Abstract
Introduction
Despite the development of new therapies for advanced prostate cancer, it remains the most common cause of cancer and the second leading cause of cancer death in men. It is critical to develop novel agents for the treatment of prostate cancer, particularly those that target aspects of androgen receptor (AR) signalling or prostate biology other than inhibition of androgen synthesis or AR binding. Neoadjuvant pharmacodynamic studies allow for a rational approach to the decisions regarding which targeted therapies should progress to phase II/III trials. CDK4/6 inhibitors have evidence of efficacy in breast cancer, and have been shown to have activity in preclinical models of hormone sensitive and castrate resistant prostate cancer. The LEEP trial aims to assess the pharmacodynamic effects of LEE011 (ribociclib), an orally bioavailable and highly selective CDK4/6 inhibitor, in men undergoing radical prostatectomy for high-risk, localised prostate cancer.
Methods and analysis
The multicentre randomised, controlled 4:1 two-arm, phase II, open label pharmacodynamic study will recruit 47 men with high risk, localised prostate cancer who are planned to undergo radical prostatectomy. Participants who are randomised to receive the study treatment will be treated with LEE011 400 mg daily for 21 days for one cycle. The primary endpoint is the frequency of a 50% reduction in Ki-67 proliferation index from the pretreatment prostate biopsy compared to that present in prostate cancer tissue from radical prostatectomy. Secondary and tertiary endpoints include pharmacodynamic assessment of CDK4/6 cell cycle progression via E2F levels, apoptotic cell death by cleaved caspase-3, changes in serum and tumour levels of Prostate Specific Antigen (PSA), pathological regression, safety via incidence of adverse events and exploratory biomarker analysis.
Ethics and dissemination
The protocol was approved by a central ethics review committee (St Vincent’s Hospital HREC) for all participating sites (HREC/17/SVH/294). Results will be disseminated in peer-reviewed journals and at scientific conferences.
Drug supply
Novartis.
Protocol version
2.0, 30 May 2019
Trial registration number
Australian New Zealand Clinical Trials Registry (ACTRN12618000354280).
Keywords: prostatic neoplasms, neoadjuvant trial, window of opportunity trial, ribociclib, translational research
Strengths and limitations of this study.
This is the first trial evaluating the pharmacodynamic effects of CDK4/6 inhibitors in hormone sensitive prostate cancer.
This study will explore potential biomarkers for treatment response.
This trial is designed to examine anti-tumour pharmacodynamics effects of single agent ribociclib.
This trial is not designed to determine whether a short course of neoadjuvant treatment could alter oncological outcomes or recurrence rates. These will be the next steps if the trial is positive.
By utilising paired samples, this neoadjuvant proof of concept trial allows us to use relatively small sample sizes, through examining dynamic changes in the biomarkers of interest.
Introduction
Despite advances in the detection and treatment of prostate cancer, it remains the most common cause of cancer in men in the developed world and the second leading cause of cancer death.1 Over the last decade, the treatment of advanced prostate cancer has changed dramatically with new therapies including novel anti-androgens,2 3 novel taxanes,4 radioisotope therapy5 and more recently Poly(adenosine diphosphate[ADP]-ribose) polymerase (PARP) inhibitors.6 However, these agents are not curative, and it is recognised that in order to improve survival from prostate cancer, it is critical to develop novel agents, particularly those that target aspects of androgen receptor (AR) signalling or prostate biology other than inhibition of androgen synthesis or AR binding.7
CDK4/6 inhibitors for treatment of prostate cancer
One of the common driving pathways that is altered in prostate cancer, and selected for in CRPC, is aberrant cell cycle activation through the cyclin/CDK/retinoblastoma (Rb) axis, with resultant uncontrolled cellular proliferation. This axis is critically important in controlling the G1-S transition of the cell cycle. There is evidence that androgens can stimulate the increased expression of G1 cyclins and cyclin-dependent kinases and decrease the expression of CDK inhibitors. The AR may also directly contribute to the transcription of some cell cycle regulatory genes, including cyclin D3.8
By binding to CDK4 and CDK6, selective CDK inhibitors inhibit Rb phosphorylation to prevent G1-S phase transition and induce cell cycle arrest. CDK4/6 inhibitors palbociclib (PD0332991; Pfizer), ribociclib (LEE011; Novartis) and abemaciclib (LY2835219; Eli Lilly) are oral and reversible small molecule inhibitors with high selectivity for CDK4 and CDK6, with evidence of efficacy in breast cancer.9–11
In preclinical models of hormone-sensitive and castration-resistant prostate cancer, palbociclib has exhibited single agent activity, by limiting cellular proliferation and growth.12 The potential therapeutic effect was determined in both in vivo mouse xenografts and a novel ex vivo assay using primary human tumours obtained from radical prostatectomy. This ex vivo model has also shown that LEE011 significantly inhibits prostate tumour cell proliferation in a dose dependent manner (unpublished, Butler LM, 2019). This preclinical data provides evidence that CDK4/6 inhibitors achieve clinically relevant biological responses in human prostate tumours, and supports the evaluation of CDK4/6 inhibitors for treatment of prostate cancer.
Towards more rapid assessment of new therapies
Clinical trials of new drugs in the hormone sensitive phase of prostate cancer (high-risk localised prostate cancer or at relapse after localised treatment) require long follow-up due to the natural history of the disease. The interval between biopsy and surgery offers an ideal opportunity for in vivo assessment of anti-tumour activity and selection of optimal novel agents for further investigation. Both Ki67 and Cleaved Caspase 3 have been used to assess pharmacodynamic activity of novel therapies in neoadjuvant studies in prostate and breast cancer.13–15 Ki67 reduction has also been found to correlate with response in neoadjuvant studies in breast cancer,16 17 and with outcome in prostate cancer.18 However there can be significant intra-tumour Ki67 heterogeneity, particularly in high risk prostate cancer,19 as well as inter-reader variability in its measurement.20 Where possible, centralised review of Ki67 in clinical trials is advisable.18
A recent systematic review identified that a lack of a biomarker-driven strategy and failure to achieve ‘proof of concept’ in phase II trials were significantly associated with failure of cancer drugs to achieve late-stage clinical success such as US Food and Drug Administration approval.21 Neoadjuvant pharmacodynamic studies, such as the one described in this project, will allow for a more rational approach to the decisions regarding which targeted therapies should go forward into phase II/III trials.
Biomarkers for treatment response
The identification of informative biomarkers in the preclinical phase, which can be incorporated into clinical studies, is pivotal to accelerating the drug development process, and when incorporated into clinical decision-making, can maximise patient benefit and minimise harm, with judicious drug administration. Candidate biomarkers will be identified/assessed in this clinical trial and potentially validated in future trials with CDK4/6 inhibitors.
Absent or decreased staining of nuclear Rb proteins is commonly found in prostate cancer specimens, and it has been suggested that inactivation of the Rb gene may be an important event in prostate tumour progression.22 In an ex vivo model, functional Rb is required for optimal CDK4/6 inhibitor efficacy.12 There is evidence that cyclin D1 overexpression is implicated in tumourigenesis and tumour progression, and may be related to the evolution to castration resistance in prostate cancer.23 The product of the INK4A gene inactivates the G1-phase cyclin dependent kinases CDK4 and CDK6. Overexpression of p16INK4A in high-grade prostatic epithelial neoplasia is associated with early relapse in prostate cancer patients treated with radical prostatectomy.24 Given the role of cyclin D1, Rb proteins and p16INK4A in cell cycle progression, there is interest in reviewing these as biomarkers of response to CDK4/6 inhibitors. Further, induction of cyclin D1 and p16INK4A have been identified as possible pharmacodynamic endpoints on preclinical models, and can be validated in a clinical setting.12 25
Hypothesis
Primary hypothesis
We hypothesise that administration of LEE011 (Ribociclib) to men prior to undergoing radical prostatectomy will lead to a 50% reduction in Ki-67 index in 30% or more of participants treated with LEE011, compared to a 50% reduction in Ki-67 index in 10% or fewer in the control group.
Secondary hypothesis
We hypothesise that treatment with LEE011 will be associated with inhibition of CDK4/6 cell cycle progression by a decrease in the level of E2F in prostate cancer tissue, and an increased level of cleaved caspase-3 in prostate tissue indicating increased apoptotic activity.
Methods and analysis
The LEEP study is an Australian-based, multicentre, randomised controlled, phase II, open label pharmacodynamic study. The primary aim is to determine the pharmacodynamic activity of the CDK4/6 inhibitor LEE011 in men with high-risk localised prostate cancer undergoing radical prostatectomy.
Study objectives
The primary objective of this study is to determine the effect of LEE011 on tumour cell proliferation, as determined by:
The frequency of a 50% reduction in the Ki-67 proliferation index from the pretreatment prostate biopsy compared to that present in prostate cancer tissue from radical prostatectomy.
The secondary objectives are to determine:
The effect of LEE011 on CDK4/6 cell cycle progression, by measuring E2F expression in prostate tissue by immunohistochemistry and peripheral blood mononuclear cells by ELISA.
The effect of LEE011 on apoptotic cell death, by examining the frequency of a 50% increase in cleaved caspase-3 expression levels in tumour cells, measured by immunohistochemistry.
Changes in serum and tumour levels of PSA, by immunoassay.
Rates of pathological regression, assessed by histopathology, as defined by cancer cell atrophy, decreased nuclear size, increased chromatin density and pale cytoplasm.
The incidence of adverse events (Common Terminology Criteria for Adverse Events (CTCAE) v 4.03).
The tertiary objectives are to evaluate exploratory biomarkers as predictors of response to therapy. These include Rb status, cyclin D1 amplification, p16INK4a expression, PTEN loss, AR amplifications/mutations and aberrations of PI3K signalling pathways (assessed by reverse transcription-PCR and Fluorescence in situ hybridization (FISH) analysis of cancer tissue from radical prostatectomy). These will also be tested in free plasma DNA, through a novel technique that has identified these aberrations in circulating tumour DNA.26 Effects on immune system such as circulating T-cell profiling will also be assessed.
Trial oversight and monitoring
The LEEP study is a collaboration between the Chris O’Brien Lifehouse, Sydney; Royal Prince Alfred Hospital, Sydney; the University of Adelaide, Adelaide; St Vincent’s Hospital, Sydney; and the National Health and Medical Research Council (NHMRC) Clinical Trials Centre (CTC), University of Sydney.
The University of Sydney is the study sponsor. The NHMRC CTC will be responsible for study co-ordination, monitoring, data acquisition, management and statistical analysis.
Safety and efficacy endpoints will be assessed when evaluable tissue is available from 22 participants treated with LEE011 and at study completion.
Protocol amendments can only be made by the trial management committee, and must be approved by the central institutional Human Research Ethics Committee (HREC) prior to implementation.
Patient and public involvement
This research was funded through a granting process that included a consumer representative from Cancer Voices NSW. The grant, study protocol and patient information sheet/consent form were all discussed, reviewed and edited by our consumer representative. A consumer representative is a member of the trial steering committee. Following completion, a plain-English version of the results will be made available to patients via their study doctor. Results of this study will be disseminated to study participants through peer-reviewed journals, at scientific conferences and on the NHMRC CTC website.
Trial design
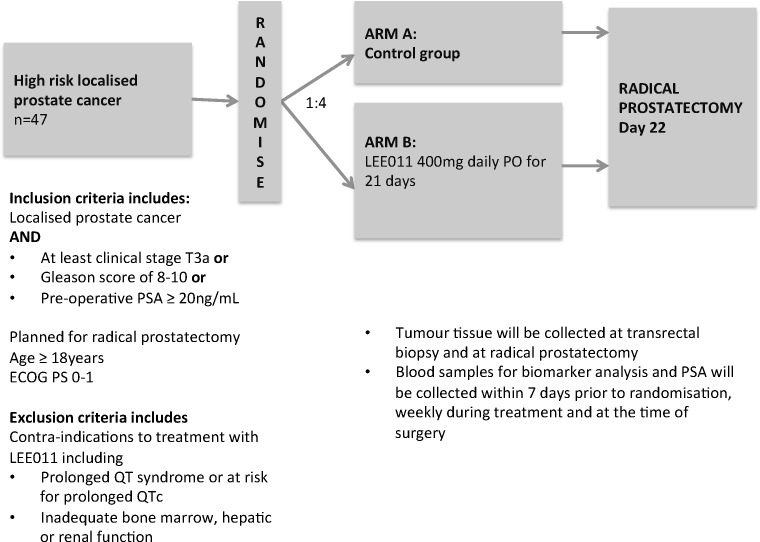
The protocol consists of a randomised, controlled 4:1 two-arm, phase II, open label pharmacodynamic study (figure 1). The trial is currently being conducted at two tertiary referral centres (Chris O’Brien Lifehouse, Sydney and St Vincent’s Health Network, Sydney) in New South Wales (NSW), Australia. There is a plan to open further sites during 2020.
Figure 1.
Study schema for randomised controlled, phase II trial of Cdk4/6 inhibitor LEE011 (ribociclib) in high-risk, localised prostate cancer. ECOG, Eastern Cooperative Oncology Group.
Inclusion criteria
Patients who fulfil all of the following characteristics will be considered eligible for enrolments:
Males ≥18 years with high-risk localised prostate cancer (at least clinical stage T3a OR Gleason score of between 8 and 10 OR Preoperative PSA ≥20 ng/mL) AND planned for radical prostatectomy.
Eastern Cooperative Oncology Group performance status of 0 or 1.
Histological confirmation of prostate cancer via a pretreatment diagnostic transrectal ultrasound (TRUS) biopsy.
Adequate bone marrow, hepatic and renal function.
Serum calcium, potassium, phosphate and magnesium within normal range or corrected with supplements.
Exclusion criteria
Patients with the following characteristics will be excluded from study enrolment:
Major surgery ≤2 weeks prior to enrolment or who have not recovered from side effects of such therapy. TRUS biopsy is not considered major surgery in this study.
Known hypersensitivity to the study drug or its excipients.
Patients with known disorders due to a deficiency in bilirubin glucuronidation (eg, Gilbert’s syndrome).
Diarrhoea ≥ CTCAE grade 2.
-
Impaired cardiac function, including any one of the following:
History (or family history) of long QT syndrome.
-
Those who already have or who are at significant risk of developing QTc prolongation, including patients with:
Long QT syndrome.
Mean QTcF ≥450 ms on baseline ECG.
Uncontrolled or significant cardiac disease including recent myocardial infarction.
Congestive heart failure, unstable angina or bradyarrhythmias.
Electrolyte abnormalities.
Clinically significant ECG abnormalities at clinician discretion.
Other clinically significant heart disease (eg, uncontrolled hypertension, history of labile hypertension or history of poor compliance with an anti-hypertensive regimen).
Clinically significant resting bradycardia (< 50 beats per minute).
Patients who are currently receiving treatment with any medication that has a relative risk of prolonging QTcF interval or inducing Torsades de Pointes and cannot be discontinued or switched to an alternative treatment prior to commencing study treatment.
Obligate use of a cardiac pacemaker.
Patients who have received prior antineoplastic therapy for advanced disease;
Prior treatment with a CDK4/6 inhibitor.
Patients who are currently receiving treatment with strong CYP3A4 inhibitors and cannot be discontinued or switched to an alternative treatment prior to commencing study treatment.
Patients receiving chronic or high-dose corticosteroid therapy.
Significant infection, including chronic active hepatitis B, hepatitis C or HIV.
Serious medical or psychiatric conditions that might limit the ability of the patient to comply with the protocol.
Investigational medical product—LEE011 (ribociclib)
This study will use LEE011, an orally bioavailable, highly selective, small-molecule inhibitor of CDK4/6 that blocks the phosphorylation of Rb protein, thereby preventing cell-cycle progression and inducing G1 phase arrest. Based on the results of preclinical toxicology studies and available clinical safety data, the main adverse reactions for LEE011 are bone marrow suppression including leucopenia, neutropenia, anaemia and thrombocytopaenia, dyspnoea, hepatic toxicity, renal toxicity, fatigue, nausea, vomiting, diarrhoea and prolongation of the QT interval. The risk of these toxicities may be amplified by concomitant administration of strong inhibitors of CYP3A4 or other combination treatments.
Randomisation
The method of randomisation will be minimisation with stratification by site. Participants will be allocated to the study treatment in a ratio of 4:1 (LEE011:control).
Recruitment and consent
Patient screening and enrolment will be overseen by the site principal investigator and performed by trained study personnel. Patients will provide written informed consent prior to study enrolment. Treatment will be planned to start within 7 days after randomisation.
Study procedures
The randomised, controlled, phase II, open label, pharmacodynamic study will assess the pharmacodynamic activity of the CDK4/6 inhibitor LEE011, in men with high-risk, localised prostate cancer.
Participants randomised to receive the study treatment will have a pretreatment multigated acquisition (MUGA) scan. Participants will receive LEE011 400 mg daily taken orally for 21 days treatment for one cycle. The scheduled surgery will occur 22 days after the first dose of LEE011 (if randomised to study drug treatment) or 22 days after randomisation (if randomised to the control group). Dose modifications are not permitted in this study. Patients who need to come off the study due to toxicity (eg, neutropenia or thrombocytopaenia) will discontinue and proceed to surgery as planned.
Local pharmacy departments will record drug receipt including a pill count to assess compliance.
Study samples will be stored at the Garvan Institute for Medical Research.
Data acquisition
Tumour tissue samples will be collected at transrectal biopsy and at radical prostatectomy. Blood samples for biomarker analysis and PSA will be collected within 7 days prior to randomisation, weekly during treatment and at the time of radical prostatectomy.
Ki-67 expression will be assessed by central pathologist review. Where possible, for all analyses, comparisons will be made between similar areas in the needle biopsy and radical prostatectomy specimens. Scoring for protein expression will be performed by two independent pathologist researchers from Royal Prince Alfred Hospital, both blinded to the treatment groups and pairings of tissue from the same patient. Discrepancies will be resolved by consensus.
Cell cycle arrest will be measured by E2F expression as determined by immunohistochemistry and scored by manual counting. Apoptotic cell death will be determined by examining the cleaved caspase-3 staining in tumour cells by immunohistochemistry and scored by manual counting. PSA levels in tumour and blood will be assessed by immunoassay, with immunohistochemistry or ELISA.
Trial data will be monitored by clinical trials programme staff from the NHMRC CTC.
Statistical considerations
Sample size estimation
Using the Simon’s two-stage design, an uninteresting rate for the true response is 10% and a clinically interesting rate which would warrant further investigation, is 30%. Based on this design, a sample size of 37 patients will have at least 90% power with 95% confidence to exclude the uninteresting rate in favour of the more clinically meaningful rate. A response is defined as ≥50% decrease in Ki-67 expression in the paired prostate biopsy baseline sample compared with the radical prostatectomy sample.
A futility analysis will be performed after 22 patients have completed one cycle of LEE011 and are evaluable for pharmacodynamic response. If there are two or fewer responses, consideration will be given to either reassessing the study design or stopping the study due to futility. When these 22 patients are assessed it is expected that there will be at least six patients in the control group, which will allow for assessment of the response to be performed in light of what is seen in the control arm. The study will then recruit an additional 15 patients in the treatment arm and four controls. This sample size allows for a modest number of drop-outs/loss to follow-up. It is anticipated that at least 33 patients in the treated cohort will be evaluable for response at study completion.
Ten untreated men will be enrolled in the control group to provide estimates of pharmacodynamic biomarkers as a basis for biological comparison, giving a total sample size of 47 patients.
It is expected that none of the control group men will have a ≥50% decrease in Ki-67. For the secondary objectives, the sample size of 10 control participants to 37 treated patients provides adequate power to detect large differences only.
Patients who do not receive study treatment, withdraw their consent or are not evaluable will be replaced.
Statistical analysis
Analysis of efficacy endpoints (ie, response, biomarkers) will include only evaluable patients. Analysis of safety endpoints (ie, toxicity) will be according to treatment received, including only patients who received at least 1 dose of the experimental treatment.
The response in each treatment arm will be summarised by the number and proportion of patients experiencing at least a 50% decrease in Ki-67 expression, with a two-tailed p value significance level of 0.05. Ki-67 levels pretreatment and post-treatment will also be summarised for each treatment arm using standard descriptive statistics. Data will be compared using McNemar’s test.
Analyses of secondary endpoints will include descriptive summaries. Continuous data will be compared using t-tests where appropriate and categorical data using χ2 tests.
Ethics and dissemination
The study will be conducted according to the Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95) annotated with Therapeutic Goods Administration Drug Safety and Evaluation Branch comments (July 2000) and in compliance with applicable laws and regulations. The study will be performed in accordance with the NHMRC Statement on Ethical Conduct in Research Involving Humans 2007 (updated May 2015), the NHMRC Australian Code for the Responsible Conduct of Research 2007 and the principles laid down by the World Medical Assembly in the Declaration of Helsinki 2008.
To this end, no patient will be recruited to the study until all the necessary approvals have been obtained, and the patient has provided written informed consent. Further, the investigator shall comply with the protocol, except when a protocol deviation is required to eliminate immediate hazard to a subject. In this circumstance, the NHMRC CTC, principal investigator and HREC must be advised immediately.
Results will be disseminated in peer-reviewed journals and at scientific conferences.
Trial status
Patient enrolment for the study commenced in November 2018 at the Chris O’Brien Lifehouse in NSW, Australia. St Vincent’s Hospital opened in late 2019, and there are plans to open several new sites in 2020. To date, eight patients have been enrolled, with anticipated enrolment to allow for the futility assessment by the first quarter in 2021.
Supplementary Material
Acknowledgments
We would like to thank Novartis for supply of LEE011 (ribociclib).
Footnotes
Twitter: @lisabutler5
LMB and LGH contributed equally.
Contributors: TS, LB and LH were responsible for manuscript writing. LH, LB, KM, JK, MS, PS, MC and LS were responsible for concept and protocol development. PS, AMJ, HW, RT, NA and LH are responsible for recruitment of study patients. All authors were responsible for final approval of the manuscript and are accountable for all aspects of the work.
Funding: This work was supported by Cancer Australia/Prostate Cancer Foundation of Australia grant number [APP1050880], Cancer Institute NSW and the Australian Prostate Cancer Research centre, NSW. Study sponsor: University of Sydney, NSW, 2006.
Competing interests: HW: lecturer and advisory boards for all of the following over the past 48 months: Astra Zeneca, Mundipharma, Janssen, Astellas, Ipsen, GSK and Boston Scientific.
Patient consent for publication: Not required.
Ethics approval: The protocol was approved at the St Vincent’s Hospital Human Research Ethics Committee and ethics review committees for all participating sites (HREC/17/SVH/294).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. De-identified raw data will be made available upon reasonable written request.
References
- 1. Cancer-Institute-NSW Cancer in New South Wales incidence and mortality; 2018.
- 2. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 4. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. The Lancet 2010;376:1147–54. 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 5. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 6. Mateo J, Carreira S, Sandhu S, et al. Dna-Repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697–708. 10.1056/NEJMoa1506859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scher HI, Buchanan G, Gerald W, et al. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 2004;11:459–76. 10.1677/erc.1.00525 [DOI] [PubMed] [Google Scholar]
- 8. Xu Y, Chen S-Y, Ross KN, et al. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res 2006;66:7783–92. 10.1158/0008-5472.CAN-05-4472 [DOI] [PubMed] [Google Scholar]
- 9. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–48. 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 10. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015;373:209–19. 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 11. Goetz MP, Toi M, Campone M, et al. Monarch 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 12. Comstock CES, Augello MA, Goodwin JF, et al. Targeting cell cycle and hormone receptor pathways in cancer. Oncogene 2013;32:5481–91. 10.1038/onc.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curigliano G, Gómez Pardo P, Meric-Bernstam F, et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast 2016;28:191–8. 10.1016/j.breast.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 14. Anantharaman A, Nguyen HG, Cooperberg MR, et al. A pharmacodynamic study of pre-prostatectomy buparlisib in men with high-risk, localized prostate cancer. JCO 2016;34:e14110–e10. 10.1200/JCO.2016.34.15_suppl.e14110 [DOI] [Google Scholar]
- 15. Armstrong AJ, Netto GJ, Rudek MA, et al. A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res 2010;16:3057–66. 10.1158/1078-0432.CCR-10-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2009;27:2630–7. 10.1200/JCO.2008.18.8391 [DOI] [PubMed] [Google Scholar]
- 17. Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167–70. 10.1093/jnci/djk020 [DOI] [PubMed] [Google Scholar]
- 18. Richardsen E, Andersen S, Al-Saad S, et al. Evaluation of the proliferation marker Ki-67 in a large prostatectomy cohort. PLoS One 2017;12:e0186852 10.1371/journal.pone.0186852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mesko S, Kupelian P, Demanes DJ, et al. Quantifying the Ki-67 heterogeneity profile in prostate cancer. Prostate Cancer 2013;2013 10.1155/2013/717080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polley M-YC, Leung SCY, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst 2013;105:1897–906. 10.1093/jnci/djt306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jardim DL, Groves ES, Breitfeld PP, et al. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. Cancer Treat Rev 2017;52:12–21. 10.1016/j.ctrv.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 22. Theodorescu D, Broder SR, Boyd JC, et al. P53, Bcl-2 and retinoblastoma proteins as long-term prognostic markers in localized carcinoma of the prostate. J Urol 1997;158:131–7. 10.1097/00005392-199707000-00040 [DOI] [PubMed] [Google Scholar]
- 23. Drobnjak M, Osman I, Scher HI, et al. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res 2000;6:1891. [PubMed] [Google Scholar]
- 24. Henshall SM, Quinn DI, Lee CS, et al. Overexpression of the cell cycle inhibitor p16INK4a in high-grade prostatic intraepithelial neoplasia predicts early relapse in prostate cancer patients. Clin Cancer Res 2001;7:544. [PubMed] [Google Scholar]
- 25. Dean JL, McClendon AK, Hickey TE, et al. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle 2012;11:2756–61. 10.4161/cc.21195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res 2015;21:2315–24. 10.1158/1078-0432.CCR-14-2666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.