Abstract
Regulated transport through the secretory pathway is essential for embryonic development and homeostasis. Disruptions in this process impact cell fate, differentiation and survival, often resulting in abnormalities in morphogenesis and in disease. Several congenital malformations are caused by mutations in genes coding for proteins that regulate cargo protein transport in the secretory pathway. The severity of mutant phenotypes and the unclear aetiology of transport protein-associated pathologies have motivated research on the regulation and mechanisms through which these proteins contribute to morphogenesis. This review focuses on the role of the p24/transmembrane emp24 domain (TMED) family of cargo receptors in development and disease.
Keywords: cargo receptor, development, disease, p24, TMED
1. Introduction
Nascent proteins are modified and transported to their final destination via the secretory pathway (Figure 1). Both regulated and constitutive transport of these cargo proteins are essential for embryonic development. In fact, a number of human diseases, including congenital malformations and cancers, arise as a result of mutated or abnormal expression of proteins in this pathway (Merte et al., 2010; Routledge et al., 2010; Garbes et al., 2015; Yehia et al., 2015; Zhang et al., 2017). The transmembrane emp24 domain (TMED) family consists of type I single-pass transmembrane proteins that are found in all eukaryotes. Members of the TMED family have emerged as important regulators of protein transport (Carney & Bowen, 2004; Dancourt & Barlowe, 2010; Jerome-Majewska et al., 2010; Zakariyah et al., 2012; Viotti, 2016; Wada et al., 2016; Hou et al., 2017; Hou & Jerome-Majewska, 2018), and although they are found in the early and late secretory pathways (Figure 2), most studies focus on the function of TMED proteins in the early secretory pathway (Figure 2a): the endoplasmic reticulum (ER), the ER–Golgi intermediate compartment (ERGIC) and the Golgi (Shevchenko et al., 1997; Dominguez et al., 1998; Fullekrug et al., 1999; Jenne et al., 2002; Chen et al., 2006; Hosaka et al., 2007; Blum & Lepier, 2008; Montesinos et al., 2012, 2013).
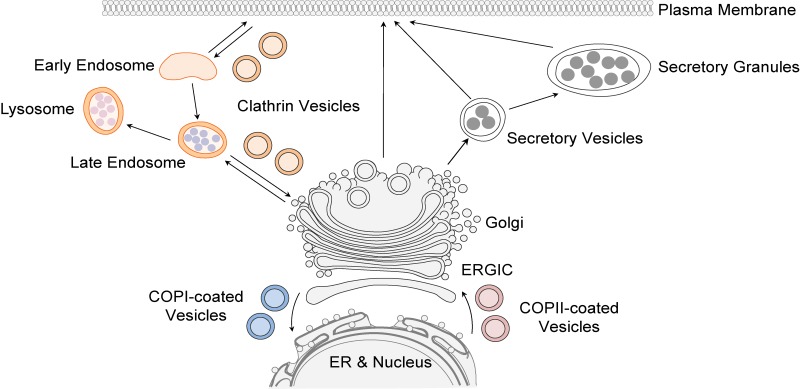
Fig. 1.
Summary of the secretory pathway. Transmembrane and secreted proteins are folded in the endoplasmic reticulum (ER) and transported to the Golgi via COPII-coated vesicles (anterograde trafficking). ER-resident or misfolded proteins are trafficked back to the ER from the Golgi via COPI-coated vesicles (retrograde trafficking). Clathrin-coated vesicles mediate a portion of post-Golgi trafficking. ERGIC = ER–Golgi intermediate compartment.
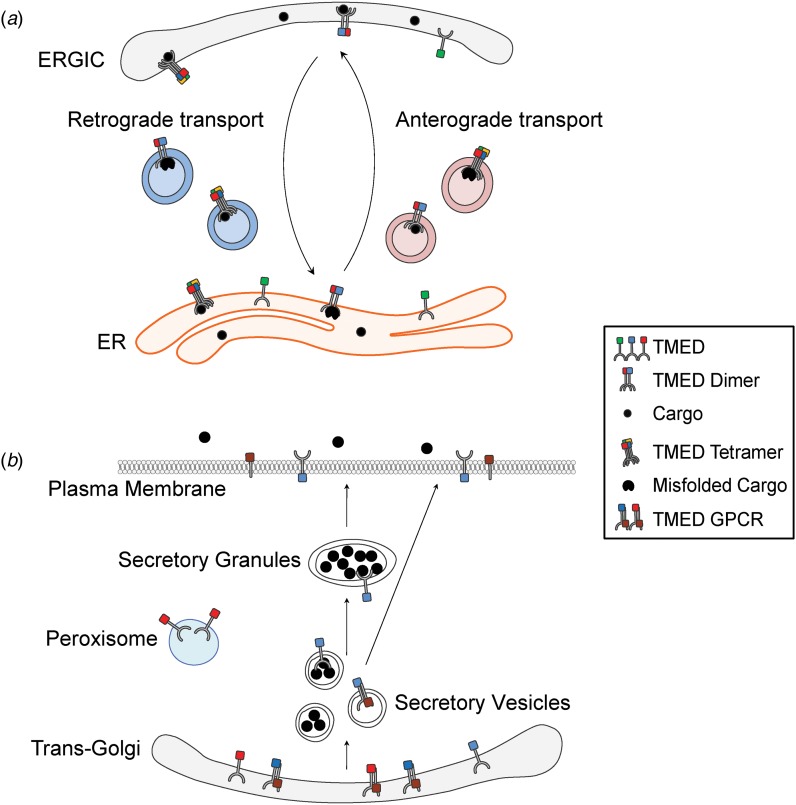
Fig. 2.
TMED proteins in the secretory pathway. (a) TMED dimers and tetramers are packaged into COPII-coated vesicles (pink) and COPI-coated vesicles (blue) and are implicated in anterograde and retrograde transport. (b) A subset of TMED proteins are also found at the plasma membrane, in secretory granules, at the trans-Golgi and in peroxisomes. ER = endoplasmic reticulum; ERGIC = ER–Golgi intermediate compartment; GPCR = G-protein-coupled receptor.
Members of the TMED family are classified into four subfamilies based on sequence homology (Table 1): α, β, γ and δ (Blum et al., 1996; Strating et al., 2009; Schuiki & Volchuk, 2012; Pastor-Cantizano et al., 2016). However, as the different TMED subfamilies have expanded independently during evolution, the number of proteins in each subfamily varies by species (Table 1) (Strating et al., 2009). Nonetheless, all TMED family members share a common structural organization (Figure 3): a luminal Golgi-dynamics (GOLD) domain, a luminal coiled-coil domain, a transmembrane domain and a short cytosolic tail (Strating et al., 2009; Nagae et al., 2016, 2017; Pastor-Cantizano et al., 2016).
Table 1.
TMED family orthologues across different organisms.
| Subfamily | Human/mouse (HGCN) | Drosophila (FlyBase) | Yeast | Caenorhabditis elegans (WormBase) | Xenopus (Xenbase) |
|---|---|---|---|---|---|
| α | TMED4 | éclair | Erp6 | tmed-4 | tmed4 |
| p24-2 | |||||
| TMED9 | Erp5 | tmed9 | |||
| tmed-12 | |||||
| TMED11 | Erp1 | ||||
| β | TMED2 | CHOp24 | Emp24 | sel-9 | tmed2 |
| CG9308 | |||||
| γ | TMED1 | tmed-1 | tmed1 | ||
| TMED3 | p24-1 | Erp4 | tmed-3 | ||
| TMED5 | opossum | Erp2 | tmed5 | ||
| tmed-13 | |||||
| CG31787 | |||||
| TMED6 | logjam | tmed6 | |||
| TMED7 | Erp3 | tmed7 | |||
| δ | TMED10 | baiser | Erv25 | tmed-10 | tmed10 |
| tmed10-like |
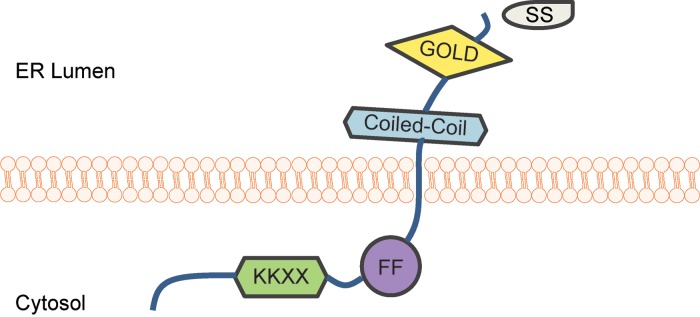
Fig. 3.
Domain structure of TMED family proteins. TMED proteins have a signal sequence (SS) that enables their translocation into the endoplasmic reticulum (ER); the SS is cleaved following ER translocation. The luminal portion of TMED proteins consists of a coiled-coil domain and a Golgi dynamics (GOLD) domain. The short cytoplasmic tail includes diphenylalanine (FF) and dilysine motifs (KK), which are important for binding to COP proteins.
During anterograde and retrograde transport, TMED proteins dimerize and interact with coatomer (COP) protein complexes to facilitate cargo selection and vesicle formation. The GOLD domain, which was expected to be involved in cargo recognition (Anantharaman & Aravind, 2002), was recently shown to mediate dimerization between TMED proteins (Nagae et al., 2016, 2017); a function previously ascribed to the coiled-coil domain. The transmembrane domain, which was predicted to mediate interactions with integral membrane proteins in order to regulate ER exit (Fiedler & Rothman, 1997), was more recently shown to interact with lipids and to aid in vesicle budding (Contreras et al., 2012; Ernst & Brügger, 2014; Aisenbrey et al., 2019). Interactions with COPI and COPII occur via conserved COP binding motifs in the cytoplasmic tail (Figure 3) (Dominguez et al., 1998; Majoul et al., 2001).
TMED proteins regulate transport of a diverse group of cargo proteins; these are summarized in Tables 2 and 3, respectively, depending on whether direct interaction with TMED proteins has or has not been shown (Anantharaman & Aravind, 2002; Theiler et al., 2014; Nagae et al., 2016, 2017). The best-characterized TMED cargo proteins are glycosylphosphatidylinositol (GPI)-anchored proteins (Marzioch et al., 1999; Muniz et al., 2000; Takida et al., 2008; Bonnon et al., 2010; Castillon et al., 2011; Fujita et al., 2011; Theiler et al., 2014; Pastor-Cantizano et al., 2016). However, members of the TMED family also interact with transmembrane and secreted proteins such as Notch and G-protein-coupled receptors, as well as with a number of Wnt ligands (Tables 2 & 3) (Stirling et al., 1992; Marzioch et al., 1999; Wen & Greenwald, 1999; Luo et al., 2007, 2011; Stepanchick & Breitwieser, 2010; Buechling et al., 2011; Port et al., 2011; Junfeng et al., 2015; Li et al., 2015). In this review, we summarize the growing body of literature indicating requirements for TMED proteins during embryogenesis and their contributions to disease.
Table 2.
Cargo interactors of TMED proteins.
| TMED protein | Cargo | Interaction location | Experimental system |
|---|---|---|---|
| TMED1 | IL1RL1 (ST2L/IL-33R) | ER, cis-Golgi | Cell culture |
| TMED2 | AGR2 | n/a | Cell culture and mouse |
| GCGR | n/a | Cell culture | |
| TMEM173 (MITA) | ER | Cell culture | |
| Wga | ER and Golgi | Drosophila | |
| CD59a | ER | Cell culture | |
| Folate receptora | ER | Cell culture | |
| Gas1pa | ER | Yeast | |
| F2R (PAR-1) | Golgi | Cell culture | |
| F2RL1 (PAR-2)a | Golgi | Cell culture | |
| P2YR (1,2,4,11) | Golgi | Cell culture | |
| OPRM1 (MOR1B) | Golgi | Cell culture | |
| CASR | ER | Cell culture | |
| SM-18 | COPI vesicles | Cell culture | |
| TMED9 | PS1-NTFa | n/a | Cell culture |
| NCTa | n/a | Cell culture | |
| APH-1a | n/a | Cell culture | |
| PEN-2a | n/a | Cell culture | |
| TMED10 | TGFBR2 | Cell membrane | Cell culture |
| TGFBR1 (ALK5) | Cell membrane | Cell culture |
a Also interacts with TMED10.
ER = endoplasmic reticulum; n/a = not applicable.
Table 3.
Proteins regulated by the TMED family.
| TMED protein | Target protein | Regulation type | Experimental system |
|---|---|---|---|
| TMED1 | Wga,b | Secretion | Drosophila |
| TMED2 | Invertasec | Secretion | Yeast |
| Kar2pc,d | Secretion | Yeast | |
| Sec13c,d | – | Yeast (genetic interaction) | |
| LIN-12/GLP-1c | Localization | Caenorhabditis elegans (genetic interaction) | |
| Fibronectin | Localization | Mouse | |
| VCAM1 | Localization | Mouse | |
| TMED3 | WNT1 | Localization, secretion | Cell culture |
| TMED4 | POMCc | Processing | Xenopus |
| TMED5 | Gas1pd | Secretion, processing | Yeast |
| WntD | Secretion | Drosophila | |
| TMED6 | Insulinc | Secretion | Cell culture |
| TMED7 | TLR-4 | Signalling | Cell culture |
| TMED9 | APP | Processing | Cell culture |
| TMED10 | CD55 (DAF) | Processing | Cell culture |
| P2YR (4) | Localization | Cell culture | |
| OPRM1 (MOR1B) | Localization | Cell culture | |
| TMEM173 (MITA) | Signalling | Cell culture | |
| Tkva | – | Drosophila (genetic interaction) |
a Also regulated by TMED4.
b Also regulated by TMED5.
c Also regulated by TMED10.
d Also regulated by TMED11.
2. TMED proteins and development
Although the TMED family is essential for normal development, with limited functional redundancy (Strating et al., 2007, 2009), the specific functions of most TMED proteins are unclear. In this section, we highlight the roles uncovered for TMED proteins during development in a variety of species (summarized in Figure 4). Mutant Tmed alleles are summarized in Table 4.
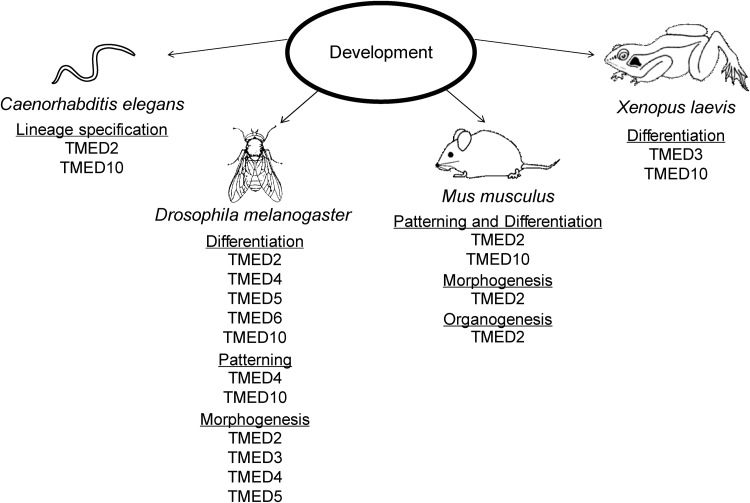
Fig. 4.
TMED family in development. TMED proteins regulate multiple developmental processes in different organisms.
Table 4.
Mutant Tmed alleles in various model organisms.
| Tmed member | Mutation type | Allele/mechanism | System | Reference |
|---|---|---|---|---|
| Sel-9 (tmed2) | Point mutations | Multiple alleles: p.V47L, p.G51R, p.G51E, p.S97N, p.W174X/antimorphic and hypomorphic | Caenorhabditis elegans | Wen and Greenwald (1999) |
| Tmed2 | Point mutation | p.A13E/protein null | Mouse | Jerome-Majewska et al. (2010) |
| β-galactosidase insertion (gene trap) | Tmed2-β-geo fusion/antimorphic | Mouse | ||
| Loj (tmed6) | P-element insertion in 5′-untranslated region and exon 2 | RNA null | Drosophila | Carney and Taylor (2003) |
| Eca (tmed4) | Premature stop codon | pW201X/antimorphic, null | Drosophila | Bartoszewski et al. (2004) |
| Deletion (300 nt) | pS7delfsx/null | Drosophila | ||
| Bai (tmed10) | Point mutation at splice site | pG124fsx/null | Drosophila | |
| Tmed10 | Exon 1 deletion | RNA/protein null | Mouse | Denzel et al. (2000) |
2.1. Caenorhabditis elegans
The Caenorhabditis elegans genome contains at least one representative member of all four TMED subfamily members (WormBase; WS270) (Table 1): there are two genes in the α subfamily (tmed-4 (Y60A3.9) and tmed-12 (T08D2.1)), one gene each in the β subfamily (sel-9 (tmed-2; W02D7.7)) and in the δ subfamily (tmed-10 (F47G9.1)) and three genes in the γ subfamily (tmed-1 (K08E4.6), tmed3 (F57B10.5) and tmed-13 (Y73B6BL.36)). Two tmed2 mutant alleles were first isolated as suppressors of the egg-laying defect associated with hypomorphic alleles of lin-12 (Table 4) (Sundaram & Greenwald, 1993). Wen and Greenwald isolated five additional tmed2 mutant alleles in a non-complementation screen (Table 4) (Wen & Greenwald, 1999) and suggested that all seven sel-9 alleles were antimorphic (dominant negative) and reduced wild-type sel-9 activity. Interestingly, six of these mutations mapped to the GOLD domain and one mutation was predicted to generate a truncated protein lacking the carboxyl region. Furthermore, though RNA interference (RNAi) knockdown of sel-9 and tmed10 did not affect viability, a truncated mutant allele of sel-9 resulted in phenotypes classified as dumpy (Dpy), uncoordinated (Unc), roller (Rol) and defective egg-laying (Wen & Greenwald, 1999). Reduced wild-type SEL-9 and TMED-10 activity allowed mutated GLP-1 protein to reach the plasma membrane and resulted in increased activity of mutated LIN-12 or GLP-1. Thus, although null alleles for tmed genes have not yet been examined in C. elegans, the phenotypes associated with mutations in sel-9 suggest a role for this family in quality control during LIN-12/Notch receptor family protein transport. These studies further indicate that the GOLD domain may play an important role in mediating interactions with LIN-12 and GLP-1.
2.2. Drosophila melanogaster
Nine TMED genes have been identified in the Drosophila genome (FlyBase; FB2019_03) (Table 1): two α (p24-2 and éclair), two β (CG9308 and CHOp24), one δ (baiser) and four γ (p24-1, logjam, opossum and CG31787)). Most of these TMED proteins are localized to the ER–Golgi interface (Boltz et al., 2007; Buechling et al., 2011; Saleem et al., 2012). Logjam (Tmed6, loj), CHOp24 (Tmed2), p24-1(Tmed3), opossum (Tmed5, opm), eclair (Tmed4, eca) and baiser (Tmed10, bai) were expressed at all developmental stages and in adult tissues (Carney & Taylor, 2003; Boltz et al., 2007; Graveley et al., 2011). CG31787 and CG9308 were expressed in a sex-dependent pattern and p24-2 was primarily expressed in adult tissues with limited or no expression in pupal and larval tissues, respectively (Boltz et al., 2007). Expression of a subset of Tmed genes during development (Tmed2, Tmed3, Tmed4, Tmed5, Tmed6 and Tmed10) suggests that they may be involved in Drosophila development beginning at the earliest stages of embryogenesis.
Mutations in Drosophila TMED family members (Table 4) and RNAi experiments indicate a role for TMED proteins in reproduction, embryonic patterning, transforming growth factor-β (TGF-β) signalling and WNT signalling (Carney & Taylor, 2003; Bartoszewski et al., 2004; Buechling et al., 2011; Port et al., 2011; Li et al., 2015). P-element insertions in the 5′-untranslated region or exon 2 of loj, the first coding exon, were shown to result in a loss of loj (Tmed6) RNA. These mutations led to oviposition defects in female Drosophila (Table 4) (Carney & Taylor, 2003) and revealed a requirement for this protein in the central nervous system and the mature egg. Bartoszewski and colleagues reported mutations in two additional Drosophila family members (Bartoszewski et al., 2004). Deletion of a large portion of the coding region of eca (tmed4) or a point mutation leading to a premature stop codon before the carboxyl domain of the protein were phenotypically indistinguishable, indicating that the carboxyl domain is essential for eca function. The group also identified mutants with a splice-site mutation in bai (tmed10), which led to a premature stop codon and a null allele. They showed that eca was required for both TGF signalling and for Wg secretion. eca and bai mutants had reduced survival, and surviving mutants showed fertility defects; similar to loj mutants, males had reduced fertility and females did not lay eggs. In addition, eca and bai genetically interacted and were both required in oocytes for dorsal–ventral patterning of embryos (Table 4) (Bartoszewski et al., 2004). This phenotype was specific for maternally deposited TKV, an orthologue of the mammalian TGF-β receptor BMPR1, and downstream of Dorsal, indicating that the two TMED proteins are required for activity of maternally deposited Drosophila BMPR1. Furthermore, although bai mutants phenocopied the temperature-sensitive thick vein phenotype of tkv mutants, it was not clear why reduced levels of TMED proteins blocked signalling by this TGF-β receptor, especially since the TKV protein was still properly localized in these mutants. Altogether, studies in Drosophila indicate that normal levels of tmed proteins are required for morphogenesis and patterning.
Multiple studies support a role for TMED proteins in WNT signalling. In an RNAi screen, eca and CHOp24 were found to be required for Wg transport (Port et al., 2011). Knockdown of these two genes resulted in loss of wing margin tissue, retention of Wg in the ER and reduced extracellular Wg. In a similar screen, Buechling et al. found that opm, CHOp24 and p24-1 were required in Wg-producing cells for canonical Wg signalling (Buechling et al., 2011). Furthermore, the group showed that maternal deposits of Opm were required for viability and WntD transport. In a more recent RNAi screen, Li et al. identified bai, CHOp24 and eca to be important in Wg-producing cells (Li et al., 2015). All three studies suggest that TMED family members are implicated in the transport of WNT proteins from the ER to the Golgi. Furthermore, eca and bai are required for both TGF and WNT signalling at different developmental stages and in different tissues, suggesting that TMED proteins function in multiple pathways and are regulated both temporally and spatially.
2.3. Xenopus laevis
The Xenopus tmed gene family is composed of nine members (Xenbase) (Table 1): two δ subfamily members (tmed10 and tmed10-like) (Strating et al., 2009), four γ subfamily members (tmed1, tmed5, tmed6 and tmed7), two α subfamily members (tmed4 and tmed9) and one β subfamily member (tmed2). The TMED family is involved in X. laevis pro-opiomelanocortin (POMC) protein biosynthesis in melanotrope cells of the intermediate pituitary gland. POMC is a precursor polypeptide that is cleaved to produce multiple peptide hormones. Six of the Tmed proteins (Tmed2, Tmed4, Tmed5, Tmed7, Tmed10 and Tmed10-like) are expressed in melanotrope cells, whereas the other two members (Tmed1 and Tmed9) are not (Rotter et al., 2002). Tmed proteins are generally localized to the Golgi compartment in Xenopus. However, the steady-state subcellular localization shifts towards a subdomain of the ER and ERGIC in biosynthetically active intermediate pituitary melanotrope cells (Kuiper et al., 2001). Furthermore, Tmed2, Tmed4, Tmed7 and Tmed10-like are upregulated in active melanotropes compared to inactive melanotropes (Rotter et al., 2002).
Tmed proteins co-localize with the major cargo protein POMC and have functionally non-redundant roles in regulating its secretion. For example, transgenic expression of Tmed4 dramatically reduces POMC transport, leading to its accumulation in ER-localized electron-dense structures (Strating et al., 2007). By contrast, transgenic expression of Tmed10-like does not induce changes in the cell's internal structure or in POMC secretion, but it does impact Golgi-based processing of POMC (Strating et al., 2007). Furthermore, transgenic expression of Tmed4 or Tmed10 disrupted pigmentation in Xenopus because of insufficient POMC secretion or abnormal POMC processing, respectively (Bouw et al., 2004; Strating et al., 2007). The Xenopus POMC studies are critical indications that Tmed4 and Tmed10 can affect different sub-compartments in the early stages of the secretory pathway and can be localized differentially depending on cell type (Kuiper et al., 2000). These studies suggest an emerging model where Tmed2, Tmed4, Tmed7 and Tmed10 function together (potentially as a tetramer) to traffic POMC through the early secretory pathway during pigment development. Since POMC neurons are also found in mammals, TMED proteins may play an evolutionarily conserved role in the development of the melanocortin system in humans.
2.4. Mouse/human
There are ten known TMED genes in mammals (Table 1): three α subfamily (Tmed4, Tmed9 and Tmed11), five γ subfamily (Tmed1, Tmed3, Tmed5, Tmed6 and Tmed7), one β subfamily (Tmed2) and one δ subfamily (Tmed10), with an additional non-functional duplicate of Tmed10 found solely in apes and humans (Blum et al., 1996; Horer et al., 1999). Individual TMED proteins appear to be more crucial in mammals during embryonic development when compared to less complex organisms. We generated two mutant mouse lines with mutations in Tmed2; one line carries a single point mutation in the signal sequence of Tmed2 and the second line has a β-galactosidase gene trap inserted in intron 3 of the gene. Both mutations resulted in a loss of TMED2 protein and embryonic lethality by embryonic day (E) 11.5 (mid-gestation) (Table 4) (Jerome-Majewska et al., 2010). Similarly, deletion of exon 1 of mouse Tmed10 results in loss of TMED10 protein and embryonic lethality at E3.5, before implantation (Denzel et al., 2000). Thus, TMED2 and TMED10 have non-redundant functions during embryogenesis.
Tmed2 mRNA is expressed in a temporal and a tissue-specific pattern in mice (Jerome-Majewska et al., 2010). At E5.5, Tmed2 is expressed in embryonic and extraembryonic regions. Expression becomes higher in the ectoplacental cone and extraembryonic ectoderm at E6.5. At E7.5, Tmed2 is observed in the ectoplacental cone, amnion, anterior neural folds and head mesoderm. At E8.5, Tmed2 expression is found in the somites, heart and tail bud. Prior to lethality by E11.5, Tmed2 homozygous null mutants are developmentally delayed (beginning at E8.5) and present with an abnormally looped heart (E9.5) and an abnormal tail bud morphology (E9.5–E10.5) (Jerome-Majewska et al., 2010). Therefore, TMED2 is important for morphogenesis of the organs in which it is expressed.
In addition, Tmed2 is essential for the formation of the placenta (Jerome-Majewska et al., 2010). At E8.5, Tmed2 mRNA is expressed in the allantois, chorionic plate and giant cells. At E9.5, Tmed2 continues to be expressed in the giant cells, spongiotrophoblasts and the forming labyrinth layer, but it is no longer expressed in the allantois. At E10.5, Tmed2 expression is similar to E9.5; however, expression in giant cells is restricted to a subset of the layer and expression can be observed in the maternal decidua. In accordance with the expression of Tmed2 in the placenta, E9.5–E10.5 placentas of Tmed2 homozygous mutants fail to form a labyrinth layer, resulting in lethality at mid-gestation, with a subset of embryos failing to undergo chorioallantoic attachment (Jerome-Majewska et al., 2010). TMED2's requirement in the placenta is cell-autonomous in the chorion and non-cell-autonomous in the allantois where it regulates fibronectin and VCAM1 localization during chorioallantoic attachment (Hou & Jerome-Majewska, 2018). These studies indicate a crucial role for TMED2 in the development of the placenta. Furthermore, TMED2 is expressed in the human placental syncytiotrophoblast, cytotrophoblast and stromal cells between the gestational ages of 5.5 and 40 weeks (Zakariyah et al., 2012). Thus, TMED2 may also be crucial during human placental development.
TMED10 is expressed in the embryonic mouse brain at E15 and expression declines with age in the developing postnatal brain (Vetrivel et al., 2008). In the rat central nervous system, TMED10 expression is found in the cortex, hippocampus and brainstem at postnatal day (P) 7 and P21 (Vetrivel et al., 2008). TMED10 is also expressed in the developing postnatal sensory epithelium of the murine inner ear at P3, but not at P14 and P30, suggesting that TMED10 may play a role in inner ear development (Darville & Sokolowski, 2018). The role, if any, of TMED10 in the pre- and post-natal development of the mammalian brain and inner ear remains to be identified.
Overall, TMED proteins are expressed in a tissue-dependent, developmental age-dependent and even sex-specific pattern, suggesting non-redundant functions across various species (Rotter et al., 2002; Carney & Taylor, 2003; Boltz et al., 2007; Jerome-Majewska et al., 2010; Hou et al., 2017). The TMED family is implicated in developmental processes such as wing morphogenesis in Drosophila, reproductive system development in C. elegans, embryonic development in rats and mice and placental development in mice and humans (Jerome-Majewska et al., 2010; Buechling et al., 2011; Hou & Jerome-Majewska, 2018). Furthermore, the role for TMED5 and TMED10 in regulating WNT and TGF-β signalling, respectively, seems to be evolutionarily conserved in Drosophila and humans (Buechling et al., 2011; Port et al., 2011). In addition, components of the Notch family of receptors could also be an evolutionarily conserved cargo of the TMED family. Our growing understanding of the involvement of the TMED family in mammalian development paves the foundation for understanding their potential roles in human diseases.
3. TMED proteins and disease
Abnormalities in the controlled transport of proteins in the secretory pathway contribute to diseases such as cancer. More specifically, aberrant expression of TMED proteins is implicated in cancer, liver disease, pancreatic disease and immune system dysregulation. Although these diseases differ greatly in symptom presentation, they reveal the importance of balanced levels of TMED proteins within cells (Figure 5). Excess TMED within the cell can cause a similar phenotype as having too little TMED protein, which makes this family challenging to study.
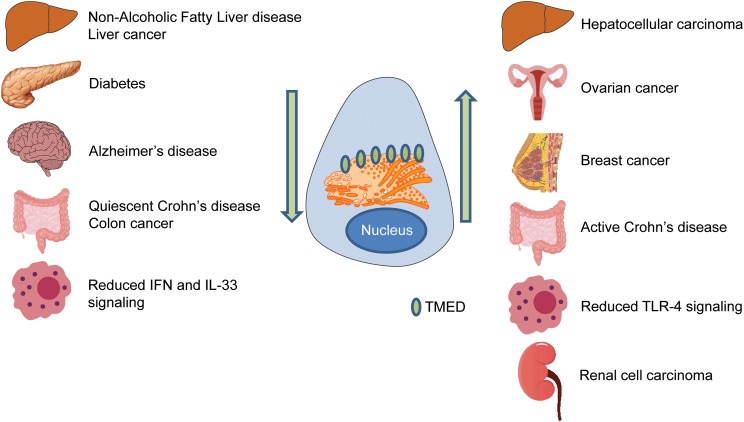
Fig. 5.
TMED family in disease. Disrupted TMED protein levels are associated with a diverse range of diseases. Arrows indicate levels of TMED protein within the cell. Organs represent different diseases associated with TMED proteins. IFN = interferon; TLR = Toll-like receptor.
3.1. TMED proteins and cancer
Current research on TMED proteins suggests an important role in cell proliferation and differentiation, especially with regards to malignancy. TMED2, the sole member of the mammalian β family, displays a cell type-specific role in cancer (Xiong et al., 2010; Shi-peng et al., 2017). In liver cells, TMED2 is required for normal cell proliferation. The aforementioned homozygous null mouse embryos, with no functional TMED2, were smaller than their littermates prior to death at mid-gestation, suggesting a requirement for TMED2 in normal cell proliferation (Table 4) (Jerome-Majewska et al., 2010). Moreover, heterozygotes were more susceptible to liver cancer compared to their wild-type littermates (Hou et al., 2017). Therefore, in liver cells, a reduction in TMED2 results in increased liver tumorigenesis and demonstrates a possible tumour suppressor-like property for TMED2. In contrast, elevated levels of TMED2 were found in ovarian carcinoma patients (Shi-peng et al., 2017), and ectopic expression of TMED2 in ovarian cancer cells resulted in increased cell proliferation and cell migration, characteristic of metastatic cells. Furthermore, Xiong and colleagues reported that ectopic expression of TMED2 accelerates cell proliferation in murine bone cells, MC3T3-E13 (Xiong et al., 2010). Elevated expression of TMED2 in breast cancer patients was identified as an unfavourable prognostic factor (Lin et al., 2019). Thus, depending on cell type, TMED2 may exert either tumour suppressor or oncogenic properties.
Much like TMED2, the role of TMED3 in cancer is cell type-specific. TMED3 expression is upregulated in a number of tumours. TMED3 was identified as a prognostic biomarker of renal cell carcinoma, since higher TMED3 expression levels were found in high-stage and -grade cohorts when compared to low-stage and -grade cohorts (Ha et al., 2019). TMED3 was also identified as a potential drug target for prostate cancer since it is elevated in patient tumour samples (Vainio et al., 2012). In addition, TMED3 expression is upregulated in human hepatocellular carcinoma tissues. Its expression was highest in metastatic hepatocellular carcinomas when compared to non-metastatic tumours. In cell culture, TMED3 promotes the proliferation, migration and invasion of breast cancer cells, MCF-7 and MDA-MB-231 (Pei et al., 2019). In fact, TMED3 knockdown inhibited hepatocellular carcinoma cell migration, whereas TMED3 overexpression enhanced cell motility. In liver cells, it is thought to promote metastasis through the IL-11/STAT3 signalling pathway, as both IL-11 and STAT3 phosphorylation levels are elevated in cells overexpressing TMED3 (Zheng et al., 2016).
However, although TMED3 seems oncogenic in hepatocellular carcinomas, renal cell carcinomas, prostate cancers and breast cancers, it appears to have tumour suppressor properties in human colon cancers. In fact, TMED3 was recovered from a genome-wide in vivo screen for metastatic suppressors in human colon cancer (Duquet et al., 2014). When TMED3 was knocked down in colon cancer cells, TMED9 was upregulated more than twofold. TMED3-mediated WNT signalling is proposed to inhibit metastasis by repressing TMED9. In the same study, loss of TMED3 resulted in increased TMED9 levels and was correlated with increased TGF-β signalling and upregulation of genes with migratory and invasive roles, while also repressing WNT signalling (Mishra et al., 2019). Thus, similarly to TMED2, the malignant properties of TMED3 are cell type-specific. Although TMED9 has not been investigated in other cancers, in colon cancer it functions as an oncogene.
TMED10 has an important role in limiting TGF-β signalling. TGF-β is a secreted cytokine that modulates cell proliferation, differentiation and apoptosis, and it is implicated in numerous cancers. TMED10 regulates the dissociation of the TGF-β heteromeric I/II receptor complex. In fact, an isolated small peptide derived from the extracellular domain of TMED10 is sufficient to antagonize TGF-β signalling, which could be used as a therapy for cancers with abnormal TGF-β signalling activity (Nakano et al., 2017).
Our understanding of the contribution of disrupted TMED levels in cancer is in its infancy. However, it appears that their role is tissue- and context-dependent. Since, TMED proteins may promote or inhibit cancer onset and progression, it is important to identify the specific contributions of individual TMED proteins. Intriguingly, TMED proteins may also form regulatory loops to regulate each other's levels during cancer progression.
3.2. Dysregulated immune responses
TMED1 interaction with ST2L, a member of the IL-R family, regulates signalling of IL-33, a proinflammatory cytokine. Following TMED1 knockdown, production of IL-6 and IL-8 – downstream targets of IL-33 – was impaired, indicating that the absence of TMED1 reduced IL-33 signalling. Surprisingly, the interaction between TMED1 and ST2L was found to occur via a protrusion of the GOLD domain of TMED1 into the cytosolic compartment (Connolly et al., 2013).
TMED2 is necessary for cellular interferon (IFN) responses to viral DNA. MITA (mediator of IRF3 activation also known as STING) has a vital role in innate immune responses to cytosolic viral dsDNA. The luminal domain of TMED2 associates with MITA and stabilizes dimerization of MITA following viral infection. Association with MITA facilitates translocation into the ER, as well as anterograde trafficking from the ER to the Golgi. Consequently, suppression or deletion of TMED2 in cells markedly increased titre volume of herpes simplex virus 1 (HSV-1) and led to impaired IFN-1 production upon HSV-1 infection. Moreover, although TMED10 knockdown led to reduced CXCL10 levels, it did not interact directly with MITA and did not disrupt the synergy of TMED2 on cGAS-MITA-mediated activation of an IFN-stimulated response element. Thus, TMED2 is essential for IFN responses to viral DNA, and it is required for the translocation, localization and dimerization of MITA (Sun et al., 2018).
Interestingly, TMED2 was recently reported to play a role in Crohn's disease through the regulation of the dimeric state of AGR2 (anterior gradient 2) (Maurel et al., 2019). Dimeric AGR2 protein acts as a sensor of ER homeostasis. In the presence of ER stress, AGR2 dimers are disrupted and AGR2 monomers are secreted to induce inflammation in the cell. Secreted AGR2 monomers may act as a systemic alarm signal for proinflammatory responses. When TMED2 levels are lower in the cell, partial homeostasis is achieved, resulting in some inflammation from the increase in monomeric AGR2. Thus, low levels of TMED2 are associated with quiescent Crohn's disease as a consequence of fewer AGR2 dimers, causing some inflammation. However, when TMED2 levels are high in the cell, homeostasis is entirely disrupted, resulting in severe acute inflammation (active Crohn's disease). In fact, Crohn's patients with severe acute inflammation had high levels of TMED2 and disrupted autophagy (Maurel et al., 2019). Since the dimeric state of AGR2 is regulated by TMED2, stable levels of TMED2 in the cell may work to suppress inflammatory responses.
TMED7, a member of the γ family, has a critical role in the negative regulation of TLR4 (Toll-like receptor) signalling. Following lipopolysaccharide (LPS) stimulation of human monocytes, TMED7 shows a biphasic increase. Moreover, TMED7 localized mostly in the Golgi and in endosomes, with increased localization to late endosomes following LPS stimulation. Homotypic interaction between TMED7 and TAG disrupts the formation of the TRIF/TRAM complex, inhibiting the MyD88-independent TLR4 signalling pathway. Thus, TMED7 may be a critical inhibitor of TLR4 signalling and innate immune signalling (Doyle et al., 2012).
It is noteworthy that only TMED7 and TMED1 have their GOLD domains in the cytosol (Doyle et al., 2012; Connolly et al., 2013). No other TMED protein has been shown to have a cytosolic GOLD domain, suggesting that this may be a unique feature of these two γ family members.
3.3. Pancreatic disease
An insulinoma is a rare neuroendocrine insulin-secreting tumour derived from pancreatic β cells. Most insulinomas are benign in that they grow exclusively at their site of origin, but a minority metastasize. The aforementioned TMED10 and pancreatic secretory granules study showed that TMED10 is localized to the plasma membrane of pancreatic β cells, where it is involved in the quality control, folding and secretion of insulin molecules (Blum et al., 1996; Hosaka et al., 2007; Zhang & Volchuk, 2010). Knockdown of TMED10 markedly impaired insulin biosynthesis and release; however, the mechanisms by which this occurs are still unclear. In fact, knockdown of TMED10 does not impact total protein levels, suggesting that the effects of TMED10 on insulin biosynthesis are independent of its other secretory pathway functions.
TMED6 has also been implicated in diabetes. Its expression was significantly lower in diabetic rats (Wang et al., 2012), and knockdown of TMED6 in mouse pancreatic Min6 β cells resulted in decreased insulin secretion (Fadista et al., 2014). TMED6 expression in pancreatic islets of patients with type 2 diabetes was reduced when compared to control islets isolated from healthy patients. Thus, TMED6 seems also to be required for normal pancreatic function and insulin secretion (Fadista et al., 2014).
3.4. Dementia and Alzheimer's disease
Alzheimer's disease is characterized by the extracellular accumulation of amyloid-β (Aβ) peptides in the brain. Amyloid precursor protein is cleaved by γ-secretases to generate Aβ, which can accumulate into a plaque, causing neurological deficits. In mouse studies, TMED10 expression was widespread throughout the grey matter of the brain, but it was predominantly localized to the presynaptic membranes of neuronal junctions. As amyloid precursor processing enzymes like γ-secretase were also localized to presynaptic membranes, the presence of TMED10 at presynaptic junctions suggests a role in Aβ secretion (Laßek et al., 2013; Liu et al., 2015). In addition, TMED10 also suppresses the transport of amyloid precursor proteins through the secretory pathway, resulting in less accumulation of fully processed amyloid precursor protein. TMED9 also reduced Aβ accumulation in the brain (Hasegawa et al., 2010). Therefore, TMED9 and TMED10 might play an important role in preventing Alzheimer's disease pathogenesis in humans (Chen et al., 2006; Hasegawa et al., 2010; Liu et al., 2015).
3.5. Liver disease
Work from our laboratory showed that Tmed2 may play a role in the progression of non-alcoholic fatty liver disease (NAFLD) (Hou et al., 2017), which is the major cause of chronic liver disease worldwide (Abd El-Kader & El-Den Ashmawy, 2015). Low TMED2 levels were associated with a corresponding decrease in TMED10 levels. In this novel NAFLD model, TMED2 was not required for activation of the tunicamycin-associated unfolded protein response. However, livers isolated from heterozygous mice had dilated ER membranes and increased levels of eIF2α, suggesting ER stress and activation of the PERK unfolded protein response (Hou et al., 2017). Histological studies of mouse livers showed that 28% of heterozygous mice displayed clinical features associated with NAFLD by the age of 6 months.
4. Future directions
Our understanding of TMED proteins in development and disease is in its infancy, and in fact little is known regarding the primate-specific TMED11. These proteins are evolutionarily conserved, and their localization and levels are tightly regulated by the cellular machinery. As discussed, individual TMED proteins are important for morphogenesis, differentiation and homeostasis in a number of organisms (Carney & Taylor, 2003; Boltz et al., 2007; Vetrivel et al., 2008; Jerome-Majewska et al., 2010; Graveley et al., 2011; Zakariyah et al., 2012; Darville & Sokolowski, 2018). However, although their roles in cargo transport are clearly important and numerous putative cargo proteins are regulated by TMED proteins (Tables 2 & 3), little is understood of the impact of most TMED proteins in development and disease. TMED proteins are found to interact with lipids and with high specificity for specific carbon chain length, begging the question of their contribution to the structure and integrity of the various organelles in which they are found. There are no unifying themes emerging on the requirement, localization or function of TMED proteins in the many organs in which they are expressed. In fact, current data challenge the longstanding notion that TMED proteins are redundant. Thus, it is imperative that individual TMED proteins be studied. In addition, TMED protein oligomerization – a longstanding assumption – is poorly understood and may also be tissue- and temporal-specific. The finding that loss of a TMED protein does not always result in loss of associating members makes a very strong case for studying how TMED proteins interact within different tissues and organisms. Finally, point mutations in the GOLD domain, deletions of large portions of the gene or premature stop codons before the carboxyl domain of TMED proteins all result in loss of function. Thus, it is enticing to speculate that variants found in human TMED genes may directly contribute to the diseases in which they are misregulated.
Acknowledgements
We would like to thank members of the Jerome-Majewska lab for comments on the manuscript.
Financial support
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2015-06699). LAJ-M is a member of the Research Centre of the McGill University Health Centre, which is supported in part by Fonds de la recherche en santé du Québec (FRQS).
Conflict of interest
None.
References
- Abd El-Kader S. M. and El-Den Ashmawy E. M. (2015). Non-alcoholic fatty liver disease: the diagnosis and management. World Journal of Hepatology 7(6), 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenbrey C., Kemayo-Koumkoua P., Salnikov E. S., Glattard E. and Bechinger B. (2019). Investigations of the structure, topology, and interactions of the transmembrane domain of the lipid-sorting protein p24 being highly selective for sphingomyelin-C18. Biochemistry 58(24), 2782–2795. [DOI] [PubMed] [Google Scholar]
- Anantharaman V. and Aravind L. (2002). The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biology 3(5), research0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewski S., Luschnig S., Desjeux I., Grosshans J. and Nusslein-Volhard C. (2004). Drosophila p24 homologues eclair and baiser are necessary for the activity of the maternally expressed Tkv receptor during early embryogenesis. Mechanisms of Development 121(10), 1259–1273. [DOI] [PubMed] [Google Scholar]
- Blum R. and Lepier A. (2008). The luminal domain of p23 (Tmp21) plays a critical role in p23 cell surface trafficking. Traffic 9(9), 1530–1550. [DOI] [PubMed] [Google Scholar]
- Blum R., Feick P., Puype M., Vandekerckhove J., Klengel R., Nastainczyk W. and Schulz I. (1996). Tmp21 and p24A, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. Journal of Biological Chemistry 271(29), 17183–17189. [DOI] [PubMed] [Google Scholar]
- Boltz K. A., Ellis L. L. and Carney G. E. (2007). Drosophila melanogaster p24 genes have developmental, tissue-specific, and sex-specific expression patterns and functions. Developmental Dynamics 236(2), 544–555. [DOI] [PubMed] [Google Scholar]
- Bonnon C., Wendeler M. W., Paccaud J. P. and Hauri H. P. (2010). Selective export of human GPI-anchored proteins from the endoplasmic reticulum. Journal of Cell Science 123(Pt 10), 1705–1715. [DOI] [PubMed] [Google Scholar]
- Bouw G., Van Huizen R. Jansen E. J. and Martens G. J. (2004). A cell-specific transgenic approach in Xenopus reveals the importance of a functional p24 system for a secretory cell. Molecular Biology of the Cell 15(3), 1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechling T., Chaudhary V., Spirohn K., Weiss M. and Boutros M. (2011). p24 proteins are required for secretion of Wnt ligands. EMBO Reports 12(12), 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney G. E. and Bowen N. J. (2004). p24 proteins, intracellular trafficking, and behavior: Drosophila melanogaster provides insights and opportunities. Biol Cell 96(4), 271–278. [DOI] [PubMed] [Google Scholar]
- Carney G. E. and Taylor B. J. (2003). Logjam encodes a predicted EMP24/GP25 protein that is required for Drosophila oviposition behavior. Genetics 164(1), 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon G. A., Aguilera-Romero A., Manzano-Lopez J., et al. (2011). The yeast p24 complex regulates GPI-anchored protein transport and quality control by monitoring anchor remodeling. Molecular Biology of the Cell 22(16), 2924–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Hasegawa H., Schmitt-Ulms G., et al. (2006). TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature 440(7088), 1208–1212. [DOI] [PubMed] [Google Scholar]
- Connolly D. J., O'Neill L. A. and McGettrick A. F. (2013). The GOLD domain-containing protein TMED1 is involved in interleukin-33 signaling. Journal of Biological Chemistry 288(8), 5616–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras F. X., Ernst A. M., Haberkant P., et al. (2012). Molecular recognition of a single sphingolipid species by a protein's transmembrane domain. Nature 481(7382), 525–529. [DOI] [PubMed] [Google Scholar]
- Dancourt J. and Barlowe C. (2010). Protein sorting receptors in the early secretory pathway. Annual Review of Biochemistry 79, 777–802. [DOI] [PubMed] [Google Scholar]
- Darville L. N. F. and Sokolowski B. H. A. (2018). Label-free quantitative mass spectrometry analysis of differential protein expression in the developing cochlear sensory epithelium. Proteome Science 16, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzel A., Otto F., Girod A., et al. (2000). The p24 family member p23 is required for early embryonic development. Current Biology 10(1), 55–58. [DOI] [PubMed] [Google Scholar]
- Dominguez M., Dejgaard K., Füllekrug J., et al. (1998). gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. Journal of Cell Biology 140(4), 751––765.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S. L., Husebye H., Connolly D. J., Espevik T., O'Neill L. A. and McGettrick A. F. (2012). The GOLD domain-containing protein TMED7 inhibits TLR4 signalling from the endosome upon LPS stimulation. Nature Communications 3, 707. [DOI] [PubMed] [Google Scholar]
- Duquet A., Melotti A., Mishra S., Malerba M., Seth C., Conod A. and Ruiz i Altaba A. (2014). A novel genome-wide in vivo screen for metastatic suppressors in human colon cancer identifies the positive WNT–TCF pathway modulators TMED3 and SOX12. EMBO Molecular Medicine 6(7), 882–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A. M. and Brügger B. (2014). Sphingolipids as modulators of membrane proteins. Biochimica et Biophysica Acta 1841(5), 665–670. [DOI] [PubMed] [Google Scholar]
- Fadista J., Vikman P., Laakso E. O., et al. (2014). Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America 111(38), 13924–13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K. and Rothman J. E. (1997). Sorting determinants in the transmembrane domain of p24 proteins. Journal of Biological Chemistry 272(40), 24739–24742. [DOI] [PubMed] [Google Scholar]
- Fujita M., Watanabe R., Jaensch N., et al. (2011). Sorting of GPI-anchored proteins into ER exit sites by p24 proteins is dependent on remodeled GPI. Journal of Cell Biology 194(1), 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J., Suganuma T., Tang B. L., Hong W., Storrie B. and Nilsson T. (1999). Localization and recycling of gp27 (hp24gamma3): complex formation with other p24 family members. Molecular Biology of the Cell 10(6), 1939–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbes L., Kim K., Riess A., et al. (2015). Mutations in SEC24D, encoding a component of the COPII machinery, cause a syndromic form of osteogenesis imperfecta. American Journal of Human Genetics 96(3), 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., et al. (2011). The developmental transcriptome of Drosophila melanogaster. Nature 471(7339), 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Liu L. and Nishimura M. (2010). Dilysine retrieval signal-containing p24 proteins collaborate in inhibiting gamma-cleavage of amyloid precursor protein. Journal of Neurochemistry 115(3), 771–781. [DOI] [PubMed] [Google Scholar]
- Ha M., Moon H., Choi D., et al. (2019). Prognostic role of TMED3 in clear cell renal cell carcinoma: a retrospective multi-cohort analysis. Frontiers in Genetics 10, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horer J., Blum R., Feick P., Nastainczyk W. and Schulz I. (1999). A comparative study of rat and human Tmp21 (p23) reveals the pseudogene-like features of human Tmp21-II. DNA Sequence 10(2), 121–126. [DOI] [PubMed] [Google Scholar]
- Hosaka M., Watanabe T., Yamauchi Y., et al. (2007). A subset of p23 localized on secretory granules in pancreatic beta-cells. Journal of Histochemistry and Cytochemistry 55(3), 235–245. [DOI] [PubMed] [Google Scholar]
- Hou W. and Jerome-Majewska L. A. (2018). TMED2/emp24 is required in both the chorion and the allantois for placental labyrinth layer development. Developmental Biology 444(1), 20–32. [DOI] [PubMed] [Google Scholar]
- Hou W., Gupta S., Beauchamp M.-C., Yuan L. and Jerome-Majewska L. A. (2017). Non-alcoholic fatty liver disease in mice with heterozygous mutation in TMED2. PLoS ONE 12(8), e0182995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne N., Frey K., Brugger B. and Wieland F. T. (2002). Oligomeric state and stoichiometry of p24 proteins in the early secretory pathway. Journal of Biological Chemistry 277(48), 46504–46511. [DOI] [PubMed] [Google Scholar]
- Jerome-Majewska L. A., Achkar T., Luo L., Lupu F. and Lacy E. (2010). The trafficking protein Tmed2/p24beta(1) is required for morphogenesis of the mouse embryo and placenta. Developmental Biology 341(1), 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junfeng H., Ming Z., Sean F., et al. (2015). The identification of novel protein–protein interactions in liver that affect glucagon receptor activity. PLoS ONE 10(6), e0129226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper R. P., Waterham H. R., Rotter J., Bouw G. and Martens G. J. (2000). Differential induction of two p24delta putative cargo receptors upon activation of a prohormone-producing cell. Molecular Biology of the Cell 11(1), 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper R. P., Bouw G., Janssen K. P., Rotter J., van Herp F. and Martens G. J. (2001). Localization of p24 putative cargo receptors in the early secretory pathway depends on the biosynthetic activity of the cell. Biochemical Journal 360(Pt 2), 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laßek M., Weingarten J., Einsfelder U., Brendel P., Muller U. and Volknandt W. (2013). Amyloid precursor proteins are constituents of the presynaptic active zone. Journal of Neurochemistry 127(1), 48–56. [DOI] [PubMed] [Google Scholar]
- Li X., Wu Y., Shen C., Belenkaya T. Y., Ray L. and Lin X. (2015). Drosophila p24 and Sec22 regulate Wingless trafficking in the early secretory pathway. Biochemical and Biophysical Research Communications 463(4), 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Liu J., Hu S. F. and Hu X. (2019). Increased expression of TMED2 is an unfavorable prognostic factor in patients with breast cancer. Cancer Management and Research 11, 2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Fujino K. and Nishimura M. (2015). Pre-synaptic localization of the γ-secretase-inhibiting protein p24α2 in the mammalian brain. Journal of Neurochemistry 133(3), 422–431. [DOI] [PubMed] [Google Scholar]
- Luo W., Wang Y. and Reiser G. (2007). p24A, a type I transmembrane protein, controls ARF1-dependent resensitization of protease-activated receptor-2 by influence on receptor trafficking. Journal of Biological Chemistry 282(41), 30246–30255. [DOI] [PubMed] [Google Scholar]
- Luo W., Wang Y. and Reiser G. (2011). Proteinase-activated receptors, nucleotide P2Y receptors, and -opioid receptor-1B are under the control of the type I transmembrane proteins p23 and p24A in post-Golgi trafficking. Journal of Neurochemistry 117(1), 71–81. [DOI] [PubMed] [Google Scholar]
- Majoul I., Straub M., Hell S. W., Duden R. and Soling H. D. (2001). KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Developmental Cell 1(1), 139–153. [DOI] [PubMed] [Google Scholar]
- Marzioch M., Henthorn D. C., Herrmann J. M., et al. (1999). Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Molecular Biology of the Cell 10(6), 1923–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M., Obacz J., Avril T., et al. (2019). Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Molecular Medicine 11(6), e10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merte J., Jensen D., Wright K., Sarsfield S., Wang Y., Schekman R. and Ginty D. D. (2010). Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nature Cell Biology 12(1), 41–46; sup pp 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Bernal C., Silvano M., Anand S. and Ruiz i Altaba A. (2019). The protein secretion modulator TMED9 drives CNIH4/TGFα/GLI signaling opposing TMED3-WNT–TCF to promote colon cancer metastases. Oncogene 38(29), 5817–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos JC, Sturm S., Langhans M., Hillmer S., Marcote M. J., Robinson D. G. and Aniento F. (2012). Coupled transport of Arabidopsis p24 proteins at the ER–Golgi interface. Journal of Experimental Botany 63(11), 4243–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J. C., Langhans M., Sturm S., Hillmer S., Aniento F., Robinson D. G. and Marcote M. J. (2013). Putative p24 complexes in Arabidopsis contain members of the delta and beta subfamilies and cycle in the early secretory pathway. Journal of Experimental Botany 64(11), 3147–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz M., Nuoffer C., Hauri H. P. and Riezman H. (2000). The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. Journal of Cell Biology 148(5), 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae M., Hirata T., Morita-Matsumoto K., Theiler R., Fujita M., Kinoshita T. and Yamaguchi Y. (2016). 3D structure and interaction of p24beta and p24delta Golgi dynamics domains: implication for p24 complex formation and cargo transport. Journal of Molecular Biology 428(20), 4087–4099. [DOI] [PubMed] [Google Scholar]
- Nagae M., Liebschner D., Yamada Y., et al. (2017). Crystallographic analysis of murine p24gamma2 Golgi dynamics domain. Proteins 85(4), 764–770. [DOI] [PubMed] [Google Scholar]
- Nakano N., Tsuchiya Y., Kako K., et al. (2017). TMED10 protein interferes with transforming growth factor (TGF)-beta signaling by disrupting TGF-beta receptor complex formation. Journal of Biological Chemistry 292(10), 4099–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Cantizano N, Montesinos J. C., Bernat-Silvestre C., Marcote M. J. and Aniento F. (2016). p24 family proteins: key players in the regulation of trafficking along the secretory pathway. Protoplasma 253(4), 967–985. [DOI] [PubMed] [Google Scholar]
- Pei J., Zhang J., Yang X., Wu Z., Sun C., Wang Z. and Wang B. (2019). TMED3 promotes cell proliferation and motility in breast cancer and is negatively modulated by miR-188-3p. Cancer Cell International 19, 75. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Port F., Hausmann G. and Basler K. (2011). A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO Reports 12(11), 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter J., Kuiper R. P., Bouw G. and Martens G. J. (2002). Cell-type-specific and selectively induced expression of members of the p24 family of putative cargo receptors. Journal of Cell Science 115(Pt 5), 1049–1058. [DOI] [PubMed] [Google Scholar]
- Routledge K. E., Gupta V. and Balch W. E. (2010). Emergent properties of proteostasis–COPII coupled systems in human health and disease. Molecular Membrane Biology 27(8), 385–397. [DOI] [PubMed] [Google Scholar]
- Saleem S., Schwedes C. C., Ellis L. L., et al. (2012). Drosophila melanogaster p24 trafficking proteins have vital roles in development and reproduction. Mechanisms of Development 129(5–8), 177–191. [DOI] [PubMed] [Google Scholar]
- Schuiki I. and Volchuk A. (2012). Diverse roles for the p24 family of proteins in eukaryotic cells. Biomolecular Concepts 3(6), 561–570. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Keller P., Scheiffele P., Mann M. and Simons K. (1997). Identification of components of trans-Golgi network-derived transport vesicles and detergent-insoluble complexes by nanoelectrospray tandem mass spectrometry. Electrophoresis 18(14), 2591–2600. [DOI] [PubMed] [Google Scholar]
- Shi-peng G., Chun-lin C., Huan W., et al. (2017). TMED2 promotes epithelial ovarian cancer growth. Oncotarget 8(55), 94151–94165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanchick A. and Breitwieser G. E. (2010). The cargo receptor p24A facilitates calcium sensing receptor maturation and stabilization in the early secretory pathway. Biochemical and Biophysical Research Communications 395(1), 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling C. J., Rothblatt J., Hosobuchi M., Deshaies R. and Schekman R. (1992). Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Molecular Biology of the Cell 3(2), 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strating J. R., Bouw G., Hafmans T. G. and Martens G. J. (2007). Disparate effects of p24alpha and p24delta on secretory protein transport and processing. PLoS ONE 2(8), e704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strating J. R., Hafmans T. G. and Martens G. J. (2009). Functional diversity among p24 subfamily members. Biology of the Cell 101(4), 207–219. [DOI] [PubMed] [Google Scholar]
- Sundaram M. and Greenwald I. (1993). Suppressors of a lin-12 hypomorph define genes that interact with both lin-12 and glp-1 in Caenorhabditis elegans. Genetics 135(3), 765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M. S., Zhang J., Jiang L. Q., et al. (2018). TMED2 potentiates cellular IFN responses to DNA viruses by reinforcing MITA dimerization and facilitating its trafficking. Cell Reports 25(11), 3086–3098.e3. [DOI] [PubMed] [Google Scholar]
- Takida S., Maeda Y. and Kinoshita T. (2008). Mammalian GPI-anchored proteins require p24 proteins for their efficient transport from the ER to the plasma membrane. Biochemistry Journal 409(2), 555–562. [DOI] [PubMed] [Google Scholar]
- Theiler R., Fujita M, Nagae M., Yamaguchi Y., Maeda Y. and Kinoshita T. (2014). The alpha-helical region in p24gamma2 subunit of p24 protein cargo receptor is pivotal for the recognition and transport of glycosylphosphatidylinositol-anchored proteins. Journal of Biological Chemistry 289(24), 16835–16843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio P., Mpindi J. P., Kohonen P., et al. (2012). High-throughput transcriptomic and RNAi analysis identifies AIM1, ERGIC1, TMED3 and TPX2 as potential drug targets in prostate cancer. PLoS ONE 7(6), e39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel K. S., Kodam A., Gong P., Chen Y., Parent A. T., Kar S. and Thinakaran G. (2008). Localization and regional distribution of p23/TMP21 in the brain. Neurobiology of Disease 32(1), 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C. (2016). ER to Golgi-dependent protein secretion: the conventional pathway. Methods in Molecular Biology 1459, 3–29. [DOI] [PubMed] [Google Scholar]
- Wada Y., Sun-Wada G. H., Kawamura N. and Yasukawa J. (2016). Membrane dynamics in mammalian embryogenesis: implication in signal regulation. Birth Defects Research. Part C, Embryo Today: Reviews 108(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang R., Jadhao S. B., et al. (2012). Transmembrane emp24 protein transport domain 6 is selectively expressed in pancreatic islets and implicated in insulin secretion and diabetes. Pancreas 41(1), 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C. and Greenwald I. (1999). p24 proteins and quality control of LIN-12 and GLP-1 trafficking in Caenorhabditis elegans. Journal of Cell Biology 145(6), 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Lu Y., Zhang L., et al. (2010). Discovery of novel cell proliferation-enhancing gene by random siRNA library based combinatorial screening. Combinatorial Chemistry & High-Throughput Screeninh 13(9), 798–806. [DOI] [PubMed] [Google Scholar]
- Yehia L., Niazi F., Ni Y., et al. (2015). Germline heterozygous variants in SEC23B are associated with Cowden syndrome and enriched in apparently sporadic thyroid cancer. American Journal of Human Genetics 97(5), 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakariyah A., Hou W., Slim R. and Jerome-Majewska L. (2012). TMED2/p24b1 is expressed in all gestational stages of human placentas and in choriocarcinoma cell lines. YPLAC Placenta 33(3):214–219. [DOI] [PubMed] [Google Scholar]
- Zhang L. and Volchuk A. (2010). p24 family type 1 transmembrane proteins are required for insulin biosynthesis and secretion in pancreatic beta-cells. FEBS Letters 584(11), 2298–2304. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yue H., Wang C., et al. (2017). Novel mutations in the SEC24D gene in Chinese families with autosomal recessive osteogenesis imperfecta. Osteoporosis International 28(4), 1473–1480. [DOI] [PubMed] [Google Scholar]
- Zheng H., Yang Y., Han J., et al. (2016). TMED3 promotes hepatocellular carcinoma progression via IL-11/STAT3 signaling. Scientific Reports 6, 37070. [DOI] [PMC free article] [PubMed] [Google Scholar]