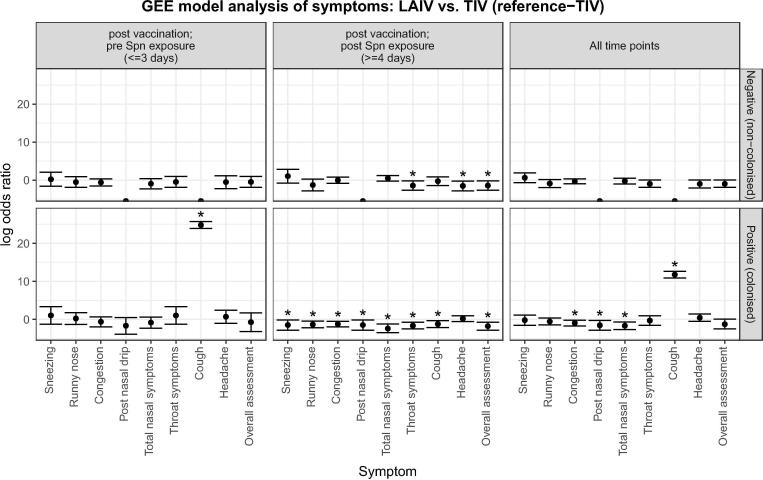
Fig. 4.
Antecedent study- Symptoms reported by participants receiving nasal LAIV (colonised/positive n = 24; non colonised/negative n = 29) compared with those receiving intramuscular TIV (colonised/positive n = 23; non colonised/negative n = 38). Timepoints are split up as until day of Spn inoculation, post Spn inoculation or all timepoints combined. Bars represent log10 transformed odds ratios with 95% confidence intervals. Asterisks denote p < 0.05 i.e. a statistically significant difference in the rate of reporting between the two arms of the trial. Odds ratios are derived from GEE model analysis (see methods), and are presented as those occurring early (left panels, at or before 3 days after vaccination and pre Spn inoculation), or late (centre panels, 4 or more days after vaccination and following Spn inoculation), or combined throughout the period of reporting (right panels). Reported symptoms are subdivided by their colonisation status: negative (no colonisation, upper panels); positive (colonised, lower panels). Spn = S. pneumoniae.