Abstract
Design
Systematic review and meta-analysis of observational studies was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for studies reporting on diabetes mellitus (DM) or metabolic syndrome (MetS) and kidney stone disease (KSD).
Objective
To examine the association between chronic hyperglycaemia, in the form of DM and impaired glucose tolerance (IGT) in the context of MetS and KSD.
Setting
Population-based observational studies. Databases searched: Ovid MEDLINE without revisions (1996 to June 2018), Cochrane Library (2018), CINAHL (1990 to June 2018), ClinicalTrials.gov, Google Scholar and individual journals including the Journal of Urology, European Urology and Kidney International.
Participants
Patients with and without chronic hyperglycaemic states (DM and MetS).
Main outcome measures
English language articles from January 2001 to June 2018 reporting on observational studies. Exclusions: No comparator group or fewer than 100 patients. Unadjusted values were used for meta-analysis, with further meta-regression presented as adjusted values. Bias was assessed using Newcastle-Ottawa scale.
Results
2340 articles were screened with 13 studies included for meta-analysis, 7 DM (three cohort) and 6 MetS. Five of the MetS studies provided data on IGT alone. These included: DM, n=28 329; MetS, n=31 767; IGT, n=12 770. Controls: DM, n=5 89 791; MetS, n=1 78 050; IGT, n=2 93 852 patients. Adjusted risk for DM cohort studies, RR=1.23 (0.94 to 1.51) (p<0.001). Adjusted ORs for: DM cross-sectional/case-control studies, OR=1.32 (1.21 to 1.43) (p<0.001); IGT, OR=1.26 (0.92 to 1.58) (p<0.0001) and MetS, OR=1.35 (1.16 to 1.54) (p<0.0001). There was no significant difference between IGT and DM (cross-sectional/case-control), nor IGT and MetS. There was a moderate risk of publication bias. Statistical heterogeneity remained significant in adjusted DM cohort values and adjusted IGT (cross-sectional/case-control), but non-signficant for adjusted DM (cross-sectional/case-control).
Conclusion
Chronic hyperglycaemia increases the risk of developing kidney stone disease. In the context of the diabetes pandemic, this will increase the burden of stone related morbidity and mortality.
PROSPERO registration number
CRD42018093382
Keywords: urolithiasis; diabetes & endocrinology; other metabolic, e.g. iron, porphyria
Strengths and limitations of this study.
Largest systematic review and meta-analysis examining the risk of chronic hyperglycaemic states and kidney stone disease (KSD), with bias analysis.
Meta-analysis of cohort studies examining diabetes mellitus (DM) demonstrates an increased risk of KSD of 1.23 (0.94 to 1.51) (p<0.001) over the general population.
There was a moderate risk of publication bias.
Statistical heterogeneity remained significant in adjusted DM cohort values and adjusted impaired glucose tolerance.
No data on stone type.
Introduction
Kidney stone disease (KSD) is a painful and costly condition1 where precipitates of normal urinary solutes aggregrate to form stones of varying sizes and compositions.2 Incidence of acute urolithiasis is rising worldwide,3–6 with corresponding rises in surgical treatment rates7 and morbidity8 9 although mortality has declined.8 10 Five-year recurrence rates have been reported as high as 50%.11 Long-term problems associated with recurrent KSD are decreased quality of life, missed work days,12 disabling pain, need for repeated operations, complications including infection and acute kidney injury,13 14 as well as long-term increased risk of developing chronic kidney disease.15
Patients with diabetes mellitus (DM)16 and metabolic syndrome (MetS)17 have been identified as carrying a higher risk of developing KSD. The global prevalence of both conditions has risen to pandemic levels9 18 seemingly in parallel with KSD.19 There is overlap between the two conditions, with impaired glucose tolerance (IGT), or pre-diabetes being one of the five components of the ‘metabolic syndrome’.20 Although the pathophysiology with respect to KSD is yet to be definitively described, patients with either MetS or DM have been shown to have increased urinary acidification and produce more uric acid stones than controls. Notably, with rising body mass index (BMI) in both diabetic and non-diabetic patients, the incidence of uric acid stones rises, while calcium oxalate stones fall.21 22
Previous systematic reviews have examined either DM16 or MetS17 23 in isolation. These studies performed either no meta-analysis,17 or else their heterogeneity/sensitivity analyses were limited.16 23 Given the overlap between the two conditions we aimed to perform a systematic review and meta-analysis of the existing literature on both DM and MetS with complete sensitivity, bias and heterogeneity analyses.
Evidence acquisition
Search strategy and study selection
Population – Chronic hyperglycaemics (diabetes mellitus, impaired glucose tolerance in the context of metabolic syndrome) and those with metabolic syndrome.
Comparator – Those without hyperglycaemia (DM/IGT) or metabolic syndrome, respectively.
Outcome – KSD – all compositions.
Study design – Systematic review and meta-analysis of published observational studies (cohort, case-control and cross-sectional).
Inclusion criteria
All articles written in the English language.
Adults (>18 years).
All articles reporting on risk of developing kidney stone disease in diabetes mellitus (type 1 and type 2) in comparison to general population.
All articles reporting on risk of developing kidney stone disease in patients with metabolic syndrome in comparison to general population.
Risk in risk ratio (RR), HR, OR or prevalence ratio (PR) with 95% CIs.
Exclusion criteria
Older studies using the same data as a more recent study – longest follow-up used.
Studies exclusively using patients with kidney stone disease – unable to calculate risk.
Studies with less than 100 patients – likely to be underpowered.
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.24 The search strategy was conducted to find relevant studies from Ovid MEDLINE without revisions (1996 to June 2018), Cochrane Library (2018), CINAHL (1990 to June 2018), ClinicalTrials.gov, Google Scholar and individual journals including the Journal of Urology, European Urology and Kidney International. The review was registered prospectively with PROSPERO.
Terms used included: ‘Diabetes’, ‘Diabetes mellitus’, ‘metabolic syndrome’, ‘urolithiasis’, ‘nephrolithiasis’, ‘kidney’, ‘uret*’, ‘ston*’, ‘calcul*’. Boolean operators (AND, OR) were used to refine the search.
The search was limited to English language articles between January 2001 and June 2018. Only published data were used.
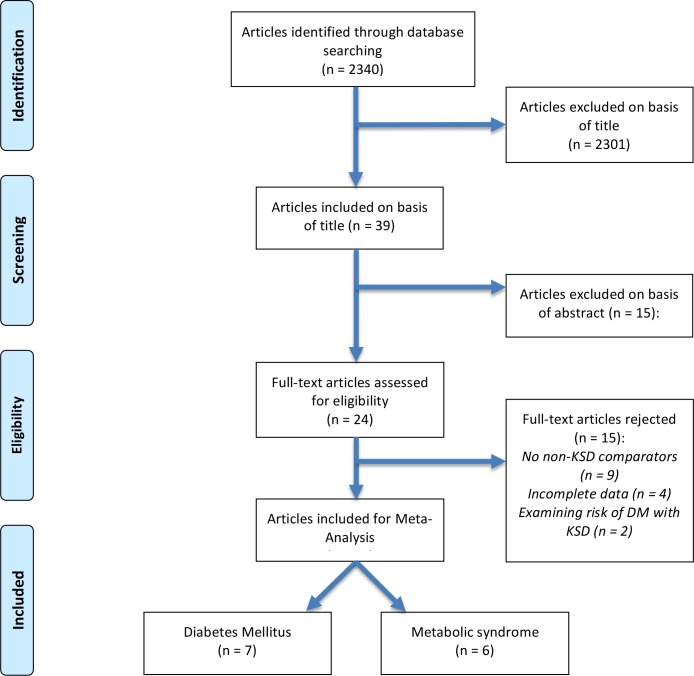
Two reviewers (RG and AA) identified all studies. All studies that appeared to fit the inclusion criteria were included for full review. Each reviewer independently selected studies for inclusion in the review (see figure 1). If there was disagreement, PR and BS made final decision on inclusion.
Figure 1.
PreferredReporting Items for Systematic Reviews and Meta-Analyses flow diagram for article selection. DM, diabetes mellitus; KSD, kidney stone disease.
Data extraction and assessment of quality
The following variables were extracted from each study: first author, year of publication, type of study, sample size, age, country, male:female ratio, ascertainment of DM/IGT/MetS/KSD, type of DM, number of patient reporting/presenting with stone disease for diabetes mellitus, metabolic syndrome and specifically IGT in the context of MetS (given the common mechanism – hyperglycaemia and insulin resistance).
Risk of KSD in RR, HR, OR or PR with 95% CIs was also extracted. HR and RR, and OR and PR, were considered the same and are presented as RR and OR, respectively. Unadjusted and adjusted risk values were extracted from the studies. Adjustment factors were recorded. If adjusted values were missing then the study was removed from the adjusted meta-analysis.
Cross-sectional and case-control studies were pooled as there were no case-control studies for MetS, and two case-control studies for DM, only one of which gave adjusted values.
Data were collated using Microsoft Excel (V.12.2.4). Level of evidence was assessed and study bias was analysed using the Newcastle-Ottawa bias assessment tool.25
Data sharing
Data has been uploaded to PROSPERO or can be obtained, on reasonable request, by emailing the corresponding author.
Statistical methods
Risk is presented with a 95% CI as RR for cohort studies and OR for case-control (CaCo) and cross-sectional (XS) studies. Statistical heterogeneity was tested for using I2, Tau2 and Cochran’s Q. P values <0.05 were considered statistically significant, I2 values were interpreted according to chapter 9.5.2 of the Cochrane Handbook. Heterogeneity was also tested with ‘leave-one-out’ analyses. Publication bias was assessed with Egger’s test and ‘trim and fill’ analysis. Meta-regression analysis was performed, adjusting for age and gender. Student's t-statistic is used for df.
Statistical analyses and figures were generated in R (R foundation for statistical computing, Vienna, Austria) with the metafor package.26
Evidence synthesis
Fifteen studies were included in the systematic review from an initial search total of 2340 (see figure 1). Articles excluded on the basis of title were 2301, 15 on the basis of abstract and 15 on reading the full text. This left 13 studies, 7 examining DM and 6 examining IGT in the context of MetS. Inter-rater reliability as assessed by Cohen’s kappa was 0.95.
Demographics of included studies
Diabetes mellitus
Seven studies were included examining DM.27–33 Three were cohort,27–29 three were case-control30–32 and three were cross-sectional.27 29 33 Taylor et al 27 and Akoudad et al 29 performed both cross-sectional and prospective cohort studies with their cohorts. The studies were conducted in Turkey, Taiwan and USA. They sampled varying populations, from hospital inpatients to national patient data. Patients with type 1 DM were included in all but one of the studies32 (see table 1).
Table 1.
Study demographics
| DM | Study | Study type | Country | Sample | Controls | Metabolic syndrome definition | Diabetes mellitus ascertainment | KSD ascertainment | M:F (%) | Mean age |
| Cohort | Taylor et al 27 | Prospective cohort | USA | NHS I (1980–2000: 20-year f/u)+II (1991–2001: 20-year f/u) (female nurses), HPFS participants (1986–2000: 14-year f/u) (Health Professionals Follow-up Study - all male) – ‘diabetics’, those with known KSD excluded | NHS I+II, HPFS participants - non-diabetics | N/A | Biennial health questionnaire with supplementary questionnaire on symptoms, diagnostic tests and treatment - DM diagnosis corroborated by medical record review. T1 (≥2 episodes of ketonuria/ketoacidosis) and T2 included. | Biennial health questionnaire and medical record review for corroboration - incident stone with pain/haematuria | NHS: Entirely female HPFS: Entirely male |
NHS I: 48.6; NHS II: 37.6; HPFS: 60.9 |
| Chen et al 28 | Retrospective cohort | Taiwan | National Health Insurance system database - prospectively maintained - patients with DM (T1+T2) (2000–2007: 7 years f/u). Known KSD excluded at start. | Without DM and excluding patients who developed DM in follow-up period | N/A | At least three outpatient visits for DM from 2000 to 2002 with corresponding health insurance records; ICD-9-CM 250; A-code A181. T1+T2 included | Health insurance records; ICD9-CM 592; A-code A352, excluding bladder stones. Only new stones included | 50:50 | N/A | |
| Akoudad et al 29 | Prospective cohort | USA | ARIC study participants: Visit 3 (1993–1995) to 2005 with incident KSD (patient reported physician diagnosis of KSD at baseline excluded). F/U – mean 10.8 years. | Without incident KSD | N/A | Receiving diabetic medication, OGTT with FPG>110 mg/dL, FPG>126 mg/dL, patient reported physician diagnosis. Unclear T1/T2 differentiation. | ICD-9 codes: 592, 592.0, 592.1, 592.9, 274.11 on discharge summaries | 42:58 | 60.0±5.7 (calculated) | |
| CaCo | Lieske et al 31 | Case-control | USA | Rochester, Olmsted County, Minnesota residents with electronically documented KSD - random sample of results of electronic medical record search of Mayo Clinic and Olmsted Clinic databases (original search n>7000) | Patients without electronic documentation of KSD, matched for age, sex and calendar year of stone | N/A | Electronic medical records using codes: ICD-9 codes 250, 357.2, 362.0, 366.41, 648.0 (gestational DM), 648.8, 790.2, 791.5, 962.3. No clear differentiation between T1+T2. | Electronic medical records using codes: ICD-9-CM 592, 594, 275.11 with case review | 62: 38 | 45.0±18 |
| Davarci et al 32 | Case-control | Turkey | Hospital outpatients with urolithiasis attending single centre between 2008–2009, T1DM excluded | Without urolithiasis | N/A | Receiving diabetic medication, OGTT with FPG>110 mg/dL, FPG>126 mg/dL. T1 excluded | USS, AXR, patient reported | 47.5:52.5 | 49.0±10 | |
| XS | Meydan et al 30 | Cross-sectional with matching | Turkey | Diabetic hospital attendees, unclear if inpatients or outpatients | Non-diabetic hospital attendees, unclear if inpatients or outpatients - matched for age | N/A | Unclear how defined. Included both T1 and T2. | History of KSD, XR/USS – if any positive confirmed with IVU | Cases: 30:70 Controls: 21:79 |
Cases: 57±10 Controls: 56±9 |
| Taylor et al 27 | Cross-sectional | USA | Baseline characteristics: NHS I (1980) + II (1991) (female nurses), HFPS participants (1986) (male health professionals) - diabetics | Baseline characteristics: NHS I+II, HFPS participants - non-diabetics | N/A | Biennial health questionnaire with supplementary questionnaire on symptoms, diagnostic tests and treatment - DM diagnosis corroborated by medical record review | Biennial health questionnaire and medical record review for corroboration - kidney stone history | 22:78 | NHS I: 48.6; NHS II: 37.6; HFPS: 60.9 | |
| Akoudad et al 29 | Cross-sectional | USA | ARIC study participants: Visit 3 (1993–1995), patient reported physician diagnosis of KSD | Without KSD | N/A | Receiving diabetic medication, OGTT with FPG>110 mg/dL, FPG>126 mg/dL, patient reported physician diagnosis | Patient reported physician diagnosis | 44:56 (calculated) | 60.0±5.7 (calculated) | |
| Weinberg et al 33 | Cross-sectional | USA | NHANES participants 2007–2010 with T2DM | Without DM | N/A | Self- reported history of DM, use of glucose-lowering medications (insulin or oral hypoglycemics), and self-reported diabetic comorbidities. T2 only. | Patient reported answer to: ‘have you ever had a kidney stone?’ | N/A | N/A | |
| MetS | IGT/DM ascertainment | |||||||||
| XS | Rendina et al 34 | Cross-sectional | Italy | Single centre inpatients between 2004–2005 - those with MetS or IGT. Exclusions: acute/chronic renal failure, abnormal renal anatomy, hyperthyroidism, hyperparathyroidism, treatment for osteoporosis, metabolic bone disorders, neoplasia | Those without MetS or IGT | American Heart Association; National Heart, Lung, and Blood Institute: three or more of: (1) Waist circumference >102 cm in men, >88 cm in women. (2) fasting serum triglycerides >1.7 mmol/L or treatment. (3) fasting serum HDL <1.03 mmol/L in men,<1.30 mmol/L in women or treatment. (4) systolic >130 mm Hg or diastolic >85 mm Hg or treatment. (5) fasting serum glucose >5.6 mmol/L or treatment | Fasting serum glucose >5.6 mmol/L or treatment | Questionnaire re: symptoms of renal colic and ultrasonography | 49:51 | 63.8±15.8 |
| West et al 35 | Cross-sectional | USA | NHANES III participants (1988–1994) - those with metabolic syndrome/impaired glucose tolerance | two or fewer MetS traits/no MetS traits | American Heart Association; National Heart, Lung, and Blood Institute as per Rendina et al | Fasting serum glucose >5.6 mmol/L or treatment | Self report of physician diagnosis | 48:52 | 58.8±17.1 | |
| Jeong et al 37 | Cross-sectional | South Korea | Single centre - health promotion patients - those with IGT or MetS | Unclear - ?those without MetS or IGT | NCEP ATP III; American Heart Association; National Heart, Lung, and Blood Institute - three or more of: Systolic >130 mm Hg, diastolic >85 mm Hg, random blood glucose >110 mg/dL, random serum triglycerides >150 mg/dL, random serum HDL <40 mg/dL in men or <50 mg/dL in women, obese range waist circumference | Fasting blood glucose >110 mg/dL | Radiological records (ultrasound and CT) | 60:40 | 50.0±10.4 | |
| Jung et al 36 | Cross-sectional | South Korea | Single Centre - patients recruited to health promotion centre to undergo metabolic + KSD screen - study group - those with impaired glucose tolerance and those with metabolic syndrome | Unclear - ?patients without impaired glucose tolerance or metabolic syndrome | NCEP ATP III - three or more of: Systolic >130 mm Hg, diastolic >85 mm Hg, random blood glucose >110 mg/dL, random serum triglycerides >150 mg/dL, random serum HDL <40 mg/dL in men o r<50 mg/dL in women, obese range waist circumference | Fasting blood glucose >110 mg/dL | Ultrasonography | 55:45 | 44.9±11.5 | |
| Kim et al 38 | Cross-sectional | South Korea | Single centre - health promotion patients - those with IGT or MetS | Unclear - ?those without MetS or IGT | NCEP ATP III; American Heart Association; National Heart, Lung, and Blood Institute - three or more of: Systolic >130 mm Hg, diastolic >85 mm Hg, random blood glucose >110 mg/dL, random serum triglycerides >150 mg/dL, random serum HDL <40 mg/dL in men or <50 mg/dL in women | Fasting blood glucose >110 mg/dL | Ultrasonography | 58:42 | 42.3±8.4 | |
| Lee et al 39 | Cross-sectional | Taiwan | Single centre - men undergoing free health screening - those with MetS/DM | Unclear - ?those without MetS or DM | Three of the five following criteria: patients were defined as having MetS by the presence of at least three of five of the following criteria: waist circumference (WC) 90 cm, high-density lipoprotein (HDL) cholesterol 540 mg/dL, triglyceride (TG) 150 mg/ dL, blood pressure (BP) 130/85 mm Hg or diagnosed hypertension on therapy and fasting blood glucose (FBG) 4100 mg/dL or have a diagnosis of T2DM. | T2DM - fasting BGL >126 mg/dL | (a) Characteristic clinical findings diagnosed by a physician with available medical records; (b) evidence of kidney stones from ultrasonography judged by an investigator (urologist); (c) operative history of stones removal from kidney. | 100:0 | 55.6±4.6 |
CaCo = Case Control; XS = Cross-Sectional; OGTT = Oral Glucose Tolerance Test; FPG = Fasting Plasma Glucose; AXR = Abdominal X-Ray; IVU = Intravenous Urogram; NCEP ATP III = National Cholesterol Education Programme Adult Treatment Programme 3rd Iteration.
BGL, blood glucose level; DM, diabetes mellitus; f/u, follow-up; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IGT, impaired glucose tolerance; KSD, kidney stone disease; MetS, metabolic syndrome; N/A, not available; NHANES, National Health and Nutrition Examination Survey; NHS, National Health Service; T1, type 1 diabetes mellitus; T2, type 2 diabetes mellitus.
The male to female ratio and mean age for each study is detailed in table 1. DM and KSD ascertainment ranged from the patient reporting the diagnosis to International Classification of Diseases (ICD) codes in medical records.
Overall there were 618 120 patients, of which 28 329 (4.6%) had DM. These figures include 17 577 patients with DM in cohort studies with 348 036 controls (see table 2) and 10 752 patients with DM in case-control or cross-sectional studies with 241 755 controls (see table 3). In the cohort studies, 1312 (7.5%) of patients with DM developed KSD compared with 11 516 (3.3%) of controls. In the case-control and cross-sectional studies, 1097 (10.2%) of diabetics had KSD compared with 11 985 (5.0%) of controls. Study reported risk is detailed in tables 2 and 3.
Table 2.
DM cohort studies
| Cohort | Study | Baseline DM, n | Controls, n | With DM, person-years | Without DM, person-years | DM with KSD, n (% of DM) | Control with KSD, n (% of no DM) | Study reported unadjusted risk (95% CI) | Study reported adjusted risk (95% CI) | Adjusted for |
| DM | Taylor et al 27 2005: NHS I (younger female) | 1409 | 93 758 | 65 566 | 1 371 080 | 109 (7.7%) | 1578 (1.7%) | RR 1.45 (1.20 to 1.77) |
RR 1.29 (1.05 to 1.58) |
Age, BMI, thiazide use, fluid intake, alcohol use, calcium supplementation and diet |
| Taylor et al 27 2005: NHS II (older female) | 891 | 101 877 | 12 291 | 824 076 | 40 (4.5%) | 1491 (1.5%) | RR 1.86 (1.36 to 2.56) |
RR 1.60 (1.16 to 2.21) |
Age, BMI, thiazide use, fluid intake, alcohol use, calcium supplementation and diet | |
| Taylor et al 27 2005: HPFS (male) | 1391 | 46 062 | 21 676 | 450 984 | 44 (3.2%) | 1426 (3.1%) | RR 0.76 (1.56 to 1.03) |
RR 0.81 (0.59 to 1.09) |
Age, BMI, thiazide use, fluid intake, alcohol use, calcium supplementation and diet | |
| Chen et al 28 | 12 257 | 96 781 | 75 975 | 607 842 | 1096 (8.9%) | 6950 (7.2%) | HR 1.22 (1.15 to 1.30) |
HR 1.18 (1.10 to 1.27) |
Age, sex, occupation, urbanisation, income and UTIs | |
| Akoudad et al 29 | 1629 | 9558 | N/A | N/A | N/A | N/A | N/A | HR 1.98 (1.20 to 3.28) |
Age, sex, race, waist circumference, hypertension, triglyceride level, uric acid, gallstones | |
| Total | 17 577 | 348 036 | 253 365 | 3 253 982 | 1289 (8.1%) | 11 445 (3.4%) |
HPFS = Healthcare Professionals Follow-up Study (all male)
BMI, body mass index; DM, diabetes mellitus; KSD, kidney stone disease; N/A, not available; NHS, National Health Service; RR, risk ratio; UTIs, urinary tract infections.
Table 3.
DM and IGT case-control and cross-sectional studies
| DM | Study | Study population (DM), n | Controls, n | DM with KSD, n (% of DM) | Control with KSD, n (% of No DM) | Study reported unadjusted risk (95% CI) | Study reported adjusted risk (95% CI) | Adjusted for |
| CaCo | Lieske et al 31 | 3561 | 3561 | 335 (9.4%) | 268 (7.5%) | OR 1.29 (1.09 to 1.53) |
OR 1.22 (1.03 to 1.46) |
Age, sex, year of diagnosis, DM, hypertension and obesity |
| Davarci et al 32 | 23 | 177 | 14 (17.5%) | 66 (37.3%) | RR 1.63 (1.12 to 2.39) |
N/A | N/A | |
| XS | Meydan et al 30 | 321 | 115 | 84 (26.2%) | 14 (12.2%) |
OR 2.5
(1.39 to 4.71) (calculated) |
N/A | N/A |
| Taylor et al 27 2005: NHS I (younger female) | 1473 | 74 266 | 64 (4.3%) | 2029 (2.7%) | RR 1.55 (1.20 to 1.99) |
RR 1.38 (1.06 to 1.79) |
Age, BMI, thiazide use, fluid intake, alcohol use, calcium supplementation and diet | |
| Taylor et al 27 2005: NHS II (older female) | 949 | 94 485 | 58 (6.1%) | 3093 (3.3%) | RR 1.84 (1.41 to 2.41) |
RR 1.67 (1.28 to 2.20) |
Age, BMI, thiazide use, fluid intake, alcohol use, calcium supplementation and diet | |
| Taylor et al 27 2005: HFPS (male) | 1568 | 47 737 | 177 (11.3%) | 4002 (8.4%) | RR 1.21 (1.03 to 1.42) |
RR 1.31 (1.11 to 1.54) |
Age, BMI, thiazide use, fluid intake, alcohol use, calcium supplementation and diet | |
| Akoudad et al 29 | 1812 | 10 349 | 183 (18.8%) | 1629 (14.6%) | N/A | PR 1.27 (1.08 to 1.49) |
Age, sex, race, region, waist circumference, triglycerides, hypertension, uric acid, gallstones | |
| Weinberg et al 33 | 1045 (estimated) | 11 065 (estimated) | 182 (17.1%) (estimated) | 884 (8.0%) (estimated) | OR 2.44 (1.84 to 3.25) |
OR 1.76 (1.33 to 2.32) |
Age, sex, race, smoking history, BMI | |
| Sub Total | 10 752 | 241 755 | 1097 (10.2%) | 11 985 (5.0%) | ||||
| IGT in context of MetS | Impaired glucose tolerance (IGT) only n (% of total) | IGT with KSD, n (% of IGT) | ||||||
| XS | Rendina et al 34 | 317 (14.9%) | 1815 (calculated estimate) | 43 (13.6%) | 177 (8.7%) (calculated estimate) | N/A | Male: OR 1.1 (0.5 to 2.4) Female: OR 1.1 (0.3 to 1.8) |
Age, waist circumference, high serum triglycerides, low serum HDL, hypertension |
| West et al 35 | 1260 (8.5%) | 7268 (calculated estimate) | 17 (1.3%) | 71 (1.0%) |
OR 1.39
(0.81 to 2.36) (calculated) |
OR 1.27 (0.77 to 2.10) (One metabolic syndrome component) | Sex, race, socioeconomic status, gout, thiazide use, allopurinol use | |
| Jeong et al 37 | 6929 (19.9%) (Quintile 5 -≥104 mg/dL) | 13 700 (Quintile 1 -≤85 mg/dL) | 211 (3.0%) | 240 (1.8%) | OR 1.57 (1.26 to 1.95) |
OR 1.09 (0.87 to 1.37) |
Age, sex, metabolic syndrome components, MetS status | |
| Jung et al 36 | 4192 (10.3%) | 28 692 (calculated estimate) | 102 (2.4%) | 450 (1.6%) (calculated estimate) | 1.26 (1.12 to 1.42) |
OR 1.30 (1.03 to 1.64) |
Age, GFR, serum urate, phosphorous and calcium | |
| Kim et al 38 | N/A | N/A | N/A | N/A | Male: OR 1.18 (1.10 to 1.26) Female: OR 1.26 (1.12 to 1.42) |
Male: OR 1.03 (0.97 to 1.11) Female: OR 1.02 (0.90 to 1.16) |
Age, serum creatinine, serum urate, past medical history of KSD | |
| Lee et al 39 | 72 (11.3%) (DM) | 622 | 14 (19.4%) | 71 (11.7%) |
OR 1.87
(0.99 to 3.53) (calculated) |
N/A | N/A | |
| Sub Total | 12 770 (6.1%) | 52 097 | 387 (3.2%) | 1009 (1.9%) | ||||
| Total | 23 522 | 293 852 | 1484 (6.3%) | 12 994 (4.4%) |
BMI, body mass index; DM, diabetes mellitus; GFR, glomerular filtration rate; HDL, high-density lipoprotein; KSD, kidney stone disease; MetS, metabolic syndrome; N/A, not available; NHS, National Health Service; PR, prevalence ratio; RR, risk ratio.
Metabolic syndrome
There were six studies34–39 examining metabolic syndrome, of which five provided data on chronic hyperglycaemia (IGT/DM).34–37 39 All of these studies were cross-sectional. These took place in Italy, South Korea, Taiwan and USA. The samples ranged from hospital inpatients to representative population-based studies, which were representative of target populations (see table 1).
The male to female ratio and mean age for each study is detailed in table 1. MetS and KSD ascertainment ranged from the patient-reported diagnosis to ICD codes in medical records.
Overall there were 209 817 patients, of whom 31 767 (17.8%) had MetS, 12 770 (6.1%) had IGT only (see table 4); 2258 (7.1%) of those with MetS had KSD, compared with 7593 (4.3%) of controls and 387 (3.2%) of those with IGT had KSD, compared with 1009 (1.9%) of controls (see table 3). Unfortunately control population had to be calculated from the OR for some of the studies,34–36 therefore the figures for IGT are estimates. Study reported risk is detailed in tables 3 and 4.
Table 4.
MetS cross-sectional studies
| MetS | Study | Total participants, n | Metabolic syndrome, n (% of total) | Controls, n | Metabolic syndrome with KSD, n (% of MetS) | Control with KSD, n (%) | Study reported unadjusted risk (95% CI) | Study reported adjusted risk (95% CI) | Adjusted for |
| XS | Rendina et al 34 | 2132 | 725 (34.0%) | 1407 | 112 (15.4%) | 108 (7.7%) | OR 2.2 (1.7 to 2.9) |
OR 2.0 (1.3 to 3.0) |
Age, sex, history of KSD |
| West et al 35 | 14 870 | 4952 (33.3%) | 9921 | 628 (12.7%) | 363 (3.7%) | OR 2.13 (1.74 to 2.62) |
OR 1.52 (1.22 to 1.89) |
Sex, race, socioeconomic status, gout, thiazide use, allopurinol use | |
| Jeong et al 37 | 34 895 | 4602* (13.2%) | 30 293 | 177 (3.8%) | 662 (2.2%) | OR 1.71 (1.45 to 2.03) |
1.25 (1.03 to 1.50) |
Sex, race, socioeconomic status, gout, thiazide use, allopurinol use | |
| Jung et al 36 | 40 687 | 7803 (19.2%) | 32 884 | 166 (2.1%) | 443 (1.3%) | N/A | OR 1.36 (1.13 to 1.64) |
Age, GFR, serum urate, phosphorous and calcium | |
| Kim et al 38 | 116 536 | 13 416 (11.5%) | 103 120 | 1129 (8.4%) | 5978 (5.8%) | OR 1.33 (1.24 to 1.44) |
OR 1.11 (1.03 to 1.20) |
Age, serum creatinine, serum urate, past medical history of KSD | |
| Lee et al 39 | 694 | 269 (42.1%) | 425 | 46 (17.1%) | 39 (9.2%) | N/A | OR 1.83 (1.14 to 2.93) |
Age | |
| Total | 209 814 | 31 767 (15.1%) | 178 050 | 2258 (7.1%) | 7593 (4.3%) |
*Discrepancy between text and table.
DM, diabetes mellitus; GFR, glomerular filtration rate; KSD, kidney stone disease; MetS, metabolic syndrome; N/A, not available.
Meta-analysis
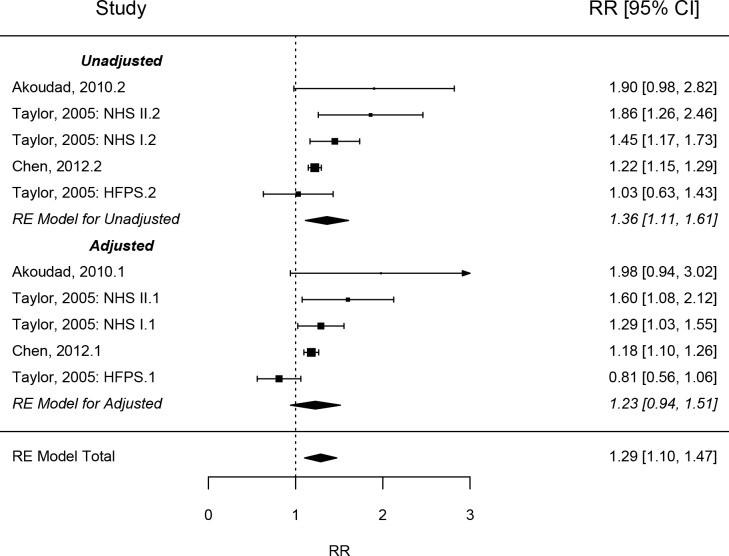
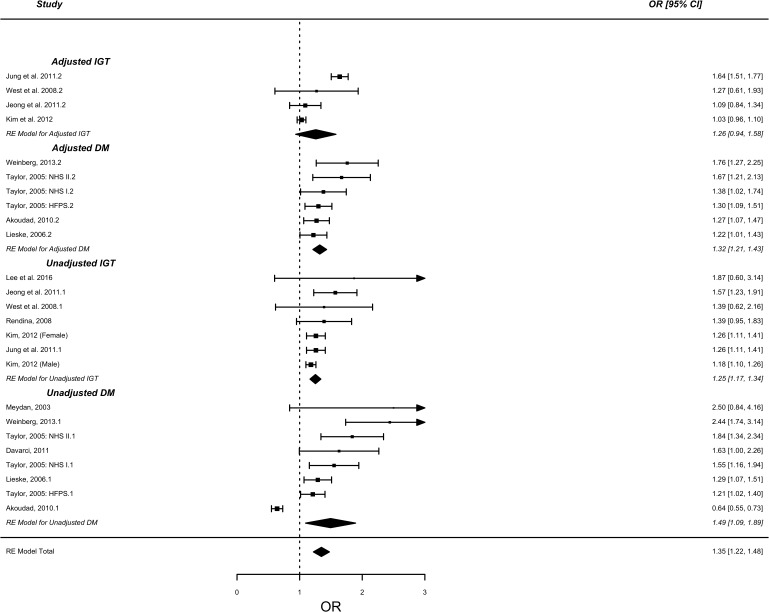
Tests for overall unadjusted effect in those with DM demonstrated significantly higher risk of KSD (RR=1.66 (95% CI: 1.27 to 2.18, p<0.001). Subgroup analyses by study type demonstrated significantly higher risk of KSD in patients with DM in cohort studies in both unadjusted (1.36, 95% CI: 1.11 to 1.60, p<0.001) (see figure 1) and adjusted risk (RR=1.23, 95% CI: 0.94 to 1.51, p<0.001) (see figure 2). Significantly increased risk was also demonstrated in cross-sectional/case-control studies in both unadjusted (OR=1.49, 95% CI: 1.09 to 1.89, p<0.0001) and adjusted risk (OR=1.32, 95% CI: 1.21 to 1.43, p<0.001) (see figure 3). IGT in the context of MetS demonstrated significantly increased risk in both unadjusted (OR=1.25, 95% CI: 1.16 to 1.54, p<0.0001) and adjusted risk (OR=1.26, 95% CI: 0.94 to 1.58) [see figure 3]. Combining DM case-control and cross-sectional studies with IGT demonstrated significantly increased risk in both unadjusted (OR=1.38, 95% CI: 1.18 to 1.59, p<0.0001) and adjusted risk (OR=1.32, 95% CI: 1.17 to 1.49, p<0.0001).
Figure 2.
Forest plot analysis – diabetes mellitus cohort. NHS, NationalHealth Service; RR, risk ratio.
Figure 3.
Forest plot analysis – diabetes mellitus + impaired glucose tolerance cross-sectional and case-control studies. NHS, National Health Service.
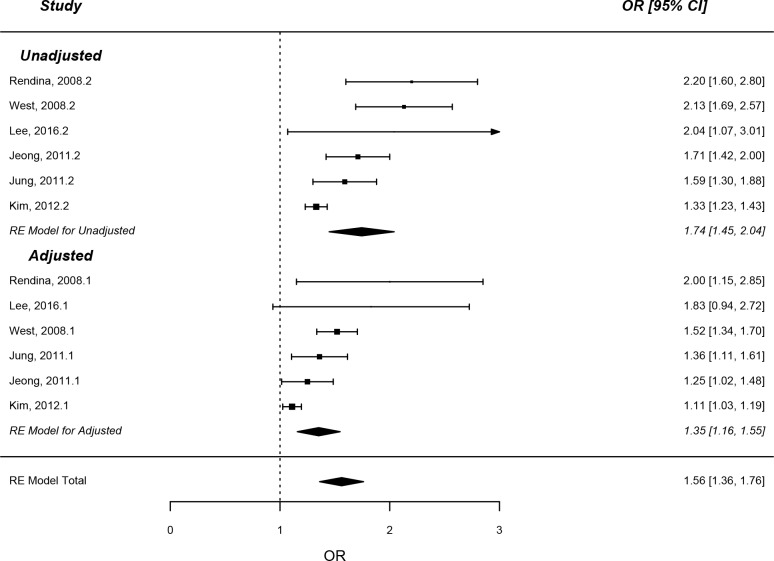
Cross-sectional studies examining MetS also demonstrated significantly increased risk of KSD in both unadjusted (OR=1.74, 95% CI: 1.45 to 2.04, p<0.0001) and adjusted (OR=1.35, 95% CI: 1.16 to 1.54, p<0.0001) (see figure 4) values.
Figure 4.
Forest plot analysis – metabolic syndrome (cross-sectional).
Heterogeneity and sensitivity analysis
There was borderline significant statistical heterogeneity between DM cohort studies in unadjusted risk (Tau2=0.042, Cochran’s Q=9.50, p=0.05, I2=62.3%), however there was significant heterogeneity when risk was adjusted (Tau2=0.070, Cochran’s Q=13.70, p=0.008, I2=80.2%).
There was significant statistical heterogeneity between DM case-control/cross-sectional studies in unadjusted risk (Tau2=0.258, Cochran’s Q=104.67, p<0.0001, I2=93.2%), however this was non-significant for adjusted risk (Tau2=0.00, Cochran’s Q=6.46, p=0.26, I2=0.0%).
There was non-significant statistical heterogeneity between IGT cross-sectional studies for unadjusted risk (Tau2=0.003, Cochran’s Q=7.18, p=0.30, I2=21.6%), however this was significant for adjusted risk (Tau2=0.086, Cochran’s Q=62.21, p<0.0001, I2=92.7%).
Combination of cross-sectional IGT studies with cross-sectional/case-control DM studies demonstrated significant heterogeneity for both unadjusted (Tau2=0.11, Cochran’s Q=160.10, p<0.0001, I2=91.2%) and adjusted risk (Tau2=0.044, Cochran’s Q=75.4, p<0.001, I2=81.2%). However, there was no statistical difference between subgroups for either unadjusted (I2=0%, p=0.54) or adjusted risk (I2=0%, p=0.60).
There was significant statistical heterogeneity between MetS cross-sectional studies for both unadjusted risk (Tau2=0.092, Cochran’s Q=26.08, p<0.0001, I2=79.5%), and adjusted risk (Tau2=0.034, Cochran’s Q=22.71, p<0.001, I2=72.7%).
Publication bias and quality of evidence
Leave-one-out analysis did not identify any studies that significantly changed the RR or OR for DM with and without IGT inclusion, nor for MetS.
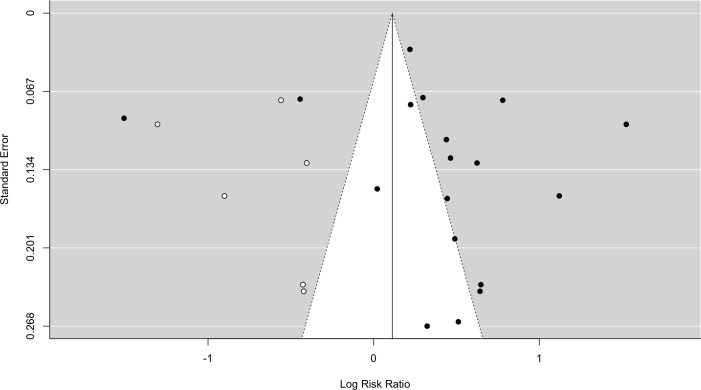
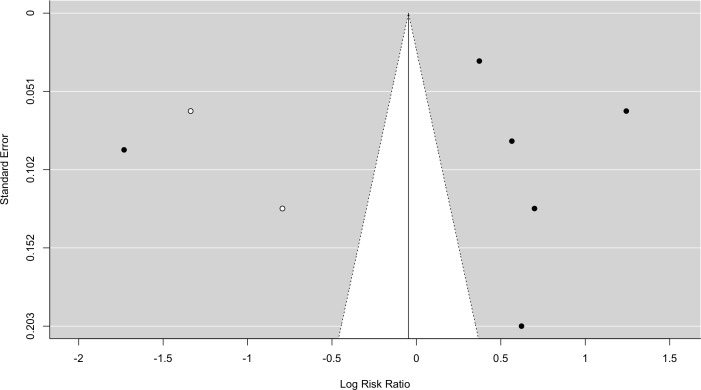
Trim and fill analysis did no demonstrate any missing studies for DM without IGT (SE=2.21). Inclusion of IGT with DM demonstrated six missing studies (SE=2.75) (see figure 5). The analysis demonstrated lack of negative studies. Trim and fill analysis of MetS demonstrated two missing studies (SE=1.78) (see figure 6), both negative.
Figure 5.
Funnel plot - diabetes mellitus with impairedglucose tolerance. Black dots=included studies, white dots=missing studies identified on ‘trim and fill analysis’.
Figure 6.
Funnel plot - metabolic syndrome. Black dots=included studies, white dots=missing studies identified on ‘trim and fill analysis’.
Egger’s regression demonstrated no significant results for: DM without IGT (z=0.81, p=0.42), DM with IGT (z=0.85, p=0.40) or MetS (z=0.15, p=0.88).
Overall there was a moderate risk of bias. All but two studies27 28 had scores greater than 7 on examination with the Newcastle-Ottawa quality assessment scale (see tables 5–7). Broadly taking in all studies there were no sample size calculations or demonstrable levels of response. None of the cohort studies provided Consolidated Standards of Reporting Trials diagrams nor did they provide loss to follow-up data in the text.
Table 5.
Bias analysis of cohort studies
| DM/MetS | Cohort | Newcastle-Ottawa quality assessment scale | |||
| Study | Selection (four stars total) |
Comparability (two stars total) |
Outcome (three stars total) |
Total (out of 9) |
|
| DM | Taylor et al 27 | *** | ** | ** | 7 |
| Akoudad et al 29 | **** | ** | *** | 9 | |
| Chen et al 28 | *** | ** | *** | 8 | |
DM, diabetes mellitus; MetS, metabolic syndrome.
Table 6.
Bias analysis of cross-sectional studies
| DM/MetS | Cross-sectional | Newcastle-Ottawa quality assessment scale | |||
| Study | Selection (five stars total) |
Comparability (two stars total) |
Outcome (three stars total) |
Total (out of 10) |
|
| DM | Meydan et al 30 | 0 | 0 | ** | 2 |
| Taylor et al 27 | ** | ** | ** | 6 | |
| Akoudad et al 29 | *** | ** | ** | 7 | |
| Weinberg et al 33 | *** | ** | ** | 7 | |
| MetS | Rendina et al 34 | *** | * | *** | 7 |
| West et al 35 | **** | ** | ** | 8 | |
| Jeong et al 37 | *** | ** | *** | 8 | |
| Kim et al 38 | *** | ** | *** | 8 | |
| Lee et al 39 | ** | * | *** | 6 | |
DM, diabetes mellitus; MetS, metabolic syndrome.
Table 7.
Bias analysis of case-control studies
| DM/MetS | Case-control | Newcastle-Ottawa quality assessment scale | |||
| Study | Selection (four stars total) |
Comparability (two stars total) |
Exposure (three stars total) |
Total (out of 9) |
|
| DM | Lieske et al 31 | **** | ** | ** | 8 |
| Davarci et al 32 | * | * | *** | 5 | |
DM, diabetes mellitus; MetS, metabolic syndrome.
Discussion
In this review and meta-analysis DM carried a significantly increased risk of developing KSD in cohort studies with a low risk of bias. Cross-sectional and case-control studies also demonstrate significantly increased likelihood of having KSD in those who have DM with a moderate risk of bias. IGT in the context of MetS carries a similar likelihood to DM in cross-sectional studies.
MetS carries a similar likelihood to DM and IGT in the context of MetS, with little difference between each in terms of adjusted ORs, again with a moderate risk of bias.
This is the first systematic review and meta-analysis to examine DM and MetS together. The results are highly significant although are limited by heterogeneity, despite meta-regression analysis. The results for DM are likely to be reflective of the true situation given that there were no missing studies identified on ‘trim and fill’ analysis. The situation for IGT and MetS may not be reflective given some negative studies were identified, and therefore there is a risk of publication bias.
The main strength in this study is the cohort studies examining DM, which have long follow-up periods and demonstrate highly significant results with a low risk of bias, despite suffering from significant statistical heterogeneity. This may be the result of differing adjustments between studies.
The case-control and cross-sectional studies examining DM were of variable quality but demonstrated highly significant results, similar to the cohort studies. Direct comparison between cohorts and these studies is difficult due to the differing outcome measure
There was no differentiation between type 1 and type 2 DM in most studies. It is unclear if type 1 confers the same risk as type 2.
It was unclear from the studies whether IGT was considered in isolation or in combination with other MetS components, nor was it clear whether the comparator groups contained those with MetS components, without reaching the required three components needed for diagnosis. This risks falsely lowering the risk associated with IGT due to the comparisons with other potential KSD risk factors.
Statistical heterogeneity demonstrated in most of the analyses may be due to ascertainment of KSD, variability in study populations and design and publication bias. There were significant variations in KSD ascertainment from patient-reported to medical notes to radiologically proven. Some studies may therefore under-report the true number of stones.
Variability in study populations and design (cohort, cross-sectional and case-control) ranged from hospital attendees in a single centre to large regional or national cohort studies. The effect of this variability is somewhat negated by dividing the studies by study design and analysing each separately.
DM cohort study adjusted values although the overall figure was significant the CI includes 1, therefore this could represent type 1 error.
Publication bias was low in this study with trim and fill analyses demonstrating few missing studies (mostly for MetS) and leave-one-out analysis not demonstrating any significantly heterogeneous studies.
The most common stone composition in all KSD formers is calcium oxalate, followed closely by calcium phosphate, together comprising around 85% of all stones. Uric acid stones are third, accounting for 12% in men, 7% in women, while the far rare cystine stones account for less than 1% in either gender.40 Both DM and MetS have been linked to increased uric acid stone formation, while calcium stone formation remains static, seemingly un-influenced by either DM or MetS.41
The increased risk of KSD in DM is thought to be secondary to two factors, glycaemic control (common to both types 1 and 2 and impaired glucose tolerance) and insulin resistance (as seen in type 2 DM and MetS). Hyperglycaemia has been demonstrated to increase urinary calcium,42 43 phosphorous,42 43 uric acid44 45 and oxalate46 secretion. Whereas increased insulin resistance increases renal ammonium secretion47 and decreased urinary pH,46 which in turn increases urinary calcium and uric acid secretion48 while decreases urinary citrate49 (an alkalizing agent), compounding urinary acidification. Together these mechanisms lead to increased risk of precipitation and subsequent formation of uric acid stones.
Notably, Chung et al 50 and Weikert51 in prospective cohort studies demonstrated patients who suffered from KSD were more likely to develop DM over a 5-year period than those who did not form stones. This muddies the water, giving a ‘chicken and egg’ scenario. It could be that KSD is a symptom of an underlying systemic metabolic disorder, or something intrinsic to KSD formers increases the risk of metabolic derangement. The former is more likely given the evidence for biochemical disruption in urinary excretions prior to stone formation.
Metabolic syndrome has been defined multiple times,52 however all definitions are in agreement that it comprises a combination of insulin resistance, hypertension and dyslipidaemia. Insulin resistance in metabolic syndrome is the same mechanism resulting in type 2 diabetes and thus the findings of urinary acidification,48 53 increased risk of uric acid secretion53 and uric acid stone formation48 via the pathophysiology described above are the same.
In this review a small, although non-significant increase in risk suffering from heterogeneity, was associated with MetS versus IGT/DM. This may be attributable to the other components of MetS.
There is conflicting evidence about hypertension and a possible link to increased risk of KSD35 and vice versa.54 A prospective cohort study by Cappuccio et al 55 demonstrated a significantly increased crude risk of hypertensives developing KSD than non-hypertensives. However, when observing the difference between stone formers and non-stone formers, the stone formers had no significant difference in blood pressure. It was noted that the hypertensives were significantly heavier, older and had higher BMI’s. Madore et al in consecutive studies on both genders,54 56 demonstrated there was no increased risk compared with non-hypertensive individuals when age, BMI and electrolyte intake were adjusted for. Akoudad et al 27 in their prospective cohort study demonstrated an increased risk of KSD with hypertension. However on multivariate analysis the effect was rendered non-significant. Perhaps the risk found by Cappuccio was confounded by the presence of metabolic syndrome, which at the time of publication was not defined.20 Hypertension is more likely indicative of underlying metabolic disturbance than having a truly lithogenic effect.
Dyslipidaemia, defined as hypercholesterolaemia, low serum high-density lipoprotein and high serum triglycerides20 has also been associated with increased risk of KSD.57 However, when adjusted in multivariate analysis the association is lost.57 Moreover, the only demonstrable biochemical abnormality after multivariate analysis is high urinary uric acid. Therefore the risk associated with dyslipidaemia is due to insulin resistance instead.
Renal lipotoxicity, defined as lipid accumulation in non-adipose tissues, has been linked to decreased ammonium secretion and therefore lower pH in rat models.58 However, this observation has yet to be reflected in humans. Renal lipotoxicity may represent the endpoint of chronic dyslipidaemia.
The addition of renal lipotoxicity to insulin resistance may explain the seemingly increased risk of KSD observed in patients with MetS versus IGT. Further studies are required to accurately demonstrate the underlying mechanism.
The rise in prevalence of DM and MetS is well documented and is now perceived as a global pandemic.9 18 KSD prevalence has risen in parallel.3 5 6 The Global Burden of Disease study9 10 demonstrated morbidity and absolute mortality associated with KSD has increased, perhaps due to the pandemic of DM/MetS,19 although age standardised mortality rates have decreased globally,. The effect is marked in higher income countries, but is attenuated in lower-middle income countries.8 10 This may be attributable to lack of availability of prompt intervention in developing countries, leading to later presentation and invasive treatments including nephrectomy.59–61 Following surgical treatment, management to prevent recurrence is recommended,13 again this may not be available in developing countries.
In this review, those with impaired glucose tolerance (pre-diabetes) had an increased likelihood of KSD, which was similar to those with DM in cross-sectional/case-control studies, although this may be suffering from publication bias and the real situation may be that the likelihood of KSD in IGT is lower than DM. Indeed, The NationalHealth and Nutrition Examination Survey III cross-sectional study33 demonstrated with increasingly poor glycaemic control led to increasing likelihood of KSD as determined by fasting plasma glucose and glycosylated haemoglobin. Given the evidence suggesting those with DM or MetS are at increased risk of developing KSD measures to improve glycaemic control should be examined for their efficacy in KSD prevention in this ‘at-risk’ population. It should be noted that the stone type in those with DM or MetS is most commonly calcium oxalate, however although still small, the proportion of urate stones increases in these related populations.22 62
Clarity is required on the risk in type 1 diabetics and future studies should differentiate these patients from type 2. Further prospective examination of DM and MetS should be undertaken to accurately portray whether additional risk is posed by MetS over DM and quantify this. Tight glycaemic control and weight loss should be explored in primary prevention studies for both MetS and DM, given the common pathophysiological mechanism. Further investigation is required to demonstrate if these patient are at increased risk of recurrence.
The risk of developing kidney stones is significantly increased in populations with chronic hyperglycaemia. This has global implications with rising morbidity and absolute mortality attributable to stones and is likely to increase the health and economic burden on patients and healthcare providers. Tight glycaemic control and weight loss are low-cost and non-invasive measures, which should be investigated for their primary preventative effect on KSD in these populations and included as part of the long-term management of kidney stone disease.
Supplementary Material
Footnotes
Twitter: @RobertGeraght16, @endouro
Contributors: RG performed the search, statistical analysis and wrote the manuscript. AA performed the search and reviewed the manuscript. PC, BS and PR edited the manuscript and critiqued the statistical analysis. BS and PR decided whether or not to include studies as the senior authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Data are available upon reasonable request.
References
- 1. Lotan Y. Economics and cost of care of stone disease. Adv Chronic Kidney Dis 2009;16:5–10. 10.1053/j.ackd.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 2. Sakhaee K. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int 2009;75:585–95. 10.1038/ki.2008.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pearle MS, Calhoun EA, Curhan GC, et al. Urologic diseases in America project: urolithiasis. J Urol 2005;173:848–57. 10.1097/01.ju.0000152082.14384.d7 [DOI] [PubMed] [Google Scholar]
- 4. Yasui T, Okada A, Hamamoto S, et al. Pathophysiology-based treatment of urolithiasis. Int J Urol 2017;24:32–8. 10.1111/iju.13187 [DOI] [PubMed] [Google Scholar]
- 5. Edvardsson VO, Indridason OS, Haraldsson G, et al. Temporal trends in the incidence of kidney stone disease. Kidney Int 2013;83:146–52. 10.1038/ki.2012.320 [DOI] [PubMed] [Google Scholar]
- 6. Yasui T, Iguchi M, Suzuki S, et al. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology 2008;71:209–13. 10.1016/j.urology.2007.09.034 [DOI] [PubMed] [Google Scholar]
- 7. Geraghty RM, Jones P, Somani BK. Worldwide trends of urinary stone disease treatment over the last two decades: a systematic review. J Endourol 2017;31:547–56. 10.1089/end.2016.0895 [DOI] [PubMed] [Google Scholar]
- 8. Bayne D, Chi T, Harris C, et al. PD47-02 global trends in urolithiasis morbidity and mortality from 1990-2010. Journal of Urology 2016;195:e1170–1. 10.1016/j.juro.2016.02.2690 [DOI] [Google Scholar]
- 9. Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 10. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med 1989;111:1006–9. 10.7326/0003-4819-111-12-1006 [DOI] [PubMed] [Google Scholar]
- 12. Bensalah K, Tuncel A, Gupta A, et al. Determinants of quality of life for patients with kidney stones. J Urol 2008;179:2238–43. discussion 2243 10.1016/j.juro.2008.01.116 [DOI] [PubMed] [Google Scholar]
- 13. Pearle MS, Goldfarb DS, Assimos DG, et al. Medical management of kidney stones: AUA guideline. J Urol 2014;192:316–24. 10.1016/j.juro.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 14. Türk C, Petřík A, Sarica K, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol 2016;69:475–82. 10.1016/j.eururo.2015.07.041 [DOI] [PubMed] [Google Scholar]
- 15. Saucier NA, Sinha MK, Liang KV, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis 2010;55:61–8. 10.1053/j.ajkd.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu LH, Kang R, He J, et al. Diabetes mellitus and the risk of urolithiasis: a meta-analysis of observational studies. Urolithiasis 2015;43:293–301. 10.1007/s00240-015-0773-5 [DOI] [PubMed] [Google Scholar]
- 17. Wong Y, Cook P, Roderick P, et al. Metabolic syndrome and kidney stone disease: a systematic review of literature. J Endourol 2016;30:246–53. 10.1089/end.2015.0567 [DOI] [PubMed] [Google Scholar]
- 18. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008;28:629–36. 10.1161/ATVBAHA.107.151092 [DOI] [PubMed] [Google Scholar]
- 19. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National heart, lung, and blood Institute/American heart association conference on scientific issues related to definition. Circulation 2004;109:433–8. 10.1161/01.CIR.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 21. Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol 2006;17:2026–33. 10.1681/ASN.2006030262 [DOI] [PubMed] [Google Scholar]
- 22. Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res 2006;34:193–9. 10.1007/s00240-006-0042-8 [DOI] [PubMed] [Google Scholar]
- 23. Besiroglu H, Otunctemur A, Ozbek E. The metabolic syndrome and urolithiasis: a systematic review and meta-analysis. Ren Fail 2015;37:1–6. 10.3109/0886022X.2014.976133 [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 25. Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa scale. World J Metaanal 2017;5:80 10.13105/wjma.v5.i4.80 [DOI] [Google Scholar]
- 26. Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 27. Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 2005;68:1230–5. 10.1111/j.1523-1755.2005.00516.x [DOI] [PubMed] [Google Scholar]
- 28. Chen H-S, Su L-T, Lin S-Z, et al. Increased risk of urinary tract calculi among patients with diabetes mellitus--a population-based cohort study. Urology 2012;79:86–92. 10.1016/j.urology.2011.07.1431 [DOI] [PubMed] [Google Scholar]
- 29. Akoudad S, Szklo M, McAdams MA, et al. Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: the ARIC study. Prev Med 2010;51:416–20. 10.1016/j.ypmed.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meydan N, Barutca S, Caliskan S, et al. Urinary stone disease in diabetes mellitus. Scand J Urol Nephrol 2003;37:64–70. 10.1080/00365590310008730 [DOI] [PubMed] [Google Scholar]
- 31. Lieske JC, de la Vega LSP, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis 2006;48:897–904. 10.1053/j.ajkd.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 32. Davarci M, Helvaci MR, Aydin M. What is the relationship between type 2 diabetes mellitus and urolithiasis? Bratisl Lek Listy 2011;112:711–4. [PubMed] [Google Scholar]
- 33. Weinberg AE, Patel CJ, Chertow GM, et al. Diabetic severity and risk of kidney stone disease. Eur Urol 2014;65:242–7. 10.1016/j.eururo.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rendina D, Mossetti G, De Filippo G, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant 2009;24:900–6. 10.1093/ndt/gfn548 [DOI] [PubMed] [Google Scholar]
- 35. West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National health and nutrition examination survey (NHANES III) 1988-1994. Am J Kidney Dis 2008;51:741–7. 10.1053/j.ajkd.2007.12.030 [DOI] [PubMed] [Google Scholar]
- 36. Jung HS, Chang IH, Kim KD, et al. Possible relationship between metabolic syndrome traits and nephrolithiasis: incidence for 15 years according to gender. Korean J Urol 2011;52:548–6. 10.4111/kju.2011.52.8.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis 2011;58:383–8. 10.1053/j.ajkd.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 38. Kim Y-J, Kim C-H, Sung E-J, et al. Association of nephrolithiasis with metabolic syndrome and its components. Metabolism 2013;62:808–13. 10.1016/j.metabol.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 39. Lee Y-C, Huang S-P, Juan Y-S, et al. Impact of metabolic syndrome and its components on kidney stone in aging Taiwanese males. Aging Male 2016;19:197–201. 10.1080/13685538.2016.1174987 [DOI] [PubMed] [Google Scholar]
- 40. Knoll T. Epidemiology, pathogenesis, and pathophysiology of urolithiasis. Eur Urol Suppl 2010;9:802–6. 10.1016/j.eursup.2010.11.006 [DOI] [Google Scholar]
- 41. Kadlec AO, Greco K, Fridirici ZC, et al. Metabolic syndrome and urinary stone composition: what factors matter most? Urology 2012;80:805–10. 10.1016/j.urology.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 42. Nagasaka S, Murakami T, Uchikawa T, et al. Effect of glycemic control on calcium and phosphorus handling and parathyroid hormone level in patients with non-insulin-dependent diabetes mellitus. Endocr J 1995;42:377–83. 10.1507/endocrj.42.377 [DOI] [PubMed] [Google Scholar]
- 43. Thalassinos NC, Hadjiyanni P, Tzanela M, et al. Calcium metabolism in diabetes mellitus: effect of improved blood glucose control. Diabet Med 1993;10:341–4. 10.1111/j.1464-5491.1993.tb00076.x [DOI] [PubMed] [Google Scholar]
- 44. Cook DG, Shaper AG, Thelle DS, et al. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J 1986;62:1001–6. 10.1136/pgmj.62.733.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gotfredsen A, McNair P, Christiansen C, et al. Renal hypouricaemia in insulin treated diabetes mellitus. Clinica Chimica Acta 1982;120:355–61. 10.1016/0009-8981(82)90376-X [DOI] [PubMed] [Google Scholar]
- 46. Eisner BH, Porten SP, Bechis SK, et al. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol 2010;183:2244–8. 10.1016/j.juro.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 47. Maalouf NM, Cameron MA, Moe OW, et al. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 2010;5:1277–81. 10.2215/CJN.08331109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abate N, Chandalia M, Cabo-Chan AV, et al. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int 2004;65:386–92. 10.1111/j.1523-1755.2004.00386.x [DOI] [PubMed] [Google Scholar]
- 49. Cupisti A, Meola M, D'Alessandro C, et al. Insulin resistance and low urinary citrate excretion in calcium stone formers. Biomed Pharmacother 2007;61:86–90. 10.1016/j.biopha.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 50. Chung S-D, Chen Y-K, Lin H-C. Increased risk of diabetes in patients with urinary calculi: a 5-year followup study. J Urol 2011;186:1888–93. 10.1016/j.juro.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 51. Weikert C, Weikert S, Schulze MB, et al. Presence of gallstones or kidney stones and risk of type 2 diabetes. Am J Epidemiol 2010;171:447–54. 10.1093/aje/kwp411 [DOI] [PubMed] [Google Scholar]
- 52. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–28. 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 53. Okamoto M, Kohjimoto Y, Iba A, et al. Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol 2010;17:996–1003. 10.1111/j.1442-2042.2010.02661.x [DOI] [PubMed] [Google Scholar]
- 54. Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis 1998;32:802–7. 10.1016/S0272-6386(98)70136-2 [DOI] [PubMed] [Google Scholar]
- 55. Cappuccio FP, Siani A, Barba G, et al. A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens 1999;17:1017–22. 10.1097/00004872-199917070-00019 [DOI] [PubMed] [Google Scholar]
- 56. Madore F, Stampfer MJ, Rimm EB, et al. Nephrolithiasis and risk of hypertension. Am J Hypertens 1998;11:46–53. 10.1016/S0895-7061(97)00371-3 [DOI] [PubMed] [Google Scholar]
- 57. Torricelli FCM, SK D, Gebreselassie S, et al. Urolithiasis/Endourology dyslipidemia and kidney stone risk. J Urol 2014;191:667–72. [DOI] [PubMed] [Google Scholar]
- 58. Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol 2008;294:F1315–22. 10.1152/ajprenal.00550.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rimtebaye K, Sillong FD, Tashkand AZA, et al. Urolithiasis: Diagnostic and Therapeutic Aspects in Urology Department of N’Djamena in Chad. OJU 2015;05:199–206. 10.4236/oju.2015.511032 [DOI] [Google Scholar]
- 60. Hounnasso PP, Avakoudjo JDG, Paré AK, et al. Symptomatic urinary lithiasis: epidemiology and management at urology department of university hospital of Cotonou. OJU 2015;05:7–12. 10.4236/oju.2015.52002 [DOI] [Google Scholar]
- 61. Marchini GS, Mello MF, Levy R, et al. Contemporary trends of inpatient surgical management of stone disease: national analysis in an economic growth scenario. J Endourol 2015;29:956–62. 10.1089/end.2015.0021 [DOI] [PubMed] [Google Scholar]
- 62. Cho ST, Jung SI, Myung SC, et al. Correlation of metabolic syndrome with urinary stone composition. Int J Urol 2013;20:208–13. 10.1111/j.1442-2042.2012.03131.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.