Abstract
Objectives
The effects of hypercapnia on regional cerebral oxygen saturation (rSO2) during surgery are unclear. We conducted a randomised controlled trial to investigate the relationship between mild hypercapnia and rSO2. We hypothesised that, compared with targeted normocapnia (TN), targeted mild hypercapnia (TMH) during major surgery would increase rSO2.
Design
A prospective, randomised, controlled trial in adult participants undergoing elective major surgery.
Setting
A single tertiary centre in Heidelberg, Victoria, Australia.
Participants
40 participants were randomised to either a TMH or TN group (20 to each).
Interventions
TMH (partial pressure of carbon dioxide in arterial blood, PaCO2, 45–55 mm Hg) or TN (PaCO2 35–40 mm Hg) was delivered via controlled ventilation throughout surgery.
Primary and secondary outcome measures
The primary endpoint was the absolute difference between the two groups in percentage change in rSO2 from baseline to completion of surgery. Secondary endpoints included intraoperative pH, bicarbonate concentration, base excess, serum potassium concentration, incidence of postoperative delirium and length of stay (LOS) in hospital.
Results
The absolute difference between the two groups in percentage change in rSO2 from the baseline to the completion of surgery was 19.0% higher in both hemispheres with TMH (p<0.001). On both sides, the percentage change in rSO2 was greater in the TMH group than the TN group throughout the duration of surgery. The difference between the groups became more noticeable over time. Furthermore, postoperative delirium was higher in the TN group (risk difference 0.3, 95% CI 0.1 to 0.5, p=0.02). LOS was similar between groups (5 days vs 5 days; p=0.99).
Conclusion
TMH was associated with a stable increase in rSO2 from the baseline, while TN was associated with a decrease in rSO2 in both hemispheres in patients undergoing major surgery. This resulted in a clear separation of percentage change in rSO2 from the baseline between TMH and TN over time. Our findings provide the rationale for larger studies on TMH during surgery.
Trial registration number
The Australian New Zealand Clinical Trials Registry (ACTRN12616000320459).
Keywords: delirium; hypercapnia; oximetry; respiration, artificial; spectroscopy, near infrared
Strengths and limitations of this study.
High internal validity due to blinding and random allocation to groups.
Frequent sampling of oximetry data throughout monitoring period.
Non-invasive nature of near-infrared spectroscopy cerebral oximetry for regional cerebral oxygen saturation (rSO2) measurements.
Study findings do not apply to emergency surgeries, intracranial surgeries or surgeries requiring one lung ventilation.
Interpretation of rSO2 depends on an assumption that rSO2 is the same in different regions of the brain.
Introduction
In patients undergoing major surgery, the effects of mild hypercapnia on regional cerebral oxygen saturation (rSO2) have not been fully examined, and any beneficial or harmful effects of hypercapnia as a therapeutic ventilation strategy to improve cerebral oxygenation are unknown. In animal models, CO2 is a well-known vasodilator, improving cerebral blood flow.1–3 The neuroprotective mechanisms of mild hypercapnia, while not completely understood, have been postulated to be a result of an increase in cerebral blood flow, enhancement of oxygen delivery, improvements in cerebral glucose use and oxidative metabolism4 5 and activation of adenosine triphosphate-sensitive potassium channels to maintain normal neuronal activity in the setting of ischaemia.6
The recent emergence of near-infrared spectroscopy (NIRS) cerebral oximetry has provided a practical method to measure rSO2 continuously and non-invasively. This technology has gained substantial supportive evidence in resuscitation, critical care and surgical applications.7–9 Numerous studies have shown that NIRS can be applied clinically in the resuscitation and cardiac surgery settings, where cerebral desaturation events can be both effectively monitored and managed.10–13 However, while absolute and relative saturation thresholds theoretically requiring prompt interventions have been proposed,14 these thresholds have not been validated, and there is a lack of consensus on the indication and timing of interventions. In patients undergoing surgery, rSO2 was reported to be higher with mild hypercapnia; however, the intraoperative temporal relationship between rSO2 and mild hypercapnia remains unclear.15
Accordingly, we conducted a randomised controlled trial to test the hypothesis that targeted mild hypercapnia (TMH), defined as the partial pressure of carbon dioxide in arterial blood (PaCO2) between 45 and 55 mm Hg, during elective major surgery would increase cerebral oxygen saturation compared with targeted normocapnia (TN), defined as PaCO2 between 35 and 40 mm Hg. As a secondary aim, we evaluated whether TMH would affect the development of postoperative delirium, a commonly reported complication that is linked to functional decline, institutionalisation and higher mortality.16–18
Methods
Ethics approval
The study was approved by the Austin Health Research and Ethics Committee on 6 January 2016 (HREC/15/Austin/488), and all participants gave written informed consent. The study was reported in accordance with the CONSORT Guidelines for reporting randomised trials.19
Trial design, setting and population
Between March 2016 and March 2017, we conducted the randomised controlled trial at the Austin Hospital, a university teaching, tertiary, metropolitan hospital at Heidelberg, Victoria. Following a preoperative assessment at the anaesthesia preadmissions clinic and the receipt of written informed consent, eligible patients undergoing elective major surgery were identified. Inclusion criteria included the following: adult patients (age over 18 years), surgery of greater than 2 hours expected duration requiring at least one overnight admission, a clinical indication for continuous blood pressure monitoring via an invasive arterial line and intermittent positive pressure ventilation via an endotracheal tube as part of standard anaesthesia care. Age criterion was modified from the previous criterion (age over 65 years) to age over 18 years to recruit patients who represent the intended study population. Exclusion criteria included patients undergoing cardiac surgery, procedures requiring one lung isolation, liver transplantation, intracranial surgery, Glascow Coma Scale less than 15, known cognitive impairment, intellectual disability or a mental illness, moderate pulmonary hypertension (mean pulmonary arterial pressure greater than 40 mm Hg) and American Society of Anesthesiology (ASA) status V.
Randomisation and blinding
An independent statistician generated a computerised sequence of 40 allocation codes, 20 for each group. A research nurse sealed the allocation codes into sequentially numbered opaque envelopes. The study participants, surgeons and all perioperative staff were blinded to treatment allocation. However, it was not possible to blind the attending anaesthetist who was responsible for the delivery of the intervention. Immediately after induction of anaesthesia, patients were randomised to either targeted mild hypercapnia (PaCO2 45–55 mm Hg) or targeted normocapnia (PaCO2 35–40 mm Hg). The end-tidal carbon dioxide (EtCO2) was titrated accordingly to achieve the desired intervention, but the anaesthetist did not have an rSO2 goal to titrate to. Data collection for all the trial outcomes was collected by an independent researcher blinded to treatment allocation. The sequence was decoded after the data were analysed. The anaesthetist delivering the intervention did not participate in the assessment of postoperative delirium.
Outcomes and data collection
The primary endpoint was the absolute difference between the TMH and TN groups in percentage change in rSO2 from baseline to completion of surgery. Secondary endpoints evaluated the effects of mild hypercapnia on the incidence of postoperative delirium, intraoperative pH, bicarbonate, base excess, serum potassium and length of hospital stay (LOS). LOS was prespecified as secondary outcome in the original study protocol. However, it was not prespecified as a secondary outcome in the prospective Australian New Zealand Clinical Trials Registry. Therefore, the trials registry was retrospectively updated to include LOS as a secondary outcome to align with the study protocol.
Measurement of rSO2
Regional cerebral oxygen saturation was collected using the Masimo O3 regional oximetry component of the Root Patient Monitor platform (O3 Masimo, Irvine, California, USA). This regional oximetry device uses NIRS and reflectance oximetry to monitor rSO2 in the brain, displaying both absolute and trend rSO2 values. The absolute oximetry value is defined as the rSO2 value measured by the oximetry probe calibrated by a fixed ratio of arterial to venous blood. In our study, only the absolute oximetry data were extracted and analysed. The accuracy of the Masimo O3 regional oximetry was investigated by Redford et al previously, and the measurement error was reported to be approximately 4% when checked against reference blood samples taken from the radial artery and internal jugular bulb vein.20 Regional cerebral oxygen saturation was measured in the two hemispheres separately, with a NIRS sensor attached to each side of patient’s forehead. The baseline rSO2 was recorded before commencing any premedication and before induction of anaesthesia. Subsequent rSO2 measurements were recorded every 2 s until the last surgical suture was sited. Data were exported as comma separated values files after surgery and processed using manually written R scripts on RStudio V.1.0.136 (see online supplementary file 1). The percentage change in rSO2 (%ΔrSO2) was computed by subtracting the baseline rSO2 value from the measured rSO2 value at all timepoints throughout surgery, multiplied by 100%. Data from the left and right forehead were analysed separately.
bmjopen-2019-029159supp001.pdf (36.4KB, pdf)
Measurement of delirium
Delirium was assessed using a validated and widely used Confusion Assessment Method (CAM) rating scale, adapted from Inouye et al, immediately on arrival to hospital, then within 18–24 hours after surgery.21 22 Diagnosis of delirium requires the presence of both acute onset with fluctuating course and inattention, together with either disorganised thinking or altered level of consciousness. A single trained interviewer, blinded to randomisation and proficient and trained in CAM, conducted all the assessments preoperatively when each patient arrived at the hospital and at 08:00 on the next day after surgery in the ward (within 18–24 hours postoperatively). The baseline cognitive function was not formally assessed with collateral history from family members or carers.
Measurement of PaCO2 and intraoperative adherence to group allocation
Immediately after tracheal intubation with a cuffed endotracheal tube, minute ventilation was adjusted to achieve an EtCO2 concentration of 45–55 mm Hg in the TMH group or 35–40 mm Hg in the TN group. Due to the presence of alveolar dead space, EtCO2 can be lower than PaCO2 by up to 5 mm Hg. Therefore, an arterial blood gas (ABG) was obtained to check PaCO2, and ventilation was further adjusted accordingly to achieve the desired PaCO2 target ranges. The PaCO2–EtCO2 gradient was then maintained throughout surgery, with the assumption that the PaCO2 would remain constant. Additional ABGs were sampled at the discretion of the anaesthetist if the gradient required re-evaluation, for example, requirements for an adjustment of the ventilation setting. Finally, at completion of surgery, an ABG was sampled to accurately document the PaCO2 value and to assess whether PaCO2 was being maintained within target values.
Arterial blood gas analysis
All arterial blood gas variables were collected by ABL80 FLEX Blood Gas Analyzer (Radiometer, Copenhagen, Denmark) with a fully automated micromode, eliminating the risk of user-induced bias or loss of accuracy with very small samples and an interference-protected lactate analyser. ABG variables include partial pressure of oxygen (PaO2), PaCO2, pH, bicarbonate concentration, base excess, lactate, haemoglobin concentration (Hb) and electrolytes such as sodium and potassium ion concentrations. The machine calculates the bicarbonate concentration using the Henderson-Hasselbalch equation and the standard base excess using the Van Slyke equation by determining changes in bicarbonate, protein anion and phosphate concentrations, with the reference points pH=7.40, PaCO2=40 mm Hg and temperature=37°C. Two or more ABG samples were measured intraoperatively, as described previously. The mean values of pH, bicarbonate concentration, base excess and serum potassium concentration from the first and the last ABG samples were considered as some of the secondary outcomes for the study. Intraoperative pH, bicarbonate and base excess are important variables that inform the acid–base status of a patient; in particular, bicarbonate and base excess are useful when determining the extent of metabolic contributions or compensation. Potassium concentration is a key physiological parameter that affects cardiac action potential conduction, and its relevance in the study is paramount, as hyperkalaemia from hypercapnic-induced acidosis is a potential complication of the intervention. Potential confounders to rSO2 measurements, such as Hb and PaO2, were recorded. Other variables, such as lactate and sodium concentration, were collected for routine clinical care, and they were not considered as part of the outcome measures.
Standardisation of care
All patients underwent a preoperative multidisciplinary team assessment, including a haematology-led, multimodal perioperative haemoglobin optimisation programme based on the National Blood Authority of Australia’s patient blood management initiatives to optimise preoperative red cell mass, minimise perioperative blood loss and tolerate postoperative anaemia.23 All participants were fasted 2 hours for clear fluids and 6 hours for solids, according to standard hospital fasting protocols. All participants received a general anaesthetic with propofol for induction, an inhalational agent for the maintenance of anaesthesia, with a 50% oxygen-to-air mixture to maintain oxygen saturations above 97%. Routine monitoring for all participants included continuous ECG, pulse oximetry, temperature, bispectral index (BIS) monitoring and neuromuscular monitoring. Adequate depth of anaesthesia was ensured by targeting BIS readings between 40 and 60. Conduct of anaesthesia, including the use of additional invasive monitoring, intraoperative medications, intravenous fluids, vasoactive medications, regional anaesthesia and intraoperative opioids, were entirely at the discretion of the attending anaesthetist. In keeping with hospital protocol, we transfused blood if the haemoglobin concentration was less than 75 g/L or less than 80 g/L in the presence of ongoing bleeding.
Sample size calculations
Based on our institution’s pilot data and reported figures, normal rSO2 values for awake patients could range from 60% to 80%,24 which we assumed to be the case at the baseline (beginning of surgery). We assumed no change in rSO2 in the control group and considered an absolute difference between the groups in percentage change in rSO2 value from the baseline to the completion of surgery of 15% to be clinically important. Thus, the absolute changes in rSO2 from the baseline to the end of surgery were hypothesised to be 0% in the control group and 12% (15% percentage change from the baseline of 80% rSO2) in the intervention group. Assuming a two-tailed threshold for statistical significance of 0.05 and SD of the absolute change of 10%, the total sample size of 40 patients (equally distributed between two groups) will yield the 0.9 power to observe a large treatment effect (Cohen’s d=1.1 or higher).
Statistical analysis
The study was reported in accordance with the Statistical Analyses and Methods in the Published Literature Guidelines.25 The statistical analysis was performed using commercial statistical software STATA/IC V.13 with a p value of 0.05 to indicate statistical significance. Figures and tables were created by manually written R scripts on RStudio V.1.0.136 (see online supplementary file 2). Fisher’s exact test was used in the analysis of all categorical variables. For continuous variables, normality was determined by the Shapiro-Wilk test and further confirmed by a manual inspection of the skewness and kurtosis of the data. Parametric continuous data were compared by the Student’s t-test, and non-parametric continuous data were compared by the Mann-Whitney U test. For normally distributed data, the results were presented as the mean (SD); and for non-parametric data, the results were presented as the median (inter-quartile range) unless otherwise stated. A more detailed longitudinal analysis of time-by-treatment interaction was also conducted using a random effect generalised least squares regression model (due to the repeated measures nature of the data) with percentage change in rSO2 at a given time point throughout the surgery as the output, the treatment group, the time (minutes from start of surgery), as well as the time-by-treatment interaction term as inputs. The duration of surgery varied between different patients, and therefore, to compare %ΔrSO2 at different time points across all the patients, the time was measured using the ‘minutes from the start of surgery’ metric. For robustness analyses, similar models adjusted for age, baseline oximetry values and preoperative Hb levels were implemented, as well as models where time was measured not in minutes but as a percentage of total surgery duration.
bmjopen-2019-029159supp002.pdf (35KB, pdf)
Patient and public involvement
Patients were involved in the study from the initial preadmission consultation appointment where the rationale of the study, potential applications of the study outcomes, data privacy and management and potential harmful effects were explained in detail. Patients were not directly involved in the development of the research question and outcome measures, and they were not involved in the design and conduct of the study. Potential burden of the intervention was not rated by the patients themselves; rather, potential harmful effects were monitored by the attending anaesthetist as part of routine clinical care. Study results and outcomes, once finalised, will be mailed out to study participants.
Results
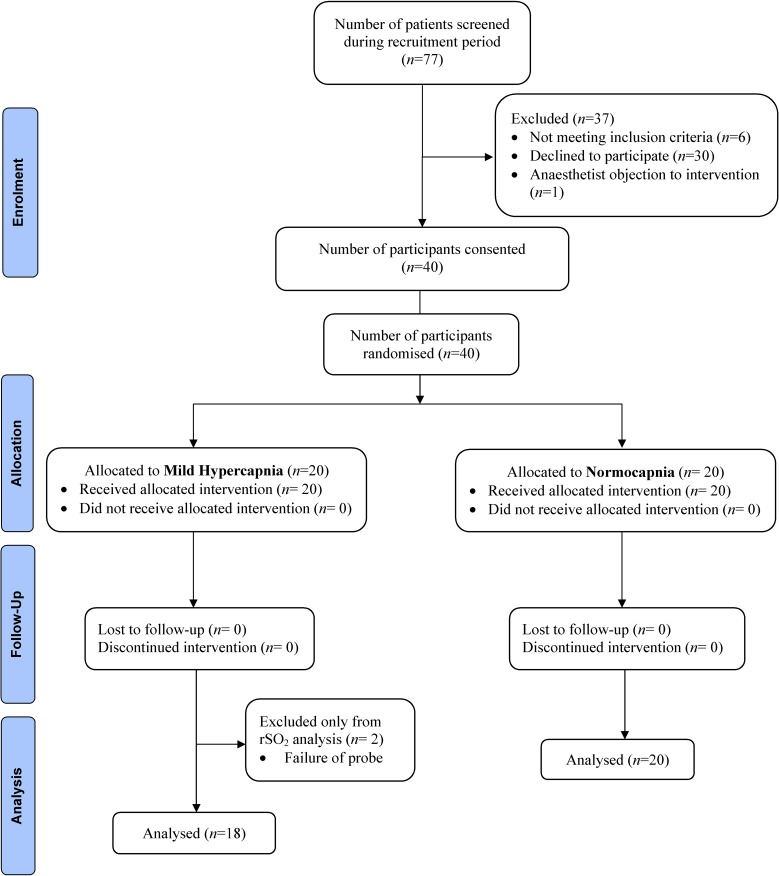
Seventy-seven participants were screened for eligibility. Thirty-seven patients were excluded because they did not meet the inclusion criteria (n=6), they declined to participate (n=30) or the anaesthetist objected to the intervention (n=1). For logistical reasons, recruitment could only be performed when the interviewer conducting the CAM testing was available. The Consolidated Standards of Reporting Trials diagram is presented in figure 1. There were no violations or breaches of the study protocol; however, two participants in the hypercapnic group had a failure of bilateral probe attachment and lead connection problem that were unable to be rectified. These patients were subsequently excluded from the analyses of oxygenation, as no rSO2 data were captured. They were included in the analysis of all other variables and endpoints. In the hypercapnic group, three participants had unilateral discontinuous oximetry readings due to intermittent signal dropout. In the normocapnic group, signal dropout occurred in two patients on the left side. The corresponding data were excluded.
Figure 1.
The progress of all participants through the trial displayed by the Consolidated Standards of Reporting Trials flow diagram.
The baseline participant and surgical characteristics are summarised in table 1.
Table 1.
Baseline patient characteristics and surgical characteristics
| TMH group (n=20) |
TN group (n=20) |
|
| Patient characteristics | ||
| Gender (male:female) | 11:9 | 12:8 |
| Age (years) | 63.7 (32–81) | 65.4 (31–81) |
| Age >65 (years) | 9 (45.0) | 11 (55.0) |
| Weight (kg) | 83.7 (56.8–110.6) | 81.2 (67.9–94.5) |
| BMI (kg/m2) | 33.6 (20.7–46.5) | 32.8 (26.8–38.8) |
| ASA status | ||
| 1 | 5 (25.0) | 2 (10.0) |
| 2 | 6 (30.0) | 4 (20.0) |
| 3 | 7 (35.0) | 10 (50.0) |
| 4 | 2 (10.0) | 4 (20.0) |
| Diabetes | 4 (22.2) | 5 (25.0) |
| COPD | 5 (27.8) | 0 (0.0) |
| Malignancy | 11 (61.1) | 7 (35.0) |
| Other comorbidities | 11 (61.1) | 16 (80.0) |
| Surgical characteristics | ||
| Duration of surgery (min) | 219.0 (123.8–303.8) | 144.0 (107.8–218.2) |
| Left baseline oximetry (%) | 68.7 (63.9–72.2) | 63.4 (57.3–69.6) |
| Right baseline oximetry (%) | 67.9 (64.6–70.3) | 64.0 (59.4–69.0) |
| Pulse oximetry (%) | 98.5 (98.1–99.0) | 98.5 (97.9–99.0) |
| LOS (days) | 5 (2.0–12.0) | 5 (1.8–11.5) |
| Type of surgery | ||
| Colorectal | 2 (10.0) | 1 (5.0) |
| Endocrine | 2 (10.0) | 2 (10.0) |
| Ear, nose and throat | 0 (0.0) | 1 (5.0) |
| Hepatobiliary | 6 (30.0) | 9 (45.0) |
| Spinal surgery | 1 (5.0) | 1 (5.0) |
| Orthopaedic | 2 (10.0) | 1 (5.0) |
| Thoracic | 5 (25.0) | 1 (5.0) |
| Urology | 2 (10.0) | 3 (15.0) |
| Vascular | 0 (0.0) | 1 (5.0) |
Data reported as number (%) or median (IQR), except for age, which is reported as mean (range).
Other comorbidities include any of the following, ischaemic heart disease, atrial fibrillation, hypertension, history of cerebral vascular disease and chronic kidney impairment.
Spinal surgery includes non-intracranial procedures.
Thoracic surgery includes procedures not requiring one lung ventilation, for example, mediastinoscopy with nodal dissection.
ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LOS, length of hospital stay.
Both groups were similar in terms of gender, age, weight, body mass index, ASA physical status and type of surgery performed. In terms of comorbidities, both groups were similar, except for the presence of chronic obstructive pulmonary disease. There was 100% compliance to the designated PaCO2 intraoperative targets. The median (IQR) PaCO2 in the TMH group and TN groups were 51.5 mm Hg (46.9–60.9) and 34.8 mm Hg (32.8–38.1), respectively (p<0.001). With regards to surgical characteristics, the duration of surgery was longer in the TMH group, with a median (IQR) duration of 219 min (124–304) versus 144 min (108–218) in the TN group, although this was not significant at the 5% level (p=0.121). PaO2 was similar between the two groups: 156.8 mm Hg (146.3–217.2) in the TMH group and 142.5 mm Hg (122.5–199.1) in the TN group (p=0.380). Oxygen saturation was similar: 98.5% in the TMH group (98.1–99.0) and 98.5% in the TN group (97.9–99.0) (p=0.834). Both groups also had similar mean arterial pressure (MAP) intraoperatively (p=0.307), similar total Hb (130.5 vs 122.2 g/L; p=0.132) and received similar total doses of intravenous morphine equivalent opioid, 21.67 mg in the TMH group (13.75–32.50) and 16.67 mg in the TN group (10.00 - 22.50) (p=0.22). In terms of intraoperative positioning of patients, one patient from each group was positioned in steep reverse Trendelenburg with minimal tilt. All other patients were positioned in the supine position with a neutral head position.
Primary endpoint
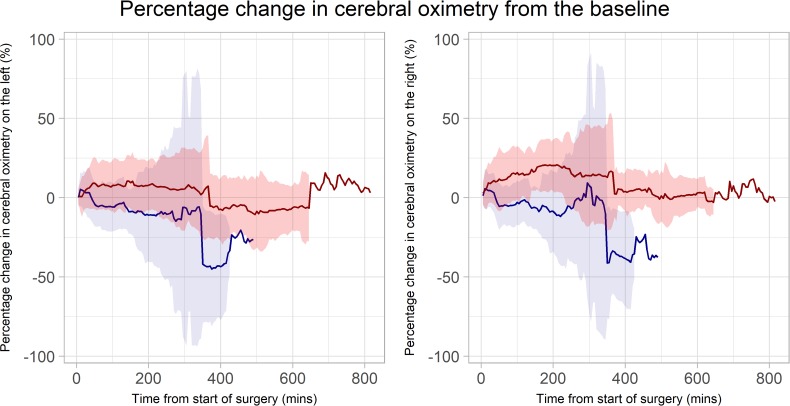
On the left hemisphere, the median (IQR) baseline oximetry was 68.7% (63.9–72.2) in the TMH group versus 63.4% (57.3–69.6) in the TN group (p=0.233). On the right hemisphere, the median (IQR) baseline oximetry was 67.9% (64.6–70.3) in the TMH group versus 64.0% (59.4–69.9) in the TN group (p=0.286). On both sides, the %ΔrSO2 was greater in the TMH group than the TN group throughout the duration of surgery (figure 2). The mean (SD) percentage changes in rSO2 from the baseline to the conclusion of the surgery in the TMH group were +8.56% (18.90%) on the left and +13.86% (18.17%) on the right; and in TN the group, they were −6.18% (17.24%) on the left and −5.48% (18.94%) on the right. The resulting treatment effects were 19% (95% CI 9.2 to 28.8; p<0.001) on the left and 19% (95% CI 10.9 to 27.0; p<0.001) on the right (table 2).
Figure 2.
The solid lines represent the mean percentage change; while the shaded areas represent the SD. The targeted mild hypercapnia group is represented by the red line and the red area; while the targeted normocapnia group is represented by the blue line and the blue area. Left: percentage change of regional cerebral oxygen saturation from the baseline on the left hemisphere. Right: percentage change of regional cerebral oxygen saturation from the baseline on the right hemisphere.
Table 2.
Percentage change in cerebral oximetry (%ΔrSO2) from baseline
| Time from start of surgery (min) | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 |
| Left | ||||||||
| TMH | 0.8 (12.9) {15} |
5.8 (12.3) {15} |
9.0 (15.9) {15} |
7.0 (14.6) {15} |
8.5 (15.4) {14} |
7.3 (14.7) {13} |
7.7 (17.4) {13} |
8.1 (14.8) {13} |
| TN | 4.7 (10.5) {18} | 3.2 (15.4) {18} |
−1.9 (14.1) {17} |
−5.6 (12.7) {17} |
−5.3 (15.2) {17} |
−5.5 (15.8) {17} |
−6.0 (15.2) {17} |
−3.6 (15.8) {14} |
| Right | ||||||||
| TMH | 6.0 (12.9) {17} |
9.8 (13.2) {17} |
10.4 (18.1) {17} |
11.1 (17.4) {17} |
13.0 (16.4) {16} |
15.6 (17.3) {15} |
14.4 (17.5) {14} |
14.1 (13.6) {14} |
| TN | 5.2 (12.6) {20} |
3.9 (11.7) {20} |
−3.3 (13.2) {19} |
−5.2 (12.1) {19} |
−5.4 (12.3) {19} |
−4.7 (14.1) {19} |
−3.8 (13.7) {18} |
−1.3 (13.9) {15} |
| Time from start of surgery (min) | 120 | 240 | 360 | 480 | 600 | 720 | Mean % difference from start to completion of surgery | 95% CI | P value (treatment) |
| Left | 19.0 | 9.2 to −28.8 | <0.001 | ||||||
| TMH | 8.1 (14.8) {13} | 6.8 (20.6) {7} | 6.4 (32.5) {4} | −8.6 (21.1) {3} | −6.1 (14.1) {3} | 6.9 {1} | |||
| TN | −3.6 (15.8) {14} | −10.4 (39.5) {5} | −43.4 (34.9) {2} | −27.8 {1} | |||||
| Right | 19.0 | 10.9 to 27.0 | <0.001 | ||||||
| TMH | 14.1 (13.6) {14} | 18.4 (23.5) {8} | 16.8 (36.8) {4} | 1.5 (14.9) {3} | 3.0 (8.7) {3} | 2.0 {1} | |||
| TN | −1.3 (13.9) {15} | −5.3 (32.6) {5} | −35.4 (26.9) {2} | −37.8 {1} |
Data reported as mean (SD) {sample size} and presented every 15 min for the first 2 hours and every 2 hours afterwards.
TMH, targeted mild hypercapnia; TN, targeted normocapnia.
On the longitudinal time-by-treatment interaction analysis, the difference in %ΔrSO2 on both left and right hemispheres between the two groups diverged with time, with the intervention group exhibiting a smaller percentage decrease over time compared with the control group (time-by-treatment interaction p<0.001 for both left and right hemispheres). We obtained very similar results on the robustness analyses when the above model was adjusted for age, baseline oximetry and preoperative Hb levels, as well as when the percentage of total duration of surgery, instead of minutes from the start of surgery, were included.
Secondary outcomes
Postoperative delirium was statistically significantly less common in the TMH group. Postoperative delirium was present in 0 out of 20 (0%) participants in the TMH group and 6 out of 20 (30%) participants in the TN group (risk difference 0.3, 95% CI 0.1 to 0.5, Fisher’s exact p=0.02) (table 3).
Table 3.
Postoperative delirium and opioid doses
| TMH group (n=20) |
TN group (n=20) |
||
| Premedication | |||
| Number of patients | 0 (0.0) | 2 (10.0) | |
| Mean midazolam dose (mg) | 0 | 1.75 | |
| Intraoperative opioid | |||
| Total dose (mg) | 21.67 (13.75–32.50) | 16.67 (10.00–22.50) | (p=0.22) |
| Received intravenous morphine | 2 (10.0) | 1 (5.0) | |
| Received intravenous fentanyl | 10 (50.0) | 14 (70.0) | |
| Received intravenous oxycodone | 9 (45.0) | 7 (35.0) | |
| Received intravenous tramadol | 4 (20.0) | 0 (0.0) | |
| Received intravenous clonidine | 0 (0.0) | 2 (10.0) | |
| Intrathecal morphine | |||
| Number of patients | 5 | 2 | |
| Mean dose (μg) | 220 | 350 | |
| Epidural analgesia | |||
| Number of patients | 0 | 0 | |
| Blood glucose level | |||
| Glucose (mmol/L) | 7.1 (7.0–7.1) | 6.6 (6.4 to 6.7) | (p=0.33) |
| Preoperative CAM | 0 (0.0–0.0) | 0 (0.0–0.0) | |
| Postoperative CAM | 0 (0.0–0.0) | 1.5 (0.0–3.0) | |
| Presence of postoperative delirium | 0 (0.0) | 6 (30.0) | (p=0.02) |
Data reported as median (IQR) or number (%).
Note some patients received two or more different intraoperative opioids.
Total dose of intraoperative opioid normalised to intravenous morphine equivalent.
CAM, Confusion Assessment Method; TMH, targeted mild hypercapnia; TN, targeted normocapnia.
In terms of acid–base variables, median intraoperative pH was statistically significantly lower (7.31 vs 7.46; p<0.001), and intraoperative bicarbonate was statistically significantly higher (25.00 vs 24.00 mEq/L; p=0.020) in the TMH group. No statistically significant differences in base excess (−1.00 vs 1.00 mmol/L; p=0.069) and potassium (3.98 vs 4.03 mEq/L; p=0.759) were observed intraoperatively. Length of hospital stay was also similar between the two groups (5 vs 5 days; p=0.988). These results are summarised in table 4.
Table 4.
Arterial blood gas values and the corresponding EtCO2
| TMH group (n=20) |
TN group (n=20) |
P value | |
| pH | 7.31 (7.27–7.33) | 7.46 (7.43–7.47) | <0.001 |
| PaO2 (mm Hg) | 156.8 (146.3–217.2) | 142.5 (122.5–199.1) | 0.380 |
| PaCO2 (mm Hg) | 51.50 (46.88–60.88) | 34.75 (32.75–38.12) | <0.001 |
| EtCO2 (mm Hg) | 46.40 (39.80–50.20) | 30.40 (28.50–32.00) | <0.001 |
| Bicarbonate (mEq/L) | 25.00 (24.00–27.75) | 24.00 (22.00–24.62) | 0.020 |
| Base excess (mmol/L) | −1.00(-2.50–0.25) | 1.00 (-0.88–2.00) | 0.069 |
| Potassium (mEq/L) | 3.98 (3.73–4.38) | 4.03 (3.58–4.31) | 0.759 |
| Total Hb (g/L) | 130.50 (118.12–140.62) | 122.25 (106.88–131.25) | 0.132 |
Data reported as median (IQR) or number (%).
EtCO2, end tidal carbon dioxide; Hb, haemoglobin concentration; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood; TMH, targeted mild hypercapnia; TN, targeted normocapnia.
Discussion
We conducted a prospective, single-centre, single-blinded, randomised controlled trial evaluating the effects of TMH and TN on rSO2 in patients undergoing major surgery. TMH led to a stable increase in both left and right NIRS-derived rSO2 from the baseline values, while TN led to a decrease in rSO2. This effect was sustained throughout surgery and became more pronounced with the passage of time. Furthermore, TMH was associated with a lower incidence of postoperative delirium within 24 hours after surgery.
While the relationship between elevated PaCO2 and cerebral blood flow (CBF) is well described,26–29 the associations between hypercapnia and higher rSO2 are poorly understood. Numerous factors, for instance, cardiac output, haemoglobin affinity for oxygen, cerebral autoregulation and the ratio of cerebral arterial to venous blood volume affect rSO2 in the setting of hypercapnia, but changes in PaCO2 and CBF, in turn, have a direct influence on these factors.30 31 To complicate the subject further, the duration of effect of hypercapnia on rSO2 is unknown. In our study, confounding variables, such as MAP, PaO2, Hb and intraoperative position, were similar between the TMH and TN groups. However, pH, which directly affects the haemoglobin affinity for oxygen via the Bohr Effect, was significantly different. Since we cannot measure the ratio of arterial to venous blood volume, it would be impetuous to comment on the mechanism behind the observed higher rSO2 values in TMH. Clinically, similar observations have been reported previously. Eastwood et al compared rSO2 values at the end of alternating hypercapnic and normocapnic periods in postcardiac arrest patients in a double cross-over study, and discovered that mild hypercapnia resulted in higher rSO2.32 When Akca et al delivered mild hypercapnia intraoperatively to investigate tissue oxygenation and its relationship with wound infection risk after surgery, cerebral oxygen saturation was found to be higher in the mild hypercapnic group.15 Similarly, rSO2 remained higher in hypercapnic patients throughout shoulder surgery, and less cerebral desaturation events were observed by Murphy et al.33 Our study is one of the few randomised controlled trials that investigated rSO2 change over time. We found that the sustained difference in rSO2 over time was a combined effect of a stable increase in rSO2 from the baseline in the TMH group and a stable decrease in rSO2 from the baseline in the TN group. In the literature, the association between normocapnia and reduced CBF and lower levels of rSO2 have been reported.34 However, the exact mechanism and associations between normocapnia and variations in rSO2 values are not entirely clear. While theoretical absolute and relative saturation thresholds requiring prompt interventions have been proposed,14 these thresholds have not been validated and there is a lack of consensus on the indication and timing of interventions. In our study, the reduction in rSO2 from the baseline was small in the majority of patients in the TN group, and the attending anaesthetists had no rSO2 target to titrate to. As a result, no interventions were performed intraoperatively in response to changes in rSO2. Comparing the TMH and TN groups, the sustained difference in percentage change in rSO2 over time is a novel finding.
Interestingly, the incidence of postoperative delirium after surgery was lower in the TMH group, while LOS remained similar between the groups. Patients who suffered from postoperative delirium were all in the TN group, but they were also older (median (IQR) age=72 (59.5–77)) and had higher ASA scores (ASA scores of 3, 2, 1, 4 and 4). Their baseline medical comorbidities and duration of surgery (median (IQR) duration of surgery=171 min (83.5–254.5)) were similar to other study participants. There has been conflicting evidence in the literature regarding the relationship between rSO2 and LOS on postoperative cognitive performance. Cognitive outcomes were similar in groups with or without NIRS-based rSO2 optimisation in a recent randomised controlled trial.14 35 On the other hand, Murkin et al found that monitoring and reacting to cerebral desaturation during coronary artery bypass surgery was associated with clinical benefits.13 Patients with shorter LOS (<10 days) had a higher mean rSO2. Intraoperative NIRS rSO2 monitoring led to a significant reduction in postoperative cognitive disturbance, confirmed by Trafidlo et al.36 Casati et al also reported that higher rSO2 led to shorter LOS and improved Mini-Mental State Examination scores in elderly patients undergoing major abdominal surgery,37 and Schoen et al found that low preoperative rSO2 was associated with a higher incidence of postoperative delirium. Among patients who started at a normal rSO2 level, those who developed delirium had a larger intraoperative drop in rSO2.38 Our findings were consistent with those of Schoen et al.; however, they need to be interpreted with caution, as the ASA scores and age were slightly higher in the TN group, and our study was not designed to quantitatively investigate postoperative cognitive performance in hypercapnia.
Implications of our findings demonstrate that TMH can be delivered reliably during major surgery, and its effects on rSO2 can be monitored with NIRS in most patients. Its delivery is reliably associated with increased levels of rSO2, and the relatively higher rSO2 is sustained over the duration of surgery, an observation that has not been reported in the literature. Furthermore, TMH may reduce the incidence of the development of immediate postoperative delirium. A clinical concern of mild hypercapnia is hypercapnic-induced acidosis and the subsequent development of hyperkalaemia. While a linear correlation between arterial carbon dioxide and plasma pH is well reported,39 the relationship between acute hypercapnia, respiratory acidosis and plasma potassium is also poorly understood.40 In the present study, we found no association between hypercapnia and serum potassium concentration, a finding also supported by others.41 We did not observe any other deleterious or adverse effects from hypercapnic-induced acidosis such as cardiac arrhythmias in our study. Interestingly, while our study was not designed to measure differences in analgesia and partial pressure of oxygen in arterial blood, we observed a 10% higher median PaO2 level in the TMH group and found that the median intraoperative analgesia requirements were also approximately 30% higher. Both arterial oxygen levels and pain have been reported to influence tissue oxygenation,42 which was not directly measured in our study. The effect of pain on cerebral oxygenation is unclear and has not been borne out in clinical studies43; further studies exploring this association are needed. Finally, we have shown that NIRS-based cerebral oximetry is a non-invasive and practical method of measuring rSO2, easily incorporated into the existing collection of routine monitoring variables, findings that are in agreement with other research groups.20 44–46
Our study has multiple strengths. Our findings have high internal validity because the study was a randomised controlled trial with concealed allocation and blinded assessment, minimising selection and ascertainment bias. The rSO2 data were exported directly to RStudio, and ABG data were analysed by the ABL Blood Gas Analyzer, rendering sampling error from data entry unlikely, thereby increasing the robustness of our findings. Sampling of continuous oximetry data resulted in a stream of oximetry data throughout the monitoring periods, maximising the details of our assessment. Although the duration of surgery was different for individual patients, oximetry data were not normalised to another time scale, enabling a fair comparison of data across the study groups. NIRS-derived rSO2 has been criticised for potential extracranial contamination that would confound true rSO2.47 However, there is sufficient evidence to support the accuracy of NIRS-derived rSO2,20 44 particularly in the case of hypercapnia, where extracranial signal interference has been shown to be insignificant, justifying its reliability.48 Moreover, as the technology was the same in both groups, any inaccuracy should not have been a source of bias.
Our study also has a number of limitations. The attending anaesthetists were not blinded due to the nature of the intervention. Nevertheless, bias was mitigated by the fact that measurements were taken directly from the cerebral oximetry machine, and the assessment of delirium was conducted by an independent researcher blinded to the intervention. The external validity of our findings was restricted by the small sample size from one single centre. The sample size calculation was based on the assumption that there were no changes in rSO2 values from the baseline in the TN group. The observed negative change can therefore impact the calculation. The strong nature of interaction between treatment and time for rSO2 outcome should be treated with caution due to the potential minor departures of the data from the linear trend. Our findings were not applicable to patients undergoing emergency surgery, intracranial surgery or surgery requiring one lung ventilation. The cerebral oximetry probes were only attached to the forehead, measuring rSO2 within the frontal cortex region, which carries the assumption that rSO2 was homogenous across every area of the brain. Quantification of device failure rate, despite being a critical consideration, cannot be described by our study design.
We did not measure cardiac output, stroke volume and systemic vascular resistance. Therefore, the effects on changes in intrathoracic pressure on cardiac output are unknown. Changes in intrathoracic pressure may have adversely impacted cardiac output, which may in turn have affected the EtCO2. However, given that the positive end-expiratory pressure was held constant in both groups, and the changes in lung tidal volumes were relatively small, the impact of intrathoracic pressure on cardiac output is likely to be small. Finally, our findings of a greater incidence of early postoperative delirium in the TN group need to be interpreted with caution, as confounders of postoperative delirium were not controlled, our study was not powered to investigate postoperative delirium, and mental state was only assessed by CAM, once preoperatively and once postoperatively. Accordingly, our findings for delirium should be viewed as hypothesis generating. Nevertheless, if we were to consider that our effect size observed (ie, risk difference of 0.3) could be due to chance and a smaller effect would be observed in a larger study, an appropriate powered randomised controlled trial for this outcome would be very feasible. If the proportion of patients with delirium in the intervention group is 10%, to achieve 90% power, the required sample size for each group would be 92.
Conclusion
In summary, TMH was associated with a stable increase in rSO2 from the baseline, while TN was associated with a decrease in rSO2 from the baseline in both hemispheres. This effect was sustained and became more pronounced with the passage of time intraoperatively.
Supplementary Material
Footnotes
Presented at: Findings of this study were presented as a poster presentation at the PostGraduate Assembly in Anesthesiology held during 8–12 December 2018 at New York, USA.
Contributors: CW and LC contributed to data collection, data analysis and manuscript write-up. DC contributed to patient recruitment, data collection and preparation of manuscript. COT, RH, DT, BP and PP contributed to patient recruitment and preparation of manuscript. DK contributed to data collection and preparation of manuscript. RB contributed to study design and preparation of manuscript. LW designed the study, contributed to patient recruitment, data collection, data analysis and preparation of manuscript.
Funding: Masimo provided the oximetry sensors used for this trial. This study conception, design, trial management, data collection, data analysis, and the writing of the manuscript, have been executed completely independently of Masimo and any other external organisations. This work was supported by the Deparment of Anaesthesia Research Fund, Austin Hospital, Heidelberg, Victoria, Australia.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Brosnan RJ, Steffey EP, LeCouteur RA, et al. . Effects of ventilation and isoflurane end-tidal concentration on intracranial and cerebral perfusion pressures in horses. Am J Vet Res 2003;64:21–5. 10.2460/ajvr.2003.64.21 [DOI] [PubMed] [Google Scholar]
- 2. Faraci FM, Breese KR, Heistad DD. Cerebral vasodilation during hypercapnia. Role of glibenclamide-sensitive potassium channels and nitric oxide. Stroke 1994;25:1679–83. 10.1161/01.STR.25.8.1679 [DOI] [PubMed] [Google Scholar]
- 3. Hino JK, Short BL, Rais-Bahrami K, et al. . Cerebral blood flow and metabolism during and after prolonged hypercapnia in newborn lambs. Crit Care Med 2000;28:3505–10. 10.1097/00003246-200010000-00026 [DOI] [PubMed] [Google Scholar]
- 4. Nakahata K, Kinoshita H, Hirano Y, et al. . Mild hypercapnia induces vasodilation via adenosine triphosphate-sensitive K+ channels in parenchymal microvessels of the rat cerebral cortex. Anesthesiology 2003;99:1333–9. 10.1097/00000542-200312000-00014 [DOI] [PubMed] [Google Scholar]
- 5. Kaku D, Giffard R, Choi D. Neuroprotective effects of glutamate antagonists and extracellular acidity. Science 1993;260:1516–8. 10.1126/science.8389056 [DOI] [PubMed] [Google Scholar]
- 6. Vannucci RC, Brucklacher RM, Vannucci SJ. Effect of carbon dioxide on cerebral metabolism during hypoxia-ischemia in the immature rat. Pediatr Res 1997;42:24–9. 10.1203/00006450-199707000-00005 [DOI] [PubMed] [Google Scholar]
- 7. Murkin JM, Arango M. Near-Infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009;103:i3–13. 10.1093/bja/aep299 [DOI] [PubMed] [Google Scholar]
- 8. Elizabeth A, Frost M. Cerebral oximetry: emerging applications for an established technology. Anesthesiol News 2012;38:10. [Google Scholar]
- 9. Steppan J, Hogue CW. Cerebral and tissue oximetry. Best Pract Res Clin Anaesthesiol 2014;28:429–39. 10.1016/j.bpa.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn A, Yang J, Inigo-Santiago L, et al. . A feasibility study of cerebral oximetry monitoring during the post-resuscitation period in comatose patients following cardiac arrest. Resuscitation 2014;85:522–6. 10.1016/j.resuscitation.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 11. Storm C, Leithner C, Krannich A, et al. . Regional cerebral oxygen saturation after cardiac arrest in 60 patients—A prospective outcome study. Resuscitation 2014;85:1037–41. 10.1016/j.resuscitation.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 12. Ito N, Nishiyama K, Callaway CW, et al. . Noninvasive regional cerebral oxygen saturation for neurological prognostication of patients with out-of-hospital cardiac arrest: a prospective multicenter observational study. Resuscitation 2014;85:778–84. 10.1016/j.resuscitation.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 13. Murkin JM, Adams SJ, Novick RJ, et al. . Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg 2007;104:51–8. 10.1213/01.ane.0000246814.29362.f4 [DOI] [PubMed] [Google Scholar]
- 14. Deschamps A, Hall R, Grocott H, et al. . Cerebral oximetry monitoring to maintain normal cerebral oxygen saturation during high-risk cardiac surgery: a randomized controlled feasibility trial. Anesthesiology 2016;124:826–36. [DOI] [PubMed] [Google Scholar]
- 15. Akça O, Liem E, Suleman M-I, et al. . Effect of intra-operative end-tidal carbon dioxide partial pressure on tissue oxygenation. Anaesthesia 2003;58:536–42. 10.1046/j.1365-2044.2003.03193.x [DOI] [PubMed] [Google Scholar]
- 16. Robinson TN, Eiseman B. Postoperative delirium in the elderly: diagnosis and management. Clin Interv Aging 2008;3:351–5. 10.2147/CIA.S2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu LL, Leung JM. Predicting adverse postoperative outcomes in patients aged 80 years or older. J Am Geriatr Soc 2000;48:405–12. 10.1111/j.1532-5415.2000.tb04698.x [DOI] [PubMed] [Google Scholar]
- 18. Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–65. 10.1056/NEJMra052321 [DOI] [PubMed] [Google Scholar]
- 19. Schulz KF, Altman DG, Moher D, et al. . Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redford D, Paidy S, Kashif F. Absolute and trend accuracy of a new regional oximeter in healthy volunteers during controlled hypoxia. Anesth Analg 2014;119:1315–9. 10.1213/ANE.0000000000000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inouye SK, van Dyck CH, Alessi CA, et al. . Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8. 10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- 22. Sharon K, Inouye MD MPH. Confusion assessment method: training manual and coding guide, Copyright 2003.
- 23. National Blood Authority Blood management guidelines: module 2 perioperative, 2012. Available: http://www.nba.gov.au/guidelines/module2/po-mod2.pdf2
- 24. Vretzakis G, Georgopoulou S, Stamoulis K, et al. . Cerebral oximetry in cardiac anesthesia. J Thorac Dis 2014;6:S60–9. 10.3978/j.issn.2072-1439.2013.10.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang TA, Altman DG. Basic statistical reporting for articles published in Biomedical Journals: The “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int J Nurs Stud 2015;52:5–9. 10.1016/j.ijnurstu.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 26. Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: carbon dioxide. Critical Care 2010;14:220 10.1186/cc8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yokoyama I, Inoue Y, Kinoshita T, et al. . Heart and brain circulation and CO2 in healthy men. Acta Physiol 2008;193:303–8. 10.1111/j.1748-1716.2008.01846.x [DOI] [PubMed] [Google Scholar]
- 28. Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol 2011;589:3039–48. 10.1113/jphysiol.2011.206052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giardino ND, Friedman SD, Dager SR. Anxiety, respiration, and cerebral blood flow: implications for functional brain imaging. Compr Psychiatry 2007;48:103–12. 10.1016/j.comppsych.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vranken NPA, Weerwind PW, Sutedja NA, et al. . Cerebral oximetry and autoregulation during cardiopulmonary bypass: a review. J Extra Corpor Technol 2017;49:182–91. [PMC free article] [PubMed] [Google Scholar]
- 31. Ševerdija EE, Vranken NPA, Simons AP, et al. . Hemodilution combined with hypercapnia impairs cerebral autoregulation during normothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2015;29:1194–9. 10.1053/j.jvca.2015.03.022 [DOI] [PubMed] [Google Scholar]
- 32. Eastwood GM, Tanaka A, Bellomo R. Cerebral oxygenation in mechanically ventilated early cardiac arrest survivors: the impact of hypercapnia. Resuscitation 2016;102:11–16. 10.1016/j.resuscitation.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 33. Murphy GS, Szokol JW, Avram MJ, et al. . Effect of ventilation on cerebral oxygenation in patients undergoing surgery in the beach chair position: a randomized controlled trial. Br J Anaesth 2014;113:618–27. 10.1093/bja/aeu109 [DOI] [PubMed] [Google Scholar]
- 34. Brian JE, Brian Johnny E MD. Carbon dioxide and the cerebral circulation. Anesthesiology 1998;88:1365–86. 10.1097/00000542-199805000-00029 [DOI] [PubMed] [Google Scholar]
- 35. Rogers CA, Stoica S, Ellis L, et al. . Randomized trial of near-infrared spectroscopy for personalized optimization of cerebral tissue oxygenation during cardiac surgery. Br J Anaesth 2017;119:384–93. 10.1093/bja/aex182 [DOI] [PubMed] [Google Scholar]
- 36. Trafidło T, Gaszyński T, Gaszyński W, et al. . Intraoperative monitoring of cerebral NIRS oximetry leads to better postoperative cognitive performance: a pilot study. International Journal of Surgery 2015;16:23–30. 10.1016/j.ijsu.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 37. Casati A, Fanelli G, Pietropaoli P, et al. . Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg 2005;101:740–7. 10.1213/01.ane.0000166974.96219.cd [DOI] [PubMed] [Google Scholar]
- 38. Schoen J, Meyerrose J, Paarmann H, et al. . Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care 2011;15:R218 10.1186/cc10454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Finsterer U, Lühr H-G, Wirth AE. Effects of acute hypercapnia and hypocapnia on plasma and red cell potassium, blood lactate and base excess in man during anesthesia. Acta Anaesthesiol Scand 1978;22:353–66. 10.1111/j.1399-6576.1978.tb01311.x [DOI] [PubMed] [Google Scholar]
- 40. Adrogué HJ, Madias NE. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med 1981;71:456–67. 10.1016/0002-9343(81)90182-0 [DOI] [PubMed] [Google Scholar]
- 41. Natalini G, Seramondi V, Fassini P, et al. . Acute respiratory acidosis does not increase plasma potassium in normokalaemic anaesthetized patients. A controlled randomized trial. Eur J Anaesthesiol 2001;18:394–400. 10.1097/00003643-200106000-00008 [DOI] [PubMed] [Google Scholar]
- 42. Akca O. Pain and tissue oxygenation. Crit Care Med 2015;43:e462–3. 10.1097/CCM.0000000000001126 [DOI] [PubMed] [Google Scholar]
- 43. Høiseth Lars Ø., Hisdal J, Hoff IE, et al. . Tissue oxygen saturation and finger perfusion index in central hypovolemia. Crit Care Med 2015;43:747–56. 10.1097/CCM.0000000000000766 [DOI] [PubMed] [Google Scholar]
- 44. MacLeod DB, Ikeda K, Vacchiano C, et al. . Development and validation of a cerebral oximeter capable of absolute accuracy. J Cardiothorac Vasc Anesth 2012;26:1007–14. 10.1053/j.jvca.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 45. Watzman HM, Kurth CD, Montenegro LM, et al. . Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology 2000;93:947–53. 10.1097/00000542-200010000-00012 [DOI] [PubMed] [Google Scholar]
- 46. Brady K, Joshi B, Zweifel C, et al. . Real-Time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010;41:1951–6. 10.1161/STROKEAHA.109.575159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology 2012;116:834–40. 10.1097/ALN.0b013e31824c00d7 [DOI] [PubMed] [Google Scholar]
- 48. Akça O, Sessler DI, Delong D, et al. . Tissue oxygenation response to mild hypercapnia during cardiopulmonary bypass with constant pump output. Br J Anaesth 2006;96:708–14. 10.1093/bja/ael093 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-029159supp001.pdf (36.4KB, pdf)
bmjopen-2019-029159supp002.pdf (35KB, pdf)