Abstract
Background
Neonatal jaundice (NNJ) is a frequent complication of glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Objectives
To estimate the prevalence of G6PD deficiency among neonates with jaundice and to assess mothers’ perception towards G6PD and NNJ.
Methods
A cross-sectional study was carried out on 487 ethnic Egyptian neonates with indirect hyperbilirubinaemia from June 2018 to July 2019. The collected data included maternal and neonatal characteristics. Laboratory investigations included serum bilirubin, reticulocyte count, ABO grouping, Rh typing and neonatal serum G6PD test. Mothers were interviewed individually using a structured, researcher-administered questionnaire to assess their perceptions of G6PD deficiency and NNJ.
Results
The prevalence of G6PD deficiency was 10.10%. Neonates with G6PD deficiency showed higher levels of serum bilirubin (p<0.001). Male gender, family history of G6PD deficiency and consanguinity were risk factors for G6PD deficiency (OR=4.27, 95% CI 1.66 - 10.99; OR=9.54, 95% CI 4.80- 18.95; OR=10.219, 95% CI 5.39 - 19.33, respectively). Mothers’ perceptions of NNJ and G6PD were low, with only 30% having good knowledge on NNJ and 17.10% on G6PD deficiency, 46.8% with positive attitude towards NNJ and 45.0% towards G6PD deficiency, and 29.9% with good practice towards NNJ and 19.9% towards G6PD deficiency.
Conclusion
G6PD deficiency seems to be an important cause of NNJ. Mothers’ perceptions of both NNJ and G6PD deficiency were low. A mass health education programme on both of these diseases is needed to ensure better and early detection, good timing of treatment, and better prevention of the triggering factors to ensure better health for children.
Keywords: epidemiology, public health, community child health, family medicine, G6PD
Strengths and limitations of this study.
The study collectively assessed the prevalence and risk factors of glucose-6-phosphate dehydrogenase (G6PD) deficiency, aside from assessing mothers’ level of knowledge, attitude and practice (KAP) towards both G6PD deficiency and neonatal jaundice (NNJ).
The study clarified the extent of change in NNJ based on previous levels published in some research articles in the same region, and also drew on how G6PD deficiency is a poorly known disease despite it being serious.
A suitable sample size was studied in a short period and allowed us to reach a large number of mothers and families.
There was a need to carry out more investigations to mothers, such as a direct Coombs test.
A post-test to assess the extent of understanding and KAP among mothers was needed; however, it was difficult to collect the same number of study participants one more time.
KAP towards these diseases needs to be assessed in the general population to ensure risk factors are identified and addressed, and we also tried to help mothers deliver and share with their families the knowledge they gained from the health education session that was conducted.
Introduction
The term ‘jaundice’ is used to describe the yellow-orange discolouration of the skin and conjunctiva due to excessive bilirubin in the skin and mucous membranes.1 2 It is not a disease, but a symptom or sign of a disease.3 Jaundice (hyperbilirubinaemia), although a common benign occurrence in the first week of life, can sometimes progress to critical levels.4 Neonatal jaundice (NNJ) is a frequent complication of glucose-6-phosphate dehydrogenase (G6PD) deficiency, which is a genetic disease more often observed in male patients as this is an X linked enzymatic deficiency. However, in female patients, it might present deficient activity levels severe enough to induce haemolysis even if they are heterozygous.5 6 African, Asian, Mediterranean and Middle-Eastern descents are most commonly affected by this disorder.7 8 The prevalence of G6PD deficiency among Egyptian neonates is 8.9%.9 G6PD enzymatic deficiency was first reported in India, and its prevalence varies a lot from place to place.9 Haemolysis in patients with G6PD deficiency can be triggered by specific agents, including certain foods, drugs and infections.6 Avoiding the triggers and starting treatment early, including intensive phototherapy, to decrease the incidence of severe haemolysis are simple and inexpensive and can be started before the symptoms appear.8–10 Counselling with regard to avoiding the risks of jaundice and triggering agents should be directed to parents of deficient newborns.10 G6PD deficiency is commonly known in Egypt as fava bean anaemia or favism (after intake of fava bean), as this enzyme deficiency increases the susceptibility of red blood cells to agents such as oxidants present in raw beans, some medications and oxidative stress caused by infections.11 There are few studies conducted on the prevalence of G6PD deficiency among Egyptian neonates with jaundice,9 as well as on the knowledge, attitude and practice (KAP) towards NNJ or G6PD deficiency.11 12 However, data on the prevalence and measurement of KAP in both NNJ and G6PD deficiency from a single study are not available. This study aimed to estimate the prevalence of G6PD deficiency among Egyptian neonates with jaundice and assess mothers’ perceptions by studying their level of KAP towards G6PD deficiency and NNJ to ensure avoidance of triggering factors.
Subjects and methods
A cross-sectional study was carried out in Egypt on 487 neonates with indirect hyperbilirubinaemia from June 2018 to June 2019 at three Egyptian neonatal and paediatric centres, medically serving their population and also receiving patients from the surrounding areas. Mothers with their neonates visiting these paediatric and neonatal centres to seek medical advice for treatment of jaundice were from some Egyptian governorates, mainly Menoufia Governorate (the place of the study) and the surrounding governorates. At 0–10 days of age, admitted term and preterm neonates with clinically evident jaundice were included in the study. The exclusion criteria were neonates with direct hyperbilirubinaemia >20% (conjugated hyperbilirubinaemia exists when more than 20% of the total bilirubin or more than 2 mg/dL is conjugated; if neither criterion is met, hyperbilirubinaemia is classified as unconjugated), metabolism errors, congenital anomalies and sepsis. All these exclusion criteria were set to be more focused on G6PD deficiency as a single cause of NNJ in this study. The collected data included maternal and neonatal characteristics such as gestational age (on admission), parity, gravidity, neonatal gender, weight and age of onset of jaundice (maternal recall). The neonates had been previously subjected to laboratory investigations, including serum bilirubin (total, direct, indirect), reticulocyte count, ABO grouping and Rh typing of the mother and the baby, Coombs test (for the baby only), and C reactive protein (as routine and to exclude sepsis). Ultraviolet-kinetic method using cellular enzyme determination reagents by spectrophotometry was used to obtain a quantitative estimation of serum G6PD from 1 mL of whole blood collected in an EDTA tube. G6PD deficiency was defined as <4.6 g/L Hb.10 The neonates were subjected to phototherapy according to phototherapy guidelines for hospitalised infants from ≥35 weeks of gestation.13 To assess perception of G6PD deficiency and NNJ, mothers of neonates with jaundice (N=487) and mothers of neonates currently with no jaundice (n=3) (excluded from the study due to very small number) were interviewed individually during admission by a trained team of data collectors. Mothers of neonates receiving medical examination received researcher-administered questionnaire. We interviewed mothers of neonates with and without jaundice while seeking medical advice. The mothers of neonates with jaundice were interviewed before informing them about having a G6PD deficient baby. The questionnaire was designed by experts in paediatrics and public health specialties, and was based on their experience in paediatrics and public involvement, as well as on published reviews of literature. A pilot study was conducted on about 30 mothers (about 6% of the calculated sample) to test the adequacy of the questionnaire with regard to content, language and time consumed to answer, and to explore the potential obstacles and difficulties with regard to execution of the study. It was also used as a tool to train the team of data collectors (five nurses and three junior staff) to avoid interobserver and intraobserver bias. Training was conducted with the working team for 2 days, followed by assessment of the degree of response to training and the quality of asking questions and of reporting data. For NNJ, the questionnaire (online supplementary questionnaire) included questions such as the mother’s knowledge on diagnosis, causes, complications and treatment. With regard to the attitude of mothers towards NNJ and its treatment, the questions included whether the mother thinks that NNJ is a worrisome condition and so on. With regard to practice, the questionnaire asked whether the mother would seek medical advice and so on. For G6PD deficiency, the questionnaire (online supplementary questionnaire) included questions such as whether the mother has ever heard about G6PD deficiency or its common term fava bean anaemia. At this point, we continued our questions using the common term. However, during data analysis, we used the scientific term to avoid misunderstanding among the readers. The questionnaire included questions such as whether G6PD deficiency is a blood disease, whether both parents have to be carriers of G6PD deficiency, whether the inheritance of G6PD deficiency is related to the baby’s gender, agents that can trigger G6PD deficiency such as fava beans and medications, pallor, shortness of breath, whether G6PD deficiency is a cause of jaundice, and whether gastrointestinal tract (GIT) symptoms such as nausea and vomiting are symptoms of G6PD deficiency. With regard to attitude, the mothers were asked whether they see this as a serious problem, whether marriage between contagious couples consanguineous couples is a cause and so on. With regard to practice, the questionnaire included questions on seeking medical advice (a general question not specifying the current condition), premarital counselling and so on. Answers were scored as 1 (for correct) and 0 (for incorrect), and participants with at least 60% correct answers were considered to have good knowledge. The correct answer was determined for any single or multiple right answers in order to help estimate the final score. Participants with at least 60% positive answers were considered to have positive attitude and practice. A health education talk, with adequate clarification of the diseases, was given to mothers by the researcher through an organised special day at a conference room.
bmjopen-2019-034079supp001.pdf (85.4KB, pdf)
Sample size calculation
Based on a previous review of literature10 which reported an 8.9% prevalence of G6PD among newborns with jaundice with nearly the same inclusion and exclusion criteria as our study, the sample size was calculated using the following equation: n=(z2×p×q)/D2, at 95% CI. The sample size was estimated to be 487 neonates with jaundice.
Statistical analysis
Data were analysed using SPSS V.22. Descriptive statistics in the form of percentage (%), mean±SD and range were performed. An independent t-test and analysis of variance were used for normally distributed quantitative variables, while χ2 was used for qualitative variables. OR was used to assess the risk of exposure: OR=1: exposure does not affect the odds of outcome; OR >1: exposure is associated with higher odds of outcome; and OR <1: exposure is associated with lower odds of outcome. Pearson’s correlation was used to assess the direction and strength of association between the variables. A p value less than 0.05 was set as statistically significant.
Patient and public involvement
This work aimed to study the prevalence of G6PD deficiency among Egyptian neonates with jaundice and mothers’ perception of these diseases. To improve the relevance of research, research oriented to patients and the public is vital. A paper-based survey asked some mothers seeking medical advice in the three neonatal and paediatric centres to submit unanswered questions regarding G6PD deficiency and NNJ. The final top 4 research priorities derived from an inperson meeting were ranked. Thirty respondents submitted 40 questions. The respondents were from urban and rural areas, and their age ranged from 20 to 40 years. The 40 questions were refined to 17 unique questions, and from this list the top 4 research questions of priority were derived, namely whether these diseases are infectious, whether they can be transmitted to the next generation, whether they are a lifelong disease and whether there is complete cure. The observers administered questionnaires to the respondents to assess their degree of response and reactivity. The interviewed mothers recommended generalising the screening over a large population and conducting it in the most crowded districts. Hence we asked them to tell every pregnant woman they suspect to have a yellow baby to seek medical advice for free in the neonatal centres where we are working and encourage them to participate in the study. The 30 women helped us recruit approximately 92 women. The rest of the sample was based on our advertisement, plus the usual patients coming on their own to the study centres. We organised a special day at a conference room to thank all participants, disseminate the results and provide an indepth group health education session about the two diseases, with special focus directed to mothers of G6PD-deficient babies on avoiding the triggering factors and seeking prompt medical advice. For other mothers, the main aim of the health education session was to correct wrong information and to build knowledge base for new mothers so they know well of these diseases and share this knowledge to their families and to others in their community.
Results
The study was conducted on 478 neonates with jaundice. Mothers’ age ranged from 22 to 39 years (31.45±4.77). Neonates were aged 0–10 days, with 69.6% male and 30.4% female. Their birth weight ranged from 2.30 to 3.50 kg. Bilirubin levels (mg/dL) included total bilirubin of 15.17±5.14, direct bilirubin of 1.08±0.38 and indirect bilirubin of 13.17±3.74. Mean Hb (g/dL) showed a good level at about 12.18±1.75 despite the low and high range (9.50–14.50). The total percentage of neonates with jaundice who needed phototherapy on admission was 4.7%. G6PD-deficient cases who needed phototherapy on admission represented about 0.20% of the total neonates with jaundice. Family history of G6PD deficiency and consanguinity were reported among 29.6% and 21.1%, respectively. ABO incompatibility and Rh incompatibility were detected in 12.9% and 9.7%, respectively (table 1). The prevalence of G6PD deficiency was 10.10% (<4.6 u/g Hb for 42 male neonates (2.88±0.95) and for 7 female neonates (4.0±0.57)) (figure 1). Neonates with G6PD deficiency showed higher levels of bilirubin (total, direct and indirect) (p<0.001). In this population of neonates with jaundice, G6PD-deficient neonates were more likely to be of male gender (OR=4.27, 95% CI 1.66 -10.99), to be born to consanguineous parents (OR=10.21, 95% CI 5.39 - 19.33) and to have family history of G6PD deficiency (OR=9.54, 95% CI 4.80 - 18.95) (table 2). A positive correlation was observed between G6PD level among G6PD-deficient neonates and time of onset of jaundice based on maternal recall (r=0.436, p=0.002). One of the interesting findings was that total bilirubin was higher in G6PD-deficient cases (23.03±2.94, CI 22.18 - 23.87, M=23, IQR: 21.3–25) than those with Rh incompatibility (15.7±4.75, CI 14.33- 17.12, M=15, IQR: 11.6–18.2) or ABO incompatibility (11.0±2.59, CI 10.49 to 11.79, M=11, IQR: 9–13) (figure 2). With regard to KAP towards NNJ and G6PD deficiency, it seems that mothers showed a somehow better perception towards jaundice in comparison with G6PD deficiency. Unfortunately KAP towards both diseases was low, as majority of mothers (95.9%) did not know the term G6PD deficiency while about 24% of them had heard about fava bean anaemia. Also 90% of them did not know that parents (both or just the mother) have to be carriers of G6PD deficiency to have an affected child. All mothers knew fava beans can trigger G6PD deficiency, while 39.3% knew that it is triggered by drugs (table 3). Almost all mothers knew about NNJ. About 70% of them thought that prematurity is a cause of NNJ, 68.6% knew that they can detect jaundice through the skin of their newborn, while 25% reported that jaundice can be detected through the sclera of their newborn. About 95% of the mothers knew that phototherapy is a method of treatment for NNJ (table 4). Good knowledge on NNJ was reported in 30% vs 17.10% on G6PD deficiency, positive attitude towards NNJ was reported in 46.8% vs 45.0% towards G6PD deficiency, and finally good practice towards NNJ was reported in 29.9% vs 19.9% towards G6PD deficiency (figure 3).
Table 1.
General characteristics of mothers and neonates with jaundice
| General characteristics | Study group (N=487) | |
| Mothers’ characteristics | ||
| Age (years), mean±SD (range) | 31.45±4.77 | (39–22) |
| Gestational age (weeks) | ||
| Mean±SD (range) | 37.71±1.05 | (41–37) |
| Median (IQR) | 37 | 37–38 |
| BMI (kg/m2), mean±SD (range) | 22.02±2.37 | (27.10–18.30) |
| Gravidity, n (%) | ||
| ≤3 | 292 | (60.0) |
| >3 | 195 | (40.0) |
| Parity, n (%) | ||
| ≤2 | 252 | (51.7) |
| >2 | 235 | (48.3) |
| Neonatal characteristics | ||
| Gender, n (%) | ||
| Male | 339 | (69.6) |
| Female | 148 | (30.4) |
| Birth weight (kg), mean±SD (range) | 2.60±0.29 | (3.50–2.30) |
| Age of neonate (days), mean±SD (range) | 4.45±0.86 | (8–3) |
| Bilirubin (mg/dL), mean±SD (range) | ||
| Total | 15.17±5.14 | (25.50–7.30) |
| Direct | 1.08±0.38 | (0.50–1.50) |
| Indirect | 13.17±3.74 | (23.15–6.40) |
| Hb (g/dL), mean±SD (range) | 12.18±1.75 | (14.50–9.50) |
| Reticulocyte count (%), mean±SD (range) | 3.38±1.30 | (6.0–1.40) |
| Age of onset of jaundice (maternal recall), mean±SD (range) | 3.45±0.85 | (7–2) |
| Need for phototherapy on admission, n (%) | ||
| G6PD deficiency | 5 | (0.20) |
| All causes | 23 | (4.7) |
| Family history of G6PD deficiency, n (%) | 144 | (29.6) |
| Consanguinity, n (%) | 103 | (21.1) |
| ABO incompatibility, n (%) | 63 | (12.9) |
| Rh incompatibility, n (%) | 47 | (9.7) |
BMI, body mass index; G6PD, glucose-6-phosphate dehydrogenase.
Figure 1.

Prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency among the group of neonates with jaundice. Hb, Hemoglobin.
Table 2.
Distribution of the G6PD groups according to bilirubin, neonate gender, family history and consanguinity
| G6PD | Test of significance | P value | OR (95% CI) | ||||
| Deficient (n=49) |
Normal (n=438) |
||||||
| Mean±SD | Mean±SD | ||||||
| Bilirubin (mg/dLl) | |||||||
| Total | 23.03±2.94 | 14.30±4.55 | t=18.40 | <0.001* | – | ||
| Direct | 1.38±0.14 | 1.02±0.41 | t=12.47 | <0.001* | |||
| Indirect | 17.02±3.45 | 12.74±3.52 | t=8.21 | <0.001 | |||
| Neonate gender | n | % | n | % | χ2=10.49 | 0.001* | 4.27 (1.66 to 10.99) 1.0 |
| Male | 42 | 85.7 | 297 | 45.0 | |||
| Female | 7 | 14.3 | 141 | 55.0 | |||
| Family history of G6PD deficiency | |||||||
| Positive | 37 | 75.5 | 107 | 24.4 | χ2=55.21 | <0.001* | 9.54 (4.80 to 18.95) 1.0 |
| Negative | 12 | 24.5 | 331 | 75.6 | |||
| Consanguinity | |||||||
| Positive | 33 | 67.3 | 70 | 16.0 | χ2=69.72 | <0.001* | 10.21 (5.39 to 19.33) 1.0 |
| Negative | 16 | 32.7 | 368 | 84.0 | |||
*Significant family history: not in the immediate family but in their relatives.
G6PD, glucose-6-phosphate dehydrogenase.
Figure 2.
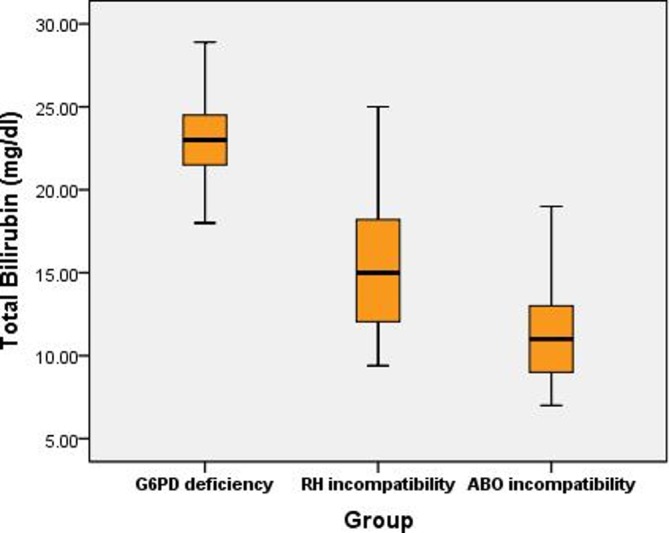
Distribution of total bilirubin among patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, Rh incompatibility and ABO incompatibility.
Table 3.
Knowledge, attitude and practice of mothers towards G6PD deficiency (N=487)
| Knowledge | n | % |
| Hearing about G6PD deficiency (per say). | ||
| Yes | 2 | 4.1 |
| No | 485 | 96 |
| Hearing about fava bean anaemia (G6PD deficiency). | ||
| Yes | 112 | 23 |
| No | 375 | 76 |
| G6PD deficiency is a blood disease. | ||
| Yes | 100 | 89 |
| No | 12 | 11 |
| I don’t know | 0 | 0 |
| Is it a hereditary disease? | ||
| Yes | 105 | 94 |
| No | 5 | 4.5 |
| I don’t know | 2 | 1.8 |
| Parents (both or just the mother) have to be carriers of G6PD deficiency to have an affected child? | ||
| Yes | 5 | 4.9 |
| No | 10 | 8.9 |
| I don’t know | 101 | 90 |
| The inheritance of G6PD deficiency related to the baby’s gender? | ||
| Yes | 10 | 8.9 |
| No | 3 | 2.7 |
| I don’t know | 99 | 88 |
| Knowing whether personally you may have a child with G6PD deficiency. | ||
| Yes | 8 | 7.1 |
| No | 85 | 76 |
| I don’t know | 29 | 26 |
| There should be a family history of G6PD deficiency to result. | ||
| Yes | 75 | 67 |
| No | 15 | 13 |
| I don’t know | 62 | 55 |
| Some medications can trigger G6PD deficiency. | ||
| Yes | 44 | 39 |
| No | 10 | 8.9 |
| I don’t know | 58 | 52 |
| Symptoms of G6PD Pallor |
||
| Yes | 10 | 8.9 |
| No | 52 | 46 |
| I don’t know | 60 | 54 |
| Nausea, vomiting, anorexia and diarrhoea | ||
| Yes | 15 | 13 |
| No | 45 | 40 |
| I don’t know | 52 | 46 |
| Dizziness | ||
| Yes | 30 | 27 |
| No | 5 | 4.5 |
| I don’t know | 77 | 69 |
| Shortness of breath | ||
| Yes | 35 | 31 |
| No | 5 | 4.5 |
| I don’t know | 72 | 66 |
| G6PD is a cause of jaundice. | ||
| Yes | 105 | 94 |
| No | 2 | 1.8 |
| I don’t know | 5 | 4.5 |
| Attitude | n | % |
| Is this a serious problem? | ||
| Yes | 100 | 89 |
| No | 5 | 4.5 |
| I don’t know | 7 | 6.3 |
| Consanguinity is the cause of the disease. | ||
| Yes | 40 | 36 |
| No | 20 | 18 |
| I don’t know | 52 | 4.3 |
| Next pregnancy should be prevented. | ||
| Yes | 3 | 2.7 |
| No | 50 | 45 |
| I don’t know | 59 | 53 |
| Follow-up of the diseased child should continue for life. | ||
| Yes | 100 | 89 |
| No | 2 | 1.8 |
| I don’t know | 10 | 8.9 |
| Practice | n | % |
| Have you been subjected to premarital counselling? | ||
| Yes | 112 | 100 |
| No | 0 | 0 |
| Have you been subjected to genetic screening? | ||
| Yes | 2 | 1.8 |
| No | 110 | 98 |
| I don’t know | 0 | 0 |
| Seeking medical advice after delivery to be assured. | ||
| Yes | 0 | 0 |
| No | 112 | 100 |
G6PD, glucose-6-phosphate dehydrogenase.
Table 4.
Knowledge, attitude and practice of mothers towards NNJ (N=487)
| Knowledge | n | % |
| Hearing about NNJ | ||
| Yes | 487 | 100 |
| No | 0 | 0 |
| Site to detect NNJ | ||
| Skin | 334 | 68.6 |
| Eye | 123 | 25.2 |
| Tongue | 30 | 6.2 |
| Causes | ||
| Prematurity | 341 | 70 |
| ABO disparity between mother and baby | 73 | 15 |
| Breast feeding | 146 | 30 |
| Infection | 139 | 33 |
| Haemolysis | 194 | 39.8 |
| Dehydration | 170 | 34.9 |
| Increased ultrasound examination during pregnancy | 292 | 60 |
| Mothers with diabetes | 73 | 15 |
| Others | 141 | 28.9 |
| Complications | ||
| Death | 146 | 30 |
| Cerebral palsy | 112 | 23 |
| Mental retardation | 170 | 34.9 |
| Handicapping | 112 | 23 |
| Hearing loss | 30 | 6.2 |
| Methods of treatment | ||
| Phototherapy | 461 | 94.7 |
| Blood exchange transfusion | 146 | 30 |
| Drugs | 399 | 82 |
| Neon lamp | 364 | 74.7 |
| Increase breast feeding | 238 | 48.9 |
| Attitude | n | % |
| NNJ is a worrisome condition? | ||
| Yes | 248 | 50.9 |
| No | 200 | 41.1 |
| I don’t know | 39 | 8 |
| Phototherapy is the best method for treatment. | ||
| Yes | 409 | 84 |
| No | 8 | 1.6 |
| I don’t know | 70 | 14.4 |
| Blood exchange transfusion is the best method for management. | ||
| Yes | 107 | 22 |
| No | 50 | 10.3 |
| I don’t know | 330 | 67.7 |
| Is it important to seek medical advice? | ||
| Yes | 450 | 92.4 |
| No | 20 | 4.1 |
| I don’t know | 18 | 13.5 |
| Practice | n | % |
| Seeking medical advice quickly if baby has NNJ. | ||
| Yes | 477 | 95.9 |
| No | 10 | 4.1 |
| I don’t know | 0 | 0 |
| Reasons for denial of medical care | n=10 | |
| Afraid of hospitalisation | 6 | 60 |
| Admission/investigation not required | 1 | 10 |
| High cost of medical care | 2 | 20 |
| Lack of transportation | 0 | 0 |
| Long hours to reach hospital | 0 | 0 |
| Time of seeking medical advice | ||
| Within 24–48 hours | 136 | 28.5 |
| >48 hours | 341 | 71.5 |
| Continuation of breast feeding | ||
| Yes | 448 | 92 |
| No | 39 | 8 |
NNJ, neonatal jaundice.
Figure 3.
Perception of glucose-6-phosphate dehydrogenase (G6PD) deficiency and neonatal jaundice (NNJ) among mothers.
Discussion
The prevalence of G6PD deficiency in neonates with jaundice was reported at 10.1%, which is within the range of the prevalence revealed in some Egyptian studies (8.9%–30.2%).9 12 The higher prevalence of G6PD deficiency among neonates with jaundice (30.2%) in El-Menshay et al 12 may be due to the small-sized purposive sample chosen to conduct the study. The wide range of G6PD deficiency prevalence in Egypt could be explained by the country’s special geographical position, being situated between three continents with different ethnic groups. The case is the same at the global scale. In Iraq, the prevalence was 10.65%,13 while in Iran it was around 9%14 and in South Brazil 7.9%.15 Neonates with G6PD deficiency showed higher levels of bilirubin, and this result is in parallel to that of Bahraini and Nigerian studies conducted by Isa et al 16 and Badejoko et al,17 respectively. Male gender is associated with a higher risk for G6PD deficiency, which is similar to the findings reported in Egypt by Abo El Fotoh and Rizk,9 Elella et al,10 and El-Menshay et al,12 in India by Sinha et al,18 and in Iran by Eghbalian and Monsef.19 Family history of G6PD deficiency and consanguinity are risk factors for G6PD deficiency, which coincide with an Egyptian study conducted by Abo El Fotoh and Rizk10 and Garg and Joag.20 On the contrary, a study conducted in Japan stated that only one case of G6PD deficiency was born to non-consanguineous Japanese parents without any family history.21 A positive correlation between G6PD deficiency and time of onset of jaundice (based on maternal recall) was found. As known, there are two peak time points for patients with jaundice to be admitted: the first is on the third day and the second is on the seventh day of life. Bimodal peaks of maximum serum bilirubin concentrations are known to occur among G6PD-deficient infants, and when the haemolytic episode starts early the elevation of serum bilirubin is anticipated to be clear and hence a course of hyperbilirubinaemia may be predicted.22 This finding is in accordance with Abo El Fotoh and Rizk,10 but in disagreement with a Turkish study conducted by Atay et al.23 Mean Hb (g/dL) showed a good level at about 12.18±1.75 despite the low and high range (9.50–14.50), and this is normal for Egyptians as the prevalence of anaemia among Egyptian pregnant women is approximately 52.5%.24 The total percentage of neonates with jaundice who needed phototherapy on admission was 4.7%. G6PD-deficient cases who needed phototherapy on admission represented about 0.20% of the total neonates with jaundice. The neonates were subjected to phototherapy according to phototherapy guidelines for hospitalised infants from ≥35 weeks of gestation.25 In G6PD deficiency, hyperbilirubinaemia is thought to be secondary to reduced hepatic conjugation and excretion of bilirubin, rather than increased bilirubin production resulting from haemolysis.18 Total bilirubin was obviously higher among G6PD-deficient cases than those with Rh or ABO incompatibility, and this finding agrees with that concluded by Das and Singh26 in India and Hussain et al 27 in Pakistan, but also in contrast to the findings obtained by Shah and Yeo28 in Singapore and Aletayeb et al 29 in Iran. KAP towards G6PD deficiency showed that majority of the mothers (95.9%) did not know the term G6PD deficiency, which does not agree with Al-Joborae,30 who found that about 91% of mothers in Iraq had heard about G6PD deficiency. In Egypt, the most commonly used term is ‘Fava bean anemia’, so in our study 23% of mothers had heard about fava bean anaemia and only 4.1% knew the term ‘G6PD deficiency anemia’. All mothers knew that fava beans can trigger an attack of haemolysis due to G6PD deficiency, hence the term, and this is in agreement with a study carried out in Bahrain by Al Arrayed and Al Hajeri.31 In this study, about 40% of mothers thought that haemolysis can be triggered by drugs, and this result is inconsistent with that obtained by Almuhaini et al.32 The current study revealed that all mothers have heard about NNJ and about 70% of them reported that prematurity causes its occurrence. This result is consistent with Magfouri et al 33 in Saudi Arabia. Jaundice can be detected in infants’ skin and sclera by 68.6% and 25% of mothers, respectively. These findings agree with that obtained by Aggarwal et al 34 in India. The study was performed on a carefully selected group of mothers aiming to evaluate their KAP. However, the study also benefited the group as most of them were first-time mothers of neonates with jaundice, so it seemed for us as the case of studying of a group of general population, besides this group will share the health education message efficiently as it is bsed on self-experience. Mothers showed a somehow better perception towards jaundice in comparison with G6PD deficiency. This is in agreement with the results reported by Boo et al 35 in Malaysia. Our results however still show poor KAP towards these diseases, which is in agreement with Goodman et al 36 in Nigeria and Alfouwais et al 37 in Saudi Arabia. In contrast to this study’s results, Al-Joborae30 in Iraq and Al Arrayed et al 32 reported that mothers had a fairly good level of awareness of G6PD deficiency. The results of this study showed some improvement in the level of knowledge in comparison with Allahony et al,38 who reported that only 18.9% of mothers had good knowledge, 48.0% had good attitude and only 25.3% had good practice towards NNJ, which reflect the effect of health education carried out to the mothers in their study and which also show that we are still in dire need of more extensive and focused health education. The results of this study showed that the risk factors for hyperbilirubinaemia were prematurity, ABO incompatibility and infection, which are in agreement with Saadat et al 39 and Rabiepoor et al 40 in Iran.
Strengths
The study collectively assessed the prevalence and risk factors of G6PD deficiency, aside from assessing the level of KAP towards G6PD deficiency and NNJ. The study clarified the extent of change in NNJ based on previous levels published in some research articles in the same region, and also drew on how G6PD deficiency is a poorly known disease despite it being serious. It is recommended that special health education sessions be provided to mothers in health centres from day 1. A suitable sample size was studied in a short period and allowed us to reach a large number of mothers and families.
Limitations
There was a need to carry out more investigations to mothers, such as a direct Coombs test. A post-test to assess the extent of understanding and KAP among the mothers was also needed; however, it was difficult to collect the same number of study participants one more time. We reached only the mothers who sought medical advice for their neonates. KAP towards these diseases needs to be assessed in the general population to ensure risk factors are identified and addressed, and we tried to help mothers deliver and share with their families the knowledge they gained from the health education session that was conducted.
Conclusion
G6PD deficiency seems to be an important cause of NNJ. Cord blood for complete blood count, direct Coombs test, blood grouping, bilirubin and G6PD screening in high-risk populations are recommended to determine earlier whether a prolonged hospital stay is needed. G6PD deficiency and NNJ are serious conditions. The study revealed that mothers’ KAP towards NNJ, despite being low, shows promising improvement. In contrast, mothers’ KAP towards G6PD deficiency is very poor. It is important to provide mass health education programmes on G6PD deficiency and NNJ to ensure better and early detection, good timing of treatment, and better prevention of the triggering factors to ensure better health for children.
Supplementary Material
Acknowledgments
The authors thank the participants for participation in the study and the team who helped in data collection.
Footnotes
Contributors: All persons who meet the authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing or revision of the manuscript. ZK conceived the idea, performed the statistical analysis, wrote the methodology and results sections, and was responsible for final revision and publishing. SHA, a family physician, wrote the manuscript and was responsible for its revision. WB, a paediatrician, received, diagnosed and collected the data. SMEH performed the lab investigations. The entire team arranged a health education session to provide explanation for both diseases in all aspects.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Institutional Review Board (IRB) of the Menoufia Faculty of Medicine approved the study. Research work was performed in accordance with the Declaration of Helsinki. A written patient consent was taken from the parents and caregivers after explaining all aspects of the study, with the right to withdraw at any time.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available on request by email (zeinabkasemy@yahoo.com).
References
- 1. Khan RS, Houlihan DD, Newsome PN. Investigation of jaundice. Medicine 2015;43:573–6. 10.1016/j.mpmed.2015.07.009 [DOI] [Google Scholar]
- 2. Azzuqa A, Watchko JF. Bilirubin concentrations in jaundiced neonates with conjunctival icterus. J Pediatr 2015;167:840–4. 10.1016/j.jpeds.2015.06.065 [DOI] [PubMed] [Google Scholar]
- 3. Brits H, Adendorff J, Huisamen D, et al. . The prevalence of neonatal jaundice and risk factors in healthy term neonates at national district hospital in Bloemfontein. Afr J Prim Health Care Fam Med 2018;10:1582 10.4102/phcfm.v10i1.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watson RL. Hyperbilirubinemia. Crit Care Nurs Clin North Am 2009;21:97–120. 10.1016/j.ccell.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 5. Beutler E. Glucose-6-Phosphate dehydrogenase deficiency: a historical perspective. Blood 2008;111:16–24. 10.1182/blood-2007-04-077412 [DOI] [PubMed] [Google Scholar]
- 6. Glader B. Hereditary hemolytic anemias due to red blood cell enzyme disorders : Greer JP, Foerster J, Rodgers GM, Wintrobe’s clinical hematology. 12th edn Philadelphia: Lippincott, Williams & Wilkins, 2009: 933. [Google Scholar]
- 7. Kaplan M, Hammerman C. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency: biochemical versus genetic technologies. Semin Perinatol 2011;35:155–61. 10.1053/j.semperi.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 8. Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician 2005;72:1277–82. [PubMed] [Google Scholar]
- 9. M Abo El Fotoh WM, Rizk MS. Prevalence of glucose-6-phosphate dehydrogenase deficiency in jaundiced Egyptian neonates. J Matern Fetal Neonatal Med 2016;29:3834–7. 10.3109/14767058.2016.1148133 [DOI] [PubMed] [Google Scholar]
- 10. Elella SA, Tawfik M, Barseem N, et al. . Prevalence of glucose-6-phosphate dehydrogenase deficiency in neonates in Egypt. Ann Saudi Med 2017;37:362–5. 10.5144/0256-4947.2017.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El-Sayed L, Tantawi H. Prevention of hemolytic crisis among G6PD children: effect of educational program intervention. J Am Sci 2012;8. [Google Scholar]
- 12. El-Menshay A, Khalifa N, Awad S. Prevalence of glucose-6-phosphate dehydrogenase deficiency in jaundiced neonates in Egypt. Aust J Basic Appl Sci 2009;3:2016–23. [Google Scholar]
- 13. Frankool WM, Al-Tu’ma FJ. Molecular basis of G6PD deficiency in hyperbilirubinemic neonates in middle Euphrates Province: Iraq. Karbala J Med 2010;3:98–102. [Google Scholar]
- 14. Karimi M, Martinez di Montemuros F, Danielli MG, et al. . Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in the Fars Province of Iran. Haematologica 2003;88:346–7. [PubMed] [Google Scholar]
- 15. Castro S, Weber R, Dadalt V, et al. . Prevalence of G6PD deficiency in newborns in the South of Brazil. J Med Screen 2006;13:85–6. 10.1258/096914106777589641 [DOI] [PubMed] [Google Scholar]
- 16. Isa HM, Mohamed MS, Mohamed AM, et al. . Neonatal indirect hyperbilirubinemia and glucose-6-phosphate dehydrogenase deficiency. Korean J Pediatr 2017;60:106–11. 10.3345/kjp.2017.60.4.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badejoko BO, Owa JA, Oseni SBA, et al. . Early neonatal bilirubin, hematocrit, and glucose-6-phosphate dehydrogenase status. Pediatrics 2014;134:e1082–8. 10.1542/peds.2014-0654 [DOI] [PubMed] [Google Scholar]
- 18. Sinha R, Sachendra B, Syed VSabid, et al. . To study the prevalence of glucose 6 phosphate dehydrogenase(G6PD) deficiency in neonates with neonatal hyperbilirubinemia and to compare the course of the neonatal jaundice in deficient versus non deficient neonates. J Clin Neonatol 2017;6:71–4. 10.4103/jcn.JCN_59_16 [DOI] [Google Scholar]
- 19. Eghbalian E, Monsef A. Evaluation of glucose-6-phosphate dehydrogenase deficiency without hemolysis in icteric newborns. Iran J Pediatr 2007;17. [DOI] [PubMed] [Google Scholar]
- 20. Garg S, Joag GG. Assessment of the prevalence of G6PD deficiency in rbcS of live new-borns born at tertiary care hospital. Int J Contemp Pediatrics 2019;6:676–82. 10.18203/2349-3291.ijcp20190710 [DOI] [Google Scholar]
- 21. Tsuzuki S, Akahira-Azuma M, Kaneshige M, et al. . A Japanese neonatal case of glucose-6-phosphate dehydrogenase deficiency presenting as severe jaundice and hemolytic anemia without apparent trigger. Springerplus 2013;2:434 10.1186/2193-1801-2-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valaes T. Severe neonatal jaundice associated with glucose-6-phosphate dehydrogenase deficiency: pathogenesis and global epidemiology. Acta Paediatr Suppl 1994;394:58–76. 10.1111/j.1651-2227.1994.tb13216.x [DOI] [PubMed] [Google Scholar]
- 23. Atay E, Bozaykut A, Ipek IO. Glucose-6-Phosphate dehydrogenase deficiency in neonatal indirect hyperbilirubinemia. J Trop Pediatr 2006;52:56–8. 10.1093/tropej/fmi042 [DOI] [PubMed] [Google Scholar]
- 24. Abu Salem M, Mahrous O O, El Shazly H, et al. . Epidemiology of iron-deficiency anemia among pregnant women in menoufia governorate, Egypt and Taiz Governorate, Yemen: A comparative study). Menoufia Med J 2016;29:1005–11. [Google Scholar]
- 25. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316. 10.1542/peds.114.1.297 [DOI] [PubMed] [Google Scholar]
- 26. Das M, Singh A. A comparative study of peak total serum bilirubin level in neonates with ABO incompatibility, Rh incompatibility and G6PD deficiency. IOSR J Dental Med Sci 2018;10:21–3. [Google Scholar]
- 27. Hussain M, Irshad M, Kalim M, et al. . Glucose-6-Phosphate dehydrogenase deficiency in jaundiced neonates. JMPI 2010;94:122–96. [Google Scholar]
- 28. Shah VA, Yeo CL. Identifying risk of neonatal hyperbilirubinaemia and early discharge for glucose-6-phosphate dehydrogenase deficient newborns in Singapore. Ann Acad Med Singapore 2007;36:1003–9. [PubMed] [Google Scholar]
- 29. Aletayeb S, Dehdashtian M, Aramesh M, et al. . Outcome of jaundice in neonates with ABO and Rh blood incompatibility and glucose-6-phosphate dehydrogenase deficiency. Biomed Res 2017;28:3440–4. [Google Scholar]
- 30. Al-Joborae S. Extent of knowledge of mothers of neonates with G6PD deficiency in Hilla City. J Babylon Univ/ Pure Appl Sci 2015;23:1542–50. [Google Scholar]
- 31. Al Arrayed S, Al Hajeri A. Public awareness of glucose-6-phosphate dehydrogenase (G6PD) deficiency. Bahrain Medical Bulletin 2011;33. [Google Scholar]
- 32. Almuhaini MS, Alruzayhi MK, Alwassel AI. Public awareness of glucose-6-phosphate dehydrogenase (G6PD. 4 Deficiency Causes and Prevalence Factors: The Journal of Middle East and North Africa Sciences, 2018. http://oaji.net/pdf.html?n=2017/2705-1517398879.pdf [Google Scholar]
- 33. Magfouri H, Aqeel A, Maashi A, et al. . Mothers' perception of neonatal jaundice in Jazan region, KSA. J Clin Neonatol 2019;8:116–9. [Google Scholar]
- 34. Aggarwal B, Agrawal A, Chaudhary P, et al. . Attitude beliefs, and practices of postnatal mothers in a tertiary care hospital in Uttarakhand, India. Indian J Child Health 2017;4:603–8. [Google Scholar]
- 35. Boo NY, Gan CY, Gian YW, et al. . Malaysian mothers' knowledge & practices on care of neonatal jaundice. Med J Malaysia 2011;66:239–43. [PubMed] [Google Scholar]
- 36. Goodman O, Kehinde O, Odugbemi B, et al. . Neonatal jaundice: knowledge, attitude and practices of mothers in Mosan-Okunola community, Lagos, Nigeria. Niger Postgrad Med J 2015;22:158–63. 10.4103/1117-1936.170741 [DOI] [PubMed] [Google Scholar]
- 37. Alfouwais N, Seada L, Alahmadi R, et al. . Assessment of knowledge, attitude and practice of Saudi parents towards neonatal jaundice (NNJ). Egypt J Hospital Med 2018;70:1686–94. [Google Scholar]
- 38. Allahony DM, Hegazy NN, Kasemy ZA, et al. . Mothers' perception toward neonatal jaundice in Kafr El-batanoon village, Menoufia, Egypt. Menoufia Med J 2016;29:743–8. [Google Scholar]
- 39. Saadat SH, Naderi S, Zare S, et al. . Epidemiologic study of jaundice in newborns with jaundice in the first 24 hours of birth in children's hospital and Shariati hospital of Bandar Abbas in 2010-2014. J Res Med Dental Sci 2018;6:113–7. [Google Scholar]
- 40. Rabiepoor S, Gheibi S, Jafari S. To study the knowledge and attitude of postnatal mothers on neonatal jaundice in Motahari Hospital, Iran. Clin Med Res 2014;3:1–5. 10.11648/j.cmr.20140301.11 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034079supp001.pdf (85.4KB, pdf)



