Abstract
Objectives
The present study investigated (1) trends in the prevalence and incidence of knee osteoarthritis over a 20-year period (1996–2015); (2) trends in multimorbidity and (3) trends in drug prescriptions.
Design
Registry-based study.
Setting
Primary healthcare, Flanders, Belgium.
Participants
Data were collected from Intego, a general practice-based morbidity registration network. In the study period between 1996 and 2015, data from 440 140 unique patients were available.
Outcome measures
Trends in prevalence and incidence rate of knee osteoarthritis were computed using joinpoint regression analysis. The mean disease count was calculated to assess trends in multimorbidity. In addition, the number of drug prescriptions was identified by the Anatomical Therapeutic Chemical Classification code and trends were equally recorded with joinpoint regression.
Results
The total age-standardised prevalence of knee osteoarthritis increased from 2.0% in 1996 to 3.6% in 2015. An upward trend was observed with an average annual percentage change (AAPC) of 2.5 (95% CI 2.2 to 2.9). In 2015, the prevalence rates in the 10 year age groups from the 45–54 years age group onwards were 3.1%, 5.6%, 9.0% and 13.9%, to reach 15.0% in people aged 85 years and older. The incidence remained stable with 3.75‰ in 2015 (AAPC=−0.5, 95% CI −1.4 to 0.5). The mean disease count significantly increased from 1.63 to 2.34 (p<0.001) for incident cases with knee osteoarthritis. Finally, we observed a significantly positive trend in the overall prescription of acetaminophen (AAPC=6.7, 95% CI 5.6 to 7.7), weak opioids (AAPC=4.0, 95% CI 0.9 to 7.3) and glucosamine (AAPC=8.6, 95% CI 2.4 to 15.1). Oral non-steroidal anti-inflammatory drugs were most prescribed, with a prevalence rate of 29.8% in 2015, but remained stable during the study period (AAPC=0.0, 95% CI −1.1 to 1.1).
Conclusions
Increased prevalence, multimorbidity, and number of drug prescriptions confirm an increased burden of knee osteoarthritis. In future, these trends can be used to prioritise initiatives for improvement in care.
Keywords: knee, musculoskeletal disorders, primary care, public health
Strengths and limitations of this study.
The Intego open registry, with primary care data over a 20-year-time period (1996–2015), is representative for the Flemish population and lends itself for trend analyses.
Estimates on the prevalence and incidence of knee osteoarthritis are scarce for primary care settings. This study defines knee osteoarthritis when it becomes a healthcare problem for the patient.
Data completeness depends on the quality of registration of the participating general practitioners. To this end, only optimal registration practices are included in the Intego database.
The lack of data verification and misclassification is minimalised because new diagnoses are automatically linked to International Classification of Primary Care Version 2 and International Statistical Classification of Diseases and Related Health Problems 10thRevision codes.
Introduction
Osteoarthritis (OA) is the most common joint disease and is expected to become the fourth leading cause of disability worldwide by 2020.1 OA mainly affects the joints of the knees, hips, hands, facets and feet, but knee OA accounts for 83% of the total OA burden.2 The prevalence of knee OA varies according to the definition: from subjective (population-based) assessments to clinical and radiographic definitions, often with low levels of concordance between them.3 However, estimates on the prevalence of knee OA are scarce for primary care settings.4
At present, the purposes of conservative knee OA treatment are to alleviate pain, to improve the function of the joint and to slow down joint damage by pharmacological and non-pharmacological means.5 All patients should be offered the following core conservative interventions: information to enhance their understanding about OA, advice to exercise and to achieve weight loss for people who are obese or overweight.6 7 Pharmacological management is dominated both by acetaminophen and by non-steroidal anti-inflammatory drugs (NSAIDs).5 8 9 The presence of multimorbidity may also affect choices in the pharmacological management, since multimorbidity and polypharmacy are closely related.6 10 11 OA has one of the highest rates of multimorbidity for patients who are managed in general practice.12 13 Common multimorbidities in patients with knee OA are cardiovascular diseases, diabetes mellitus, chronic obstructive pulmonary disease and obesity.14 Nevertheless, multimorbidity-adapted management protocols are being developed and provide tailored guidance for pharmacological management and exercise therapy.5 7 Numerous reports indicate that the number of people suffering from chronic diseases, multimorbidity and polypharmacy continues to increase, but those studies are mainly based on cross-sectional studies in different populations.15 Time trends in the prevalence of multimorbidity and polypharmacy are scare.16 17 The Flemish primary care-based Intego database offers the opportunity to extract ‘real-world’ data and evaluate time trends.
The aims of the present study were (1) to evaluate time trends in the prevalence and incidence of patients with knee OA managed in general practice; (2) to assess trends in multimorbidity and (3) to assess trends in drug prescriptions over a 20-year period.
Methods
Data source
This trend analysis study was performed using Intego, a general practice-based morbidity registration network in Flanders, Belgium.18 The Intego database comprises data extracted from electronic health records (EHR) of general practitioners (GPs), all using the medical software programme Medidoc (Corilus NV, Aalter, Belgium).19 Systematic collection of data started in 1994. In 2015, 111 GPs of 48 practices evenly spread throughout Flanders collaborated in the Intego project. GPs applied for inclusion in the registry. Before acceptance of their data, registration performance was audited using a number of algorithms that compared their results with those of all other applicants. Only the data of the practices with an optimal registration performance were included in the database. The design, selection process, quality control procedures and comparability with other (inter)national registration networks were described in detail previously.18 The Intego GPs prospectively and routinely registered all new diagnoses using computer-generated keywords internally linked to codes together with new drug prescriptions, as well as laboratory test results, some background information (including gender and year of birth) and some biomedical parameters (ie, blood pressure, height, weight, smoking status and mortality). With specially framed extraction software, new data were encrypted and collected from the GPs’ personal computers and entered into a central database. Registered data were continuously updated and historically accumulated for each patient. New diagnoses were classified according to a detailed thesaurus and automatically linked to the International Classification of Primary Care (ICPC-2) and International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10).20 Drugs were classified according to the WHO’s Anatomical Therapeutic Chemical classification system.21
Study population
For the present study, data over a 20-year time interval from 1 January 1996 to 31 December 2015 were used. Since Intego is an open registry, the amount of unique patients changes every year. The yearly contact group (YCG), defined as the number of unique patients who consult their GP in a given year, was used to describe the population at risk (denominator) in this study.22 Throughout the study period, 79 GP practices provided their data, with 72% contributing for 15 or more years (see online supplementary file 1). This study was reported in accordance with the REporting of studies Conducted using Observational Routinely-collected health Data checklist specific to observational studies using routinely collected health data.23
bmjopen-2019-031734supp001.pdf (55KB, pdf)
Measures
Data on prevalence and incidence
Patients with knee OA were identified based on an ICPC-2 coded diagnosis in their EHR. The prevalence of a population is the proportion of the population with the disease at a specified time. Unlike incidence rates, which focus on new events, prevalence focuses on existing states. Because of the design of Intego (no episode registration and no recording of cure), prevalence rates could only be calculated on incurable chronic diseases, such as knee OA.18 The incidence in Intego is calculated as the number of new cases of disease divided by the person-time magnitude. Calculating disease prevalence and incidence requires both a numerator (number of events or persons with a disease) and a matching denominator (the ‘population at risk’ being studied). Determining primary care practice denominators is challenging.24 In this study, the YCG was used as denominator for all time trend analyses.22
Data on multimorbidity
The Intego registry captures the historical diagnoses of an included patient, and not just the diagnoses made in the years the data were send to the repository. This means that all information on comorbid diseases is integrated at the time of patient’s inclusion. There are several instruments available to calculate multimorbidity, for example, the Carlson Index, the Cumulative Illness Rating Scale, the Index of Coexistent Diseases and the Kaplan Index.25–28 For this study, the disease count was calculated for all incident cases with knee OA (ie, at the time when knee OA was registered as a diagnosis). For this disease count, a list of chronic diseases based on the paper by Knottnerus et al was used.29 For the presence of chronic kidney disease, the glomerular filtration rate was based on the closest creatinine measurement in the 2 years before or after presentation with knee OA diagnosis (online supplementary file 2: ICPC codes for diagnosis and multimorbidity).
bmjopen-2019-031734supp002.pdf (58.2KB, pdf)
Data on drug prescriptions
The prescription of medication for knee OA, including acetaminophen, oral and topical anti-inflammatory drugs, cox-2 selective anti-inflammatory drugs, weak and strong opioids, parenteral glucocorticoids, parenteral hyaluronic acid and glucosamine was extracted from Intego for all prevalent cases with knee OA (online supplementary file 3: used Anatomical Therapeutic Chemical (ACT) Classification System codes). Prescription of medication was considered positive if it was prescribed at least once a year.
bmjopen-2019-031734supp003.pdf (12.6KB, pdf)
Statistical analysis
Descriptive statistics, with frequency distribution and percentages, were used to measure the prevalence (/100 patients) and incidence (/1000 patient years at risk) of patients with knee OA. Data were stratified by gender and 10-year age cohorts, starting from 25 with 85 years and older as the last cohort. The rates were age standardised by taking the Flemish population of the year 1996 as reference population.30 Additionally, possible time trends were analysed in the age-standardised cohorts with joinpoint regression analysis.31 Joinpoint analysis identifies the best-fitting point, where a statistically significant change (called the ‘joinpoint’) occurs, and determines the trends between joinpoints. Joinpoint regression allows us to identify the time point(s) of follow-up at which trends significantly change.32 A minimum number of three observations from a joinpoint to either end of the data, and a minimum number of four observations between two joinpoints were required.33 The annual percentage change (APC) is proposed to summarise and compare the rates of changes between successive change points.34 In the final model, the joinpoint analysis also provides an average annual percentage change (AAPC) as an average of APC estimates.34 This means that trends over a specific period were described by the APC, while trends over the whole 1996–2015 period were summarised using the AAPC. Analysis was performed with the Joinpoint Regression Program (V.3.5.3, released in May 2013 and available at http://surveillance.cancer.gov/joinpoint). This programme starts with the minimum number of joinpoint (eg, zero joinpoints, which is a straight line) and tests whether more joinpoints are statistically significant and must be added to the model. This enables the user to test that an apparent change in trend is statistically significant.
Trends in the multimorbidity profile for incident cases with knee OA were explored over four time intervals of 5 years (1996–2000, 2001–2005, 2006–2010 and 2011–2015) by the Cochran-Armitage test and the Jonckheere-Terpstra test. The Cochran-Armitage test for trend analysis is a modified Pearson’s χ2 test to assess the association between binary and ordinal categories (eg, between multimorbidities and time intervals). The Jonckheere-Terpstra trend test was used to analyse trends for continuous variables (eg, between age and time intervals).35
Over the same 20-year time period, trends in drug prescriptions for prevalent cases with knee OA were analysed using joinpoint regression analysis, as described above. Two-sided p values less than 0.05 were considered to indicate statistical significance. Analyses were performed using R Software V.3.3.2 (Free Software Foundation, Boston, Massachusetts, USA).
Patient involvement
No patients were involved in defining the research question or the outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in the dissemination of the results.
Results
Demographic characteristics and trends in the prevalence and incidence of patients with knee OA (1996–2015)
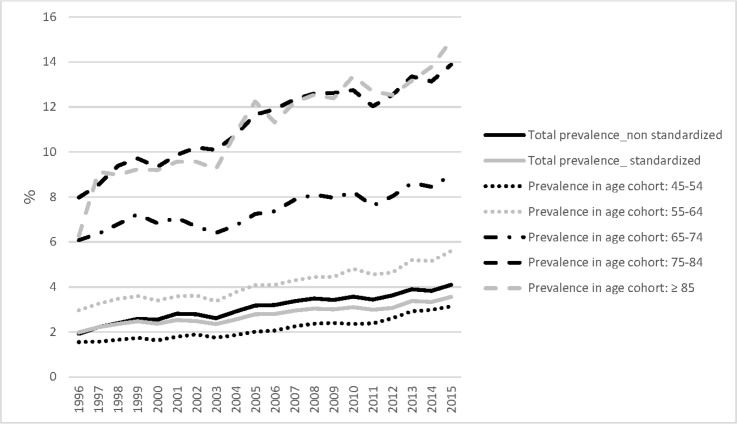
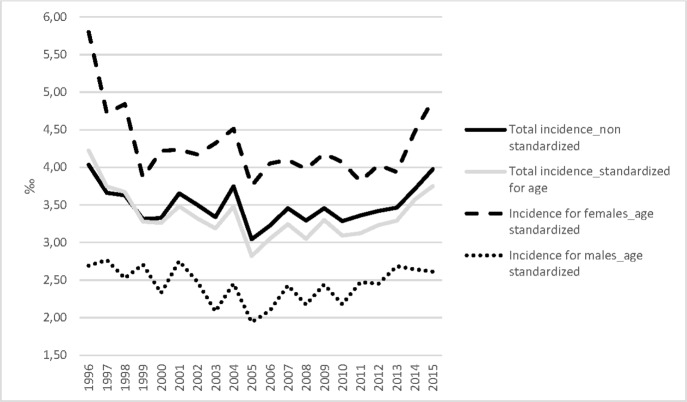
Between 1 January 1996 and 31 December 2015, the Intego database included data on 440 140 unique patients. During the study period, the YCG varied between 81 763 and 151 971 people (see online supplementary file 4 for the exact number per year). Table 1 shows the demographic characteristics of the patients with knee OA by gender and age cohorts. The age-standardised prevalence of knee OA increased by 79% from 1.99% in 1996 to 3.56% in 2015 (AAPC=2.5, 95% CI 2.2 to 2.9, figure 1, online supplementary file 5). Woman have a higher prevalence than men do, but over the 20 years of the study men have a higher relative increase in prevalence (AAPC=3.1, 95% CI 2.7 to 3.5 for men vs AAPC=2.4, 95% CI 2.0 to 2.7 for women). Figure 2 presents the observed and modelled long-term time trends in prevalence by gender. The age-standardised incidence of patients with knee OA remained stable with 4.23‰ in 1996 and 3.75‰ in 2015 (AAPC=−0.5, 95% CI −1.4 to 0.5), but showed a positive trend between 2006 and 2015 from 3.05‰ to 3.75‰, respectively (APC=1.9, 95% CI 0.4 to 3.5) (figure 3). Between 2006 and 2015, this positive trend was higher for men (APC=2.5, 95% CI 0.5 to 4.5) than for women (APC=1.9, 95% CI 0.4 to 3.5; figure 3).
Table 1.
Demographic characteristics and trends in prevalence and incidence of patients with knee osteoarthritis in the Intego registry (1996–2015)
| Year 1996* |
Year 2015* |
Overall trend† | Trend 1 | Trend 2 | Trend 3 | ||||
| % | % | AAPC (95% CI) | Years | APC (95% CI) |
Years | APC (95% CI) |
Years | APC (95% CI) |
|
| Prevalence | |||||||||
| Total | 1.99 | 3.56 | 2.5 (2.2 to 2.9) | 1996–2015 | 2.5 (2.2 to 2.9) | ||||
| Men | 1.32 | 2.59 | 3.1 (2.7 to 3.5) | 1996–2015 | 3.1 (2.7 to 3.5) | ||||
| Women | 2.64 | 4.55 | 2.4 (2.0 to 2.7) | 1996–2015 | 2.4 (2.0 to 2.7) | ||||
| Prevalence by age group‡ | |||||||||
| 25–34 | 0.68 | 1.82 | 4.7 (3.7 to 5.6) | 1996–2007 | 7.7 (6.4 to 9.1) | 2007–2015 | 0.6 (−0.9 to 2.1) | ||
| 35–44 | 0.70 | 2.21 | 5.5 (4.3 to 6.7) | 1996–2011 | 4.5 (3.6 to 5.4) | 2011–2015 | 9.5 (4.3 to 15.0) | ||
| 45–54 | 1.55 | 3.14 | 4.0 (3.3 to 4.8) | 1996–2011 | 3.4 (2.8 to 4.0) | 2011–2015 | 6.5 (3.2 to 10.0) | ||
| ≥45 | 3.68 | 7.42 | 2.8 (2.5 to 3.2) | 1996– 2015 | 2.8 (2.5 to 3.2) | ||||
| ≥45 Males | 2.53 | 5.64 | 3.9 (3.6 to 4.3) | 1996– 2015 | 3.9 (3.6 to 4.3) | ||||
| ≥45 Females | 5.26 | 9.03 | 2.4 (2.0 to 2.7) | 1996– 2015 | 2.4 (2.0 to 2.7) | ||||
| 55–64 | 2.96 | 5.60 | 3.0 (2.6 to 3.4) | 1996–2015 | 3.0 (2.6 to 3.4) | ||||
| 65–74 | 6.08 | 8.97 | 1.7 (1.3 to 2.2) | 1996–2015 | 1.7 (1.3 to 2.2) | ||||
| 75–84 | 7.80 | 13.9 | 2.6 (2.0 to 3.2) | 1996–2007 | 3.6 (2.7 to 4.5) | 2007–2015 | 1.2 (0.2 to 2.1) | ||
| ≥85 | 6.27 | 15.0 | 3.0 (2.4 to 3.5) | 1996–2015 | 3.0 (2.4 to 3.5) | ||||
| Incidence | |||||||||
| Total | 0.42 | 0.38 | −0.5 (−1.4 to 0.5) | 1996–2006 | −2.6 (−4.0 to − 1.1) | 2006–2015 | 1.9 (0.4 to 3.5) | ||
| Men | 0.27 | 0.26 | −0.2 (−1.4 to 1.1) | 1996–2006 | −2.5 (−4.4 to − 0.5) | 2006–2015 | 2.5 (0.5 to 4.5) | ||
| Women | 0.58 | 0.49 | −0.5 (−2.4 to 1.4) | 1996–1999 | −8.7 (−16.2 to − 0.6) | 1999–2013 | −0.4 (−1.2 to 0.5) | 2013–2015 | 11.8 (−3.3 to 29.3) |
| ≥45 | 0.79 | 0.69 | 0.0 (−1.4 to 1.4) | 1996–2011 | −1.3 (−2.2 to −0.3) | 2011–2015 | 4.7 (−1.5 to 11.4) | ||
| ≥45 Males | 0.44 | 0.51 | 0.6 (−0.4 to 1.6) | 1996–2015 | 0.6 (−0.4 to 1.6) | ||||
| ≥45 Females | 1.11 | 0.81 | −1.9 (−3.7 to 0) | 1996–1999 | −11 (−21.4 to 0.7) | 1999–2015 | 0.0 (−0.9 to −0.8) | ||
Statistically significant differences for (average) annual percentage change (APC) are indicated in bold.
*These percentages are standardised for the total Flemish population.
†Joinpoint regression modelling was used to estimate (average) APC in prevalence and incidence trends. Three possible trends were calculated during the 20-year study period.
‡Standardisation was possible for the total population, but not for specific age cohorts.
AAPC, average annual percentage change.
Figure 1.
The standardised and non-standardised prevalence of patients with knee osteoarthritis by age cohorts in the Intego registry (1996–2015). Standardisation was performed by taking the Flemish population of the year 1996 as reference population.
Figure 2.
An overview of the observed and modelled trends in prevalence for men and women in the Intego registry (1996–2015). Observed (bullets) and modelled (trend line) age-standardised average annual percentage change (AAPC) in prevalence with 95% CIs for time trends for patients with knee osteoarthritis in Intego register, 1996–2015. The AAPC is significantly different from zero at alpha=0.05.
Figure 3.
The standardised and non-standardised incidence of patients with knee osteoarthritis in the Intego registry (1996–2015). Standardisation was performed by taking the Flemish population of the year 1996 as reference population.
bmjopen-2019-031734supp004.pdf (37.3KB, pdf)
bmjopen-2019-031734supp005.pdf (30KB, pdf)
Trends in multimorbidity in newly diagnosed patients with knee OA (1996–2015)
In the 20-year study period, the mean age at diagnosis of knee OA remained stable (p=0.384) with 55.3 years in 1996 and 56.9 years in 2015, respectively, while a non-significant decline was found in the proportion of women in this period (65%–62%, p=0.052). Additionally, the disease burden was defined by calculating the mean disease count of patients with knee OA.29 This mean disease count showed a significant increase in the study period ranging from 1.6 to 2.3 (p<0.001), meaning that the multimorbidity of patients with knee OA increased. In this study, the following other diseases increased significantly: the proportions of patients with diabetes (6%–15%, p<0.001), cardiovascular events (21%–27%, p<0.001), depression (9%–13%, p=0.009) and obesity (5%–8%, p<0.001). Hypertension, gastrointestinal ulcer and renal failure remained stable. Additionally, we noted that the proportion of patients with knee OA and cancer (2%–3%, p<0.001), asthma (8%–17%, p<0.001) and substance abuse (0%–2%, p<0.001) increased significantly during the study period, while the proportion with osteoporosis remained stable (table 2).
Table 2.
Trends in multimorbidity of patients with knee osteoarthritis (OA) in the Intego registry (1996–2015)
| Variables | 1996–2000 | 2001–2005 | 2006–2010 | 2011–2015* | P value** |
| Mean age (±SD) | 55.3 (21.9) | 57.6 (20.5) | 57.8 (19.8) | 56.9 (19.8) | 0.384 |
| Women, n (%) | 972 (65%) | 1234 (65%) | 1419 (64%) | 1412 (62%) | 0.05224 |
| Incidence, n | 1503 | 1912 | 2202 | 2288 | |
| Multimorbidity, n (%) | |||||
| Hypertension | 359 (24%) | 485 (25%) | 623 (28%) | 593 (26%) | 0.0756 |
| Diabetes | 93 (6%) | 161 (8%) | 252 (11%) | 346 (15%) | <0.001 |
| CV events | 323 (21%) | 480 (25%) | 597 (27%) | 614 (27%) | <0.001 |
| GI complication (ulcer) | 28 (2%) | 60 (3%) | 59 (3%) | 61 (3%) | 0.3585 |
| Renal failure | 23 (2%) | 70 (4%) | 71 (3%) | 66 (3%) | 0.1025 |
| Depression | 141 (9%) | 230 (12%) | 259 (12%) | 287 (13%) | 0.009 |
| Obesity | 74 (5%) | 101 (5%) | 145 (7%) | 191 (8%) | <0.001 |
| Osteoporosis | 57 (4%) | 81 (4%) | 107 (5%) | 103 (5%) | 0.2303 |
| Cancer | 29 (2%) | 60 (3%) | 59 (3%) | 61 (3%) | <0.001 |
| Asthma | 125 (8%) | 205 (11%) | 328 (15%) | 392 (17%) | <0.001 |
| Substance abuse | 4 (0%) | 22 (1%) | 31 (1%) | 48 (2%) | <0.001 |
| Disease burden, n (±SD)*** | 1.63 (1.81) | 1.84 (2.00) | 2.18 (2.20) | 2.34 (2.35) | <0.001 |
Multimorbidity was measured for all incident cases with knee OA (ie, at the time when knee OA was registered as a diagnosis).
*Four time intervals of 5 years were defined to evaluate trends for all incident patients with knee OA.
†P-value for multimorbidity was calculated with the Cochran-Armitage trend test; p-value for age was calculated with the Jonckheere-Terpstra trend test.
‡The full list of diseases to calculate this mean disease burden is presented in online supplementary file 2.
CV, cardiovascular; GI, gastrointestinal.
Trends in prescriptions for patients with knee OA (1996–2015)
The prescription of acetaminophen (AAPC=6.7, 95% CI 5.6 to 7.7), weak opioids (AAPC=4.0, 95% CI 0.9 to 7.3) and glucosamine (AAPC=8.6, 95% CI 2.4 to 15.1) for patients with knee OA increased during the study period (table 3). The prevalence of patients with knee OA who were prescribed acetaminophen was lower than those with oral NSAIDs (19.2% vs 29.4% in 2015; 5.3% vs 28.4% in 1996). The prescription of oral, topical and cox-2 selective NSAIDs remained stable for both genders during the study period. The use of strong opioids showed a strong increase between 1996 and 2003 (AAPC=9.0, 95% CI 2.5 to 16), but then decreased slightly in the period from 2003 to 2015 (AAPC=−2.0, 95% CI 3.7 to −0.3).
Table 3.
Trends in medication use of patients with knee osteoarthritis in the Intego registry (1996–2015)
| Group/medication | Prev. in 1996 | Prev. in 2015 | Overall trend | Trend 1 | Trend 2 | Trend 3* | |||
| AAPC (95% CI) | Years | APC (95% CI) |
Years | APC (95% CI) |
Years | APC (95% CI) |
|||
| Acetaminophen | 5.3 | 19.2 | 6.7 (5.6 to 7.7) | 1996–2010 | 8.0 (6.8 to 9.2) | 2010–2015 | 3.1 (0.3 to 5.9) | ||
| Males | 5.2 | 17.4 | 5.8 (4.9 to 6.6) | ||||||
| Females | 5.4 | 20.2 | 7.0 (5.8 to 8.3) | 1996–2010 | 8.7 (7.3 to 10.1) | 2010–2015 | 2.7 (−0.6 to 6.0) | ||
| Oral NSAID (exclusion cox-2) | 28.4 | 29.4 | 0.0 (−1.1 to 1.1) | 1996–2002 | −1.0 (−3.5 to 1.6) | 2002–2008 | 2.4 (−0.1 to 5.0) | 2008–2015 | −1.2 (−2.4 to 0.1) |
| Males | 28.6 | 28.8 | 0.5 (−0.2 to 1.2) | 1996–2009 | 1.1 (0.4 to 1.9) | 2009–2015 | 0.5 (−0.2 to 1.2) | ||
| Females | 28.3 | 29.6 | 0.3 (−0.1 to 0.8) | ||||||
| Cox-2 selective NSAID | 3.0 | 2.3 | −7.7 (−36.0 to 33.0) | 2000–2004 | −2.7 (−29.3 to 33.9) | 2004–2007 | −48.4 (−93.5 to 309.5) | 2007–2015 | 11.8 (−3.4 to 29.5) |
| Males | 2.2 | 1.8 | −13.3 (−19.3 to − 6.7) | ||||||
| Females | 3.4 | 2.7 | −7.3 (−34.0 to 30.1) | 2000–2004 | −3.3 (−29.1 to 32.0) | 2004–2007 | −47.2 (−92.2 to 257.6) | 2007–2015 | 12.0 (−2.9 to 29.2) |
| Topical NSAID | 7.8 | 5.9 | −1.0 (−2.4 to 0.4) | 1996–2003 | −4.7 (−8.1 to − 1.2) | 2003–2015 | 1.2 (−0.0 to 2.4) | ||
| Males | 9.3 | 5.8 | −0.9 (−2.2 to 0.5) | ||||||
| Females | 7.1 | 5.9 | −0.8 (−2.3 to 0.7) | 1996–2003 | −4.3 (−8.0 to − 0.4) | 2003–2015 | 1.3 (−0.0 to 2.6) | ||
| Weak opioids | 2.8 | 6.1 | 4.0 (0.9 to 7.3) | 1996–1998 | 36.3 (0.4 to 85.2) | 1998–2009 | −0.9 (−2.3 to 0.5) | 2009–2015 | 4.0 (1.6 to 6.4) |
| Males | 1.5 | 5.2 | 2.9 (1.5 to 4.4) | ||||||
| Females | 3.3 | 6.7 | 2.8 (−0.0 to 5.7) | 1996–2000 | 14.7 (1.6 to 29.4) | 2000–2008 | −3.2 (−6.4 to 0.2) | 2008–2015 | 3.5 (0.6 to 6.4) |
| Strong opioids | 2.5 | 4.3 | 1.9 (−0.4 to 4.3) | 1996–2003 | 9.0 (2.5 to 16.0) | 2003–2015 | −2.0 (−3.7 to − 0.3) | ||
| Males | 1.7 | 3.6 | −0.2 (−2.0 to 1.6) | ||||||
| Females | 2.9 | 4.7 | 2.3 (0.3 to 4.3) | 1996–2003 | 10.0 (4. 4 to 15.9) | 2003–2015 | −2.0 (−3.4 to − 0.5) | ||
| Parenteral glucocorticoids | 9.1 | 8.1 | −0.7 (−1.8 to 0.5) | 1996–2005 | −2.1 (−3.5 to − 0.7) | 2005–2012 | 2.7 (0.8 to 4.7) | 2012–2015 | −4.1 (−9.2 to 1.2) |
| Males | 8.1 | 8.6 | 0.8 (0.0 to 1.6) | ||||||
| Females | 9.6 | 7.9 | −1.3 (−2.6 to 0.0) | 1996–2003 | −3.8 (−6.0 to − 1.5) | 2003–2012 | 2.0 (0.6 to 3.4) | 2012–2015 | −5.1 (−10.7 to 0.8) |
| Glucosamine* | 0.6 | 1.8 | 8.6 (2.4 to 15.1) | 2001–2004 | 64.1 (25.0 to 115.3) | 2004–2011 | −9.6 (−14.3 to − 4.4) | 2011–2015 | 9.8 (−0.6 to 21.2) |
| Males | 0.1 | 1.8 | 17.3 (−18.8 to 69.5) | 2001–2003 | 212.4 (−83.1 to 5664.3) | 2003–2015 | −0.4 (−4.8 to 4.2) | ||
| Females | 0.9 | 1.8 | 6.8 (0.4 to 13.7) | 2001–2004 | 56.7 (18.3 to 107.5) | 2004–2011 | −10.0 (−15.3 to − 4.3) | 2011–2015 | 8.2 (−3.6 to 21.4) |
Bold: indicates that the (average) annual percentage change (APC) is significantly different from zero at the alpha= 0.05 level.
*Three possible time trends were computed with the joinpoint regression analysis. The corresponding time cohorts and APC are mentioned in these three columns.
†Glucosamine: registration starts from 2001; cox-2 selective non-steroidal anti-inflammatory drug (NSAID) starts from 2000.
AAPC, average annual percentage change; Prev, prevalence.
Discussion
This study presents estimates of knee OA prevalence and incidence based on a large morbidity registration network for general practice in Belgium. During the 20-year study period, the age-standardised prevalence of knee OA significantly increased while the age-standardised incidence rate remained stable. During the study period, patients with knee OA experienced higher multimorbidity, as shown by almost a doubling of the disease count. Oral NSAIDs were most frequently prescribed for the prevalent patients with knee OA, while prescription of acetaminophen, weak opioids and glucosamine showed an overall positive trend.
This study shows that the prevalence rate of knee OA significantly increased even after standardisation of the study population. General practice morbidity registration networks in other European countries show similar rates for knee OA: in the Netherlands, an overall prevalence of 3.4% and incidence of 3.2‰ was registered in 2016.36 In our study, we found similar rates with 3.56% and 3.75‰, respectively, for the year 2015. In the UK, the estimated proportion of people who sought treatment for knee OA is high: 18% of the population aged 45 and over consulted their GP for knee OA.37 The latter study also found that OA is the most common musculoskeletal condition in older people and that just over half of all patients consulting their GP about OA have knee OA. In our study, we found a consultation prevalence of 21% for the same reference year (2010) and age cohorts. In the near future, the number of people with knee OA is expected to rise considerably because of an ageing population and obesity trends.38 Nevertheless, the increasing prevalence of knee OA in general practice registration could also be attributed to other factors, for example: better access to general practice, more awareness of the public of preventive medicine, better diagnostics, better registration and higher demands and expectations of older people to remain physically active. Future qualitative research with different stakeholders could assess these possible explanations.
OA is one of the diseases with the highest rate of multimorbidity, with reported rates of 68%–85%.39 40 Coexisting disorders may worse pain and bring additional impairments, which necessitate adaptations to the conservative management of knee OA.14 41 In our study, knee OA was also strongly associated with the following multimorbidities: asthma, cancer, depression and substance abuse. The substantial contribution of OA to multimorbidity and frailty should be recognised, further investigated and needs extra attention in general practice management of long-term conditions.
Pharmacological management of knee OA in general practice is dominated both by acetaminophen and by NSAIDs, as they are both recommended in evidence-based guidelines.5 7–9 In Intego, we look at the GP’s prescription and not the actual drug use by the patient. Although the review by Machado et al suggested that acetaminophen has little clinical benefit in OA, guidelines recommend starting with acetaminophen, because of the adverse side effect profile of NSAIDs.42 In our study, NSAIDs were the most frequently prescribed pain drug for prevalent patients with knee OA. Verkleij et al observed the effects of medication on 104 patients with knee OA in general practice. They demonstrated no significant difference regarding knee pain and knee function between patients taking diclofenac or acetaminophen.43 Furthermore, the discrepancy between drug prescription by the professional and drug use by the patient can be accumulated by the over the counter availability of acetaminophen and some oral NSAID in Belgium. Over the counter availability could be considered as part of self-care to reduce the burden on healthcare systems and increase people’s choice to take informed treatment decisions, but the medical outcome resulting from therapeutic options bypassing the physician prescription stays a major issue.44 If acetaminophen should remain the ‘first-line’ pharmacological treatment for patients with a new episode, the effects of acetaminophen and the role in patients with multimorbidity should be further investigated.45
Strengths and limitations
The major strengths of this study are the long-term follow-up data of a practice-based morbidity registration network in general practice. Intego covers more than 2% of the Flemish population, representative in terms of age and gender.18 Deckers et al updated an inventory of primary care surveillance networks in Europa and formulated minimal standard criteria for these networks.46 When fulfilling identical minimal criteria, networks can provide comparable estimates of morbidity, ultimately leading to improved national and European surveillance. For continuous surveillance networks, they advise that a sufficient sample size is approximately 1% of the population, which will allow the study of common diseases.46 Longitudinal data in registry-based studies are used to track the natural history of diseases over time and enable us to perform time-to-event analyses. In addition, general practices have to pass three quality criteria before being accepted as participants in Intego, what results in a reliable morbidity database.18 Important attributes of most patient registries are their large sample size and data variability.47 A few limitations must also be considered. Lack of data verification is a common problem in registry-based studies with longitudinal data of large sample size. In Intego, the lack of data verification and misclassification is minimalised because new diagnoses are automatically linked to ICPC-2 and ICD-10 codes with a detailed thesaurus, individual patients are followed over time and their history is taken into account. The change for misclassification for knee OA was higher in younger age cohorts. If diagnoses are not mutually exclusive, then they count for one. Second, we are aware that accurate coding is always a risk for possible underdiagnosis. The difference between early-onset knee OA and chronic established that knee OA cannot be established with the ICPC codes. Standardised coding for OA should be adopted in general practice to accurately describe the extent of the condition and to maximise the conservative management options to improve quality of life. Third, there is no obligation for patients to be registered with a particular GP in Belgium. Therefore, it can be difficult to define ‘the population at risk’ for epidemiological studies in general practice. In Intego, the YCG was used as denominator for all trend analyses. Importantly, mortality data are lacking in Intego. Therefore, patients in the incidence analysis are considered at risk until the diagnosis or until December 31 of any specific year to compensate for possible overestimation in this registry-based study. Furthermore, to calculate the total prevalence and incidence rates, we used the total YCG as the denominator. Since age is an important risk factor to develop knee OA, younger unaffected individuals are probably over-represented in the total population. This could result in an underestimation of the total prevalence and incidence rates. Therefore, we also provide these rates for all age cohorts in tables and supplementary files. Finally, obesity and smoking status could not be reliably assessed from the Intego database, because of insufficient registration in the patient files. To date, the information on socioeconomic status on patient level in the Intego register cannot yet be extracted for data analysis. This information is available on practice level and based on the postal code. However, since GP practices in Flanders often take care of patients living in neighbouring municipalities and people living within a specific postal code can have a different socioeconomic status, we in general do not use this information in our analyses. Quality improvement initiatives should make GPs more aware of the necessity of properly recording up-to-date patient variables, such as body mass index, in the EHR because of their growing importance in patient-tailored management strategies. Patient portals and remote access to their own medical health record are future initiatives, where the patient could play a more central role to help the GP in keeping these parameters more up-to-date by shared responsibility.48
Conclusion and recommendations
In conclusion, increased prevalence, multimorbidity and number of drug prescriptions, together with the young age at incidence, confirm the high burden of knee OA. Our registry-based study represents knee OA diagnoses at a time it becomes a health issue for patients. Professionals face more difficulties in their conservative management options due to rising multimorbidity. In future, these health trends can be used to prioritise initiatives for improvement in care.
Supplementary Material
Footnotes
Contributors: PM and DS performed the analyses, and DS, PV, RH, MS, FL and BV wrote the manuscript. DS, MS and BV are responsible for the study concept, design, the recruitment of subjects and acquisition of data. All authors participated in the interpretation of the data. All authors approved the final version of the manuscript.
Funding: The Flemish Government (Ministry of Health and Welfare) funds Intego on a regular basis. This work would not have been possible without the collaboration of all general practitioners of the Intego network. The authors hereby state the independence of the researchers from the funders.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Intego project was presented to the Belgian Privacy Commission (no SCSZG/13/079) and approved by the ethical review board of the Medical School of the Catholic University of Leuven (No. ML 1723). This permission completely covered the current investigation. In the Intego protocol, participating GP practices have to inform their patients that the practice participates in a morbidity registration network. Patients can choose to opt out for the possibility of their anonymised data extraction.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The dataset supporting the conclusions of this article is held at the University of Leuven, Belgium, and can be shared upon contacting the corresponding author.
References
- 1. Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. The Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. The Lancet 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Litwic A, Edwards MH, Dennison EM, et al. Epidemiology and burden of osteoarthritis. Br Med Bull 2013;105:185–99. 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bedson J, Jordan K, Croft P. The prevalence and history of knee osteoarthritis in general practice: a case-control study. Fam Pract 2005;22:103–8. 10.1093/fampra/cmh700 [DOI] [PubMed] [Google Scholar]
- 5. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence (NICE) Osteoarthritis: care and management (quality standard 87). Available: http://guidance.nice.org.uk/qs87 [Accessed 22 Oct 2019].
- 7. Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 8. Hochberg MC, Altman RD, April KT, et al. American College of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- 9. Kingsbury SR, Gross HJ, Isherwood G, et al. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology 2014;53:937–47. 10.1093/rheumatology/ket463 [DOI] [PubMed] [Google Scholar]
- 10. Harding PA, Holland AE, Hinman RS, et al. Physical activity perceptions and beliefs following total hip and knee arthroplasty: a qualitative study. Physiother Theory Pract 2015;31:107–13. 10.3109/09593985.2014.959581 [DOI] [PubMed] [Google Scholar]
- 11. de Rooij M, Steultjens MPM, Avezaat E, et al. Restrictions and contraindications for exercise therapy in patients with hip and knee osteoarthritis and comorbidity. Physical Therapy Reviews 2013;18:101–11. 10.1179/1743288X12Y.0000000056 [DOI] [Google Scholar]
- 12. Kadam UT, Jordan K, Croft PR. Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consulters in England and Wales. Ann Rheum Dis 2004;63:408–14. 10.1136/ard.2003.007526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Oostrom SH, Picavet HSJ, de Bruin SR, et al. Multimorbidity of chronic diseases and health care utilization in general practice. BMC Fam Pract 2014;15:61 10.1186/1471-2296-15-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reeuwijk KG, de Rooij M, van Dijk GM, et al. Osteoarthritis of the hip or knee: which coexisting disorders are disabling? Clin Rheumatol 2010;29:739–47. 10.1007/s10067-010-1392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freid VM, Bernstein AB, Bush MA. Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief 2012;100:1–8. [PubMed] [Google Scholar]
- 16. van den Akker M, Vaes B, Goderis G, et al. Trends in multimorbidity and polypharmacy in the Flemish-Belgian population between 2000 and 2015. PLoS One 2019;14:e0212046 10.1371/journal.pone.0212046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract 2008;14:28–32. 10.1080/13814780802436093 [DOI] [PubMed] [Google Scholar]
- 18. Truyers C, Goderis G, Dewitte H, et al. The Intego database: background, methods and basic results of a Flemish General practice-based continuous morbidity registration project. BMC Med Inform Decis Mak 2014;14:48 10.1186/1472-6947-14-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanbeselaere V, Truyers C, Elli S, et al. Association between atrial fibrillation, anticoagulation, risk of cerebrovascular events and multimorbidity in general practice: a registry-based study. BMC Cardiovasc Disord 2016;16:61 10.1186/s12872-016-0235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okkes IM, Becker HW, Bernstein RM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a Nomenclature and a terminology for ICPC-2. Fam Pract 2002;19:543–6. 10.1093/fampra/19.5.543 [DOI] [PubMed] [Google Scholar]
- 21. WHO Collaborating Centre for Drug Statistics Methodology ATC/DDD index, 2010. Available: http://www.whocc.no/atc_ddd_index [Accessed 19 Oct 2019].
- 22. Bartholomeeusen S, Kim C-Y, Mertens R, et al. The denominator in general practice, a new approach from the Intego database. Fam Pract 2005;22:442–7. 10.1093/fampra/cmi054 [DOI] [PubMed] [Google Scholar]
- 23. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational Routinely-collected health data (record) statement. PLoS Med 2015;12:e1001885 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greiver M, Williamson T, Bennett T-L, et al. Developing a method to estimate practice denominators for a national Canadian electronic medical record database. Fam Pract 2013;30:347–54. 10.1093/fampra/cms083 [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26. Shah AN, Vail TP, Taylor D, et al. Comorbid illness affects hospital costs related to hip arthroplasty: quantification of health status and implications for fair reimbursement and surgeon comparisons. J Arthroplasty 2004;19:700–5. [DOI] [PubMed] [Google Scholar]
- 27. Linn BS, Linn MW, Gurel LEE. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–6. 10.1111/j.1532-5415.1968.tb02103.x [DOI] [PubMed] [Google Scholar]
- 28. Piccirillo JF, Lacy PD, Basu A, et al. Development of a new head and neck Cancer–Specific comorbidity index. Arch Otolaryngol Head Neck Surg 2002;128:1172–9. 10.1001/archotol.128.10.1172 [DOI] [PubMed] [Google Scholar]
- 29. Knottnerus JA, Metsemakers J, Hoppener P, et al. Chronic Illness in the Community and the Concept of ‘Social Prevalence’. Fam Pract 1992;9:15–21. 10.1093/fampra/9.1.15 [DOI] [PubMed] [Google Scholar]
- 30. Truyens C, Elli S, Goderis G, et al. [Dutch: 20 year general practice in Flanders (1994-2013)]. Leuven: Acco, 2015. [Google Scholar]
- 31. Kim H-J, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 32. Rea F, Pagan E, Compagnoni M, et al. Joinpoint regression analysis with time-on-study as time-scale. Application to three Italian population-based cohort studies. Epidemiology Biostatistics and Public Health 2017;14:1–8. [Google Scholar]
- 33. Yu B, Barrett MJ, Kim H-J, et al. Estimating joinpoints in continuous time scale for multiple change-point models. Comput Stat Data Anal 2007;51:2420–7. 10.1016/j.csda.2006.07.044 [DOI] [Google Scholar]
- 34. Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med 2009;28:3670–82. 10.1002/sim.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alan A. Categorical data analysis. 2nd edn Wiley, 2002. [Google Scholar]
- 36. Nivel database for primary care morbidity registration in the Netherlands. Available: https://www.nivel.nl/nl/ [Accessed 19 Oct 2019].
- 37. Osteoarthritis in general practice: data and perspectives, 2013. Available: www.arthritisresearchuk.org [Accessed October 19, 2019].
- 38. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 39. de Rooij M, van der Leeden M, Cheung J, et al. Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: a randomized controlled trial. Arthritis Care Res 2017;69:807–16. 10.1002/acr.23013 [DOI] [PubMed] [Google Scholar]
- 40. van Dijk GM, Veenhof C, Schellevis F, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord 2008;9:95 10.1186/1471-2474-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calders P, Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;47:805–13. 10.1016/j.semarthrit.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 42. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015;350:h1225 10.1136/bmj.h1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verkleij SPJ, Luijsterburg PAJ, Willemsen SP, et al. Effectiveness of diclofenac versus paracetamol in knee osteoarthritis: a randomised controlled trial in primary care. Br J Gen Pract 2015;65:e530–7. 10.3399/bjgp15X686101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO/EDM/QSM/00.1 Who guidelines for the regulatory assessment of medicinal products for use in self-medication, 2000. Available: https://apps.who.int/medicinedocs [Accessed 11 Sep 2019].
- 45. Conaghan PG. Nsaids or paracetamol for short-term treatment of mild to moderate knee pain in early osteoarthritis: are they equivalent? Evid Based Med 2016;21:14 10.1136/ebmed-2015-110289 [DOI] [PubMed] [Google Scholar]
- 46. Deckers JGM, Paget WJ, Schellevis FG, et al. European primary care surveillance networks: their structure and operation. Fam Pract 2006;23:151–8. 10.1093/fampra/cmi118 [DOI] [PubMed] [Google Scholar]
- 47. Trotter JP. Patient registries: a new gold standard for "real world" research. Ochsner J 2002;4:211–4. [PMC free article] [PubMed] [Google Scholar]
- 48. Alpert JM, Krist AH, Aycock RA, et al. Designing User-Centric patient portals: clinician and patients' uses and Gratifications. Telemed J E Health 2017;23:248–53. 10.1089/tmj.2016.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031734supp001.pdf (55KB, pdf)
bmjopen-2019-031734supp002.pdf (58.2KB, pdf)
bmjopen-2019-031734supp003.pdf (12.6KB, pdf)
bmjopen-2019-031734supp004.pdf (37.3KB, pdf)
bmjopen-2019-031734supp005.pdf (30KB, pdf)