Abstract
Objective
This study aimed to assess the cost-effectiveness of telehealthcare in heart failure patients as add-on to usual care.
Design
A cost-utility analysis was conducted from a public payer perspective alongside the randomised controlled TeleCare North trial.
Setting
The North Denmark Region, Denmark.
Participants
The study included 274 heart failure patients with self-reported New York Heart Association class II-IV.
Interventions
Patients in the intervention group were provided with a Telekit consisting of a tablet, a digital blood pressure monitor, and a scale and were instructed to perform measurements one to two times a week. The responsibility of the education, instructions and monitoring of the heart failure (HF) patients was placed on municipality nurses trained in HF and telemonitoring. Both groups received usual care.
Outcome measures
Cost-effectiveness was reported as incremental net monetary benefit (NMB). A micro-costing approach was applied to evaluate the derived savings in the first year in the public health sector. Quality-adjusted life-years (QALY) gained were estimated using the EuroQol 5-Dimensions 5-Levels questionnaire at baseline and at a 1-year follow-up.
Results
Data for 274 patients were included in the main analysis. The telehealthcare solution provided a positive incremental NMB of £5164. The 1-year adjusted QALY difference between the telehealthcare solution and the usual care group was 0.0034 (95% CI: −0.0711 to 0.0780). The adjusted difference in costs was -£5096 (95% CI: −8736 to −1456) corresponding to a reduction in total healthcare costs by 35%. All sensitivity analyses showed the main results were robust.
Conclusions
The TeleCare North solution for monitoring HF was highly cost-effective. There were significant cost savings on hospitalisations, primary care contacts and total costs.
Trial registration number
ClinicalTrials.gov: NCT02860013.
Keywords: health economics, heart failure, telemedicine, health informatics
Strengths and limitations of this study.
This study should be relevant for decision-makers at the national healthcare level as well as at the clinical level.
It is the first economic evaluation of telehealthcare in heart failure patients that strictly follows international guidelines for health economic evaluation alongside clinical trials.
Precise assessment of the economic costs was allowed through patient specific data and detailed registration of operational as well as capital costs of telehealthcare.
No evidence was provided, however, on the long-term cost-effectiveness or on the explanation of what components of the intervention were actually effective or whether the effect was contingent on the intervention in its entirety.
Trial-based economic evaluations are limited by truncated time horizons, difficulty in generalising to other settings, and failure to incorporate evidence from other trials or observational studies.
Introduction
Heart failure (HF) is a common chronic disease with an estimated global prevalence of approximately two per cent.1–3 In Denmark, approximately 9000 patients are diagnosed with HF each year. The incidence increases with higher age, and it has been estimated that one in five individuals will develop HF during their lifetime.2–4 In total, the condition is conservatively estimated to affect approximately 66 000 citizens in Denmark, and about five per cent of all Danish citizens above the age of 75 have been diagnosed with HF.4 5 The prevalence of HF is, however, expected to rise in the future due to, among others, a higher prevalence of predisposing factors, such as hypertension, diabetes and obesity but also due to the increased longevity of patients with HF, which is likely the result of an improved treatment of the condition.1 2 6
HF symptoms include dyspnoea, fatigue, lethargy and oedema.3 4 The severity of patients’ HF is often described according to the New York Heart Association (NYHA) functional classification system, which may be used by patients to classify the severity of their HF according to their own experience of the condition. Class I indicates that the condition does not limit physical activity and that ordinary activity does not cause any symptoms. In higher classes, the symptoms reported are increasingly more severe; thus, in class IV, patients cannot perform physical activity without experiencing symptoms, or they experience symptoms even at rest.3 7 HF is believed to impair patients’ health-related quality of life (HRQoL) compared with individuals without the condition, and the condition entails a substantially increased mortality.2 8–10 In addition to the personal burden that HF entails, the condition also causes a substantial burden on healthcare systems worldwide, accounting for approximately two per cent of total healthcare expenditures.2 11 Hospitalisations are recognised as the primary driver for the total costs related to HF, though outpatient visits also constitute a substantial part.2 12
In 2016, the European Society of Cardiology published updated guidelines for the diagnosis and treatment of acute and chronic HF,13 emphasising the beneficial impact of continuous monitoring of, among others, biomedical parameters to enable the detection of the development of complications and disease progression that may prompt changes to patients’ disease management. In the guidelines, telehealthcare is mentioned as a possible means of monitoring patients.13 Evidence suggests that telehealthcare in different forms may be beneficial in the management of HF, both for the improvement of patients’ HRQoL but also in the prevention of, for example, hospitalisations and all-cause mortality.14–16 Findings, however, are inconsistent,13 17 which might be ascribed to the fact that the components of the investigated telehealthcare solutions differ. Effectively, this heterogeneity makes the various telehealthcare solutions incomparable in terms of their design, effectiveness and, consequently, cost-effectiveness.15–17 A number of reviews16–18 have requested more high-quality studies of the health economic consequences of telehealthcare interventions. To our knowledge, however, up until now, no cost-effectiveness analyses of telehealthcare in HF patients have been conducted according to international good practice guidelines for the economic evaluation alongside clinical trials.19 20
In Denmark, a national strategy has been formulated for the introduction of telehealthcare as a means of reducing healthcare costs while also providing patients with greater HRQoL and the feeling of improved control of their disease.21 22 In this respect, the North Denmark Region has played a major role in the formulation of the national strategy by performing prelaunch, large-scale randomised controlled trials and health economic evaluations and national business cases as decision-support for the nationwide implementation.23 24 In the wake of the first TeleCare North trial directed at patients suffering from chronic obstructive pulmonary disease (COPD), which was executed in 2014 to 2015,23 24 the TeleCare North Heart Failure (HF) trial was launched in 2016 with the purpose of evaluating the effectiveness and cost-effectiveness of a telehealthcare solution directed at patients with HF.25 The purpose of this economic evaluation is to evaluate the cost-effectiveness of the TeleCare North HF solution, comparing the impact on costs and effects (ie, quality-adjusted life-years (QALYs)) with that of the usual practice for the treatment of HF in Denmark.
Methods
The cost-utility analysis was conducted in accordance with international guidelines for health economic evaluations alongside clinical trials.19 20 26 All clinical and cost data for the analysis were collected alongside the TeleCare North HF trial, and the time horizon for the analysis was restricted to a 1-year period. A Danish public healthcare sector perspective was applied, including costs accumulating under the auspices of the regional healthcare (ie, pre-hospital services and inpatient and outpatient services in somatic and psychiatric healthcare), municipality-based health and social care (eg, home care services and rehabilitation) and primary healthcare (eg, general practice and physiotherapy) and costs associated with purchases of prescription medicine at Danish pharmacies. Costs associated with patient-paid or relative-paid transportation and productivity costs were not included.
The trial protocol presenting the design of the TeleCare North HF Trial and associated economic evaluation has previously been published.25 The participants in the intervention group received patient education and telehealthcare equipment for continuous monitoring of physiological measurements. Patients in the intervention group were provided with a Telekit consisting of a tablet, a digital blood pressure monitor and a scale, and were instructed to perform measurements one to two times a week. The responsibility for the education, instructions and monitoring of the HF patients was placed on municipality nurses trained in HF and telemonitoring. The nurses were given the authority to intervene and change medication if, for instance, measurements indicated a deterioration in the patient’s health. The specialised nurses could contact the heart failure clinic at the central university hospital for guidance regarding specific patient issues. Patients in the control group received the usual care, where general practitioners were responsible for the monitoring of the patients (see the online supplementary appendix A for elaboration).
bmjopen-2019-031670supp001.pdf (79.2KB, pdf)
The result of the economic evaluation is expressed as the incremental net monetary benefit (NMB) = (),27 where ΔQALY is the incremental health-related benefit and ΔCost is the incremental costs. Under the assumption of a cost-effectiveness threshold (Rt) of £20 000 per QALY gained, an incremental NMB >0 indicates that the telehealthcare solution is cost-effective compared with the usual care.26
The cost-effectiveness of the telehealthcare solution is estimated for a 12-month period starting 30 days after participant enrolment in the study. This 30-day ‘blanking period’ for both groups was introduced from the day of referral to accommodate that participants in the intervention group would only receive the telehealthcare solution belatedly compared with the referral date and therefore effectively did not receive any intervention in this period. The difference in follow-up length was accommodated for in the estimation of cost and effect accumulation by weighting the accumulation by the lengths of the follow-up of individual participants to represent a 12-month follow-up. The enrolment period started on 1 September 2016 and the follow-up period ended on 4 March 2018.
Cost accumulation
All costs are presented in 2018 values in British Pounds Sterling (£). The Danish consumer price index for healthcare products and services28 was used to adjust the cost data from 2016 and 2017 to the price level in 2018. Costs were estimated in Danish Krone (DKK) and subsequently converted, based on a conversion rate of DKK 827.19 per £100 from 31 December 2018.29
Healthcare service use and healthcare costs
Patient-specific costs related to healthcare service use were estimated based on register data. In Denmark, all citizens are provided with a unique personal identification number at birth or immigration, which enables the linkage of information from various registers at the individual level. Information on patients’ gender, birthday, migration status and vital status was retrieved from the Danish Civil Registration System.30
Information on patients’ use of prescription medicine was retrieved from the Danish National Prescription Registry. The costs related to prescription medicine were valued at pharmacy selling prices excluding value added tax.
Patients’ contacts with general practice were identified through the National Health Insurance Service Register.31 32 The costs associated with the contacts to general practice are registered in the registry and based on fees quoted in a collective agreement negotiated with the Danish Medical Association.33
Information on patient hospitalisations was retrieved from the Danish National Patient Registry, which holds information on all inpatient, outpatient and emergency hospitalisations in somatic and psychiatric wards in Denmark.34 In the registry, each contact is valued according to the designated diagnosis-related group used for reimbursement, the actual procedures performed and the length of stay in relation to the contact.
Estimates of the resource consumption of community care services in the municipalities were based on detailed registrations from 4 of the 11 contributory municipalities (the administrative units for tax-financed local health and social care). For patients in both groups, registrations included all local care activities, such as personal care, practical help, home nursing, rehabilitation and telehealthcare activities. To increase generalisability to other settings in Denmark, the registered time consumption for standard care activities was valued using the national average effective hourly wage of the municipality nurses without managerial responsibility.35 Time consumption in relation to rehabilitation consisting of physiotherapy was valued using the national average effective hourly wage of the municipality and regional physiotherapists without managerial responsibility.35 Days of respite care in relation to rehabilitation were valued according to the estimated expenses of a day in care homes (see online supplementary appendix B).36 37
bmjopen-2019-031670supp002.pdf (93.1KB, pdf)
Information on trial participants’ healthcare service use and healthcare costs was retrieved for 12 months following their individual study start-up date (30 days after the date of their enrolment). Information on health service use and healthcare costs was retrieved for the participants from 12 months before the study start date for each participant to control for differences in healthcare utilisation before the start of the intervention.
Telehealthcare intervention costs
The administrative office for TeleCare North provided a detailed registration of all intervention costs (see online supplementary appendix B). Capital costs included the development of software and hardware modifications for the Telekit, the delivery of and the Telekit itself and one-time start-up costs related to the education of patients and healthcare professionals. In the analysis, capital costs were annuitised over a period of 5 years with a discount rate of four per cent per annum and included as equivalent annual costs. The useful equipment lifetime and applied discount rate are in accordance with what applies for ‘other information technology equipment’ in Danish capital accounting.38 39 Operational costs included, among other things, maintenance, support and licenses. The daily work with continuous monitoring of the patients was included in the municipalities’ registrations of healthcare service use and healthcare costs described above.
Software development and hardware configuration were valued as prices paid to an external supplier, reflecting actual tenders. The Telekit was valued based on the expected purchase price if the intervention were to be implemented and used in real-life practice following the results of the TeleCare North HF trial. The delivery of hardware, running costs related to licenses, handling of assets, data charges and substitution of malfunctioning equipment were valued as the price negotiated and paid to the external supplier.
Before the trial, various meetings and educational seminars were held to train healthcare professionals in the use of the telehealthcare solution and monitoring duties and to increase their general knowledge on the management of HF, rehabilitation and palliation. Participants in these meetings and seminars included general practitioners and regional and municipality nurses. In addition, meetings were held informing project managers, key persons and healthcare professionals on the telehealthcare solution and the implementation of the intervention. The per-patient costs of educating healthcare professionals and others were estimated based on the planned time spent in the meetings, the number of participants at the meetings and the average effective hourly wage of the participants. The applied average effective hourly wages were estimated based on national average wages to increase generalisability to the other regions in Denmark.35
Costs of modifications of the hardware, software development and education for healthcare professionals and management staff were allocated to all HF patients who would be offered the telehealthcare solution in the North Denmark Region. The number of HF patients in the North Denmark Region was estimated to be 6700, given an estimated prevalence of 66 000 HF patients in Denmark4 and that approximately 10% of the Danish population resides in the North Denmark Region.
The annual operational costs of telehealthcare were allocated to the estimated number of HF patients and other patients using the regional telehealth system in the North Denmark Region (10 500 patients24). The operational costs were valued as prices paid.
Measure of effectiveness
Information on patients’ HRQoL was collected from questionnaires at baseline and at the end of the follow-up. Index scores for participants’ HRQoL were estimated based on the EuroQol 5-Dimensions 5-Levels (EQ-5D-5L) questionnaire. Currently, however, there are no Danish societal weights estimated for the EQ-5D-5L questionnaire for which reason the responses in the EQ-5D-5L questionnaire were used to predict responses in the EuroQol 5-Dimensions 3-Levels (EQ-5D-3L) questionnaire by applying a response mapping approach.40 41 Danish societal weights for the EQ-5D-3L questionnaire were subsequently applied.42 Information on mortality was retrieved from the Danish Registry of Causes of Death, which holds information on all causes of death in Denmark. Information on participants’ HRQoL and relevant demographic characteristics were collected at baseline at participant enrolment in the outpatient clinics or after the participant returned home, if preferred by the patient.25 Irrespective of where the data were collected, the time of collection was dated to be 30 days after the date of their enrolment. At the end of the follow-up, the EQ-5D-5L questionnaire was sent in paper form to patients’ home addresses from the trial administration office. A prepaid return envelope was included. The response was dated to the end of follow-up (4 March 2018).43
Linear interpolation of the utility scores from baseline to follow-up was performed to estimate the QALY gain and was scaled to represent the QALY gain within 1 year. The utility score for patients who died during follow-up was set to 0 at the time of death.
Analysis
Missing data management
In accordance with good research practice guidelines within effectiveness and cost-effectiveness studies, the primary analysis was performed according to the intention-to-treat principle and imputation was performed to account for missing data.19 20 44 45 Imputations of missing data for the primary analysis was performed in accordance with the methods for multiple imputations described by Faria et al.20 A full description of the imputation approach is provided in the online supplementary appendix C.
bmjopen-2019-031670supp003.pdf (46KB, pdf)
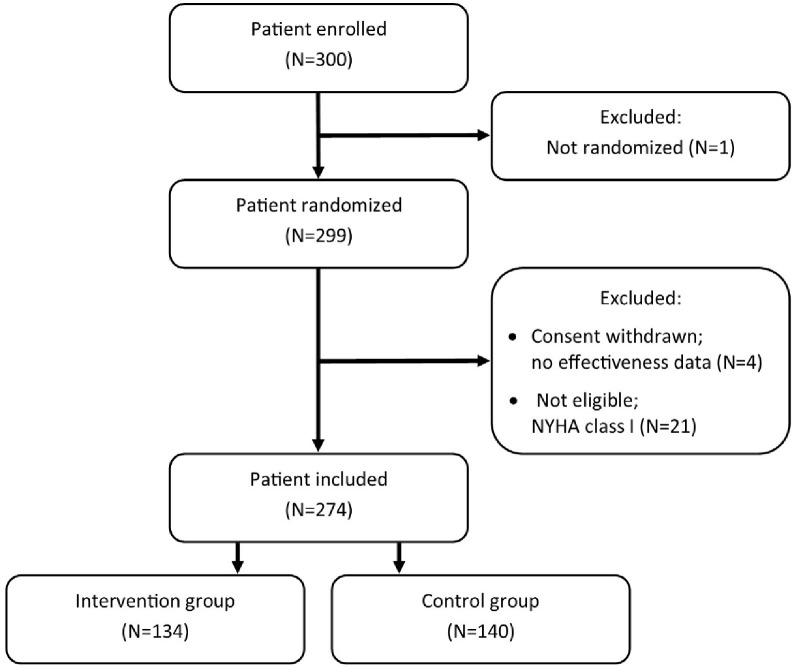
In total, 299 participants were enrolled in the trial (intervention group n=145, control group n=154) (see figure 1). One patient was enrolled but was never randomised to any treatment group and, therefore, not included in the study. Four patients did not return questionnaires at either baseline or follow-up due to withdrawal shortly after enrolment for which reason no data were available on them except basic registry information. As no effectiveness data were available for these patients and they did, de facto, not participate in the study, they were excluded from analyses in accordance with guidelines on post-randomisation exclusion.46 Furthermore, 21 patients with a self-reported NYHA I classification were wrongfully included in the randomisation. Given the eligibility criteria of the trial, these patients were excluded from the primary analysis. For the primary analysis, the intervention group included 134 patients, and the control group included 140 patients.
Figure 1.
Flowchart of exclusion of patients for the economic evaluation.
Cost-utility analysis
For descriptive statistics, all data are reported as means and SEs, and differences in means between the intervention and the control group are presented as raw, unadjusted differences. P values for between-group differences have been evaluated by a Student’s t-test for continuous variables and a Pearson’s X2 test for binary and multinomial variables. Statistical significance was assumed for p values<0.05, and all significance tests were two-tailed.
The estimates of incremental costs and QALYs between the intervention group and the control group were based on a seemingly unrelated regression analysis. This regression method is recommended and widely used in economic evaluation because cost and HRQoL is normally correlated.47 In the primary analysis, both total costs and QALYs were adjusted for group allocation, age, gender, baseline EQ-5D-3L summary score, total costs in the year preceding the study start date, self-reported NYHA classification at baseline, the self-reported length of HF diagnosis at study start, education level, relationship status and the presence of self-reported smoking, diabetes mellitus, psychological disorder, COPD, cancer and musculoskeletal disorder. The estimations were performed using the mi estimate, cmdok: sureg command in Stata.
The deterministic incremental NMB was estimated using the treatment beta coefficients from the seemingly unrelated regressions, and a probabilistic sensitivity analysis was performed to evaluate the decision uncertainty. A scatter plot of incremental cost-effectiveness was generated based on 10 000 simulations. The simulations were based on random draws from the estimated treatment effect on cost and QALY accumulation and their associated SEs. The incremental costs were expected to assume a gamma distribution and the QALYs were expected to assume a Gaussian distribution.
All statistical analyses were performed in Stata, V.15.1.
Sensitivity analyses
Both the primary analysis and sensitivity analyses were performed with and without adjustment. For deterministic sensitivity analyses, three different scenarios were investigated;
Scenario I: A complete case analysis, that is, an analysis in which information on all outcome variables and variables used for adjustment were available.
Scenario II: An analysis including all patients that were enrolled in the study, including patients with a self-reported NYHA classification of I.
Scenario III: To evaluate whether results were driven by a minority of patients with very high resource consumption, a sensitivity analysis was performed in which the upper 10 per cent of patients with the highest resource consumption before imputation were excluded before imputation.
Scenario analysis II and III were both based on imputed data sets.
Patient and public involvement
Patient and public involvement in the project was organised by the TeleCare North project organisation placed within the regional healthcare administration. This included open seminars/meetings with patients, relatives, healthcare providers and others. A special homepage was designed with relevant information for patients and relatives, hospitals, municipalities and general practitioners, respectively. The TeleCare North project organisation also organised the development of the educational programme for patients and healthcare providers in all sectors. The research-based evaluation of the project was presented in public for all interested citizens free of charge. At the local political and public administrative levels, the project was followed and discussed in relevant fora with participation from all municipalities and the region.
Results
There were no statistically significant differences in baseline characteristics between the two groups, and missingness in variables was also fairly distributed between them (table 1).
Table 1.
Participant baseline characteristics. P values for differences have been evaluated by Student’s t-test for continuous variables and Pearson’s X2 test for binary and multinomial variables
| Study population | Telehealthcare solution | Control group | Raw between-group difference | P value for difference |
| No of patients, n (%) | 134 (49 %) | 140 (51 %) | ||
| Age, mean (SD), y* | 67.21 (11.51) | 67.30 (11.78) | −0.09 | 0.95 |
| Sex, female, %* | 18.91 (n=24) | 20.71 (n=29) | −1.8 | 0.56 |
| Relationship status | 0.14 | |||
| Missing, % | 1.49 (n=2) | 0.71 (n=1) | ||
| Living with somebody, % | 75.76 (n=100) | 67.63 (n=94) | 8.13 | |
| Living alone, % | 24.24 (n=32) | 32.37 (n=45) | −8.13 | |
| Education | 0.67 | |||
| Missing, % | 2.23 (n=3) | 1.43 (n=2) | ||
| Primary (<3 years), % | 65.65 (n=86) | 68.12 (n=94) | −2.47 | |
| Secondary (>3 years), % | 34.35 (n=45) | 31.88 (n=44) | 2.47 | |
| Smoking, (yes)* % | 23.31 (n=31) | 17.14 (n=24) | 6.17 | 0.20 |
| Self-reported duration of HF | ||||
| Missing, % | 5.97 (n=8) | 6.43 (n=9) | ||
| Mean (SD), y | 5.27 (7.45) | 5.47 (7.13) | −0.20 | 0.82 |
| Median, y | 2 | 2 | 0 | |
| NYHA score at baseline, mean (SD) | 2.55 (0.69) | 2.50 (0.61) | 0.05 | 0.53 |
| Missing, % | 4.48 (n=6) | 5.00 (n=7) | ||
| NYHA class II, % | 56.25 (n=72) | 56.39 (n=75) | −0.14 | |
| NYHA class III, % | 32.81 (n=42) | 37.59 (n=50) | −5.41 | |
| NYHA class IV, % | 10.94 (n=14) | 6.02 (n=8) | 4.92 | |
| Self-reported comorbidity, %* | 41.04 (n=55) | 41.43 (n=58) | −0.39 | 0.95 |
| Diabetes, % | 13.43 (n=18) | 19.29 (n=27) | −5.86 | 0.19 |
| COPD, % | 16.42 (n=22) | 15.71 (n=22) | 0.71 | 0.87 |
| Psychological disorder, % | 2.24 (n=3) | 2.14 (n=3) | 0.10 | 0.96 |
| Musculoskeletal disorder, % | 16.42 (n=22) | 15.71 (n=22) | 0.71 | 0.87 |
| Cancer, % | 6.72 (n=9) | 7.14 (n=10) | −0.42 | 0.89 |
| Baseline EQ-5D-3L index score, mean (SD) | 0.7073 (0.1514) | 0.7078 (0.1465) | 0 | 0.98 |
| Missing, % | 5.22 (n=7) | 0.7 (n=1) | ||
| Baseline historical costs excluding municipality costs (£), mean (SD)* | 18 587.52 (21 605.38) | 19 560.00 (23 491.52) | −972.48 | 0.72 |
| Baseline historical municipality costs (£), mean (SD) | 122.24 (303.18) | 479.88 (1585.97) | −357.64 | 0.07 |
| Missing, % | 49.25 (n=66) | 50.71 (n=71) |
*Variable has no missing values.
£, British Pounds Sterling; COPD, chronic obstructive pulmonary disease; EQ-5D-3L, EuroQol 5-Dimensions 3-Levels; HF, heart failure; NYHA, New York Heart Association.
Within the 1-year follow-up, the group receiving the telehealthcare solution had a consistently lower resource consumption across all healthcare cost categories compared with the group receiving usual care, leading to a total raw difference of -£5668 (table 2). Thus, the usage of telemedicine reduces total healthcare costs by 35% (5668 off a base of 16 241 British Pounds Sterling). This lower mean cost per patient was primarily driven by lower costs associated with hospitalisations (intervention group £5055 vs control group £9064, p value=0.01).
Table 2.
Unadjusted mean costs per patient in the intervention group and the control group, respectively, partitioned into cost categories over the 12-month follow-up (2018 £). For all cost categories, data are complete except for the municipality costs of which 50% missing (n=137). The costs associated with the telehealthcare solution is based on deterministic estimates
| Cost category | Mean costs (SE), £ | |||
| Telehealthcare solution (n=134) |
Control group (n=140) | Raw between-group difference (£) | P value for difference | |
| Hospital contacts | ||||
| Hospitalisations | 5055.13 (1027.31) | 9063.65 (1217.95) | −4008.52 | 0.01 |
| Outpatient contacts | 3163.53 (264.85) | 4191.29 (644.82) | −1027.76 | 0.15 |
| Psychiatric outpatient contacts | 13.72 (5.95) | 62.46 (39.20) | −48.74 | 0.23 |
| Primary care contacts | 469.26 (44.37) | 600.36 (40.43) | −131.10 | 0.03 |
| Pharmacy purchases | 972.25 (94.01) | 1076.57 (81.31) | −104.32 | 0.40 |
| Municipality costs (home care, rehabilitation, monitoring in relation to the telehealthcare solution, etc) | 681.61 (137.16) | 1246.88 (461.78) | −565.27 | 0.25 |
| Healthcare costs, excluding costs of the telehealthcare solution | 10 355.50 | 16 241.21 | −5,885.71 | 0.01 |
| Costs of the telehealthcare solution, excluding costs of monitoring: | ||||
| Software development and support*/† | 0.27 | 0 | 0.27 | |
| Basic operation: surveillance, support of health professionals, server licenses, etc‡ | 8.47 | 0 | 8.47 | |
| Running development of apps, system updates, etc‡ | 1.76 | 0 | 1.76 | |
| Education of healthcare professionals*/† | 3.04 | 0 | 3.04 | |
| Telekit, including initial delivery and patient education* | 122.36 | 0 | 122.36 | |
| Annual operational costs: licenses, sim card data, substitution of faulty equipment, etc | 82.15 | 0 | 82.15 | |
| Total costs (including costs of the telehealthcare solution) | 10 573.55 | 16 241.21 | −5,667.66 | 0.01 |
*Annuitised over a 5-year period with a discount rate of four per cent.
†Costs divided among the expected number of HF patients in the North Denmark Region (6700 patients).
‡Costs divided among the expected number of HF and COPD patients in the North Denmark Region (10 500 patients24). See appendix B for further information.
£, British Pounds Sterling; apps, applications; COPD, chronic obstructive pulmonary disease; HF, heart failure.
In the primary analysis, the 1-year adjusted QALY difference between the telehealthcare solution and the usual care group was 0.0034 (95% CI: −0.0711 to 0.0780), indicating an insignificant gain in HRQoL for patients receiving the telehealthcare solution (table 3). The adjusted baseline utility score was similar across the two groups (0.7079 for control and 0.7075 for intervention). The mortality was similar between both groups, with five deaths in the control group and seven deaths in the intervention group.
Table 3.
Incremental costs (£) and quality-adjusted life years after 12-month follow-up
| Scenario | N | Incremental costs, £ (95% CI) | Incremental QALYs (95% CI) | Net monetary benefit, £* |
| Primary analysis, adjusted† | 274 | −5095.92 (−8736.33 to −1455.51) |
0.0034 (−0.0712 to 0.0780) |
5163.98 |
| Primary analysis, unadjusted‡ | 274 | −5539.10 (−9483.26 to −1594.95) |
−0.0005 (−0.0723 to 0.0714) |
5530.04 |
| Scenario I: | ||||
| Complete case analysis, adjusted† | 89 | −1609.85 (−7036.27 to 3816.57) |
−0.0239 (−0.0605 to 0.0127) |
1131.62 |
| Complete case analysis, unadjusted‡ | 94 | −2752.84 (−8438.59 to 2932.91) |
−0.0157 (−0.0536 to 0.0221) |
3570.69 |
| Scenario II: | ||||
| Incl. NYHA class I patients, adjusted† | 295 | −4572.69 (−8030.66 to −1114.73) |
−0.0037(-0.0736 to 0.0663) | 4498.88 |
| Incl. NYHA class I patients, unadjusted‡ | 295 | −4857.43 (−8587.98 to −1126.88) |
−0.0061 (-0.0730 to 0.0609) | 4736.20 |
| Scenario III: | ||||
| Excl. top 10th percentile resource-heavy patients, leaving out municipality costs, adjusted† | 247 | −3060.50 (−4836.08 to −1284.93) |
−0.0096 (-0.0949 to 0.0756) | 2867.62 |
| Excl. top 10th percentile resource-heavy patients, leaving out municipality costs, unadjusted‡ | 247 | −3181.34 (−5103.28 to −1259.40) |
−0.0130 (-0.0944 to 0.0683) | 2921.12 |
*Estimated based on an expected cost-effectiveness threshold of £20 000 per QALY.
†Seemingly unrelated regression, adjustment for group allocation, age, gender, baseline EQ-5D-3L summary score, total costs in the year preceding the study start date, self-reported NYHA classification at baseline, the self-reported length of HF diagnosis, education level, relationship status and the presence of self-reported smoking, diabetes mellitus, psychological disorder, COPD, cancer and musculoskeletal disorder.
‡Seemingly unrelated regression with intervention group as the only predictor.
£, British Pounds Sterling; COPD, chronic obstructive pulmonary disease; EQ-5D-3L, EuroQol 5-Dimensions 3-Levels; Excl., excluding; HF, heart failure; Incl., including; NYHA, New York Heart Association; QALYs, Quality-adjusted life-years.
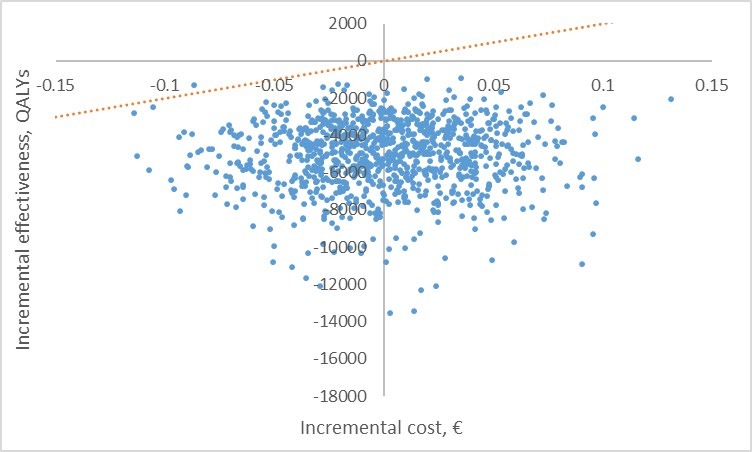
The adjusted difference in costs was -£5096 (95% CI: −8736 to −1456), indicating a significantly lower total mean cost per patient in the telehealthcare solution group. Based on the incremental cost and QALY estimates and an assumed cost-effectiveness threshold of £20 000 per QALY,27 the telehealthcare solution provides a positive incremental NMB of £5164, indicating that the telehealthcare solution is cost-effective. The unadjusted analysis also indicates that the telehealthcare solution provides a significant cost saving (-£5539 (95% CI: −9483 to −1595)) and an insignificant impact on patients’ QALY gain (−0.0005 (95% CI: −0.0723 to 0.0714)) and therefore is cost-effective (NMB = £5530). The result of the probabilistic sensitivity analysis is shown in the incremental cost-effectiveness scatter plot in figure 2. The incremental cost-effectiveness distribution disperses across the southwest and southeast quadrant of the incremental cost-effectiveness plane in agreement with the QALY gain associated with the telehealthcare solution being insignificant but the incremental negative cost being significant.
Figure 2.
Incremental cost-effectiveness scatter plot based on the probabilistic sensitivity analysis. The dotted line indicates a cost-effectiveness threshold of £20 000 per quality-adjusted life-year (QALY).
All scenario analyses showed the same result with telehealthcare associated with lower costs and an insignificant impact on patients’ HRQoL (table 3). Across the adjusted and unadjusted sensitivity analyses, the cost-effectiveness result is relatively robust, with all analyses indicating a positive incremental NMB of the telehealthcare solution compared with usual practice.
Discussion
The principal finding of this study is that the investigated telehealthcare solution is highly cost-effective for the treatment of HF patients in the Danish setting. High-quality economic evaluations of telehealthcare solutions in the management of HF have been requested16–18 and, to our knowledge, the present study is the first economic evaluation of telehealthcare in HF patients that strictly follows international guidelines for health economic evaluation alongside clinical trials.19 Thus, a particular strength of this study was the micro-costing approach, including the availability of information on patient-specific resource usage from the Danish registers. The majority of information on patients’ resource consumption was retrieved from well-validated Danish registers, ensuring the validity of the registrations with no missing data in these parameters. For the resource consumption in the municipalities, data from 4 out of the 11 participatory municipalities were applicable. The four municipalities were relatively large (making for approximately 50% of the total participant sample), and thus, the representativeness of their organisation and consequently costs for smaller municipalities is debatable. However, municipality costs only constitute a minor share of the total costs (cf. table 3); for which reason it could be suspected that even if the estimate of municipality costs is not representative for all participatory municipalities, the cost-effectiveness conclusion would not be markedly affected.
In the present cost-utility analysis, the impact on patients’ QALY gain was insignificant across all analyses. The increased sensitivity, which could have been achieved by using the 5-L questionnaire,19 may effectively have been watered down when predicting the 3-L responses from the 5-L responses and applying the 3-L weights. This might provide an explanation of why it was not possible to observe any substantial differences in QALY accumulation between the two intervention groups, which could otherwise have been expected given findings in previous studies.16
In general, telehealthcare interventions and the studies of them are relatively heterogeneous, making a comparison of them difficult.15 16 In a Cochrane review from 2015,16 structured telephone support and telemonitoring for HF patients were found to reduce all-cause mortality and HF-related hospitalisations. In addition, the impact on patients HRQoL and cost accumulation was inconsistent, emphasising the difficulties of evaluating and comparing the cost-effectiveness of telehealthcare interventions aggregately.16
Only a few papers report on cost savings in relation to telehealthcare within this field. These studies do not have economic evaluation as their primary aim and do not strictly follow proper practice guidelines for economic evaluations.19 26 Nevertheless, they all point in the same direction of potentially huge savings.16–18 Frederix et al 48 reported insignificant long-term savings of approximately 27% from an initial 6 month telehealthcare intervention. Jiménez-Marrero et al 49 report savings of approximately 38% for a subgroup of HF patients with a left ventricular ejection fraction >40. Comín-Colet et al 50 report savings of approximately 45%, mainly driven by a significant reduction in hospitalisations between the telehealthcare group and control group. An economic modelling study by Liu et al 51 also points in the same direction of possible savings from telehealthcare interventions directed at intermediate-risk and high-risk patients over a 1- to 5-year window. Their results suggest the economic viability of telehealthcare programme for the management of chronic HF, but emphasised the importance of risk stratification in such programme.51 In our study, however, the severity of HF did not seem to be important, as there appeared to be only minor differences in cost savings depending on whether patients reporting being in NYHA class I were included or not. A particular difference between our study and other studies may also have been the level of organisational learning and knowledge management, as the TeleCare North trial builds on many years of experience with telehealthcare solutions from previous trials23 24 as well as the national implementation of telehealth programme for COPD in Denmark decided in 2015.22
As the design of the TeleCare North HF trial and the components of the telehealthcare intervention was somewhat similar to that of the TeleCare North COPD trial,23 25 the present study anticipated that the economic evaluation would essentially produce results similar to that of Udsen et al.24 In agreement with the economic evaluation by Udsen et al,24 no significant difference in QALY accumulation between the intervention groups was observed in this study. In contrast, the present study found telehealthcare to produce substantial cost savings, which contrasts with the added costs associated with telehealthcare found by Udsen et al.24 The difference is that telehealthcare is cost-effective for HF patients but not all COPD patients. This discrepancy indicates that the cost-effectiveness of telehealthcare interventions, to a large degree, depends on the recipient patient group, making it difficult to comment on the cost-effectiveness of telehealthcare interventions as a whole. The characteristics of specific patient groups ought, therefore, to be incorporated when telehealthcare interventions are designed and implemented.
The impact of the TeleCare North solution of patients quality of life measured with the Short-Form 36 (SF-36) questionnaire’s physical and mental component summary scores and the Kansas City Cardiomyopathy Questionnaire 12 score are published elsewhere.52 It was only possible to detect a small but significant positive change in the SF-36 mental component summary score. Thus, with respect to the impact on patients’ HRQoL, the telehealthcare solution cannot be characterised as an unqualified success. It could, however, be hypothesised that the currently applied methods of measurement of effect are too insensitive to detect any beneficial impact especially on patients’ mental well-being, as suggested by the positive impact on the SF-36 mental component summary score but none of the other measures. It is possible that the positive impact of telehealthcare does not manifest itself as an impact on patients’ HRQoL but rather their opinions and beliefs, which subsequently affects their healthcare-seeking behaviour.
Given the complexity and multiple purposes of the intervention under investigation,25 it is possible that conventional measures of effectiveness, such as HRQol and QALY, do not sufficiently capture all potential effects. It is possible that impacts on other parameters could have been observed, such as patients’ satisfaction, self-perceived risk of dying, comfort, ability to reduce anxiety through telephone contact with a well-known local nurse and an increased sense of capability among others. It could be considered whether the slightly narrow focus on patients’ HRQoL and QALY in the present analysis represent an appropriate evaluation approach to this particular kind of complex intervention.
Though the present economic evaluation found the telehealthcare solution to be highly cost-effective, questions remain as to why this result was achieved. Thus, it remains unclear what components of the intervention were actually effective or whether the effect is contingent on the intervention in its entirety. In the design phase of future trials on the effectiveness and cost-effectiveness of complex interventions such as telehealthcare, early consideration of mechanisms of action and programme theory53 ought to be introduced to improve our understanding of why some interventions may prove effective and cost-effective and others not. This may increase the cost-effectiveness of future telehealth solutions.
Supplementary Material
Acknowledgments
The authors would like to thank all of the participants in TeleCare North Heart Failure for their time and effort when filling out questionnaires and reporting physical measurements. Likewise, a thank you goes out to all healthcare professionals involved in the execution of the trial, the North Denmark Region and the municipalities for their goodwill in making this trial possible. A special thank you to Kuno Strand Kudajewski, project manager for the TeleCare North Heart Failure Trial at the North Denmark Region for helping with information on cost data, and to Simon Lebech Cichosz for the lengthy keying of the EQ-5D questionnaire data.
Footnotes
Contributors: Author contributions: LHE is the primary investigator for economic evaluation in the TeleCare North Heart Failure Trial; LHE planned the design of the economic evaluation and stands as a guarantor of the statistical quality for the evaluation as a whole. LHE, LH, MBJ, SSS and ASV contributed equally to the detailed planning and design of the analyses in the economic evaluation. LH was mainly responsible for data management. All analyses were performed by collaboration by all authors, and ASV drafted the paper in collaboration with the other authors. All authors had full access to the data and accept responsibility for the integrity of the data and the data analyses. All authors met regularly during the data analysis period and contributed equally to the interpretation and presentation of data. All authors reviewed and approved the manuscript prior to submission.
Funding: The study was funded by the Danish Agency for Digitalisation under the Danish Ministry of Finance the North Denmark Region.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The trial has been authorised by the Danish Data Protection Agency. The study is being conducted in accordance with the Helsinki declaration. The trial has been presented to the Ethical Committee for Medical Research in the North Denmark Region; this committee decided that no ethical approval was necessary.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No additional data available.
References
- 1. Savarese G, Lund LH, Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden . Global public health burden of heart failure. Card Fail Rev 2017;03:7 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inamdar A, Inamdar A, Clin Med J. Heart failure: diagnosis, management and utilization. J Clin Med 2016;5:62 10.3390/jcm5070062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madelaire C, Gislason G, Danmark Hi. Socioøkonomiske forskelle i forekomst, dødelighed og behandling [Heart Failure in Denmark 2017 - socioeconomic differences in prevalence, mortality, and treatment]; 2017.
- 5. Dansk Selskab for Almen Medicin Kronisk hjertesvigt/kronisk systolisk hjerteinsufficiens [Chronic heart failure/chronic systolic heart failure]. DSAM Guidel 2013. [Google Scholar]
- 6. Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–59. 10.1161/CIRCRESAHA.113.300268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dansk Cardiologisk Selskab, Behandlingsvejledning . 5. Kronisk hjertesvigt [Treatment Guidelines, Chronic Heart Failure], 2017. Available: https://www.cardio.dk/chf#55-behandling [Accessed 16 Nov 2018].
- 8. Polikandrioti M. Health failure and health related quality of life. Clin Pract Epidemiol ment Health 2005;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 2002;87:235–41. 10.1136/heart.87.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Comín-Colet J, Anguita M, Formiga F, et al. Health-Related quality of life of patients with chronic systolic heart failure in Spain: results of the VIDA-IC study. Revista Española de Cardiología 2016;69:256–71. 10.1016/j.rec.2015.07.030 [DOI] [PubMed] [Google Scholar]
- 11. Shafie AA, Tan YP, Ng CH. Systematic review of economic burden of heart failure. Heart Fail Rev 2018;23:131–45. 10.1007/s10741-017-9661-0 [DOI] [PubMed] [Google Scholar]
- 12. Brorholt G, Jakobsen M, Hauge AM, et al. En helhjertet indsats – En artikelbaseret klinisk, patientnær og sundhedsøkonomisk kortlægning af hjerte-kar-området [A whole-hearted effort - a paper-based clinical, patient-relevant and health economic survey of the cardiovascular area]. København K 2018.
- 13. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016;18 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 14. Bashi N, Karunanithi M, Fatehi F, et al. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res 2017;19:e18 10.2196/jmir.6571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dierckx R, Inglis SC, Clark RA, et al. Telemedicine in heart failure: new insights from the Cochrane meta-analyses. Eur J Heart Fail 2017;19:304–6. 10.1002/ejhf.759 [DOI] [PubMed] [Google Scholar]
- 16. Inglis SC, Clark RA, Dierckx R, et al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015;116 10.1002/14651858.CD007228.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gensini GF, Alderighi C, Rasoini R, et al. Value of Telemonitoring and telemedicine in heart failure management. Card Fail Rev 2017;3:1 10.15420/cfr.2017:6:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grustam AS, Severens JL, van Nijnatten J, et al. Cost-Effectiveness of telehealth interventions for chronic heart failure patients: a literature review. Int J Technol Assess Health Care 2014;30:59–68. 10.1017/S0266462313000779 [DOI] [PubMed] [Google Scholar]
- 19. Ramsey SD, Willke RJ, Glick H, et al. Cost-Effectiveness analysis alongside clinical trials II-An ISPOR good research practices Task force report. Value Health 2015;18:161–72. 10.1016/j.jval.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 20. Faria R, Gomes M, Epstein D, et al. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157–70. 10.1007/s40273-014-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Digitaliseringsstyrelsen Telemedicin - en nøgle til fremtidens sundhedsydelser. National handlingsplan for udbredelse af telemedicin - kort fortalt [Telehealthcare - a key to future healthcare services. A national action plan for the introduction of telehealthcare - in brief]. København K; 2012.
- 22. Sundhedsdatastyrelsen Telemedicin og telesundhed [Telemedicine and telehealth], 2018. Available: https://sundhedsdatastyrelsen.dk/da/rammer-og-retningslinjer/telemedicin-og-telesundhed [Accessed 21 Dec 2018].
- 23. Udsen FW, Lilholt PH, Hejlesen O, et al. Effectiveness and cost-effectiveness of telehealthcare for chronic obstructive pulmonary disease: study protocol for a cluster randomized controlled trial. Trials 2014;15:1–7. 10.1186/1745-6215-15-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Witt Udsen F, Lilholt PH, Hejlesen O, et al. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish 'TeleCare North' cluster-randomised trial. BMJ Open 2017;7:e014616 10.1136/bmjopen-2016-014616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cichosz SL, Ehlers LH, Hejlesen O. Health effectiveness and cost-effectiveness of telehealthcare for heart failure: study protocol for a randomized controlled trial. Trials 2016;17:4–9. 10.1186/s13063-016-1722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drummond MF, Schulpher M, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed Oxford: Oxford University Press, 2015. [Google Scholar]
- 27. Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? a binary choice analysis. Health Econ 2004;13:437–52. 10.1002/hec.864 [DOI] [PubMed] [Google Scholar]
- 28. Statistics Denmark Pris111: consumer price index ((2015=100) by commodity group and unit, 2018. Available: http://www.statistikbanken.dk/PRIS8 [Accessed December 5, 2018].
- 29. EUROinvestor Valutakurser [Exchange rates], 2018. Available: https://www.valutakurser.dk/ [Accessed 15 Feb 2019].
- 30. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 31. Sundhedsdatastyrelsen Sygesikringsregisteret (SSR) [National Health Insurance Service Register], 2017. Available: https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sundhedsoekonomi-og-finansiering/sygesikringsregisteret [Accessed November 20, 2018].
- 32. Andersen JS, Olivarius NDF, Krasnik A. The Danish National health service register. Scand J Public Health 2011;39:34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 33. Danish Medical Association Honorartabel 01-10-2018 [Fee table 2018-10-01], 2018. Available: https://www.laeger.dk/sites/default/files/honorartabel_2018_oktober_web.pdf
- 34. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Statistics Denmark Statistikbanken - LONS20: Løn efter arbejdsfunktion, sektor, aflønningsform, lønmodtagergruppe, lønkomponenter og køn [Statistikbanken - LONS20: Wages estimated based on job function, sector, payment form, employment group, wage component and gender], 2018. Available: http://statistikbanken.dk/statbank5a/default.asp?w=1920 [Accessed 19 Nov 2018].
- 36. Ministry of Finance Frit valg og kvalitet - afregningsmodeller på de kommunale serviceområder [Liberty of choice and quality - models of settlements in the municipal service areas] Ministry of Finance; 2003. [Google Scholar]
- 37. Jakobsen M, Klausen E, Kolodziejczyk C. Omkostninger ved blodprop i hjernen og blødninger blandt patienter med atrieflimren i Danmark - en registerbaseret ’cost of illness’-analyse [The costs of stroke and hemorrhages amongst patients with atrial fibrillation - a register-based cost of illness. Copenhagen: KORA, 2014. [Google Scholar]
- 38. 7 Nov 2018 Agency for Modernisation - Ministry of Finance Levetider [Service lives]. Den Økonomiske Adm Vejl [Economic Adm Instr; 2018. https://modst.dk/oekonomi/oeav/regnskabsregler/generelle-bogfoeringsbestemmelser/levetider/
- 39. Ministry of Finance Den samfundsøkonomiske diskonteringsrente [The economic discount rate]; 2018: 1–19.
- 40. van Hout B, Janssen MF, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708–15. 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 41. The EuroQol Group EQ-5D-5L Crosswalk index value calculator for windows; 2018.
- 42. Wittrup-Jensen KU, Lauridsen J, Gudex C, et al. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health 2009;37:459–66. 10.1177/1403494809105287 [DOI] [PubMed] [Google Scholar]
- 43. Sundhedsdatastyrelsen Dødsårsagsregisteret (DAR) [Danish Registry of Causes of Death] n.d. Available: https://sundhedsdatastyrelsen.dk/dar [Accessed 12 Nov 2018].
- 44. Gupta SK. Intention-To-Treat concept: a review. Perspect Clin Res 2011;2:109 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fergusson D, Aaron SD, Guyatt G, et al. Post-Randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652–4. 10.1136/bmj.325.7365.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nixon RM, Thompson SG. Methods for incorporating covariate adjustment, subgroup analysis and between-centre differences into cost-effectiveness evaluations. Health Econ 2005;14:1217–29. 10.1002/hec.1008 [DOI] [PubMed] [Google Scholar]
- 48. Frederix I, Vanderlinden L, Verboven A-S, et al. Long-Term impact of a six-month telemedical care programme on mortality, heart failure readmissions and healthcare costs in patients with chronic heart failure. J Telemed Telecare 2019;25:1877463 10.1177/1357633X18774632 [DOI] [PubMed] [Google Scholar]
- 49. Jiménez-Marrero S, Yun S, Cainzos-Achirica M, et al. Impact of telemedicine on the clinical outcomes and healthcare costs of patients with chronic heart failure and mid-range or preserved ejection fraction managed in a multidisciplinary chronic heart failure programme: a sub-analysis of the iCOR randomized trial. J Telemed Telecare 2018:1357633X18796439 10.1177/1357633X18796439 [DOI] [PubMed] [Google Scholar]
- 50. Comín-Colet J, Enjuanes C, Verdú-Rotellar JM, et al. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: results of a randomized controlled trial. J Telemed Telecare 2016;22:282–95. 10.1177/1357633X15600583 [DOI] [PubMed] [Google Scholar]
- 51. Liu SX, Xiang R, Lagor C, et al. Economic modeling of heart failure telehealth programs: when do they become cost saving? Int J Telemed Appl 2016;2016:1–9. 10.1155/2016/3289628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cichosz SL, Udsen FW, Hejlesen O. The impact of telehealth care on health-related quality of life of patients with heart failure: results from the Danish TeleCare North heart failure trial. J Telemed Telecare 2019:1357633X1983271 10.1177/1357633X19832713 [DOI] [PubMed] [Google Scholar]
- 53. Pawson R, Tilley N. Realistic evaluation. London: Sage, 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031670supp001.pdf (79.2KB, pdf)
bmjopen-2019-031670supp002.pdf (93.1KB, pdf)
bmjopen-2019-031670supp003.pdf (46KB, pdf)