Abstract
Introduction
Chemotherapy may cause infertility in young survivors of breast cancer. Various fertility preservation techniques increase the likelihood of survivors becoming genetic mothers. Disclosure of cancer diagnosis may impact decision making about fertility preservation. This protocol will develop and test the effectiveness of a web-based decision aid for helping women with breast cancer to make well-informed choices about fertility preservation.
Methods and analysis
This study will be conducted in three phases using mixed methods. In phase I, the aim is to develop a web-based patient decision aid (PDA) in French with a steering committee and using a focus group of five women already treated for breast cancer. In phase II, the face validity of the decision aid will be assessed using questionnaires. In phase III, the PDA will be assessed by a two-arm randomised controlled trial. This will involve a quantitative evaluation of the PDA in clinical practice comparing the quality of the decision-making process between usual care and the PDA. The primary outcome will be informed choice and its components. The secondary outcomes will be decisional conflict and anxiety. Data will be collected during and after an oncofertility consultation. Phase III is underway. Since September 2018, 52 participants have been enrolled in the study and have completed the survey. We expect to have results by February 2020 for a total of 186 patients.
Ethics and dissemination
This study protocol was approved by the Ouest V Research Ethics Board. Results will be spread through peer-reviewed publications, and reported at suitable meetings.
Trial registration number
The ClinicalTrials.gov registry. (NCT03591848).
Keywords: breast cancer, decision-making process, fertility preservation, informed choice, study protocol, web-based decision aid
Strengths and limitations of this study.
To our knowledge, this study will lead to the development of the first French language patient decision aid aiming to support the decision-making process concerning fertility preservation in young women diagnosed with breast cancer.
The major strength of the present investigation will be the number of patients expected to be included.
Investigators will not be blinded to the intervention, which represents a limitation.
The study is specifically focused on patients with breast cancer. Therefore, the results will be not applicated to other malignancies.
Background
Fertility preservation
Breast cancer is the most common malignant disease among women. According to cancer statistics in France, 2344 women below the age of 40 were diagnosed with breast cancer in 2012.1 Recent advances in treatments have markedly improved survival rates, leading to a group of young female survivors. However, the fertility potential of most of them will be reduced as a result of the direct gonadotoxicity of chemotherapeutic agents, combined with physiological ovarian ageing during the treatment.2 3 Fertility in young survivors is a crucial issue for their quality of life.4 5 Therefore, the possible risk of future infertility and uncertainty about being able to have a biological child are major causes for concern5 and may lead to significant and distressing outcomes of their cancer treatment.5 6
Fertility preservation (FP) is now considered a right in France for people who have been diagnosed with cancer. It can help them to rebuild their self-esteem as it provides them with the possibility of pursuing parenthood like everyone else. In practice, the concept of healing has undergone a major evolution, since the overall aspirations of a person are now taken into account. Healing no longer focuses only on the sick organ or body; it now comprises a person’s psychological, professional, social and emotional well-being. The family environment and social balance must, therefore, become an integral part of the missions of healthcare providers.
Over the past decade, various FP techniques have been developed in order to increase the chances of young cancer survivors of becoming genetic mothers.7 8 Various options are available according to the patient’s clinical profile. In France, since 2004, FP has been subject to a bioethics law,9 as well as in the two previous cancer plans.10 11 FP counselling is systematically offered to all patients of reproductive age diagnosed with a disease that could negatively impact fertility as a result of its natural history and/or its treatment. In addition, the recent recommendations of the American Society of Clinical Oncology state that oncofertility counselling should be considered as standard care at the time of cancer diagnosis in all young women.12 13
Current FP options include medical treatments for ovarian protection, oocyte and/or embryo cryopreservation after controlled ovarian stimulation or after in vitro maturation (IVM) and ovarian tissue cryopreservation.13 Ovarian stimulation for oocyte or embryo cryopreservation is the only established method for female FP.13 However, the duration of stimulation as well as follicle-stimulating hormone induced hyperestradiolaemia may constitute limitations in some clinical situations. Although experimental, alternative techniques should, therefore, be offered. Such techniques include the retrieval of immature oocytes without stimulation, followed by their IVM,14 and/or the surgical removal of a piece of ovarian cortex for cryopreservation.15–17 In addition, the administration of gonadotrophin-releasing hormone agonists during chemotherapy is also a method of ovarian protection.18
Patient decision making
Decisions making about FP can be very complex and time-consuming for patients. Indeed, oncofertility counselling takes place only a few days after disclosure of the cancer diagnosis,19 and FP techniques should be performed before starting any gonadotoxic treatment. During the consultation, a state of emotional confusion and shock may hinder women’s grasp of what they are told about current scientific progress. They may find it difficult to see beyond the disease, especially when they may not have thought about motherhood until then.20 The complex emotional situation, the need to make the right decision in a short space of time and conflicting ideas about life and death may mean that the decision about FP is a difficult one to take. Weighing up the risks and benefits of the various FP options may seem a mammoth task. Furthermore, other factors may have a bearing on decision making: lack of referral from an oncologist; limited information about fertility; fear of delaying cancer treatment or exacerbating the malignancy; deciding which treatment is more suitable for them; the possible consequences of a future pregnancy; their personal situation and the cost of FP treatments.21
Hershberger et al investigated the decision-making process of women with regard to FP and identified four main phases that precede the active formulation of a decision.22 During the identification phase, they grasp the knowledge necessary for decision making, often with a devastating awareness of their cancer and its consequences. The reflection phase allows them to participate actively in the formulation of a decision about FP by establishing their preferences and values. There are no good or bad options: the assessment of the risks and benefits of each FP option depends on each women’s personal values.23 During this phase, they feel the need to seek more information about FP, and sometimes need to hear from other women who have experienced the same situation. They often want to hear about the experiences of others before making their own decision. Once the decision has been taken, they usually commit to the process whole-heartedly. Given the complexity of this multistep decision-making process, patients should receive the right support to make the most appropriate decision for their situation.
However, current evidence suggests that women do not feel that they get adequate support in making these decisions.5 24 25 In addition, the lack of information about FP may impact long-term quality of life26 and increase ‘decisional regret’, defined as ‘distress or remorse after a healthcare decision’.27 Health professionals in France are required by a law passed in 2002 to provide patients with quality information about the potential risks and benefits of healthcare.28 This obligation is also laid out in the priorities of the 2014–2019 cancer plan.10
Patient decision aids
Use of a patient decision aid (PDA) could be a way to provide more support to patients and improve the quality of the decision-making process. According to the International Patient Decision Aids Standard (IPDAS) Collaboration,24 29 30 PDAs are tools that ‘provide information on the options and help patients clarify and communicate the personal value they associate with different features of the options’. Commonly used before making a decision, their aim is to allow patients to prepare their discussion with healthcare providers and to guide them in making informed choices about what is best for them. Although they do not replace discussion between professionals and patients or allow one option to be chosen rather than another, they improve the quality of decision making.31 PDAs come in various forms, such as pamphlets, audio guides, tables or interactive multimedia sites, and may be used before or during the consultation, their permanent accessibility being an important advantage.
A recent Cochrane review of 105 studies involving 31 043 participants showed that PDAs increase patients’ knowledge of treatment options and congruency between informed values and care.31 Furthermore, they decrease decisional conflict.31 In the field of female FP, some interventions have been evaluated and led to similar results.30 32–38 Two PDAs are available for patients with breast cancer in English and Dutch, focusing on level of knowledge and on decisional conflict, respectively.35–37 Nevertheless, since medical guidelines may differ from one country to another and very few French patients have sufficient mastery of English or Dutch, a PDA in French was needed.
Objectives
Therefore, the present study has the following aims: (1) present the development of a French web-based PDA designed for young women presenting with breast cancer in collaboration with oncofertility specialists, cancer experts and patients; (2) evaluate its validity regarding its structure, content and language; (3) compare the quality of the decision-making process between patients receiving conventional oral information supplied by a fertility specialist (‘IRIS’ group) and those having access to the PDA before and during oncofertility counselling (‘DECISIF’ group) through a randomised controlled trial.
Methods and analysis
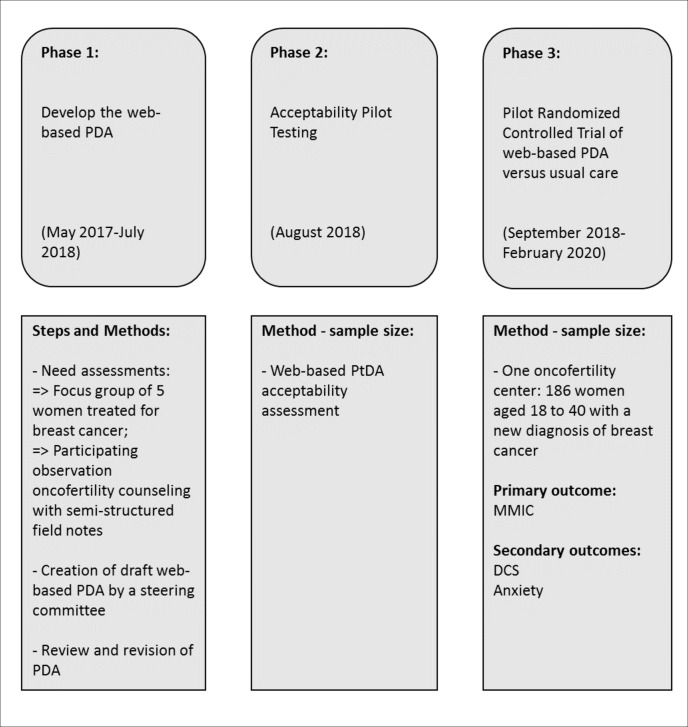
This study will be conducted in three phases (figure 1).
Figure 1.
Study flow diagram. DCS, Decisional Conflict Scale; MMIC, Multidimensional Measure of Informed Choice; PDA, patient decision aid.
Phase 1: development of the web-based PDA (May 2017–July 2018)
The objective of the first phase was to develop the web-based PDA with the input of a steering committee and an expert in health informatics.
Steering committee
The steering committee comprised women treated for breast cancer, gynaecologists, oncologists, embryologists, midwives, psychologists and web designers. Regular meetings were organised to develop the content and format of the PDA according to the Ottawa Decision Support Framework.
Needs assessment
The needs of patients with breast cancer in oncofertility counselling were analysed by qualitative research with a focus group approach in which data about opinions and needs with regard to the oncofertility care pathway were collected. Hence, five women treated for breast cancer 18–24 months before and who had benefited from cryopreservation of oocytes, embryos or ovarian tissue in a University Hospital in Paris were interviewed.
Creation of the web-based PDA
Based on the needs assessment and a literature review, the web-based PDA was originally developed by members of the steering committee in 2017 under the auspices of the IPDAS quality framework, key performance indicator of PDA.29 It was designed to serve as a decision aid for women with breast cancer regarding FP techniques, in addition to standard oncofertility counselling. Named ‘FertiEll’P’, the tool is accessible via a secure weblink from a computer or handheld tablet (www.fertiellp-onco.com).
The PDA begins by raising the awareness of patients that they will have a decision to make about FP procedures.29 It explains the procedure of an oncofertility consultation and the definition and objectives of a PDA. It contains the following: general information about pelvic anatomy, ovarian function and the evaluation of ovarian reserve; data about ovarian ageing and the impact of breast cancer therapies on female fertility; a description of available FP options, including their advantages, drawbacks and possible uncertainties; information regarding alternative options (oocyte donation, adoption, life without children); patients’ testimonials; a test designed to check whether the patient has sufficient knowledge to make an informed choice; questions concerning the patient’s personal values in order to clarify their priorities.
The PDA is divided into three sections. The first is called PETUNIA and concerns the freezing of oocytes, embryos and/or ovarian tissue. It is available before the FP consultation to all women fulfilling the research protocol criteria. The second, ROSE, explains the different FP options and the possibility of not freezing oocytes, embryos or ovarian tissue. This section is available during and after the FP consultation only for women whose oncological team did not contraindicate controlled ovarian stimulation. MAGNOLIA, the third section, explains the various FP options including that of not freezing oocytes, embryos or ovarian tissue. It is available during and after the FP consultation only for women whose oncological team did contraindicate controlled ovarian stimulation.
Phase 2: acceptability pilot testing (August 2018)
The purpose of phase 2 was to assess the face validity of the PDA. A descriptive study was conducted to check whether the content, format and vocabulary were acceptable for patients and their families. Ten women and five health professionals were recruited at Antoine Beclere University Hospital as follows: five women with breast cancer who had had their oocytes, embryos or ovarian tissue frozen 18 –24 months previously; five women aged 18–40 with a primary diagnosis of breast cancer and who had not yet received cancer treatment; five health professionals (psychologist, embryologist, oncologist, surgeon and gynaecologist).
An evaluation questionnaire was completed anonymously by each participant after reading the PDA. The PDA was then modified according to the results obtained.
Phase 3: evaluation of the web-based PDA (September 2018–February 2020)
Objectives and hypothesises
The primary objective of this phase will be to determine whether a web-based PDA is able to help women with breast cancer to make an informed choice among FP options. The secondary objective will be to assess the level of decisional conflict and anxiety of patients. We hypothesise that it will improve women’s decision making and increase the number of women making informed choices. Finally, we hypothesise a decrease in decisional conflict thanks to the use of the PDA. These positive effects of the intervention should reduce patients’ anxiety.
Study design and setting
The investigation will be a single-centre randomised controlled study and will be conducted in the FP department of an academic hospital in France.
Study period
The study will be conducted from September 2018 to February 2020.
Study population
The trial will enrol women aged 18–40, with a primary diagnosis of breast cancer, who have not yet started any cancer treatment. Patients will have to be able to read, write and speak French. Women presenting metastatic, relapsing breast cancer or current pregnancy will not be eligible. Furthermore, protected adults will not be eligible.
Sample size and sampling design
A sample size of 186 participants, that is, 93 in each arm, is planned. The required sample size was calculated in order to detect a difference of 20% (50%–70%) in the informed choice among women included in the IRIS and DECISIF groups and by using a two-sided test at a significance level of 0.05% and 80% power. Based on our experience, we estimate that half of the patients seen in fertility counselling today make an informed decision. We believe that a 20% increase in the number of patients who make an informed choice with the PDA would be acceptable in this context.
Allocation of subjects to study and control arms
After being referred by oncological centres, participants will be invited to participate in the study by a physician in reproductive medicine. After providing written informed consent, they will be randomly assigned to one of the two study arms using blind and secure allocation by a sealed envelope system (figure 2). The block size of the randomisation is four. Participants will be free to withdraw at any time during the study, and this will not affect their clinical treatment.
Figure 2.
Trial flow diagram. PDA, patient decision aid.
Usual care control group (IRIS)
Participants who are randomly assigned to the control group will receive standard oral information from a gynaecologist, an embryologist and a midwife. The information provided will relate to pelvic anatomy, ovarian function and the evaluation of ovarian reserve, ovarian ageing, the impact of breast cancer therapies on female fertility, available FP options and alternative options, such as oocyte donation, adoption and life without children. After this consultation, they will complete the designed and validated questionnaire that addresses knowledge, deliberation, preferences and values, decisional conflict and anxiety. For ethical reasons of equality of access to information, women in this arm will have access to the web-based PDA after the oncofertility consultation if they wish, once the questionnaire is completed.
Intervention group (DECISIF)
Participants randomly assigned to the intervention group will receive an email with a link and a password to connect to the section ‘PETUNIA’ of the web-based PDA. The day of the oncofertility consultation, the ‘MAGNOLIA’ section or the ‘ROSE’ section of the PDA will be used by medical staff to explain the different options and whether the oncological team contraindicates controlled ovarian stimulation or not. After this consultation, the women will complete the designed and validated questionnaire that addresses knowledge, deliberation, preferences and values, decisional conflict and anxiety.
Study variables including primary and secondary outcome variables
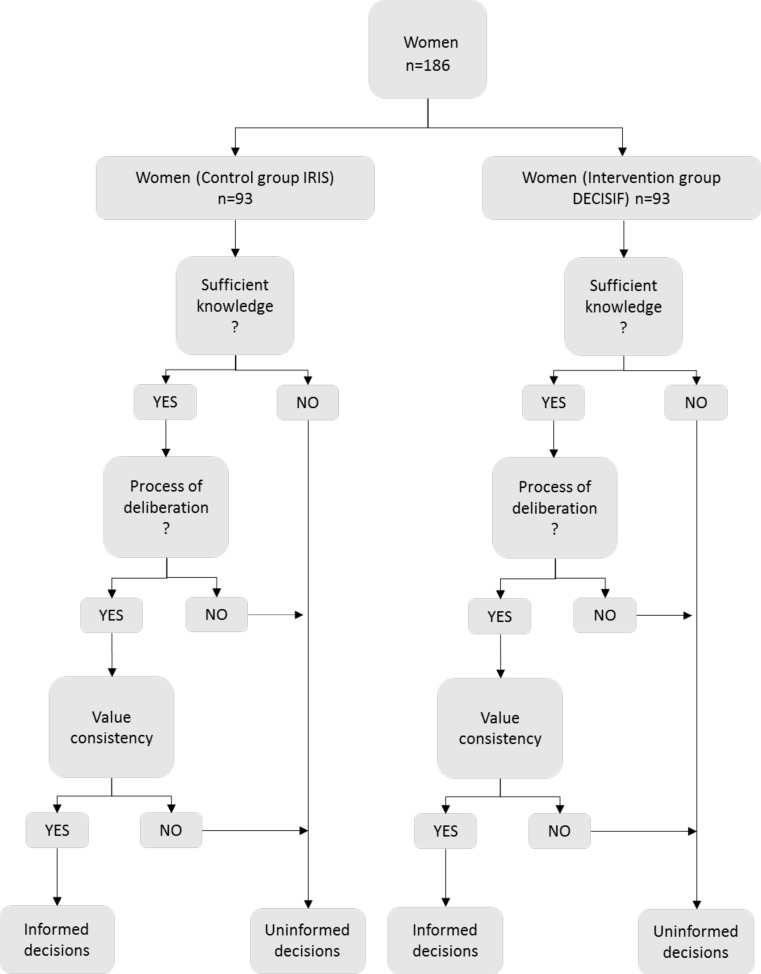
We speculate that patients in the DECISIF group will make a more informed choice than those in the IRIS group. Not only will the PDA increase knowledge about fertility biology, options and involvement preferences, but we also believe that it will improve the concordance between the reasoning of the patients concerning their ‘best choice’ and their final choice. Regarding the definition of the term ‘informed choice’, O’Connor et al,39 interalia, define an informed choice as being ‘a choice that is based on relevant knowledge, consistent with the decision-maker’s values and behaviourally implemented’ (figure 3). On the other hand, some definitions of the term include a process of deliberation about the alternatives and a more thorough weighing up of the pros and cons.40–44
Figure 3.
Informed decision making: knowledgeable, deliberated, value consistent.
Primary outcome measure
The primary outcome is the degree of patient autonomy for making decisions, according to the Multidimensional Measure of Informed Choice (MMIC).45 This quantitative instrument was developed by Marteau et al to evaluate informed choice in prenatal screening for Down’s syndrome. Validated in 2002, it is considered as a reliable and valid measure of informed choice.46 The original MMIC consisted of an eight-item knowledge scale, a four-item attitude scale and the two alternatives (to do or not to do). Since there is no standard measure of informed choice for FP, we adapted the MMIC by including the concept of deliberation in decision making. Indeed, van den Berg et al developed a measure of informed choice including the three theoretically founded dimensions: knowledge, value consistency and deliberation.47
Knowledge about fertility and FP techniques was measured by a 10-question survey designed with previously used instruments48–50 and the expertise of the medical team. We ranked sufficient knowledge as correct answers on at least 70% of items.51 Concerning attitude, we included three of the four items used in the original MMIC scale (beneficial/harmful, important/unimportant, good/bad). The item (pleasant/unpleasant) being viewed as irrelevant in the context of breast cancer, we selected two other items (desirable/undesirable, reassuring/disturbing), as previously validated in other modified MMIC scales.52 We also included a deliberation scale to evaluate the alternatives, thinking about the consequences, and weighing up the pros and cons.52
Based on Marteau’s and van den Berg’s work and in a context of FP, an informed choice may be considered to be made when a woman: (1) has sufficient relevant knowledge about fertility and FP techniques; (2) has positive attitudes towards engaging in FP; (3) engages in deliberated decision making and (4) starts the process of FP. It is also made when a woman: (1) has sufficient relevant knowledge about FP options; (2) has a negative attitude towards engaging in FP; (3) engages in a deliberated decision-making process and (4) does not start the process of FP.
Secondary outcomes measures
The Decisional Conflict Scale is used to assess health-related decisions across divergent health conditions. It consists in 16 items which define the degree of patient uncertainty about options, the factors contributing to this uncertainty and satisfaction with decision making.39 53 Items are scored on a Likert scale as follows: 0=‘strongly agree’; 1=‘agree’; 2=‘neither agree nor disagree’; 3=‘disagree’; 4=‘strongly disagree’. Scores range from 0 (no decisional conflict) to 100 (extremely high decisional conflict).39
The six items of State and Trait Anxiety Inventory,54 55 a reliable and sensitive measure of anxiety, will be used to assess emotional disturbance. This inventory consists of six items, indicating the lowest level of anxiety (1=not at all) and 4 the highest (4=very much), with a reverse rating item. Scores >50 indicate a high level of anxiety.
Data collection, handling and analysis
Data will be collected electronically by two researchers and stored on a secure server at the university hospital. The server offers robust security to ensure privacy and technological controls and is accessible with restricted permission. All original forms will be stored at the study site. A clinical research officer mandated by the sponsor will ensure that the research is carried out properly, and that the data generated in writing is collected, documented, recorded and reported, in accordance with standard operating procedures. We will conduct a descriptive analysis of the women participating in this research. Possible basic differences between the two arms will be statistically tested. For the primary outcome, a statistical analysis will be performed on an intention-to-treat basis: all randomised women will be included for analysis in the group assigned at randomisation. The impact of the tool on the primary outcome will be analysed using the χ2 test. For the secondary outcomes, we will use the χ2 test to analyse the binary variables and a two-tailed t-test for continuous variables, with a significance level of 5%.
Patient involvement
Women were involved at several stages of the study, including the design of the trial. They were included in the development and pilot testing of the PDA. We received input from patients who had been treated for breast cancer.
Ethics and dissemination
Informed consent will be obtained from all participants. Each participant will receive a written informed consent form and information sheet. At the end of the research project, the results will be submitted for presentation at meetings. These innovative data will also be proposed for publication in peer-reviewed journals.
Discussion
We describe here the development and evaluation of a web-based PDA designed for patients with breast cancer and who are candidates for FP. This system was developed to address the objective of improving patients’ information and medical care. The past 40 years have seen ideological changes in the medical practice of decision making. Patterns have shifted from a ‘paternalist’ approach, characterised by a passive role of the patient, to a concept of ‘shared decision making’, in which mutual agreement is reached between patients and health professionals. This concept of ‘shared decision making’ is based on enhancing patient participation for decisions about their personal health.56
However, available data show that patients do not feel that they have the support that they need in decision making regarding FP in a context of cancer. The goal of a web-based PDA is to improve patients’ quality of life by helping them to make better quality decisions. Indeed, we think that a PDA will increase knowledge about fertility options, information and involvement preferences. Furthermore, the deployment of this PDA on the national level could reduce inequality in access to care and to adverse events and improve the observance of FP treatments. Once the PDA has been evaluated, it will be widely promoted and made available to all participants, patients and professionals through a wide range of media (social and print), websites, professional organisations and scientific societies.
Trial status
Phase III is underway. As of September 2018, 52 participants have been enrolled in the study and have completed the survey. We expect to have a total of 186 patients by February 2020. No publication containing the results of this study has already been published or submitted for review. Patient inclusions are still ongoing.
Supplementary Material
Acknowledgments
Thanks to Ray Cooke for copyediting the manuscript.
Footnotes
Contributors: AB, MG, GM and NS participated in the design of the study, wrote the first draft of the manuscript, developed the PDA and applied for funding. RM and GG participated in the design of the study and critical review of the manuscript. All authors read and approved the final manuscript.
Funding: The study was supported by the French Agency of Biomedicine, grant number (50/18 000 euros).
Disclaimer: Trial sponsor: « Assistance Publique des Hôpitaux de Paris », contributing to the design and management of the study.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study protocol (V.1.1, 30/04/18) was approved by the Ouest V Research Ethics Board (protocol reference 18/003-2).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bossard N B-FF. Estimation nationale de l'incidence et de la mortalité par cancer en France entre 1980 et 2012 Partie 1 - Tumeurs solides. Saint-Maurice (France): Institut de veille sanitaire, 2013. [Google Scholar]
- 2. Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 2001;7:535–43. 10.1093/humupd/7.6.535 [DOI] [PubMed] [Google Scholar]
- 3. Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol 2012;26:379–90. 10.1016/j.bpobgyn.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 4. Howard-Anderson J, Ganz PA, Bower JE, et al. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:386–405. 10.1093/jnci/djr541 [DOI] [PubMed] [Google Scholar]
- 5. Peate M, Meiser B, Hickey M, et al. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat 2009;116:215–23. 10.1007/s10549-009-0401-6 [DOI] [PubMed] [Google Scholar]
- 6. Mancini J, Rey D, Préau M, et al. Infertility induced by cancer treatment: inappropriate or no information provided to majority of French survivors of cancer. Fertil Steril 2008;90:1616–25. 10.1016/j.fertnstert.2007.08.064 [DOI] [PubMed] [Google Scholar]
- 7. Frydman R, Grynberg M. Introduction: female fertility preservation: innovations and questions. Fertil Steril 2016;105:4–5. 10.1016/j.fertnstert.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 8. Bastings L, Baysal Ö, Beerendonk CCM, et al. Deciding about fertility preservation after specialist counselling. Hum Reprod 2014;29:1721–9. 10.1093/humrep/deu136 [DOI] [PubMed] [Google Scholar]
- 9. Loi n° 2004-800 du 6 août 2004 relative la bioéthique (1), 2011. Available: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000441469 [Accessed 13 Jan 2019].
- 10. Institut National du Cancer Plan cancer 2014-2019; 2014.
- 11. Le plan cancer 2009-2013, 2009. Available: https://www.e-cancer.fr/Plan-cancer/Les-Plans-cancer-de-2003-a-2013/Le-Plan-cancer-2009-2013 [Accessed 11 Apr 2019].
- 12. Lee SJ, Schover LR, Partridge AH, et al. American Society of clinical oncology recommendations on fertility preservation in cancer patients. JCO 2006;24:2917–31. 10.1200/JCO.2006.06.5888 [DOI] [PubMed] [Google Scholar]
- 13. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. JCO 2018;36:1994–2001. 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 14. Grynberg M, El Hachem H, de Bantel A, et al. In vitro maturation of oocytes: uncommon indications. Fertil Steril 2013;99:1182–8. 10.1016/j.fertnstert.2013.01.090 [DOI] [PubMed] [Google Scholar]
- 15. Gremeau A-S, Andreadis N, Fatum M, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril 2012;98:355–60. 10.1016/j.fertnstert.2012.04.046 [DOI] [PubMed] [Google Scholar]
- 16. Buckett WM, Chian R-C, Holzer H, et al. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol 2007;110:885–91. 10.1097/01.AOG.0000284627.38540.80 [DOI] [PubMed] [Google Scholar]
- 17. In vitro maturation: a committee opinion. Fertil Steril 2013;99:663–6. 10.1016/j.fertnstert.2012.12.031 [DOI] [PubMed] [Google Scholar]
- 18. Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-Releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual Patient–Level data. JCO 2018;36:1981–90. 10.1200/JCO.2018.78.0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Comtet M, Sonigo C, Valdelièvre C, et al. Préservation de la fertilité dans le cancer du sein : où en est-on en 2014 ? Bull Cancer 2015;102:443–53. 10.1016/j.bulcan.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 20. Trèves R, Grynberg M, Parco Sle, et al. Female fertility preservation in cancer patients: an instrumental tool for the envisioning a postdisease life. Future Oncol 2014;10:969–74. 10.2217/fon.13.265 [DOI] [PubMed] [Google Scholar]
- 21. Jones G, Hughes J, Mahmoodi N, et al. What factors hinder the decision-making process for women with cancer and contemplating fertility preservation treatment? Hum Reprod Update 2017;23:433–57. 10.1093/humupd/dmx009 [DOI] [PubMed] [Google Scholar]
- 22. Hershberger PE, Finnegan L, Pierce PF, et al. The decision-making process of young adult women with cancer who considered fertility cryopreservation. J Obstet Gynecol Neonatal Nurs 2013;42:59–69. 10.1111/j.1552-6909.2012.01426.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Connor AM, Légaré F, Stacey D. Risk communication in practice: the contribution of decision AIDS. BMJ 2003;327:736–40. 10.1136/bmj.327.7417.736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thewes B, Meiser B, Taylor A, et al. Fertility- and Menopause-Related information needs of younger women with a diagnosis of early breast cancer. JCO 2005;23:5155–65. 10.1200/JCO.2005.07.773 [DOI] [PubMed] [Google Scholar]
- 25. Partridge AH, Gelber S, Peppercorn J, et al. Web-Based survey of fertility issues in young women with breast cancer. JCO 2004;22:4174–83. 10.1200/JCO.2004.01.159 [DOI] [PubMed] [Google Scholar]
- 26. Vadaparampil ST, Christie J, Quinn GP, et al. A pilot study to examine patient awareness and provider discussion of the impact of cancer treatment on fertility in a registry-based sample of African American women with breast cancer. Support Care Cancer 2012;20:2559–64. 10.1007/s00520-012-1380-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brehaut JC, O'Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making 2003;23:281–92. 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- 28.Loi n° 2002-303 du 4 mars 2002 relative aux droits des malades et la qualité du système de santé (1); 2002. [PubMed]
- 29. Elwyn G, O'Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision AIDS: online international Delphi consensus process. BMJ 2006;333:417 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones GL, Hughes J, Mahmoodi N, et al. Observational study of the development and evaluation of a fertility preservation patient decision aid for teenage and adult women diagnosed with cancer: the cancer, fertility and me research protocol. BMJ Open 2017;7:e013219 10.1136/bmjopen-2016-013219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stacey D, Légaré F, Col NF, et al. Decision AIDS for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014;28 10.1002/14651858.CD001431.pub4 [DOI] [PubMed] [Google Scholar]
- 32. Woodard TL, Hoffman AS, Covarrubias LA, et al. The pathways fertility preservation decision aid website for women with cancer: development and field testing. J Cancer Surviv 2018;12:101–14. 10.1007/s11764-017-0649-5 [DOI] [PubMed] [Google Scholar]
- 33. Ehrbar V, Urech C, Rochlitz C, et al. Fertility preservation in young female cancer patients: development and pilot testing of an online decision aid. J Adolesc Young Adult Oncol 2018;7:30–6. 10.1089/jayao.2017.0047 [DOI] [PubMed] [Google Scholar]
- 34. Ehrbar V, Urech C, Rochlitz C, et al. Randomized controlled trial on the effect of an online decision aid for young female cancer patients regarding fertility preservation. Hum Reprod 2019;34:1726–34. 10.1093/humrep/dez136 [DOI] [PubMed] [Google Scholar]
- 35. Garvelink MM, ter Kuile MM, Fischer MJ, et al. Development of a decision aid about fertility preservation for women with breast cancer in the Netherlands. J Psychosom Obstet Gynaecol 2013;34:170–8. 10.3109/0167482X.2013.851663 [DOI] [PubMed] [Google Scholar]
- 36. Peate M, Meiser B, Friedlander M, et al. Development and pilot testing of a fertility decision aid for young women diagnosed with early breast cancer. Breast J 2011;17:112–4. 10.1111/j.1524-4741.2010.01033.x [DOI] [PubMed] [Google Scholar]
- 37. Peate M, Meiser B, Cheah BC, et al. Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br J Cancer 2012;106:1053–61. 10.1038/bjc.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garvelink MM, ter Kuile MM, Louwé LA, et al. Feasibility and effects of a decision aid about fertility preservation. Hum Fertil 2017;20:104–12. 10.1080/14647273.2016.1254821 [DOI] [PubMed] [Google Scholar]
- 39. O’Connor A, O’Brien-Pallas LL. McFarland GK, McFarlane EA, Nursing diagnosis and intervention. Toronto: C.V. Mosby Company, 1989: 573–88. [Google Scholar]
- 40. Wiley J. Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons, Ltd, 2001. [Google Scholar]
- 41. Bekker HL, Hewison J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial. Prenat Diagn 2004;24:265–75. 10.1002/pd.851 [DOI] [PubMed] [Google Scholar]
- 42. Kohut RJ, Dewey D, Love EJ. Women's knowledge of prenatal ultrasound and informed choice. J Genet Couns 2002;11:265–76. 10.1023/A:1016378415514 [DOI] [PubMed] [Google Scholar]
- 43. Rimer BK, Briss PA, Zeller PK, et al. Informed decision making: what is its role in cancer screening? Cancer 2004;101:1214–28. 10.1002/cncr.20512 [DOI] [PubMed] [Google Scholar]
- 44. Green J, Hewison J, Bekker H, et al. Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review. Health Technol Assess 2004;8 10.3310/hta8330 [DOI] [PubMed] [Google Scholar]
- 45. Michie S, Dormandy E, Marteau TM. The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns 2002;48:87–91. 10.1016/S0738-3991(02)00089-7 [DOI] [PubMed] [Google Scholar]
- 46. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect 2001;4:99–108. 10.1046/j.1369-6513.2001.00140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van den Berg M, Timmermans DRM, ten Kate LP, et al. Informed decision making in the context of prenatal screening. Patient Educ Couns 2006;63:110–7. 10.1016/j.pec.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 48. Adams E, Hill E, Watson E. Fertility preservation in cancer survivors: a national survey of oncologists’ current knowledge, practice and attitudes. Br J Cancer 2013;108:1602–15. 10.1038/bjc.2013.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lampic C, Svanberg AS, Karlström P, et al. Fertility awareness, intentions concerning childbearing, and attitudes towards parenthood among female and male academics. Hum Reprod 2006;21:558–64. 10.1093/humrep/dei367 [DOI] [PubMed] [Google Scholar]
- 50. Hickman LC, Fortin C, Goodman L, et al. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018;23:130–8. 10.1080/13625187.2018.1455085 [DOI] [PubMed] [Google Scholar]
- 51. Ames AG, Jaques A, Ukoumunne OC, et al. Development of a fragile X syndrome (FXS) knowledge scale: towards a modified multidimensional measure of informed choice for FXS population carrier screening. Health Expect 2015;18:69–80. 10.1111/hex.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lewis C, Hill M, Skirton H, et al. Development and validation of a measure of informed choice for women undergoing non-invasive prenatal testing for aneuploidy. Eur J Hum Genet 2016;24:809–16. 10.1038/ejhg.2015.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benedict C, Thom B, N Friedman D, et al. Young adult female cancer survivors' unmet information needs and reproductive concerns contribute to decisional conflict regarding posttreatment fertility preservation. Cancer 2016;122:2101–9. 10.1002/cncr.29917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tluczek A, Henriques JB, Brown RL. Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait anxiety inventory. J Nurs Meas 2009;17:19–28. 10.1891/1061-3749.17.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait anxiety inventory (STAI). Br J Clin Psychol 1992;31:301–6. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 56. Breitsameter C. Medical decision-making and communication of risks: an ethical perspective. J Med Ethics 2010;36:349–52. 10.1136/jme.2009.033282 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.