Abstract
Objectives
To assess the prevalence of undiagnosed diabetes and pre-diabetes in the healthy population in the Mollerussa cohort. As a secondary objective, to identify the variables associated with these conditions and to describe the changes in glycaemic status after 1 year of follow-up in subjects with pre-diabetes.
Design
Prospective observational cohort study.
Setting
General population from a semi-rural area.
Participants
The study included 583 participants without a diagnosis of diabetes recruited between March 2011 and July 2014.
Results
The prevalence of undiagnosed diabetes was 20, 3.4% (95% CI 2.6 to 4.2) and that of pre-diabetes was 229, 39.3% (37.3 to 41.3). Among those with pre-diabetes, 18.3% had isolated impaired fasting plasma glucose (FPG) (FPG: 100 to <126 mg/dL), 58.1% had isolated impaired glycated haemoglobin (HbA1c) (HbA1c 5.7 to <6.5) and 23.6% fulfilled both criteria. Follow-up data were available for 166 subjects; 41.6%(37.8 to 45.4) returned to normoglycaemia, 57.6% (57.8 to 61.4) persisted in pre-diabetes and 0.6% (0 to 1.2) progressed to diabetes. Individuals with pre-diabetes had worse cardiometabolic risk profiles and sociodemographic features than normoglycaemic subjects. In the logistic regression model, variables significantly associated with pre-diabetes were older age (OR; 95% CI) (1.033; 1.011 to 1.056), higher physical activity (0.546; 0.360 to 0.827), body mass index (1.121; 1.029 to 1.222) and a family history of diabetes (1.543; 1.025 to 2.323). The variables significantly associated with glycaemic normalisation were older age (0.948; 0.916 to 0.982) and body mass index (0.779; 0.651 to 0.931).
Conclusions
Among adults in our region, the estimated prevalence of undiagnosed diabetes was 3.4% and that of pre-diabetes was 39.3%. After a 1-year follow-up, a small proportion of subjects (0.6%) with pre-diabetes progressed to diabetes, while a high proportion (41.6%) returned to normoglycaemia. Individuals with pre-diabetes who returned to normoglycaemia were younger and had a lower body mass index.
Keywords: prediabetes, undiagnosed diabetes, prediabetes prevalence
Strengths and limitations of this study.
This was a population-based study of a small cohort that included a representative sample of a non-previously studied population of a semi-rural area in Catalonia.
We did not perform an oral glucose tolerance test, which is a common test in most studies but is a time-consuming and expensive procedure.
The small number of cases of undiagnosed diabetes precluded further statistical analyses on this topic.
Background
Diabetes mellitus, a public health concern with an increasing incidence worldwide, is a great threat to general health and is leading to increased morbidity and mortality. These effects are mainly occurring because diabetes is a disorder of glucose metabolism that affects multiple organ systems and is associated with various microvascular and macrovascular complications and several non-vascular complications. Additionally, a large group of subjects do not fulfil the diabetes criteria but have intermediate glycaemic variables, between normal and diabetes, and are thus classified as having pre-diabetes. One of the most commonly used definitions of pre-diabetes is that of the 2010 American Diabetes Association (ADA) criteria:1 2 (a) impaired fasting plasma glucose (IFG), defined as fasting plasma glucose (FPG) between 100 and <126 mg/dL (5.6 to 5.9 mmol/L); (b) impaired glucose tolerance (IGT), defined as a 2-hour plasma glucose value after a 75 g oral glucose tolerance test (OGTT) between 140 and <200 mg/dL (7.8 to 11.0 mmol/L) or (c) glycated haemoglobin (HbA1c) levels between 5.7% and <6.5% (39 to 46 mmol/mol).
Pre-diabetes is becoming increasingly important as it represents a high risk of developing type 2 diabetes (T2D) and cardiovascular diseases.2 3 Moreover, individuals with pre-diabetes are phenotypically quite similar to patients with T2D. That is, they tend to be older, with a higher body mass index (BMI) and higher blood pressure than people with normal glucose tolerance; in addition, they tend to have insulin resistance and dyslipidaemia.4 Additionally, multiple risk factors, such as family history, gestational diabetes and certain ethnicities as well as combined risk factors such as metabolic syndrome, are known to predispose subjects to a higher risk for pre-diabetes and its progression to T2D.5 Based only on IGT, the worldwide prevalence of pre-diabetes among adults has been estimated by the International Diabetes Federation to be 7.3% in 2017, with half of these individuals (49%) being younger than 50 years.6 The National Diabetes Statistics Report in the USA reported that the total crude prevalence of diabetes was 9.4% (30.3 million, 2017 US population), with 23.8% undiagnosed and an additional 33.9% with pre-diabetes.7
In Spain, according to data from the Di@bet.es study, based on OGTT, FPG and HbA1c, 13.8% of the adult population, adjusted for age and sex, had diabetes, and of these individuals up to 6% had undiagnosed diabetes. Furthermore, an additional 14.8% of individuals presented with some type of pre-diabetic state, 3.4% based on IFG, 9.2% based on IGT and 2.2% with disturbances in both, after adjusting for age and sex.8 9 According to the ADA, up to 70% of people with pre-diabetes will develop overt diabetes throughout their lives.10 11 Moreover, each year, 5% to 10% of subjects with pre-diabetes will eventually develop overt diabetes, and according to some studies, this percentage can reach up to 18% per year; however, this rate may vary with the definition of pre-diabetes and population characteristics.12–15 It has been shown that over 3 to 5 years, approximately 25% of subjects progress to T2D, 25% return to a normal state of glucose tolerance and 50% remain in the pre-diabetic state.16 Thus, the early diagnosis and screening of pre-diabetes are essential steps towards the prevention of its progression or at least the delay of the onset of T2D.
The primary aim of this study was to assess the prevalence of undiagnosed diabetes and pre-diabetes in the healthy population in the Mollerussa cohort. As a secondary objective, we aimed to assess the variables associated with these conditions and to describe the changes in glycaemic status after 1 year of follow-up in subjects with pre-diabetes.
Methods
Subjects
This was a prospective population-based cohort study from the semi-rural area of Mollerussa in Catalonia (northeast Spain) selected between March 2011 and July 2014. The description of the cohort and the procedures performed were initially published as a cohort profile.17 Briefly, the database of the Catalan Health Institute (ICS) through its Primary Care Electronic Clinical Station (Estació Clínica Electronica d’Atenció Primaria – eCAP) was used to select the population sample. All population is passively included in the Primary Care Electronic Clinical record according to the Spanish health system, which is based on the principles of universality, free access, equity and fairness of financing.18 Then, from a total population of 24 666 potentially eligible individuals in the healthcare area (subjects older than 25 years and attending any Primary Healthcare Centre in the same health area), 2226 subjects were randomly selected using a randomiser programme (SPSS software V.16.0 for Windows; SPSS), following the principles of simple random sampling, and were then invited to participate by telephone contact. Based on their willingness to join the study, exclusion criteria, consent and baseline laboratory data, 594 subjects aged ≥25 years were finally included.17 The exclusion criteria included a previous diagnosis of diabetes (type 1 diabetes, T2D or any specific subtype of diabetes), treatment with oral antidiabetic drugs or the use of metformin for other conditions. In addition, subjects with cardiovascular disease (heart disease, heart failure, aortic stenosis), cancer, kidney disease, anaemia, hepatitis, gastrointestinal diseases, recent abdominal surgery, chronic obstructive pulmonary disease, chronic infectious diseases, use of systemic glucocorticoids or beta blockers or major psychiatric disorders with psychotic symptoms were excluded from the study. Subjects were considered to have hypertension or dyslipidaemia if they were using anti-hypertensive or lipid-lowering agents. Pre-diabetes was defined as any of the following abnormal glycaemic variables: FPG 100 to <126 mg/dL or HbA1c 5.7 to <6.5%; diabetes was defined as FPG ≥1256 mg/dL or HbA1c ≥6.5%. Normal glycaemic status was defined by FPG <100 mg/dL and HbA1c <5.7 according to the 2010 ADA criteria.1 Eleven subjects without baseline HbA1c or FPG measurements were excluded. Subjects with pre-diabetes at baseline (n=229) underwent a second visit 12 months after the baseline visit, and 166 (72.5%) of them had relevant information at follow-up.
A fasting blood sample was taken to determine glucose, HbA1c, total cholesterol, high density lipoprotein (HDL)-cholesterol, low density lipoprotein (LDL)-cholesterol, triglycerides, renal function and other parameters following standard protocols.17 The Fatty Liver Index (FLI) was calculated with the equation developed by Bedogni et al.19 Insulin resistance was calculated by the homeostatic model assessment-2 insulin resistance (HOMA2-IR), beta cell function (HOMA2-ß) and insulin sensitivity (HOMA2-S) data were calculated with a HOMA2 calculator released by the Diabetes Trials Unit, University of Oxford: HOMA Calculator. This calculator is available at: http://www.dtu.ox.ac.uk/homacalculator/ (updated 11 October 2017).20 The estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.21
Sociodemographic variables were recorded by researchers following a protocol for the inclusion of patients using a standardised baseline questionnaire during the clinical interview. In all cases a physical examination (including weight, height, blood pressure and waist circumference) was carried out by trained research staff. Education level and physical activity were assessed according to the International Standard Classification of Education22 and the Spanish-validated International Physical Activity Questionnaire,23 respectively. We classified the education level as low level (studied until primary school) and high level (secondary high school education or higher). Physical activity was classified as sedentary or active (not regularly vs regularly active).
The study protocol was conducted following the Declaration of Helsinki. All study participants signed an informed consent form.
Sample size
The sample size was determined based on an estimated pre-diabetes prevalence of 35.5% and 38% using HbA1c levels and the 2010 ADA criteria, respectively.1 24 25 It was estimated that a random sample of 505 subjects was sufficient to assess an estimated prevalence of approximately 30% with a 95% CI and an error of ±4%.17
Statistical methods
Descriptive statistics of the mean (SD) or median (IQR) were estimated for quantitative variables with a normal or non-normal distribution, respectively. Qualitative variables were assessed using absolute and relative frequencies. Normally distributed data were analysed using the Shapiro-Wilk test. Comparisons between groups of all variables were performed to evaluate the differences. Student’s t-test, analysis of variance, the Mann-Whitney test or the Kruskal-Wallis test were used to assess the differences between groups. The X2 test or Fisher’s exact test were used to determine differences in qualitative variables. Tukey's correction was applied to account for multiple tests. Multivariate logistic regression models were used to determine the association of variables with pre-diabetes, isolated FPG, isolated HbA1c and both FPG and HbA1c at baseline with covariables that were clinically or statistically associated. In the pre-diabetes model, the variables used were age, sex, education level, physical activity, dyslipidaemia, hypertension, family history of diabetes, BMI, waist, glomerular filtration rate and Fatty Liver Index. A stepwise method with selection of variables by backward elimination was used to build the final logistic regression model to predict the normalisation of the glycaemic state; in all models, the goodness-of-fit assumption was tested by the Hosmer-Lemeshow test. The predictive accuracy of the logistic regression model for normalisation was checked by receiver-operating characteristic (ROC) curves and the area under the ROC curve (AUCROC). ORs with corresponding 95% CIs are shown, and statistical significance was established as a p value <0.05. Data management and all analyses were performed using R statistical software, V.3.3.1, and SPSS software (V.22, IBM, SPSS, Chicago, Illinois, USA).
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Out of the 594 individuals recruited, complete data on FPG and HbA1c were available from 583 (98.1%). The prevalence of undiagnosed diabetes was 20 subjects, 3.4% (95% CI 2.7 to 4.2), and the prevalence of pre-diabetes was 229 subjects, 39.3% (37.3 to 41.3). Furthermore, the prevalence based on isolated FPG was 7.2%, and that based on isolated HbA1c was 22.8%, while based on the criteria of both FPG and HbA1c, the prevalence was 9.3% (figure 1).
Figure 1.
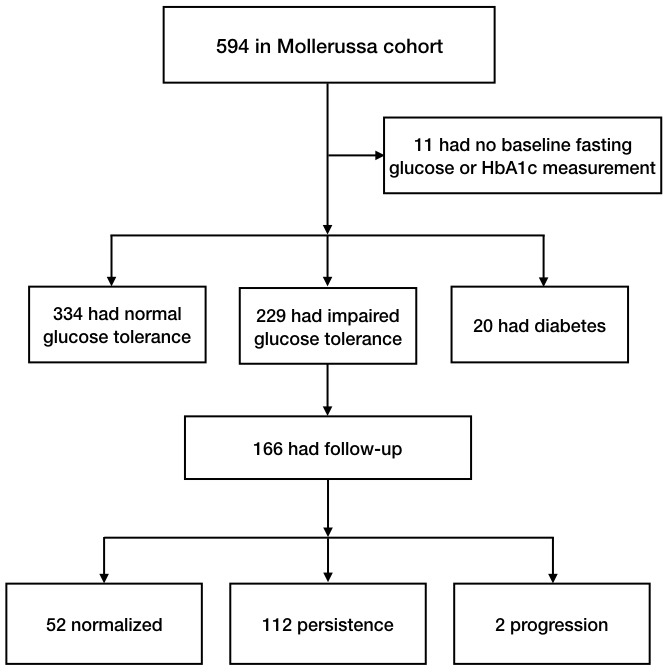
Flow diagram of subjects at baseline and after follow-up. HbA1c, glycated haemoglobin.
The differences of clinical and sociodemographic characteristics between normoglycaemic with pre-diabetic and diabetic groups are shown in table 1. Except for sex, family history of diabetes, current smoking status, alcohol consumption status, triglycerides and HDL-cholesterol levels, there were significant differences in the majority of parameters, including age and BMI, between the three groups.
Table 1.
Clinical and sociodemographic differences among glycaemic status groups of the Mollerussa cohort
| Normoglycaemia FPG <100 mg/dL and HbA1c <5.7% |
Pre-diabetes FPG 100 to <126 mg/dL, or HbA1c 5.7% to <6.5% |
Diabetes FPG≥126 mg/dL or HbA1c ≥6.5% |
Mean difference NG vs PD (95% CI) | Mean difference NG vs DM (95% CI) | |
| N | 334 | 229 | 20 | – | – |
| Sex, women | 193 (57.8%) | 135 (59.0%) | 13 (65.0%) | 1.2 (-7.1 to 9.4) | 7.2 (-14.3 to 28.8) |
| Age, years | 47.1 (12.8) | 54.6 (12.3) | 61.2 (13.6) | 7.5 (5.4 to 9.6) | 14.2 (8.4 to 19.9) |
| BMI, kg/m2 | 25.3 (4.27) | 27.5 (4.75) | 30.2 (5.48) | 2.1 (1.4 to 2.9) | 4.9 (2.9 to 6.9) |
| BMI categories | |||||
| Normal weight | 160 (50.0%) | 67 (30.2%) | 4 (20.0%) | −18.6 (-26.6 to -10.7) | −27.9 (-46.2 to -9.6) |
| Overweight | 120 (37.5%) | 106 (47.7%) | 5 (25.0%) | 10.4 (2.1 to 18.6) | −10.9 (-30.6 to 8.7) |
| Obesity | 40 (12.5%) | 49 (22.1%) | 11 (55.0%) | 9.4 (3.1 to 15.8) | 43.0 (20.9 to 65.1) |
| Waist, cm | 91.9 (11.9) | 97.0 (12.3) | 101 (16.8) | 5.1 (2.9 to 7.2) | 9.3 (3.8 to 14.9) |
| SBP, mm Hg | 119 (16.3) | 126 (16.6) | 130 (18.6) | 6.7 (3.9 to 9.5) | 10.9 (3.5 to 18.5) |
| DBP, mm Hg | 75.7 (10.0) | 78.2 (9.88) | 78.0 (9.24) | 2.5 (0.8 to 4.2) | 2.3 (-2.2 to 6.8) |
| Hypertension | 37 (11.1%) | 49 (21.4%) | 9 (45.0%) | 10.3 (4.0 to 16.6) | 33.9 (11.9 to 55.9) |
| Dyslipidaemia | 27 (8.08%) | 39 (17.0%) | 5 (25.0%) | 8.9 (3.2 to 14.6) | 16.9 (-2.3 to 36.1) |
| Family history DM | 94 (29.6%) | 78 (37.0%) | 8 (42.1%) | 5.9 (-1.9 to 13.7) | 11.8 (-10.1 to 33.9) |
| Education, high level | 265 (82.6%) | 145 (65.0%) | 11 (55.0%) | −16.0 (-23.6 to -8.4) | −24.3 (-46.6 to -2.1) |
| Physical activity | 243 (75.9%) | 141 (63.2%) | 10 (50.0%) | −11.2 (-19.1 to -3.3) | −22.7 (-45.2 to -0.3) |
| Current smoker | 82 (24.6%) | 63 (27.5%) | 3 (15.0%) | 3.0 (-4.4 to 10.4) | −9.5 (-25.9 to 6.8) |
| Alcohol, g/day | 8.33 (13.9) | 12.3 (21.3) | 10.6 (17.2) | 4.0 (0.9 to 6.9) | 2.2 (-5.8 to 10.3) |
| FPG, mg/dL | 86.6 (7.04) | 97.0 (11.2) | 119 (15.2) | 10.4 (8.8 to 11.9) | 32.6 (28.4 to 36.8) |
| HbA1c, % | 5.25 (0.26) | 5.80 (0.29) | 6.26 (0.54) | 0.6 (0.5 to 0.6) | 1.0 (0.9 to 1.1) |
| HbA1c, mmol/mol | 33.8 (2.81) | 39.9 (3.12) | 45.0 (5.92) | 6.1 (5.6 to 6.6) | 11.1 (9.7 to 12.5) |
| eGFR mL/min/1.73 m2 | 96.6 (14.1) | 90.4 (15.9) | 85.5 (18.1) | −6.2 (-8.7 to -3.7) | −11.2 (-17.9 to -4.4) |
| Triglycerides, mg/dL | 104 (90.0) | 111 (63.2) | 116 (47.3) | 6.6 (-6.8 to 19.9) | 11.2 (-24.7 to 47.1) |
| T-cholesterol, g/dL | 197 (38.2) | 205 (32.4) | 214 (31.1) | 7.9 (1.9 to 14.0) | 16.9 (0.7 to 33.1) |
| HDL, mg/dL | 58.7 (15.0) | 58.8 (14.3) | 64.6 (17.6) | 0.1 (-2.4 to 2.6) | 5.9 (-0.86 to 12.6) |
| LDL, mg/dL | 119 (31.4) | 125 (29.4) | 126 (27.7) | 6.3 (1.1 to 11.4) | 7.5 (-6.3 to 21.4) |
| Insulin, µU/mL | 7.99 (3.78) | 10.1 (5.46) | 16.2 (17.1) | 2.1 (1.2 to 3.0) | 8.2 (5.8 to 10.7) |
| Fatty Liver Index | 34.0 (26.9) | 44.3 (28.6) | 59.4 (33.0) | 10.3 (5.5 to 15.0) | 25.4 (12.8 to 38.0) |
| HOMA2-β | 104 (31.8) | 97.7 (31.9) | 89.0 (48.4) | −6.3 (-11.8 to -0.8) | −14.9 (-29.7 to -0.2) |
| HOMA2-S | 118 (52.1) | 94.0 (43.4) | 63.3 (26.7) | −23.8 (-32.0 to -15.6) | −54.4 (-76.3 to -32.6) |
| HOMA2-IR | 1.03 (0.48) | 1.33 (0.72) | 2.16 (2.08) | 0.3 (0.2 to 0.4) | 1.1 (0.8 to 1.4) |
Mean (SD) and n (%).
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein cholesterol; HOMA2-ß, homeostatic model assessment-2 beta cell function; HOMA2-IR, homeostatic model assessment-2 insulin resistance; HOMA2-S, homeostatic model assessment-2 insulin sensitivity; LDL, low-density lipoprotein cholesterol; NG, normoglycaemia; PD, pre-diabetes; SBP, systolic blood pressure; T-cholesterol, total cholesterol.
We observed an association in age, BMI, waist circumference, systolic and diastolic blood pressure, alcohol consumption status, hypertension, dyslipidaemia, triglycerides, total cholesterol, LDL-cholesterol, insulin test, FLI and HOMA2-IR, which were higher in individuals with pre-diabetes than in individuals with normoglycaemia and were higher in the diabetic group than in the pre-diabetic group. On the other hand, physical activity, education level, eGFR, HOMA2-β and HOMA2-S exhibited a negative trend between the same groups. In the pre-diabetic group, 41.9% had impaired FPG and 81.7% had impaired HbA1c. On the other hand, among the newly identified diabetic subjects, up to 80% met the FPG criteria and 85% met the HbA1c criteria. The prevalence of pre-diabetes increased with increasing age, with percentages of 17.4%, 28.6%, 46.4%, 50% and 52.9% in participants aged <35 years, 36 to 45 years, 46 to 55 years, 56 to 65 years and >65 years, respectively. Regarding BMI categories of normal weight (BMI <25 kg/m2), overweight (BMI 25 to 29.9 kg/m2) and obese (BMI >30 kg/m2), the prevalence of pre-diabetes was 29%, 45.9%, and 49%, respectively (online supplementary file 1 figure 1).
bmjopen-2019-033332supp001.pdf (972.7KB, pdf)
Table 2 shows the characteristics of pre-diabetic individuals by glycaemic state: isolated FPG, isolated HbA1c and both altered FPG and HbA1c. Thus, among the 229 subjects with pre-diabetes, 42 (18.3%) had abnormal isolated FPG, 133 (58.1%) had abnormal isolated HbA1c and 54 (23.6%) had both abnormal FPG and HbA1c. Patients with both abnormal FPG and HbA1c were older, had larger waist circumferences, had increased FLI and HOMA2-IR, were more likely to be overweight or obese and have hypertension and had lower HOMA2-S. The isolated FPG group had a higher proportion of subjects with a family history of diabetes, higher alcohol consumption, higher levels of total cholesterol and LDL-cholesterol and lower levels of HDL-cholesterol, although none of these differences were statistically significant. Finally, the isolated HbA1c group had an elevated HOMA2-β. Although there were no statistically significant differences, the proportion of men was higher in the isolated FPG group, whereas the proportion of women was higher in the isolated HbA1c and both FPG and HbA1c groups. Among the three groups, no statistically significant differences were found regarding the following variables: sex, dyslipidaemia, family history of diabetes, education level, physical activity, current smoking status, alcohol consumption, triglycerides, total cholesterol, HDL-cholesterol or LDL-cholesterol.
Table 2.
Clinical and sociodemographic characteristics by glycaemic status of the individuals with pre-diabetes
| Impaired HbA1c 5.7% to <6.5% | Impaired FPG 100 to <126 mg/dL |
HbA1c 5.7% to <6.5% and FPG 100 to <126 mg/dL | P overall | P HbA1c vs FPG | P HbA1c vs both | P FPG vs both | |
| N | 133 | 42 | 54 | – | – | – | – |
| Sex, women | 84 (63.2%) | 19 (45.2%) | 32 (59.3%) | 0.12 | 0.181 | 0.74 | 0.369 |
| Age, years | 53.4 (12.4) | 50.6 (11.8) | 60.6 (10.5) | < 0.001 | 0.388 | 0.001 | < 0.001 |
| BMI, kg/m2 | 25.8 (24.5 to 28.9) | 27.8 (24.5 to 30.6) | 27.5 (25.6 to 30.5) | 0.056 | 0.534 | 0.036 | 0.534 |
| BMI categories | 0.018 | 0.107 | 0.05 | 0.032 | |||
| Normal weight | 43 (33.1%) | 16 (41.0%) | 8 (15.1%) | ||||
| Overweight | 64 (49.2%) | 12 (30.8%) | 30 (56.6%) | ||||
| Obesity | 23 (17.7%) | 11 (28.2%) | 15 (28.3%) | ||||
| Waist, cm | 95.0 (88.0 to 102) | 98.0 (90.0 to 106) | 101 (95.0 to 107) | 0.008 | 0.232 | 0.006 | 0.333 |
| SBP, mm Hg | 124 (16.1) | 129 (15.5) | 128 (18.4) | 0.169 | 0.296 | 0.29 | 0.991 |
| DBP, mm Hg | 78.0 (9.44) | 79.5 (12.0) | 77.9 (9.39) | 0.674 | 0.675 | 0.999 | 0.723 |
| Hypertension | 21 (15.8%) | 9 (21.4%) | 19 (35.2%) | 0.014 | 0.542 | 0.019 | 0.32 |
| Dyslipidaemia | 25 (18.8%) | 4 (9.52%) | 10 (18.5%) | 0.358 | 0.515 | 1 | 0.515 |
| Family history DM | 43 (34.1%) | 18 (48.6%) | 17 (35.4%) | 0.265 | 0.471 | 1 | 0.471 |
| Education, high level | 91 (69.5%) | 23 (59.0%) | 31 (58.5%) | 0.252 | 0.455 | 0.455 | 1 |
| Physical activity | 88 (67.2%) | 21 (53.8%) | 32 (60.4%) | 0.281 | 0.547 | 0.68 | 0.68 |
| Current smoker | 38 (28.6%) | 14 (33.3%) | 11 (20.4%) | 0.338 | 0.693 | 0.496 | 0.496 |
| Alcohol, g/day | 2.92 (0.00 to 15.2) | 7.42 (0.90 to 16.3) | 1.53 (0.00 to 17.9) | 0.369 | 0.336 | 0.735 | 0.336 |
| FPG, mg/dL | 89.2 (6.89) | 106 (4.97) | 109 (5.96) | < 0.001 | < 0.001 | < 0.001 | 0.14 |
| HbA1c, % | 5.80 (5.70 to 6.00) | 5.40 (5.40 to 5.57) | 5.95 (5.80 to 6.10) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| HbA1c, mmol/mol | 39.9 (38.8 to 42.1) | 35.5 (34.7 to 37.4) | 41.5 (39.9 to 43.2) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 93.6 (79.6 to 103) | 93.2 (79.7 to 107) | 89.3 (73.1 to 97.2) | 0.076 | 0.556 | 0.073 | 0.073 |
| Triglycerides, mg/dL | 88.0 (72.0 to 134) | 86.5 (67.0 to 130) | 106 (74.5 to 132) | 0.332 | 0.729 | 0.304 | 0.304 |
| Total cholesterol, mg/dL | 205 (34.5) | 209 (28.6) | 203 (29.8) | 0.689 | 0.767 | 0.947 | 0.677 |
| HDL-cholesterol, mg/dL | 58.0 (51.0 to 69.0) | 52.0 (45.0 to 65.8) | 57.0 (51.0 to 66.0) | 0.128 | 0.141 | 0.755 | 0.18 |
| LDL-cholesterol, mg/dL | 125 (32.2) | 133 (25.5) | 120 (23.5) | 0.114 | 0.278 | 0.593 | 0.096 |
| Insulin, µU/mL | 8.00 (6.10 to 10.0) | 9.90 (6.90 to 15.9) | 10.9 (7.90 to 15.6) | < 0.001 | 0.01 | < 0.001 | 0.577 |
| Fatty Liver Index | 34.4 (16.9 to 59.2) | 42.2 (17.7 to 73.6) | 53.8 (32.2 to 73.0) | 0.016 | 0.373 | 0.011 | 0.378 |
| HOMA2-β | 96.6 (81.5 to 122) | 81.7 (64.5 to 118) | 82.8 (63.0 to 108) | 0.001 | 0.034 | 0.002 | 0.693 |
| HOMA2-S | 98.0 (77.2 to 127) | 75.0 (47.2 to 107) | 67.5 (47.5 to 91.3) | < 0.001 | 0.003 | < 0.001 | 0.564 |
| HOMA2-IR | 1.00 (0.80 to 1.30) | 1.30 (0.90 to 2.15) | 1.50 (1.10 to 2.10) | < 0.001 | 0.005 | < 0.001 | 0.545 |
Significant values are shown in bold. Mean (SD), median (IQR) and n (%).
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA2-β, homeostatic model assessment-2 beta cell function; HOMA2-IR, homeostatic model assessment-2 insulin resistance; HOMA2-S, homeostatic model assessment-2 insulin sensitivity; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Pre-diabetes follow-up
Of the 229 individuals with pre-diabetes at baseline, 166 (72.5%) had clinical and laboratory data after 12 months of follow-up. Of them, 52 (41.6%) returned to a normal glycaemic status, 112 (57.6%) persisted in their state of pre-diabetes and only 2 (0.6%) progressed to diabetes. Table 3 shows the outcome of the follow-up of the isolated FPG, HbA1c and both FPG and HbA1c groups.
Table 3.
Outcomes at follow-up of patients with different altered glucose metabolism statuses at baseline
| Variables | Baseline | N with follow-up | Follow-up | ||
| Normalised | Persisted | Progressed | |||
| Pre-diabetes | 229 (39.3%) | 166 (90.7%) | 52 (41.6%) | 112 (57.8%) | 2 (0.6%) |
| Isolated FPG | 42 (7.2%) | 3 (1.8%) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| Isolated HbA1c | 133 (22.8%) | 114 (68.7%) | 47 (41.3%) | 67 (58.7%) | 0 (0%) |
| Both altered | 54 (9.3%) | 49 (29.5%) | 4 (8.2%) | 44 (89.8%) | 1 (2%) |
FPG, fasting plasma glucose; HbA1c, glycated haemoglobin.
Association of pre-diabetes with glycaemic status
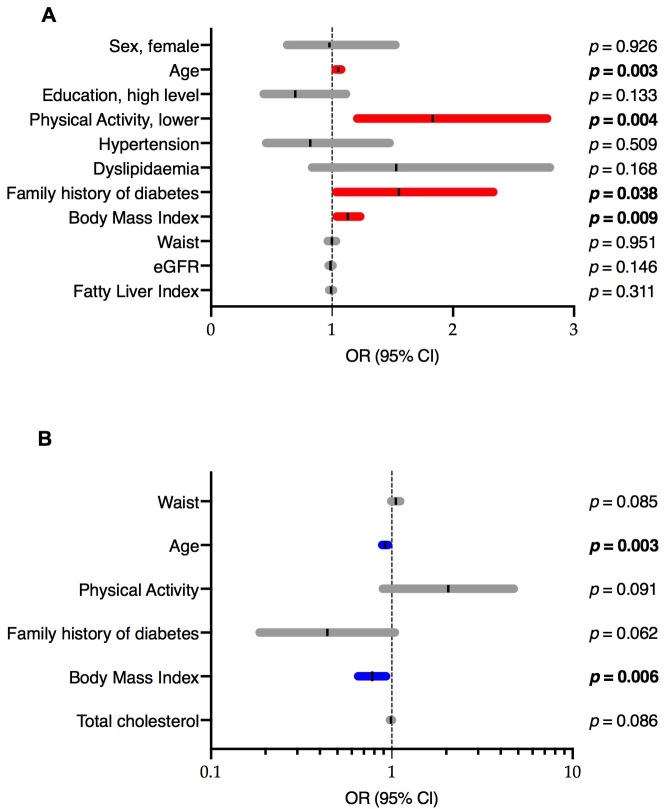
The multivariate logistic regression model of pre-diabetes versus normoglycaemia showed that the variables associated with pre-diabetes were older age (OR; 95% CI) (1.033; 1.011 to 1.056), higher physical activity levels (0.546; 0.360 to 0.827), higher BMI (1.121; 1.029 to 1.222) and a family history of diabetes (1.543; 1.025 to 2.323) (figure 2A). The models for isolated FPG alterations, isolated HbA1c alterations and both FPG and HbA1c alterations are shown in online supplementary file 2 tables 1, 2 and 3, respectively. The variables associated with isolated FPG were older age (1.032; 1.008 to 1.057), higher physical activity levels (0.535; 0.318 to 0.899) and a family history of diabetes (1.798; 1.067 to 3.028). On the other hand, the only variable associated with impaired HbA1c was older age (1.048; 1.029 to 1.067). Finally, in the model for altered FPG and HbA1c, the variables associated were older age (1.056; 1.026 to 1.086) and high FLI (1.031; 1.002 to 1.061).
Figure 2.
Multivariate logistic regression models: (a) Model of pre-diabetes versus normoglycaemic state in the Mollerussa cohort at baseline. Significant p values are shown in BOLD. Hosmer-Lemeshow test p=0.295. (b) Model of normalised versus persisted in subjects with follow-up data. Significant p values are shown in BOLD. Hosmer-Lemeshow test p=0.931. eGFR, estimated glomerular filtration rate.
bmjopen-2019-033332supp002.pdf (34.3KB, pdf)
Prediction of normalisation
Logistic regression model, as described in the methods section, starting with the variables age, sex, waist circumference, BMI, hypertension, physical activity, family history of diabetes, education level, total cholesterol, HDL-cholesterol, FLI and HOMA2-IR, was performed to identify factors independently associated with the prediction of glycaemic status normalisation (online supplementary file 2 table 4). The variables that predicted glycaemic normalisation were older age (0.948; 0.916 to 0.982) and BMI (0.779; 0.651 to 0.931) (figure 2B); this model had a good predictive ability (AUCROC 0.77; p<0.001) (online supplementary file 3 figure 2).
bmjopen-2019-033332supp003.pdf (1MB, pdf)
Discussion
We found that the prevalence of undiagnosed diabetes was 3.4%, and the prevalence of pre-diabetes was 39.3% in this semi-rural population in Catalonia (northeast Spain). The prevalence of pre-diabetes was three-fold higher based on HbA1c than that based on FPG. Subjects with pre-diabetes defined by both HbA1c and FPG criteria had unfavourable clinical and sociodemographic profiles related to increased cardiovascular risk. These factors were older age; abdominal obesity; higher triglycerides; increased FLI and a higher proportion of overweight, obesity and hypertension. In our population, age was the variable most strongly associated with pre-diabetes based on all specific glycaemic status variables: isolated impaired FPG, isolated impaired HbA1c or both impaired FPG and HbA1c. Other variables associated with pre-diabetes were lower physical activity levels, a family history of diabetes and obesity. Finally, the characteristics related to normalisation at follow-up were younger age and lower BMI.
The prevalence of pre-diabetes and undiagnosed diabetes in our healthy population were within the ranges found in other population studies defining pre-diabetes based on the 2010 ADA criteria, using FPG and/or HbA1c. Among these studies, a large national Chinese study (with 170 287 subjects) showed a prevalence of pre-diabetes of 35.7% and a prevalence of undiagnosed diabetes of 6.9%.26 In a study of the Caribbean population, the corresponding figures were 44.1% for pre-diabetes and 7.3% for undiagnosed diabetes.27 In England, based on HbA1c levels, the pre-diabetes prevalence was 35.5% in the adult population in 2011.24 In these studies, the prevalence of pre-diabetes was higher in older, overweight and obese participants.24 26 27 Many other studies found this relationship of age and obesity with the risk and incidence of diabetes.28–31
In the 1999 to 2002 National Health and Nutrition Examination Survey (NHANES), the prevalence of undiagnosed diabetes was 2.8%, and up to 26% of the participants had IFG.32 However, the age-standardised prevalence of pre-diabetes based on HbA1c and FPG combined was similar in the periods between 1999 and 2002 and 2003 and 2006 at 29.2% and 29.3%, respectively, but increased significantly to 36.2% in the period between 2007 and 2010.33 This prevalence continued to increase to as high as 38% in 2012 among adults from the USA.25 The change in the prevalence of pre-diabetes over time occurred because of a significant change in elevated HbA1c, whereas the prevalence based on elevated FPG was similar over this period.33 Thus, in our population, as in the NHANES study, HbA1c was the most significant contributor to pre-diabetes prevalence, followed by FPG, which is in concordance with the findings in the Caribbean population27 and discordant with the reports from the NHANES study between 2011 and 2014 in which they reported that FPG was the most significant contributor to pre-diabetes prevalence followed by HbA1c.34 Our results show that individuals with isolated impaired HbA1c when diagnosed with pre-diabetes might have a slightly better cardiometabolic risk profile than those with isolated FPG, while those individuals with both impaired FPG and HbA1c had the worst cardiovascular risk. These results are in line with the findings of the prospective observational study in the primary care setting of a Spanish cohort with pre-diabetes (PREDAPS) of our group.35 36
Additionally, two meta-analyses found that among individuals with pre-diabetes based on the ADA criteria, all-cause and cardiovascular disease (CVD) mortality were increased37 and that the risk of cardiovascular disease increased independently of the glucose assessment in comparison to the risk of normoglycaemic subjects.38 Moreover, a recent study concluded that those who returned to normoglycaemia from FPG-defined or HbA1c-defined pre-diabetes were not at reduced risk of future CVD or death.39 Studies of shorter duration, over 3 to 5 years, have shown that approximately 25% of subjects progress to diabetes, 25% return to a normal state of glucose tolerance and 50% remain in the pre-diabetic state;16 after 1 year, 18.8% of subjects with pre-diabetes returned to normoglycaemia and approximately 30% with abnormal FPG, 29.1% with abnormal HbA1c and 7.6% with abnormalities in both FPG and HbA1c returned to a normal state of glucose tolerance.40 In our findings from a 1-year follow-up, the rate of reversion from pre-diabetes to normoglycaemia was approximately 40%, and approximately 60% of participants remained in the pre-diabetic state. On the other hand, lifestyle modifications, such as weight loss and increased physical activity, among other factors associated with pre-diabetes, reduced the risk of diabetes among these subjects.13 41 According to these reports, in our study, lower BMI was a factor that was independently associated with the normalisation of the glycaemic state, and an active lifestyle decreased the risk of having pre-diabetes.
The results of this study need to be interpreted in light of its strengths and weaknesses. First, the number of participants in our study is smaller in comparison to other studies. In addition, the study may not be representative of urban areas in our region. Thus, the results may not be generalisable to other territories with different population characteristics in our country. However, the Mollerussa cohort is a representative sample of the region, which is a specific semi-rural area that has never been specifically investigated. Second, our study sample is probably healthier than the general population, as we excluded subjects with already known diabetes and other comorbidities, a lower number of subjects were counted in the denominator, thus resulting in a higher prevalence of this condition. Third, we did not assess glucose tolerance through an oral glucose tolerance test, which is common in most population studies. Although this assay is sensitive, it is also less specific for identifying subjects who could develop diabetes.42 Furthermore, the oral glucose tolerance test has a low reproducibility and is a rather time-consuming and expensive procedure.9 43 Conversely, HbA1c and FPG are cost-effective and more convenient for patients. Currently, FPG is an accepted screening method to detect diabetes and pre-diabetes. HbA1c improves the sensitivity of FPG in the detection of early T2D in high-risk subjects32 44 and is a better predictor of CV events than FPG.45 Fourth, we only followed up those participants with pre-diabetes. Thus, we could not analyse the probability of changing from normoglycaemia to pre-diabetes or diabetes in this study. Finally, it is probable that the use of the WHO pre-diabetes criteria in our study would have resulted in a smaller proportion of subjects who returned to a normal glycaemic state. The WHO established a normal concentration of FPG between 110 and <126 mg/dL.46
Conclusions
For the first time, our study provides information on the prevalence of diabetes and pre-diabetes in the Mollerussa healthcare area, a Mediterranean semi-rural area in northeast Spain. Individuals with pre-diabetes had a more unfavourable cardiometabolic risk profile than normoglycaemic subjects. Moreover, individuals with abnormalities in both criteria used to diagnose pre-diabetes had the worst risk profile. Finally, after 1 year of follow-up, few people progressed to diabetes, while more than 40% returned to a normal glycaemic state and nearly 60% persisted in the pre-diabetic state. These results suggest that the use of both FPG and HbA1c criteria in clinical practice could help identify people with high diabetes and cardiovascular risk. Moreover, the identification of individuals with pre-diabetes provides an opportunity for intervention through lifestyle modification and pharmacological treatments not only to reduce the development of diabetes.
Supplementary Material
Acknowledgments
The authors thank Jordi Real for their valuable assistance in conducting the statistical analysis. CIBER of Diabetes and Associated Metabolic Diseases is an initiative from Instituto de Salud Carlos III (Plan Nacional de I+D+I and Fondo Europeo de Desarrollo Regional).
Footnotes
Contributors: MF, EC and DM conceived and designed the study; MBV, JFN and MMC participated in the study design; MBV, MF, NA, MGC, NM, AM and CC collected the data; EC and JRM performed the statistical analyses; MF, EC, MMC and DM wrote the manuscript. All authors critically reviewed the manuscript and approved the final version to be published.
Funding: This study was funded by Institut Universitari d’Investigació en Atenció Primària Jordi Gol (IDIAP Jordi Gol).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The project was approved by the Ethics Committee of the Primary Health Care University Research Institute (IDIAP) Jordi Gol (PI12/043) Barcelona, Spain.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Readers may contact Dr Didac Mauricio (didacmauricio@gmail.com) regarding the data.
References
- 1. Olson DE, Rhee MK, Herrick K, et al. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184–9. 10.2337/dc10-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association 2. classification and diagnosis of diabetes. Diabetes Care 2017;40:S11–24. 10.2337/dc17-S005 [DOI] [PubMed] [Google Scholar]
- 3. Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med 2016;241:1323–31. 10.1177/1535370216654227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrannini E. Definition of intervention points in prediabetes. Lancet Diabetes Endocrinol 2014;2:667–75. 10.1016/S2213-8587(13)70175-X [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S13–28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 6. International diabetes Federation diabetes atlas. 8th ED, 2017. Available: http://www.diabetesatlas.org/ [Accessed Jun 2019].
- 7. Centers for Disease Control and Prevention National diabetes statistics report, 2017. Atlanta, GA: Centers for disease control and prevention, US. Dept of Health and Human Services; 2017. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html [Google Scholar]
- 8. Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia 2012;55:88–93. 10.1007/s00125-011-2336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mata-Cases M, Artola S, Escalada J, et al. Consenso sobre La detección Y El manejo de la prediabetes. Grupo de Trabajo de Consensos Y Guías Clínicas de la Sociedad Española de diabetes. Revista Clínica Española 2015;215:117–29. 10.1016/j.rce.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 10. Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–9. 10.2337/dc07-9920 [DOI] [PubMed] [Google Scholar]
- 11. Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. The Lancet 2012;379:2279–90. 10.1016/S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 13. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramachandran A, Snehalatha C, Mary S, et al. The Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97. 10.1007/s00125-005-0097-z [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–12. 10.1016/j.diabres.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 16. Paulweber B, Valensi P, Lindström J, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 2010;42 Suppl 1:S3–36. 10.1055/s-0029-1240928 [DOI] [PubMed] [Google Scholar]
- 17. Vilanova MB, Falguera M, Marsal JR, et al. Prevalence, clinical features and risk assessment of pre-diabetes in Spain: the prospective Mollerussa cohort study. BMJ Open 2017;7:e015158–8. 10.1136/bmjopen-2016-015158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernal-Delgado E, García-Armesto S, Oliva J, et al. Spain: health system review. Health Syst Transit 2018;20:1–179. [PubMed] [Google Scholar]
- 19. Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:44–7. 10.1186/1471-230X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2. 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. OECD/Eurostat/UNESCO Institute for Statistics ISCED 2011 operational manual: guidelines for classifying national education programmes and related qualifications. Paris: OECD Publishing, 2015. [Google Scholar]
- 23. Roman-Viñas B, Serra-Majem L, Hagströmer M, et al. International physical activity questionnaire: reliability and validity in a Spanish population. Eur J Sport Sci 2010;10:297–304. 10.1080/17461390903426667 [DOI] [Google Scholar]
- 24. Mainous AG, Tanner RJ, Baker R, et al. Prevalence of prediabetes in England from 2003 to 2011: population-based, cross-sectional study. BMJ Open 2014;4:e005002–8. 10.1136/bmjopen-2014-005002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–9. 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515–6. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unwin N, Howitt C, Rose AMC, et al. Prevalence and phenotype of diabetes and prediabetes using fasting glucose vs HbA1c in a Caribbean population. J Glob Health 2017;7:1–11. 10.7189/jogh.07.020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soriguer F, Rojo-Martínez G, Almaraz MC, et al. Incidence of type 2 diabetes in southern Spain (Pizarra study). Eur J Clin Invest 2008;38:126–33. 10.1111/j.1365-2362.2007.01910.x [DOI] [PubMed] [Google Scholar]
- 29. DECODE Study Group Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–9. 10.2337/diacare.26.1.61 [DOI] [PubMed] [Google Scholar]
- 30. Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–10. 10.2337/diab.46.4.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burke JP, Williams K, Gaskill SP, et al. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996. Arch Intern Med 1999;159:1450–6. 10.1001/archinte.159.13.1450 [DOI] [PubMed] [Google Scholar]
- 32. Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National health and nutrition examination survey 1999-2002. Diabetes Care 2006;29:1263–8. 10.2337/dc06-0062 [DOI] [PubMed] [Google Scholar]
- 33. Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National health and nutrition examination surveys, 1999-2010. Diabetes Care 2013;36:2286–93. 10.2337/dc12-2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Menke A, Casagrande S, Cowie CC. Contributions of A1c, fasting plasma glucose, and 2-hour plasma glucose to prediabetes prevalence: NHANES 2011-2014. Ann Epidemiol 2018;28:681–5. 10.1016/j.annepidem.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 35. Giráldez-García C, Sangrós FJ, Díaz-Redondo A, et al. Cardiometabolic Risk Profiles in Patients With Impaired Fasting Glucose and/or Hemoglobin A1c 5.7% to 6.4%. Medicine 2015;94:e1935–8. 10.1097/MD.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franch-Nadal J, Caballería L, Mata-Cases M, et al. Fatty liver index is a predictor of incident diabetes in patients with prediabetes: the PREDAPS study. PLoS One 2018;13:e0198327–17. 10.1371/journal.pone.0198327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953 10.1136/bmj.i5953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levitan EB, Song Y, Ford ES, et al. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? Arch Intern Med 2004;164:2147–55. 10.1001/archinte.164.19.2147 [DOI] [PubMed] [Google Scholar]
- 39. Vistisen D, Kivimäki M, Perreault L, et al. Reversion from prediabetes to normoglycaemia and risk of cardiovascular disease and mortality: the Whitehall II cohort study. Diabetologia 2019;62:1385–90. 10.1007/s00125-019-4895-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giráldez-García C, García Soidán FJ, Serrano Martín R, et al. Evolución de pacientes Con prediabetes en Atención Primaria de Salud (PREDAPS): resultados del primer año de seguimiento. Diabetes practica 2014;5:1–48. [Google Scholar]
- 41. Díaz-Redondo A, Giráldez-García C, Carrillo L, et al. Modifiable risk factors associated with prediabetes in men and women: a cross-sectional analysis of the cohort study in primary health care on the evolution of patients with prediabetes (PREDAPS-Study). BMC Fam Pract 2015;16 10.1186/s12875-014-0216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Unwin N, Shaw J, Zimmet P, et al. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–23. 10.1046/j.1464-5491.2002.00835.x [DOI] [PubMed] [Google Scholar]
- 43. Gossain VV, Aldasouqi S. The challenge of undiagnosed pre-diabetes, diabetes and associated cardiovascular disease. Int J Diabetes Mellit 2010;2:43–6. 10.1016/j.ijdm.2009.10.004 [DOI] [Google Scholar]
- 44. Droumaguet C, Balkau B, Simon D, et al. Use of HbA1c in predicting progression to diabetes in French men and women: data from an epidemiological study on the insulin resistance syndrome (DESIR). Diabetes Care 2006;29:1619–25. 10.2337/dc05-2525 [DOI] [PubMed] [Google Scholar]
- 45. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–11. 10.1056/NEJMoa0908359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Ginebra: World Health Organization, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033332supp001.pdf (972.7KB, pdf)
bmjopen-2019-033332supp002.pdf (34.3KB, pdf)
bmjopen-2019-033332supp003.pdf (1MB, pdf)