Abstract
Introduction
In the UK and Ireland, severe and complex obesity is managed in specialist weight management services (SWMS), which provide multicomponent lifestyle interventions to support weight loss, and use of medication if available. Liraglutide 3 mg (LIRA 3 mg) is an effective weight-loss medication, but weight loss in individual patients is variable, and its efficacy has not been assessed in SWMS. This study aims to investigate whether a targeted prescribing pathway for LIRA 3 mg with multiple prespecified stopping rules could help people with severe obesity and established complications achieve ≥15% weight loss in order to determine whether this could be considered a clinically effective and cost-effective strategy for managing severe and complex obesity in SWMS.
Methods and analysis
In this 2-year, multicentre, open-label, real-world randomised controlled trial, 384 adults with severe and complex obesity (defined as body mass index ≥35 kg/m2 plus either prediabetes, type 2 diabetes, hypertension or sleep apnoea) will be randomised via a 2:1 ratio to receive either standard SWMS care (n=128) or standard SWMS care plus a targeted prescribing pathway for LIRA 3 mg with prespecified stopping rules at 16, 32 and 52 weeks (n=256).
The primary outcome is to compare the proportion of participants achieving a weight loss of ≥15% at 52 weeks with a targeted prescribing pathway versus standard care. Secondary outcomes include a comparison of (1) the weight loss maintenance at 104 weeks and (2) the budget impact and cost effectiveness between the two groups in a real-world setting.
Ethics and dissemination
The Health Research Authority and the Medicines and Healthcare products Regulatory Authority in UK, the Health Products Regulatory Authority in Ireland, the North West Deanery Research Ethics Committee (UK) and the St Vincent’s University Hospital European Research Ethics Committee (Ireland) have approved the study. The findings of the study will be published in peer-reviewed journals.
Trial registration number
ClinicalTrials.gov—Identifier: NCT03036800.
European Clinical Trials Database—Identifier: EudraCT Number 2017-002998-20.
Keywords: complex obesity, liraglutide 3 mg, saxenda, weight loss, specialist weight management services
Strengths and limitations of this study.
Large, multicentre, real-world, randomised controlled trial.
Assessment of the clinical effectiveness and cost effectiveness of a targeted prescribing pathway with three stopping rules for liraglutide (LIRA) 3 mg to optimise its use in obesity services.
The study will include only patients with severe and complex obesity (body mass index ≥35 kg/m2 plus either prediabetes, type 2 diabetes, hypertension or sleep apnoea) referred to a specialist weight management service (SWMS).
Some participants will stop using LIRA 3 mg due to the second and third stopping rules despite achieving clinically significant weight loss and metabolic benefits.
The study has been deliberately designed as open label; however, randomisation to control (standard care) group, without the opportunity to receive LIRA 3 mg, may be a disincentive to adherence to the SWMS programme and attendance to study visits for some participants.
Introduction
Obesity is a complex disease characterised by increased hunger and reduced satiety.1 Severe and complex obesity is defined as a body mass index (BMI) ≥35 kg/m2 with at least one major obesity-related complication.2 Around 10% of the adult population in England has a BMI ≥35 kg/m2,3 and many have established complications such as type 2 diabetes (T2D),4 hypertension5 and sleep apnoea,4 imposing colossal direct and indirect healthcare costs.6
Lifestyle interventions are considered cornerstones for the management of obesity.7 Despite the impressive results from intensive lifestyle interventions in Look Ahead study,8 and more recently the Diabetes Remission Clinical Trial (DIRECT) and Counterweight-Plus studies,9 10 which used intensive and structured weight management programmes to achieve weight loss and T2D remission in real-life community settings, lifestyle interventions still commonly only achieve an average of 5% wt loss11 12 and long-term weight maintenance remains a challenge.13 14 Although weight loss as little as 5% does produce metabolic improvements, it is not enough to make a difference to the lives of most people with severe and complex obesity. Maximal benefits for the treatment of obesity-associated complications are obtained with weight loss above 15%.15 Pharmacotherapy for obesity can support some people to achieve these results,16–18 but currently it is not used frequently enough to be considered a cornerstone treatment.7
Severe and complex obesity is managed in specialist weight management services (SWMS) in the UK and Ireland.7 11 19 20 SWMS consist of a multidisciplinary team typically led by a medical clinician with expertise in obesity management and/or a specialist dietician and supported by specialist physiotherapists, psychologists and nurses; these services offer intensive lifestyle interventions with similar components to those used by DIRECT and Look Ahead studies, and can be supported by pharmacotherapy.7 Orlistat is the only weight loss medication approved by the National Institute for Health and Care Excellence (NICE) for use in SWMS; however, its side effects and limited effectiveness18 21 have reduced the penetrance of its usage. SWMS support is usually required for up to 1 year, before patients may be offered bariatric surgery.11
Liraglutide 3.0 mg (LIRA 3 mg), a glucagon-like peptide-1 receptor analogue, was approved in 2015 by the European Medicines Agency (EMA) for the treatment of obesity in combination with lifestyle intervention after multiple, large, phase 3 randomised controlled trials demonstrated its safety and efficacy for weight loss, weight maintenance and improvement of obesity-related complications.17 22–24
The average weight loss in the Satiety and Clinical Adiposity - Liraglutide Evidence (SCALE) Obesity and Prediabetes trial at 1 year after initiation of treatment with LIRA 3 mg was 8.0%±6.7% compared with 2.6%±5.7% for patients in the placebo group.22 The same data also suggest that after 1 year of therapy, LIRA 3 mg can result in ≥15% wt loss in 14% of patients, compared with 3.5% of patients treated with placebo.22 Nevertheless, SCALE trials were placebo controlled, included patients with BMI as low as 27 kg/m2, and typically combined LIRA 3 mg with moderate-intensity lifestyle interventions.17 22–25 These limitations make it difficult to predict the clinical effectiveness of LIRA 3 mg for the treatment of severe and complex obesity in SWMS. Addressing these limitations, Wadden et al in a pragmatic, single-centre, open-label study demonstrated that by intensifying the diet and behavioural therapy in combination with LIRA 3 mg, 28% of participants were able to achieve ≥15% wt loss at 12 months compared with 12% with intensive behavioural therapy alone.26 Therefore, the current evidence suggests that LIRA 3 mg could help a number of patients with severe and complex obesity referred to SWMS to achieve significant weight reduction, and this is highly likely to generate substantial health benefits and long-term cost savings, especially if weight loss can be maintained, as demonstrated at the 3-year results of the SCALE Prediabetes study.25
Currently, LIRA 3 mg has not been approved by NICE for use in management of severe and complex obesity in the UK. NICE guidelines take into account both the clinical and the cost effectiveness of treatment. EMA has approved LIRA 3 mg with a single stopping rule of 5% wt loss at 16 weeks after initiation of treatment, based on a post-hoc analysis of the SCALE trials demonstrating that ‘early responders’ (defined as those achieved ≥4% wt loss after 16 weeks of LIRA 3 mg) were more likely to achieve clinically significant weight loss at 1 year compared with ‘early non-responders’ (10.8% vs 3.0% mean weight loss in those without T2D and 8.5% vs 3.1% mean weight loss in those with T2D).27 Based on the EMA single stopping rule, it is estimated that 62%–77% of patients with obesity referred to SWMS will be suitable to continue long-term on LIRA 3 mg.27 However, only 9%–21% of those who achieve ≥5% wt loss at 16 weeks will obtain maximal benefit (≥15% wt loss) from LIRA 3 mg.27 Taking into account the cost of the medication, it is unlikely that NICE or other equivalent organisations will approve long-term treatment with LIRA 3 mg for all patients who currently present for obesity treatment in SWMS and achieve the single EMA stopping rule. A different and more pragmatic use of LIRA 3 mg in SWMS is needed in order for the medication to be targeted to those who will benefit most from using it.
In this paper, we describe the rationale and methodology of a study investigating the effectiveness and cost of a targeted prescribing pathway (with multiple prespecified stopping rules) for the use of LIRA 3 mg in SWMS settings for treatment of severe and complex obesity. The targeted prescribing pathway aims to optimise the use of LIRA 3 mg in ‘early responders’ (in accordance with the EMA stopping rule) to the combination of lifestyle intervention plus LIRA 3 mg as well as identifying patients who are most likely to benefit more from continued long-term prescription of LIRA 3 mg (ie, those who are able to achieve ≥15% wt loss at 1 year). This approach aims to direct the use of this medication to patients with severe and complex obesity that will have substantial benefit from it, and at the same time to optimise the health economic outcomes (cost effectiveness and budget impact of SWMS) associated with the use of LIRA 3 mg.
Aim and objectives
The aim of the present study is to compare the clinical effectiveness, the cost effectiveness and the budget impact (on SWMS) of a targeted prescribing pathway for LIRA 3 mg (with prespecified stopping rules) plus SWMS standard care versus the SWMS standard care alone.
The primary objective will be to compare the proportion of participants with severe and complex obesity achieving weight loss ≥15% at 52 weeks using a targeted prescribing pathway (use of LIRA 3 mg according to a prespecified protocol) in combination with SWMS standard care versus SWMS standard care alone.
The secondary objectives (see also online supplementary appendix 1) are to compare the targeted prescribing pathway plus SWMS standard care versus SWMS standard care alone in terms of
bmjopen-2019-034137supp001.pdf (78.1KB, pdf)
improving obesity-related complications (prediabetes, T2D, hypertension, obstructive sleep apnoea, dyslipidaemia, depression) at 52 and 104 weeks.
referral rates to other obesity interventions at 52 and 104 weeks.
long-term weight maintenance (defined as the proportion of participants maintaining weight loss of ≥15% at 104 weeks among those who achieved ≥15% wt loss at 52 weeks).
budget impact on a SWMS at 52 and 104 weeks.
estimated cost effectiveness of treatment over 104 weeks.
direct healthcare costs in terms of admissions, frequency and cost of appointments at 52 and 104 weeks.
safety-related outcomes at 52 and 104 weeks.
adherence to treatment at 16, 32, 52 and 104 weeks.
patient satisfaction and quality of life at 52 and 104 weeks.
Methods and analysis
The protocol of this clinical trial follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines.28
Study design
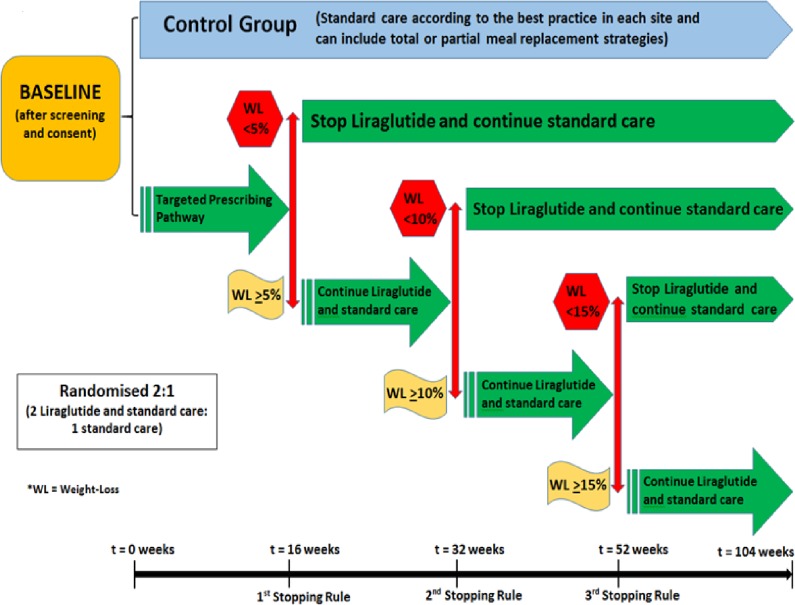
The current study (Effectiveness and cost of integrating a pragmatic pathway for prescribing liraglutide 3.0 mg in obesity services (STRIVE study)) is a phase 4, investigator-initiated, 2-year, parallel, two group, multicentre, open-label, real-world, randomised controlled trial taking place in the UK and Ireland. The total duration of participation will be 104 weeks (±2 weeks). In total, 384 patients with severe and complex obesity who are referred to a SWMS will be randomised through a validated online system (sealedenvelope.com) provided through the Leicester Clinical Trials Unit (LCTU) in a 2:1 fashion (2 intervention: 1 control) to either the intervention (SWMS standard care plus targeted prescribing pathway) or control arm (SWMS standard care; see also figure 1). Randomisation will be stratified by centre and BMI (>45 kg/m2; <45 kg/m2).
Figure 1.
Study design.
The study is intentionally designed to reflect a pragmatic ‘real-world’ scenario and each SWMS provider may require a different number of visits for their programme. However, study appointments for data collection, medication titration reviews, application of the stopping rules of LIRA 3 mg and dispensing of the medication will be standardised for all of the study sites.
The first 52 weeks of the study will determine whether using the targeted prescribing pathway of LIRA 3 mg plus standard care in a SWMS setting will result in more participants achieving ≥15% wt loss compared with participants in the control group (standard care alone). The second 52 weeks of the study will assess whether patients who lose ≥15% of their baseline weight by the first 52 weeks with the targeted prescribing pathway plus standard care are more likely to maintain ≥15% wt loss for another 52 weeks compared with patients in the control group (standard care alone) who were also able to achieve ≥15% wt loss by the first 52 weeks. Further measurements on budget impact of SWMS and cost effectiveness for both treatment groups will be assessed and compared.
Control (standard care)
Across the UK and Ireland, each region has a SWMS. This service includes a clinician-led multidisciplinary team approach, potentially including a specialist physician, dietitian, nurse, psychologist and physiotherapist/physical activity physiologist. The accessibility and the workflow of the SWMS are broadly similar for all the five participating sites in this study.
Participants in the control group will follow the best medical care provided by the SWMS at the relevant site. This typically involves dietary advice to reduce energy intake (and may include a period using formula-diet meal replacement or total diet replacement), accompanied—if available—by a physical activity programme, both supported by behavioural change techniques with regular professional contacts. The nature of the standard care will vary between the different SWMS at each site. Clinician input will include the medical assessment of participants for severe and complex obesity and the prescription of antiobesity drugs (ie, orlistat) as per local SWMS policy. Participants will remain in the SWMS in line with NICE guidance throughout the duration of the research study. Participants may be offered treatment options within the duration of the study, including bariatric surgery, as per NICE guidance and according to the decision of the local SWMS multidisciplinary team.
Intervention (targeted prescribing pathway plus standard care)
Participants in the intervention arm will receive the same SWMS care with the control arm. Additionally, at baseline, LIRA 3 mg will be prescribed to all of the participants in the intervention arm. Dose escalation of LIRA will occur in accordance with the summary of product characteristics (SPC) from 0.6 mg to a maximum of 3.0 mg. Participants will be withdrawn from receiving LIRA 3 mg if the doses are not tolerated during or following the titration period.
Participants will also be informed about the prespecified weight loss criteria in order to continue receiving treatment with LIRA 3 mg. Participants in the targeted prescribing pathway will be prescribed LIRA 3 mg for the duration of the study, unless they do not meet the predefined weight loss targets at each stopping point (after 16, 32 and 52 weeks, see also figure 1).
Targeted prescribing pathway stopping rules
First stopping rule
After 16 weeks (±14 days) on the medication, only those participants who have lost ≥5% of their baseline weight will be offered further treatment with LIRA 3 mg for another 16 weeks.
Second stopping rule
After 32 weeks (±14 days) on the medication, only those participants who have lost ≥10% of their baseline weight and are still on treatment with LIRA 3 mg will be offered another 20 weeks on LIRA 3 mg.
Third stopping rule
After 52 weeks (±14 days) on the medication, only those participants who have lost ≥15% of their baseline weight and are still on treatment with LIRA 3 mg will be offered another 52 weeks on LIRA 3 mg.
Participants who fail to reach the thresholds to continue LIRA 3 mg treatment, or who have withdrawn from LIRA 3 mg due to intolerance, adverse effects or inability to titrate up to a maximum dose of LIRA 3 mg, will continue to be offered the standard care provided by the relevant SWMS. The conditions for participants’ withdrawal from the study as well as for temporary and/or permanent treatment discontinuation for LIRA 3 mg (outside prespecified stopping rules) are described in online supplementary appendix 2.
bmjopen-2019-034137supp002.pdf (18.3KB, pdf)
Study outcomes
Primary outcome
The primary outcome is the proportion (%) of participants with severe and complex obesity achieving weight loss ≥15% of baseline weight at 52 weeks after randomisation with the standard care provided in SWMS versus a targeted prescribing pathway of LIRA 3 mg with prespecified stopping rules plus the standard care provided in SWMS.
Secondary measurements and outcomes
A range of secondary outcomes including anthropometrics, weight maintenance, obesity-associated comorbidities, budget impact, cost effectiveness, safety/adverse events, compliance of patients with treatment and referrals to other obesity interventions between the two groups will be measured and assessed throughout the study. These are listed in online supplementary appendix 1.
Study population
Five culturally and racially diverse sites in different geographical areas (Dublin, Glasgow, Leicester, Liverpool and London) will be used to reflect typical SWMS settings across the UK and Ireland.
Potentially eligible patients who have agreed to participate in the SWMS will be identified by the clinical care team and invited for screening. Patients will be allowed at least 24 hours to consider their participation to the study. Full inclusion and exclusion criteria are listed in box 1. Each site will plan to recruit up to 77 subjects over the 24-month recruitment period.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
be aged between 18 and 75 years old (inclusive)
understand written and spoken English
be able to give informed consent
have a body mass index ≥35 kg/m2
have been referred to the specialist weight management service in one of the participating sites
have a stable body weight (less than 5 kg self-reported change during the previous 12 weeks)
-
have at least one of prediabetes, type 2 diabetes (T2D), hypertension and/or obstructive sleep apnoea, as defined below:
prediabetes (defined as established diagnosis of impaired fasting glycaemia from general practitioner (GP) and/or established diagnosis of impaired glucose tolerance from GP and/or HbA1C 42–47 mmol/mol (6%–6.4%) without glucose-lowering medications, at a blood test during the last 6 months)
T2D (defined as established diagnosis of T2D from GP and/or HbA1C≥48 mmol/mol (≥6.5%) at a blood test during the last 6 months and/or being treated with any combination of lifestyle, metformin, sulphonylureas, thiazolidinediones or sodium-glucose co-tranporter-2 inhibitors)
hypertension treated (defined as being on antihypertensive treatment with or without a diagnosis of hypertension from GP) or untreated (defined as systolic blood pressure ≥140 mm Hg at two consecutive visits at the specialist weight management service clinic)
obstructive sleep apnoea (on Continuous Positive Airway Pressure (CPAP) or established diagnosis of Apnoea Hypopnoea Index≥15 at sleep studies during the last 12 months).
Exclusion criteria
Diagnosis of type 1 diabetes
T2D with treatment on Dipeptidyl peptidase-4 (DPP-4) inhibitors or insulin currently
Treatment with glucagon-like peptide-1 (GLP-1) receptor agonists within the last 6 months and/or have a history of GLP-1 receptor agonist intolerance.
Treatment with antiobesity drugs within 12 weeks prior to randomisation
eGFR ≤30 mL/min/1.73 m2 on serum testing over the last 26 weeks
Females referred to the clinic because of fertility problem
Females of childbearing potential who are pregnant, breast feeding or intend to become pregnant or are not using or willing to use adequate contraceptive methods during the study period
Have terminal illness
Are not primarily responsible for their own care
Any other significant disease or disorder, which in the opinion of the investigator, may either put the participants at risk or may influence the result of the study or the participant’s ability to participate
Untreated or uncontrolled hypothyroidism/hyperthyroidism defined as thyroid-stimulating hormone >6 mIU/L or <0.4 mIU/L
Family or personal history of multiple endocrine neoplasia type 2 or familial medullary thyroid carcinoma (FMTC)
Personal history of non-FMTC
History of chronic pancreatitis or idiopathic acute pancreatitis
Amylase levels three times higher than the upper normal range
Obesity induced by other endocrinologic disorders (eg, Cushing’s Syndrome)
Current or history of treatment with medications that may cause significant weight gain, within 12 weeks prior to randomisation, including systemic corticosteroids (except for a short course of treatment, that is, 7–10 days), atypical antipsychotic and mood stabilisers (eg, clozapine, olanzapine, valproic acid and its derivatives, and lithium)
Initiation of antidepressants during the last 12 weeks
Previous surgical treatment for obesity (excluding liposuction if performed >1 year before trial entry)
History of other severe psychiatric disorders
History of known or suspected abuse of alcohol and/or narcotics
History of major depressive episode during the last 2 years
Simultaneous participation in other clinical trials of investigational drugs, lifestyle or physical activity interventions. Patients will only be able to take part following participation in a previous clinical trial after a wash-out period of 16 weeks.
Study procedures
Study visits
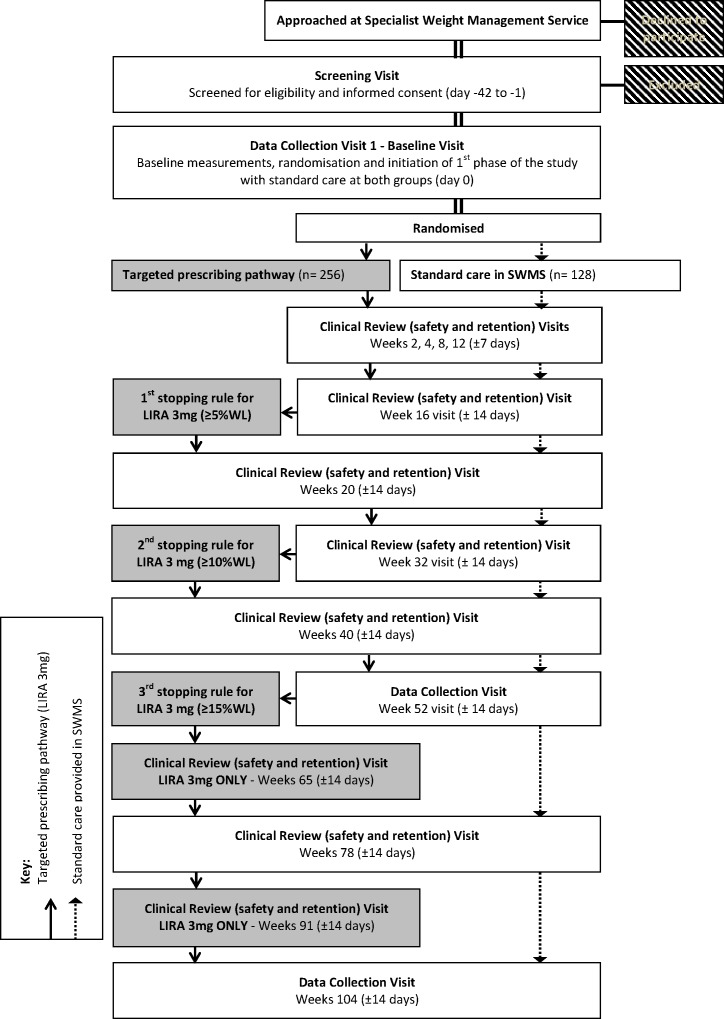
A participant flowchart is demonstrated in figure 2 and the study visit outline and measurement plan for each visit is included in online supplementary appendix 3, table 1. Participants in both groups will attend 13 visits during the 2-year trial. Participants in the intervention group who continue on LIRA 3 mg during the second year of the study will attend two additional visits for safety, monitoring of side effects, compliance and medication supply.
Figure 2.
Participant flowchart. LIRA, liraglutide; SWMS, specialist weight management service; WL, weight loss.
bmjopen-2019-034137supp003.pdf (226.4KB, pdf)
Screening and consent visit (day −42 to −1)
A study clinician will determine the eligibility of potential participants and obtain written informed consent (see online supplementary appendix 4). Following this, demographics, medical and surgical history, anthropometrics (height, weight, waist circumference), blood pressure and pulse rate, a comprehensive clinical examination and routine laboratory investigations will be performed (see online supplementary appendix 3, table 1). Female participants of childbearing potential will undergo a urine pregnancy test. Urine albumin creatinine ratio (ACR) will be analysed for participants with prediabetes, T2D and hypertension.
bmjopen-2019-034137supp004.pdf (636.7KB, pdf)
All participants will undergo an assessment with the King’s College Obesity Staging System (see online supplementary appendix, table 2), STOP-Bang Questionnaire and Epworth Sleepiness Score. Participants at risk of undiagnosed OSA (defined by STOP-Bang Score of ≥5) will be referred to sleep clinic for further investigation. Participants with established OSA on CPAP therapy will have their notes reviewed to determine compliance with CPAP therapy and whether it was a ‘successful’ treatment according to the following criteria: (1) CPAP usage ≥4 hours/night=successful, (2) CPAP usage <4 hours/night=struggling, and (3) CPAP usage ≤1 hour/night=failure.
bmjopen-2019-034137supp005.pdf (51.3KB, pdf)
Data collection and safety visits (baseline, weeks 52 and 104)
During the baseline visit, eligibility will be confirmed by the study clinician at each site based on the results and investigations from screening visit. Following this, only eligible participants will be randomised and allocated into either group (open-label study). Participants allocated to the targeted prescribing pathway plus standard care (intervention group) will be prescribed LIRA 3 mg and reminded of the prescribing pathway medication stopping rules at weeks 16, 32 and 52. Participants will receive training to confidently administer the Investigational Medical Product. Female participants of childbearing potential will be reminded to use effective contraception throughout the study.
A full description of the outcome measures and the procedures during baseline visit, week 52 and week 104, is shown in online supplementary appendix 3, table 1. Serious adverse events (SAE) and adverse events (AE) as well as changes in medication or diseases will be recorded during every visit from consent to the end of study. Moreover, participant adherence to the SWMS programme will be monitored (completers will be defined as those who attended >70% of scheduled visits in the SWMS during the first 52 weeks).
At weeks 52 and 104, blood tests for biochemical outcomes will be performed for all participants in the study as well as urine ACR for participants with T2D, prediabetes and hypertension.
At week 104, the study clinician will evaluate all the participants who experience significant weight regain in either treatment group and may consider a recommendation to tier 4 service for bariatric surgery. For ‘responders’ on LIRA 3 mg, the clinician will recommend that the participant’s GP may consider prescribing LIRA 3 mg long term, depending on the local prescribing practice.
Clinical review visits (safety and retention; weeks 2, 4, 8, 12, 16, 20, 32, 40 and 78)
Participant’s eligibility and consent for continuation in the study will be confirmed. Measurements of outcomes during these visits including reporting potential AEs/SAEs are detailed in online supplementary appendix 3, table 1. Participants on LIRA 3 mg will be given advice on dose titration (at weeks 2 and 4) as per SPC and will be monitored for any possible side effects and adherence with the daily injection (by examining medication returns).
At week 32, blood tests for biochemical outcomes will be performed for all participants in the study as well as urine ACR for participants with T2D, prediabetes and hypertension.
Additional clinical review (safety and retention) visits for LIRA 3 mg group (weeks 65 and 91)
Two additional visits will take place only for participants in the intervention group who remain eligible and continue to be offered treatment with LIRA 3 mg in the second year. The visits will be identical to the other clinical review visits as described above, with the intention to provide participants with a new prescription of LIRA 3 mg, discuss adherence with the treatment and record any AE/SAEs.
Treatment of trial participants
Study medication
Participants randomised to LIRA 3 mg group will be instructed to take daily subcutaneous injection of LIRA (Saxenda) while continuing their usual medication. Participants will be given specific instructions to adhere to the titration policy of LIRA 3 mg in accordance with the SpC. LIRA 3 mg will be prescribed to participants for a maximum of 106 weeks (4-week dose titration, 100-week maintenance dose, ±2-week visit window).
Compliance and accountability: participants will be asked to return all unused investigational products and vials/packages to the pharmacy at each study visit. Compliance and concordance with LIRA 3 mg will be evaluated and discussed at each study visit based on tolerability and returned vials of medication. Participants will be defined as treatment compliant if they self-administer at least 70% of planned doses.
Harms
Throughout the STRIVE study, all the reported AEs, SAEs and other unintended effects of trial interventions or trial conduct will be collected, assessed, reported and managed according to the Good Clinical Practice—International Conference of Harmonisation (GCP-ICH) guidelines.
Data collection, management and confidentiality
Details on data collection, management and confidentiality for the STRIVE study are provided in online supplementary appendix 6.
bmjopen-2019-034137supp006.pdf (103.5KB, pdf)
Statistics
Sample size
Based on previous studies, it is anticipated that at 1 year approximately 5% of the participants in the standard care group will have achieved ≥15% wt loss (likely range: 3%–5%).17 22 An achievable target for ≥15% wt loss at 52 weeks in the intervention group (standard care plus LIRA 3 mg with prespecified stopping rules) is 16% (likely range: 14%–20%).17 22 Accounting for 25% drop out, 5% alpha and a 2:1 randomisation ratio with higher proportion of participants being randomised to the intervention group; we would need to recruit 384 participants (256 intervention group; 128 standard care) to have 80% power to detect a significant difference between the groups in participants achieving ≥15% wt loss at 1 year.
Statistical methods and analysis
The primary analysis will compare the proportion of participants achieving ≥15% wt loss at 52 weeks (primary outcome) after randomisation between the two study arms using a logistic regression model with adjustment for stratification factors (site and BMI). The adjusted proportion (95% CI) of participants achieving ≥15% wt loss will be estimated by group. The primary analysis will be based on the complete case population. This is defined as all randomised participants who have data available for the outcome being analysed, according to the study group to which they were randomised at baseline.
The secondary analyses of the primary outcome will be based on intention-to-treat (ITT) and per-protocol analyses. The ITT population is defined as all randomised participants in the study and the per protocol population is defined as all randomised participants who were compliant with their treatment group. Treatment compliance for the control group is defined as completion at least 70% of the planned contacts in the SWMS. Participants in the intervention group are defined as treatment compliant if they complete at least 70% of the planned contacts in the SWMS, and take at least 70% of their planned doses of LIRA 3 mg as stipulated by the prescribing pathway. In all analyses, participants will be analysed within the treatment arm to which they were allocated at baseline. More specifically, participants within the intervention group who were taken off LIRA 3 mg due to prespecified stopping rule will remain in the intervention group. Participants who have undergone bariatric surgery during the first 52 weeks of the study will be excluded from the complete cases population analysis (primary analysis) and the per-protocol analysis for the primary outcome (but they will be included at the ITT analysis). Missing primary outcome data will be imputed for the ITT analyses; categorically, we will classify those participants as ‘non-responders’ (did not achieve ≥15% wt loss at 52 weeks). The characteristics of those with missing outcome data will be compared with those who have completed follow-up.
Secondary outcomes measured at 52 and 104 weeks will be analysed based on the complete cases population. Binary outcomes will be analysed using logistic regression models and summarised using proportion (95% CI). Continuous outcomes will be compared using linear regression models and summarised as mean with standard deviation (95% CI). If continuous outcomes are non-normally distributed, the most suitable regression model will be selected. Only the complete cases population will be used for the secondary outcomes to reduce the number of models. Participants who have undergone bariatric surgery during the first 52 weeks of the study will be excluded from the analyses for secondary outcomes at 52 weeks. Moreover, participants who have undergone bariatric surgery during the study period will be excluded from the analyses for secondary outcomes at 104 weeks. Data on treatment adherence, safety (including AEs), and treatment satisfaction will be summarised and tabulated.
In addition, we will perform a responder analysis which will repeat the analyses of the secondary outcomes with the intervention group restricted to those participants who responded to the targeted prescribing pathway (those achieving ≥15% wt loss at 52 weeks after randomisation). This allows us to statistically compare the outcomes of ‘super’ responders within the targeted prescribing pathway to all the participants who received standard care in the control group.
Health economic input
A state-transition Markov cohort model has been developed for the cost-effectiveness analysis, with health states encompassing the possible comorbidities associated with obesity and documented to respond to weight loss. A thorough review of the literature was conducted to identify such conditions and inform transition probabilities in the model.
The model projects development of T2DM, myocardial infarction, stroke, asthma cancer or mortality in the long term (up to lifetime horizon) based on short-term effects of interventions in surrogate outcomes—BMI, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol and (for patients with T2D) glycated haemoglobin (HbA1c). Effects on surrogate outcomes are translated into lifetime risks through risk-prediction models.
Treatment effects are applied incrementally to the efficacy of the targeted prescribing pathway group. Effect on weight, in terms of BMI % reduction, is applied at every subsequent cycle starting from 3 months after treatment start for as long as the cohort remains on treatment and over the predefined catch-up time post treatment. Note that a natural increase in weight is applied each year to all interventions considered, including no treatment.
Treatment can be discontinued at 16 weeks, 32 weeks or at 52 weeks (based on the prespecified stopping rule). Discontinuation causes the cohort to receive the standard care provided via the SWMS at the control group. Thus, if the discontinuation is allowed at 16 weeks, ‘non-responders’ will have the same effect of the cohort in the control group, and ‘responders’ will have the effect of the targeted prescribing pathway in the clinical trial.
Discontinuation of treatment beyond 52 weeks causes BMI, and other cardiometabolic risk factors, to revert to the levels projected under the no treatment option. Reversal takes place over a given number of cycles (years). Costs and quality-of-life outcomes are applied to the health state in which the cohort resides at each particular moment in time or once off to events. Costs and health benefits (life years, quality-adjusted life years, years T2D free, years cancer free, etc.) are summed up for the time horizon of the model and results reported as incremental cost-effectiveness ratios. The risk of acquiring obesity-related comorbidities has been taken from published epidemiological studies.
Patient and public involvement
Patients and public were not involved in the development of the research questions or the design of this study. However, on completion of the study, all participants will be informed for the results of the study.
Ethics and dissemination
Ethical issues
Approval for the protocol (and any subsequent substantial amendments) has been obtained from the Medicines and Healthcare products Regulatory Agency (UK Competent authority) and Health Products Regulatory Authority (Irish Competent Authority). This protocol has been approved by the Health Research Authority (HRA) and ethical approval as a Clinical Trial of an Investigational Medicinal Product study was granted by the North West Deanery National Research Ethics Service (NRES) committee (17/NW/0517) in UK (29 September 2017) and from the St Vincent’s University Hospital European Research Ethics Committee (EUREC) (2017-002998-20) in Ireland. This manuscript details the fourth version of the protocol approved on 8 June 2018.
The study is conducted in accordance with the principles laid down by the 18th World Medical Assembly (Helsinki, 1964) and all applicable amendments laid down by the World Medical Assemblies, as well as in full conformity with the GCP-ICH guidelines (CPMP/ICH/135/95) July 1996. Ethical and research governance approval has been obtained for this study through the HRA, appropriate regulatory bodies and National Health Service Trust prior to any participant activity.
Protocol amendments
All changes to the study protocol were reviewed by the North West Deanery NRES committee in UK and from the St Vincent’s Hospital EUREC in Ireland and then reported to the sponsor and funder. The participating sites and coinvestigators were sent regular emails with updates on the study recruitment timeline and any major protocol changes during the enrolment period. All significant protocol changes were noted in ClinicalTrials.gov.
Trial oversight and governance
The study sponsor is the University of Leicester (UK) and the study is managed by the LCTU and is overseen by trial steering committee (TSC) comprising independent chair and a group of experts. This study is registered at the http://www.clinicaltrials.gov (NCT03036800) as well as on the European Clinical Trials Database (EudraCT Number 2017-002998-20) before the enrolment of the first participant and monitored by the University of Leicester.
Safety of the participants will be independently monitored by the Data Monitoring and Ethics Committee, which will make recommendations to the TSC for appropriate action in the interest of the participants.
Current status and time scale
Recruitment started in November 2017 and the last patient last visit should complete by December 2021. To date, 380 patients have been screened and consented, of which 351 were eligible to participate and 337 have been randomised to the study.
Dissemination plan
The results of the study will be presented in national and international meetings and will be submitted for publication to the relevant Obesity/General Medical peer-reviewed journals. Acknowledgement of any supporting organisations, including funders, University of Leicester and the LCTU, will be included.
Supplementary Material
Footnotes
Contributors: DP: coinvestigator at University of Leicester, trial coapplicant, primary author of manuscript, coauthor of the approved study protocol. WA-N: coauthor at University College Dublin, contributed to writing the manuscript and approved the final version. JL: coauthor at University of Liverpool, contributed to writing the manuscript and approved the final version. JC: provided critical appraisal of current manuscript and approved final version. ML: principal investigator at University of Glasgow, trial coapplicant, coauthor for approved study protocol, contributed to trial set-up and design, provided critical appraisal of the manuscript and approved the final version. ClR: principal investigator at University of Dublin, coauthor of the approved study protocol, contributed to trial set-up and design, provided critical appraisal of the manuscript and approved the final version. BM: principal investigator at Guy’s and St Thomas’ NHS Foundation Trust, trial coapplicant, coauthor for approved study protocol, contributed to trial set-up and design, provided critical appraisal of the manuscript and approved the final version. DO: principal investigator at University of Dublin, trial coapplicant, coauthor for approved study protocol, provided critical appraisal of the manuscript and approved the final version. DW: principal investigator at University of Leicester, trial coapplicant, contributed to trial set-up and design, coauthor for approved study protocol, provided critical appraisal of the manuscript and approved the final version. JW: principal investigator at University of Liverpool, trial coapplicant, contributed to trial set-up and design, coauthor for approved study protocol, provided critical appraisal of the manuscript and approved the final version. MJD: chief investigator, trial coapplicant, contributed to trial set-up and design, coauthor for approved study protocol, corresponding author, provided critical appraisal of the manuscript and approved the final version.
Funding: This study is investigator-initiated and the investigators received a grant from Novo Nordisk to enable them to conduct the study. Novo Nordisk provided also the medication for the study. The funder (Novo Nordisk) did not contribute to study design and will not contribute to data collection, analyses and interpretation of the results.
Competing interests: DP is funded from an NIHR Clinical Lectureship and reports grants from the Novo Nordisk UK Research Foundation outside submitted work; WA-N is funded by the Irish Research Council’s Postdoctoral Enterprise Partnership Scheme and reports personal fees from Novo Nordisk outside the submitted work; JL has nothing to disclose; JC reports participation at the Novo Nordisk Emerging Obesity Leaders Programme which included registration and travel expenses to the European Congress of Obesity 2019, Glasgow, funded by Novo Nordisk; ML reports grants and personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Eli Lilly, personal fees from Counterweight, personal fees from Roche, outside the submitted work; ClR reports grants from Science Foundation Ireland, grants from Health Research Board, during the conduct of the study; other from Novo Nordisk, other from GI Dynamics, personal fees from Eli Lilly, grants and personal fees from Johnson and Johnson, personal fees from Sanofi Aventis, personal fees from Astra Zeneca, personal fees from Janssen, personal fees from Bristol-Myers Squibb, personal fees from Boehringer-Ingelheim, outside the submitted work; BM reports grants from Novo Nordisk, during the conduct of the study; grants and personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Boheringher Ingelheim, personal fees from MSD, outside the submitted work; DO has nothing to disclose; DW has nothing to disclose; JW reports grants from Novo Nordisk, during the conduct of the study; grants, personal fees and other from AstraZeneca, other from Astellas, personal fees and other from Boehringer Ingelheim, other from Janssen, personal fees and other from Mundipharma, personal fees and other from Napp, personal fees and other from Lilly, personal fees and other from Sanofi, other from Wilmington Healthcare, outside the submitted work; MJD reports grants from Novo Nordisk, during the conduct of the study; personal fees from Novo Nordisk, personal fees from Sanofi-Aventis, personal fees from Lilly, personal fees from Merck Sharp & Dohme, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Janssen, personal fees from Servier, personal fees from Mitsubishi Tanabe Pharma Corporation, personal fees from Takeda Pharmaceuticals International, grants from Sanofi-Aventis, grants from Lilly, grants from Boehringer Ingelheim, grants from Janssen, outside the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bray GA, Kim KK, Wilding JPH, et al. . Obesity: a chronic relapsing progressive disease process. A position statement of the world obesity Federation. Obes Rev 2017;18:715–23. 10.1111/obr.12551 [DOI] [PubMed] [Google Scholar]
- 2. Welbourn R, Hopkins J, Dixon JB, et al. . Commissioning guidance for weight assessment and management in adults and children with severe complex obesity. Obes Rev 2018;19:14–27. 10.1111/obr.12601 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad A, Laverty AA, Aasheim E, et al. . Eligibility for bariatric surgery among adults in England: analysis of a national cross-sectional survey. JRSM Open 2014;5:204253331351247 10.1177/2042533313512479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gatineau M, Hancock C, Holman N. Public health England adult obesity and type 2 diabetes, 2014. [Google Scholar]
- 5. Nieto FJ, Young TB, Lind BK, et al. . Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. sleep heart health study. JAMA 2000;283:1829–36. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 6. Scarborough P, Bhatnagar P, Wickramasinghe KK, et al. . The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006-07 NHS costs. J Public Health 2011;33:527–35. 10.1093/pubmed/fdr033 [DOI] [PubMed] [Google Scholar]
- 7. Wilding JPH. Beyond lifestyle interventions: exploring the potential of anti-obesity medications in the UK. Clin Obes 2018;8:211–25. 10.1111/cob.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Look AHEAD Research Group Eight-Year weight losses with an intensive lifestyle intervention: the look ahead study. Obesity 2014;22:5–13. 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lean MEJ, Leslie WS, Barnes AC, et al. . Primary care-led weight management for remission of type 2 diabetes (direct): an open-label, cluster-randomised trial. The Lancet 2018;391:541–51. 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 10. McCombie L, Brosnahan N, Ross H, et al. . Filling the intervention gap: service evaluation of an intensive nonsurgical weight management programme for severe and complex obesity. J Hum Nutr Diet 2019;32:329–37. 10.1111/jhn.12611 [DOI] [PubMed] [Google Scholar]
- 11. Jennings A, Hughes CA, Kumaravel B, et al. . Evaluation of a multidisciplinary tier 3 weight management service for adults with morbid obesity, or obesity and comorbidities, based in primary care. Clin Obes 2014;4:254–66. 10.1111/cob.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alkharaiji M, Anyanwagu U, Donnelly R, et al. . Tier 3 specialist weight management service and pre-bariatric multicomponent weight management programmes for adults with obesity living in the UK: a systematic review. Endocrinol Diab Metab 2019;2:e00042 10.1002/edm2.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;99:14–23. 10.3945/ajcn.113.070052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med 1993;119:688–93. 10.7326/0003-4819-119-7_Part_2-199310011-00012 [DOI] [PubMed] [Google Scholar]
- 15. Cefalu WT, Bray GA, Home PD, et al. . Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors' expert forum. Diabetes Care 2015;38:1567–82. 10.2337/dc15-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montesi L, El Ghoch M, Brodosi L, et al. . Long-Term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes 2016;9:37 10.2147/DMSO.S89836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wadden TA, Hollander P, Klein S, et al. . Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the scale maintenance randomized study. Int J Obes 2013;37:1443–51. 10.1038/ijo.2013.120 [DOI] [PubMed] [Google Scholar]
- 18. Douglas IJ, Bhaskaran K, Batterham RL, et al. . The effectiveness of pharmaceutical interventions for obesity: weight loss with orlistat and sibutramine in a United Kingdom population-based cohort. Br J Clin Pharmacol 2015;79:1020–7. 10.1111/bcp.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Institute for Health and Care Excellence Obesity: identification, assessment and management. Available: https://www.nice.org.uk/guidance/cg189/chapter/1-recommendations [Accessed 12 Feb 2019]. [PubMed]
- 20. Hughes CA. The rewards and challenges of setting up a tier 3 adult weight management service in primary care. Br J Obes 2015;1:25–31. [Google Scholar]
- 21. Torgerson JS, Hauptman J, Boldrin MN, et al. . XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–61. 10.2337/diacare.27.1.155 [DOI] [PubMed] [Google Scholar]
- 22. Pi-Sunyer X, Astrup A, Fujioka K, et al. . A randomized, controlled trial of 3.0 Mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 23. Davies MJ, Bergenstal R, Bode B, et al. . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the scale diabetes randomized clinical trial. JAMA 2015;314:687–99. 10.1001/jama.2015.9676 [DOI] [PubMed] [Google Scholar]
- 24. Blackman A, Foster GD, Zammit G, et al. . Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes 2016;40:1310–9. 10.1038/ijo.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. le Roux CW, Astrup A, Fujioka K, et al. . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. The Lancet 2017;389:1399–409. 10.1016/S0140-6736(17)30069-7 [DOI] [PubMed] [Google Scholar]
- 26. Wadden TA, Walsh OA, Berkowitz RI, et al. . Intensive behavioral therapy for obesity combined with liraglutide 3.0 Mg: a randomized controlled trial. Obesity 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujioka K, O'Neil PM, Davies M, et al. . Early weight loss with liraglutide 3.0 Mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity 2016;24:2278–88. 10.1002/oby.21629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan A-W, Tetzlaff JM, Altman DG, et al. . Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034137supp001.pdf (78.1KB, pdf)
bmjopen-2019-034137supp002.pdf (18.3KB, pdf)
bmjopen-2019-034137supp003.pdf (226.4KB, pdf)
bmjopen-2019-034137supp004.pdf (636.7KB, pdf)
bmjopen-2019-034137supp005.pdf (51.3KB, pdf)
bmjopen-2019-034137supp006.pdf (103.5KB, pdf)