Abstract
Beckwith-Wiedemann syndrome (BWS) and Silver-Russell syndrome (SRS) are two imprinting disorders associated with opposite molecular alterations in the 11p15.5 imprinting centres. Their clinical diagnosis is confirmed by molecular testing in 50–70% of patients. The authors from different reference centres for BWS and SRS have identified single patients with unexpected and even contradictory molecular findings in respect to the clinical diagnosis. These patients clinically do not fit the characteristic phenotypes of SRS or BWS, but illustrate their clinical heterogeneity. Thus, comprehensive molecular testing is essential for accurate diagnosis and appropriate management, to avoid premature clinical diagnosis and anxiety for the families.
Keywords: Beckwith-Wiedemann syndrome, molecular testing, Silver-Russell syndrome, unexpected results
1. Introduction
Beckwith-Wiedemann syndrome (BWS) and Silver-Russell syndrome (SRS) are congenital imprinting disorders, associated with oppositely altered parent of origin-specific expression of two neighbouring clusters of imprinted genes on Chr11p15.5 (Soellner et al., 2017 b) (Figure 1). SRS affects approximately 1:50,000 individuals, with characteristic features including pre- and post-natal growth restriction, relative macrocephaly and prominent forehead, early feeding difficulties, and body asymmetry (Wakeling et al., 2016). BWS, or the recently-described Beckwith-Wiedemann Spectrum (BWSp) affects approximately 1:10,500 individuals, and its clinical features include macroglossia, anterior abdominal wall defects, prenatal and/or postnatal overgrowth, tumour predisposition and lateralized overgrowth (Brioude et al., 2018). Due to their clinical heterogeneity, for both syndromes clinical scoring systems are a prerequisite for a more directed diagnostic protocol and clinical management (Wakeling et al., 2016; Brioude et al., 2018).
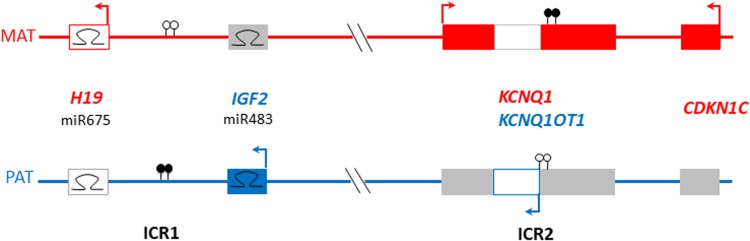
Fig. 1.
Schematic of 11p15 region indicating common imprinting disturbances (DNA methylation imbalances) associated with Beckwith-Wiedemann syndrome (BWS) and Silver-Russell syndrome (SRS).
Filled lollipops: methylated imprinting control region (IC); empty lollipops: unmethylated IC; hairpins: microRNA; filled oblongs: coding genes; outline oblongs: noncoding RNA; red denotes genes expressed from maternal allele; blue denotes genes expressed from paternal allele; grey denotes genes not expressed from the allele shown.
Over 50% of SRS cases are caused by loss of paternal allele methylation (LOM) of imprinting centre 1 (IC1 or H19/IGF2:IG-DMR), whereas gain of maternal allelic methylation at IC1 (GOM) can be identified in 5–10% of BWS cases. However, in BWS 50% of cases show loss of maternal allele methylation of imprinting centre 2 (IC2 or KCNQ1OT1:TSS-DMR). Sequence variants in CDKN1C and IGF2, as well as copy number variants or mosaic segmental uniparental disomy affecting chromosome 11p15.5, are also associated with BWS and SRS. In 10% of SRS patients, maternal uniparental disomy of chromosome 7 can be detected. Mosaic methylation disturbances of IC1 and IC2 are frequent (Wakeling et al., 2016; Brioude et al., 2018) with strong differences in distribution between different tissues (Azzi et al., 2015), thereby challenging genetic testing and probably leaving several patients without molecular diagnosis. A significant fraction of children with IC1 and/or IC2 LOM have multi-locus imprinting disturbances (MLID), that is, aberrant imprinting marks at additional imprinted loci (for review see Sanchez-Delgado et al., 2016).
To identify the major molecular changes, first-line testing for BWS and SRS is recommended to include DNA methylation analysis of IC1 and IC2 (Eggermann et al., 2016). In fact, the majority of patients exhibit the disease-specific (epi)mutations in 11p15, but single individuals referred with symptoms consistent with BWS or SRS show molecular changes inconsistent with the clinical diagnosis, or even consistent with its molecular ‘mirror’, thus posing challenges for interpretation, diagnostic reporting and genetic counselling.
Here we describe selected cases from different European laboratories where apparent ambiguities have arisen in BWS/SRS diagnosis, to offer a precedent for interpretation and reporting. By considering the clinical data and the reason for referral and the molecular findings, we suggest to categorize these cases into three groups. Examples for each category are presented in Table 1.
Table 1.
Cases with reported discrepancy between clinical referral and molecular diagnosis of Beckwith-Wiedemann syndrome (BWS) and Silver-Russell syndrome (SRS).
| Case | Clinical referral | Asymmetry | First molecular result1 | MLID1 | Clinical features2 | Reference |
|---|---|---|---|---|---|---|
| A: Isolated asymmetry | ||||||
| 1 | BWS | Yes | IC1 LOM | No | Hemihypertrophy and facial asymmetry. Tongue left side larger than right. At WG 41: birth length 52 cm (P46), weight 3000 g (P0). | |
| 2 | BWS | Yes | IC1 LOM | No | Asymmetry of limbs. Slightly elevated AFP. At WG 40: birth weight 2570 g (P0), 47 cm (P1), OFC 33.5 cm (P5). At 1 year: height 75 cm (P15), weight 8.8 kg (P28) | |
| 3 | BWS | Yes | IC1 LOM | No | Isolated hemihypertrophy, normal growth (but shorter than her monozygous twin), methylation indices near normal in blood but lower in fibroblasts | |
| 4 | BWS | Yes | IC1 LOM | No | IUGR, relative macrocephaly, hemihyperplasia (legs, arms, kidneys) elevated AFP initially | |
| 5 | BWS | Yes | IC1 LOM | NK | Isolated hemihypertrophy | |
| 6 | BWS | Yes | IC1 LOM | NK | Features of BWS (unspecified) | |
| 7 | BWS | Yes | IC1 LOM | NK | Hemihypertrophy (clinical diagnosis reassessed as hemiatrophy after molecular diagnosis) | |
| 8 | BWS | Yes | IC1 LOM | NK | Asymmetry | |
| 9 | Asymmetry | Yes | IC1 LOM | NK | Hemihypertrophy of limbs (left leg), optic hypoplasia left eye | |
| 10 | Asymmetry | Yes | IC1 LOM | NK | Hemihypertrophy of right side | |
| 11 | Asymmetry | Yes | IC1 LOM | NK | Hemihypertrophy of limbs | |
| 12 | Asymmetry | Yes | IC1 LOM | NK | Isolated asymmetry | Russo et al. (2016) |
| 13 | Asymmetry | Yes | IC1 LOM | NK | Not given | |
| 14 | Asymmetry | Yes | IC1 LOM | NK | Not given | |
| 15 | Asymmetry | Yes | IC1 LOM | NK | Hemihypotrophy left arm | |
| 16 | Asymmetry | Yes | IC1 LOM | NK | Hemihypertrophy of limbs; at WG 39 birth length 48 cm, birth weight 2724 g, OFC 33 cm, ear anomalies NH-CSS: 1/6 | |
| B: features of SRS with or without asymmetry | ||||||
| 17 | SRS | Yes | IC2 LOM | NK | IUGR, micrognathia, psychomotor delay, blue sclerae | |
| 18 | SRS | Yes | IC2 LOM | No | IUGR, PNGR, relative macrocephaly, prominent forehead, triangular facies, thyroid carcinoma | |
| 19 | SRS | NK | IC2 LOM | No | Features of SRS (unspecified) | |
| 20 | SRS | NK | IC2 LOM | No | IUGR, PNGR, no relative macrocephaly, anterior midline defect, transient hypoglycaemia | Murphy et al. (2012) |
| 21 | SRS | No | IC2 LOM | No | IUGR, PNGR, no relative macrocephaly, developmental delay, radioulnar synostosis | Turner et al. (2010) |
| 22 | SRS | Yes | IC2 LOM | No | IUGR, asymmetry, feeding difficulties, excessive sweating | |
| 23 | SRS | Yes | Upd(11)pat | No | Features of SRS (unspecified) | |
| 24 | SRS | No | IC2 LOM | No | IUGR, short stature, 5th finger clinodactyly | |
| C: Multi-locus imprinting disorder | ||||||
| 25 | BWS | No | IC1 LOM | Yes | ICSI, postnatal macrosomia, macroglossia, umbilical hernia, leg length discrepancy | Tee et al. (2013) |
| 26 | SRS | Yes | IC1 + IC2 LOM | Yes | IUGR (birth weight <4th centile), cleft lip and palate, feeding difficulties, mild developmental delay, behavioural difficulty | Docherty et al. (2015) |
| 27 | SRS | Yes | IC1 + IC2 LOM | Yes | Discordant monozygous twin. Mild IUGR and PNGR, mild motor delay, renal dysplasia | Begemann et al. (2011) |
| 28 | SRS | Yes | IC1 + IC2 LOM | Yes | Birth weight 2.3 kg at 40 WG: short stature, asymmetry, normal development | |
| 29 | SRS | NK | IC1 + IC2 LOM | Yes | IUGR, PNGR | |
| 30 | BWS | Yes | IC1 + IC2 LOM | Yes | Macrosomia, macroglossia, naevus flammeus, developmental delay | Begemann et al. (2018) |
| 31 | SRS | No | IC1 + IC2 LOM | Yes | In vitro fertilization, one of fraternal triplets. NH-CSS 6/6 | Begemann et al. (2018) |
| 32 | SRS | No | IC1 + IC2 LOM | Yes | BW at 27 WG 465 g, OFC 32 cm. PNGR, respiratory support for two months, gastric tube feeding for first year. Microcephaly, precocious puberty, dysmorphism. Developmental delay. 47,XXY | Begemann et al. (2018) |
| 33 | BWS | No | IC2 LOM | Yes | Fetus ascertained at 16 WG with 9 mm hernia, 22 × 14 mm omphalocoele containing intestine, vacuolated placenta | |
| 34 | BWS? | No | IC2 LOM | Yes | Fetal ascertainment: induced abortion at 19 WG. Omphalocele, shortened humeri, mesenchymal placenta | Soellner et al. (2017 a) |
In the majority of cases, methylation specific multiplex ligation-dependent probe amplification (MS-MLPA) based kits were used for diagnostic purposes.
Clinical details given at the referral of DNA samples for molecular testing. Not given: no additional clinical information provided at referral. AFP: alpha-foetoprotein; BW: birth weight; ICSI: intra-cytoplasmic sperm injection; IUGR: intrauterine growth restriction; LOM: loss of methylation; MLID: multi-locus imprinting disturbance; NH-CSS Netchine-Harbison clinical scoring system; NK: not known (clinical data not reported or molecular analysis not performed); OFC: occipitofrontal circumference; PNGR: postnatal growth restriction; upd(11)pat: paternal uniparental disomy of chromosome 11; WG: weeks of gestation.
2. Clinical referral of BWS or isolated asymmetry; molecular diagnosis of IC1 LOM
In three cases (patients 1, 2, 6), the initial clinical suspicion of BWS based on some key features according to the recent consensus guidelines (Brioude et al., 2018) had to be revised after molecular diagnosis of a IC1 LOM. As this finding is the characteristic epimutation for SRS, two of the patients (patients 1, 2) were clinically re-evaluated, but did not fulfil the clinical Netchine-Harbison Score (NHS) for SRS (one out of six items each; Wakeling et al., 2016). Interestingly, two of the patients showed more or less normal growth. In the majority of patients with IC1 LOM, asymmetry was the major symptom provoking molecular testing (e.g., cases 10–16).
Asymmetry is one of the key features of both BWS and SRS, but it can be difficult to clinically distinguish hemihypertrophy from hemihypotroply/hemiatrophy, particularly if other clinical features are lacking. Isolated lateralized overgrowth (ILO) in the presence of an 11p15 molecular anomaly is within the BWSp. ILO is sufficient to prompt BWS testing (Brioude et al., 2018), and some European diagnostic laboratories have historically logged all cases of ILO for BWS first-line testing by 11p15 DNA methylation analysis. According to current consensus guidelines, isolated asymmetry is insufficient to warrant SRS testing (Wakeling et al., 2016). Thus, in cases referred solely for asymmetry, identification of a molecular defect normally associated with a clinical diagnosis of SRS may be unexpected, but it is not discrepant.
3. Clinical features of SRS (with or without asymmetry); molecular diagnosis consistent with BWS
Some individuals with growth restriction, with or without additional SRS features, were referred for SRS diagnosis but received molecular diagnosis consistent with BWS – in the majority, IC2 LOM. Molecular SRS testing is commonly requested as an exclusion diagnosis for growth-restricted children, and in these cases, parallel testing of IC1 and IC2 occasionally diagnoses IC2 LOM. Our data confirm that IC2 LOM in BWS is not strongly associated with overgrowth (Brioude et al., 2018), but that in some cases it is associated with growth restriction (Unpublished data from authors IN, FB, DJM, IKT), which when associated with body asymmetry can prompt initial clinical diagnosis of SRS. Growth restriction associated with IC2 LOM may expand the clinical spectrum of BWSp.
4. Clinical referral for diagnosis of SRS or BWS; molecular diagnosis of MLID
Of eight postnatal referrals with MLID, six had clinical diagnoses of SRS and two of BWS, which may reflect: (a) ascertainment bias for referrals meeting specific clinical criteria; (b) the relative likelihood of imprinting disturbance restricting rather than enhancing growth; (c) mosaic LOM in different tissues, with the critical imprinting disturbance eluding detection in the tissue analysed (Azzi et al., 2015). Two cases were ascertained prenatally. One case (patient 33) was referred for 11p15.5 methylation testing after detection of omphalocele and vacuolated placenta, with normal growth parameters. Methylation specific multiplex ligation-dependent probe amplification (MS-MLPA) analysis revealed LOM of IC1, IC2 and the GNAS/GNAS-AS locus. Another case (patient 34) was ascertained with omphalocele, shortened humeri and mesenchymal placenta, and showed LOM of IC2, GRB10 and MEST loci (Soellner et al., 2017a). To our knowledge these are the first reported prenatal diagnoses of MLID.
MLID is detectable in approximately 25% of BWS and 10% of SRS cases with IC2 or IC1 LOM, respectively, and being mosaic by nature, may elude detection in diagnostic samples. Because MLID may result from underlying genetic changes, and may alter genetic counselling and perinatal as well as clinical management (Soellner et al., 2017 a, b), it should be considered in individuals with discrepant molecular and clinical diagnoses.
5. Conclusion
The compilation of data from patients with unexpected molecular findings confirms the urgent need to apply comprehensive molecular tests targeting different imprinted loci to identify unexpected and/or overlapping molecular changes, and thereby to contribute to the discovery of the causative (epi)mutations in patients with unspecific phenotypes. As these examples show, the application of clinical scoring systems and the clinical evaluation can be a major prerequisite for a more directed diagnostic testing strategy, but some patients might be missed if the decision about molecular testing is strictly based on fulfilment of clinical criteria. We want to emphasize that in patients with inconclusive clinical features the communication of a clinical diagnosis should be delayed until molecular confirmation is available because a premature diagnosis might cause anxiety to the families.
The discrepancy between clinical and molecular features of BWS and SRS is an infrequent occurrence. Though objective numbers are lacking, these cases probably represent ⩽1% of diagnostic referrals. Prompt, sensitive and comprehensive molecular testing is essential for accurate diagnosis, appropriate management and genetic counselling, for these as for all imprinting disorders.
Acknowledgements
The authors were members of the former COST Action BM1208.
Author ORCIDs
Thomas Eggermann, 0000-0002-8419-0264
Ethics and consent of participate
All participants gave a written informed consent to participate in research studies. The study has been approved by the ethical committee of the University Hospital Aachen, Germany (EK-302-16).
Declaration of interest
None.
References
- Azzi S, Steunou V, Tost J, Rossignol S, Thibaud N, Das Neves C, Le Jule M, Habib WA, Blaise A, Koudou Y, Busato F, Le Bouc Y and Netchine I. (2015). Exhaustive methylation analysis revealed uneven profiles of methylation at IGF2/IC1/H19 11p15 loci in Russell Silver syndrome. Journal of Medical Genetics 52, 53–60. [DOI] [PubMed] [Google Scholar]
- Begemann M, Rezwan FI, Beygo J, Docherty LE, Kolarova J, Schroeder C, Buiting K, Chokkalingam K, Degenhardt F, Wakeling EL, Kleinle S, González Fassrainer D, Oehl-Jaschkowitz B, Turner CLS, Patalan M, Gizewska M, Binder G, Bich Ngoc CT, Chi Dung V, Mehta SG, Baynam G, Hamilton-Shield JP, Aljareh S, Lokulo-Sodipe O, Horton R, Siebert R, Elbracht M, Temple IK, Eggermann T and Mackay DJG. (2018). Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. Journal of Medical Genetics 55, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M, Spengler S, Kanber D, Haake A, Baudis M, Leisten I, Binder G, Markus S, Rupprecht T, Segerer H, Fricke-Otto S, Mühlenberg R, Siebert R, Buiting K and Eggermann T. (2011). Silver-Russell patients showing a broad range of IC1 and IC2 hypomethylation in different tissues. Clinical Genetics 80, 83–88. [DOI] [PubMed] [Google Scholar]
- Brioude F, Kalish JM, Mussa A, Foster AC, Bliek J, Ferrero GB, Boonen SE, Cole T, Baker R, Bertoletti M, Cocchi G, Coze C, De Pellegrin M, Hussain K, Ibrahim A, Kilby MD, Krajewska-Walasek M, Kratz CP, Ladusans EJ, Lapunzina P, Le Bouc Y, Maas SM, Macdonald F, Õunap K, Peruzzi L, Rossignol S, Russo S, Shipster C, Skórka A, Tatton-Brown K, Tenorio J, Tortora C, Grønskov K, Netchine I, Hennekam RC, Prawitt D, Tümer Z, Eggermann T, Mackay DJG, Riccio A and Maher ER. (2018). Expert consensus document: clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nature Reviews. Endocrinology 14, 229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty LE, Rezwan FI, Poole RL, Turner CLS, Kivuva E, Maher ER, Smithson SF, Hamilton-Shield JP, Patalan M, Gizewska M, Peregud-Pogorzelski J, Beygo J, Buiting K, Horsthemke B, Soellner L, Begemann M, Eggermann T, Baple E, Mansour S, Temple IK and Mackay DJ. (2015). Mutations in NLRP5 are associated with reproductive wastage and multi-locus imprinting disorders in humans. Nature Communications 6, 8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann K, Bliek J, Brioude F, Algar E, Buiting K, Russo S, Tümer Z, Monk D, Moore G, Antoniadi T, Macdonald F, Netchine I, Lombardi P, Soellner L, Begemann M, Prawitt D, Maher ER, Mannens M, Riccio A, Weksberg R, Lapunzina P, Grønskov K, Mackay DJ and Eggermann T. (2016). EMQN best practice guidelines for the molecular genetic testing and reporting of chromosome 11p15 imprinting disorders: Silver-Russell and Beckwith-Wiedemann syndrome. European Journal of Human Genetics 24, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Mackay D and Mitchell EA. (2012). Beckwith Wiedemann imprinting defect found in leucocyte but not buccal DNA in a child born small for gestational age. BMC Medical Genetics 13, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S, Calzari L, Mussa A, Mainini E, Cassina M, Di Candia S, Clementi M, Guzzetti S, Tabano S, Miozzo M, Sirchia S, Finelli P, Prontera P, Maitz S, Sorge G, Calcagno A, Maghnie M, Divizia MT, Melis D, Manfredini E, Ferrero GB, Pecile V and Larizza L. (2016). A multi-method approach to the molecular diagnosis of overt and borderline 11p15.5 defects underlying Silver-Russell and Beckwith-Wiedemann syndromes. Clinical Epigenetics 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Delgado M, Riccio A, Eggermann T, Maher ER, Lapunzina P, Mackay DJG and Monk D. (2016). Causes and consequences of multi-locus imprinting disturbances in humans. Trends in Genetics 32, 444–455. [DOI] [PubMed] [Google Scholar]
- Soellner L, Begemann M, Degenhardt F, Geipel A, Eggermann T and Mangold E. (2017a). Maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring. European Journal of Human Genetics 25, 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soellner L, Begemann M, Mackay DJ, Grønskov K, Tümer Z, Maher ER, Temple IK, Monk D, Riccio A, Linglart A, Netchine I and Eggermann T. (2017 b). Recent advances in imprinting disorders. Clinical Genetics 91, 3–13. [DOI] [PubMed] [Google Scholar]
- Tee L, Lim DH, Dias RP, Baudement MO, Slater AA, Kirby G, Hancocks T, Stewart H, Hardy C, Macdonald F and Maher ER. (2013). Epimutation profiling in Beckwith-Wiedemann syndrome: relationship with assisted reproductive technology. Clinical Epigenetics 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CL, Mackay DJ, Callaway JL, Docherty LE, Poole RL, Bullman H, Lever M, Castle BM, Kivuva EC, Turnpenny PD, Mehta SG, Mansour S, Wakeling EL, Mathew V, Madden J, Davies JH and Temple IK. (2010). Methylation analysis of 79 patients with growth restriction reveals novel patterns of methylation change at imprinted loci. European Journal of Human Genetics 18, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling EL, Brioude F, Lokulo-Sodipe O, O'Connell SM, Salem J, Bliek J, Canton AP, Chrzanowska KH, Davies JH, Dias RP, Dubern B, Elbracht M, Giabicani E, Grimberg A, Grønskov K, Hokken-Koelega AC, Jorge AA, Kagami M, Linglart A, Maghnie M, Mohnike K, Monk D, Moore GE, Murray PG, Ogata T, Petit IO, Russo S, Said E, Toumba M, Tümer Z, Binder G, Eggermann T, Harbison MD, Temple IK, Mackay DJ and Netchine I. (2016). Diagnosis and management of Silver-Russell syndrome: first international consensus statement. Nature Reviews. Endocrinology 13, 105–124 [DOI] [PubMed] [Google Scholar]