Abstract
Introduction
Hepatitis E virus (HEV) is a leading cause of acute viral hepatitis in the developing world and is a public health problem, in particular among pregnant women, where it may lead to severe or fatal complications. A recombinant HEV vaccine, 239 (Hecolin; Xiamen Innovax Biotech, Xiamen, China), is licensed in China, but WHO calls for further studies to evaluate the safety and immunogenicity of this vaccine in vulnerable populations, and to evaluate protection in pregnancy. We are therefore conducting a phase IV trial to assess the effectiveness, safety and immunogenicity of the HEV 239 vaccine when given in women of childbearing age in rural Bangladesh, where HEV infection is endemic.
Methods and analysis
Enrolment of a target of approximately 20 000 non-pregnant women, aged 16–39 years, started on 2 October 2017 in Matlab, Bangladesh. Sixty-seven villages were randomised by village at a 1:1 ratio to receive either the HEV vaccine or the control vaccine (hepatitis B vaccine). A 3-dose vaccination series at 0, 1 and 6 months is ongoing, and women are followed up for 24 months. The primary outcome is confirmed HEV disease among pregnant women. After vaccination, participants are requested to report information about clinical hepatitis symptoms. Participants who become pregnant are visited at their homes every 2 weeks to collect information about pregnancy outcome and to screen for clinical hepatitis. All suspected hepatitis cases undergo laboratory testing for diagnostic evaluation. The incidence of confirmed HEV disease among pregnant and non-pregnant women will be compared between the HEV vaccinated and control groups, safety and immunogenicity of the vaccine will also be evaluated.
Ethics and dissemination
The protocol was reviewed and approved by the International Centre for Diarrhoeal Disease Research, Bangladesh Research Review Committee and Ethical Review Committee, and the Directorate General of Drug Administration in Bangladesh, and by the Regional Ethics Committee in Norway. This article is based on the protocol version 2.2 dated 29 June 2017. We will present the results through peer-reviewed publications and at international conferences.
Trial registration number
The trial is registered at clinicaltrials.gov with the registry name “Effectiveness Trial to Evaluate Protection of Pregnant Women by Hepatitis E Vaccine in Bangladesh” and the identifier NCT02759991.
Keywords: immunology, epidemiology, hepatobiliary disease
Strengths and limitations of the study.
This trial is conducted in a defined population covered by a long-term demographic surveillance registry, in a site that has a well-established clinical and laboratory infrastructure and effective referral systems.
Women are visited in their homes for 7 days following vaccination to follow-up and record details of any adverse events.
Active surveillance of acute hepatitis is conducted through home visits every 2 weeks to all the pregnant participants during their pregnancy.
Dried blood spots are used to assess immunogenicity of the vaccine, simplifying blood collection and making storage of 40 000 samples feasible in a community setting.
A limitation of this trial design is the lack of blood sampling at the end of follow-up; thus, we will not be able to explore the occurrence of asymptomatic hepatitis E.
Introduction
Background and rationale
Hepatitis E virus (HEV) is an enteric RNA virus causing outbreaks or endemics in developing countries with poor sanitation and is one of the leading causes of acute hepatitis worldwide.1 Pregnant women are particularly vulnerable to HEV infection, with a high rate of maternal mortality, miscarriage, premature delivery and stillbirth.2
An effective vaccine could prevent symptomatic HEV infection in vulnerable people. Only two HEV vaccines have been evaluated in human clinical trials, namely rHEV (GlaxoSmithKline), and HEV 239 (Hecolin, Xiamen Innovax Biotech, China), of which only the latter has undergone further commercial development.3 Zhu et al studied this vaccine in a large phase III clinical study in China, where 100 000 healthy men and women (aged 16–65 years) received either the HEV vaccine or a hepatitis B vaccine (HBV).4 They found more than 90% protection against symptomatic HEV infection. The adverse events related to the vaccine were few and mild, and there were no vaccine-related serious adverse events (SAEs). The vaccine is currently only licensed in China for people 16 years and older, and is recommended for individuals with a high risk of HEV infection.
WHO Strategic Advisory Group of Experts made a working group to review the evidence on the HEV 239 vaccine and make recommendations for its use. They concluded that knowledge gaps prevents recommendation of the vaccine in endemic countries, and that further studies should evaluate the safety and immunogenicity of this vaccine in children, the elderly, persons with underlying diseases or conditions such as immunosuppression or liver disease, and immunogenicity and protection in pregnant women.3 5
HEV comprises eight genotypes, of which mainly four infect humans.6 HEV genotypes 1 and 2 dominate human infections in developing countries. Genotypes 3 and 4 primarily infect animals that can further transmit the virus to humans, causing illness in both developed and developing countries. Genotype 4 predominates in mainland China, where the previous phase III vaccine trial was conducted, but there are limited data on protection of the vaccine against the other genotypes. A small study investigated immunogenicity against genotypes 1–4 after vaccination with p239 and found that in humans, IgG antibodies reacted slightly stronger against genotypes 1 and 2 versus 3 and 4, which could be due to the presence of genotype-specific neutralising antibodies.7 However, the vaccine still needs to be tested in other geographical areas to fully evaluate efficacy against all the genotypes that frequently cause illness in humans.3
We are currently conducting a phase IV cluster-randomised clinical trial (2017–ongoing) with the HEV 239 vaccine to provide more data on the effectiveness of the vaccine on genotype 1 and the outcome in pregnant women, and on safety of the vaccine. The trial is conducted in a rural area of Bangladesh where genotype 1 is predominant, and includes women aged 16–39 years, allowing us to evaluate effectiveness and immunogenicity of the vaccine among women who become pregnant following vaccination.
Objectives
Primary objective
To determine the effectiveness of the HEV 239 vaccine given to women of childbearing age in rural Bangladesh in preventing HEV disease during pregnancy.
Secondary objectives
To determine the safety of HEV vaccine in Bangladeshi women of childbearing age.
To determine the immunogenicity of HEV vaccine in Bangladeshi women of childbearing age.
To determine the effectiveness of HEV vaccine in preventing HEV disease in non-pregnant Bangladeshi women of childbearing age.
To assess the anti-HEV IgG levels before and 1 month after the last dose of vaccine for primary vaccine response and record if any HEV disease occurs.
To assess the feasibility, acceptability and cost effectiveness of HEV vaccination of women of childbearing age in rural Bangladesh.
To investigate acute HEV cases virologically, clinically and immunologically in relation to outcome.
Trial design
This study is a phase IV cluster-randomised, double-blinded trial on the safety and effectiveness of three doses of the HEV 239 vaccine in women of childbearing age (16-39 years) in Bangladesh. In this phase IV study, we will assess the safety, acceptability, immunogenicity and effectiveness of Hepatitis E vaccine. It is not required to conduct a phase III study in Bangladesh when it has already been conducted elsewhere and the results are published, which showed that the vaccine is safe and highly efficacious. In a cluster randomised trial, groups of people rather than individuals are randomly allocated to the interventions under investigations. The unit of allocation in this trail is a ‘Village’. Participants are sampled at day 0 and day 210 and followed up as described in table 1.
Table 1.
Participant timeline in the study
| Contact with participant | |||||||||
| Visit 1 | Visit 2 | Visit 3–9 | Visit 10 | Visit 11–17 | Visit 18 | Visit 19–25 | Visit 26 | Visit 27 |
|
| Days | −1 | 0 | 1–7 | 30* | 31–37* | 180* | 181–187* | 210* | 2½ years |
| Enrolment | |||||||||
| Invitation visit | X | ||||||||
| Eligibility screening | X | X | X | ||||||
| Informed consent | X | ||||||||
| Demographics (age, height, weight, BMI), medications/medical history |
X | X | X | X | X | ||||
| Interventions | |||||||||
| Vaccination | X | X | X | ||||||
| Blood sample† | X | X† | |||||||
| Surveillance | |||||||||
| Hepatitis surveillance (active and passive) | Throughout the study period | ||||||||
| Pregnancy surveillance | Throughout the study period | ||||||||
| Assessments | |||||||||
| Pregnancy home visit | Every 2 weeks | ||||||||
| Physical examination | X | ||||||||
| Harms/safety | |||||||||
| Immediate reactions | X | X | X | ||||||
| Home visit | X | X | X | X | |||||
| SAE | At any time following first vaccine dose | ||||||||
| Participant reporting of AEs | At any time following first vaccine dose | ||||||||
| Withdrawal | At any time following enrolment | ||||||||
*±2 days.
†Dried blood spots (DBSs) are collected before vaccination and 1 month after last vaccine dose, or earlier if off study.
AE, adverse event; BMI, body mass index; SAE, serious adverse events.
Methods and analysis
Study setting
The study field site for this clinical trial is located in Matlab in rural Bangladesh, where HEV is endemic.8 International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) has maintained a field research site in this area for more than 50 years with an ongoing health and demographic surveillance system covering the entire population. The icddr,b field site comprises 67 villages, with a total population of about 116 000 and a well-established infrastructure including healthcare services (hospital and local health clinics), laboratory facilities and effective referral systems.
Randomisation and blinding
All 67 villages were randomised by village at a 1:1 ratio to receive either HEV vaccine or HBV vaccine. All participants, investigators and field staff are blinded. The vaccine administrators, however, cannot be blinded since the dose of the licensed HBV vaccine in Bangladesh is different for women below and above 18 years, while the HEV vaccine dose is identical for all age groups. Each village is allocated to two vaccine codes (one for 16–18 years and another for 19–39 years) with eight codes in total; two codes for each vaccine in each age group. To achieve similar distributions of the codes for the two vaccines in different age groups, computerised allocation was employed taking the population size of the villages into consideration. The randomisation was rerun until the groups were balanced in size (<200 in difference). An independent statistician from Johns Hopkins University performed the randomisation. The vials were labelled with the eight respective codes by Incepta Pharmaceuticals, Bangladesh, who fill finished and bottled the vaccine shipped in bulk from China.
The treatment allocation code is being securely kept under lock and key and may be broken by the investigator only in case of SAEs for which knowledge of the participant’s treatment assignment may influence her or the clinical care or if the event is unexpected and suspected to be causally related to the investigational product. Code breaking will be reported to the clinical monitor and Data Safety and Monitoring Board (DSMB) within 24 hours. The participant may continue in the study with protocol-specified follow-up despite unblinding, unless she fulfils any protocol-defined exclusion criteria.
Recruitment and enrolment
All eligible women in the study area, identified through existing Matlab surveillance system, are being approached at their homes by designated study staff who inform about the nature of the study according to the International Conference on Harmonization (ICH) of Good Clinical Practice in their local language, and the participants may inquire about the details of the study. Eligibility is further assessed according to the inclusion and exclusion criteria in box 1.
Box 1. Hepatitis E virus Bangladesh study inclusion and exclusion criteria.
Inclusion criteria
Healthy non-pregnant female, aged 16–39 years at time of first vaccination
Living in the International Centre for Diarrhoeal Disease Research, Bangladesh field site in Matlab, Bangladesh
Willingly giving written informed consent
Exclusion criteria
Pregnancy (visible or verbal report on date of last menstruation or urine for pregnancy test)
History of severe allergic reaction to a vaccine or a vaccine component
Received another vaccine or immunoglobulin within 2 weeks
Serious chronic diseases (medical assessment)
Acute or chronic infectious disease (medical assessment)
Fever >38°C (axillary temperature)
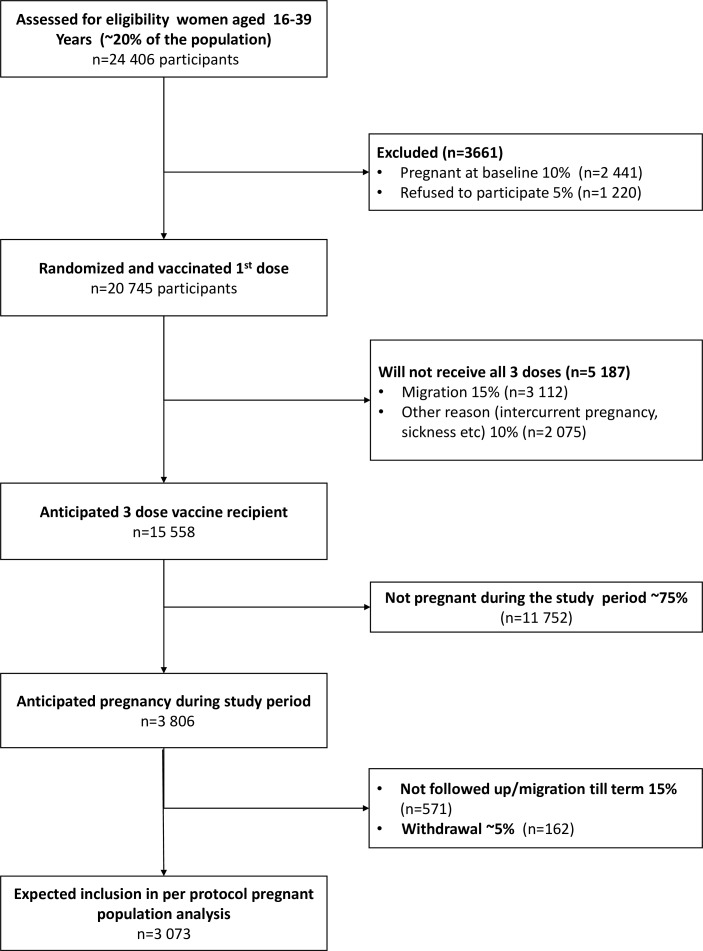
A urinary pregnancy test is offered to women who have missed a period. Eligible participants visit a fixed site clinic for enrolment, where they sign a consent form. In the case of 16–17 year-old participants, their legal guardians sign an assent form in place of the consent form. A case report form (CRF) with an identification number is then created for each participant. Confidentiality of personal identifiers is maintained by keeping names noted in primary data instruments in secure files and by removing names from the computerised dataset for analysis. A flow chart of anticipated participant recruitment is shown in figure 1.
Figure 1.
Anticipated participants enrolment flow chart.
Withdrawal
The participants and/or their legal guardians are informed that participation is voluntary, and that they may withdraw consent at any time, without giving a reason and without prejudice to further treatment. Participants who withdraw may demand destruction of samples and deletion of data concerning themselves. Participants may be discontinued from the study by the investigator at any time in case of safety reasons or significant protocol deviations. The reason for withdrawal will be recorded in the CRF. If withdrawal is due to an adverse event, appropriate follow-up will be arranged.
Interventions
The HEV 239 vaccine is based on a 239 amino acid long recombinant HEV peptide, corresponding to amino acids 368–606 of open reading frame 2 that encodes the capsid protein of HEV. The amino acid sequence is derived from a genotype 1 Chinese HEV strain. HEV 239 was developed and is produced by Innovax. The vaccine is expressed in Escherichia coli,9 and contains 30 µg of the purified protein absorbed to 0.8 mg of aluminium hydroxide suspended in 0.5 mL of buffered saline. The control vaccine is a commercial HBV (Hepa-B) produced by Incepta. Each 0.5 mL dose (for age 16–18 years) contains 10 µg of hepatitis B surface antigen absorbed on aluminium hydroxide gel equivalent to Al3+ 0.25 mg. A 1.0 mL dose for older persons contains 20 µg of hepatitis B surface antigen absorbed on aluminium hydroxide gel equivalent to Al3+ 0.5 mg. We chose this control vaccine for the following reasons: it will benefit the target population who have not received this vaccine through the child immunisation programme; the dosing regimen is the same as the HEV vaccine; and it was the control vaccine in the phase III trial,4 facilitating comparison with previous results.
Innovax donated bulk HEV vaccine for the trial, while Incepta filled the HBV and HEV vaccines in identical, single dose vials, respectively. Both bulk vaccines and finished products were quality tested and labelled according to ICH-Good Manufacturing Practice.
Innovax donated bulk vaccine to Incepta, Bangladesh maintaining cold chain through courier. Incepta prepared the HEV single-dose vaccine vials. We maintained proper cold chain from Incepta to field sites.
The vaccines are administered intramuscularly in the deltoid muscle of either arm, in a 3-dose regimen on day 0, at 1 month and at 6 months (table 1).
Follow-up/surveillance
The full study period will last for 2½ years after enrolment of the final participant (Table 1).
Hepatitis surveillance
Participant get an immunisation card with identification numbers and a phone number, and are instructed to contact the investigator immediately if they experience jaundice or have any of the following symptoms for at least 3 days: fatigue, loss of appetite, abdominal discomfort, abdominal pain in the right upper quadrant, nausea or vomiting. This passive surveillance for hepatitis is ongoing throughout the full study period for all participants. After each dose of vaccine, field staff visited participant’s household daily for 7 days. Then, all participants are visited by field staff for hepatitis surveillance weekly and will be continued till the end of the study. All women who became pregnant after any dose are visited every 2 weeks to collect information about pregnancy outcomes and to screen for clinical hepatitis. In addition, message on hepatitis symptoms is also being sent on mobile phones to all participants (>80% of households have cell phone access). Suspected cases will be referred to Matlab hospital for clinical and laboratory examination including tests of liver function and virological causes of hepatitis (A, B, C, E), and eventual treatment. If HEV disease is confirmed by the presence of anti-HEV IgM or HEV RNA, blood samples will be analysed for relevant biochemical, microbiological and immunological markers. This includes viral load, HEV subtypes, other hepatitis infections, antibody titer, cellular immune response, cytokines, alanine transaminase (ALT), International normalized ratio (INR) and albumin. Blood samples will be taken at least two times a week until recovery in order to assess the dynamic in viral and host factors during the illness. The patient’s symptoms and signs will also be recorded regularly in this period. The last study visit to pregnant women is 14 days after delivery, to record information on the health status of the child.
Adverse events surveillance
After each vaccination dose, participants are observed for 30 min and visited at their homes by a field worker for seven consecutive days. During these visits, participants are specifically asked about any local reactions (eg, erythema, swelling, induration and pain at the injection site) and systemic symptoms (eg, nausea, malaise, myalgia, arthralgia, headache and fever). Participants are also asked to report any significant symptoms after the last vaccine dose, that is, at least 2 years until the last study visit. After this visit, they are advised to report to the study team about any possible SAEs or signs of hepatitis, even after completion of the study.
Outcomes
The primary outcome is clinical HEV disease among pregnant women. HEV disease is defined by illness lasting for at least 3 days, abnormal serum ALT level of ≥2.5 times upper limit of normal, detection of IgM anti-HEV in serum collected within 1 month after onset, and/or the presence of HEV RNA and/or ≥4-fold rise of IgG anti-HEV levels in paired sera. All acute hepatitis cases are also tested for the presence of markers of other viral hepatitis (A, B, C). Secondary outcomes are confirmed HEV disease in non-pregnant women, safety and immunogenicity. Safety outcomes include all local and systemic adverse events, which are recorded in the participant case report form during the study period. Investigation of vaccine-induced immune responses will include anti-HEV IgG on all participants, together with additional antibody and cellular responses in plasma and peripheral blood mononuclear cell (PBMC) samples taken from a subset of 50 participants. A possible protective anti-HEV IgG level will be sought. An antibody response to vaccination will be defined as a ≥4-fold rise of IgG anti-HEV levels in an individual’s post vaccination sample (1 month after the last dose) compared with baseline sample.4 Data on pregnancy outcome, including complications during delivery and health status of mother and child, will be collected on all participants on a pregnancy report form and analysed together with records of eventual HEV disease and type, time and number of vaccine doses.
Sample size and power calculations
The sample size was calculated to estimate total vaccine protection of pregnant participants in a per protocol (PP) analysis (figure 1). The following assumptions are based on data from Matlab health and demographic surveillance system and previous HEV research studies from the region.8 We expect that 10% of women will not be included because of baseline pregnancy, and that the refusal rate will be 5%. After randomisation, there will be 15% loss of person time because of migration out of the study site, and 10% will not complete three vaccine doses for other reasons. During follow-up after the last vaccine dose, we assume that 16% of women (followed over a mean of 16 months) will become pregnant and reach term before the end of surveillance; an additional 9% of women (followed over a mean of 9 months) will be followed for an average of half full term (4.5 months). This predicts that 20% of the dose 3 recipients will have ‘completed’ pregnancies, 3806 are expected to become pregnant after dose 3 and 3073 to be followed to term. Follow-up of the remaining pregnancies will be right censored by termination of follow-up. We expect 15% of the pregnant dose 3 recipients to be lost to follow-up because of migration out, while 5% are expected to withdraw from the study. We expect the design effect for this cluster randomised trial to be 2. We assume that 22% of the participants are HEV seropositive at baseline, indicating protection against HEV infection.8 Furthermore, 6% of seronegative pregnant women are expected to become infected during pregnancy,8 of which we assume 35% to be symptomatic.8 The protective efficacy of a 3-dose regimen of HEV vaccine against symptomatic infections is >95%.4 We therefore need a sample size of 20 745 women at baseline to achieve 80% power at p<0.05 (two tailed) for the analyses in pregnant women. Further, our study will have 90% power to show >95% protective efficacy against HEV disease among non-pregnant women. Our study is not powered to directly evaluate perinatal and maternal death, because of the low rates of these outcomes in Matlab. However, given the substantial evidence linking maternal and perinatal mortality to HEV disease in pregnancy,2 demonstration of vaccine protection against confirmed HEV disease in pregnancy will provide strong evidence that vaccination is likely to prevent maternal and perinatal deaths.
Data collection, management and analysis
The field staff are entering the required data into the paper-based CRFs through interviewing during vaccination and home visits, and by reviewing medical records when applicable. The data are further transcribed to an electronic data capturing system (developed by icddr,b using Oracle data base) within a week of the clinic visit. This system will automatically check data to detect errors and inconsistencies. The data in the system are reviewed weekly by the analyst programmer, and any data deleted from the main database will be saved in a shadow table. Data are stored in the Oracle database system in a central server at Matlab. An electronic database backup is made weekly by the data management team, and the final database is sent to the icddr,b data archive system.
Health registry data for study participants and their previous pregnancy outcome are also being collected. Field staff and medical officers are checking these data with the family record book. They are checking the participant data with the eligible participant list after every visit. The data analyst manager is verifying entered data biweekly. Any inconsistencies are resolved with the field staff and medical officers.
All completed CRFs and other documents are stored in a locked cabinet with limited personnel access. The CRF register in the data management centre at Matlab keeps track of CRF movement between the file cabinet and the data management centre. The log book contains the columns; participant ID, receipt date, visit number, number of pages, name of staff, purpose of file movement, return date, name of staff and any relevant remarks. All informed consent forms are being filed and kept separately in a locked cabinet. Data are entered in electronic data base. A dedicated data management team will be responsible for data entry, cleaning, analysis and data archiving in deidentified way.
Blood samples are being analysed by icddr,b and/or Norwegian Institute of Public Health (NIPH), according to predefined standard operating procedures. Additional blood will be stored in the research biobank at icddr,b in case reanalysis is needed.
Statistical methods
Analyses
In the primary analysis, the risk of confirmed HEV disease in pregnant women who received the HEV vaccine will be compared with the risk in women who received the HBV vaccine using Cox regression with shared frailty,10 to adjust for the design effect of cluster randomisation. Demographic and other baseline characteristics will be compared between the HEV and HBV vaccination clusters, for all participants, and separately for pregnant participants, to assess the degree to which randomisation was achieved. Unbalanced covariates (eg, occupation, age, body mass index (BMI), hepatitis B disease, socioeconomic information, education, sanitation and water use, distance from river, season, baseline HEV IgG antibodies) may be included in the models. Subgroup analyses will be performed to evaluate effectiveness in participants who are negative/positive at baseline for anti-HEV IgG antibodies, respectively, and subgroup analyses for effectiveness will also be performed per number of doses received. Additionally, subgroup analyses will be carried out to explore effectiveness in participants within different BMI and age intervals. Safety analyses of all local and SAEs (eg, pain, swelling, redness, fever, myalgia and headache) will be performed using generalised estimating equations for logistic regression to account for cluster randomisation
Analysis sets
The full analysis set (FAS) population will include all randomised participants who received at least one dose of either the study or the control vaccine. The PP population will include participants who were randomised and received three doses of the study or control vaccine, respectively, and provided blood samples according to the protocol schedule (table 1). Pregnant FAS and PP populations are defined as participants from the respective populations with confirmed pregnancies during the study period. The primary effectiveness analysis will be performed on the pregnant PP population. Secondary effectiveness analyses will be performed on the pregnant FAS population, as well as the non-pregnant FAS and PP populations, with the same methods as the primary analysis. Safety analyses will be performed on the FAS population.
Data and safety monitoring
Data monitoring
An independent DSMB with no competing interests was appointed to provide the icddr,b Ethical Review Committee (ERC) with an overall scientific, safety and ethical appraisal of the study. The DSMB also informs this committee regarding the progression of the study, with special attention to the safety of the participants. They convene at least once annually, and make recommendations directly to the ERC Chairperson. As described in the ERC guidelines, the principal investigator prepares a report to the DSMB before each meeting, describing the accumulated adverse events and SAEs and a summary of the current data for inclusion, progress and deviations from the protocol or planned procedures. Any reports from the monitor regarding quality control are also included. The DSMB is also responsible for detailed reviews of all the SAEs. More details can be found in the DSMB Charter included in the Trial Master file in the trial office in Bangladesh.
No interim analyses have been planned, and no stopping guidelines have been created for the trial. In the event of serious safety issues, the DSMB will meet to provide recommendations regarding termination of the trial. The steering committee will have the final decision to terminate the study.
Harms
All adverse events observed or reported are logged in the relevant participant CRF by the study staff. The following information is registered: description of the adverse event (in precise standard medical terminology), time of onset and resolution, severity (mild/moderate/severe; according to Common Terminology Criteria for Adverse Events version 4.0), outcome, assessment of the causality with study drug and action taken with study drug.
All SAEs are communicated to the DSMB within 24 hours after awareness by the study staff. SAE reports are initially sent to the Chairperson of the DSMB and the ERC with copy to sponsor/principal investigator. All adverse events and SAEs are being followed up to resolution unless the event is considered by the investigator to be unlikely to resolve due to underlying disease. We will endeavour that all events are resolved, even if they continue after study completion.
Auditing
The sponsor (NIPH) has appointed an independent local clinical monitor to ensure that the study is conducted according to protocol, standard operating procedures, GCP and regulatory requirements, and to verify that investigators are collecting and reporting quality data. In addition, a monitor from NIPH is auditing the study. The monitors are periodically monitoring on site, including at study initiation and at closeout. The monitors check the informed consent process, reporting of adverse events and other safety data, CRF completeness and adherence to the study protocol for at least 10% of the study participants randomly distributed between the 67 study villages. They also monitor maintenance of regulatory documents, study supply accountability, facilities and equipment.
Patient and public involvement statement
The public was not involved in the development of the research question nor the study design.
Ethics and dissemination
The protocol has been approved by the icddr,b ERC and Regional Ethics Committee in Norway.
Protocol amendments
Any significant amendments and/or new versions of the study protocol will be notified to and approved by the competent authority and the ethics committees in both countries according to EU and national regulations before changes are implemented.
Confidentiality
All study-related information is being kept confidential, under lock and key, or on secure servers in case of digital information. The study monitor, the ERC and any law-enforcing agency will have access to this information only in the event of necessary inspection. The samples may be sent outside the country for analysis, and preserved for 5 years where applicable; however, any personal identifiable information will be held and processed under secured conditions with access limited to preidentified staff. Remaining blood samples will be disposed of after completing testing for all participants in the study.
Access to data
The study principal investigator, the coinvestigators in the study and the data analyst manager will have access to the final trial dataset.
Dissemination policy
On study completion and finalisation of the study report, we will submit the study results to the competent authority and ethics committees according to national regulations, and publish the results in peer-reviewed journals. We will report the trial in accordance with the Consolidated Standards of Reporting Trials guidelines, with authorship based on the ICMJE recommendations.
Supplementary Material
Acknowledgments
icddr,b acknowledges with gratitude to the Research Council of Norway for supporting this research through the GLOBVAC program. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. The authors thank all the members of the research team at icddr,b who helped with participant recruitment, vaccination, sampling and follow up, and the laboratory team at icddr,b who performed laboratory procedures. The local clinical monitor (Wasif Ali Khan) is thanked for monitoring the trial. The authors also thank the data safety monitoring board (Kazi Zulifiquer Mamun, S M Shamsuzzaman, Saria Tasnim, Md. Nur Haque Alam, and Hanne M. Nøkleby), and the steering committee (Shams El Arifeen, Rashidul Haque, Ingeborg Aaberge and Geir Bukholm) for their important contributions throughout the trial. A special thanks to Innovax for their donation of the HEV vaccine. NIPH is the sponsor for this clinical trial (Contact name: Susanne Dudman. Address: Norwegian Institute of Public Health, Postboks 222 Skøyen, 0213 Oslo, Norway).
Footnotes
Twitter: @Asma Aziz
Presented at: The authors presented a poster of the study at 10th Conference on Global Health and Vaccination (GLOBVAC) Research in Trondheim, Norway in 2017.
Contributors: JDC, KZ, SD, KS-J, FQ, MY, SS, JO, ESG, JLD, QN, AR, PKS, JK and CHJ contributed to study design. KZ, JDC, AA, MK, SD, KS-J, JO, SS, TRB, MR, WH, JLD and CHJ were involved in trial management. CHJ, KS-J, SD, JDC, KZ, SS, JLD, JO and ESG contributed in manuscript writing and editing. KZ, AA, MK and WH responsible for managing the field teams/logistics of the study. All authors read and approved the final manuscript.
Funding: The Research Council of Norway supported this study through the GLOBVAC program, project number 248143. Xiamen Innovax Biotech, China, provided the bulk HEV 239 vaccine. SGD is supported by both NIPH and University of Oslo, KSJ and JLD are additionally supported by NIPH, who also provided additional support from statisticians, technical engineers, vaccine experts and access to laboratory facilities and instruments. The design, management, analysis and reporting of the study are entirely independent of the vaccine manufacturers.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: This trial is approved by the icddr,b Research Review Committee and Ethical Review Committee, and by the Directorate General of Drug Administration in Bangladesh. Further, it has been approved by the Regional Ethics Committee in Norway.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012;55:988–97. 10.1002/hep.25505 [DOI] [PubMed] [Google Scholar]
- 2. Bergløv A, Hallager S, Weis N. Hepatitis E during pregnancy: maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J Viral Hepat 2019;26:1240–8. 10.1111/jvh.13129 [DOI] [PubMed] [Google Scholar]
- 3. WHO Hepatitis E vaccine: who position paper; 2015: 185–200. [PubMed]
- 4. Zhu F-C, Zhang J, Zhang X-F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. The Lancet 2010;376:895–902. 10.1016/S0140-6736(10)61030-6 [DOI] [PubMed] [Google Scholar]
- 5. Meeting of the strategic Advisory group of experts on immunization, April 2014 –- conclusions and recommendations. Wkly Epidemiol Rec 2014;89. [PubMed] [Google Scholar]
- 6. Smith DB, Simmonds P. Classification and genomic diversity of Enterically transmitted hepatitis viruses. Cold Spring Harb Perspect Med 2018;8:a031880 10.1101/cshperspect.a031880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen J, Behloul N, Dai X, et al. Immunogenicity difference between two hepatitis E vaccines derived from genotype 1 and 4. Antiviral Res 2016;128:36–42. 10.1016/j.antiviral.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 8. Labrique AB, Zaman K, Hossain Z, et al. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am J Epidemiol 2010;172:952–61. 10.1093/aje/kwq225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang J, Zhang X-F, Zhou C, et al. Protection against hepatitis E virus infection by naturally acquired and vaccine-induced immunity. Clin Microbiol Infect 2014;20:O397–405. 10.1111/1469-0691.12419 [DOI] [PubMed] [Google Scholar]
- 10. Therneau TM, Grambsch PM. Modeling survival data: extending the COX model. New York: Springer, 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.