Abstract
Introduction
Intertrochanteric fractures are common fragility injuries in the elderly. Surgical fixation using intramedullary devices are one of the widely used management options. To date, evidence demonstrating the effects of lag screw configuration and the mode of lag screw locking in these devices is lacking. The purpose of this study is to investigate whether the lag screw configuration (single vs integrated dual interlocking screw) and the mode of lag screw locking (static vs dynamic) of a femoral nail device result in differences in clinical and functional outcomes.
Methods and analysis
A multicentre, pragmatic, single-blinded randomised controlled trial (RCT) with a three-arm parallel group design is proposed. Nine-hundred patients with intertrochanteric fractures (A1 and A2 AO/OTA) will be randomised to fracture treatment using a Gamma3 nail (Stryker; proximally dynamic) or a Trigen Intertan nail (Smith & Nephew) in a dynamic or static lag screw configuration. The primary outcome measure consists of radiological evidence of construct failure within 6 months following surgery, with failure being defined as breakage of the femoral nail or distal locking screw, a change in tip-apex distance of more than 10 mm or lag screw cut-out through the femoral head. Secondary outcomes include surgical data (operation time, fluoroscopy time), complications (surgical site infection, reoperation, patient death), return to mobility and home circumstances, functional independence, function and pain. Patients who are able to walk independently with or without a mobility aid and are able to answer simple questions and follow instructions will be asked to participate in three dimensional gait analysis at 6 weeks and 6 months to assess hip biomechanics from this cohort. Additional secondary measures of gait speed, hip range of motion, joint contact and muscle forces and gross activity monitoring patterns will be obtained in this subgroup.
Ethics and dissemination
The Central Adelaide Local Health Network Human Research Ethics Committee has approved the protocol for this RCT (HREC/17/RAH/433). The results will be disseminated via peer-reviewed publications and presentations at relevant conferences.
Trial registration number
ACTRN12618001431213.
Keywords: clinical trials, fracture fixation, intramedullary nailing, hip, orthopaedic & trauma surgery, trauma management
Strengths and limitations of this study.
Multicentre, pragmatic, single-blinded randomised controlled trial.
The first study to investigate the effects of femoral nail lag screw locking mode on clinical and functional outcomes.
The first study to collect three-dimensional motion capture data from the patients postoperatively.
Powered to detect differences in device failure between the three parallel arm groups in a large sample size (900).
Limitations of the study include an unpredictable loss to follow-up from death or failure to attend.
Introduction
Background and rationale
Proximal femur fractures are a highly prevalent injury in the elderly,1 2 with an estimated 1.31 million fractures occurring worldwide each year.3 4 With a growing elderly population resulting from an increasing life expectancy,5 there is an increasing global incidence of these fractures,5–9 projected to reach 6.26 million by the year 2050.10 Fractures within the intertrochanteric region represent approximately half of all proximal femur fractures.11 Treatment typically consists of surgical fixation using either intramedullary (IM; eg, proximal femoral nail (Synthes)) or extramedullary (eg, Dynamic Hip Screw, Synthes) fixation devices.
Since its introduction in the 1990s, IM fixation has become increasingly popular,12 with increasing trends towards this device preference recorded in the USA13 and Australia.14 Numerous types of IM fixation devices are available for clinical use,15 however the optimum implant choice remains unknown.16 While there is evidence to support the use of these devices in the treatment of intertrochanteric fractures, the evidence demonstrating whether variations in design characteristics influence patient clinical outcomes is conflicting.12 17–19 As no rationale behind implant selection can be drawn from the literature, there is considerable diversity regarding the choice of implant between clinicians.20
The Gamma3 nail (Stryker) is a well-established and widely used current generation single lag screw IM device12 which shows good clinical and radiographic outcomes.21–23 However, complications still exist, with the most frequently reported complication being cut-out of the lag screw through the femoral head,24–26 with an incidence rate ranging between 4% and 8%.27–29 The Trigen Intertan nail (Smith & Nephew) is a similar current generation IM device, featuring a dual lag screw configuration comprised of a larger superior lag screw and a smaller screw integrated within the superior screw.30 Together, this interlocking dual-oval shaped composite screw mechanism allows for linear compression of the fragments at the fracture site while providing high rotational stability.31 32 Clinical studies evaluating the Intertan nail against other single screw devices have recorded a significant reduction in the occurrences of implant failure, fracture site non-union, mal-union, lag screw cut-out and uncontrolled varus fracture collapse.30 31 33–35 Several authors have postulated the reduced complication rate being attributed to the design of this nail.30 Moreover, ex vivo biomechanical studies have demonstrated superior biomechanical results with the Intertan nail.36–39 However, despite the Gamma3 and Trigen Intertan nails, both being well-established implant choices used in the treatment of these fractures, very little direct comparative clinical evidence exists between these nails.
A meta-analysis by Ma et al 40 found only nine papers, four of which included the Gamma3 and the Intertan. Of these four, three were randomised controlled trials (RCTs) comparing the two devices, but with relatively small cohort sizes. From these studies, the Intertan nail was shown to result in a lower incidence of implant cut-out and femoral fractures which was of statistical significance. No statistically significant differences in time to union and postoperative complications were found between devices. Ma et al highlighted that a limitation of this statistical analysis was the relatively small sample size of the studies included, and indicated a need for more high-quality RCTs to yield a more convincing test power.40 Hence, the literature reveals limited evidence of whether design characteristics of femoral nails affect clinical and patient outcomes in the treatment of trochanteric fractures.
In addition to the choice of the IM implants, other aspects of these devices used in the practical management of intertrochanteric fractures need further evaluation. This includes the mode of lag screw fixation (static or dynamic). Technically, both the Gamma3 and Intertan nails can be used in static or dynamic modes of the lag screw. In the dynamic mode, fracture collapse occurs under physiological loading, resulting in macro and micromotion of the fracture fragments as well as compression/apposition of fracture fragments,41–43 desired to stimulate fracture healing. However, excessive sliding of the lag screw has been shown by some authors to lead to mechanical complications and negatively affect patient function.44–46
While there is evidence highlighting a reduced risk of lag screw cut-out when using a sliding lag screw in extramedullary devices,45 47–50 there is a paucity of similar evidence relating to the use of IM devices. One study compared the static and dynamic modes of the proximal lag screw in the Gamma3 nail in 80 patients.51 From this study, no statistically or clinically significant difference in Harris Hip Scores (HHS), time to fracture healing or length of hospital stay was found. No such comparative evidence exists for the Intertan nail. Moreover, no clinical studies to date, comparing one IM device to another has made any note of which mode of the lag screw was employed. Consequently, considerable variance in practice can be seen between clinicians.
For the Gamma3 nail, it is suggested by Stryker in their operative technique guide that the device has to be used in the dynamic mode, with the use of the nail in a static mode considered off label.52 For the Intertan nail (Smith & Nephew), this decision is stated in their surgical technique guide as optional with both modes considered on label,53 and left to the operating surgeons decision.
Considering the substantial costs attributed to the management of intertrochanteric fractures, we believe that more evidence is required to evaluate the effectiveness of a single or dual screw femoral nail, as well the use of these devices in the static and dynamic modes. Moreover, no previous studies have compared postoperative lower extremity biomechanics in patients with intertrochanteric fracture treated with these devices. This proposed multicentre, parallel, three-arm RCT has been designed to fill these gaps in knowledge, and will include a two-way comparison between the Gamma3 (dynamic) and Intertan (dynamic) nails, as well as the Intertan (dynamic) and Intertan (static) nails.
Objectives
Primary objective
The aim of this RCT is to investigate if there are differences in failure rates between the surgical management of intertrochanteric fractures using a single screw or dual screw femoral nail, as well as when using a femoral nail in either the static or dynamic modes of the lag screw.
It is hypothesised there will be no difference in failure rates between patients managed with a single screw or dual screw femoral nail device. It is also hypothesised that there will be no difference in failure rates between patients managed with a femoral nail in the static or dynamic mode of the lag screw.
Secondary objectives
Several secondary will also objectives will also be studied for this RCT to evaluate the effectiveness of the devices used by quantifying and drawing inferences from observed differences between treatment groups in the following:
Intraoperative surgical data (operation time, fluoroscopy time, blood loss, tip-apex distance (TAD)).
Pain within 6 months after surgery (visual analogue scale (VAS) Pain Score).
Patient function (Functional Independence Measure (FIM)) and hip function (HHS) within 6 months after surgery.
Postoperative hip biomechanics using objective measures from gait analysis up to 6 months after surgery.
Trial design
The Proximal Femoral Nail Unlocked versus Locked study is a multicentre, pragmatic (as defined by the Pragmatic-Explanatory Continuum Indicator Summary 2 Tool),54 single-blinded RCT with a three-arm parallel group design.
Methods and analysis
Patients will be randomised using an online computerised sequence generation service to test if there is a difference in outcomes between the treatment interventions. Recruitment, medical and surgical data collection will take place at the Royal Adelaide Hospital and Queen Elizabeth Hospital with other sites added later as required for participant numbers. Radiographic images will be collected at a diagnostic imaging practice and three-dimensional (3D) motion capture data will be conducted at The University of Adelaide, South Australia.
This RCT has been registered with the Australian New Zealand Clinical Trials Registry. Trial registration data are shown in table 1.
Table 1.
Trial registration data
| Data category | Information |
| Primary registry and trial identifying number | https://www.anzctr.org.au, ACTRN12618001431213 |
| Date of registration in primary registry | 27/08/2018 |
| Secondary identifying numbers | None |
| Source of monetary or material support | Smith & Nephew Pty Ltd |
| Primary sponsor | Royal Adelaide Hospital, Department of Orthopaedics & Trauma Contact person: MR (mark.rickman@sa.gov.au) Smith & Nephew Inc Orthopaedic Division (SN) |
| Secondary sponsor | University of Adelaide, Centre for Orthopaedic & Trauma Research Contact person: AS (arjun.sivakumar@adelaide.edu.au) Contact person: DT (dominic.thewlis@adelaide.edu.au) |
| Contact for public queries | MR (mark.rickman@sa.gov.au) |
| Contact for scientific queries | DT (dominic.thewlis@adelaide.edu.au) |
| Public title | Evaluating the treatment methods of proximal femur fractures in elderly patients with trauma |
| Scientific title | A multicentre, single-blinded prospective RCT of the Gamma3 intramedullary nail to the unlocked and locked Intertan intramedullary nail for the treatment of proximal femur fractures |
| Countries of recruitment | Australia |
| Health problem studied | Proximal femur fracture |
| Interventions | Gamma3 trochanteric nail (unlocked proximally) Trigen Intertan Trochanteric nail (unlocked proximally) Trigen Intertan Trochanteric nail (locked proximally) |
| Key inclusion and exclusion criteria | Inclusion criteria: traumatic extracapsular hip fracture, closed injury, patient aged over 60 years, ability to be followed for up to 6 months, presentation to hospital within 14 days of injury Exclusion criteria: patients with concomitant injuries affecting treatment and rehabilitation of the affected limb, patients with associated neurovascular injuries requiring immediate surgery, patients where consent is refused, patients with limited English proficiency including family members |
| Study type | RCT |
| Date of first enrolment | 05/09/2018 |
| Target sample size | 900 |
| Recruitment status | Recruiting |
| Primary Outcome | Construct failure (time point: up to 6 months after intervention) |
| Key secondary outcomes | Incidence of Injury specific complications (time point: 6 months) Functional independence (time point: 6 months) Reoperation incidence (time point: 6 months) Return to mobility circumstances (time point: 6 months) Hip joint range of motion (time point: 6 months) Hip joint contact forces (time point: 6 months) Postoperative hip muscle function (abductors, flexors, extensors; time point: 6 months) |
RCT, randomised controlled trial.
This study protocol was developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials statement.
Patient and public involvement
Patients and public were not involved in the design, conduct or reporting of this study.
Eligibility
Patients over 60 years of age presenting to any of the participating hospitals with an isolated, closed intertrochanteric fracture will be recruited against the following eligibility criteria:
Inclusion criteria
Traumatic intertrochanteric femur fracture (A1 and A2 AO/OTA) where a decision has been made for surgical management using a femoral nail.
Closed injury.
Patients aged over 60 years.
Presentation to hospital within 14 days of injury.
Exclusion criteria
Patients with concomitant injuries affecting treatment and rehabilitation of the affected limb.
Patients with associated neurovascular injuries requiring immediate surgery.
Patients with limited English proficiency including family members.
Patients where consent is refused.
All eligible patients will be provided with a study information sheet and consent form by the hospital medical staff (online supplementary appendix). If eligible patients are not able to consent due to cognitive impairment, consent will be sought from the family, in the same manner that consent for surgery and anaesthesia occurs currently (online supplementary appendix). Randomisation will then occur once consent has been obtained.
bmjopen-2019-032640supp001.pdf (159.2KB, pdf)
Randomisation and blinding
Patients will be randomised via a computerised generation system managed by the Griffith University’s Clinical Trial Unit (Griffith University, QLD, Australia), allocating patients to three study groups of equal weights using random block sizes of 6 and 9. Randomisation will be stratified by site (three categories), gender (two categories) and cognitive function via Abbreviated Mental Health Test Score (AMTS; two categories). Randomisation of the next subject will be computer-generated at the time of request by a medical research officer at the hospital via the online randomisation system.
Patients will be blinded to their allocation until the conclusion of the trial to reduce bias in patient-reported outcome measures. The statistician performing the analysis will also be blinded to the group allocation. Surgeons and researchers will not be blinded to allocation.
Standard treatment pathway
The clinical pathway for recruited patients will be unchanged from the routine for each institution; surgery is typically carried out within 24–48 hours, and no changes will be necessary to any part of the surgical episode with the exception of the individual device used and mode of proximal locking as directed by the randomisation outcome. Training and observation will be provided to all surgeons throughout the duration of this study, from senior surgeons competent with the use of both devices; throughout the duration of the study it is anticipated that a large number of surgeons will carry out the procedures, using both devices at all sites. This adds to the pragmatic nature of the study. All fractures will be compressed proximally at the time of surgery, just prior to the nail being either locked proximally or left unlocked. Similarly, postoperative management will remain unchanged from routine, including discharge timing and destination. All patients will be mobilised with full weight bearing as soon as possible after surgery.
Allocated interventions
A total of 900 patients with intertrochanteric fractures (31A1 and 31A2 AO/OTA) will be randomised to receive one of the three femoral nail interventions.
Gamma3 (Stryker; locked proximally).
Intertan (Smith & Nephew; unlocked proximally).
Intertan (Smith & Nephew; locked proximally).
Participant flow timeline
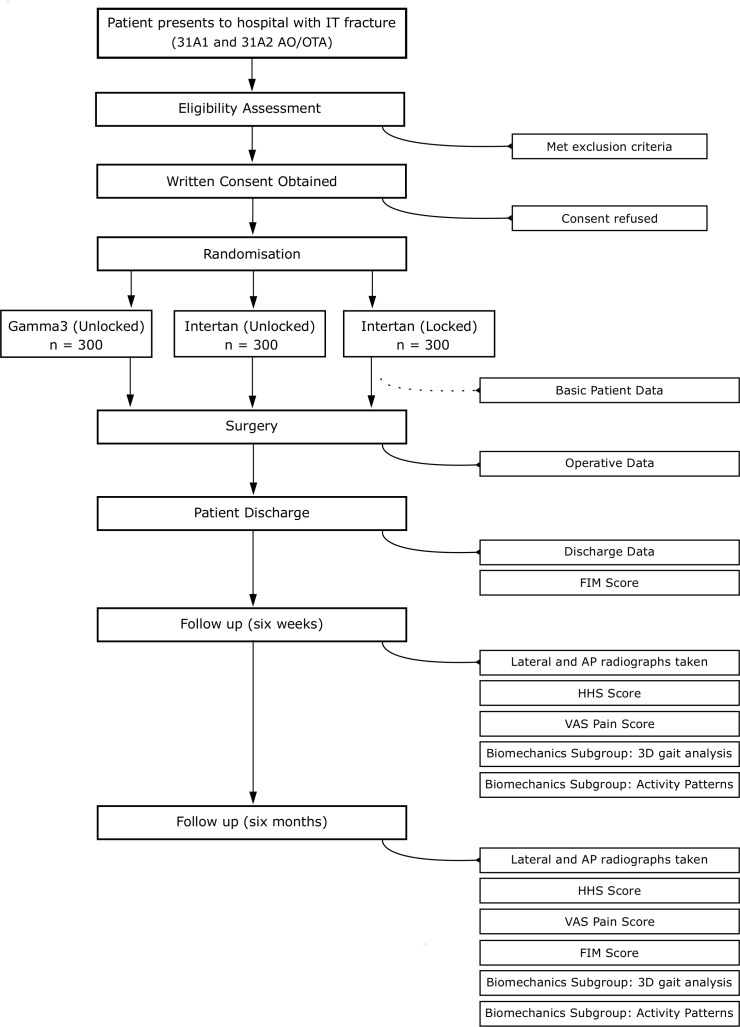
A succinct summary of the patient timeline is described by figure 1. Once a patient has been recruited and randomised, a baseline patient registration assessment will be completed through the use of an online form. Surgery will then proceed at the earliest available opportunity as per routine for the hospital. Nail diameters are all fixed at 11 mm for the Gamma3 nail, and 11.5 mm for the Intertan nail with the nail centrum collum diaphyseal angle at 125°. Following surgery, an operative information form will be completed by the operating surgeon using another online form. On patient discharge, medical staff will complete a patient discharge online form which includes a clinical assessment of FIM Score. Following discharge, follow-up appointments will be scheduled to coincide with 6 weeks and 6 months with appointment letters and X-ray referrals sent from the Royal Adelaide Hospital. A week prior to each patients appointment, patients will be called to confirm their appointments or reschedule, if required. Anteroposterior (AP) and lateral hip radiographs will be taken, followed by a clinical examination with an orthopaedic and trauma specialist, where measures of hip pain (VAS from 0 to 10) and hip function (HHS) will be recorded. At the 6 month follow-up, AP and lateral hip radiographs will be taken, followed by a similar clinical examination with an orthopaedic and trauma specialist. At this appointment, a FIM Score will be recorded in addition to VAS and HHSs.
Figure 1.
Patient flow diagram. 3D, three dimensional; AP, anteroposterior; FIM, functional independence measure; HHS, harris hip score; VAS, visual analogue scale.
Patients who are able to walk independently with or without a mobility aid and are able to answer simple questions and follow instructions will be included in a ‘biomechanics subgroup’ where 3D gait analysis will be performed at the 6 weeks and 6 month follow-ups immediately following the clinical examination. After the gait analysis, patients will be provided with a wrist worn activity monitor (GeneActiv Original, Activinsights Ltd, Kimbolton, UK, 100 Hz) to wear for 7 days at a time, providing information on 24 hours gross physical activity patterns of these patients. Patients will be asked to complete a sleep log during this period to better distinguish sedentary time from sleep. After 7 days, patients will post the monitors back via prepaid return envelopes. Patients living rurally and unable to attend follow-up appointments will have X-ray appointments organised at locations convenient to them collected over the phone by an orthopaedics and trauma specialist. Patients presenting to clinics reporting complications, will be reviewed by a clinician and radiographs taken, as per standard procedure. In these events, the occurrence of these complications is recorded against the patient’s hospital number.
Outcomes
Primary outcome measure
The primary outcome measure is radiological evidence of device–bone construct failure at any point up to 6 months following surgery and will be assessed via AP and lateral radiographs. Failure will be defined as the occurrence of any of the following:
Breakage (mechanical fracture) of the femoral nail.55
Breakage (mechanical fracture) of the distal locking screw.
Protrusion of lag screw through the cortex of the femoral head (cut-out).56
A change in TAD of more than 10 mm.
The TAD,57 measured from the tip of the lag screw to the apex of the femoral cortex in lateral and AP radiographs, is generally used in clinical practice and is desirable for this measurement to be under 25 mm. TADs larger than 25 mm have been shown to serve as an accurate indicator of future protrusion of a lag screw through the femoral head (cut-out).58 The TAD has been shown to be reproducible to within 2–3 mm between measurements57 59 and highlighted to change by 2–3 mm over time.60 A change in TAD of more than 10 mm has therefore been selected as a reference level which represents failure of the IM device to maintain fracture stability.
Secondary outcome measures
Secondary outcomes will also assess differences in the effectiveness between the interventions using several measures, including the following.
Femoral neck shortening
Femoral neck shortening will be measured from the AP radiograph along the long axis of the femur. This is a frequently used measure after surgical treatment of hip fractures61 62 and is regarded a reliable measure.63
Functional independence: FIM Score
The FIM Score is a widely used instrument for measuring the severity of patient disability and dependence in rehabilitation medicine.64 It has been demonstrated as a validated and reliable measure65 with good interrater reliability of the total score (Intraclass correlation coefficient of 0.96).66
Pain: VAS
Pain will be assessed using a VAS67 of categorical values from 0 to 10, with 0 indicating no pain and 10 indicating excruciating pain. The VAS pain score is a commonly used and validated measurement for patient-reported acute pain.68
Hip function: HHS
Hip function will be assessed by a clinician using the HHS to evaluate hip function and disability across domains of pain, function, absence of deformity and range of motion.69 The HHS is a well performing70 and frequently used clinician-based outcome measure that has shown high reliability and validity in evaluating hip function.71 72
Perioperative data
Perioperative data recorded in this trial will include surgery time, fluoroscopy time, intraoperative TAD, length of hospital stay, union time and intraoperative complications, all of which are commonly reported as valid measures across a number of RCTs evaluating femoral nail devices.40 73–77 Intraoperative blood loss will also be recorded, however the reliability of this measure is unclear due to its underestimation during hip fracture surgery.78
Injury/surgery-specific complications
Surgical complications not only affect clinical outcome parameter, but appear to be a significant and often long-term predictor of patient postoperative psychosocial outcomes.79 80 Complications recorded will include the number and type of injury and surgery-specific events and complications including, technical complications, surgical site infection, unplanned surgery and death up to 1 year following surgery. This has been reliably collected in previous studies.81 82
Reoperation
The number of patients presenting to the clinic requiring reoperation will be recorded in this trial. The rate of reoperation is a reliable measure in assessing quality of medical treatment.83
General medical complications
In this study, the number of patients suffering from general medical complications will be recorded. This has been collected and reported as a valid measure in the literature.80
Secondary outcome measures: biomechanics subgroup
Physical mobility: Timed up and go
Physical mobility will be assessed using the timed up and go (TUG) test which has been widely used in the literature84 and noted to be a practical and reliable performance indicator of physical mobility.85 The validity of the TUG has been highlighted with its correlation with a number of mobility and performance measures such as the BBS86 and gait speed85 87 with normative reference values available.88
Hip biomechanics and function: 3D motion analysis
Using a 10 camera motion capture system (Vicon Motion Systems Ltd, Oxford, UK, 100 Hz), 3D kinematic data will be collected as patients are asked to walk short distances between two marked points at their own comfortable pace. A set of 49 retroreflective markers will be placed on anatomical landmarks of each patient to identify positions of joints, in line with standardised position and coordinate system protocols established by the International Society of Biomechanics.89 In addition to the recorded 3D marker trajectories using the above motion capture setup, ground reaction forces will be measured via two force platforms (AMTI Optima, Watertown, Massachusetts, USA, 2000 Hz) as well as superficial muscle activity (ie, activation, timing and amplitude) using passive surface electromyography electrodes (Delsys, Boston, Massachusetts, USA, 2000 Hz, contact material 99.99% silver, interbar spacing 10 mm, common mode rejection ratio >80 dB). These electrodes will be placed on the hamstring (biceps femoris), gluteal muscles (gluteus maximus and gluteus medius), quadriceps (rectus femoris and vastus medialis) and hip adductor (adductor longus) of each leg, in line with ‘Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles’ (SENIAM) guidelines. This standardisation ensures reliability in using 3D motion capture for the measurement gait parameters.90
An OpenSim91 model will be scaled using the 3D motion data alongside the Musculoskeletal Atlas Programme software to produce patient-tailored musculoskeletal models.92 Dynamic simulations will be run on the musculoskeletal models using OpenSim to calculate objective outcome measures from gait analysis including (but not limited to):
Hip range of motion.
Hip joint contact forces.
Hip muscle force (simulated).
Gait speed
Gait speed will be calculated from the motion capture trials as a valid and reliable measure of physical performance during gait, commonly reported in the literature.93
24-Hour activity patterns
Twenty-four hours activity monitoring data over a 7-day period will be collected at the 6 week and 6 month time points, using wearable accelerometers at the wrist (GeneActiv Original, Activinsights Ltd, Kimbolton, UK, 100 Hz). Patients will be asked to wear these activity monitors, at all times besides bathing. To better distinguish sedentary time from sleep time, patients will also be asked to fill in sleep logs. Physical activity measured using wrist-worn accelerometers has been strongly correlated to gross motor activity patterns measured using waist-worn monitors.94 Wrist-worn accelerometers will be used as opposed to waist-worn or ankle-worn accelerometers for better compliance.
Data management
Outcome data will be entered electronically and stored in a password-protected shared drive and backed up weekly to a password-protected folder on the University of Adelaide’s network. Only investigators will have access to the data.
Sample size
Gamma3 nail failure rates in the literature have been reported to vary from 2% to 15%.95 96 We opted for a conservative failure estimate of 7.5% as it represents the mid-range of reported data.97 A clinically significant difference between the two groups would be a difference of 5% between the two intervention groups. Therefore using a significance level of 0.05 and power of 80%, allowing for 30% loss to follow-up at 6 months (including 10% mortality) and a 1.5 variance inflation factor to allow for repeated measurements over time, results in a requirement for 300 patients in each of the three groups (control group—unlocked Gamma3 nail, and each of the intervention groups—unlocked and locked Intertan nail).
Statistical analysis
The primary outcome measure of device failure is considered a binary outcome (device failed/did not fail). A binary logistic regression model will be performed to assess the association between the outcome of device failure and the predictor of device type (Gamma3 unlocked, Intertan unlocked and Intertan locked). Confounders of AMTS and gender will also be included in the model as covariates as they were stratification factors in the randomisation. Post-hoc comparisons will result in ORs, 95% CIs, comparison p values and a global p value.
Some secondary outcomes are measured over two time periods. The FIM Score is measured at patient discharge and at 6 months. Therefore, a linear mixed-effects model will be used for the outcome of FIM Score and the interaction of time and device type, adjusting for repeated measurements over time as a random effect. A logarithmic transformation of the outcome may be necessary. Similarly, as pain (VAS) and HHS are measured at 6 weeks and 6 months, linear mixed-effects models will be used for these outcomes. For perioperative continuous outcomes, including surgery time, fluoroscopy time, intraoperative TAD, length of hospital stay and fracture union time, the association with device type will be investigated using a linear regression. For dichotomous secondary outcomes, including intraoperative complications, injury-specific complication rates, reoperation rates and general medical complication rates, the association with device type will be investigated using binary logistic regression. Stratification variables AMTS and gender will be included as covariates in all secondary regression models.
For the biomechanics subgroup, secondary measures of gait speed, hip range of motion, hip muscle forces and joint contact forces will be measured at 6 weeks and 6 months. Three linear mixed-effects models will be used with the device type (Gamma3 unlocked, Intertan unlocked and Intertan locked) as a fixed factor with timepoint as a repeated measure and the interaction of time and device type. Post-hoc pairwise comparisons will then be used to identify the differences in the outcomes between the timepoints of 6 weeks and 6 months.
An intention-to-treat analysis will be performed (and as randomised analysis to deal with protocol non-adherence). Missing data will be handled on the basis of each outcome—if a patient is missing outcome data for a particular regression, they will be excluded from that regression. However, if they are not missing data for the remaining outcomes, they will be included in those analyses. The use of linear mixed-effects models also retains patient data when there is missing data from only one time period. Evidence for a statistically significant difference will be accepted as p<0.05. The statistical software that will be used is SAS V.9.4 (SAS Institute Inc, Cary, North Carolina, USA).
Trial oversight
The overall oversight of the trial will be under the responsibility of the head of the Department for Orthopaedics and Trauma at the Royal Adelaide Hospital and supported by the University of Adelaide’s Centre for Orthopaedic & Trauma Research. A Trial Steering Committee (TSC) and Data Safety and Monitoring Committee (DSMC) will be set up. The TSC will comprise of the chief investigator and associate investigator and will provide overall supervision. the DSMC will comprise of an associate investigator, clinicians and database management staff at the Royal Adelaide Hospital. The DSMC and TSC will meet prior to commencing the trial with further meetings arranged depending on the trial requirements.
Supplementary Material
Footnotes
Contributors: MR, DT and AS contributed to the design and implementation of the study. AL will contribute to data collection and SE will contribute to the statistical analysis of the data. AS will be responsible for data collection, processing and analysis of the biomechanics subgroup. AS, AL, MR, DT and SE contributed to the writing of this manuscript. All investigators will communicate any protocol modifications such that amendments can be made to the relevant parties (ethics committee, trial registry).
Funding: This project will be funded through an investigator initiated research grant awarded by Smith & Nephew Inc Orthopaedic Division. DT is supported by a Career Development Fellowship (ID: 1126229) from the National Health and Medical Research Council.
Competing interests: IP is owned by the Royal Adelaide Hospital/University of Adelaide.
Patient consent for publication: Not required.
Ethics approval: This protocol has received ethics approval by the Central Adelaide Local Health Network Human Research Ethics Committee and will be conducted in accordance to the NHMRC National Statement of Ethical Conduct in Human Research. This clinical trial has been registered on the Australia New Zealand Clinical Trials Registry (ANZCTR): ACTRN12618001431213.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Boulton C, Bunning T, Johansen A, et al. National hip fracture database: annual report 2017. United Kingdom, 2017. [Google Scholar]
- 2. Butt FF, Hussain AS, Khan AM, et al. Implants for extracapsular neck of femur fracture dynamic hip screw versus intramedullary nailing. J Ayub Med Coll Abbottabad 2017;29:697–701. [PubMed] [Google Scholar]
- 3. Harvey N, McCloskey E. Gaps and solutions in bone health: a global framework for improvement. International Osteoporosis Foundation (IOF), 2016. [Google Scholar]
- 4. Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 2004;15:897–902. 10.1007/s00198-004-1627-0 [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Odén A, McCloskey EV, et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 2012;23:2239–56. 10.1007/s00198-012-1964-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kannus P, Parkkari J, Sievänen H, et al. Epidemiology of hip fractures. Bone 1996;18:S57–63. 10.1016/8756-3282(95)00381-9 [DOI] [PubMed] [Google Scholar]
- 7. Lippuner K, Golder M, Greiner R. Epidemiology and direct medical costs of osteoporotic fractures in men and women in Switzerland. Osteoporos Int 2005;16(Suppl 2):S8–17. 10.1007/s00198-004-1696-0 [DOI] [PubMed] [Google Scholar]
- 8. Lyons AR. Clinical outcomes and treatment of hip fractures. Am J Med 1997;103:S51–64. discussion S-4S 10.1016/S0002-9343(97)90027-9 [DOI] [PubMed] [Google Scholar]
- 9. Lakstein D, Hendel D, Haimovich Y, et al. Changes in the pattern of fractures of the hip in patients 60 years of age and older between 2001 and 2010: a radiological review. Bone Joint J 2013;95-B:1250–4. 10.1302/0301-620X.95B9.31752 [DOI] [PubMed] [Google Scholar]
- 10. Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int 1992;2:285–9. 10.1007/BF01623184 [DOI] [PubMed] [Google Scholar]
- 11. Jacob J, Desai A, Trompeter A. Decision Making in the Management of Extracapsular Fractures of the Proximal Femur - is the Dynamic Hip Screw the Prevailing Gold Standard? Open Orthop J 2017;11:1213–7. 10.2174/1874325001711011213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knobe M, Gradl G, Ladenburger A, et al. Unstable intertrochanteric femur fractures: is there a consensus on definition and treatment in Germany? Clin Orthop Relat Res 2013;471:2831–40. 10.1007/s11999-013-2834-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anglen JO, Weinstein JN, American Board of Orthopaedic Surgery Research Committee . Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of orthopaedic surgery database. J Bone Joint Surg Am 2008;90:700–7. 10.2106/JBJS.G.00517 [DOI] [PubMed] [Google Scholar]
- 14. ANZHFR Australia & New Zealand Hip Fracture Registry: Annual Report 2018, 2018. [Google Scholar]
- 15. Sambandam SN, Chandrasekharan J, Mounasamy V, et al. Intertrochanteric fractures: a review of fixation methods. Eur J Orthop Surg Traumatol 2016;26:339–53. 10.1007/s00590-016-1757-z [DOI] [PubMed] [Google Scholar]
- 16. Parker MJ, Handoll HHG. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev 2008:CD000093 10.1002/14651858.CD000093.pub4 [DOI] [PubMed] [Google Scholar]
- 17. Socci AR, Casemyr NE, Leslie MP, et al. Implant options for the treatment of intertrochanteric fractures of the hip. Bone Joint J 2017;99-B:128–33. 10.1302/0301-620X.99B1.BJJ-2016-0134.R1 [DOI] [PubMed] [Google Scholar]
- 18. Yu J, Zhang C, Li L, et al. Internal fixation treatments for intertrochanteric fracture: a systematic review and meta-analysis of randomized evidence. Sci Rep 2016;5:18195 10.1038/srep18195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giordano V, Ribeiro DN, Tinoco RG, et al. A survey of current practices and preferences for internal fixation of trochanteric fractures of the femur in Brazil. Cureus 2018;10:e2286 10.7759/cureus.2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie B, Wu D, Yang Z, et al. Comparison of intramedullary fixation and arthroplasty for the treatment of intertrochanteric hip fractures in the elderly: a meta-analysis. Medicine 2017;96:e7446 10.1097/MD.0000000000007446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giessauf C, Glehr M, Bernhardt GA, et al. Quality of life after pertrochanteric femoral fractures treated with a γ nail: a single center study of 62 patients. BMC Musculoskelet Disord 2012;13:214 10.1186/1471-2474-13-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kempf I, Grosse A, Taglang G, et al. Gamma nail in the treatment of closed trochanteric fractures. results and indications of 121 cases. Orthop Traumatol Surg Res 2014;100:75–83. 10.1016/j.otsr.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 23. Yang Y-H, Wang Y-R, Jiang S-D, et al. Proximal femoral nail antirotation and third-generation gamma nail: which is a better device for the treatment of intertrochanteric fractures? Singapore Med J 2013;54:446–50. 10.11622/smedj.2013152 [DOI] [PubMed] [Google Scholar]
- 24. Schupfner R, Käsmann LT, Wagner W, et al. Complications in Treatment of 31-A Fractures with Trochanteric Gamma Nail (TGN) Versus Gamma3 Nail (G3N) - A Review of 217 Cases. Open Orthop J 2016;10:389–95. 10.2174/1874325001610010389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Georgiannos D, Lampridis V, Bisbinas I. Complications following treatment of trochanteric fractures with the gamma3 nail: is the latest version of gamma nail superior to its predecessor? Surg Res Pract 2014;2014:143598:1–6. 10.1155/2014/143598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Unger AC, Wilde E, Kienast B, et al. Treatment of trochanteric fractures with the gamma3 nail - methodology and early results of a prospective consecutive monitored clinical case series. Open Orthop J 2014;8:466–73. 10.2174/1874325001408010466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varela-Egocheaga JR, Iglesias-Colao R, Suárez-Suárez MA, et al. Minimally invasive osteosynthesis in stable trochanteric fractures: a comparative study between Gotfried percutaneous compression plate and gamma 3 intramedullary nail. Arch Orthop Trauma Surg 2009;129:1401–7. 10.1007/s00402-009-0955-0 [DOI] [PubMed] [Google Scholar]
- 28. Schipper IB, Marti RK, van der Werken C. Unstable trochanteric femoral fractures: extramedullary or intramedullary fixation. review of literature. Injury 2004;35:142–51. 10.1016/S0020-1383(03)00287-0 [DOI] [PubMed] [Google Scholar]
- 29. Queally JM, Harris E, Handoll HHG, et al. Intramedullary nails for extracapsular hip fractures in adults. Cochrane Database Syst Rev 2014:CD004961 10.1002/14651858.CD004961.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruecker AH, Rupprecht M, Gruber M, et al. The treatment of intertrochanteric fractures: results using an intramedullary nail with integrated cephalocervical screws and linear compression. J Orthop Trauma 2009;23:22–30. 10.1097/BOT.0b013e31819211b2 [DOI] [PubMed] [Google Scholar]
- 31. Serrano R, Blair JA, Watson DT, et al. Cephalomedullary nail fixation of Intertrochanteric femur fractures: are two proximal screws better than one? J Orthop Trauma 2017;31:577–82. 10.1097/BOT.0000000000000967 [DOI] [PubMed] [Google Scholar]
- 32. Knobe M, Gradl G, Buecking B, et al. Locked minimally invasive plating versus fourth generation nailing in the treatment of AO/OTA 31A2.2 fractures: A biomechanical comparison of PCCP(®) and Intertan nail(®). Injury 2015;46:1475–82. 10.1016/j.injury.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 33. Zhang C, Xu B, Liang G, et al. Optimizing stability in AO/OTA 31-A2 intertrochanteric fracture fixation in older patients with osteoporosis. J Int Med Res 2018;46:1767–78. 10.1177/0300060518761504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seyhan M, Turkmen I, Unay K, et al. Do PFNA devices and Intertan nails both have the same effects in the treatment of trochanteric fractures? A prospective clinical study. J Orthop Sci 2015;20:1053–61. 10.1007/s00776-015-0750-4 [DOI] [PubMed] [Google Scholar]
- 35. Gavaskar AS, Tummala NC, Srinivasan P, et al. Helical blade or the integrated lag screws: a matched pair analysis of 100 patients with unstable trochanteric fractures. J Orthop Trauma 2018;32:274–7. 10.1097/BOT.0000000000001145 [DOI] [PubMed] [Google Scholar]
- 36. Nüchtern JV, Ruecker AH, Sellenschloh K, et al. Malpositioning of the lag screws by 1- or 2-screw nailing systems for pertrochanteric femoral fractures: a biomechanical comparison of gamma 3 and intertan. J Orthop Trauma 2014;28:276–82. 10.1097/BOT.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 37. Santoni BG, Nayak AN, Cooper SA, et al. Comparison of femoral head rotation and varus collapse between a single lag screw and integrated dual screw Intertrochanteric hip fracture fixation device using a cadaveric Hemi-Pelvis biomechanical model. J Orthop Trauma 2016;30:164–9. 10.1097/BOT.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 38. Kubiak EN, Bong M, Park SS, et al. Intramedullary fixation of unstable intertrochanteric hip fractures: one or two lag screws. J Orthop Trauma 2004;18:12–17. 10.1097/00005131-200401000-00003 [DOI] [PubMed] [Google Scholar]
- 39. Kouvidis GK, Sommers MB, Giannoudis PV, et al. Comparison of migration behavior between single and dual lag screw implants for intertrochanteric fracture fixation. J Orthop Surg Res 2009;4:16 10.1186/1749-799X-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma J-X, Kuang M-J, Fan Z-R, et al. Comparison of clinical outcomes with InterTan vs gamma nail or PFNA in the treatment of intertrochanteric fractures: a meta-analysis. Sci Rep 2017;7:15962 10.1038/s41598-017-16315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allen WC, Piotrowski G, Burstein AH, et al. Biomechanical principles of intramedullary fixation. Clin Orthop Relat Res 1968;60:13–20. 10.1097/00003086-196809000-00003 [DOI] [PubMed] [Google Scholar]
- 42. Kyle RF. Biomechanics of intramedullary fracture fixation. Orthopedics 1985;8:1356–9. 10.3928/0147-7447-19851101-08 [DOI] [PubMed] [Google Scholar]
- 43. Perren SM. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop Relat Res 1979:175–96. [PubMed] [Google Scholar]
- 44. Fang C, Gudushauri P, Wong T-M, et al. Increased fracture collapse after Intertrochanteric fractures treated by the dynamic hip screw adversely affects walking ability but not survival. Biomed Res Int 2016;2016:1–8. 10.1155/2016/4175092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoo J-H, Kim T-Y, Chang J-D, et al. Factors influencing functional outcomes in United intertrochanteric hip fractures: a negative effect of lag screw sliding. Orthopedics 2014;37:e1101–7. 10.3928/01477447-20141124-58 [DOI] [PubMed] [Google Scholar]
- 46. Loch DA, Kyle RF, Bechtold JE, et al. Forces required to initiate sliding in second-generation intramedullary nails. J Bone Joint Surg Am 1998;80:1626–31. 10.2106/00004623-199811000-00009 [DOI] [PubMed] [Google Scholar]
- 47. Mahomed MN, Harrington IJ, Hearn TC. Biomechanical analysis of the Medoff sliding plate. J Trauma 2000;48:93–100. 10.1097/00005373-200001000-00016 [DOI] [PubMed] [Google Scholar]
- 48. Euler E, Betz A, Schweiberer L. The treatment of trochanteric and femoral neck fractures using the dynamic hip screw (DHS). Orthop Traumatol 1992;1:246–58. 10.1007/BF02620365 [DOI] [Google Scholar]
- 49. Choueka J, Koval KJ, Kummer FJ, et al. Biomechanical comparison of the sliding hip screw and the dome plunger. Effects of material and fixation design. J Bone Joint Surg Br 1995;77-B:277–83. 10.1302/0301-620X.77B2.7706347 [DOI] [PubMed] [Google Scholar]
- 50. Dodds SD, Baumgaertner MR. The sliding hip screw. Curr Opin Orthop 2004;15:12–17. 10.1097/00001433-200402000-00005 [DOI] [Google Scholar]
- 51. Zhu Y, Meili S, Zhang C, et al. Is the lag screw sliding effective in the intramedullary nailing in A1 and A2 AO-OTA intertrochanteric fractures? A prospective study of sliding and None-sliding lag screw in Gamma-III nail. Scand J Trauma Resusc Emerg Med 2012;20:60 10.1186/1757-7241-20-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trauma S. Gamma3 trochanteric nail 170 & 180 operative technique. 33 Schönkirchen, Germany: Stryker, 2016. [Google Scholar]
- 53. Nephew S. Trigen Intertan trochanteric antegrade nail: operative technique. Tenessee, USA: Smith & Nephew, 2012: 31. [Google Scholar]
- 54. Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 55. Mavrogenis AF, Panagopoulos GN, Megaloikonomos PD, et al. Complications after hip nailing for fractures. Orthopedics 2016;39:e108–16. 10.3928/01477447-20151222-11 [DOI] [PubMed] [Google Scholar]
- 56. Bojan AJ, Beimel C, Taglang G, et al. Critical factors in cut-out complication after gamma nail treatment of proximal femoral fractures. BMC Musculoskelet Disord 2013;14:1 10.1186/1471-2474-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baumgaertner MR, Curtin SL, Lindskog DM, et al. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Joint Surg Am 1995;77:1058–64. 10.2106/00004623-199507000-00012 [DOI] [PubMed] [Google Scholar]
- 58. Geller JA, Saifi C, Morrison TA, et al. Tip-apex distance of intramedullary devices as a predictor of cut-out failure in the treatment of peritrochanteric elderly hip fractures. Int Orthop 2010;34:719–22. 10.1007/s00264-009-0837-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Bruijn K, den Hartog D, Tuinebreijer W, et al. Reliability of predictors for screw cutout in intertrochanteric hip fractures. J Bone Joint Surg Am 2012;94:1266–72. 10.2106/JBJS.K.00357 [DOI] [PubMed] [Google Scholar]
- 60. Aihara LJ, Nanni RA, Carvalho MS, et al. Late postoperative analysis of the tip-apex distance (TAD) in pertrochanteric fractures: is there an accommodation of the implant within the bone? Injury 2017;48(Suppl 4):S54–6. 10.1016/S0020-1383(17)30776-3 [DOI] [PubMed] [Google Scholar]
- 61. Felton J, Slobogean GP, Jackson SS, et al. Femoral neck shortening after hip fracture fixation is associated with inferior hip function: results from the faith trial. J Orthop Trauma 2019;33:487–96. 10.1097/BOT.0000000000001551 [DOI] [PubMed] [Google Scholar]
- 62. Zielinski SM, Keijsers NL, Praet SFE, et al. Femoral neck shortening after internal fixation of a femoral neck fracture. Orthopedics 2013;36:e849–58. 10.3928/01477447-20130624-13 [DOI] [PubMed] [Google Scholar]
- 63. Marmor M, Nystuen C, Ehemer N, et al. Accuracy of in situ neck-shaft angle and shortening measurements of the anatomically reduced, varus malreduced and shortened proximal femur: can we believe what we see on the postoperative films? Injury 2012;43:846–9. 10.1016/j.injury.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 64. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the functional independence measure. Arch Phys Med Rehabil 1994;75:127–32. [PubMed] [Google Scholar]
- 65. Ottenbacher KJ, Hsu Y, Granger CV, et al. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996;77:1226–32. 10.1016/S0003-9993(96)90184-7 [DOI] [PubMed] [Google Scholar]
- 66. Hamilton BB, Laughlin JA, Fiedler RC, et al. Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 1994;26:115–9. [PubMed] [Google Scholar]
- 67. Katz J, Melzack R. Measurement of pain. Surg Clin North Am 1999;79:231–52. 10.1016/S0039-6109(05)70381-9 [DOI] [PubMed] [Google Scholar]
- 68. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011;152:2399–404. 10.1016/j.pain.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 69. Nilsdotter A, Bremander A. Measures of hip function and symptoms: Harris hip score (HHS), hip disability and osteoarthritis outcome score (HOOS), Oxford hip score (OHS), Lequesne index of severity for osteoarthritis of the hip (LISOH), and American Academy of orthopedic surgeons (AAOS) Hip and Knee Questionnaire. Arthritis Care Res 2011;63:S200–7. 10.1002/acr.20549 [DOI] [PubMed] [Google Scholar]
- 70. Frihagen F, Grotle M, Madsen JE, et al. Outcome after femoral neck fractures: a comparison of Harris hip score, Eq-5d and Barthel index. Injury 2008;39:1147–56. 10.1016/j.injury.2008.03.027 [DOI] [PubMed] [Google Scholar]
- 71. Söderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? 2001: 189–97. [DOI] [PubMed] [Google Scholar]
- 72. Hoeksma HL, Van Den Ende CHM, Ronday HK, et al. Comparison of the responsiveness of the Harris hip score with generic measures for hip function in osteoarthritis of the hip. Ann Rheum Dis 2003;62:935–8. 10.1136/ard.62.10.935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hopp S, Wirbel R, Ojodu I, et al. Does the implant make the difference ? - Prospective comparison of two different proximal femur nails. Acta Orthop Belg 2016;82:319–31. [PubMed] [Google Scholar]
- 74. Berger-Groch J, Rupprecht M, Schoepper S, et al. Five-year outcome analysis of Intertrochanteric femur fractures: a prospective randomized trial comparing a 2-Screw and a Single-Screw Cephalomedullary nail. J Orthop Trauma 2016;30:483–8. 10.1097/BOT.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 75. Su H, Sun K, Wang X. A randomized prospective comparison of Intertan and gamma3 for treating unstable intertrochanteric fractures. Int J Clin Exp Med 2016;9:8640–7. [Google Scholar]
- 76. Wu D, Ren G, Peng C, et al. InterTan nail versus gamma3 nail for intramedullary nailing of unstable trochanteric fractures. Diagn Pathol 2014;9:191 10.1186/s13000-014-0191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Macefield RC, Boulind CE, Blazeby JM. Selecting and measuring optimal outcomes for randomised controlled trials in surgery. Langenbecks Arch Surg 2014;399:263–72. 10.1007/s00423-013-1136-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Budair B, Ahmed U, Hodson J, et al. Are we all guilty of under-estimating intra-operative blood loss during hip fracture surgery? J Orthop 2017;14:81–4. 10.1016/j.jor.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pinto A, Faiz O, Davis R, et al. Surgical complications and their impact on patients' psychosocial well-being: a systematic review and meta-analysis. BMJ Open 2016;6:e007224 10.1136/bmjopen-2014-007224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lawrence VA, Hilsenbeck SG, Noveck H, et al. Medical complications and outcomes after hip fracture repair. Arch Intern Med 2002;162:2053–7. 10.1001/archinte.162.18.2053 [DOI] [PubMed] [Google Scholar]
- 81. Flikweert ER, Wendt KW, Diercks RL, et al. Complications after hip fracture surgery: are they preventable? Eur J Trauma Emerg Surg 2018;44:573–80. 10.1007/s00068-017-0826-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim K-H, Han KY, Kim KW, et al. Local postoperative complications after surgery for Intertrochanteric fractures using Cephalomedullary nails. Hip Pelvis 2018;30:168–74. 10.5371/hp.2018.30.3.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matre K, Havelin LI, Gjertsen J-E, et al. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop Relat Res 2013;471:1379–86. 10.1007/s11999-012-2728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Herman T, Giladi N, Hausdorff JM. Properties of the 'timed up and go' test: more than meets the eye. Gerontology 2011;57:203–10. 10.1159/000314963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 86. Bennie S, Bruner K, Dizon A, et al. Measurements of balance: comparison of the timed "up and go" test and functional reach test with the berg balance scale. J Phys Ther Sci 2003;15:93–7. 10.1589/jpts.15.93 [DOI] [Google Scholar]
- 87. Freter SH, Fruchter N. Relationship between timed 'up and go' and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil 2000;14:96–101. 10.1191/026921500675545616 [DOI] [PubMed] [Google Scholar]
- 88. Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther 2006;29:64–8. 10.1519/00139143-200608000-00004 [DOI] [PubMed] [Google Scholar]
- 89. Wu G, Siegler S, Allard P, et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion--part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech 2002;35:543–8. 10.1016/S0021-9290(01)00222-6 [DOI] [PubMed] [Google Scholar]
- 90. Tsushima H, Morris ME, McGinley J. Test-Retest reliability and inter-tester reliability of kinematic data from a three-dimensional gait analysis system. J Jpn Phys Ther Assoc 2003;6:9–17. 10.1298/jjpta.6.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Delp SL, Anderson FC, Arnold AS, et al. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 2007;54:1940–50. 10.1109/TBME.2007.901024 [DOI] [PubMed] [Google Scholar]
- 92. Zhang J, Fernandez J, Hislop-Jambrich J, et al. Lower limb estimation from sparse landmarks using an articulated shape model. J Biomech 2016;49:3875–81. 10.1016/j.jbiomech.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 93. Kline Mangione K, Craik RL, Lopopolo R, et al. Predictors of gait speed in patients after hip fracture. Physiother Can 2008;60:10–18. 10.3138/physio/60/1/10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dieu O, Mikulovic J, Fardy PS, et al. Physical activity using wrist-worn accelerometers: comparison of dominant and non-dominant wrist. Clin Physiol Funct Imaging 2017;37:525–9. 10.1111/cpf.12337 [DOI] [PubMed] [Google Scholar]
- 95. Bojan AJ, Beimel C, Speitling A, et al. 3066 consecutive gamma nails. 12 years experience at a single centre. BMC Musculoskelet Disord 2010;11:133 10.1186/1471-2474-11-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoffmann S, Paetzold R, Stephan D, et al. Biomechanical evaluation of interlocking lag screw design in intramedullary nailing of unstable pertrochanteric fractures. J Orthop Trauma 2013;27:483–90. 10.1097/BOT.0b013e3182a1f54b [DOI] [PubMed] [Google Scholar]
- 97. Abram SGF, Pollard TCB, Andrade AJMD. Inadequate 'three-point' proximal fixation predicts failure of the Gamma nail. Bone Joint J 2013;95-B:825–30. 10.1302/0301-620X.95B6.31018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032640supp001.pdf (159.2KB, pdf)