two studies reported on the outcome induction of labour (Doany 1997;Magann 1998b). Compared to vaginal/intracervical prostaglandins, membrane sweeping may, on average, have little to no effect on the risk of an induction of labour (average RR 0.90, 95% CI 0.56 to 1.45, 2 studies, 157 participants, low‐certainty evidence; Analysis 2.2). Heterogeneity was low between the trials contributing data (Tau² 0.00, I² = 0%, P = 0.79).
2.2. Analysis.
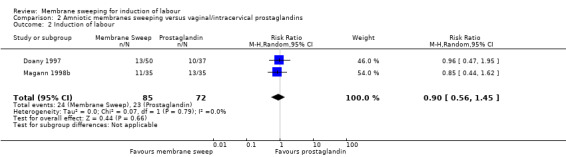
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 2 Induction of labour.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
No studies reported on subgroup analysis by parity for the outcome induction of labour.
Cervical status
No study reported data for a favourable cervix. One study reported data for an unfavourable cervix and one study reported data for unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.07, P = 0.79, I² = 0%), suggesting that cervical status does not modify intervention effect. However, we note no studies contributed data to the favourable subgroup and only one contributed data to the unfavourable and unknown cervical status subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 14.2).
14.2. Analysis.
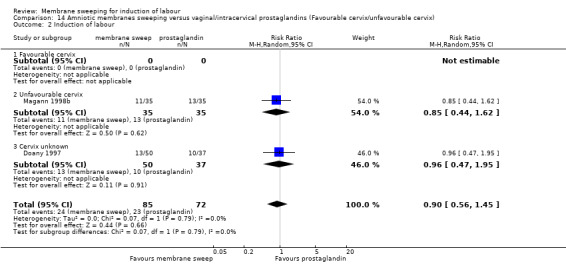
Comparison 14 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins (Favourable cervix/unfavourable cervix), Outcome 2 Induction of labour.
2.3 Caesarean section
Three studies reported on the outcome caesarean section (Doany 1997; Magann 1998b; Magann 1999). Compared to vaginal/intracervical prostaglandins, membrane sweeping may have, on average, little to no effect on the risk of a caesarean section (average RR 0.69, 95% CI 0.44 to 1.09, 3 studies, 339 participants, low‐certainty evidence; Analysis 2.3). Heterogeneity was low between the trials contributing data (Tau² 0.0, I² = 0%, P = 0.87).
2.3. Analysis.
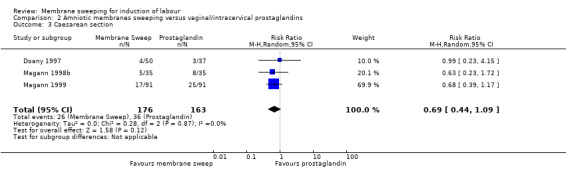
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 3 Caesarean section.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
No studies reported on subgroup analysis by parity for the outcome caesarean section.
Cervical status
No study reported data for a favourable cervix. Two studies reported data for an unfavourable cervix and one study reported data for unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.26, P = 0.61, I² = 0%), suggesting that cervical status does not modify intervention effect. However, we note no studies contributed data to the favourable subgroup, two contributed data to the unfavourable and one to the unknown cervical status subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 14.3.).
14.3. Analysis.
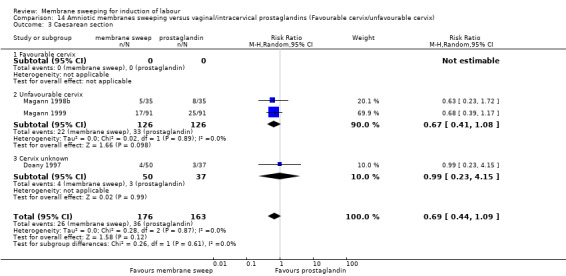
Comparison 14 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins (Favourable cervix/unfavourable cervix), Outcome 3 Caesarean section.
2.4 Spontaneous vaginal birth
Two studies reported on the outcome spontaneous vaginal birth (Magann 1998b; Magann 1999). Compared to vaginal/intracervical prostaglandins, membrane sweeping may have, on average, little to no effect on the risk of a spontaneous vaginal birth (average RR 1.12, 95% CI 0.95 to 1.32, 2 studies, 252 participants, low‐certainty evidence; Analysis 2.4). Heterogeneity was low between the trials contributing data (Tau² 0.0, I² = 0%, P = 0.79).
2.4. Analysis.
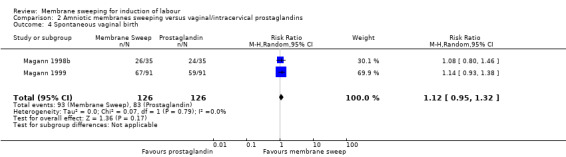
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 4 Spontaneous vaginal birth.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
No studies reported on subgroup analysis by parity for the outcome spontaneous vaginal birth.
Cervical status
No study reported data for a favourable cervix. Two studies reported data for an unfavourable cervix and no study reported data for unknown cervical status for the outcome spontaneous vaginal birth. Therefore, tests for subgroup interaction effects were not possible (Analysis 14.4).
14.4. Analysis.
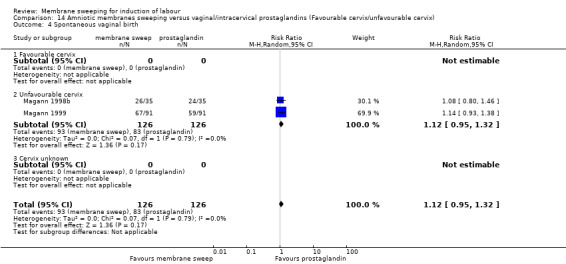
Comparison 14 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins (Favourable cervix/unfavourable cervix), Outcome 4 Spontaneous vaginal birth.
2.5 Uterine hyperstimulation with/without FHR changes
No studies reported on the outcome uterine hyperstimulation with/without FHR changes.
2.6 Maternal death or serious maternal morbidity
One study reported on the outcome maternal death or serious maternal morbidity (Doany 1997). Compared to vaginal/intracervical prostaglandins, membrane sweeping may have, on average, little to no effect on the risk of a maternal death or serious maternal morbidity (average RR 0.93, 95% CI 0.27 to 3.21, 1 study, 87 participants, low‐certainty evidence; Analysis 2.5).
2.5. Analysis.
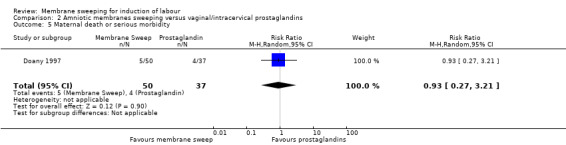
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 5 Maternal death or serious morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
No studies reported on subgroup analysis by parity for the outcome maternal death or serious maternal morbidity.
Cervical status
No studies reported data for a un/favourable cervix for the outcome maternal death or serious maternal morbidity.
2.7 Neonatal death or serious neonatal perinatal morbidity
Two studies reported on the outcome neonatal death or serious neonatal perinatal morbidity (Doany 1997; Magann 1999). Compared to vaginal/intracervical prostaglandins, membrane sweeping may have, on average, little to no effect on the risk of a neonatal death or serious neonatal perinatal morbidity (average RR 0.40, 95% CI 0.12 to 1.33, 2 studies, 269 participants, low‐certainty of evidence; Analysis 2.6). Heterogeneity was low between the trials contributing data (Tau² 0.0, I² = 0%, P = 0.43).
2.6. Analysis.
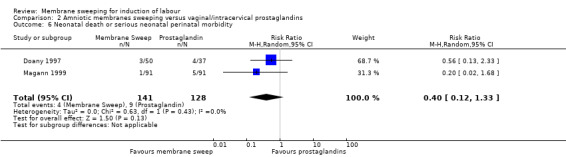
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 6 Neonatal death or serious neonatal perinatal morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
No studies reported on subgroup analysis by parity for the outcome neonatal death or serious neonatal perinatal morbidity.
Cervical status
No studies reported data for a favourable cervix. One study reported data for an unfavourable cervix and one study reported data for unknown cervical status. The test for subgroup differences indicates that there is no statistically significant subgroup effect (Chi² = 0.61, P = 0.44, I² = 0%), suggesting that cervical status does not modify intervention effect. However, we note no studies contributed data to the favourable subgroup and one contributed data to both the unfavourable and the unknown cervical status subgroups, meaning that the analysis may not be able to detect subgroup differences (Analysis 14.5).
14.5. Analysis.
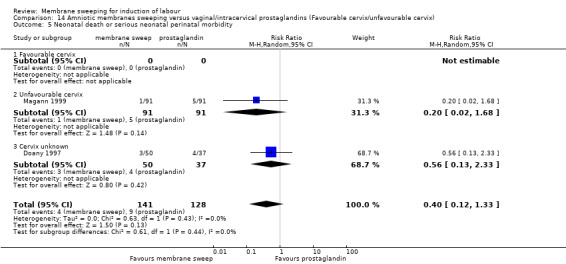
Comparison 14 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins (Favourable cervix/unfavourable cervix), Outcome 5 Neonatal death or serious neonatal perinatal morbidity.
Secondary outcomes
2.8 Instrumental vaginal birth
Three studies reported on the outcome instrumental vaginal birth (Doany 1997;Magann 1998b; Magann 1999). Compared to vaginal/intracervical prostaglandins, membrane sweeping may, on average, have little to no effect on the risk of an instrumental vaginal birth (average RR 1.57, 95% CI 0.59 to 4.14, 3 studies, 339 participants, low‐certainty evidence; Analysis 2.7). There was moderate heterogeneity between the trials contributing data (Tau² 024, I² = 31%, P = 0.24).
2.7. Analysis.
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 7 Instrumental vaginal birth.
2.9 Epidural analgesia
No studies reported on the outcome epidural analgesia.
2.10 Postpartum haemorrhage
No studies reported on the outcome postpartum haemorrhage.
2.11 Uterine rupture
No studies reported on the outcome uterine rupture.
2.12 Augmentation of labour
One study reported on the outcome augmentation of labour (Doany 1997). Compared to vaginal/intracervical prostaglandins, membrane sweeping may, on average, have little to no effect on the risk of an augmentation of labour (average RR 0.78, 95% CI 0.47 to 1.30, 1 study, 87 participants, low‐certainty evidence; Analysis 2.8).
2.8. Analysis.
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 8 Augmentation of labour.
2.13 Apgar score less than seven at five minutes
Three studies reported on the outcome Apgar score less than seven at five minutes (Doany 1997;Magann 1998b; Magann 1999). Compared to vaginal/intracervical prostaglandins, membrane sweeping may, on average, have little to no effect on the risk of an Apgar score less than seven at five minutes (average RR 0.87, 95% CI 0.13 to 5.77, 3 studies, 339 participants, low‐certainty evidence; Analysis 2.9). Heterogeneity was low between the trials contributing data (Tau² 0.0, I² = 0%, P = 0.46).
2.9. Analysis.
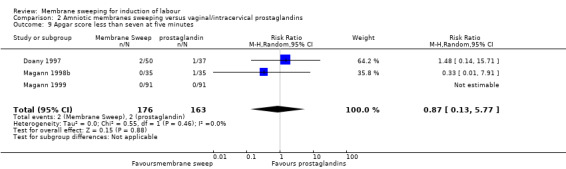
Comparison 2 Amniotic membranes sweeping versus vaginal/intracervical prostaglandins, Outcome 9 Apgar score less than seven at five minutes.
2.14 Neonatal encephalopathy
No studies reported on the outcome neonatal encephalopathy.
Sensitivity analyses
All included studies for this comparison were judged to have an unclear risk for allocation concealment (selection bias) and were therefore excluded from sensitivity analysis.
Comparison 3: Amniotic membrane sweeping versus intravenous oxytocin +/‐ amniotomy
Only one study, with 104 participants (Salamalekis 2000) compared membrane sweeping with oxytocin.
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots.
Primary outcomes
3.1 Spontaneous onset of labour
The one included study (Salamalekis 2000) reported on spontaneous onset of labour within this comparison. Compared to intravenous oxytocin +/‐ amniotomy, membrane sweeping may, on average, have little to no effect on the risk of a spontaneous onset of labour (average RR 1.32, 95% CI 88 to 1.96, 1 study, 69 participants, low‐certainty evidence; Analysis 3.1).
3.1. Analysis.
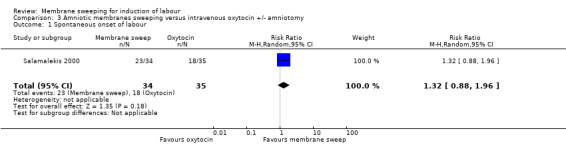
Comparison 3 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy, Outcome 1 Spontaneous onset of labour.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
The one included study in this comparison (Salamalekis 2000) did not report data for multiparous women, but did report data for primiparous women for the outcome spontaneous onset of labour (Analysis 10.1).
10.1. Analysis.
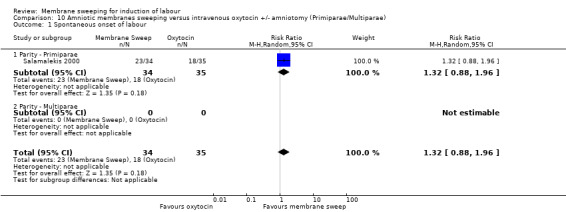
Comparison 10 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Primiparae/Multiparae), Outcome 1 Spontaneous onset of labour.
Cervical status
Salamalekis 2000 did not report data for a favourable cervix, but reported data for an unfavourable cervix for the outcome spontaneous onset of labour (Analysis 15.1.).
15.1. Analysis.
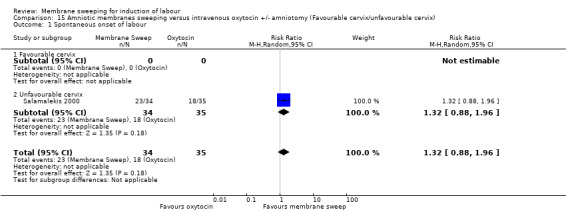
Comparison 15 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Favourable cervix/unfavourable cervix), Outcome 1 Spontaneous onset of labour.
3.2 Induction of labour
Salamalekis 2000 reported on Induction of labour. Compared to intravenous oxytocin +/‐ amniotomy, membrane sweeping may, on average, have little to no effect on the risk of an induction of labour (average RR 0.51, 95% CI 0.05 to 5.42, 1 study, 69 participants, low‐certainty evidence; Analysis 3.2).
3.2. Analysis.
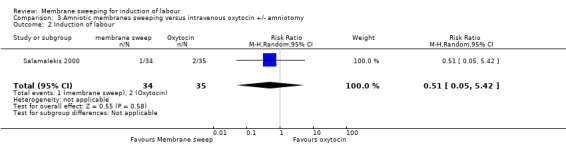
Comparison 3 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy, Outcome 2 Induction of labour.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Salamalekis 2000 did not report data for multiparous women, but did report data for primiparous women for the outcome induction of labour (Analysis 10.2).
10.2. Analysis.
Comparison 10 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Primiparae/Multiparae), Outcome 2 Induction of labour.
Cervical status
Salamalekis 2000 did not report data for a favourable cervix, but did report data for an unfavourable cervix for the outcome induction of labour (Analysis 15.2).
15.2. Analysis.
Comparison 15 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Favourable cervix/unfavourable cervix), Outcome 2 Induction of labour.
3.3 Caesarean section
Salamalekis 2000 reported on caesarean section within this comparison. Compared to intravenous oxytocin +/‐ amniotomy, membrane sweeping may, on average, have little to no effect on the risk of a caesarean section (average RR 0.69, 95% CI 0.12 to 3.85, 1 study, 69 participants, low certainty of evidence; Analysis 3.3).
3.3. Analysis.
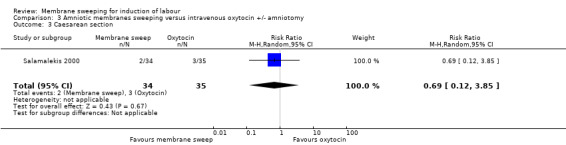
Comparison 3 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy, Outcome 3 Caesarean section.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Salamalekis 2000 did not report data for multiparous women, but did report data for primiparous women for the outcome caesarean section (Analysis 10.3).
10.3. Analysis.
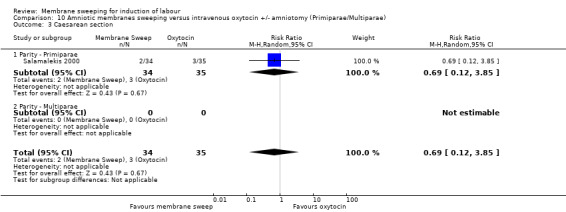
Comparison 10 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Primiparae/Multiparae), Outcome 3 Caesarean section.
Cervical status
Salamalekis 2000 did not report data for a favourable cervix, but did report data for an unfavourable cervix for the outcome caesarean section (Analysis 15.3.).
15.3. Analysis.
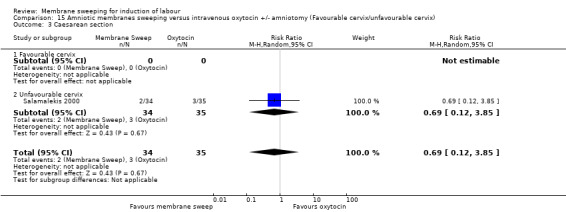
Comparison 15 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Favourable cervix/unfavourable cervix), Outcome 3 Caesarean section.
3.4 Spontaneous vaginal birth
Salamalekis 2000 did not report on the outcome spontaneous vaginal birth.
3.5 Uterine hyperstimulation with/without FHR changes
Salamalekis 2000 did not report on the outcome uterine hyperstimulation with/without FHR changes.
3.6 Maternal death or serious maternal morbidity
Salamalekis 2000 reported on the outcome maternal death or serious maternal morbidity; however, no event was reported for the outcome (Analysis 3.4.).
3.4. Analysis.
Comparison 3 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy, Outcome 4 Maternal death or serious morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Salamalekis 2000 reported on the outcome maternal death or serious maternal morbidity; however no events were reported for the outcome (Analysis 10.4).
10.4. Analysis.
Comparison 10 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Primiparae/Multiparae), Outcome 4 Maternal death or serious morbidity.
Cervical status
Salamalekis 2000 reported on the outcome maternal death or serious maternal morbidity; however no events were reported for the outcome (Analysis 15.4.).
15.4. Analysis.
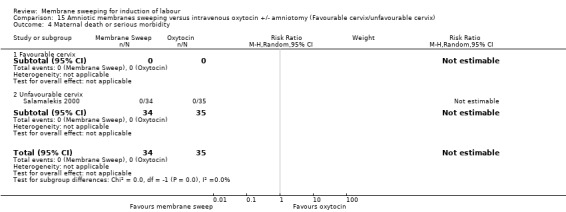
Comparison 15 Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Favourable cervix/unfavourable cervix), Outcome 4 Maternal death or serious morbidity.
3.7 Neonatal death or serious neonatal perinatal morbidity
The included study did not report on the outcome neonatal death or serious neonatal perinatal morbidity .
Secondary outcomes
3.8 Instrumental vaginal birth
The included study did not report on the outcome instrumental vaginal birth.
3.9 Epidural analgesia
The included study did not report on the outcome epidural analgesia.
3.10 Postpartum haemorrhage
The included study did not report on the outcome postpartum haemorrhage.
3.11 Uterine rupture
The included study did not report on the outcome uterine rupture.
3.12 Augmentation of labour
The included study did not report on the outcome augmentation of labour.
3.13 Apgar score less than seven at five minutes
The included study did not report on the outcome Apgar score less than seven at five minutes.
3.14 Neonatal encephalopathy
The included study did not report on the outcome neonatal encephalopathy.
Sensitivity analyses
Sensitivity analyses were not possible as only one study with an unclear risk for allocation concealment (selection bias) was included for this comparison.
Comparison 4: Amniotic membrane sweeping versus amniotomy only
We found no studies which compared membrane sweeping with amniotomy only.
Comparison 5: Amniotic membrane sweeping versus vaginal/oral misoprostol
Two studies providing data for 160 women compared membrane sweeping with vaginal/oral misoprostol (Adeniji 2013; Salmanian 2012). Adeniji 2013 compared a single membrane sweep with a single 50 μg misoprostol tablet given orally on an outpatient basis. Salmanian 2012 compared membrane sweeping with intravaginal PG E1 (misoprostol). Salmanian 2012 is a conference abstract and contributed no data. Adeniji 2013 excluded women from the study who had a history of a previous caesarean section or a uterine scar, Salmanian 2012 included multiparous and nulliparous women, no exclusion criteria were reported.
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots.
Primary outcomes
5.1 Spontaneous onset of labour
Neither study reported on the outcome spontaneous onset of labour.
5.2 Induction of labour
Neither study reported on the outcome induction of labour.
5.3 Caesarean section
One study (Adeniji 2013) reported on caesarean section within this comparison. Compared to vaginal/oral misoprostol, membrane sweeping may, on average, have little to no effect on the risk of a caesarean section (average RR 0.82, 95% CI 0.31 to 2.17, 1 study, 96 participants, low‐certainty evidence; Analysis 5.1).
5.1. Analysis.
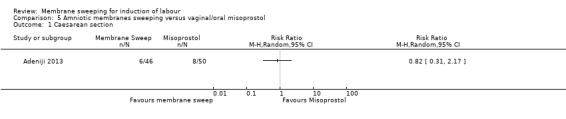
Comparison 5 Amniotic membranes sweeping versus vaginal/oral misoprostol, Outcome 1 Caesarean section.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Neither study reported on subgroup analysis by parity for the outcome caesarean section.
Cervical status
Neither study reported data for a favourable cervix. One study reported data for an unfavourable cervix for the outcome caesarean section (Analysis 17.1).
17.1. Analysis.
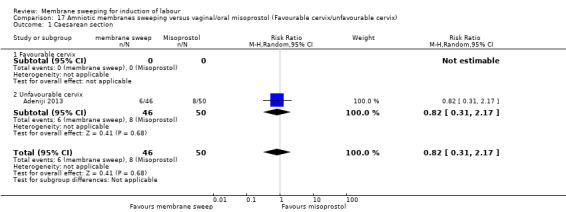
Comparison 17 Amniotic membranes sweeping versus vaginal/oral misoprostol (Favourable cervix/unfavourable cervix), Outcome 1 Caesarean section.
5.4 Spontaneous vaginal birth
Neither study reported on the outcome spontaneous vaginal birth.
5.5 Uterine hyperstimulation with/without FHR changes
Neither study reported on the outcome uterine hyperstimulation with/without FHR changes.
5.6 Maternal death or serious maternal morbidity
Neither study reported on the outcome maternal death or serious maternal morbidity.
5.7 Neonatal death or serious neonatal perinatal morbidity
Neither study reported on the outcome neonatal death or serious neonatal perinatal morbidity.
Secondary outcomes
5.8 Instrumental vaginal birth
Neither study reported on the outcome instrumental vaginal birth.
5.9 Epidural analgesia
Neither study reported on the outcome epidural analgesia.
5.10 Postpartum haemorrhage
Neither study reported on the outcome postpartum haemorrhage.
5.11 Uterine rupture
Neither study reported on the outcome uterine rupture.
5.12 Augmentation of labour
Adeniji 2013 reported on augmentation of labour within this comparison (average RR 1.81, 95% CI 1.00 to 3.28, 1 study, 96 participants, low‐certainty evidence; Analysis 5.2). As the 95% CI for the RR includes the null value of 1 and given the small study size, we conclude that it is unlikely that there is, on average, a difference between groups for the outcome augmentation of labour.
5.2. Analysis.
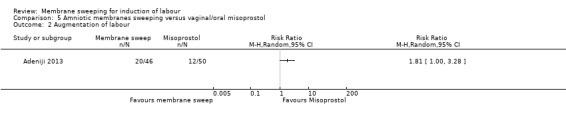
Comparison 5 Amniotic membranes sweeping versus vaginal/oral misoprostol, Outcome 2 Augmentation of labour.
5.13 Apgar score less than seven at five minutes
One study reported on Apgar score less than seven at five minutes within this comparison (Adeniji 2013); however, no events were reported (Analysis 5.3).
5.3. Analysis.
Comparison 5 Amniotic membranes sweeping versus vaginal/oral misoprostol, Outcome 3 Apgar score less than seven at five minutes.
5.14 Neonatal encephalopathy
Neither study reported on the outcome neonatal encephalopathy.
Sensitivity analyses
We planned to exclude all studies at high or unclear risk of bias for either sequence generation and/or allocation concealment, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011). One trial (Adeniji 2013) was judged to be of low risk of bias and included in a sensitivity analysis. On sensitivity analyses, all pre‐specified outcomes confirmed results in the same direction as the main analyses.
Comparison 6: Amniotic membrane sweeping versus mechanical methods (including extra‐amniotic Foley catheter)
We found no studies which compared amniotic membrane sweeping with mechanical methods.
Comparison 7: One frequency of amniotic membrane sweeping versus another frequency of amniotic membrane sweeping
We found one study providing data for 355 women which compared once weekly membrane sweep with twice‐weekly membrane sweep and a sham procedure (Putnam 2011).
Assessment of reporting biases
As there were less than 10 studies in the meta‐analysis, we did not investigate reporting biases (such as publication bias) using funnel plots.
Primary outcomes
7.1 Spontaneous onset of labour
The one included study (Putnam 2011) did not report on this outcome.
7.2 Induction of labour
Putnam 2011 reported on Induction of labour within this comparison. There were no differences, on average, between groups for the outcome induction of labour (average RR 1.19, 95% CI 0.76 to 1.85, 1 study, 234 participants, low‐certainty evidence; Analysis 7.1).
7.1. Analysis.
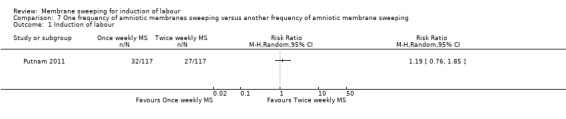
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 1 Induction of labour.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Putnam 2011 did not report on subgroup analysis by parity for the outcome induction of labour.
Cervical status
Putnam 2011 did not report data for a favourable cervix, but did report data for an unfavourable cervix for the outcome induction of labour (Analysis 18.1.).
18.1. Analysis.
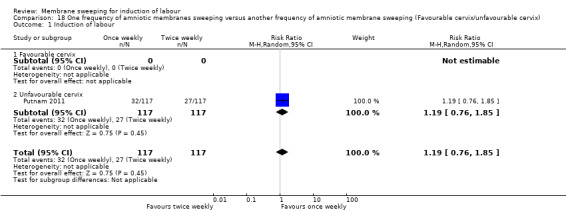
Comparison 18 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping (Favourable cervix/unfavourable cervix), Outcome 1 Induction of labour.
7.3 Caesarean section
Putnam 2011 reported on caesarean section within this comparison. There were no differences, on average, between groups for the outcome caesarean section (average RR 0.93, 95% CI 0.60 to 1.46, 1 study, 234 participants, low‐certainty evidence; Analysis 7.2).
7.2. Analysis.
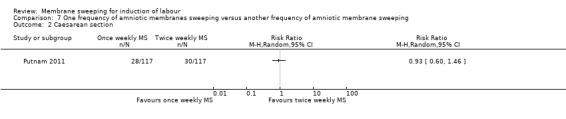
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 2 Caesarean section.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Putnam 2011 did not report on subgroup analysis by parity for the outcome caesarean section.
Cervical status
Putnam 2011 did not report data for a favourable cervix, but did report data for an unfavourable cervix for the outcome caesarean section (Analysis 18.2).
18.2. Analysis.
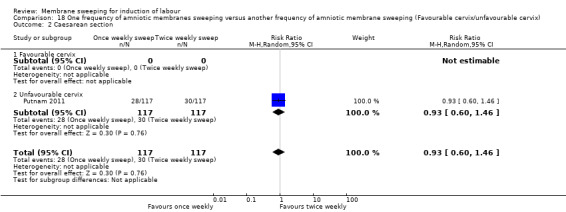
Comparison 18 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping (Favourable cervix/unfavourable cervix), Outcome 2 Caesarean section.
7.4 Spontaneous vaginal birth
Putnam 2011 reported on spontaneous vaginal birth within this comparison. There were no differences, on average, between groups for the outcome spontaneous vaginal birth (average RR 1.00, 95% CI 0.86 to 1.17, 1 study, 234 participants, moderate‐certainty evidence; Analysis 7.3).
7.3. Analysis.
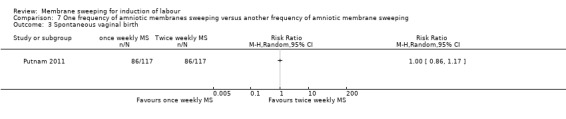
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 3 Spontaneous vaginal birth.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Putnam 2011 did not report on subgroup analysis by parity for the outcome spontaneous vaginal birth.
Cervical status
Putnam 2011 did not report data for a favourable cervix, but did report data for an unfavourable cervix for the outcome spontaneous vaginal birth (Analysis 18.3).
18.3. Analysis.
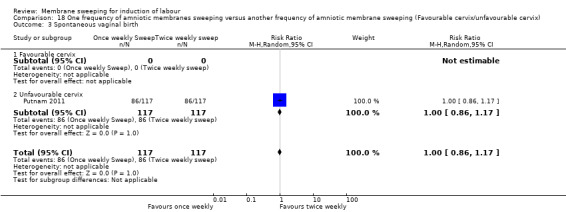
Comparison 18 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping (Favourable cervix/unfavourable cervix), Outcome 3 Spontaneous vaginal birth.
7.5 Uterine hyperstimulation with/without FHR changes
No studies reported on the outcome uterine hyperstimulation with/without FHR changes.
7.6 Maternal death or serious maternal morbidity
Putnam 2011 reported on maternal death or serious maternal morbidity within this comparison. There were no differences, on average, between groups for the outcome maternal death or serious maternal morbidity (average RR 0.78, 95% CI 0.30 to 2.02, 1 study, 234 participants, low‐certainty evidence; Analysis 7.4).
7.4. Analysis.
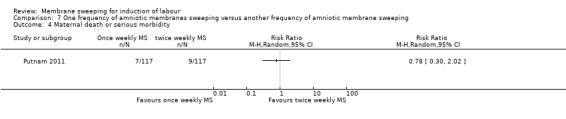
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 4 Maternal death or serious morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Putnam 2011 did not report on subgroup analysis by parity for the outcome maternal death or serious maternal morbidity.
Cervical status
Putnam 2011 did not report data for a favourable cervix, but did report data for an unfavourable cervix for the outcome maternal death or serious maternal morbidity (Analysis 18.4).
18.4. Analysis.
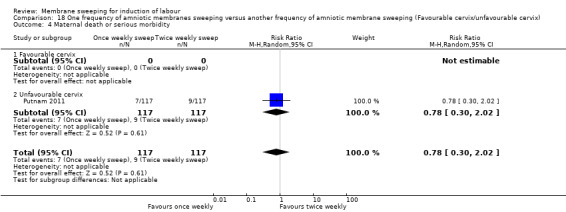
Comparison 18 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping (Favourable cervix/unfavourable cervix), Outcome 4 Maternal death or serious morbidity.
7.7 Neonatal death or serious neonatal perinatal morbidity
Putnam 2011 reported on neonatal death or serious neonatal perinatal morbidity within this comparison. There were no differences, on average, between groups for the outcome neonatal death or serious neonatal perinatal morbidity (average RR 2.00, 95% CI 0.18 to 21.76, 1 study, 234 participants, low‐certainty evidence; Analysis 7.5).
7.5. Analysis.
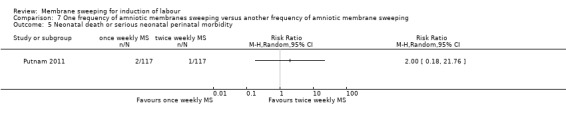
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 5 Neonatal death or serious neonatal perinatal morbidity.
Subgroup analysis
The results were analysed by the clinical categories of parity and cervix favourability.
Parity
Putnam 2011 did not report subgroup analysis by parity for the outcome neonatal death or serious neonatal perinatal morbidity .
Cervical status
Putnam 2011 did not report data for a favourable cervix, but reported data for an unfavourable cervix for the outcome neonatal death or serious neonatal perinatal morbidity (Analysis 18.5).
Secondary outcomes
7.8 Instrumental vaginal birth
Putnam 2011 reported on instrumental vaginal birth within this comparison. There were no differences, on average, between groups for the outcome instrumental vaginal birth (average RR 3.00, 95% CI 0.32 to 28.42, 1 study, 234 participants, low‐certainty evidence; Analysis 7.6).
7.6. Analysis.
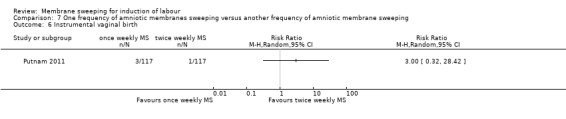
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 6 Instrumental vaginal birth.
7.9 Epidural analgesia
Putnam 2011 did not report on the outcome epidural analgesia.
7.10 Postpartum haemorrhage
Putnam 2011 did not report on the outcome postpartum haemorrhage.
7.11 Uterine rupture
Putnam 2011 did not report on the outcome uterine rupture.
7.12 Augmentation of labour
Putnam 2011 did not report on the outcome augmentation of labour.
7.13 Apgar score less than seven at five minutes
Putnam 2011 reported on Apgar score less than seven at five minutes within this comparison. There were no differences, on average, between groups for the outcome Apgar score less than seven at five minutes (average RR 0.20, 95% CI 0.01 to 4.12, 1 study, 234 participants, low‐certainty evidence; Analysis 7.7).
7.7. Analysis.
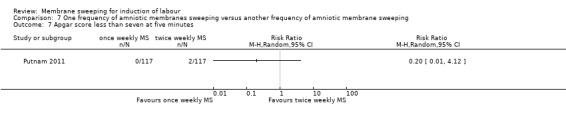
Comparison 7 One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping, Outcome 7 Apgar score less than seven at five minutes.
7.14 Neonatal encephalopathy
Putnam 2011 did not report on the outcome neonatal encephalopathy.
Sensitivity analyses
Only one study with an unclear risk for allocation concealment (selection bias) was included for this comparison, therefore no sensitivity analyses were undertaken.
Measures of satisfaction
1. Woman’s satisfaction
Three studies providing data for 675 women reported on maternal satisfaction (Adeniji 2013; Boulvain 1998; de Miranda 2006). Forty‐three per cent of women (n = 26) in a study comparing membrane sweeping to oral misoprostol indicated that they felt positive about membrane sweeping (Adeniji 2013). Boulvain 1998 reported that 86.8% (n = 79) of women in the membrane sweeping group would recommend the intervention to a friend requiring induction of labour and 77.3% (n = 68) believed that the advantages of membrane sweeping outweighed the disadvantages. Few women (9.2%, n = 8) believed the procedure was not helpful for induction of labour. de Miranda 2006 reports that 88% (n = 312) of women questioned in the postnatal period would choose membrane sweeping in a next pregnancy. Women described varying degrees of discomfort while receiving a membrane sweep. It was described as ‘not painful’ by 31% (n = 111), ‘somewhat painful’ by 51% (n = 179), while 17% (n = 60) considered it ‘painful’ or ‘very painful’. However, 88% (n = 210) of women who reported pain would choose membrane sweeping again in the next pregnancy.
2. Cost
Two studies reporting data for 290 women reported on a cost analysis (Magann 1998b; Magann 1999). Both studies compared membrane sweeping with vaginal/intracervical prostaglandins. Magann 1998b found that induction of labour in the prostaglandin and control groups were significantly more expensive that the membrane sweeping group. This study reported a cost per person (US dollars) of approximately $692 in the control group, $476 per person in the membrane sweeping group and $1207 per person in the prostaglandin group. Magann 1999 compared membrane sweeping with intracervical prostaglandins. This study examined the total antenatal and intrapartum cost for membrane sweeping compared with intracervical prostaglandins. It reported that the prostaglandin group had total antenatal and intrapartum costs approximately 44% higher than the membrane sweeping group (membrane sweeping = $40,672 versus prostaglandin = $91,244). These figures show significant cost savings with membrane sweeping, however with only two relatively small studies focusing on a single comparison further research is recommended in this area.
Discussion
Summary of main results
We included randomised and quasi‐randomised trials comparing membrane sweeping used for third trimester labour induction with placebo/no treatment or other methods listed on a predefined list of labour induction methods. We included 44 studies (20 new to this update), reporting data for 6940 participants.
Amniotic membrane sweeping versus no treatment/sham
Forty studies (6540 participants) compared membrane sweeping with no treatment or a sham treatment. We found women randomised to membrane sweeping may, on average, be more likely to experience spontaneous onset of labour (low‐certainty evidence) and may, on average, be less likely to experience an induction of labour (low‐certainty evidence). However, these findings should be interpreted with caution as on sensitivity analysis, we found no difference between groups for the outcomes spontaneous onset of labour and induction of labour.
There may, on average, be little to no difference between groups for the following outcomes caesarean section (moderate‐certainty evidence), spontaneous vaginal birth (moderate‐certainty evidence), maternal death or serious morbidity (low‐certainty evidence), neonatal death or serious neonatal perinatal morbidity (low‐certainty evidence), instrumental vaginal birth, postpartum haemorrhage (low‐certainty evidence), augmentation of labour (low‐certainty evidence) and Apgar score less than seven at five minutes (low‐certainty evidence). The outcomes uterine hyperstimulation with/without fetal heart rate (FHR) changes, uterine rupture and neonatal encephalopathy were not reported on in this comparison.
On sensitivity analyses, all pre‐specified outcomes with the exception of spontaneous onset of labour and induction of labour were consistent with overall summary effect estimates.
Amniotic membrane sweeping versus vaginal/intracervical prostaglandins
Four studies (480 participants) compared membrane sweeping with vaginal/intracervical prostaglandins. Two studies included women with an unfavourable cervix only. We found, on average, little to no difference, between groups for the outcomes spontaneous onset of labour (low‐certainty evidence), induction of labour (low‐certainty evidence), caesarean section (low‐certainty evidence), spontaneous vaginal birth(low‐certainty evidence), maternal death or serious maternal morbidity (low‐certainty evidence), instrumental vaginal birth (low‐certainty evidence), augmentation of labour (low‐certainty evidence) or Apgar score less than seven at five minutes (low‐certainty evidence). No studies reported on the outcomes uterine hyperstimulation with/without FHR changes, epidural analgesia, postpartum haemorrhage, uterine rupture or neonatal encephalopathy.
Amniotic membrane sweeping versus intravenous oxytocin +/‐ amniotomy
One study (104) participants) compared membrane sweeping with oxytocin. We found, on average, little to no difference between the groups for the outcomes spontaneous labour (low‐certainty evidence), induction of labour (low‐certainty evidence) or caesarean section (low‐certainty evidence).
The included study did not report on the outcomes spontaneous vaginal birth, uterine hyperstimulation with/without FHR changes, neonatal death or serious neonatal perinatal morbidity, instrumental vaginal birth, epidural analgesia, postpartum haemorrhage, uterine hyperstimulation, uterine rupture, augmentation of labour, Apgar score less than seven at five minutes or neonatal encephalopathy. The study reported on the outcome maternal death or serious morbidity but no event was recorded.
Amniotic membrane sweeping versus amniotomy only
We found no studies which compared membrane sweeping with amniotomy only.
Amniotic membrane sweeping versus vaginal/oral misoprostol
Two studies (160 women) compared membrane sweeping with vaginal/oral misoprostol (Adeniji 2013; Salmanian 2012). However, the studies used different forms of misoprostol for their analyses. One compared a single membrane sweep with a single 50 μg misoprostol tablet given orally (Adeniji 2013); the other compared membrane sweeping with intravaginal PG E1 (misoprostol) (Salmanian 2012). Salmanian 2012 contributed no data to outcomes included in this review. Adeniji 2013 compared membrane sweeping versus oral misoprostol.
We found, on average, little to no difference between groups for the outcomes caesarean section (low‐certainty evidence) and Apgar score less than seven at five minutes (low‐certainty evidence). Adeniji 2013 reported on the outcome augmentation of labour. As the 95% confidence interval for the relative risk included the null value of 1, we found insufficient evidence to support a difference.
Neither study reported on the outcomes spontaneous onset of labour, Induction of labour, spontaneous vaginal birth, uterine hyperstimulation with/without FHR changes, maternal death or serious maternal morbidity, neonatal death or serious neonatal perinatal morbidity, instrumental vaginal birth, epidural analgesia, postpartum haemorrhage, uterine rupture or neonatal encephalopathy.
Amniotic membrane sweeping versus mechanical methods (including extra‐amniotic Foley catheter)
We found no studies which compared membrane sweeping with mechanical methods.
One frequency of amniotic membrane sweeping versus another frequency of amniotic membrane sweeping
We found one study (355 women) which compared once‐weekly membrane sweep with twice‐weekly membrane sweep and a sham procedure. We found on average, little to no difference, between groups for the outcomes induction of labour (low‐certainty evidence), caesarean section (low‐certainty evidence), spontaneous vaginal birth (moderate‐certainty evidence), maternal death or serious morbidity (low‐certainty evidence), neonatal perinatal death or serious morbidity (low‐certainty evidence), instrumental vaginal birth (low‐certainty evidence) and Apgar score less than seven at five minutes (low‐certainty evidence) between the groups. The outcomes spontaneous onset of labour epidural analgesia, postpartum haemorrhage, uterine hyperstimulation with/without FHR changes, uterine rupture, augmentation of labour and neonatal encephalopathy were not reported in this study.
Woman’s satisfaction
Three studies reported on maternal satisfaction with membrane sweeping. A significant majority of women reported positively on their experiences, stating that they felt the potential advantages of the intervention outweighed the disadvantages and would in general recommend the intervention to a friend. While a cohort of women questioned in the postnatal period described membrane sweeping as painful, the majority (88%, n = 312) reported that they would choose membrane sweeping again in future pregnancies (de Miranda 2006).
Cost
Two relatively small studies reported a cost analysis for membrane sweeping (Magann 1998b; Magann 1999). Both studies were undertaken in hospital‐based settings in the USA and compared amniotic membrane sweeping with vaginal/intracervical prostaglandins. These studies reported a significant cost per person difference between pharmacological induction of labour and membrane sweeping.
Overall completeness and applicability of evidence
This review includes 44 trials, reporting data for 6940 participants. Forty studies compared membrane sweeping with no treatment, four compared sweeping with prostaglandins, two compared sweeping with oral misoprostol, one compared sweeping with oxytocin and one compared differing frequencies of membrane sweeping. Six studies reported more than one comparison.
Of the 44 trials included in this review, 18 (18/44) reported on the outcome 'Spontaneous onset of labour', 16 (16/40) reported on the outcome 'Induction of labour', 34 (34/44) reported on the outcome 'Caesarean section', 27 (27/44) reported on the outcome 'Spontaneous vaginal delivery' and 23 (23/40) reported on the outcome 'Instrumental vaginal birth'. The assessment of these outcomes in particular are intrinsic to a comprehensive evaluation of membrane sweeping for of induction of labour and it is surprising that so few trials reported on these, particularly as all relevant data for these outcomes are often recorded routinely in women’s health care.
Four studies reported data for the comparison membrane sweeping versus vaginal/intracervical prostaglandins, one study reported data for the comparison membrane sweeping versus intravenous oxytocin +/‐ amniotomy, two studies reported data for the comparison membrane sweeping versus vaginal/oral misoprostol and one study reported data for the comparison of different frequencies of membrane sweeping. No studies reported on the comparison membrane sweeping versus amniotomy only or the comparison membrane sweeping versus mechanical methods. The limited data are insufficient to evaluate the efficacy of membrane sweeping for these comparisons.
Included studies comprised of women from 36 to 42 weeks’ gestation with varying intensities of membrane sweeping. Questions remain as to whether there is an optimal number of membrane sweeps and the timings and gestation of these to promote spontaneous onset of labour. One study (1/44) provided data for the comparison of different frequencies of membrane sweeping. The data available are insufficient to evaluate the efficacy of this comparison.
Maternal perception of discomfort during membrane sweeping is cited routinely when discussing membrane sweeping yet only three studies (3/44) collected data on maternal satisfaction. These limited data are insufficient to meaningfully discuss women’s satisfaction with membrane sweeping for induction of labour.
While membrane sweeping potentially offers a cost‐effective method of preventing a formal induction of labour, there were limited data available to evaluate this. Two studies (2/44) reported a cost analysis with both comparing membrane sweeping with vaginal/intracervical prostaglandins. No cost analysis was provided for any other comparisons.
Quality of the evidence
This review includes 44 trials, undertaken in hospital settings from a wide range of economic and geographical regions. Overall, the risk of bias was assessed as unclear risk of bias in most domains. Thirty‐one of the 44 included studies were found to have an unclear or high risk of bias for allocation concealment and 15 were found to have an unclear or high risk of bias for random sequence generation. All 44 studies in our review were judged to be of high risk of performance bias. Clinicians were not blinded to the intervention in any study and it is unclear (and unlikely in our view) in most studies whether or not study participants were blinded post allocation. Thirty‐four studies were found to have an unclear risk of detection bias primarily due to a lack of methodological detail. Nine studies were found to have an unclear or high risk of attrition bias with 20 having an unclear or high risk of bias for selective reporting.
Evidence was assessed using the GRADE approach. Evidence was downgraded for risk of serious bias when evidence of study design limitations were found. Evidence was downgraded for risk of serious inconsistency when evidence of inconsistency (statistical heterogeneity) was present and remained unexplained after exploration of a priori hypotheses that might explain heterogeneity. Evidence was assessed for imprecision by calculating the optimal information size (OIS) and using this to make judgements. Evidence was downgraded if the OIS criterion was not met.
For our comparison membrane sweeping versus no treatment/sham, our GRADE assessments in the majority were found to be of low certainty. Two outcomes were assessed to be of moderate certainty (caesarean section and spontaneous vaginal birth). We downgraded for serious bias due to evidence of study design limitations in all trials, serious inconsistency and for serious imprecision due to the total (cumulative) sample size being less than the OIS. See Table 1.
For our comparison membrane sweeping versus vaginal/intracervical prostaglandins, our GRADE assessments were overall found to be of low certainty. We downgraded for serious bias due to evidence of study design limitations in all trials and for serious imprecision due to the total (cumulative) sample size being less than the OIS. See Table 2.
For our comparison membrane sweeping versus intravenous oxytocin+/‐ amniotomy, our GRADE assessments were low certainty for all outcomes. This comparison included one trial and we downgraded for serious bias due to evidence of study design limitations in this trial. We downgraded for serious imprecision due to a small sample size with the confidence interval crossing the line of no effect. We downgraded for serious imprecision in one outcome due to a small sample size with no events recorded. See Table 3.
For our comparison membrane sweeping versus vaginal/oral misoprostol, our GRADE assessments were low certainty for all outcomes. This comparison included one trial and we downgraded for serious bias due to evidence of study design limitations in this trial. We downgraded for serious imprecision due to the total (cumulative) sample size being less than the OIS. See Table 4.
No study reported on the comparison membrane sweeping versus mechanical methods (including extra‐amniotic Foley catheter).
For our comparison one frequency of membrane sweeping versus another frequency of membrane sweeping, our GRADE assessments were low certainty. This comparison included one trial and we downgraded for serious bias due to evidence of study design limitations in this trial. We downgraded for serious imprecision due to the total (cumulative) sample size being less than the OIS. See Table 5.
Potential biases in the review process
A potential source of bias related to the lack of blinding within all the included trials. All 44 studies in our review were judged to be of high risk of performance bias. Clinicians were not blinded to the intervention in any study and it is unclear (and unlikely in our view) in most studies if study participants were blinded. Lack of participant blinding may also have had an effect on the reporting of maternal satisfaction with membrane sweeping.
Michel Boulvain is a principle investigator in one of the included studies (Boulvain 1998) and is the principle author of the original 2005 Cochrane Review ‘Membrane sweeping for induction of labour’ (Boulvain 2005). Michel's study was independently reviewed by two review authors for inclusion and risk of bias and extracted data. A third author independently reviewed the study and extracted data where any conflict was unresolved.
While review authors have differed in the course of conducting this systematic review, we have made every effort to reach consensus and endeavoured to minimise any potential bias. Two review authors independently reviewed studies for inclusion and risk of bias and extracted data. A third author independently reviewed studies and extracted data where any conflict was unresolved.
Agreements and disagreements with other studies or reviews
Guidelines by bodies including NICE (NICE 2008), the Society of Obstetricians and Gynaecologists of Canada (SOGC 2013), the Department of Health, South Australia (Queensland DOH 2017) and the World Health Organization (World Health Organization 2011) state that women should be offered the option of membrane sweeping at or near term. The NICE guidelines state that a membrane sweep should be offered to nulliparous women at term gestation and women who have had one or more infants at 41 weeks’ gestation. In addition, it recommends that women be offered further membrane sweeps during their antenatal visits if labour does not commence (NICE 2008).
Recent studies have supported elective pharmacological induction of labour to lower the risk of caesarean section. However, these studies compared induction of labour with expectant management only, with none evaluating the potential effects of membrane sweeping on the process (Grobman 2018; Middleton 2018; Wood 2014). In addition, a 2018 Cochrane Systematic Review ‘Induction of labour for improving birth outcomes for women at or beyond term’ (Middleton 2018) compared induction of labour with expectant management but did not include membrane sweeping as a method of induction of labour in its analysis.
Authors' conclusions
Implications for practice.
Membrane sweeping is probably effective in increasing the likelihood of achieving a spontaneous onset of labour. When compared to expectant management, it potentially reduces the risk of formal induction of labour. The majority of women report positive experiences and would recommend the intervention to a friend suggesting women find membrane sweeping acceptable as a method of preventing a formal induction of labour. Two small studies report that membrane sweeping potentially offers significant savings in healthcare costs.
Implications for research.
Included studies comprised of women from 36 to 42 weeks’ gestation with varying intensities of membrane sweeping. None examined the potential effect of differing gestations to commence membrane sweeping and only one reported a comparison of differing frequencies of membrane sweep. Questions remain as to the optimal gestation to commence and frequency for membrane sweeping to prevent post‐term pregnancy. Future research could address the potential impact gestation may have on the success of membrane sweeping. In addition, any potential effect the intensity of the intervention, i.e. multiple or single membrane sweeps has on this process could be evaluated.
Two small studies reported on membrane sweeping in women who were group B streptococcus positive. While no additional maternal or fetal risk was noted with membrane sweeping, further research would potentially provide data to inform health policy.
Women’s perceptions and satisfaction with membrane sweeping are intrinsic to its clinical use. Our review found that few studies explored women’s views of membrane sweeping. Further research is needed to assess women’s overall views and acceptability of membrane sweeping. In addition, we recommend that clinician’s views and acceptability of membrane sweeping, a fundamental factor to its use clinically, could also be explored.
Few studies reported on the cost‐effectiveness of membrane sweeping (two relatively small studies). It would be helpful to have a cost‐effectiveness analysis of the overall incurred costs, including intrapartum, postnatal and neonatal care, associated with the use of membrane sweeping to prevent post‐term pregnancy. In addition, a health economic analysis of membrane sweeping relative to expectant management and other methods of induction of labour to prevent post‐term pregnancy would provide valuable data to inform health policy.
What's new
| Date | Event | Description |
|---|---|---|
| 5 March 2020 | Amended | The full title for the Health Service Executive (HSE), Ireland, has been added to Elaine Finucane's declaration of interest and also to the acknowledgements. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 25 February 2019 | New citation required and conclusions have changed | Membrane sweeping is probably effective in achieving a spontaneous onset of labour. When compared to expectant management, it potentially reduces the risk of formal induction of labour and caesarean section. However, evidence is of low certainty. |
| 25 February 2019 | New search has been performed | We searched for evidence on 25 February 2019. Twenty new studies have been added for this update. Two studies previously excluded (Gemer 2001; McColgin 1993), are now included. The review now includes a total of 44 studies reporting data for 6940 women. On reflection of peer review feedback and in consultation with the Cochrane Pregnancy and Childbirth editorial team, data were analysed using the random‐effects model. Within the primary outcome 'Neonatal death or serious neonatal perinatal morbidity', 'probable or definite neonatal sepsis' was specified as suitable for inclusion following peer review. |
| 31 July 2009 | Amended | Search updated. Ten new reports added to Studies awaiting classification (de Miranda 2006a; Hill 2006a; Hill 2008b; Hill 2008b; Ifnan 2006b; Imsuwan 1999a; Kashanian 2006a; Kaul 2004a; Tan 2006a; Yildirim 2008a). |
| 18 September 2008 | Amended | Converted to new review format. |
| 9 November 2004 | New search has been performed | We have added two new trials (Dare 2002; Wong 2002) , one new ongoing trial (Manidakis 1999) and a new report of Magann 1998b. We have excluded four new trials (Bergsjo 1989; Foong 2000; Gemer 2001a; McColgin 1993a). |
Acknowledgements
This review is an update of a 2005 review conducted by Michel Boulvain, Catalin M Stan and Olivier Irion. This review was supported by Health Research Board, Ireland (HRB) through a HRB Cochrane Fellowship. We acknowledge gratefully the support of the University of Limerick Hospitals Group and the Nursing and Midwifery Planning and Development Unit West/Midwest of the Health Service Executive, Ireland (HSE). We thank Cochrane Ireland and the Cochrane Pregnancy and Childbirth who provided support for this update.
We thank Olivier Irion and Catalin Stan for their contributions to previous versions of this review.
As part of the pre‐publication editorial process, this protocol/review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Everett F Magann, Professor of Obstetrics and Gynecology, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA; Johanna Quist‐Nelson, MD, Thomas Jefferson University Hospital, Philadelphia, USA.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and the WHO ICTRP
membrane(s) AND sweep(ing)
membrane(s) AND strip(ping)
Appendix 2. Methodological quality of trials
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially‐sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
Data and analyses
Comparison 1. Amniotic membranes sweeping versus no treatment/sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 17 | 3170 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.08, 1.34] |
| 2 Induction of labour | 16 | 3224 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.56, 0.94] |
| 3 Caesarean section | 32 | 5499 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.04] |
| 4 Spontaneous vaginal birth | 26 | 4538 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 5 Maternal death or serious morbidity | 17 | 2749 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.20] |
| 6 Neonatal death or serious neonatal perinatal morbidity | 18 | 3696 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 7 Instrumental vaginal birth | 22 | 3888 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.25] |
| 8 Epidural analgesia | 9 | 2162 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.97, 1.33] |
| 9 Postpartum haemorrhage | 5 | 760 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.39] |
| 10 Augmentation of labour | 9 | 2011 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.17] |
| 11 Apgar score less than seven at five minutes | 10 | 1958 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.51, 2.40] |
Comparison 2. Amniotic membranes sweeping versus vaginal/intracervical prostaglandins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 3 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.98, 1.57] |
| 2 Induction of labour | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.45] |
| 3 Caesarean section | 3 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.09] |
| 4 Spontaneous vaginal birth | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.32] |
| 5 Maternal death or serious morbidity | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.27, 3.21] |
| 6 Neonatal death or serious neonatal perinatal morbidity | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.12, 1.33] |
| 7 Instrumental vaginal birth | 3 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.59, 4.14] |
| 8 Augmentation of labour | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.30] |
| 9 Apgar score less than seven at five minutes | 3 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.13, 5.77] |
Comparison 3. Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.96] |
| 2 Induction of labour | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.42] |
| 3 Caesarean section | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.85] |
| 4 Maternal death or serious morbidity | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Amniotic membranes sweeping versus vaginal/oral misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Augmentation of labour | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Apgar score less than seven at five minutes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of labour | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Caesarean section | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Spontaneous vaginal birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Maternal death or serious morbidity | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Neonatal death or serious neonatal perinatal morbidity | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Instrumental vaginal birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Apgar score less than seven at five minutes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 17 | 3170 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.08, 1.34] |
| 1.1 Parity ‐ Multiparae | 2 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.92, 1.20] |
| 1.2 Parity ‐ Primiparae | 3 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.15, 1.72] |
| 1.3 Parity ‐ unknown | 12 | 2362 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.05, 1.38] |
| 2 Induction of labour | 17 | 3271 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.92] |
| 2.1 Parity ‐ Primiparae | 5 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.14, 0.85] |
| 2.2 Parity ‐ Multiparae | 2 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.47] |
| 2.3 Parity ‐ unknown | 11 | 2368 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.58, 1.06] |
| 3 Caesarean section | 32 | 5499 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.03] |
| 3.1 Primiparae | 4 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.41, 2.21] |
| 3.2 Multiparae | 4 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.19] |
| 3.3 Parity ‐ unknown | 25 | 4421 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.07] |
| 4 Spontaneous vaginal birth | 26 | 4538 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 4.1 Primiparae | 3 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.18] |
| 4.2 Multiparae | 4 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.82, 1.75] |
| 4.3 Parity ‐ unknown | 20 | 3529 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 5 Maternal death or serious morbidity | 17 | 2749 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.20] |
| 5.1 Parity ‐ Primiparae | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Parity ‐ Multiparae | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Parity ‐ unknown | 15 | 2580 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.20] |
| 6 Neonatal death or serious neonatal perinatal morbidity | 18 | 3696 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 6.1 Parity ‐ Primiparae | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.18, 1.83] |
| 6.2 Parity ‐ Multiparae | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Parity ‐ unknown | 17 | 3596 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.23] |
8.3. Analysis.
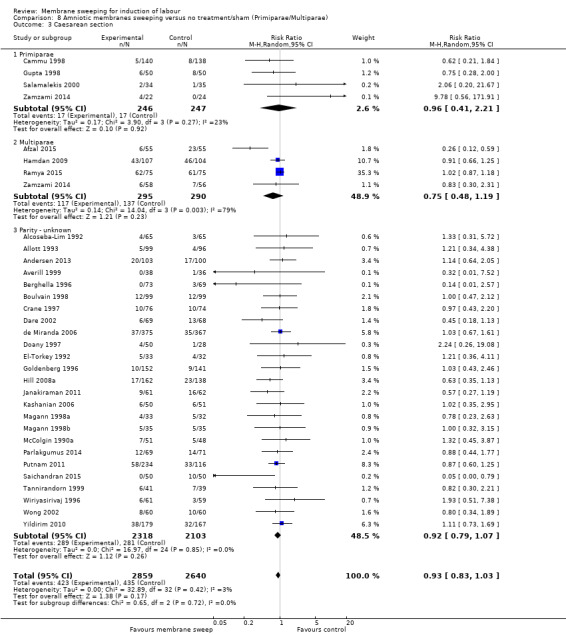
Comparison 8 Amniotic membranes sweeping versus no treatment/sham (Primiparae/Multiparae), Outcome 3 Caesarean section.
Comparison 10. Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Primiparae/Multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.96] |
| 1.1 Parity ‐ Primiparae | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.96] |
| 1.2 Parity ‐ Multiparae | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Induction of labour | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.42] |
| 2.1 Parity ‐ Primiparae | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.42] |
| 2.2 Parity ‐ Multiparae | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.85] |
| 3.1 Parity ‐ Primiparae | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.85] |
| 3.2 Parity ‐ Multiparae | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal death or serious morbidity | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Parity ‐ Primiparae | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Parity ‐ Multiparae | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Amniotic membranes sweeping versus no treatment/sham Favourable cervix/unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 17 | 3170 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.08, 1.34] |
| 1.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Unfavourable cervix | 5 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.05, 2.47] |
| 1.3 Cervix unknown | 12 | 2470 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.04, 1.32] |
| 2 Induction of labour | 16 | 3224 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.58, 0.95] |
| 2.1 Favourable cervix | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.66, 1.41] |
| 2.2 Unfavourable cervix | 4 | 589 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.85] |
| 2.3 Cervix unknown | 13 | 2539 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.08] |
| 3 Caesarean section | 32 | 5499 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.04] |
| 3.1 Favourable cervix | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.35, 2.95] |
| 3.2 Unfavourable cervix | 7 | 1170 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 3.3 Cervix unknown | 24 | 4228 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 1.00] |
| 4 Spontaneous vaginal birth | 26 | 4538 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 4.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Unfavourable cervix | 5 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.15] |
| 4.3 Cervix unknown | 21 | 3783 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.08] |
| 5 Maternal death or serious morbidity | 17 | 2749 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.20] |
| 5.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Unfavourable cervix | 4 | 885 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.04] |
| 5.3 Cervix unknown | 13 | 1864 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.60] |
| 6 Neonatal death or serious neonatal perinatal morbidity | 18 | 3696 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 6.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Unfavourable cervix | 1 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.58] |
| 6.3 Cervix unknown | 17 | 3350 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
Comparison 14. Amniotic membranes sweeping versus vaginal/intracervical prostaglandins (Favourable cervix/unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 3 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.98, 1.57] |
| 1.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Unfavourable cervix | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.13, 1.76] |
| 1.3 Cervix unknown | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Induction of labour | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.45] |
| 2.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Unfavourable cervix | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.62] |
| 2.3 Cervix unknown | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.47, 1.95] |
| 3 Caesarean section | 3 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.09] |
| 3.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Unfavourable cervix | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.41, 1.08] |
| 3.3 Cervix unknown | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.23, 4.15] |
| 4 Spontaneous vaginal birth | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.32] |
| 4.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Unfavourable cervix | 2 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.32] |
| 4.3 Cervix unknown | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Neonatal death or serious neonatal perinatal morbidity | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.12, 1.33] |
| 5.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Unfavourable cervix | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.02, 1.68] |
| 5.3 Cervix unknown | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.13, 2.33] |
Comparison 15. Amniotic membranes sweeping versus intravenous oxytocin +/‐ amniotomy (Favourable cervix/unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.96] |
| 1.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Unfavourable cervix | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.96] |
| 2 Induction of labour | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.42] |
| 2.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Unfavourable cervix | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.42] |
| 3 Caesarean section | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.85] |
| 3.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Unfavourable cervix | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.12, 3.85] |
| 4 Maternal death or serious morbidity | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Unfavourable cervix | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Amniotic membranes sweeping versus vaginal/oral misoprostol (Favourable cervix/unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.31, 2.17] |
| 1.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Unfavourable cervix | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.31, 2.17] |
Comparison 18. One frequency of amniotic membranes sweeping versus another frequency of amniotic membrane sweeping (Favourable cervix/unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of labour | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.85] |
| 1.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Unfavourable cervix | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.85] |
| 2 Caesarean section | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.46] |
| 2.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Unfavourable cervix | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.46] |
| 3 Spontaneous vaginal birth | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.86, 1.17] |
| 3.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Unfavourable cervix | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.86, 1.17] |
| 4 Maternal death or serious morbidity | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.30, 2.02] |
| 4.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Unfavourable cervix | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.30, 2.02] |
Comparison 19. Amniotic membranes sweeping versus mechanical methods (Favourable cervix/unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal death or serious neonatal perinatal morbidity | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.18, 21.76] |
| 1.1 Favourable cervix | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Unfavourable cervix | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.18, 21.76] |
19.1. Analysis.
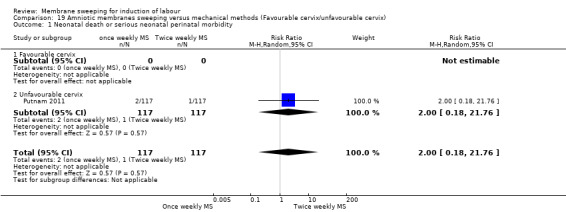
Comparison 19 Amniotic membranes sweeping versus mechanical methods (Favourable cervix/unfavourable cervix), Outcome 1 Neonatal death or serious neonatal perinatal morbidity.
Comparison 20. Amniotic membranes sweeping versus no treatment/sham‐ sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous onset of labour‐sensitivity analysis | 6 | 1884 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.98, 1.18] |
| 2 Induction of labour‐ sensitivity analysis | 6 | 1879 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.24] |
| 3 Caesarean section‐sensitivity analysis | 10 | 2480 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 4 Spontaneous vaginal birth‐sensitivity analysis | 9 | 2379 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.97, 1.06] |
| 5 Maternal death or serious morbidity ‐ sensitivity analysis | 4 | 661 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.57, 2.59] |
| 6 Neonatal death or serious neonatal perinatal morbidity ‐ sensitivity analysis | 7 | 1941 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.65, 1.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adeniji 2013.
| Methods | Prospective randomised controlled trial | |
| Participants |
Setting: antenatal clinic, Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Nigeria. Duration of study: 3 years (between April 2007 and March 2010) Inclusion criteria: “singleton live fetus, post‐term pregnancy from 40 weeks and 1 day to 40 weeks and 9 days, intact fetal membranes, Bishops score ≤ 5 and cephalic presentation”. Page 5. Exclusion criteria: “post‐term pregnancies of > 40 weeks and 10 days, multiple pregnancies, grand multiparity, cephalopelvic disproportion, previous caesarean section or a uterine scar, fetal malpresentation, fetal distress, placenta praevia, antepartum haemorrhage, premature rupture of the membranes and medical disorders.” Page 5. Parity: mixed, both nulliparous and multiparous included in the study. Page 5. Bishop score: not recorded |
|
| Interventions |
Oral misoprostol group (OM) (N = 50): “a single 50 ug misoprostol tablet orally on an outpatient basis.” Page 5. Membrane stripping group (MS) (N = 46): “had MS once only at the antenatal clinic. Patients with unyielding cervices preventing access into the cervical canal were termed 'failed MS'.” Page 5. “All patients in both groups who did not go into spontaneous labour after 48 hours were categorised as 'failed labour induction' and together with the women with post‐term pregnancies of > 40 weeks and 10 days managed according to our departmental protocol of cervical ripening and labour induction (transcervical Foley catheter or intravaginal misoprostol) to ensure delivery before 42 weeks' gestation.” Page 5. |
|
| Outcomes | Spontaneous labour Vaginal delivery Caesarean section Apgar score < 7 at 5 minutes Women’s satisfaction Oxytocin augmentation |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: yes; “were recruited after giving informed consent”. Page 5. Ethical approval: “The institutional ethical review committee approved the study”. Page 5. Email sent to author 28 August 2017 requesting study data and subgroup data Re‐sent 20 September 2017, no reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer‐generated random numbers were used for patient allocation”, page 5. |
| Allocation concealment (selection bias) | Low risk | “sealed opaque envelopes containing papers marked OM or MS (50 each) were placed in a box, thoroughly mixed and then numerically labelled.”, “ were allocated sequential numbers in order of recruitment…and the correspondingly numbered envelope was opened”, page 5. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: not discussed. Blinding of personnel: partial blinding. “attending obstetricians in the labour ward were blinded to the labour‐inducing agents used in the study groups.” (Page 5). Unclear if all other personnel involved were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n = 4 (8%) patients in nulliparous group could not have MS owing to inability to gain access to the cervical canal and were removed from analysis |
| Selective reporting (reporting bias) | Unclear risk | Rates for hospital admission not reported explicitly |
| Other bias | Low risk | No other bias indicated. |
Afzal 2015.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Department of Obstetrics & Gynaecology, Benazir Bhutto hospital, Rawalpindi, Pakistan. Duration of study: Jan 2008 to Dec 2008. Inclusion criteria: “Singleton second pregnancy with previous one lower segment transverse cesarean section, having longitudinal lie and cephalic presentation at 37 weeks of gestation confirmed by ultrasonography were included in the study. There was no absolute indication of cesarean section in present pregnancy.” page 386. Exclusion criteria: “Patients with any contraindication for vaginal delivery like cephalopelvic disproportion, breech and placenta previa, maternal medical disorders necessitating urgent delivery like severe pre‐eclampsia were excluded from the study.” page 386. Parity: not recorded Bishop score: not recorded |
|
| Interventions |
Membrane stripping (n = 55): “Membrane sweeping was started a 37 weeks and was done every 3rd day till she went into the labor or she reached 41 weeks. Even at 41 weeks of gestation if she did not go into labor, induction with prostaglandin or elective lower segment cesarean section was done depending upon the bishop score.” Page 386. Control group (N = 55): women “were not subjected to such membrane sweeping and spontaneous onset of labor was awaited till 41 weeks. After 41 weeks induction with prostaglandin or elective lower segment cesarean section was done depending upon the bishop score.” Page 386. |
|
| Outcomes | Normal vaginal delivery Caesarean section Assisted vaginal delivery Spontaneous onset of labour before 41 weeks |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: yes; “Informed consent was taken from each patient” page 386. Ethical approval: not stated Email sent to author 28 July 2017 requesting further information Resent 20 September 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “were randomly allocated to Group‐A (sweeping of membrane) and Group‐B (no intervention)” page 386. Insufficient information given to inform a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given to inform a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported, but unlikely that clinicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias noted. Protocol not available. |
| Other bias | Low risk | No other bias noted. Protocol not available. |
Alcoseba‐Lim 1992.
| Methods | Prospective randomised controlled trial. | |
| Participants |
Setting: Chong Hua Hospital, Cebu City, Philippines. Duration of study: 6 months (1 August 1991 to 31 October 1992) Inclusion criteria: women of 38 weeks' gestation based on “declared last menstrual period and the fundal height at each prenatal visit.” The “result of the ultrasound done before 26 weeks age of gestation was used to confirm age of gestation”. Page 139. Exclusion criteria: “Uncertain dates for gestational age (with size dates discrepancy not confirmed by ultrasound < 26 weeks). Abnormal fetal presentations. History of vaginal spotting during the course of current pregnancy (suspects of low‐lying placenta, placenta previa).” Patients who had a history of a “previous caesarean section who did not want to try vaginal birth”. Page 140. Parity: mixed. Both nulliparous and multiparous women included (% presented in Table 2 of manuscript page 140). 28/65 (43.1%) nulliparous women in membrane sweeping group versus 24/65 (36.9%) nulliparous women in control group. 37/65 (56.9%) multiparous women in membrane sweeping group versus 41/65 (63.1%) multiparous women in the control group. Bishop score: (% presented in Table 2 of manuscript page 140) Bishop score at initial visit: Stripped Non stripped </= 4 61 40 > 4 4 25 |
|
| Interventions |
Membrane stripping(n = 65): patients “undergo membrane stripping once every week until delivery.” “Accomplished by digital separation of the chorionic membrane from the lower uterine segment with one or two circumferential passes.” “In patients with long and closed cervices, the cervix was digitally stretched until stripping could be accomplished” Page 139 Control group (n = 65): weekly “pelvic examination and bishop scoring was done”. Page 139 All the patients were examined by the same examiner. Page 139 |
|
| Outcomes | Spontaneous vaginal delivery Low forceps delivery Caesarean section Chorioamnionitis Meconium staining |
|
| Notes |
Funding: Nestle Phils, Medichem Pharmaceuticals Inc, Pfeizer Trial authors’ declaration of interest: none stated Informed consent obtained: not stated Email sent 28 August 2017 requesting further information. Resent 20 September 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not method reported “ the subjects were then randomly assigned to a group”. Page 139 |
| Allocation concealment (selection bias) | Unclear risk | No evidence of allocation concealment given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding of participants or personnel demonstrated. Participants: no reported. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No evidence of blinding of outcome assessment demonstrated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | It is noted that page 141 of study states “Vaginal spotting was observed in 20(30.7%)”. However table 5, page 141 reports: spotting n = 17 (26.2%) |
| Other bias | High risk | Imbalanced groups for initial Bishop score, page 141. |
Allott 1993.
| Methods | Prospective randomised controlled trial | |
| Participants |
Setting: antenatal clinic of district general hospital, UK. Page 898 Duration of study: 18 months. Page 898 Inclusion criteria: “Beyond 40 weeks gestation as determined by mid‐trimester ultrasound scanning.” “Pregnancies in which no risk factors such as intra‐uterine growth restriction or hypertension had been detected”. Page 898 Exclusion criteria: “Those presenting with a closed cervix were not included in the trial as the cervix has to be potentially sweepable” Page 898 Parity: mixed. Both nulliparous and multiparous women included (% presented in Table 1 of manuscript page 899). 43/99 (43.4%) nulliparous women in membrane sweeping group versus 44/96 (45.8%) nulliparous women in control group. 56/99 (56.6%) multiparous women in membrane sweeping group versus 52/96 (54.2%) multiparous women in the control group. Bishop score: Score ≤ 6 and Score ≥ 7 recorded |
|
| Interventions |
Membrane stripping(n = 99): a vaginal examination was performed to assess the Bishop score. “The sweep was performed by inserting the examiners index finger as far through the internal cervical os as possible and rotating twice through 360 degrees”. Page 898 Control group (n = 96): “A vaginal examination was performed to assess the Bishop score”. Page 898 “After the initial intervention there were no further differences in management” between the groups“. “All were assessed by the same person to minimise subjective differences”. All women were given a deadline date for labour induction in the absence of a spontaneous onset. A minimum gap of 4 days was planned between the examination and the induction in all cases. Sweeping of membranes or Bishop's score performed by the principal investigator. Page 899 |
|
| Outcomes | Spontaneous vaginal delivery Induction of labour Caesarean section operative vaginal birth Apgar score < 6 at 5 minutes serious neonatal infection Serious neonatal outcomes Epidual in labour Maternal pyrexia?? number of women starting spontaneous labour reported for every day between day 1 to day 7 after randomisation. |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: Dr. D. Elbourne, Oxford perinatal epidemiology unit advised in study design. Mr. A. Smith helped in preparation of manuscript. Informed consent obtained: “all gave informed consent” Ethical approval: unclear; “after reading an explanatory document as stipulated by the district ethical committee” Email for further information sent 28 August 2017. Resent 20 September 2017. No reply to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Using a computer generated list of random numbers, women were randomised to a membrane sweep or no further procedure. A sealed envelope was opened for each woman after entry into the trial”. Page 898. |
| Allocation concealment (selection bias) | Unclear risk | “A sealed envelope was opened for each woman after entry into the trial” It is not reported if envelope was opaque, sequential or numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: not discussed Binding of personnel: “All were assessed by the same person (H.A.) to minimise subjective differences in evaluation”. Page 898. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information given to make informed judgement.However it is noted that caesarean section data unclear. Table 3, page 901 figures differ from written report. |
| Other bias | Low risk | No other bias noted. |
Andersen 2013.
| Methods | Randomised controlled trial | |
| Participants |
Setting: delivery wards at Hvidovre University Hospital, Odense University Hospital & Roskilde University Hospital, Denmark. Duration of study: 1 January 2007 – 31 November 2009 Inclusion criteria: “Healthy women with an uncomplicated spontaneous singleton pregnancy, a cephalic presentation, intact fetal membranes and with Danish spoken” “pregnancy week 41+2‐41+4”. “whenever an acupuncture certified midwife was available” “Gestational ages were estimated using fetometric ultrasound parameters obtained before 22 weeks of gestation”. Page 556 Exclusion criteria: “Women treated with any kind of acupuncture and women treated with sweeping of the fetal membranes within the last 2 weeks before the study were excluded”. Page 556 Parity: mixed, both primiparous and multiparous women included in this study Bishop score: median/mean Bishop score recorded |
|
| Interventions | “Women in the active groups were treated twice during 41+3‐41+5 weeks of pregnancy or on the nearest working day”. “The women in the control group received the usual control with CTG during week 41+3” “certified acupuncturists performed the acupuncture. Experienced midwives performed the sweeping of the fetal membranes” Page 556 Acupuncture (n = 104): acupuncture needles placed bi‐laterally at points LI4 (Augmentation of uterus contractions), ST 36 (Improves strength of the body, immune system and nutrient uptake), LR 3 (calming, reduces pain), BL 60 (augmentation of contractions), BL 31, BL 32, GV 20 (mental calming), SP 6. Electrical stimulation performed at points BL31(has impact on gynaecologic organs), BL 32 (has impact on gynaecologic organs) and SP6 (induction of labour, augmentation of contractions, and has an effect on difficult births combined with LI 4 and LR 3. Needles were left in place for at least 30 minutes. Stimulation was performed at a frequency of 8 0 Hz medium. Page 556 Sweeping (n = 103): “performed by circulating the investigating fingers three times between the lower membranes and their attachment to the cervix, separating membranes and the cervix as much as possible. If membrane sweeping was not possible because of a closed cervix, cervical massage was performed by moving the cervix in relation to the pregnancy” Page 556 Acupuncture and sweeping (n = 100): “treated twice during 41+3‐41+5 weeks of pregnancy or on the nearest working day”. Page 556 Control (n = 100): “Usual control with CTG during week 41+3” “In women not delivered by week 42+0, a midwife blinded regarding which group the woman was allocated to induced labour on the nearest working day” page 556 |
|
| Outcomes | Spontaneous onset of labour Caesarean section Instrumental vaginal delivery Epidural analgesia PPH (as defined by the trial authors) Apgar score less than 7 at 5 minutes Augmentation pH < 7.05 |
|
| Notes |
Trial authors’ declaration of interest: none declared Funding: not reported Consent: “written consent” given. page 556 Ethical approval: “Danish Scientific Ethical Committee approved the research” Page 556 Email with request for further information sent 28 July 2017. Resent 20 September 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer‐randomization system accessible through a telephone line (voice response)” Page 556 “two women were not randomised because of difficulties with the telephone connection to the computer randomisation system” Page 556 |
| Allocation concealment (selection bias) | Low risk | “computer‐randomization system accessible through a telephone line (voice response)” Page 556 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: “Randomization was performed just before (the same day) the treatment was initiated” “Treatment could not be hidden from the pregnant women” Page 556 Personnel: allocation only blinded to midwife performing induction of labour if woman not in spontaneous labour at 42+0 weeks' gestation. “However women “occasionally might have told the midwife” their allocated group. Page 556. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. 10 women were excluded post randomisation. “4 women declined further participation when informed of group” N = 4 women discontinued (n = 3) or did not receive (n = 1) intervention because of staff shortages, page 556. < 20% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. No protocol available. |
| Other bias | Low risk | No protocol available.N = 4 women discontinued (n = 3) or did not receive (n = 1) intervention because of staff shortages, page 556. |
Averill 1999.
| Methods | Randomised controlled trial | |
| Participants |
Setting: not reported Duration of study: 1 year Inclusion criteria: “patients with reliable GA and a candidate for vaginal delivery.” page 47S Exclusion criteria: none stated Parity: not recorded Bishop score: not recorded |
|
| Interventions |
Membrane stripping group (N = 38): weekly membrane stripping, page 47S Control group (N = 36): weekly cervical exam “Patients were randomized to WMS or a weekly cervical exam” page 47S |
|
| Outcomes | Caesarean section | |
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “signed the consent” page 47S Ethical approval: none declared Email sent to Dr. Averill requesting full study 10 April 2017. Resent 30 July 2017. No response to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomized“ page 47S |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence of attrition bias.“4 were lost to follow up” unknown whether pre or post randomisation. Page 47S. |
| Selective reporting (reporting bias) | Low risk | Maternal age, mean GA, Bishop score < 7 recorded as outcome but not reported. Page 47S. |
| Other bias | Low risk | Abstract only available. However, no other bias noted. |
Berghella 1996.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: Chinatown Health Clinic affiliated with New York Downtown Hospital. New York, USA. Page 927 Duration of study: 1 July 1991 to 30 October 1991, when the first author was the sole obstetrical provider for the clinic, and from 1 July 1993 to 30 October 1993, when the second author was the sole obstetrical provider for the clinic. Page 927 Inclusion criteria: 38 weeks' gestation “all patients included in the study were low risk. Exact gestational age was verified either by a pelvic examination during the first 12 menstrual weeks to confirm size appropriate for dates, by an ultrasound examination before the 20th week, or both”. Page 927 Exclusion criteria: “Patients who presented after 20 weeks”, “multiple pregnancy, placenta previa, low‐lying placenta, non vertex presentation, fetal growth restriction, and any medical complication of pregnancy, such as hypertension and insulin‐dependent diabetes.” “Patients with long, closed cervices that did not allow stripping”. Page 927 Parity: mixed, both nulliparous and multiparous women included. (Table 1 Page 928) Bishop score: "Bishop scores were recorded for all patients." (Table 1 Page 928) |
|
| Interventions |
Duration of study: 1 July 1991 to 30 October 1991, when the first author was the sole obstetrical provider for the clinic, and from 1 July 1993 to 30 October 1993, when the second author was the sole obstetrical provider for the clinic. Page 927 Setting: Chinatown Health Clinic affiliated with New York Downtown Hospital. New York, USA. Page 927 Membrane stripping: n = 73 weekly stripping of membranes starting at 38 weeks' gestational age. “Stripping of membranes was performed uniformly by both authors by separating an approximately 2 cm to 3 cm section of the lower membranes from its cervical attachment with at least two circumferential passes of the index finger.” Stripping was repeated weekly according to randomisation until delivery occurred. Page 928 Control group:n = 69 “Weekly gentle cervical examinations” “gentle cervical examinations were repeated weekly according to randomisation until delivery occurred.”Page 928 Bishop scores were recorded for all patients. Page 928 |
|
| Outcomes | Spontaneous vaginal delivery: Vacuum Low forceps Primary caesarean section |
|
| Notes |
Funding: none declared. Trial authors’ declaration of interest: none declared. Informed consent obtained: “signed informed Internal Review Board consent forms and were randomized” Page 927 Ethical approval: not stated Email sent to Dr Vincenzo Berghella requesting information for subgroup analysis. Sent 10 August 2017 and 28 August 2017 No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomized using computer generated numbers from opaque, sealed envelopes.” Page 927 |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment with “opaque, sealed envelopes.” Page 927. Not stated if numbered or sequential. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: blinding of patients not reported. Personnel: clinicians not blinded “These time frames were chosen so that only one investigator would perform all the examinations in a given period.” Page 927 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding details given in study. “After all the patients had delivered, the data were analyzed for statistical differences using the two‐sample t test, the Mann‐Whitney test, the generalized Fisher exact test, or x2, as appropriate.” Page 928 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias.It is noted that 7 patients “initially included in the study were excluded because of long closed cervices not amenable to stripping” page 928 |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | High risk | There is disparity in the study numbers as shown in table 1 page 928: Control group (n = 69): Primiparous n = 43 multiparous n = 26 Sweep group (n = 73): Primiparous n = 35 multiparous n = 38 Also as stated in the study “the original Bishop scores of the two groups were not recorded and compared, so this small study could have been biased by dissimilar patient characteristics in the two groups.” Page 929 |
Boulvain 1998.
| Methods | Randomised controlled clinical trial | |
| Participants |
Setting: 3 tertiary care hospitals of the province of Quebec, Canada. Page 35 Duration of study: 17 months(1 April 1995 to 1 October 1996). Page 35 Inclusion criteria: included if eligible for a “non‐urgent medical indication for induction of labour and a single fetus in cephalic presentation. Non‐urgent medical indication for induction included: post‐term pregnancy, hypertension, diabetes, fetal growth retardation without signs of fetal distress, or other medical complications of pregnancy. Post‐term pregnancy was defined as gestational age > 287 days when formal induction of labour was scheduled”. ‘Only women at term (≥ 266 days) were included in the trial’. Written informed consent must have been obtained. Gestational age was calculated from the last menstrual period and an ultrasound examination carried out in the middle trimester. Induction date between 3 and 7 days after randomisation. A date for formal induction of labour was given prior to randomisation, at least 3 days and not later than 1 week after inclusion. Page 35 Exclusion criteria: “Women presenting with placenta praevia, abnormal cervical discharge, or contraindications to vaginal delivery were excluded.” Page 35 Parity: mixed, both nulliparous and multiparous women included. Page 36 (Table 1) Bishop score: recorded (not available for 2 women, 1 in each group) Page 36 (Table 1) |
|
| Interventions |
Membrane stripping (n = 99): “examination began with assessment of the Bishop score, followed by the intervention. Physicians were requested to report the characteristics of the cervix (dilatation 0‐3 points effacement 0‐3, station 0‐3, consistency 0‐2, position 0‐2) before performing the intervention’˜. Sweeping of the membranes consisted in circular movements of the examining finger between the lower segment of the uterus and the fetal membranes. When the membranes could not be reached, physicians were requested to attempt to gently dilate the cervix. If this manoeuvre was successful, sweeping was performed. If the cervix acted as a barrier to the examining finger, cervical massage was performed” Page 35 Control group (n = 99): women in the control group had only a vaginal examination for Bishop scoring. Page 35 |
|
| Outcomes | Epidural Spontaneous vaginal delivery Forceps/vacuum delivery Caesarean section Apgar ≤ 7 at 5 minutes Neonaltal infection Neonatal convulsions Formal induction of labour Evaluation of pain during examination: VAS (n = 87‐87) PPI (n = 94‐92) labour agentry scale (n = 90‐85) |
|
| Notes |
Funding: study was supported by grant number 6605‐4645‐ 401 of NHRDP, Health Canada. Dr Boulvain received salary support from Astra Pharma. Dr Fraser receives salary support from the Medical Research Council of Canada. Dr Marcoux holds a Health Research Scholarship from Health Canada. Page 39 Trial authors’ declaration of interest: none stated. Informed consent obtained: yes Page 35 Ethical approval: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated list of random numbers, with randomly permuted blocks of six and eight, stratified by hospital” Page 35. |
| Allocation concealment (selection bias) | Low risk | “the allocations were contained in a series of opaque, sealed and consecutively numbered envelopes, kept in the delivery unit” “clerk opened the next envelope and informed the doctor of the woman’s allocation” Page 35 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: unclear if women blinded. Personnel: clinician not blinded Page 35 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Obstetric data were abstracted from the hospital charts by a research assistant who was unaware of the treatment allocation”. Page 36. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The analysis was based on “Intention to treat”. However it was noted that “Two women in the control group were excluded after randomisation: one withdrew her consent and the other failed to meet the main inclusion criteria in that she was not scheduled for induction of labour” Page 36 |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias noted. |
| Other bias | Low risk | No other bias noted. |
Cammu 1998.
| Methods | Randomised controlled trial | |
| Participants |
Setting: antenatal clinic of a university teaching hospital, Belgium. Duration of study: not stated. Inclusion criteria: “nulliparous with a singleton fetus in cephalic presentation and having no detected risk factors, such as hypertensive disorders, diabetes mellitus or intrauterine growth retardation. The women were recruited at 39 completed weeks of gestation. Gestational age had been determined in all the women by ultrasound. Third trimester ultrasound examination had been performed to exclude placenta praevia, abnormal fetal presentation and fetal growth retardation” Page 42 Exclusion criteria: limited to nulliparous women because they are at greater risk of failed induction and dystocia and their pregnancies and labour are not influenced by previous birth experience. Third trimester ultrasound examination had been performed to exclude placenta praevia, abnormal fetal presentation and fetal growth retardation. Page 42 Parity: only nulliparous women included Bishop score: Initial Bishop Score: Mean Bishop score on admission to labour ward |
|
| Interventions |
Membrane sweeping: (n = 140) “sweeping of the membranes” on a weekly basis. This involved “digital separation of 2‐3 cm of the membranes from the lower uterine segment” was “performed at every visit, rotating the finger at least twice through 360 degrees. A closed cervix was stretched digitally until membrane sweeping could be carried out. A closed cervix that would not admit a finger was vigorously massaged.” Page 42 Control group: (n = 138) “normal digital examination on a weekly basis.” “The study was carried out by two certified gynaecologists with more than ten years of experience and by an assistant in training. Induction of labour was planned from 41 completed weeks onwards. If labour had to be induced for medical reasons before 41 weeks, the woman was not excluded from the study group to which she had been assigned. Page 42 |
|
| Outcomes | Spontaneous labour Augmented labour Induced labour Epidural analgesia Instrumental delivery Caesarean section Apgar < 7 at 5 minutes Atrerial cord blood < 7.1 |
|
| Notes |
Funding: none stated Trial authors’ declaration of interest: none stated Informed consent obtained: not stated Ethical approval: the protocol was approved by the university medical ethics committee Email sent 30 August 2017 Reply 30 August 2017 "At 39 completed weeks of gestation women were asked to participate in a RCT. A list of random numbers was generated by a computer. Numbered sealed envelopes containing the treatment allocations were kept by the attending nurse of the antenatal clinic and were opened after entry to the trial." "The trial was conducted in a University Hospital and none of the patients was private. Patients followed a standardized labour induction protocol and women were delivered by residents under supervision. Delivery room midwives and attending physicians (obstetricians) were unaware of the treatment allocations after randomisation." "Only primiparous women were included in the study." "Mean Bishop score at randomisation in the sweeping group was 3.35 (SD 1.8) and in the control group 3.39 (SD 1.6). Mean Bishop score on admission to the labour ward was 7.7 (SD 1.9) in the sweeping group and 7.2 (SD 2)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A list of random numbers was generated by a computer.” Page 42 |
| Allocation concealment (selection bias) | Low risk | “Numbered sealed envelopes containing the treatment allocations were kept by the attending nurse of the antenatal clinic and were opened after entry to the trial”. Page 42. Not reported if opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: blinding of participants not discussed. Personnel: during labour “Midwives and obstetricians were unaware of the treatment allocations after randomisation”. Page 42 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Labour was managed by nurse midwives. The women were delivered by residents who were supervised by certified obstetricians. Midwives and obstetricians were unaware of the treatment allocations after randomisation”. Page 42 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N = 287‐9 = 278 “An additional nine women were excluded after randomisation for various reasons: multipara (n = 4), spontaneous rupture of the membranes before randomisation (n = 2), vaginismus (n = 2) and unexpected non vertex presentation (n = l)” < 20% Page 42 |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting, however no trial protocol available. |
| Other bias | Low risk | No other bias noted. |
Crane 1997.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Grace General Hospital, Newfoundland, Canada. Duration of study: 18 months Inclusion criteria: “low risk (as defined by the Newfoundland antenatal form), at 38‐40 completed weeks ‘gestation based on firm dates (last menstrual period) or early ultrasound (at or before 18 weeks‘ gestation).” Written informed consent. Page 586 Exclusion criteria: exclusion criteria included important medical diseases, pregnancy complications (including bleeding, hypertension, or preterm labour), evidence of fetal growth restriction, history of perinatal mortality or low birthweight infant, uncertain dating, premature rupture of membranes (PROM), abnormal presentation, placenta previa, scheduled caesarean delivery, or any other contraindication to vaginal delivery. Page 586 Parity: mixed, both nulliparous and multiparous women included (% presented in Table 1 of manuscript page 587). Bishop Score: Bishop scores were recorded for all patients (% presented in Figure 1 of manuscript page 587). |
|
| Interventions | “The groups were stratified based on the status of the cervix at pelvic examination (opened versus closed), with randomization within the strata.” Page 586 Membrane stripping (n = 76): “after the status of the cervix was determined (i.e. whether it admitted a fingertip through the internal OS). Those assigned to the sweeping‐membranes group underwent sweeping, whereby as much membrane as possible was separated from the lower uterine segment by sweeping the examiner’s index finger twice in a circumferential manner. If the examiner was unable to pass a fingertip through the cervix, vigorous cervical massage was performed, defined as firmly rubbing the external OS in a circular manner with the examining index finger.”Page 587 Control group (n = 74): “the control group had an internal examination only.” Page 587 |
|
| Outcomes | Spontaneous onset labour Induction Mode of birth Spontaneous Forceps/vacuum Caesarean Analgesia in labour: Epidural Apgar score < 7 at 5 minutes Neonatal infection |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “consent for enrolment was sought. Written informed consent was obtained from all subjects” Ethical approval: “the study was approved by the Human Investigation Committee of Memorial University of Newfoundland as well as the hospital.” Email sent requesting further information: Email received 8 September 2017 "With regards to our study, participants and personnel were not blinded. Outcome assessment was not blinded. We no longer have the original data file for this study. At the time the study was completed and published (1997) out ethics board required retention of research data for 10 years. We have since moved to a new site and in this move some research files older than 10 years were destroyed." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “random‐number tables by blocks of six, using opaque, sealed, sequentially numbered envelopes. The groups were stratified based on the status of the cervix at pelvic examination (opened versus closed), with randomization within the strata.". Page 586 |
| Allocation concealment (selection bias) | Low risk | “random‐number tables by blocks of six, using opaque, sealed, sequentially numbered envelopes." “The envelope was opened by the attending nurse during the internal examination by an investigator, after the status of the cervix was determined”. Page 586 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel: not blinded. “The envelope was opened by the attending nurse during the internal examination by an investigator, after the status of the cervix was determined” But clinicians aware of group allocation prior to intervention/no intervention. Page 586 Participants: not blinded. This bias was confirmed by Dr. Crane on 8 September 2017 in an email stating, “participants and personnel were not blinded. Outcome assessment was not blinded.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was not blinded “Medical records were reviewed after delivery to record these variables.” This bias was confirmed by Dr. Crane on 8 September 2017 in an email stating, “participants and personnel were not blinded. Outcome assessment was not blinded.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias noted. |
| Other bias | Low risk | No evidence of other bias. Protocol not available |
Dare 2002.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Owolowo University teaching hospitals, Ile‐lfe, Nigeria Duration of study: 18 months (1 January 1998 to 31 May 2000) Inclusion criteria: “Singleton gestation in the cephalic presentation at 38 weeks gestation, early confirmation of pregnancy by ultrasonography and no contraindications to vaginal delivery” Page 283 Exclusion criteria: “closed cervix not amenable to stripping at 38 weeks gestation, placenta praevia, medical complications of pregnancy such as insulin dependent diabetes mellitus, rupture of fetal membranes, unexplained vaginal bleeding, intrauterine growth restriction or a prior uterine incision” Page 283 Parity: mixed. both nulliparous and multiparous women included (% presented in Table 1 of manuscript page 284). Bishop score: recorded (% presented in Table 1 of manuscript page 284). |
|
| Interventions |
Membrane sweep (n = 69): “membrane stripping” “Stripping of the membranes was performed by separating approximately 2‐3cm of chorionic membranes from the lower uterine segment using two circumferential passes of the examining finger” Page 283 Control group (n = 68): “gentle cervical examination” Page 283 “All patients were examined by the same person to minimise subjective differences in evaluation. Bishop scores were recorded for all patients” Membranes stripping or gentle cervical examination, performed by 1 clinician. |
|
| Outcomes | Spontaneous vaginal delivery Assisted delivery Caesarean section Chorioamnionitis Apgar score < 7 at 5 minutes Neonatal death (congenital heart defects) |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained:“all candidates gave signed informed consent before randomization” Ethical approval: yes; “This study was approved by the hospital ethical committee on human investigation” Email sent 30 August 2017, 26 October 2017. No reply to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer‐generated random schedule”. Page 283 |
| Allocation concealment (selection bias) | Low risk | “The allocation of assignment was concealed by placement in a numbered, opaque sealed envelope which was drawn in consecutive order”. Page 283 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not discussed Personnel: “examined by the same person to minimise subjective differences in evaluation” Page 283 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias.“One hundred and sixty‐nine women were eligible for the study of whom 11 (6%) declined to participate. Of the 158 who signed the consent, nine were lost to follow‐up and 12 were excluded because of long, closed cervices not amenable to stripping” < 20%". Page 284 |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | Low risk | No evidence of other bias. |
de Miranda 2006.
| Methods | Randomised controlled trial | |
| Participants |
Setting: midwifery practices, the Netherlands. Duration of study: June 2000 to March 2003. Inclusion criteria: “low risk (single fetus in cephalic presentation, no pregnancy complications or risk factors and no contraindications to normal vaginal delivery), with a reliable gestational age of 41 weeks (range 40+6to41+3)” Page 403. Exclusion criteria: “history of blood loss after the first trimester or suspicion of loss of amniotic fluid during pregnancy.”Page 403 Parity: mixed,both nulliparous and multiparous women included (Table 1 page 404). Bishop score: not recorded. |
|
| Interventions |
Membrane stripping (N = 375) "Women allocated to the control group received routine monitoring. To prevent prostaglandin release, vaginal examination was not performed in the control group until the onset of labour. In addition, we asked the midwives to refrain from advice regarding sexual intercourse as a way of stimulating labour onset, regardless of the allocation." Page 403 Control group (N = 367) "Women allocated to sweeping received routine monitoring as well, followed by a vaginal examination for assessment of the cervical ripeness (Bishop score (BS)) and immediate sweeping. Sweeping was performed by separating the lower membranes as much as possible from their cervical attachment, with 3 circumferential passes of the examining fingers. When sweeping was not possible because the cervix was closed, cervical massage was performed. Massage of the cervical surface was performed with circular pushing and massaging movements of the fore finger and middle finger for approximately 15 seconds. Sweeping was repeated every 48 hours, with a maximum of 3 times, until labour commenced or 42 weeks of gestation was reached. The midwives explained to the women who had been swept that blood‐stained mucus or painful contractions could occur." Page 403 |
|
| Outcomes | Spontaneous onset of labour < 42 weeks Spontaneous onset of labour ≥ 42 weeks labour induction total Epidural Spontaneous vaginal delivery Forceps delivery Vacuum delivery Caesarean section Augmentation of labour Adverse neonatal outcomes Perinatal death Women’s perception of sweep |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “A written informed consent was obtained at the antenatal visit of 41 weeks” Page 403 Ethical approval:“The ethics committee of the Academic Medical Center of Amsterdam approved the trial” Page 403 Email sent 30 August 2017 requesting data for subgroup analysis. Reply received 31 August 2017...follow‐up email sent 20 September 2017 Subgroup data received 26 October 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “blocked randomisation using 30 blocks of 25,26 with a variable allocation ratio of 12:13 or 13:12” Page 403 |
| Allocation concealment (selection bias) | Low risk | “The allocations were placed within consecutively numbered, opaque, sealed envelopes. A box containing the agreed number of randomisations (variable for each centre) was then sent to the midwifery practices where they were kept.” Page 403 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel: “The participating midwives were unaware of the randomisation method.” Does not reference blinding for intervention. Page 403 Participants: not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | After every randomisation, the numbered envelope containing the allocation card was posted to the trial coordinator together with a randomisation form containing the date of randomisation, the allocation group and the subject characteristics.” Page 403 “Data concerning prenatal care, obstetric intervention, delivery and infant condition were recorded on a case report form (CRF).” “The midwives asked all women to complete the questionnaires.” Page 403 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. “Primary analysis was by intention to treat, i.e. three women allocated to sweeping, who did not receive the intervention, and 19 women randomised to the control group, who were nevertheless swept, were analysed according to the allocated group.” < 20% (375 in the sweeping group and 367 in the control group). Page 404 |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias noted. |
| Other bias | Low risk | No other bias noted. |
Doany 1997.
| Methods | Double‐blinded placebo‐controlled study | |
| Participants |
Setting: UCLA Medical Center, California, USA Duration of study: not stated Inclusion criteria: “Singleton pregnancy in the cephalic presentation who were referred for fetal surveillance at 287 days of gestation or more”. “Reactive nonstress test, amniotic fluid index (AFI) between 5 cm and 25 cm. Fetal weight between 2500 g and 4500 g and uterine contractions less frequent than every 5 mins” Page 72 Exclusion criteria: “No prenatal care, previous uterine surgery, acute or chronic medical or psychiatric illness or drug use” Page 72 Bishop score: Bishop score ≤ 6 recorded. |
|
| Interventions | Women were randomised to 1 of 4 treatment groups The treatments were administered at 287 days (41 weeks) and 294 days (42 weeks) of gestation, then every 3–4 days until 307 days (43 weeks and 6 days) of gestation. The assigned treatment was given at each visit after a reactive NST, a normal AFI and a Bishop score. Page 72 Group 1: n = 28 no membrane stripping and placebo gel Group 2: n = 37 no membrane stripping and 4 mL (0.5 mg/mL PGE2 gel) Group 3: n = 50 membrane stripping or cervical massage and placebo gel Group 4: n = 28 membrane stripping or cervical massage and 4 mL (0.5 mg/mL PGE2 gel) “The examining finger was introduced into the cervical canal and a total of three circumferential sweeps were made between the lower uterine segment and the chorionic membranes.” “When the cervical canal was not accessible, the cervical canal was pulled anteriorly and massaged.” “This was followed by placing 4 mL of an unlabeled gel, containing either a placebo or 2mg of PGE2, via syringe, in the posterior vaginal fornix” “both patients and staff were blinded to the type of gel administered” “After treatment patients underwent continuous external fetal and uterine monitoring….for 1 hour” If there was no sign of fetal distress the patients were allowed to go home. Page 72 “Management of study patients in labour and delivery was not controlled and thus was physician dependent. Physicians managing labour were blinded to the study group assignment.” Patients were admitted to labour ward when they had “clear changes in both effacement and dilatation of the cervix or if they are in the active phase of labour defined by cervical effacement > 80% & cervical dilatation ≥4cm.” Page 72 |
|
| Outcomes | Spontaneous labour Induction of labour Caesarean section Operative vaginal delivery 5‐minute Apgar < 7 Amnionitis Hemorrhage Probable sepsis (neonate) Oxytocin augmentation Pre‐eclampsia |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: not stated Ethical approval: “approval from our institutional Human Subject for Research Committee” Emailed for further information 28 August 2017; 8 January 2018. No reply to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomized, by table of random numbers, into one of four treatment groups”. Page 72 |
| Allocation concealment (selection bias) | Unclear risk | No information given on concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: unclear risk of bias. “Both patients and staff were blinded to the type of gel administered.” Unclear if blinded to membrane sweep. Personnel: high risk of bias. “Physicians managing labor were blinded to the study group assignment.” Page 72. Personnel blinded to gel administered, however clinician not blinded to membrane sweep. “The mixture, with a final PGE2concentra‐tion of 0.5 mg/mL, was placed in syringes of 4‐mL allocations. The placebo gel consisted of hydroxyethyl cellulose gel mixed with an inert emulsion (Fattibase, Paddock Labs, Inc., Minneapolis, MN) to produce a gel indistinguishable from the PGE2mix, and was similarly placed in syringes of 4‐mL allocations. All gel samples were stored in a freezer at 25to07C, and were updated weekly. The gel samples were thawed at room temperature for 10 min prior to administration” Page 72 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information given to inform judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias noted. The following discrepancy was noted “the only complication which was statistically more prevalent was preeclampsia, which occurred in 7/64(11%) of PGE2‐gel‐receiving subjects, groups II and IV” n = 65 in these groups not 64 as stated (10.7% v’s 10.9%). Page 73. However we judged this discrepancy as unlikely to make a clinically important difference |
| Other bias | High risk | Group sizes are imbalanced: group I = 28 group II = 37 group III = 50 group IV = 28 Unequal number of women in the 4 groups, reasons for imbalance not explained in the methods section. Author contacted, no reply received to date. |
El‐Torkey 1992.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Antenatal clinic, district maternity hospital, UK Duration of study: June 1990 to March 1991 Inclusion criteria: pregnant women between 41 and 42 weeks' gestation. "women who opted for induction of labour were randomly allocated to undergo sweeping of the membranes or to act as controls". Deadline date for labour induction given after randomisation. Page 456 Exclusion criteria: none stated Parity: mixed. Both nulliparous and multiparous women included. Bishop score: cervix > 4 cm at first exam |
|
| Interventions |
Membrane stripping (n = 33): “As much of the membranes as possible was separated from the lower segment” “If cervix would not admit a finger it was massaged vigorously to encourage prostaglandin release”. “Sweeping of the membranes was performed by one of the authors (M.E‐T.).” “After allocation the subjects were given a date for formal induction of labour”Page 456 Control group (n = 32): no vaginal examination. Page 456 |
|
| Outcomes | Spontaneous onset of labour: Epidural Mode of birth Caesarean section Forceps Spontaneous Neonatal outcomes Apgar < 6 at 5 minutes Serious neonatal infection Neonatal perinatal death |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: no, only women in sweeping group were "informed of the purpose of the trial". page 456 Ethical approval: no,”formal ethical approval of the study was not sought” Unable to contact either author. Unable to locate current place of work or email address. Hospital trial was set in now closed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by “random permuted blocks”. Page 456. |
| Allocation concealment (selection bias) | Unclear risk | The randomisation codes were placed in opaque sealed envelopes which “were kept in the antenatal clinic”. Page 456. However not noted if envelopes were sequential or sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: "Those who were randomized to sweeping were informed of the purpose of the trial and the procedure". "The women randomized to the control group were not aware that they were taking part". Page 456. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. “Because of this marked difference in the proportions of subjects achieving spontaneous labour the trial was stopped before 110 women were recruited. The decision to stop the trial was made by the authors themselves, the decision being based on the statistical stopping rule for randomized trials (Pocock,1983)” |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias noted. |
| Other bias | Low risk | No evidence of other bias noted. |
Gemer 2001.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Israel Duration of study: not reported "fifty patients" Inclusion criteria: not reported Exclusion criteria: not reported Parity: not reported |
|
| Interventions | N = 50 2 groups Group 1: membrane sweep Group 2: intracervical PGE2 0.5 mg gel |
|
| Outcomes | Change in Bishop score Active labour with 24 hours Birth within 24 hours |
|
| Notes | M Boulvain excluded this study based on inadequate method of concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to inform judgement "50 women were randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: while not reported, highly likely that it is not possible to blind. Personnel: partially blinded, "A Bishop score was assigned by a blinded examiner prior to and 24 hours following the procedure" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to inform judgement. |
| Other bias | Low risk | No other sources of bias noted |
Goldenberg 1996.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: antenatal Unit, Department of Obstetrics and Gynecology, The Chaim Sheba Medical Center, Israel. Page 130 Duration of study: 17 months (1 January 1992 to 30 June 1993). Page 130 Inclusion criteria: all term patients who arrived at the unit and had a history of regular periods. This “unit accepts low‐risk pregnant women and routinely does follow‐up by means of a non‐stress test and ultrasonographic evaluation at ≥ 38 weeks to decrease mortality and morbidity of the fetus. The gestational age was ascertained by using the last‐known menstrual period, ultrasound examination before 10 weeks’ gestation, and no size/date discrepancy by uterine size assessment.” “A non‐stress test, blood pressure and urine analysis are routinely carried out on all the patients of the antenatal unit. Only low‐risk pregnant patients who fulfilled the above criteria underwent stretching of the cervix and Stripping of the fetal membranes.” Page 130 Exclusion criteria: "None refused inclusion" Page 130 Parity: mixed. Both nulliparous and multiparous women included (table 1 page 130 of study). Bishop score: Baseline Bishop score recorded (Table 1 page 130). Bishop score at 38‐40 weeks recorded (Table 3 page 133). Bishop score at 41‐43 weeks recorded (Table 3 page 133). |
|
| Interventions |
Membrane stripping: n = 152. "The procedure was performed once at term by 2 of the authors (M.G. and D.B.) using clean examination gloves and an obstetric cream. Stretching of the cervix and vagina was accomplished as described by Ferguson (3), and stripping of the membranes was accomplished by digital separation of the membranes from the lower uterine segment with 1 or 2 circumferential rotations." Page 130 Control group: n = 141 “A pelvic examination was performed by palpating the cervix for Bishop’s scoring”. “The interval from the procedure to spontaneous labor was recorded, defining spontaneous labor as labor on self‐admission of the patients to the delivery room due to painful regular contractions occurring twice every 10 min, or more frequently. A cervical dilatation of 2‐3 cm on entry to the labor ward was considered arbitrary, to indicate the active phase of labor in women who were admitted, or rupture of the fetal membrane at term with contractions.” Page 130 |
|
| Outcomes | Augmentation Amnionitis Caesarean section Maternal febrile morbidity Apgar score < 7 at 5 minutes |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “Informed consent was obtained from all the patients”. Page 130 Ethical approval: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “All patients were assigned by computer randomization to a stretching/stripping group or to a non‐stretching/stripping group” page 130 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel: “The procedure was performed once at term by two of the authors (M.G. and D.B.)” Page 130 Participants: blinding of participants not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 302 participants enrolled. 9 lost to follow‐up when they requested "to halt the procedure" page 130. 293 participants randomised. Intervention group n = 152, Control group n = (150‐9) 141. It is noted that “An additional nine patients from the stretching/stripping group were excluded because of difficulty in performing the procedure." page 130. |
| Selective reporting (reporting bias) | Unclear risk | Mode of delivery is a stated outcome, however only caesarean section is reported on, Page 130. Fetal outcome post delivery only reported as “postpartum complications…not statistically different”, no detailed data given, Page 130. |
| Other bias | Low risk | No evidence of other bias |
Gupta 1998.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: Antenatal clinic of the Department of Obstetrics and Gynaecology, PGIMER, Chandigarh, India. Duration of study: not stated Inclusion criteria: women with “confirmed gestational age, early confirmation of pregnancy, cephalic presentation and with no contraindication for vaginal delivery“ ”at 38 weeks gestation” and “informed consent" received. Ultrasound was done to assess the fetal growth parameters, biophysical profile and placental localization (Page 116). Exclusion criteria: “Women with closed cervix at 38 weeks gestation; known medical disease or medical complications of pregnancy; multiple pregnancy; hydramnios; premature rupture of membranes PROM; vaginal or cervical infection; low lying placenta; intrauterine fetal death; malpresentation; patients in labor; and major degree of cephalopelvic disproportion.” Ultrasound was done to assess the fetal growth parameters, biophysical profile and placental localization (Page 116). Parity: only primigravida included in the study. Bishop score: (Table I, Page 117). Bishop score < 6 Bishop score ≥ 6 |
|
| Interventions |
Membrane stripping: n = 50 “stripping of membranes was done by digital separation of 2/3 cm of chorionic membranes from lower uterine segment using two circumferential passes of the examining fingers. Thereafter, all patients were followed weekly till delivery or scheduled induction. At onset of labor repeat cervical swabs were taken and placental membranes sent for bacterial culture studies” (Page 116). Control group: n = 50 “Only pelvic examination” (Page 116). Under aseptic precautions all patients were examined by the same person to minimise subjective difference in evaluation |
|
| Outcomes | Spontaneous onset of labour Vaginal delivery total Spontaneous vaginal birth Assisted vaginal delivery Caesarean section Acute fetal distress Still birth Meconium aspiration TTN Chorioamnionitis Neonatal infection |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “informed consent was taken” Ethical approval: not stated Email sent requesting further information. Reply 31 August 2017 stating author retired. No contact details available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was done using a computer generated list of random numbers”, page 116. |
| Allocation concealment (selection bias) | Unclear risk | “a sealed envelope was opened for each women after entry into the trial.”, page 116. Does not report if the envelope was sequential, opaque or numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported Personnel: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. No evidence of reporting bias. |
| Other bias | Low risk | No evidence of other bias. |
Hamdan 2009.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Outpatient clinic, University hospital, Kuala Lumpur, Malaysia. Duration of study: 3.5 year period. 2002 to 2005 Inclusion criteria: “Women with one transverse lower segment cesarean scar, a singleton pregnancy, cephalic presentation, intact membranes, and gestational age more than 36 weeks who were agreeable to VBAC and passed specialist assessment for VBAC”. Page 746 Exclusion criteria: “obstetric contraindications to VBAC (e.g. placenta previa, suspected macrosomia, suspected cephalopelvic disproportion, abnormal fetal lie, and obstructive pelvic masses).” Page 746 Parity: only multiparous women included. Bishop score: Bishop score at each session recorded (session 1 to 5). |
|
| Interventions |
Membrane stripping (N = 108): “Immediately after randomization, women assigned to “sweep” had their cervix stretched and membranes stripped from the lower uterine segment in the manner as previously described.” Page 746 Control group (N = 105): “Women assigned to “no sweep” had a gentle vaginal examination for their Bishop score. Page 746 “Weekly follow‐up sessions based at the antenatal clinic with the investigators were arranged to repeat membrane sweeping or vaginal examination until delivery. The Bishop score was recorded at each session In our center, induction of labor for prolonged pregnancy is typically offered at 41 weeks of gestation.19 Induction of labor for diabetes that required drug treatment is offered at 38 weeks and for gestational diabetes adequately controlled by diet, induction of labor is offered at 40 weeks.20 Upon prelabor rupture of membranes, women were offered either immediate uterine stimulation, typically with oxytocin, or expectant inpatient management for up to 24 hours.21 All women with a previous cesarean delivery who were offered formal induction of labor were counselled about a higher risk of scar rupture and of unplanned cesarean delivery and the option of a planned repeat cesarean delivery was given.” Page 746 |
|
| Outcomes | Spontaneous onset of labour Induction of labour Caesarean section Spontaneous vaginal delivery Augmentation of labour Instrumental delivery Caesarean delivery PPH Epidural analgesia Umbilical cord artery PH < 7.1 Apgar score 6 or less at 5 minutes |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “All participants provided written informed consent.” Ethical approval: ethical approval for the trial was obtained from the Medical Ethics Committee of the University of Malaya Medical Center, page 746 Emailed 30 August 2017 requesting further information sent.Resent 20 September 2027. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “prepared by an author (M.H.) in blocks of 50 using a computer‐generated randomization sequence (available online at http://www.random.org/).” Page 746 |
| Allocation concealment (selection bias) | Low risk | “sequential opening of numbered sealed opaque envelopes indicating “Sweep” or “No Sweep.” Only investigators aware of allocation. Page 746 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: “Blinding of participants and delivery providers was effected by a policy of not revealing allocated treatment to them unless requested for an important clinical need. There was no request to unblind during the trial. Page 746 Personnel: Only investigators aware of allocation. However it appears investigators preformed membrane sweep. All participants received standard management by delivery providers.” Page 746 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Collected by authors who are noted to be blind until data analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported. “Analysis by intention to treat”. Page 747 |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias noted. Protocol not available |
| Other bias | Low risk | No evidence of other bias noted. Protocol not available |
Hill 2008a.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Tripler Army Medical Center, Honolulu, Hawaii, USA. Duration of study: March 2006 to May 2007 Inclusion criteria: “All patients had confirmation of gestational age by first‐trimester crown rump length or mid second trimester biometry assessment. Singleton pregnancy, cephalic presentation, and anticipated vaginal delivery.” Page 1314 Exclusion criteria: “Three categories: indications for labor induction, indications for cesarean delivery, and contraindications to membrane sweeping. Included multiple gestation, placenta previa, placental abruption, pregestational or gestational diabetes, chronic or gestational hypertension, preeclampsia, any pregnancy with an indication for induction other than impending postmaturity, any pregnancy for which a cesarean delivery was planned, history of preterm delivery, history of vasa previa, active cervical infection, third‐trimester vaginal bleeding, mullerian anomalies, severe fetal anomalies, and active genital herpes infection.” Page 1314 Parity: mixed. Both nulliparous and multiparous women included. Bishop score: only cervical dilatation recorded |
|
| Interventions |
Membrane stripping (N = 162): “she received a cervix examination at every visit from 38 weeks of gestation until delivery. If the cervix was dilated, the provider swept a finger in a 360‐degree fashion inside the cervix, thereby separating the lower uterine segment from the amniotic sac. If the cervix was closed, it was massaged as described by prior authors.” Page 1314 Control group (N = 138): “a weekly cervix examination was performed from 38 weeks of gestation until delivery. Special effort was made on this examination not to stretch or manipulate the cervix.” Page 1314 |
|
| Outcomes | Vaginal delivery Caesarean delivery Chorioamnionitis Endomyometritis Labour induction Spontaneous labour Neonatal infection |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained:“written informed consent” Ethical approval: not stated Email sent requesting information on subgroup analysis 30 August 2017. Limited reply received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “a computer‐generated randomizer program” Page 1314. “Participants were randomly assigned to receive either weekly membrane sweeping or no membrane sweeping for the duration of the pregnancy after 38 0/7 weeks gestational age” Page 1314 |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment not reported.“Participants were not informed as to the group allocation.” Page 1314 “Each patient was identified by a computer‐generated sequential number that was placed in her chart” Page 1314 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel: not blinded. “Each patient was identified by a computer‐generated sequential number that was placed in her chart. Upon seeing a patient who was enrolled in the trial during a routine prenatal appointment, the clinician would enter the participant number into a Web‐based program that would tell the provider whether to sweep or not to sweep the membranes. These data were not included in the patient chart. A computer log was kept of all access through the program to the patient identifier to ensure no one but the clinician seeing the patient for routine obstetric appointments accessed her group assignment. Providers who admitted the patient to the labor and delivery unit were also blinded to the patient’s group allocation.” Page 1314 Participants: “Participants were not informed as to the group allocation.” It was understood that many patient would realize which intervention they were receiving, but we felt that not informing the patients of their group allocation would increase the quality of the blinding process…” data were not included in the patient chart” Page 1314 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded: “The same restrictions were placed on the authors of this article until the end of the trial and the completion of all data collection. All data were collected and all chart analysis was done by the primary author, who was also blinded to the group allocations. Unblinding did not occur until the time of data analysis.” Page 1314 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias noted. All outcomes reported for “Intent to treat basis”. |
| Other bias | Low risk | No evidence of other bias noted. |
Imsuwan 1999.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Department of Obstetrics and Gynecology, Phramongkutklao Hospital, Bangkok. Thailand Duration of study: not stated Participants randomised: N = 284 Inclusion criteria: “Gestational age of 38 weeks who attended antenatal clinic at Phramongkutklao Hospital.” page 267 Exclusion criteria: not reported Parity: “Only gravida women included in this study”. No further details reported. Page 267 Bishop score: not reported |
|
| Interventions |
Group 1: “first group had pelvic examination alone”. Page 267 Group 2:“ pelvic examination with membrane stripping beginning at 38 weeks gestation and continuing weekly till the onset of labor or reaching 42 complete weeks” Page 267 |
|
| Outcomes | Delivery post 41 complete weeks' gestation. Page 267 | |
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: not stated Ethical approval: not stated Email sent 25 May 2017 Reply 8 June 2017 Dr. Tanapat "Thank you for your interest in this article, I do not have a copy of the reprint with me however I will contact Dr. Imsuvan who is a staff at the Department of Obstetrics and Gynecology, Phramongkutklao Hospital and the RTCOG for you to see if they have a copy of the article. You can also go to web site of The Royal Thai College of Obstetricians and Gynecologists (RTCOG) to search their journal or as their staff to find the article for you.” Further email sent 14 June 2017. RTCOG replied 2 August 2017 with copy of abstract. Full study never published per RTCOG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Eligible gravidas were randomized” page 267 |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Maternal and fetal complications stated as trial outcomes but data not supplied. Page 267. Protocol not available. |
| Other bias | Low risk | No evidence of other bias |
Janakiraman 2011.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Outpatients obstetric clinic, USA Duration of study: not stated Participants randomised: N = 123 Inclusion criteria: “All women who presented to an outpatient obstetrics clinic who were >/= 37 weeks, were candidates for vaginal delivery and qualified for GBS prophylaxis were offered enrolment.” (Page S41). Parity: mixed. Both nulliparous and multiparous women included (Page S41). Bishop score: not stated |
|
| Interventions |
Membrane stripping (N = 61): in the intervention group sweeping was attempted at each visit (Page S41). Control group (N = 62): no membrane sweeping was attempted. Standard CDC protocol antibiotic prophylaxis was given (Page S41). |
|
| Outcomes | Vaginal delivery LTCS labour induction Chorioamnionitis Composite neonatal outcome |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: not stated Ethical approval: not stated Email requesting further information sent 11 April 2017 Reply 26 April 2017 "The women in the membrane sweep group that were not swept were mostly because they had a closed cervix (they were randomized before a cervix exam was done) The women that were in the no sweep group that were swept usually had their membrane swept because of provider or patient preference." Further information requested. Reply received 8 September 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomized using random number generation and block randomization” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not blinded. Personnel: not blinded. “No blinding was attempted” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “No blinding was attempted” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Abstract of conference proceeding. “7 women withdrew from the study or were lost to follow‐up” (4/61 women from the intervention group, 3/62 women from the control group) < 20%. |
| Selective reporting (reporting bias) | Unclear risk | Abstract of conference proceeding. Full trial not available per author. 3 (4.9%) women in the control group received 1 membrane sweep (table). 19 (31.7%) of women in membrane sweep group received no sweep. |
| Other bias | Low risk | Abstract of conference proceeding. Full trial not available per author. However, no other bias noted. |
Kashanian 2006.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Akbarabadi Teaching Hospital in Tehran, Iran Duration of study: not reported Participants randomised: N = 122 Inclusion criteria: “gestational age of 39 weeks (with dates determined on the basis of the last menstrual periods and ultrasound performed during the 1st trimester), singleton gestation, vertex presentations, and intact membranes”.” (Page 42) Exclusion criteria: “clinically significant vaginal bleeding, placenta previa, severe cervicitis, evidence of spontaneous labor (more than three painful contractions in 10 min), a known contraindication to labor induction (e.g., prior vertical uterine incision, acute fetal compromise, active herpes), systemic disorder, decreased fetal movements, any sign of fetal distress and any high‐risk pregnancy, or inability to give informed consent.” (Page 42) Parity: mixed. Both nulliparous and multiparous women included (Page 42). Bishop score: baseline Bishop score mean +/‐SD recorded |
|
| Interventions |
Membrane stripping (N = 50): “Sweeping was performed by one of the investigators. Sweeping was performed based on a standard method. As much of the membranes as possible was separated from the lower segment. If the cervix did not allow a finger, it was massaged for 2 min to stimulate prostaglandin release. The women were observed for a few hours after the procedure and were discharged, if they were well. The patients were instructed to admit to the labor ward, if they had leaking, labor pain, or excessive vaginal bleeding” (Page 42). Control group (N = 51): “only vaginal examination for determining Bishop score. Vaginal examination was performed by the same investigator for both groups. “ “Women were admitted to the labor ward whenever they had labor pain. In others, pregnancies were followed till 41 weeks, in case of lack of labor pain, induction was started to terminate labor.” (Page 42). |
|
| Outcomes | Puerperal fever Caesarean section |
|
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: “written informed consent” Ethical approval: “approval from the Hospital Ethics Committee” Unable to contact author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “four parts, block random using sealed, sequentially distributed envelopes to which the letters A, B, C, and D had been allocated”, page 42. |
| Allocation concealment (selection bias) | Low risk | “sealed, sequentially distributed envelopes to which the letters A, B, C, and D had been allocated: the letters A and C to the sweeping group and the letters B and D to the control group; the patients chose the envelopes which were opened by the investigator, and according to the letters, the group of patients was determined”, Page 42. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: unclear if participants blinded once allocated to groups. “the patients choose the envelopes, which were opened by the investigator” Page 42 Personnel: not blinded. "the patients chose the envelopes which were opened by the investigator, and according to the letters, the group of patients was determined”, Page 42. “Sweeping was performed by one of the investigators, and vaginal examination also was performed by the same investigator for the control group.” “Follow‐up of the patients was performed by another investigator who was blinded to the groups of patients; therefore, at this stage, neither the investigator nor the patients knew which was the study group.” Page 42. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Follow‐up of the patients was performed by another investigator who was blinded to the groups of patients; therefore, at this stage, neither the investigator nor the patients knew which was the study group.” page 42. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Twenty‐one women who did not give birth in our hospital were excluded from the study”, < 20%. N = 122 Intervention group = 50 (60‐10) Control group = 51 (62‐11) Page 42. |
| Selective reporting (reporting bias) | Unclear risk | “Data regarding premature rupture of membranes, abnormal bleeding during hospitalization, Bishop score, timing of delivery, mode of delivery, and birth weight were collected.” For mode of delivery only data given for caesarean section |
| Other bias | Low risk | No evidence of other bias |
Magann 1998a.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Obstetric clinics at the Naval Medical Center in San Diego, California, and the University of Mississippi Medical Center in Jackson Mississippi,USA (page 891). Duration of study: not stated Participants randomised: N = 65 (79 women met the Bishop score inclusion criteria. 14 of these women were excluded for a positive fetal fibronectin test result). Inclusion criteria: “uncomplicated singleton pregnancies and were candidates for a vaginal delivery at 39 weeks’ gestation”. All women who had “Vertex presentation, no placenta previa, or other contraindications to a vaginal delivery” were invited to participate. Gestational age was determined on the basis of the patients last menstrual period, initial examination, first auscultation of fetal heart tones with an ultrasound stethoscope (Medason, Newark, Calif), ultrasonography, or both performed before 20 weeks’ gestation. Negative fetal fibronectin test result and a Bishop score ≤ 4 (page 891). Exclusion criteria: women whose “estimated date of confinement was uncertain was not included in this study”. History of previous caesarean section (page 891). Parity: mixed. Both nulliparous and multiparous women included (Table 1, page 891). Bishop score: both baseline Bishop score and Bishop score at delivery (mean +/‐SD) recorded |
|
| Interventions |
Membrane stripping (n = 33): “Examination every 3 days with membrane sweeping and Bishop score determination”. “Membrane sweeping was performed by placing a finger through the cervix and performing 2 circumferential sweeps with the examining finger. If the cervix would not admit a finger, the examining finger was placed into the cervix every 3 days until the sweeping could be performed.” (page 891). Control group (n = 32): “Gentle vaginal examination only every 3 days with a Bishop score assigned.” “Examinations were continued every 3 days until spontaneous labor, rupture of the membranes, or the patient completed 41 weeks’ gestation at which time all remaining patients were admitted to labor and delivery for labor induction.” (page 891). . |
|
| Outcomes | Spontaneous labour Induction at 42 weeks Augmentation of labour Mode of birth Vaginal delivery Caesarean section |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: yes: “After signing an informed consent form before the 39‐week pelvic examination” Ethical approval: “This study was approved by the Investigational Review Board at the Naval Medical Center in San Diego and the University of Mississippi Medical Center in Jackson, Mississippi.” page 891. Unable to source contact details for Dr Magann |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “These cards had been obtained from a random number table and placed the patients in one of two groups.” Page 891. |
| Allocation concealment (selection bias) | Unclear risk | “a card was drawn from a consecutive series of sealed opaque envelopes.” Page 891. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not blinded. Personnel: not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting bias. |
| Other bias | Low risk | Author has treated induction of labour and augmentation in labour as mutually exclusive events, e.g. if a woman has a pharmacological induction of labour with further interventions to augment contractions this still included in the data for induction of labour. Control group n = 32, 18 women had IOL at 42 weeks. A further 7/14 women had augmentation. |
Magann 1998b.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: Naval Medical Center, San Diego, California, USA (Page 1279). Duration of study: 6 months (March 1996 to September 1996) (Page 1279). Participants randomised: n = 105 Inclusion criteria: no contraindication to a vaginal delivery. Bishop score ≤ 4. Uncomplicated pregnancy. ≥ 41 weeks' gestation. Informed consent signed (Page 1279). Exclusion criteria: contraindication to a pelvic examination, i.e. placenta praevia, rupture of membranes (Page 1279). Parity: mixed. Both nulliparous and multiparous women included (Page 1280, Table II). Bishop score: Bishop score at entry (mean +/‐SD) recorded |
|
| Interventions |
Membrane sweeping group: n = 35 “daily membrane stripping performed” (Page 1280). Prostaglandin group: n = 35 “0.5mg of prostaglandin E2 (PGE2) gel placed into the cervix on a daily basis as an outpatient.” (Page 1280). Control group: n = 35 “gentle daily cervical examination” “All patients were examined to determine Bishop scoring by one of the two examiners who were blinded to group assessment.” “If the Bishop score totaled ≥8 or the patient reached the forty second week of pregnancy the patient was admitted for induction of labour.” All patients received a modified biophysical profile (NST and amniotic fluid index) every 3 days except for those women in the prostaglandin group who had daily biophysical profiling after the insertion of the intracervical prostaglandin (Page 1280). |
|
| Outcomes | Spontaneous onset of labour Formal induction of labour Induction at 42 weeks Spontaneous vaginal delivery Caesarean section delivery Forceps delivery Apgar < 7 at 5 minutes Cost analysis |
|
| Notes |
Funding: “Departments of Obstetrics and Gynecology, Naval Medical Center and University of Mississippi Medical Center. Supported in part by the Vicksburg Hospital Medical Foundation.” page 1279. Trial authors’ declaration of interest: none declared Informed consent obtained: yes Ethical approval: “study was approved by the Institutional Review Board” page 1280. Unable to source contact details for Dr Magann |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “patients were randomly assigned to one of the groups by drawing the next in a series of opaque sealed envelopes that had been generated from a random number table”, page 1280. |
| Allocation concealment (selection bias) | Unclear risk | "by drawing next in series of opaque sealed envelopes” page 1280. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported Personnel: “All patients were examined to determine Bishop scoring by one of the two examiners who were blinded to group assessment.” Further blinding of personnel not discussed, page 1280. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | Low risk | No evidence of other bias. |
Magann 1999.
| Methods | Randomised control trial. | |
| Participants |
Setting: antenatal diagnostic unit, USA. Duration of study: 18 months (January 1995 until June of 1996) (Page 88). Participants randomised: N = 182. Inclusion criteria: > 41 weeks, “a singleton pregnancy, vertex presentation, intact membranes, reassuring antenatal assessment, no contraindication to a vaginal delivery, and a Bishop score of ≤ 4.” (Page 88). Exclusion criteria:“patients whose gestational age was uncertain” and “women not desiring to participate.” (Page 89). Parity: mixed. Both nulliparous and multiparous women included (% presented in Table 2 of manuscript page 89). Bishop score: Bishop score at trial entry and admission to labour ward (mean +/‐SD) recorded. |
|
| Interventions |
Membrane sweeping (n = 91): “daily membrane sweeping.” “The technique for membrane sweeping involved the separation of the membranes from the lower uterine segment with two circumferential sweeps of the examining finger. If the cervix did not permit entrance of the examining finger, the cervix was stretched by the examining finger daily until membrane stripping could be accomplished.” (Page 89). Dinoprostone group (n = 91): “daily placement of a dinoprostone(prostaglandin E2) vaginal suppository (Cervidil).”(releasing 0.3 mg/hour over 12 hours).“Women in the dinoprostone group had daily nonstress tests and amniotic fluid evaluation following placement of the prostaglandin. Patients were discharged from the hospital after a reassuring assessment and if any contractions were present after the contractions had begun to decrease in intensity and frequency. All patients were instructed to return to labor and delivery for regular contractions, rupture of membranes, fever, or decreased fetal movement.” (page 89). “All patients were examined by one of two examiners, blinded to group assignment to determine the daily Bishop score. Following the examination, the membranes were either stripped or the vaginal suppository was placed. Patients were examined on a daily basis until spontaneous labor, rupture of membranes, a Bishop score of $8 occurred (at which time patients were admitted for labor induction), or 42 weeks was attained, at which time all remaining patients were admitted for labor induction.” (Page 89). |
|
| Outcomes | Labour Induction at 42 weeks Postpartum endometritis Cost Mode of birth Spontaneous vaginal Caesarean section Forceps Neonatal outcome Apgar score < 7 at 5 minutes NBICU admission |
|
| Notes |
Funding: “Supported in part by the Vicksburg Hospital Medical Foundation” Trial authors’ declaration of interest: none declared Informed consent obtained: “all participants signed an informed consent before entrance into the study” page 89. Ethical approval: yes,“This study was approved by the Institutional Review Board.” page 89. Unable to source contact details for Dr Magann |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomly assigned to one of two groups by drawing a card, generated from a table of random numbers”, page 89. |
| Allocation concealment (selection bias) | Unclear risk | “sealed in an opaque envelope”, page 89. Not stated if numbered or sequential. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: blinding of patients not discussed. Personnel: “All patients were examined by one of two examiners, blinded to group assignment to determine the daily Bishop score” (Page 89). Blinding of clinicians post initial assessment not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of clinicians post initial assessment not discussed. Not stated if person collecting the data was blinded to the interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | Low risk | No evidence of other bias. |
McColgin 1990a.
| Methods | A prospective randomised controlled trial. | |
| Participants |
Setting: USA Duration of study: not stated. Participants randomised: N = 103. Inclusion criteria: women at term (38 to 42 weeks' gestation) with gestational age ascertained by menstrual dates, early examination, and sonography before 20 weeks. Women with closed cervix were included (Page 811). Exclusion criteria: uncertain dates, abnormal fetal presentations, known medical complications of pregnancy, low lying placenta, placenta praevia, scheduled repeat caesarean section, or no desire to participate (Page 811). Parity: mixed. Both nulliparous and multiparous women included. No further data given. Bishop Score: unfavourable Bishop score (≤ 5) recorded. |
|
| Interventions |
Membrane stripping (n = 51): weekly stripping of the membranes “digital separation from the lower uterine segment with 1 or 2 circumferential passes. Normally 1‐2cm of the membranes was separated from the lower uterine segment.” “In patients with long closed cervices”… “the cervix was digitally “stretched” until membrane stripping could be accomplished” (Page 811). Control group (n = 48): “weekly pelvic examination without membrane stripping” to assess cervix for Bishop scoring. All patients were examined every week in the same manner until admitted to labour/delivery ward or advanced beyond 42 weeks completed gestation. Two of the authors (SWM and JCU) performed almost all the membrane stripping and assignment of Bishops score (> 98%) (Page 811). |
|
| Outcomes | Caesarean section Forceps of vacuum Spontaneous vaginal delivery Chorioamnionitis Augmentation Oxytocin post SROM (induction of labour) Delivery within 1 week |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: Department of Obstetrics and Gynaecology, United States Airforce Hospital, Tyndall Air Force base, Florida, USA. Department of Obstetrics and Gynaecology, University of Mississippi Medical Center, Jackson, Mississippi, USA (page 811). Informed consent obtained: yes “and obtaining informed consent” (page 811) Ethical approval: not stated. Unable to contact Dr McColgin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “we prospectively assigned patients at term (38‐42 weeks’ gestation)”, page 811. Unable to contact authors. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Method of randomisation not described Not stated if sealed, opaque envelopes used/or other method of allocation concealment. Unable to contact author. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Paticipants: not reported. Unable to contact author. Personnel: not blinded. “two authors (SWM and JCM performed almost all the membrane striping and assignment of Bishop’s score (> 98%).”, page 812. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported if person collecting the data was blinded to the interventions. Unable to contact author. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. 4 exclusions (2 patients in non‐stripped arm received stripping, 1 with pre‐eclampsia and 1 with breech presentation). N = 99 (103‐4) < 20%. |
| Selective reporting (reporting bias) | High risk | Data for age, parity, Bishop scores and gestational age were recorded but are not reported in study, page 812. Maternal and neonatal complications stated as trial outcomes but not reported in data. |
| Other bias | Low risk | No evidence of other bias |
McColgin 1990b.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: University of Mississippi Medical Center, Jackson, Mississippi, USA. Duration of study: enrolment = March 1998 to June 1999 (Page 679). Participants randomised: N = 209. Inclusion criteria: 38 weeks' gestation. “Gestational age was ascertained by uterine size and by ultrasound before 20 weeks' gestation with no size dates discrepancy.” (Page 678). Exclusion criteria: uncertain gestational dating criteria, nonvertex presentation, a known medical complication of pregnancy, vaginal or cervical infection. Placenta praevia, low lying placenta (Page 678). Exclusions after randomisation (29 women). Past history of caesarean section (17) in both groups. In the stripping group, 5 women were excluded for various reasons (abnormal presentation (2), dates unclear (1), pain (1), breast cancer (1)). In the control group, 7 women were excluded for various reasons (labour induction for maternal fetal indications (3), non vertex (1), dates (1), inadvertent stripping (1), renal disease (1)) (Page 679). Parity: mixed. Both nulliparous and multiparous women included (Table 1 page 679). Bishop score: initial Bishop score recorded (Mean ± SEM). Weekly Bishop scores collected in study but data not provided. |
|
| Interventions |
Membrane stripping (n = 90): “Stripping of the membranes was accomplished by digital separation of 2‐3cm of the membranes from the lower uterine segment using 2 circumferential passes of the examining finger. In patients with long and closed cervices, the cervix was “stretched” digitally until membrane stripping could be accomplished.” (Page 678). Control group (n = 90): “Pelvic examination was performed by atraumatic assessment of the cervix for Bishop scoring” (Page 678). Bishop score was recorded for all patients (Page 678). All patients were examined every week in the same manner until delivery/scheduled induction or advanced beyond 42 weeks completed gestation (≥ 294 days). |
|
| Outcomes | Maternal Infection Fetal death (double nuchal cord) Mode of delivery: data not reported |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: “Informed consent was obtained” Ethical approval: not stated. Unable to contact Dr McColgin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “assigned by computer randomisation”, page 678. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not stated. Personnel: “two authors (S.W.M. and J.C.M. performed almost all the membrane striping and assignment of Bishop’s score (>98%).” No further information on blinding of personnel given, page 679. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if person collecting the data was blinded to the interventions, therefore, insufficient information to inform judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 209 women initially recruited, 29 were excluded in total (< 20%). Although VBAC (vaginal birth after caesarean section) or history of a caesarean section were not listed in the exclusion criteria, 17 women with a history of caesarean section wanting a VBAC were excluded “when it became apparent that caesarean deliveries and post term pregnancies were unfairly biased against the control group in this select population” page 679 |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias |
| Other bias | Low risk | No evidence of other bias. |
McColgin 1993.
| Methods | Randomised controlled trial | |
| Participants |
Setting: University of Mississippi Medical Center, Jackson, Mississippi, USA. Duration of study: 6 months (Page 72). Participants randomised: N = 30. Inclusion criteria: > 38 weeks' gestation (gestational age was ascertained from known last menstrual period, early assessment by ultrasonography before 20 weeks' gestation, and no size‐dates discrepancy.) (Page 72). Exclusion criteria: uncertain gestational dating criteria, known medical complications of pregnancy, findings of cervical or vaginal infection, low‐lying placenta (or placenta previa), or non‐vertex presentation (Page 72). Parity: mixed. |
|
| Interventions |
Three arms Membrane sweep (n = 10) Control with Bishop evaluation (n = 10) Control without cervical evaluation (n = 10) |
|
| Outcomes | No clinical outcomes reported | |
| Notes | Study reported on uterine contractile activity; change in phospholipase A2 activity and prostaglandin F2α | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Thirty patients were randomly divided” “by means of a computer generated list of envelopes” page 72 |
| Allocation concealment (selection bias) | Unclear risk | Sequentially assigned “list of envelopes” page 72 not reported if opaque or numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: while not reported, highly likely that it is not possible to blind. Personnel: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | No protocol. Outcomes stated in methods reported in results |
| Other bias | Low risk | No evidence of other bias |
Netta 2002.
| Methods | Randomised prospective controlled trial | |
| Participants |
Setting: New York, USA Duration of study: not reported Participants randomised: N = 98 Inclusion criteria: “36 weeks gestation with uncomplicated pregnancy” Ultrasound confirmation of gestational age (Page S221). Exclusion criteria: with“no evidence of placenta previa” (Page S221). Parity: mixed. Both nulliparous and multiparous women included (Page S221). Bishop score: not stated |
|
| Interventions |
Membrane stripping (n = 44): “weekly CMS beginning at 38 weeks” (cervical membrane stripping)(Page S221). Control group (n = 54): “cervical exams deferred until labour” (Page S221). “All patients underwent vaginal‐rectal cultures for GBS at the time of recruitment” (Page S221) |
|
| Outcomes | Nulliparous induction Neonatal infections |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: not stated Ethical approval: not stated Email sent requesting further information 8 August 2017. Resent 18 August 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported “a randomised prospective study was performed”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: clinicians not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 98 women "Completed the protocol", 44 = membrane stripping 54 = control group. Attrition not reported. Authors only reported data on the primiparous women, so the denominators are 20 and 27, respectively. Data not provided for 51 of 98 women = 52%. Author contacted no reply to date. |
| Selective reporting (reporting bias) | High risk | Data collected for gestational age at delivery, mode of delivery, PROM, labour induction, maternal carriage rate of GBS and neonatal outcomes. Overall rates of gestational age at delivery, mode of delivery and PROM not provided. IOL rates only reported for nulliparous women. |
| Other bias | Low risk | Conference abstract only. No protocol available. Author contacted. No reply to date. However, no other bias noted. |
Parlakgumus 2014.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Baskent University, Adana, Turkey Duration of study: February 2011 to March 2011. Participants randomised: N = 165. Inclusion criteria: “Low risk women at 38+0 ‐ 39+0 weeks of gestation.” “Gestational age was confirmed with dating ultrasound” (Page 683). Exclusion criteria: “History of uterine surgery including caesarean section, presentations other than cephalic, multiple pregnancy and contraindications to membrane sweeping which included placenta praevia, placental abruption, rupture of the membranes, active bleeding and labour.” (Page 683). Parity: mixed. Both nulliparous and multiparous women were included (Table 1 page 685). Bishop score: Bishop score < 5 recorded |
|
| Interventions |
Membrane stripping (N = 69) "Swept the membranes in the sweeping group, by separating the lower membranes as much as possible from their cervical attachment, with a 360 degree pass of the examining fingers" (Page 684). Control group (N = 71) “Cervical length was measured (cervix1) in both groups by examiner 1 and the Bishop Score was determined in the control group and sweeping was performed in the sweeping group by examiner 2. Two days later the patients had another cervical length measurement (cervix 2) by examiner 1, blinded to the group and results of the examiner 2” (Page 684). |
|
| Outcomes | Spontaneous vaginal delivery Caesarean section Induction of labour |
|
| Notes |
Funding: Baskent University Foundation Huriye Ayse Parlakgumus Trial authors’ declaration of interest: not stated Informed consent obtained: yes “written informed consent” (Page 683). Ethical approval: yes “The study protocol was approved by the local ethics committee” "Helsinki declaration" (page 683). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “sealed envelopes which included treatment allocations were prepared”, page 683. |
| Allocation concealment (selection bias) | Unclear risk | “sealed envelopes which included treatment allocations were prepared” “women in both groups selected an envelope”, page 683. Study does not state if envelopes were opaque or sequential. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants:“the patients were also blinded to the group they were allocated to. However because of discomfort women felt during sweeping, total blinding was not possible”, page 684. Personnel: incomplete blinding. “Examiner 1 …assessed the bishop score in the control group and swept the membranes in the sweeping group”, page 684. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Examiner 1: cervical length was measured (cervix1) in both groups by examiner 1 before women opened the envelopes that gave allocation. “examiner 1, blinded to the groups which the patients were allocated to”, page 681. Examiner 2: “opened the envelopes, assessed the Bishop score in the control group and swept the membranes in the sweeping group”, page 682. Examiner 1: 2 days later the patients had another cervical length measurement (cervix 2) by” examiner 1 blinded to the groups which the patients were allocated to”, page 682 “Data on delivery were retrieved from patient files and in cases of missing data, the women were contacted by the phone and other hospital records were searched”, page 685. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting Authors reported they ”may have performed the second cervical scan too early.”…”if measured at “later time, we could have found more significant results”, page 687. |
| Other bias | Low risk | No evidence of other bias |
Putnam 2011.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Obstetrics/gynecology clinic, Naval Medical Center, USA. Duration of study: January 2005 to June 2008. Participants randomised: N = 389 Inclusion criteria: “Women at 39 weeks ± 2 days gestation with an unfavorable cervix, a singleton pregnancy, ≥18 years of age, reliable pregnancy dating that included a first trimester ultrasound, ultrasound confirming that the placenta was clear of the cervix, and who had no contraindication to a vaginal delivery” (Page 288). Exclusion criteria: Bishop’s score was ≥ 4, contraindication to a vaginal delivery (Page 288). Parity: mixed. Both nulliparous and multiparous women were included (Table 1 page 290). Bishop score: Bishop score at recruitment (Table I, Page 290) and admission to labour ward (Table II, Page 291) recorded. |
|
| Interventions |
Control group (n = 117): group I “cervix examined weekly but did not have their membranes swept” (Page 288). Membrane stripping 1 x/week (n = 119): Group II: “weekly membrane sweeping” (Page 288). Membrane stripping 2 x/week (n = 119): Group III: “twice‐weekly membrane sweeping.” (Page 288). “The technique of membrane sweeping was defined as separating the fetal membranes from the lower uterine segment with two circumferential sweeps by the examining finger. If the cervix did not permit entrance of the finger on examination, the finger was placed into the cervix and two circumferential sweeps were done. This was done serially depending on the frequency of the group assignment until entrance of the examining finger could be accomplished. Women in the control group had their cervix examined and the Bishops’ score recorded every 7 days. Group I women had their membranes swept every 7 days and Group II women had their membranes swept every 3–4 days. Membrane sweeping was continued according to the assigned frequency until 41 weeks of gestation. At 41 weeks, all remaining women were admitted to the hospital for labor induction.” (Page 288). |
|
| Outcomes | Induction of labour Vaginal delivery Caesarean delivery Chorioamnionitis Instrumental vaginal delivery Apgar score < 7 at 5 minutes |
|
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: not stated. Ethical approval: yes,“study was approved by the Chief of Navy Bureau of Medicine and Surgery, Washington, DC, through the local Clinical Investigation Program (International Review Board)” (Page 288). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The method of randomization and group assignment was determined by drawing a card from a sealed opaque envelope”, page 288. |
| Allocation concealment (selection bias) | Unclear risk | “The method of randomization and group assignment was determined by drawing a card from a sealed opaque envelope that would assign the participants to Group I (control), Group II (once‐weekly sweeping), or Group III (twice‐weekly sweeping). The cards were prepared in blocks of 30 envelopes”, page 288. Not reported if envelopes were sequential or numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: partially blinded. “this study could not be blinded to the membrane sweeping investigator but was blinded to all other providers and to the investigator collecting data on each participant”, page 288. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “this study could not be blinded to the membrane sweeping investigator but was blinded to all other providers and to the investigator collecting data on each participant”, page 288 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
Ramya 2015.
| Methods | Randomised controlled trial | |
| Participants |
Setting:“antenatal outpatient department of Mahatma Gandhi Medical College and Research Institute”, India (Page 1). Duration of study: January 2011 to June 2012 Participants randomised: N = 150 Inclusion criteria: “women with one previous caesarean section with non‐recurrent indications, singleton pregnancy and cephalic presentation, gestational age of 39 weeks, intact membrane and candidates willing for VBAC.” (Page 1). Exclusion criteria: “multiple gestations, malpresentations, placenta praevia, abruptioplacentae, suspected cephalo‐pelvic disproportion, gestational diabetes, chronic or gestational hypertension, pre eclampsia, gestational age less than 39 weeks, H/O premature ruptures of membranes, vasa praevia, congenital anomalies, any previous abortions, More than one transverse lower segment caesarean scar, Previous classical caesarean scar, any other uterine surgeries related to gynaecology.” (Page 1). Parity: multiparous women were included with history of a previous caesarean section (Table 1 page 2). Bishop score: "pre swiping Bishop score recorded" (Table 1 page 2). |
|
| Interventions |
Membrane stripping (N = 75): “During vaginal examination, if cervix admitted one finger, the foetal membranes were separated from the cervix and the lower uterine segment as far as possible by sweeping a finger through 360 degrees. When the cervix was closed attempts to stretch the cervix open or cervical massage was performed. Sweeping was done at 39 and 40 weeks.” (Page 1). Control group (N = 75): “gentle vaginal examination was done once at 39 weeks for Bishop scoring and no further examination was done till the onset of labour (Page 2). All the cases were monitored by daily Non Stress Test, amniotic fluid index was measured once in every three days till onset of labour or 41 weeks. Any condition requiring immediate delivery was excluded from the study and was managed according to the institutional protocol (Page 2). |
|
| Outcomes | Spontaneous onset of labour Vaginal birth after caesarean section Caesarean section Oxytocin augmentation Instrumental vaginal delivery |
|
| Notes | 23/75 in control group and 21/75 in Membrane sweeping group had caesarean section on maternal request. Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: yes, “informed written consent”. Ethical approval: yes,“Ethical committee clearance”. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation not reported “were randomly assigned” page 1 (abstract). |
| Allocation concealment (selection bias) | Low risk | “reassigned into two groups by the sequential opening of numbered sealed opaque envelopes indicating a “sweep” or “No Sweep”, page 1 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. Unlikely that clinicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting noted. |
| Other bias | Low risk | No evidence of other bias. |
Saichandran 2015.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Hospital setting, India. Duration of study: not reported. Participants randomised: N = 100 Inclusion criteria: “a) uncomplicated singleton pregnancies with cephalic presentation and intact membranes, b) candidates for vaginal delivery, c) gestational age 40 + 0 weeks and d) primigravida/primipara.” (Page 1883). Exclusion criteria: “scarred uterus or speculum findings suggestive of vaginal infection” (Page 1883). Parity: mixed. Both nulliparous and multiparous women were included (Table I, Page 1883). Bishop Score: < 5, > 5 recorded. Data given in hours from last sweep to spontaneous labour and delivery (Table 4, Page 1884) |
|
| Interventions |
Membrane stripping (n = 48): “In the study group vaginal examination was performed for pelvic assessment and Bishop Score. During examination if the cervix is admitting a finger the fetal membranes are separated from the cervix and lower uterine segment as far as possible by sweeping a finger through 360 degrees. When the cervix is closed, attempts to stretch the cervix open or cervical massage was performed. Similar procedure was repeated every 48 hours till 41 ± 0 weeks (i.e. 40 ± 0, 40±3, and 40 ± 5) or until labor commenced.” (Page 1883). Control group (n = 50): “no pelvic examination was performed till the onset of labour or time of induction i.e. 41 ± 0 weeks. This is to avoid stimulation with cervical examination which can also raise the prostaglandin concentration causing ripening of the cervix.” Both the groups were monitored by NST (daily) and AFI (once in every 3 days). Any conditions warranting immediate delivery were excluded from the study and were managed according to the institute protocol (Page 1883). |
|
| Outcomes | Spontaneous onset of labour Induction of labour Vaginal delivery LSCS Augmentation Perinatal death |
|
| Notes | “Out of the fifty in the study group, 2 were excluded due to requirement of immediate induction of labor after the first sweeping were excluded from the final analysis” (Page 1883). This data were included in over all study number and induction of labour outcome. Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: yes, “informed consent was obtained” (Page 1883). Ethical approval: yes,“The ethical committee of our medical college approved the study” (Page 1883). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, “The participants of the study were allocated randomly by”, page 1883. |
| Allocation concealment (selection bias) | Unclear risk | “The participants of the study were allocated randomly by the use of sealed opaque envelops for study and control groups.”, page 1883. No comment regarding sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N = 100. Intervention group = 48 (50‐2) Control = 50. “Two among the study group, who required immediate induction of labor after the first sweeping were excluded from the final analysis”, Page 1883. |
| Selective reporting (reporting bias) | High risk | Primary outcome measure of “ ….. any maternal or fetal complication” not reported, page 1883. All other outcomes appear to have been reported. |
| Other bias | Low risk | No evidence of other bias. |
Salamalekis 2000.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: University of Athens “Areteion” hospital, Athens, Greece. Duration of study: not reported. Participants randomised: N = 104 Inclusion criteria: nulliparous, gestational age between 40 ‐41 weeks (281 to 287 days), singleton pregnancy and cephalic presentation. Bishop score ≤ 5. Uneventful pregnancy with gestational age determined clinically and by ultrasound during their 1st trimester (Page 241). Exclusion criteria: no maternal complications (hypertension, diabetes) or the fetus (congenital anomalies, growth retardation) (Page 241). Parity: primiparous women only included. Bishop score: initial Bishop score (Table I, Page 241) and Bishop score on admission to labour ward (Table II, page 242) recorded. |
|
| Interventions |
Membrane stripping (N = 34): “Sweeping of the membrane with a bishop score ≤ 5. During the procedure the examiners fingers were inserted as far as possible through the internal os, separating the membranes from the lower uterine segment and rotating 360◦.” (Page 241). Oxytocin uterine stimulation (n = 35): “Uterine stimulation with very low doses of Oxytocin for 6 hours. A diluted oxytocin infusion of 10 IU per 1000 mL of Ringers lactate solution was prepared and I.V. infusing was initiated with 0.5mU/min which was doubled hourly, reaching a maximum of 4mU/min. All these patients had continuous cardiotocographic monitoring throughout the 6 hour infusing period.” (Page 241). Control group (N = 35): “Gentle vaginal examination.” (Page 241). All patients were “followed up for 4 days after the vaginal examination or sweeping of the membranes and were filed in a fetal movement chart.”. “When signs of labour were noted they were transferred to the labour ward” (Page 241). |
|
| Outcomes | Spontaneous onset of labour Chorioamnionitis Caesarean section Induction of labour |
|
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: not stated Ethical approval: not stated Email sent 28/08/17, 2 November 2017 requesting further information. No reply to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Type of randomisation not reported. ”our randomly selected study” page 241 |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. It was not possible to blind the clinician who gave the intervention. It is unclear if the same clinician was there at the birth or made the decisions that might affect outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make informed decision. Trial protocol not available. |
| Other bias | Low risk | No evidence of other bias. |
Salmanian 2012.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Islamic Republic of Iran. Duration of study: not reported. Participants randomised: N = 60 Inclusion criteria: “pregnant women (gestational age >40w), primigravida and gravida 2” other inclusion criteria not reported (Page S811). Exclusion criteria: not reported. Parity: mixed. Both nulliparous and multiparous women included (primigravida and gravida 2), however no data provided (Page S811). Bishop Score: mean of Bishop score change recorded only. Baseline and final Bishop scores not recorded (Page S811). |
|
| Interventions |
Group A (N = not reported): membrane stripping Group B (N = not reported): PGE2 |
|
| Outcomes | Data supports subgroup analysis only | |
| Notes |
Funding: none declared Trial authors’ declaration of interest: none declared Informed consent obtained: not stated Ethical approval: not stated Email sent 5 June 2017 requesting further data. Email sent 28 September 2017 requesting further details. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not blinded. Personnel: unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Conference abstract only. |
| Other bias | Low risk | No protocol available, conference abstract only. However, no other bias noted. |
Tannirandorn 1999.
| Methods | Randomised controlled trial. | |
| Participants |
Duration of study: November 1994 to March 1995 (patients were enrolled). Setting: Antenatal clinic, Department of Obstetrics and Gynaecology, King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Participants randomised: N = 96. Inclusion criteria: gestation between 39 and 40 weeks verified by known last normal menstrual period, early confirmation through size and ultrasound prior to 20 weeks' gestation and no size/date discrepancy during antenatal visits (Page 230). Exclusion criteria: uncertain dates, abnormal fetal presentations, unengaged fetal head, known medical complications of pregnancy, placenta praevia known lower genital tract infections, history of a previous caesarean section or no desire to participate in the study (Page 230). Parity: mixed. Both nulliparous and multiparous women included (Page 230). |
|
| Interventions |
Membrane stripping (n = 41): in the membrane stripping group: “Stripping of the membranes was done by digital separation of 2‐3cm of the membranes from the lower uterine segment using two circumferential passes of the examining finger under aseptic technique. In those patients with long closed cervices randomised to the stripping group the cervix was stretched digitally until membrane stripping could be accepted” This intervention was performed weekly along with a gentle pelvic examination for Bishop scoring (Page 230). Control group (n = 39): in the control group: a weekly “gentle pelvic examination for Bishop scoring was given.” “The authors performed all membrane stripping and assignment of Bishop scores after standardisation of the technique.” If gestational age reached > 42 completed weeks (> 294 days) without spontaneous onset of labour, the patients were admitted into the hospital for fetal monitoring and induction was performed with either Prostin E2 vaginal tablet or IV oxytocin (Page 230). |
|
| Outcomes | Spontaneous vaginal delivery Caesarean section Forceps delivery Puerperal morbidity PPH Chorioamnionitis |
|
| Notes |
Funding: none declared. Trial authors’ declaration of interest: none declared. Informed consent obtained: yes "obtaining informed consent" (Page 230). Ethical approval: yes "the protocol was approved by the ethical committee of the faculty of medicine Chulalongkorn Hospital" (Page 230). Email sent 17 August 2017 and 28 August 2017 requesting further information. No reply to date |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “assigned to one of two groups according to a table of random numbers”, page 230. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: blinding of participants not reported. Personnel: “Only the authors performed all membrane stripping and assignment of Bishop scores after standardization of the technique.”, page 230. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N = 80 (96 were recruited, 16 were excluded. Of those excluded 7 had lower genital tract infections, 4 delivered at another hospital, 3 could not perform membrane sweeping and 2 did not participate in the study) < 20%. Page 230. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make informed decision. |
| Other bias | Low risk | No evidence of other bias. |
Ugwu 2014.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Nigeria Teaching Hospital (UNTH), Enugu, Nigeria Duration of study: February 2012 – November 2012 Participants randomised: N = 134 Inclusion criteria: “All uncomplicated singleton pregnancies at a gestational age of 40–41 weeks, without uterine contractions” (Page 30). Exclusion criteria: “unsure of date, pre‐conception irregular menstrual cycle, evidence of any contraindication to vaginal delivery, medical diseases in pregnancy, and term premature rupture of membranes.” (Page 30). Parity: mixed. Both nulliparous and multiparous women were included in this study. Bishop score: Pre‐recruitment Bishops score was recorded (Table I, Page 32). |
|
| Interventions |
Membrane stripping (n = 67): “membranes stripped under aseptic procedure in the antenatal clinic of the hospital without hospital admission. With the woman in dorsal position, initial cervical assessment for the Bishop score was carried out. Thereafter, the investigator’s examining finger was introduced into the cervical os. Then, the fetal membranes were digitally separated from the lower uterine segment by two circular movements of the introduced finger. Where the membranes could not be reached, digital stretching of the cervix was attempted, followed by membrane sweeping, when successful. In cases of failed digital cervical stretching or unfavorable cervix (low bishop score), cervical massaging in the vaginal fornices was performed for 10 s. Each participant in the membrane sweeping group was observed for 1 h in the clinic after the procedure. Prophylactic antibiotics were not administered after the stripping of membranes.” (Page 30). Control group (N = 67): “vaginal examination only to assess the initial Bishop score.” (Page 30). |
|
| Outcomes | Spontaneous vaginal delivery Assisted vaginal delivery Caesarean section Apgar < 7 at 5 minutes Chorioamnionitis Spontaneous labour within 72 hours of intervention Formal induction of labour |
|
| Notes |
Funding: none declared. Trial authors’ declaration of interest: none declared. Informed consent obtained: yes “written informed consent” (Page 30). Ethical approval: yes.“obtained from the Institutional Review Board of the UNTH, Enugu.” (Page 30). Author contacted 8 August 2017 to clarify trial data Further email sent 28 September 2017. Author reply as follows: (1.) Question: Can you please clarify why there were 2 sets of random numbers (1 to 134) and how these were used to conceal allocation? Ans: First, by 2 sets of random numbers we meant...a set of 67 random numbers for intervention group (labelled A) and another set of 67 random numbers for control group (labelled B), making a total of 134. Each envelop containing a 5 x 5 cm white paper labelled either ‘‘A’’ for intervention group or ‘‘B’’ for control group, was opaque and sealed. They were kept by a third party (neither the researchers nor the patients) who did not know about the research objectives. (2.) Question: The data for the following outcomes are reported for only the women who did not go post‐term (> 41+3). Is it possible for you to provide the outcome data on all women so it may be included in our review? Spontaneous vaginal birth Caesarean section Instrumental vaginal delivery Augmentation of labour Apgar score less than 7 at 5 minutes Answer: "Unfortunately our study was not designed to include intention to treat analysis. So, we limited our data collection and analysis to women who delivered before "post‐term" (41+3)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
“…computer‐based random sequence generator…” also “…two sets of random numbers (1 to 134) corresponding to the intervention and control groups…” Email sent to author to clarify: Question: Can you please clarify why there were 2 sets of random numbers (1 to 134) and how these were used to conceal allocation? Answer 17 August 2017: "First, by 2 sets of random numbers we meant...a set of 67 random numbers for intervention group (labelled A) and another set of 67 random numbers for control group (labelled B), making a total of 134." |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were labelled sequentially from 1 to 134 by the statistician; each numbered envelope contained a 5 9 5 cm white paper labelled either ‘‘A’’ for intervention group or ‘‘B’’ for control group, corresponding to appropriate number set described above. The envelopes were kept by a medical intern (third party), blinded to the study’s objectives. Furthermore, serial numbers 1–134 were consecutively assigned to each recruited woman following an informed consent. Page 30. Email sent to author to clarify. Answer 17 August 2017: ”Each envelop containing a 5 x 5 cm white paper labelled either ‘‘A’’ for intervention group or ‘‘B’’ for control group, was opaque and sealed. They were kept by a third party (neither the researchers nor the patients) who did not know about the research objectives.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants: not reported Blinding of personnel: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Eleven participants delivered outside the study centre and were lost to follow‐up" < 20%. Author reports 17/08/2017: "Unfortunately our study was not designed to include intention to treat analysis". |
| Selective reporting (reporting bias) | High risk | Reported data did not include women (membranes sweeping n = 10, and control n = 24) whose pregnancies progressed to post‐term pregnancy. Author contacted for clarity: Reply 17/08/2017: "Unfortunately our study was not designed to include intention to treat analysis. So, we limited our data collection and analysis to women who delivered before "post‐term" (41+3)." |
| Other bias | Low risk | No evidence of other bias |
Weissberg 1977.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Jackson Memorial Hospital, Miami Florida, USA Duration of study: not reported Participants randomised: n = 91 Inclusion criteria: ≥ 37 weeks' gestation (Judged from the date of the last menstrual period and uterine size) (Page 125). Exclusion criteria: none stated Parity: mixed. Both nulliparous and multiparous women were included in this study. Bishop score: baseline Bishop score was recorded at randomisation (Table II, Page 126) |
|
| Interventions |
Membrane stripping (n = 46): “Digital separation of the membranes from the lower uterine segment as far as possible with the examining finger.” (Page 125). Control group (n = 45): “Finger inserted into the vagina to palpate the cervix for Bishop scoring without any stripping of the membranes away from the uterus” (Page 125). All women were examined by the same examiner and evaluated as to the length of gestation, estimated fetal size and status of the cervix utilising the Bishop scoring system.” The procedure was considered to have failed if they did not go into labour within 48 hours of their pelvic examinations (Page 125). |
|
| Outcomes | spontaneous labour within 48 hours | |
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: not stated Ethical approval: not stated Unable to locate contact details for author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “forty‐six randomly selected patients underwent digital stripping of membranes”, page 125. No further detail reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “all the hospital charts were reviewed after delivery and the clinical data were extracted and placed on punch cards and appropriately analysed with the aid of a computer”, Page 125. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Stated outcome postpartum morbidity not reported. No protocol available. Insufficient information to make informed decision. |
| Other bias | Low risk | No evidence of other bias. No protocol available |
Wiriyasirivaj 1996.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Antenatal clinic, Maharaj Nakorn Chiang Mai University Hospital, Thailand. Duration of study: 4 October 1994 to 4 November 1994. Participants randomised: N = 120 Inclusion criteria: 38 weeks' gestation with, “certain dates assessed by known last menstrual period, early assessment by uterine size, or examination by ultrasound before 28weeks' gestation. Vertex presentation, ability to attend follow‐up visits. Intention to deliver at the Maharaj Nakorn Chiang Mai University hospital.” Page 767 Exclusion criteria:“previous caesarean section, known medical, surgical or obstetric complications of pregnancy that would preclude vaginal delivery.” Size‐date discrepancy during antenatal visits. Placenta praevia or low lying placenta as assessed by ultrasound.” Page 767 Parity: mixed. Both nulliparous and multiparous women included. Bishop score: initial Bishop score recorded. |
|
| Interventions | “gentle pelvic examinations were done in both groups to assess the status of the cervix by Bishop scoring.”Page 767“only one obstetrician performed membrane stripping and Bishop scoring in all patients” Page 768 Membrane stripping (N = 61): “Membranes were stripped by digital separation from the lower uterine segment as far as possible, using a gloved examining finger”. “Unfavourable cervices were stretched digitally as much as possible, or until membrane stripping could be accommodated” Page 767 Control group (N = 59): “gentle pelvic examination for Bishop scoring” Page 768 “Gentle pelvic examinations for Bishop scoring was continued weekly in both groups, whereas the study group also had the membranes stripped weekly until the onset of labour. If gestational age reached 42 completed weeks without spontaneous onset of labour, formal induction was scheduled with either prostaglandin vaginal suppository or intravenous oxytocin drip.” Page 768 |
|
| Outcomes | Intrapartum fever Oxytocin Method of delivery Spontaneous Forceps Vacuum Caesarean Postpartum fever PPH Chorioamnionitis |
|
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: yes, “After giving informed consent, subjects were assigned to one of two groups” Ethical approval: yes, “The study was approved by the ethical committee of the Faculty of Medicine, Chiang Mai University” Email requesting further data sent 30 August 2017. Resent 20 September 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “subjects were assigned to one of two groups according to a table of random numbers. A simple randomization scheme was prepared by a research nurse before the trial began” Page 767 |
| Allocation concealment (selection bias) | Unclear risk | “the code for each patient was kept in a sealed, black opaque envelope”. Not reported if envelopes were sequential or numbered. Page 767 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported. Personnel: not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Protocol not available |
| Other bias | Low risk | No evidence of other bias. Protocol not available |
Wong 2002.
| Methods | Prospective randomised controlled trial | |
| Participants |
Setting: The Princess Margaret hospital, A regional obstetric unit in Hong Kong. Page 632 Duration of study: 18 months (1 July 1998 to 31 December 1999). Page 632 Participants randomised: N = 120 (133 eligible, 13 refused to participate) Inclusion criteria: “All pregnant women beyond 40 weeks of gestation, with dates determined by last menstrual periods and ultrasound performed before 26 weeks.” Page 632 Exclusion criteria: “Women with previous uterine scar, uncertain gestational age, women who refused to participate, or those who have other indications requiring early induction of labour were excluded” Page 632 Parity: mixed. Both nulliparous and multiparous women included.“Patients were stratified into two groups, namely, nulliparous and multiparous, before randomisation.”(Table 1 of manuscript page 634). However results not reported according to parity. Bishop score: not recorded |
|
| Interventions |
Membrane stripping: n = 60 “Sweeping was performed by four obstetricians using a standardised method” `”As much of the membranes as possible were separated from the lower segment. If the cervix would not admit a finger it was massaged for two minutes to encourage prostaglandin release” Page 633 Control group: n = 60 “ "Women allocated into the control group did not have any form of vaginal examination”(Page 633). One woman in the control group had sweeping of membranes instead of no intervention. |
|
| Outcomes | Spontaneous onset of labour: Induction of labour Epidural Caesarean section Forceps delivery Vacuum delivery Spontaneous vaginal delivery Serious neonatal infection Neonatal perinatal death. |
|
| Notes |
Funding: funded by the Hong Kong Society of Obstetricians and Gynaecologists (Page 635). Trial authors’ declaration of interest: none stated Informed consent obtained: not stated Ethical approval: yes, “study was approved by the Hospital Ethical Committee” (Page 632). Email sent 30/08/18 and 28 September 2017 requesting further information. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Six different blocks of 20 randomisation codes generated by computer” Page 633. |
| Allocation concealment (selection bias) | Unclear risk | Page 633 “..were placed in opaque sealed envelopes. Three separate blocks of randomisation codes were kept for the nulliparous and the other three blocks for multiparous pregnant women. Envelope was opened after a date for formal induction was given”. Not reported if envelopes were sequential or numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported Personnel: clinicians not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | “Sweeping was unintentionally performed in one woman randomised to the control group”, page 633. Although women were stratified by parity and subgroup analysis completed no results were reported according to parity. Author contacted for further data, no reply to date. |
| Other bias | Low risk | No evidence of other bias. |
Yaddehige 2015.
| Methods | Randomised controlled trial | |
| Participants |
Setting: hospital setting, Sri Lanka. Duration of study: not reported. Participants randomised: N = 160 Inclusion criteria: not discussed Exclusion criteria: not discussed Parity: mixed. Both nulliparous and multiparous women were included Bishop score: Bishop score measured at commencement of the study and at 48 hours post intervention. Only data for mean Bishop score post intervention recorded, page 5. |
|
| Interventions |
Group 1: cervical massage group. Page 5 Group 2: membrane sweeping group. Page 5 Group 3: control group (no intervention). Page 5 |
|
| Outcomes | No data reported for outcomes. Subgroup analysis only. | |
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: not stated Ethical approval: not stated Emailed Dr Yaddehige for further data 10 April 2017, 12 April 2017 and 6 June 2017. Email resent 20 September 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation described…“Participants were randomly assigned to “ Page 5 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported Personnel: not reported. Unlikely clinicians blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only. No data given on attrition provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make informed decision. No data on mean Bishop score 48 hours post intervention given. No baseline Bishop score reported and no specific data given on all other outcomes |
| Other bias | Low risk | Abstract only. Did not provide methodological reasoning to satisfy any of the other risk of bias domains. However, no other bias noted. |
Yasmeen 2014.
| Methods | Randomised controlled trial | |
| Participants |
Setting: “Labour room Gyne unit”, department of obstetrics and gynecology, BVH, Bahawalpur, Pakistan. Duration of study: February, 2013 to August, 2013. Participants randomised: N = 60 Inclusion criteria: “Patients of para 2 and para 5 with age from 25 to 35 years, Uncomplicated single cephalic term pregnancy, Candidates for vaginal delivery and patients with 40‐41 weeks estimated gestational age (by early pregnancy scan)”. Page 876 Exclusion criteria: “primigravidae, grand multipara, high risk pregnancy and patients presentation other than cephalic”. Page 876 Parity: only multiparous women included. Bishop score: not recorded |
|
| Interventions |
Membrane stripping (N = 30): “sweeping membrane was done.” “digital separation of 2‐3cm of the membranes from lower uterine segment by rotating the finger at least twice through 360 degrees was done. A closed cervix was stretched digitally until membrane sweeping could be carried out. A closed cervix that would not admit a finger was vigorously massaged. Women who underwent sweeping was told that spotting or blood stained cervical mucus may appear.” Page 876 Control group (N = 30): “no sweeping was done.” Page 876 |
|
| Outcomes | Spontaneous labour within 48 hours | |
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: yes, “after informed consent” Page 879. Ethical approval: not stated Email sent to clarify data on 12 April 2017. Email re‐sent 30 August 2017 to request further information. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported…”patients were randomized” Page 876 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not discussed. Personnel: not discussed. Unlikely clinicians have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make informed decision.Main stated outcome was proportion of women achieving spontaneous labour within 48 hours. This is not clearly reported. Email sent to author to clarify on 12 April 2017. No reply to date. |
| Other bias | Low risk | No information given on first 4 domains. However, no evidence of other bias. |
Yildirim 2010.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Bakirkoy Maternity and Pediatric Diseases Training and Research Hospital, Istanbul, Turkey Duration of study: October 2006 and July 2007. Participants randomised: N = 351 Inclusion criteria: “a single live fetus in cephalic presentation, gestational age between 38 and 40 weeks as determined by the last menstrual period or by a first‐ or second‐trimester ultrasound scan, no previous cesarean section or any uterine surgery, a Bishop score < 4 in the presence of a closed cervix and no contraindication to vaginal birth”. Page 682 Exclusion criteria: “previous cesarean delivery and uterine surgery, intrauterine fetal death, twin pregnancies, estimated fetal weight 44500g, known gross fetal anomalies or breech presentation”. Page 682. Parity: mixed. Both nulliparous and multiparous women included. Women who agreed to participate were first stratified into nulliparous and multiparous groups. Bishop score: cervical status and Bishop score (median, IR) recorded. |
|
| Interventions | “Pelvic examinations were performed to assess the status of the cervix by Bishop scoring. Transvaginal ultrasonographic measurement of cervical length was performed with the standard longitudinal view of the cervix while the patient’s bladder was empty. The probe was placed in the vagina approximately 3 cm proximal to the cervix to avoid distortion of its position or shape and a sagittal view of the cervix, with the echogenic endocervical mucosa along the length of the canal, was obtained. Three measurements were obtained using a Voluson 730 Expert ultrasound machine (GE Medical Systems Kretztechnik, Zipf, Austria) equipped with a 4–11 MHz probe. The shortest measurement was recorded” Page 682 Membrane stripping (N = 179): “Sweeping was performed by separating the lower membrane as much as possible from its cervical attachment, with three circumferential passes of the examining fingers. When sweeping was not possible because the cervix was closed, cervical massage was performed. Massage of the cervical surface was performed with circular pushing and massaging movements of the forefinger and middle finger for approximately 30 s. Sweeping was performed by only one of the investigators, and vaginal examination also was performed by the same investigator for the control group.” Page 682 “The women were observed for a few hours after membrane sweeping and, if they were well, they were discharged. The women were warned to expect a ‘show’ and were allowed to go home with a fetal movement chart. They were instructed to go to the labor ward if they experienced decreased fetal movement, rupture of the membranes or excessive vaginal bleeding or suspected the onset of labor.” Page 682 Control group (N = 167): vaginal examination. After the initial intervention, there were no further differences in management between the sweeping group and control group. All women were given a deadline date for labour to be induced in the absence of spontaneous onset. Thereafter, all patients were followed weekly until delivery or scheduled induction, and sweeping was not repeated. Page 682 |
|
| Outcomes | Spontaneous onset of labour Vaginal delivery Caesarean section Maternal infection Maternal discomfort Neonatal mortality |
|
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: yes "Written informed consent to participate in the study was obtained from all women who entered the study" Ethical approval: the hospital ethics committee approved the study. Email requesting further information sent 30 August 2017. Resent 20 September 2017. No reply to date. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “First stratified into nulliparous and multiparous groups”. “Randomisation was carried out by using sealed opaque envelopes with a piece of paper inside marked ‘Sweep’ or ‘No Sweep’. Envelopes were prepared in blocks of 20 (10 sweep and 10 no sweep) for each stratified group. Envelopes were then shuffled and placed in boxes marked ‘nulliparous’ and ‘multiparous’. Boxes were refilled as required with blocks of 20 envelopes.” Page 682 |
| Allocation concealment (selection bias) | High risk | “The investigator was not blinded to the allocation procedure.” “using sealed opaque envelopes with a piece of paper inside marked ‘Sweep’ or ‘No Sweep’.” “For random assignment to treatment groups, an envelope was withdrawn from the appropriate box and allocated to the woman. Once allocated, an envelope was discarded if a woman chose to withdraw, or there was an error in recruitment” Page 682. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel: not blinded “The allocated envelope was opened by the clinician performing the initial vaginal examination just prior to that examination.” page 682 Participants: Study states “therefore, at this stage, neither the investigator nor the patients knew the identity of the study group”. However it also states that “The procedure allocation was recorded in the woman’s chart.” Page 682 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | A sticker bearing the identification of the randomised woman was affixed to the paper marked ‘Sweep’ or ‘No Sweep’, and the paper was placed in a sealed drop box until unblinding at the end of the study. “Follow‐up of the patients was performed by another investigator who was blinded to which group the patients were in; therefore, at this stage, neither the investigator nor the patients knew the identity of the study group. However “The procedure allocation was recorded in the woman’s chart.” Page 682 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias.“Data were analysed on an intent‐to‐treat basis” Page 682 |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias. |
| Other bias | Low risk | No evidence of other bias. |
Zamzami 2014.
| Methods | Randomised controlled trial | |
| Participants |
Setting: Antenatal clinic, King Abdulaziz University Hospital, Jeddah, Saudi Arabia. Duration of study: 1 January 2011 to 1 January 1 2012 Participants randomised: N = 160 Inclusion criteria: “singleton pregnancy, cephalic presentation, and anticipated vaginal delivery (Page 30). Exclusion criteria: “indications for induction of labor, indications for cesarean section, and contraindications to membrane sweeping, such as multiple gestation, placenta previa, placental abruption, history of preterm delivery, vasa previa, active cervical infection, Mullerian anomalies, severe fetal anomalies and active herpes infection.” (Page 30). Parity: mixed. Both nulliparous and multiparous women included (Table II, Page 32). Bishop score: Bishop score (Initial), mean SD (Table II, Page 32). Bishop score on admission to LW, mean SD |
|
| Interventions |
Membrane stripping (N = 80): "All membrane sweeping group was performed by one clinician investigator and women allocated to control group received routine monitoring; in each case, the cervix was dilated and the health provider swept a finger in a 360° manner inside the cervix, thereby separating the lower uterine segment from the amniotic sac. If the cervix was closed, it was massaged digitally.” Modified Bishop scoring were determine as the following; cervical dilatation, effacement and fetal station" (Page 31). Control group (N = 80): no sweep (Page 31). All pregnant women “both groups” who did not enter spontaneous labor or remaining undelivered at 41 weeks' gestation were being admitted and underwent for induction of labour. |
|
| Outcomes | Induction (at 41 weeks) Spontaneous labour (< 41 weeks) SVD Vacuum delivery Caesarean section Apgar score < 7 PPH |
|
| Notes |
Funding: not stated Trial authors’ declaration of interest: not stated Informed consent obtained: yes, “provided written informed consent from all participants.” (Page 31). Ethical approval: yes "approved by the Biomedical Ethics Research Committee and Human Investigation “according to principles of Helsinki Declaration” at King Abdulaziz University" (Page 30). Email sent 28 August 2017 requesting information. Resent 10 September 2017. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Women were assigned randomly at 38 weeks“ “using computer‐generated numbers”, page 31. |
| Allocation concealment (selection bias) | Low risk | “allocation concealed in opaque sealed envelopes that were drawn in order.”, page 31. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants: not reported Personnel: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of attrition bias. 80 women excluded pre randomisation (60 declined to participate, 20 did not meet inclusion criteria). All outcomes reported on “intention to treat” analysis, page 31. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting noted. All outcomes reported in methods reported in results, page 33. |
| Other bias | Low risk | No evidence of other bias |
AFI: amniotic fluid index CS: low segment caesarean section CMS: cervical membrane stripping CTG: cardiotocography GA: gestational age GBS: group B Streptococcus IU: international unit IV: intravenous LW: labour ward NST: non stress test PGE2: prostaglandin E2 PPH: postpartum haemorrhage PPI: present pain index PROM: prelabour rupture of membrane RCT: randomised controlled trial SD: standard deviation SEM: standard error of the mean TTN: transient tachypnea of the newborn VAS: visual analogue scale VBAC: vaginal birth after caesarean section
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Harmi 2015 | Sweeping of membranes was evaluated as an addition to induction of labour with oxytocin, amniotomy or prostaglandins. Quote: "Women were assigned to having their membranes "swept" or "not swept" at the initiation of labor induction" |
| Bergsjo 1989 | Randomised comparison of sweeping of membranes and oxytocin (94 women) versus expectant management with surveillance (94 women) in women with post‐term pregnancy (at or beyond 42 weeks of gestation). |
| Day 2009 | Quote: "A prospective, randomized controlled trial was performed" "who were undergoing labor induction after 34 weeks were screened. Eligible women were randomly assigned to membrane sweeping at the time of labor induction (case) or no sweeping with the first vaginal exam (control)." Intervention commenced at 34 weeks' gestation. Confirmed with author through email 18 April 2017. |
| Foong 2000 | Sweeping of membranes was evaluated as an addition to oxytocin, amniotomy or prostaglandins. Method of concealment of the allocation is unclear. The results of this study suggested that sweeping of membranes during the induction of labour process reduces the risk of caesarean section (8/124 versus 17/124, P = 0.06). This effect was more apparent in nulliparous women who had cervical ripening with prostaglandins (unfavourable cervix) (3/48 versus 12/55, P = 0.01). |
| Ifnan 2006 | Quote: "women admitted for normal delivery requiring induction of labour with singleton live pregnancy" "randomized into two groups for cervical ripening by Foley's catheter ballooning method (group‐A) and by hydrostatic membrane sweeping (group‐B)". Our review defines membrane sweeping as the clinician inserting 1 or 2 fingers into the cervix and detaching the inferior pole of the membranes from the lower uterine segment in a circular motion (Boulvain 2005) |
| Kaul 2004 | This study was excluded as the gestational age of participants was outside the parameters of our review PICO. Quote: "Sixty women with singleton pregnancy and ascertained gestational age between 34 and 38 weeks,Bishop score ‐6 were randomized either to membrane stripping or cerviprime gel instillation." |
| Laddad 2013 | This study was excluded as it uses a mechanical device, intra‐cervical Foley catheter, rather than a digital sweep by a clinician, as defined in the review protocol to facilitate membrane sweeping. Quote: "A randomized, prospective study" "patients at term with a Bishop's score < 3 with various indications for induction were randomly allocated to receive (200 pts) intra‐cervical Foley's catheter or PGE2 gel (200 pts)" |
| Park 2013 | The study examines the effect of concurrent membrane sweeping with dinoprostone. This combination does not satisfy the review protocol. |
| Park 2015 | The study examines the effect of concurrent oxytocin with membrane sweeping. This combination does not satisfy the review protocol. |
| Shravage 2009 | This study contains 2 groups Group 1: membrane sweep + cerviprime Group 2: no sweep + cerviprime The study only examines the effect of membrane sweeping when combined with cerviprime. This combination does not satisfy the review protocol. |
| Swann 1958 | Method of allocation: women had to be allocated to 1 of the following groups: (1) stripping; (2) insertion of the finger in the cervix; (3) vaginal examination. 1 in every 3 women had to be allocated in turn to each group. Despite this schedule (not concealed to the resident in charge) that would have produced balanced groups, 147 women were allocated to membrane stripping, 29 to 'finger control' and 45 to 'Bishop score only'. This major imbalance, together with the inadequate method of randomisation, raises the suspicion of a selection bias. In addition, outcome measures were poorly defined and results difficult to interpret. |
| Tan 2006 | The study examines the effect of membrane sweeping when combined with either dinoprostone pessary or amniotomy, quote: "randomly assigned to receive membrane sweeping or no membrane sweeping at initiation of formal labor induction with either dinoprostone pessary or amniotomy.". This combination does not satisfy the review protocol. |
Characteristics of ongoing studies [ordered by study ID]
Leong 2017.
| Trial name or title | Membrane sweeping versus transcervical Foley catheter for induction of labour in women with previous caesarean delivery |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Two groups Group 1: membrane sweeping Membrane sweeping involves the insertion of a digit past the internal cervical os followed by 3 circumferential passes of the digit causing separation of the membranes from the lower uterine segment. When the cervix is closed, a massage of the cervical surface for 15 to 30 seconds will be performed instead. Membrane sweeping will be undertaken twice a day at 8 to 10 hours apart. Group 2: transcervical Foley catheter for induction of labour in women with previous caesarean delivery Transcervical Foley catheter No. 18 F will be inserted under aseptic technique into the endocervical canal surpassed beyond the internal os. The balloon will be inflated with 60 mL of sterile water and the catheter is plastered to patient's thigh with gentle traction. The catheter will be checked for its position and the traction at 6‐hour intervals. If it were expelled spontaneously, it would not be re‐inserted. Otherwise, the catheter will be removed after 24 hours. |
| Outcomes |
Primary outcome measures Achievement of favourable cervix (Bishop score of 8 or more) within 48 hours of induction of labour (time frame: from the time of commencing induction until the time whereby the cervix becomes favourable (Bishop score of 8 or more), assessed up to 48 hours). The number of women who achieve Bishop score of 8 or more within 48 hours of induction of labour Secondary outcome measures
|
| Starting date | 31 October 2017 |
| Contact information | Yong Soon Leong, Ministry of Health, Malaysia Email: yongsoonleong@moh.gov.my |
| Notes | Trial completed. Email sent 26/06/2019 requesting trial data. Reply received 26/06/19 from Dr Leong stated: "I regret to inform you that it is not feasible for us, at the moment, to provide you the information and findings about the trial" |
Manidakis 1999.
| Trial name or title | Prostaglandins versus stripping of membranes in management of pregnancy beyond 40‐41 weeks |
| Methods | |
| Participants | Women beyond 40 weeks of gestation with an unfavourable cervix |
| Interventions |
Three groups Group 1: daily prostin‐E2 1.5 to 3 mg at 41 weeks for 3 days Group 2: twice‐weekly 2 to 3 minute ‘non vigorous’ membrane stripping at 40 weeks Group 3: quote: “expectant management with twice weekly cervical examination” |
| Outcomes | Induction of labour with other methods. |
| Starting date | Reported as a pilot study during a meeting in 1999. |
| Contact information | |
| Notes |
Pathiraja 2014.
| Trial name or title | Induction of multiparous women at term using different methods: prostaglandin E2 (dinopristone) vaginal gel, intracervical Foley catheter insertion and sweeping of membrane: an open‐label, randomised controlled trial. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Four arms Arm 1 (prostaglandin group): dinoprostone gel 2 mg will be inserted following initial cervical assessment. If the cervix is unfavourable after 6 hours a second dose of prostaglandin (2 mg) will inserted. Fetal well‐being will be monitored by CTG at 3 hours and 5 hours after insertion of prostaglandin. Arm 2 (Foley catheter group): the Foley catheter balloon will inserted through the cervical canal and the catheter bulb dilated with 60 mL of normal saline done. Sufficient cervical dilatation will result in the catheter dropping out. The Foley catheter will be kept for a maximum of 48 hours. Fetal well‐being will be monitored by CTG and daily Doppler assessment. Arm 3 (membrane sweeping group): the sweeping of membrane will done once daily till 41 weeks. Fetal well‐being will be monitored by CTG at 3 hours after membrane sweeping and daily Doppler assessment. Arm 4 (control group): spontaneous onset of labour will be awaited with fetal monitoring done daily by 20 minutes CTG and daily Doppler assessment. |
| Outcomes |
Primary outcomes
Secondary outcomes
Need for admission to a neonatal intensive care unit (NICU). |
| Starting date | Anticipated start date 15 October 2014 |
| Contact information | Dr. P.D.M. Pathiraja Registrar in Obstetrics and Gynaecology New unit for Obstetrics and Gynaecology Teaching Hospital, Peradeniya 0812388261 0772532828 madushan_pathi@yahoo.com |
| Notes | Email requesting trial information sent. No reply to date. |
Sharma 2012.
| Trial name or title | Induction of labour in women with previous one caesarean section. Prosprctive double blind randomised control trial comparing the effect of mifepristone with sweeping stretching and trans‐cervical Folley's catheterization. |
| Methods | |
| Participants | Pregnant females, age 18 to 40 years of age with a singleton pregnancy, previous 1 low segment caesarean section, no other uterine scar or previous rupture. Gestation beyond 40 weeks and cephalic presentation. |
| Interventions | Group 1: no details reported in trial report. Group 2: women in this group will have initial assessment of Bishop score by senior consultant and receive 400 mg of mifepristone at 40 weeks 5 days gestation and will be re‐assessed at 24 hours and 48 hours later by senior consultant (blinded to the group of patient). If patient goes into labour this will be accounted for. Any time if Bishop score is more than 6, amniotomy will be performed followed by oxytocin infusion. If Bishop score is still less than 6 after 48 hours they will be induced with oxytocin. Group 3: women in this group will be inserted with trans‐cervical catheter after initial cervical assessment (Foley catheter number 16 filled with 30 mL of normal saline) at 40 weeks 5 days gestation and will be advised to pull the catheter every 20 minutes for 1 minute each. Foleys catheter will be removed after 6 hours, if it does not come out on its own. These women will be re‐assessed vaginally after 24 hours or earlier if catheter comes out, if Bishop score is more than 6, amniotomy will be performed followed by oxytocin infusion otherwise re‐assessed at 48 hours and induced with oxytocin. |
| Outcomes |
|
| Starting date | States "open to recruitment" 11 April 2017 |
| Contact information | DR RPGMC KANGRA Aat TANDA (HP) Proff and Head, OBG, DR
RPGMC KANGRA AT TANDA (HP)
Kangra
HIMACHAL PRADESH
176001
India Tel: 91‐9218925471 Email: sureshsverma@gmail.com |
| Notes | Emailed trial authors for further information on membrane sweeping intervention. No reply to date. |
Sheffield 2018.
| Trial name or title | Membrane sweeping in early labour and delivery outcomes. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Two groups Group 1 Membrane sweeping Participants assigned to membrane sweeping will have an additional exam during their initial evaluation in which the membrane will be separated from the cervix and lower part of the uterus with a finger inserted into the cervical os. This would be done with at least 1 rotation counterclockwise and 1 rotation clockwise. Group 2 Control. Routine vaginal examination |
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
| Starting date | |
| Contact information | |
| Notes | Trial not completed. Recruitment phase due to finish 1 June 2019. |
Shipman 2000.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | Mrs Marion Shipman, Senior Clinical Audit Facilitator, Clinical Audit Department, Watford General Hospital, Vicarage Road, Watford, WD1 8HB, UK. |
| Notes |
Turgay 2018.
| Trial name or title | The effect of membrane sweeping on the delivery time and the need of induction in term pregnancy. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Two groups Group 1 Membrane sweeping Group 2 Control. No intervention |
| Outcomes |
Primary outcome measures
|
| Starting date | |
| Contact information | |
| Notes | Trial not completed, currently in recruitment phase. |
CTG: cardiotocography PG: prostaglandins
Differences between protocol and review
2019 update of the review
We have updated the methods in line with those in the standard template used by Cochrane Pregnancy and Childbirth. We have used the GRADE approach to assess the certainty of evidence and included ’Summary of findings’ tables and added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
In addition we have made the following changes.
We have added three new primary outcomes (spontaneous onset of labour, induction of labour and spontaneous vaginal delivery).
Prior to data extraction we removed the outcome of vaginal delivery not achieved within 24 hours.
We reported subgroup analysis by parity (multiparous/primiparous) and cervical favourability (favourable cervix/unfavourable cervix).
On reflection of peer review feedback and in consultation with the Cochrane Pregnancy and Childbirth editorial team, data were analysed using the random‐effects model.
Within the primary outcome 'Neonatal death or serious neonatal perinatal morbidity ', 'probable or definite neonatal sepsis' was specified as suitable for inclusion following peer review.
Contributions of authors
Elaine Finucane and Declan Devane performed inclusion/exclusion criteria to identified studies. Elaine Finucane, Declan Devane, Deirdre Murphy, Linda Biesty, Gillian Gyte, Amanda Cotter and Ethel Ryan extracted data for the included studies and completed data extraction forms. Elaine Finucane drafted the review and Declan Devane, Deirdre Murphy, Linda Biesty, Gillian Gyte, Michel Boulvain, Amanda Cotter and Ethel Ryan contributed to editing of this update.
Sources of support
Internal sources
University of Geneva, Switzerland.
-
Health Research Board, Ireland, Ireland.
Health Research Board, Ireland (HRB) Cochrane Fellowship
External sources
No sources of support supplied
Declarations of interest
Elaine M Finucane: this review was supported by Health Research Board, Ireland (HRB) through a HRB Cochrane Fellowship. We acknowledge gratefully the support of the University Of Limerick Hospitals Group and the Nursing and Midwifery Planning and Development Unit West/Midwest of the Health Service Executive, Ireland (HSE).
Deirdre J Murphy: none known.
Linda M Biesty: none known.
Gillian ML Gyte: I have received royalties from John Wiley & Sons in respect of 'A Cochrane Pocketbook ‐ Pregnancy and Childbirth' Hofmeyr GJ et al. 2008.
Amanda M Cotter: none known.
Ethel M Ryan: none known.
Michel Boulvain: Michel is a principal investigator in one of the included studies (Boulvain 1998) and was the principle author of the original 2005 Cochrane Review ‘Membrane sweeping for induction of labour’ (Boulvain 2005). He was not involved in the data collection for this update, nor in the assessment of bias.
Declan Devane: Declan is PI for a grant from the HRB to assess the feasibility of conducting a definitive randomised trial to examine the effectiveness of membrane sweeping to prevent drug‐ based induction of labour in women at or near term, to explore women and clinicians acceptability of and willingness to participate in the trial and to evaluate the effects of social media study promotion on recruitment.
Edited (no change to conclusions)
References
References to studies included in this review
Adeniji 2013 {published data only}
- Adeniji AO, Akinola SE. A comparison of orally administered misoprostol and membrane sweeping for labour induction in uncomplicated, singleton post‐term pregnancies. South African Journal of Obstetrics and Gynaecology 2013;19(1):4‐7. [Google Scholar]
Afzal 2015 {published data only}
- Afzal M, Asif U, Miraj B. Induction of labour; efficacy of sweeping of membranes at term in previous one c‐section. Professional Medical Journal 2015;22(4):385‐9. [Google Scholar]
Alcoseba‐Lim 1992 {published data only}
- Alcoseba‐Lim W, Famador‐Juario H. Stripping of the membranes to induce labor at term. Philippine Journal of Surgical Specialities 1992;47:139‐42. [Google Scholar]
Allott 1993 {published data only}
- Allott HA, Palmer CR. Sweeping the membranes: a valid procedure in stimulating the onset of labour?. British Journal of Obstetrics and Gynaecology 1993;100:898‐903. [DOI] [PubMed] [Google Scholar]
Andersen 2013 {published data only}
- Andersen BB, Knudsen B, Lyndrup J, Faelling E, Illum D, Johansen M, et al. Acupuncture and/or sweeping of the fetal membranes before induction of labor: a prospective, randomized, controlled trial. Journal of Perinatal Medicine 2013;41(5):555‐60. [DOI] [PubMed] [Google Scholar]
Averill 1999 {published data only}
- Averill KA, Scardo JA, Chauhan SP. Weekly membrane stripping to decrease the incidence of postterm pregnancy: a randomized clinical trial. Obstetrics & Gynecology 1999;93(4 Supplement):47S. [Google Scholar]
Berghella 1996 {published data only}
- *Berghella V, Rogers RA, Lescale K. Stripping of membranes as a safe method to reduce prolonged pregnancies. Obstetrics & Gynecology 1996;87(6):927‐31. [DOI] [PubMed] [Google Scholar]
- Berghella V, Mickens R. Stripping of membranes as a safe method to reduce prolonged pregnancies. XIV World Congress of Gynecology and Obstetrics (FIGO);1994 Sept 26‐30; Montreal, Canada. 1994:PO 34.16.
Boulvain 1998 {published and unpublished data}
- Boulvain M, Fraser W, Marcoux S, Fontaine J, Bazin S, Blouin D. Randomised trial of sweeping the membranes. Acta Obstetricia et Gynecologica Scandinavica 1997;76:32. [Google Scholar]
- Boulvain M, Fraser W, Marcoux S, Fontaine JY, Bazin S, Pinault JJ, et al. Does sweeping of the membranes reduce the need for formal induction of labour ? A randomised controlled trial. British Journal of Obstetrics and Gynaecology 1998;105:34‐40. [DOI] [PubMed] [Google Scholar]
Cammu 1998 {published data only}
- *Cammu H, Haitsma V. Sweeping of the membranes at 39 weeks in nulliparous women: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1998;105:41‐4. [DOI] [PubMed] [Google Scholar]
- Haitsma V, Cammu H. Is stripping of membranes useful in reducing duration of pregnancy?. Proceedings of 15th European Congress of Perinatal Medicine; 1996 Sept 10‐13; Glasgow, UK. 1996:202.
Crane 1997 {published data only}
- Crane J, Bennet K, Windrim R, Kravitz H, Young D. Prospective randomized study of sweeping membranes at term. Proceedings of the SOGC Meeting; 1996 June; Québec, Canada. 1996.
- Crane J, Bennet K, Young D, Windrim R, Kravitz H. The effectiveness of sweeping membranes at term: a randomized trial. Obstetrics & Gynecology 1997;89:586‐90. [DOI] [PubMed] [Google Scholar]
Dare 2002 {published data only}
- Dare FO, Oboro VO. The role of membrane stripping in prevention of post‐term pregnancy: a randomised clinical trial in Ile‐Ife, Nigeria. Journal of Obstetrics and Gynaecology 2002;22(3):283‐6. [DOI] [PubMed] [Google Scholar]
de Miranda 2006 {published data only}
- Miranda E, Bom JG, Bonsel GJ, Bleker OP, Rosendaal FR. Membrane sweeping and prevention of post‐term pregnancy in low‐risk pregnancies: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2006;113(4):402‐8. [DOI] [PubMed] [Google Scholar]
Doany 1997 {published data only}
- Doany W. Outpatient management of postdate pregnancy with intravaginal prostaglandin E2 and membrane stripping. American Journal of Obstetrics and Gynecology 1996;174:351. [Google Scholar]
- Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin E2 gel and membrane stripping. Journal of Maternal‐Fetal Medicine 1997;6(2):71‐8. [DOI] [PubMed] [Google Scholar]
El‐Torkey 1992 {published data only}
- El‐Torkey M, Grant JM. Sweeping of the membranes is an effective method of induction of labour in prolonged pregnancy: a report of a randomized trial. British Journal of Obstetrics and Gynaecology 1992;99:455‐8. [DOI] [PubMed] [Google Scholar]
Gemer 2001 {published data only}
- Gemer O, Kapustian V, Harari D, Sassoon E, Segal S. Sweeping of membranes vs. prostaglandin E2 gel for cervical ripening. Journal of Reproductive Medicine 2001;46:706‐8. [PubMed] [Google Scholar]
Goldenberg 1996 {published data only}
- Goldenberg M, Dulitzky M, Feldman B, Zolti M, Bider D. Stretching of the cervix and stripping of the membranes at term: a randomised controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;66(2):129‐32. [DOI] [PubMed] [Google Scholar]
Gupta 1998 {published data only}
- Gupta R, Vasishta K, Sawhney H, Ray P. Safety and efficacy of stripping of membranes at term. International Journal of Gynecology & Obstetrics 1998;60:115‐21. [DOI] [PubMed] [Google Scholar]
Hamdan 2009 {published data only}
- Hamdan M, Sidhu K, Sabir N, Omar SZ, Tan PC. Serial membrane sweeping at term in planned vaginal birth after cesarean: a randomized controlled trial. Obstetrics & Gynecology 2009;114(4):745‐51. [DOI] [PubMed] [Google Scholar]
Hill 2008a {published data only}
- Hill MJ. Safety study of membrane sweeping in pregnancy. clinicaltrials.gov/ct2/show/record/NCT00294242 (first received 17 February 2006).
- Hill MJ, McWilliams GD, Garcia D, Chen B, Munroe M, Hoeldtke NJ. The effect of membrane sweeping in uncomplicated pregnancies on prelabor rupture of membranes, a prospective randomized controlled trial. Obstetrics & Gynecology 2008;111(4 Suppl):11S. [DOI] [PubMed] [Google Scholar]
- Hill MJ, McWilliams GD, Garcia‐Sur D, Chen B, Munroe M, Hoeldtke NJ. The effect of membrane sweeping on prelabor rupture of membranes: a randomized controlled trial. Obstetrics & Gynecology 2008;111(6):1313‐9. [DOI] [PubMed] [Google Scholar]
Imsuwan 1999 {published data only}
- Imsuwan Y, Tanapat Y. Reduction of pregnancy with gestational age more than 41 weeks by membrane stripping to induce labor: a randomized controlled clinical trial. Thai Journal of Obstetrics and Gynaecology 1999;11(4):267. [Google Scholar]
Janakiraman 2011 {published data only}
- Janakiraman V, Ojo L, Sheth S, Keller J, Young H. Membrane sweeping in GBS positive patients: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S41‐2. [Google Scholar]
- Keller JM. Membrane sweeping in GBS positive patients at 37 weeks gestation: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01180023 (first received 26 May 2010).
Kashanian 2006 {published data only}
- Kashanian M, Akbarian A, Baradaran H, Samiee MM. Effect of membrane sweeping at term pregnancy on duration of pregnancy and labor induction: a randomized trial. Gynecologic and Obstetric Investigation 2006;62(1):41‐4. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Baradaran H, Meshki M. The effect of membrane sweeping at term pregnancy on the duration of pregnancy and labor induction: a randomized trial. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):226. [Google Scholar]
Magann 1998a {published data only}
- Magann EF, McNamara MF, Whitworth NS, Chauhan SP, Thorp RA, Morrison JC. Can we decrease postdatism in women with an unfavourable cervix and a negative fetal fibronectin at term by serial membrane stripping [abstract]. American Journal of Obstetrics and Gynecology 1998;178(1):S96. [DOI] [PubMed] [Google Scholar]
- Magann EF, McNamara MF, Whitworth NS, Chauhan SP, Thorpe RA, Morrison JC. Can we decrease postdatism in women with an unfavorable cervix and a negative fetal fibronectin test result at term by serial membrane sweeping?. American Journal of Obstetrics and Gynecology 1998;179:890‐4. [DOI] [PubMed] [Google Scholar]
Magann 1998b {published data only}
- Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane stripping vs dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavourable cervix [abstract]. American Journal of Obstetrics and Gynecology 1998;178(1):S30. [Google Scholar]
- Magann EF, Chauhan SP, Nevils BG, McNamara MF, Kinsella MJ, Morrison JC. Management of pregnancies beyond fourty‐one weeks' gestation with an unfavourable cervix. American Journal of Obstetrics and Gynecology 1998;178:1279‐87. [DOI] [PubMed] [Google Scholar]
Magann 1999 {published data only}
- Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane sweeping versus dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavorable cervix. Journal of Perinatology 1999;19:88‐91. [DOI] [PubMed] [Google Scholar]
McColgin 1990a {published data only}
- McColgin SW, Patrissi GA, Morrison JC. Stripping membranes at term: is it safe and efficacious?. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:100.
- McColgin SW, Patrissi GA, Morrison JC. Stripping the fetal membranes at term: is the procedure safe and efficacious?. Journal of Reproductive Medicine 1990;35(8):811‐4. [PubMed] [Google Scholar]
McColgin 1990b {published data only}
- McColgin SW, Hampton HL, McCaul JF, Howard PR, Andrew ME, Morrison JC. Stripping of membranes at term: can it safely reduce the incidence of post‐term pregnancies?. Obstetrics & Gynecology 1990;76:678‐80. [PubMed] [Google Scholar]
McColgin 1993 {published data only}
- McColgin SW, Bennet WA, Roach H, Cowan BD, Martin JN, Morrison JC. Parturitional factors associated with membrane stripping. American Journal of Obstetrics and Gynecology 1993;169:71‐7. [DOI] [PubMed] [Google Scholar]
Netta 2002 {published data only}
- Netta D, Visintainer P, Bayliss P. Does cervical membrane stripping increase maternal colonization of group B streptococcus?. American Journal of Obstetrics and Gynecology 2002;187(6):S221. [Google Scholar]
Parlakgumus 2014 {published data only}
- Parlakgumus HA, Yalcinkaya C, Haydardedeoglu B, Tarim E. The impact of sweeping the membranes on cervical length and labor: a randomized clinical trial. Ginekologia Polska 2014;85(9):682‐7. [PubMed] [Google Scholar]
Putnam 2011 {published data only}
- Putnam K, Magann EF, Doherty DA, Poole AT, Magann MI, Warner WB, et al. Randomized clinical trial evaluating the frequency of membrane sweeping with an unfavorable cervix at 39 weeks. International Journal of Women's Health 2011;3(1):287‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramya 2015 {published data only}
- Ramya V, Ghose S, Pallavee P. Membrane sweeping for vaginal birth after caesarean section and its outcome ‐ a comparative study. Journal of Clinical and Diagnostic Research 2015;9(8):QC01‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saichandran 2015 {published data only}
- Saichandran S, Arun A, Samal S, Palai P. Efficacy and safety of serial membrane sweeping to prevent post term pregnancy: a randomised study. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2015;4(6):1882‐6. [Google Scholar]
Salamalekis 2000 {published data only}
- Salamalekis E, Vitoratos N, Kassanos D, Loghis C, Batalias L, Panayotopoulos N, et al. Sweeping of the membranes versus uterine stimulation by oxytocin in nulliparous women. Gynecologic and Obstetric Investigation 2000;49:240‐3. [DOI] [PubMed] [Google Scholar]
Salmanian 2012 {published data only}
- Salmanian R, Khayamzadeh M. Prostaglandin & stripping in ripening of cervix and shortening of labor in post date pregnancies. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S811. [Google Scholar]
Tannirandorn 1999 {published data only}
- Tannirandorn Y, Jumrustanasan T. A comparative study of membrane stripping and nonstripping for induction of labor in uncomplicated term pregnancy. Journal of the Medical Association of Thailand 1999;82(3):229‐32. [PubMed] [Google Scholar]
Ugwu 2014 {published data only}
- Ugwu EO, Obi SN, Iferikigwe ES, Dim CC, Ezugwu FO. Membrane stripping to prevent post‐term pregnancy in Enugu, Nigeria: a randomized controlled trial. Archives of Gynecology and Obstetrics 2014;289(1):29‐34. [DOI] [PubMed] [Google Scholar]
Weissberg 1977 {published data only}
- Weissberg SM, Spellacy WN. Membrane stripping to induce labour. Journal of Reproductive Medicine 1977;19(3):125‐7. [PubMed] [Google Scholar]
Wiriyasirivaj 1996 {published data only}
- Wiriyasirivaj B, Vutyavanich T, Ruangsri R. A randomized controlled trial of membrane stripping at term to promote labor. Obstetrics & Gynecology 1996;87:767‐70. [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong SF, Hui SK, Choi H, Ho LC. Does sweeping of membranes beyond 40 weeks reduce the need for formal induction of labour?. BJOG: an international journal of obstetrics and gynaecology 2002;109:632‐6. [DOI] [PubMed] [Google Scholar]
Yaddehige 2015 {published data only}
- Yaddehige SS, Kalansooriya HD, Rameez MF. Comparison of cervical massage with membrane sweeping for pre‐induction cervical ripening at term‐ A randomized control trial. Sri Lanka Journal of Obstetrics and Gynaecology 2015;37(Suppl 1):5‐6, Abstract no: OP 10. [Google Scholar]
- Yaddehige SS, SLCTR/2014/001. Comparison of cervical massage with membrane sweeping for pre‐induction cervical ripening at term‐ a randomized controlled trial. slctr.lk/trials/184 (first received 9 January 2014).
Yasmeen 2014 {published data only}
- Yasmeen A, Malik AM. Outcome of sweeping membrane within 48 hours in the induction of labour in multigravidae. Pakistan Journal of Medical and Health Sciences 2014;8(4):876‐81. [Google Scholar]
Yildirim 2010 {published data only}
- Yildirim G, Gungorduk K, Idem O, Aslam H, Ceylan Y. Membrane sweeping. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):36. [DOI] [PubMed] [Google Scholar]
- Yildirim G, Gungorduk K, Karadag OI, Aslan H, Turhan E, Ceylan Y. Membrane sweeping to induce labor in low‐risk patients at term pregnancy: a randomised controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(7):681‐7. [DOI] [PubMed] [Google Scholar]
Zamzami 2014 {published data only}
- Zamzami TY, Al Senani NS. The efficacy of membrane sweeping at term and effect on the duration of pregnancy: a randomized controlled trial. Journal of Clinical Gynecology and Obstetrics 2014;3(1):30‐4. [Google Scholar]
References to studies excluded from this review
Al‐Harmi 2015 {published data only}
- Al‐Harmi J, Chibber R, Fouda M, Mohammed KZ, El‐Saleh E, Tasneem A. Is membrane sweeping beneficial at the initiation of labor induction?. Journal of Maternal‐Fetal and Neonatal Medicine 2015;28(10):1214‐8. [DOI] [PubMed] [Google Scholar]
Bergsjo 1989 {published data only}
- Bergsjo P, Huang GD, Yu SQ, Gao ZZ, Bakketeig LS. Comparison of induced versus non‐induced labor in post‐term pregnancy. A randomized prospective study. Acta Obstetricia et Gynecologica Scandinavica 1989;68:683‐7. [DOI] [PubMed] [Google Scholar]
Day 2009 {published data only}
- Day L, Fleener D, Andrews J. Membrane sweeping with labor induction ‐ a randomized controlled trial. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S47. [Google Scholar]
Foong 2000 {published data only}
- Foong L, Vanaja K, Tan G, Chua S. Effect of cervical membrane sweeping on induction of labour. Women's health into the new millennium. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 Oct 3‐6; Cape Town, South Africa. 1999:63.
- Foong LC, Vanaja K, Tan G, Chua S. Membrane sweeping in conjunction with labor induction. Obstetrics & Gynecology 2000;96:539‐42. [DOI] [PubMed] [Google Scholar]
Ifnan 2006 {published data only}
- Ifnan F, Jameel MB. Ripening of cervix for induction of labour by hydrostatic sweeping of membrane versus foley's catheter ballooning alone. Jcpsp, Journal of the College of Physicians & Surgeons ‐ Pakistan 2006; Vol. 16, issue 5:347‐50. [PubMed]
Kaul 2004 {published data only}
- Kaul V, Aggarwal N, Ray P. Membrane stripping versus single dose intracervical prostaglandin gel administration for cervical ripening. International Journal of Gynecology & Obstetrics 2004;86:388‐9. [DOI] [PubMed] [Google Scholar]
Laddad 2013 {published data only}
- Laddad ML, Kshirsagar NS, Karale AV. A prospective randomized comparative study of intra‐cervical foley's catheter insertion versus PGE2 gel for pre‐induction cervical ripening. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(2):217‐20. [Google Scholar]
Park 2013 {published data only}
- Park KH, NCT01792375. Concurrent membrane sweeping with dinoprostone versus dinoprostone in labor induction of nulliparas at term with an unfavorable cervix. clinicaltrials.gov/ct2/show/record/NCT01792375 (first received 13 February 2013).
Park 2015 {published data only}
- Park KH, NCT02618096. Concurrent oxytocin with membrane sweeping versus dinoprostone pessary in labor induction of multiparous women at term. clinicaltrials.gov/ct2/show/NCT02618096 Date first recieved: 25 September 2015.
Shravage 2009 {published data only}
- Shravage J. Effect of sweeping of membranes at initiation of formal induction of labour ‐ a randomised controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S338. [Google Scholar]
Swann 1958 {published data only}
- Swann RD. Induction of labor by stripping membranes. Obstetrics & Gynecology 1958;11:74‐8. [PubMed] [Google Scholar]
Tan 2006 {published data only}
- Tan PC, Jacob R, Omar SZ. Membrane sweeping at initiation of formal labor induction: a randomized controlled trial. Obstetrics & Gynecology. 2006;107(3):569‐77. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Leong 2017 {published data only}
- Leong YS, NCT03326557. Membrane sweeping versus transcervical Foley catheter for induction of labour in women with previous caesarean delivery. https://clinicaltrials.gov/ct2/show/NCT03326557 (first received 31 October 2017).
Manidakis 1999 {published data only}
- Manidakis G, Sifakis S, Orfanoudaki E, Mikelakis G, Prokopakis P, Magou M, et al. Prostaglandin versus stripping of membranes in management of pregnancy beyond 40‐41 weeks. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;86:S79‐80. [Google Scholar]
Pathiraja 2014 {published data only}
- Pathiraja PD, SLCTR/2014/025. Induction of multiparous women at term using different methods: Prostaglandin E2 (dinopristone) vaginal gel, intracervical foley catheter insertion and sweeping of membrane: An open‐label, randomised controlled trial. slctr.lk/trials/244 (first received 9 October 2014).
Sharma 2012 {published data only}
- Sharma C. Induction of labor in women with previous one cesarean section: prospective double blind randomized control trial comparing the effect of mifepristone with sweeping stretching and trans‐cervical folley’s catheterization. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=4745&EncHid=&modid=&compid=%27,%274745det%27 (first received 19 June 2012).
Sheffield 2018 {published data only}
- Sheffield JS, NCT03517696. Membrane sweeping in early labor and delivery outcomes. https://clinicaltrials.gov/ct2/show/NCT03517696 (first received 7 May 2018).
Shipman 2000 {published data only}
- Shipman M. The SNS trial: sweeping vs no sweeping of membranes in uncomplicated post‐date pregnancies. National Research Register http//www.update‐software.com/NRR (accessed 8 March 2000).
Turgay 2018 {published data only}
- Turgay B, NCT03591159. The effect of membrane sweeping on the delivery time and the need of induction in term pregnancy. https://clinicaltrials.gov/show/nct03591159 (first received 19 July 2018).
Additional references
ACOG 2009
ACOG 2014
Alfirevic 2014
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001338.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2016
- Alfirevic Z, Keeney E, Dowswell T, Welton N, Medley N, Dias S, et al. Which method is best for the induction of labour? A systematic review, network meta‐analysis and cost‐effectiveness analysis. Health Technology Assessment 2016;20(65):1‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Institute of Health and Welfare 2016
- Australian Institute of Health and Welfare. National core maternity indicators– stage 3 and 4 Results from 2010–2013. Available from: http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129557275 (accessed 1st September 2017).
Bakker 2013
- Bakker JJH, Goes BY, Pel M, Mol BW, Post JA. Morning versus evening induction of labour for improving outcomes. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007707] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bishop 1964
- Bishop E. Pelvic scoring for elective induction. Obstetrics & Gynecology 1964;2:266‐8. [PubMed] [Google Scholar]
Blackburn 2013
- Blackburn S. Maternal, Fetal, & Neonatal Physiology ‐ A Clinical Perspective. 3rd Edition. Missouri: Saunders Elsevier, 2013. [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caughey 2009
de Vaan 2019
- Vaan MD, Eikelder ML, Jozwiak M, Palmer KR, Davies‐Tuck M, Bloemenkamp KW, Mol BW, et al. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2019, Issue 10. [DOI: 10.1002/14651858.CD001233.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grobman 2018
- Grobman, W. A randomized trial of elective induction of labor at 39 weeks compared with expectant management of low‐risk nulliparous women. American Journal of Obstetrics and Gynaecology 2018;218(1):S601. [DOI: ] [Google Scholar]
Health Canada 2008
- Health Canada. Canadian Perinatal Health Report, 2008. Available at: http://www.phac‐aspc.gc.ca/publicat/2008/cphr‐rspc/pdf/ cphr‐rspc08‐eng. (accessed on 28th March 2017) 2008.
Health Service Executive 2016
- Health Service Executive. Irish Maternity Indicator System, IMIS National Report 2015. Available at: https://www.hse.ie/eng/services/publications/clinical‐strategy‐and‐programmes/irish‐maternity‐indicator‐system‐national‐report‐2015.pdf. (accessed on 28th March 2018).
Hedegaard 2014
- Hedegaard M, Lidegaard Ø, Wessel Skovlund C, Steinrud Mørch L, Hedegaard M. Reduction in stillbirths at term after new birth induction paradigm: results of a national intervention. BMJ Open 2014; Vol. 4, issue e005785. [DOI: 10.1136/bmjopen-2014-005785] [DOI] [PMC free article] [PubMed]
Heimstad 2008
- Heimstad RI, Romundstad PR, Salvesen KA. Induction of labour for post‐term pregnancy and risk estimates for intrauterine and perinatal death. Acta Obstetricia et Gynecologica Scandinavica 2008;87(2):247‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyer 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Middleton 2018
- Middleton P, Shepherd E, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD004945.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Childbirth Trust 2017
- The National Childbirth Trust. Maternity statistics – England. Available from: https://www.nct.org.uk/professional/research/maternity%20statistics/maternity‐statistics‐england (accessed 19th May 2018) 2017.
NHS Digital 2014
- NHS Digital. NHS Maternity Statistics ‐ England, 2013‐14. Available at:https://digital.nhs.uk/ (accessed 10th May 2017) 2014.
NICE 2008
- National Institute for Health and Care Excellence. Inducing labour. Clinical guideline [CG70]. Available from: https://www.nice.org.uk/guidance/cg70/chapter/4‐research‐recommendations (accessed 4th April 2017]. 2008.
Nippita 2015
- Nippita T, Trevena J, Patterson J, Ford J, Morris J, Roberts C. Variation in hospital rates of induction of labour: a population‐based record linkage study. BMJ Open 2015; Vol. 5, issue e008755. [DOI] [PMC free article] [PubMed]
Olesen 2003
- Olesen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to postterm delivery: a national register‐based study, 1978‐1993. American Journal of Obstetrics and Gynecology 2003;189(1):222‐7. [DOI] [PubMed] [Google Scholar]
Public Health Canada 2008
- Public Health Agency of Canada. Canadian Perinatal Health Report, 2008 Edition. Available at: file:///C:/Users/0115398s/Downloads/‐sites‐webphac‐htdocs‐archives‐cphr‐rspc08‐eng.pdf (accessed 1st June 2017). 2008.
Queensland DOH 2017
- Queensland Clinical Guidelines. Induction of labour. Available at: https://www.health.qld.gov.au/__data/assets/pdf_file/0020/641423/g‐iol.pdf (accessed 2nd May 2018) March 2017.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roos 2010
- Roos N, Sahlin L, Ekman‐Ordeberg G, Kieler H, Stephansson O. Maternal risk factors for postterm pregnancy and cesarean delivery following labor induction. Acta Obstetricia et Gynecologica Scandinavica 2010;89(8):1003‐10. [DOI: 10.3109/00016349.2010.500009] [DOI] [PubMed] [Google Scholar]
SOGC 2013
- The Society of Obstetricians and Gynaecologists of Canada. Induction of Labour SOGC Clinical Practice Guideline No. 296. Available at: https://sogc.org/wp‐content/uploads/2013/08/September2013‐CPG296‐ENG‐Online_REV‐D.pdf (accessed 4th April 2017).
South Australia DOH 2014
- SA Maternal & Neonatal Clinical Network. Clinical Guideline Induction of labour techniques. Available at:http://www.sahealth.sa.gov.au/wps/wcm/connect/ac7d37804ee4a27985598dd150ce4f37/Induction+of+labour_Clinical+Guideline_final_Dec14.pdf?MOD=AJPERES (accessed 15th October 2017) 2014.
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] Chapter10: Addressing reporting biases. Cochrane Handbook for Systematic Reviews of Interventions version5.2.0 (updated June 2017), Cochrane. 5.2.0. The Cochrane Collaboration, 2017.
Sue‐A‐Quan 1999
- Sue‐A‐Quan AK, Hannah ME, Cohen MM, Foster GA, Liston RM. Effect of labour induction on rates of stillbirth and cesarean section in post‐term pregnancies. CMAJ: Canadian Medical Association Journal 1999;160(8):1145‐9. [PMC free article] [PubMed] [Google Scholar]
The Word Bank 2018
- The World Bank. World Bank Country and Lending Groups Country Classification. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 2018.
Wood 2014
- Wood S, Cooper S, Ross S. Does induction of labour increase the risk of caesarean section? A systematic review and meta‐analysis of trials in women with intact membranes. BJOG: an international journal of obstetrics and gynaecology 2014;121(6):674‐85. [DOI] [PubMed] [Google Scholar]
World Health Organization 2000
- World Health Organization press. Managing complication in pregnancy and childbirth: a guide for midwives and doctors. Available at http://apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf (accessed 19th April 2017).
World Health Organization 2011
- World Health Organization. WHO recommendations for induction of labour. Available from: http://apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf (accessed 4th April 2017).
World Health Organization 2014
- World Health Organization. WHO recommendations for augmentation of labour. Available from: http://apps.who.int/iris/bitstream/handle/10665/112825/9789241507363_eng.pdf?sequence=1 (accessed 16/04/2017) 2014. [PubMed]
References to other published versions of this review
Boulvain 2001
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000451] [DOI] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keirse 1995
- Keirse MJNC. Stripping/sweeping membranes at term for induction of labour. [revised 03 April 1992]. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaborarion; Issue 2, Oxford: Update Software; 1995.


