Abstract
Introduction
A study based on the Danish Randomised Controlled Lung Cancer Screening Trial (DLCST) calculated the healthcare costs of lung cancer screening by comparing costs in an intervention group with a control group. Participants in both groups, however, experienced significantly increased negative psychosocial consequences after randomisation. Substantial participation bias has also been documented: The DLCST participants reported fewer negative psychosocial aspects and experienced better living conditions compared with the random sample.
Objective
To comprehensively analyse the costs of lung cancer CT screening and to determine whether invitations to mass screening alter the utilisation of the healthcare system resulting in indirect costs. Healthcare utilisation and costs are analysed in the primary care sector (general practitioner psychologists, physiotherapists, other specialists, drugs) and the secondary care sector (emergency room contacts, outpatient visits, hospitalisation days, surgical procedures and non-surgical procedures).
Design
To account for bias in the original trial, the costs and utilisation of healthcare by participants in DLCST were compared with a new reference group, selected in the period from randomisation (2004–2006) until 2014.
Setting
Four Danish national registers.
Participants
DLCST included 4104 current or former heavy smokers, randomly assigned to the CT group or the control group. The new reference group comprised a random sample of 535 current or former heavy smokers in the general Danish population who were never invited to participate in a cancer screening test.
Main outcome measures
Total healthcare costs including costs and utilisation of healthcare in both the primary and the secondary care sector.
Results
Compared with the reference group, the participants in both the CT group (offered annual CT screening, lung function test and smoking counselling) and the control group (offered annual lung function test and smoking counselling) had significantly increased total healthcare costs, calculated at 60% and 48% respectively. The increase in costs was caused by increased use of healthcare in both the primary and the secondary sectors.
Conclusion
CT screening leads to 60% increased total healthcare costs. Such increase would raise the expected annual healthcare cost per participant from EUR 2348 to EUR 3756. Cost analysis that only includes costs directly related to the CT scan and follow-up procedures most likely underestimates total costs. Our data show that the increased costs are not limited to the secondary sector.
Trial registration number
Keywords: lung cancer, mass screening, cancer screening test, CT scan, healthcare cost, illness perception
Strength and limitations of this study.
Our analysis included costs from both the primary sector and the secondary sector.
Our analysis accounted for the possible bias in the original study resulting from increased negative psychosocial consequences for participants in both the CT group and the control group after randomisation.
The risk of contamination, defined as screening in the control group (or reference group), was most likely low.
This study is not a randomised controlled trial, but we have adjusted the analysis for a number of possible confounders, in particular the socioeconomic variables.
The following costs have not been accounted for: costs in relation to days off work for CT screening and follow-up, costs of psychiatric hospitalisation, costs of medication bought without a prescription, costs of retirement and sick days and the influence of a cancer diagnosis on the insurance conditions of participants.
Introduction
There are strong incentives to screen for lung cancer: first, it is the primary cause of cancer-related deaths in the world1 2 and the aim of screening is to lower mortality by detecting and treating the cancer earlier. Second, most people diagnosed with lung cancer (80% in the USA) are former smokers3 who cannot benefit from primary prevention as they have already stopped smoking.
Lung cancer CT screening
In the USA, the National Lung Screening Trial (NLST) showed a 16% relative risk (RR) reduction in lung-cancer-specific mortality4 and 6.7% RR reduction in all-cause mortality after 6.5 years of follow-up.5 Based on these results, several American medical organisations recommend lung cancer screening,6 including the US Preventive Service Task Force (USPSTF)7 and The American Cancer Society.8 They recommend lung cancer CT screening for high-risk individuals: current smokers aged 55–74 (80 for USPSTF) with a smoking history of at least 30 pack-years; or, former smokers aged 55–74 (80 for USPSTF) who have stopped smoking within the past 15 years. As a consequence of the NLST study, lung cancer screening has now been implemented in the USA.
The European Society of Radiology (ESR) and the European Respiratory Society (ERS) also recommend lung cancer screening for high-risk individuals.6 In Europe, there have been seven randomised controlled trials (RCTs), including the Danish Lung Cancer Screening Trial (DLCST).3 So far, the European studies that have published their final mortality results were unable to find a statistically significant mortality reduction from screening.9 10 There could be a number of reasons for this finding: it could be due to lack of effect; a short follow-up period; the inclusion of more people with a lower risk (fewer pack-years of smoking) in the European trials and/or that the studies are underpowered.3 10–13
Systematic reviews have found significant heterogeneity in methodology and results of economic evaluations of lung cancer screening.14 15 Several researchers have analysed the cost-effectiveness of CT screening based on the NLST. One study estimated that the cost of preventing one lung cancer death was US$240 000.16 A second study revealed that screening with low-dose CT would cost US$81 000 per quality-adjusted life year (QALY) gained.17 Other studies found that the cost per life-year saved would be below US$19 00018 and that the average annual cost of lung cancer screening per person screened would be US$241.19 A study assessing the cost-effectiveness of CT screening within the Canadian healthcare system, estimated a cost-effectiveness of CAD 52 000 per QALY20 and another that high-risk screening would cost CAD 20 724 per QALI gained.
Using the outcomes from the NLST and cost and survival data in Australia estimated cost-effectiveness at AUD 233 000 per QALY gained.21
Two recent systematic reviews of cost-effectiveness analyses of Lung Cancer Screening concluded that it is still unclear whether or not low dose CT screening is cost effective.14 15 Several things can influence cost-effectiveness estimates among other: overdiagnosis, lead-time bias, the at-risk population, the characteristics of a smoking cessation programme and incidental findings.14 15 Only one study included incidental findings.15 17
The Danish Lung Cancer Screening Trial (DLCST)
The DLCST included 4104 current or former heavy smokers and participants were randomly assigned to the CT group or the control group. All participants took part in annual lung function testing and smoking counselling. The participants in the CT group also received an annual CT scan.12 Based on the DLCST, Rasmussen et al 22 investigated the direct and indirect costs of lung cancer screening. This study documented a statistically significant difference in total healthcare costs between the CT group and the control group when the costs of the screening programme were included. When these costs were excluded, there was no statistically significant difference between the two groups. According to the authors,22 23 three conditions might have influenced their cost estimates resulting in either an underestimation or an overestimation.
First, the participants in both the CT group and the control group experienced more negative psychosocial consequences after randomisation compared with baseline (before randomisation). This overall increased concern may have resulted in increased use of healthcare in both groups. In that case calculating the difference between costs in the two groups would then lead to underestimation of the real costs.
Second, lost income and productivity, early retirement, sick leave and use of medicines were not included in the analysis. Third, a further study on participation bias24 showed that participants in DLCST differed significantly from a representative sample of heavy smokers in the general population. The DLCST participants reported fewer negative psychosocial aspects and experienced better living conditions compared with the random sample. This bias could have resulted in underestimation or overestimation of healthcare costs.
The aim of this study, therefore, was to conduct a comprehensive analysis of healthcare costs in relation to lung cancer CT screening, using the work by Rasmussen et al 22 as a starting point. We included the costs of prescription medication in the analysis. In addition, we involved a new reference group to avoid the possible impact on healthcare cost and utilisation estimates from the control group in the original trial who experienced increased concern after randomisation, and to account for the revealed participation bias among DLCST participants.
Method
Definitions of costs
We defined ‘total costs’ as the total amount paid by the Danish state for healthcare services in the primary care sector (general practitioner (GP) contacts, GP costs, psychologist costs, physiotherapist costs, other specialist costs, prescription drug costs), plus healthcare services in the secondary care sector (emergency room contacts, outpatient visits, hospitalisation days, surgical procedures and non-surgical procedures). In addition, ‘total costs’ included the out-of-pocket costs for individual patients related to prescription drugs as well as medical reimbursement paid by the state. The cost of prescription medication paid for by the patient was included without value-added tax. We calculated total costs with the costs of the CT scan included and also excluded. Direct costs include all costs in relation to the screening procedure: the CT scan, the staff, the follow-up procedures and the housing. Indirect costs include: unintended extra healthcare use not directly related to CT screening.
Study population
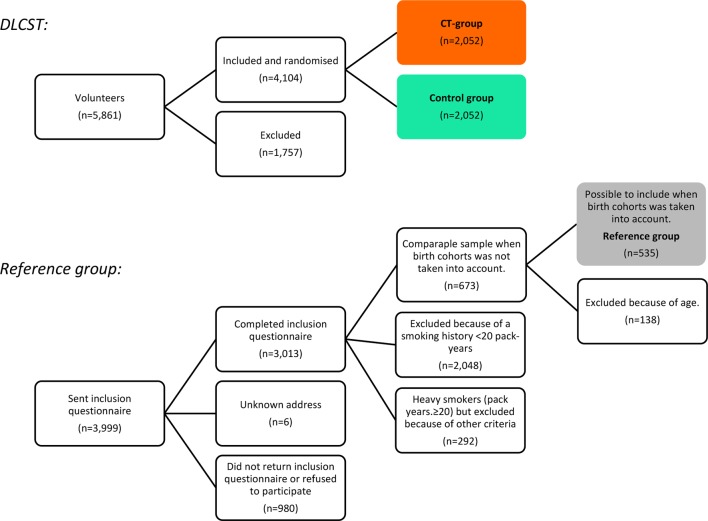
DLCST participants (the CT group and the control group) were compared with a reference group that had not been invited to screening (figure 1). The present study, therefore, included participants from two cohorts:
Figure 1.
Trial flow. DLCST, Danish Randomised Controlled Lung Cancer Screening Trial.
DLCST took place from October 2004 to March 2010. The study design has been described in detail elsewhere.12 Briefly, 4104 participants were included voluntarily in the trial. They had to be current or former heavy smokers (at least 20 pack-years) and aged 50–70 years. The participants were randomly assigned to either the CT group or the control group. No statistically significant differences in baseline socioeconomic characteristics or smoking habits were found between the two groups. All participants made annual visits to a screening clinic, where they completed questionnaires and received lung function tests and smoking counselling. In addition, the CT group received a CT scan. Participants with positive CT scans were referred for diagnostic workup.12
The reference group included 535 participants from the study ‘Participation bias in a randomised trial of screening for lung cancer’24 who met the inclusion criteria for DLCST and belonged to the same birth cohorts as DLCST participants. As previously reported, the reference group was more representative of former and heavy smokers in the general Danish population24 compared with DLCST participants, and they differed markedly with regard to socioeconomic characteristics.
The baseline characteristics of participants in DLCST (both CT and control) and in the reference group are shown in table 1. For a more comprehensive discussion of differences in characteristics, see Hestbech et al.24
Table 1.
Participant baseline characteristics in DLCST (CT and control) and the reference group
| Characteristics | N/N/N | CT group (n=2052) | Control group (n=2052) | Reference group (n=535) |
| Sex, n (%) | ||||
| Men | 2052/2052/535 | 1147 (55.9) | 1120 (54.6) | 335 (62.6) |
| Women | 905 (44.1) | 932 (45.4) | 200 (37.4) | |
| Age (years), mean (SD) | 2052/2052/535 | 57.3 (4.8) | 57.3 (4.8) | 57.0 (4.4) |
| SG, n (%) | ||||
| I | 2041/2039/489 | 155 (7.6) | 141 (6.9) | 25 (5.1) |
| II | 402 (19.7) | 410 (20.1) | 69 (14.1) | |
| III | 378 (18.5) | 378 (18.5) | 88 (18.0) | |
| IV | 545 (26.7) | 551 (27.0) | 152 (31.1) | |
| V | 265 (13.0) | 282 (13.8) | 110 (22.5) | |
| Employed, SG uncertain | 182 (8.9) | 168 (8.2) | 25 (5.1) | |
| Outside the labour market | 114 (5.6) | 109 (5.4) | 20 (4.1) | |
| School education, n (%) | ||||
| 7–9 years in school | 2047/2047/533 | 698 (34.1) | 715 (34.9) | 241 (45.2) |
| 10 years in school | 775 (37.9) | 790 (38.6) | 151 (28.3) | |
| 12–13 years in school | 553 (27.0) | 532 (26.0) | 100 (18.8) | |
| Other | 21 (1.0) | 10 (0.5) | 41 (7.7) | |
| Vocational education | ||||
| None | 2043/2047/531 | 187 (9.2) | 201 (9.8) | 81 (15.3) |
| Semiskilled worker | 20 (1.0) | 27 (1.3) | 36 (6.8) | |
| Vocational training | 702 (34.4) | 724 (35.4) | 133 (25.1) | |
| Short further education | 199 (9.7) | 194 (9.5) | 41 (7.7) | |
| Middle further education | 506 (24.8) | 539 (26.3) | 110 (20.7) | |
| Long further education | 264 (12.9) | 225 (11.0) | 49 (9.2) | |
| Other | 165 (8.1) | 137 (6.7) | 81 (15.3) | |
| Employment status, n (%) | ||||
| Employed | 2043/2045/533 | 1366 (66.9) | 1324 (64.7) | 236 (44.3) |
| Studying | 9 (0.4) | 12 (0.6) | 0 | |
| Job seeking | 113 (5.5) | 104 (5.1) | 17 (3.2) | |
| Retired | 555 (27.2) | 605 (29.6) | 280 (52.5) | |
| Region of residence, n (%) | ||||
| Capital region | 2037/2044/535 | 1644 (80.7) | 1653 (80.9) | 163 (30.5) |
| Region Zealand | 353 (17.3) | 349 (17.1) | 72 (13.5) | |
| Region of Southern Denmark | 29 (1.4) | 28 (1.4) | 129 (24.1) | |
| Region of Central Denmark | 8 (0.4) | 11 (0.5) | 113 (21.1) | |
| Region of Northern Denmark | 3 (0.2) | 3 (0.2) | 58 (10.8) | |
| Living alone, n (%) | ||||
| No | 2039/2034/533 | 1457 (71.5) | 1453 (71.4) | 415 (77.9) |
| Yes | 582 (28.5) | 581 (28.6) | 118 (22.1) | |
| Smoking status, n (%) | ||||
| Current smoker | 2050/2051/534 | 1544 (75.3) | 1579 (77.0) | 276 (51.7) |
| Former smoker | 506 (24.7) | 472 (23.0) | 258 (48.3) | |
| Smoking history (pack years), mean (SD) | 2051/2048/535 | 36.4 (13.4) | 35.9 (13.4) | 36.9 (17.7) |
DLCST, Danish Randomised Controlled Lung Cancer Screening Trial; SD, Standard Deviation; SG, social group.
Registries and outcomes
Denmark has a publicly financed healthcare system and all citizens have a unique identification number. Every time a person uses the healthcare system the type of contact and the cost is recorded in national registries. This was also the case for all participants of the DLCST.
There is an economic incentive for the providers to register services as their reimbursement depends on the invoice. The data for this study were extracted from these registries, namely: National Patient Registry; National Health Insurance Registry; Diagnostic Related Groups-Diagnostic Outpatient Group System Registries; Drug Registry. We chose a set of outcomes to reflect a comprehensive summary of healthcare utilisation in primary and secondary care sector (table 2).
Table 2.
Overview of outcomes and the registries used to extract data
| Outcomes: | Registers | Description of outcome | |||
| NPR | NHI | DRG-DAGS | DR | ||
| Primary care | |||||
| GP contacts | x | Number of contacts with GPs (ordinary consultations, home visits, telephone and email consultations) | |||
| GP costs | x | Healthcare costs of GPs | |||
| Other specialist MD costs | x | Healthcare costs of specialised medical doctors in primary care (excl. GPs and dentists) | |||
| Psychologist costs | x | Healthcare costs of psychologists in primary care | |||
| Physiotherapist costs | x | Healthcare costs of physiotherapists in primary care | |||
| Prescription drug costs | x | Costs of prescription drugs | |||
| Secondary care | |||||
| Hospitalisation days | x | Days in hospital, not as outpatient | |||
| Outpatient visits | x | Number of visits to outpatient clinic | |||
| Emergency room contacts | x | Number of out-of-hours contacts with GPs and all contacts with emergency rooms. | |||
| Surgical procedures | x | Number of surgical procedures in hospital | |||
| Non-surgical procedures | x | Number of non-surgical procedures and techniques in hospital. | |||
| Total costs | x | x | Total healthcare costs in primary and secondary sectors excluding costs in the psychiatric secondary sector. | ||
| Plus the price of CT scans in the study setting: EUR 238/CT scan including recruitment of participants and staff salaries. | |||||
DR, Drug Registry; DRG-DAGS, Diagnostic Related Groups-Diagnostic Outpatient Group System Registries; GP, general practitioner; MD, medical doctor; NHI, National Health Insurance Registry; NPR, National Patient Registry.
DLCST budget
The DLCST was financed by the Ministry of Health and Prevention. A grant of EUR 2.33 million covered the expenses of 9800 CT scans, including recruitment of participants and staff salaries.22 All procedures in the follow-up of participants with abnormal findings in their CT scans were billed to the Danish healthcare system and thus recorded in the public registries.22 This includes costs of any incidental findings.
Outcomes
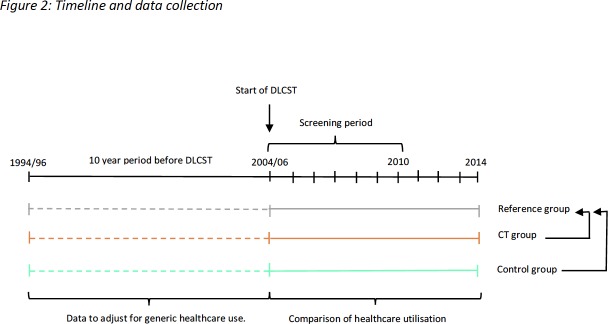
Outcomes are selected healthcare costs over the period from date of randomisation (2004/2006) until the end of 2014, death or migration (figure 2). Outcomes were annualised into outcome per year to adjust for different observation times. Participants with an observation time <12 months were excluded to avoid inflation of healthcare costs when annualising the outcomes. To make the reference group comparable to the two randomisation groups of the DLCST in this respect, a virtual randomisation date was determined by randomly selecting a date from among DLCST participants whose date of birth was in the same quarter as the participant in the reference group. Data on healthcare utilisation in the 10-year period before DLCST was used for adjustments.
Figure 2.
Timeline and data collection. DLCST, Danish Randomised Controlled Lung Cancer Screening Trial.
Statistical analysis
Outcomes that are rarely used by the population include many zero observations (eg, surgical procedures). Consequently, it is more appropriate to analyse the risk of having, for example, a surgical procedure at all in the observation period, that is, the incidence of use. Outcomes that are frequently used do not have many zero observations (eg, total costs). For these outcomes, it is more relevant to analyse the quantity of use. A multivariable analysis of the outcomes, therefore, followed a two-part model25 where separately the yearly incidence and the yearly use of the various outcomes in the two DLCST groups were compared with the reference group:
In part one, we analysed the incidence of use of selected healthcare services, or costs, in the observation period. This incidence was analysed in a Poisson regression approach so the regression parameters were equivalent to the logarithm of the RR of ever using the service;26 the logarithm of the observation period was used as an offset so that yearly incidences are compared.
In part two, we analysed the quantity of healthcare costs or services. Only the participants who used the services or had costs>0 were included in this part, and the quantity of use was analysed in a generalised linear model using a Gamma distribution and a logarithmic link function. The parameters from this model were interpreted as the logarithm of a multiplicative factor of how much more the service was used (or how much more cost was incurred) in the CT group or the control group compared with the use/cost in the reference group; the logarithm of the observation period was used as an offset so that annualised costs are compared.
Since the groups were not randomised, it was necessary to address the differences in baseline characteristics between the DLCST participants and the reference group (table 1). The following possible confounders were accounted for using multivariable regression models for the above-described two parts: sex, age, (squared), employment, living alone, socioeconomic group, smoking status, pack years, pack years squared, region of residence, Charlson’s comorbidity index and healthcare use in the 10 years prior to randomisation.
A combined multiplicative effect of being in the CT group or in the control group compared with being in the reference group was calculated by multiplying the RR from the incidence part of the model and the factor from the quantity part. This is referred to as the cumulative effect.
Statistical significance was adjusted for multiple testing by controlling the false discovery rate at 5% using the method of Benjamini- Hochberg. The level of statistical significance was then asserted at a level of 0.0202.
Total costs that might have been expected in the reference group (if the reference group had been invited for screening) were calculated by multiplying the mean total costs in the reference group with the cumulative effect in the CT and control group calculated in the two-part model.
We used SAS V.9.4 for the statistical analysis (SAS Institute, Cary, North Carolina, USA).
Patient involvement
In this study, patients were not directly involved in setting the research question, the outcome measures or in the design or implementation of the study. The research question and design, however, were informed by how participants experienced lung cancer CT screening as revealed in a qualitative study22 (ie, how they were psychosocially affected). This study assessed the burden of the intervention on participants (allocation to the CT screening group or the control group).
Ethics
All participants signed an informed consent form. Both the DLCST (number 2005-53-1083) and this project (number 2014-41-2877) have been approved by the Danish Data Protection Agency.
Results
The baseline characteristics of the three groups are shown in table 1. All of the socioeconomic characteristics are possible confounders because of comprehensive differences, and we have made adjustments accordingly.
Costs are presented in Euro (EUR), converted from Danish Kroner (DKK) using the 26 January 2016 spot rate DKK 746.22=EUR 100).
Total costs including the costs of a lung CT screening programme
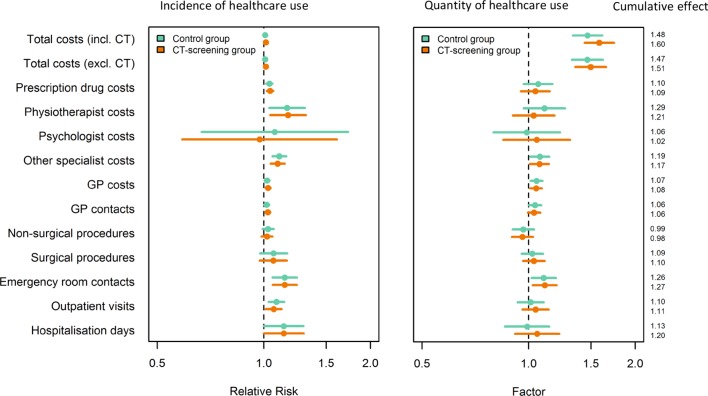
Lung CT screening increased the mean total annual healthcare costs by 60% (table 3, figure 3). Lung function tests and smoking counselling alone (as applied to the control group) increased the mean total annual healthcare costs by 48% (table 3, cumulative effect 1.48).
Table 3.
RR (incidence) and factor (quantity) of healthcare use in the DLCST groups compared with the reference group
| Total costs | Groups | RR | RR lower 95% CI | RR upper 95% CI | RR p value |
Factor (F) | F lower 95% CI | F upper 95% CI | Factor p value |
Cumulative effect |
| Total costs excl. direct cost of CT-screening programme | CONT | 1.008 | 0.994 | 1.022 | 0.284 | 1.468 | 1.325 | 1.627 | 0.000* | 1.479 |
| CT | 1.013 | 0.999 | 1.027 | 0.068 | 1.501 | 1.354 | 1.663 | 0.000* | 1.520 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Total costs incl. cost of CT-screening programme | CONT | 1.008 | 0.994 | 1.022 | 0.286 | 1.471 | 1.334 | 1.622 | 0.000* | 1.483 |
| CT | 1.012 | 0.999 | 1.026 | 0.073 | 1.585 | 1.437 | 1.747 | 0.000* | 1.604 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Secondary sector | ||||||||||
| Hospitalisation days | CONT | 1.137 | 1.000 | 1.293 | 0.050 | 0.989 | 0.856 | 1.144 | 0.884 | 1.125 |
| CT | 1.137 | 0.999 | 1.295 | 0.051 | 1.056 | 0.914 | 1.221 | 0.457 | 1.202 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Outpatient visits | CONT | 1.085 | 1.032 | 1.142 | 0.002* | 1.012 | 0.930 | 1.102 | 0.777 | 1.099 |
| CT | 1.065 | 1.012 | 1.121 | 0.016* | 1.046 | 0.961 | 1.139 | 0.301 | 1.114 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Emergency room contacts | CONT | 1.144 | 1.057 | 1.239 | 0.001* | 1.102 | 1.020 | 1.192 | 0.014* | 1.261 |
| CT | 1.144 | 1.057 | 1.239 | 0.001* | 1.110 | 1.026 | 1.200 | 0.009* | 1.270 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Surgical procedures | CONT | 1.065 | 0.975 | 1.163 | 0.163 | 1.024 | 1.098 | 1.098 | 0.511 | 1.090 |
| CT | 1.063 | 0.973 | 1.162 | 0.178 | 1.034 | 1.109 | 1.109 | 0.355 | 1.099 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Non-surgical procedures | CONT | 1.028 | 0.992 | 0.134 | 0.134 | 0.966 | 0.901 | 1.034 | 0.318 | 0.992 |
| CT | 1.020 | 0.984 | 0.278 | 0.278 | 0.96 | 0.896 | 1.028 | 0.244 | 0.979 | |
| REF | 1.000 | 1.000 | – | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Primary sector | ||||||||||
| GP contacts | CONT | 1.018 | 1.002 | 1.035 | 0.024 | 1.041 | 1.000 | 1.084 | 0.053 | 1.060 |
| CT | 1.025 | 1.010 | 1.041 | 0.002* | 1.035 | 0.994 | 1.078 | 0.094 | 1.062 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| GP costs | CONT | 1.021 | 1.005 | 1.037 | 0.009* | 1.052 | 1.013 | 1.093 | 0.009* | 1.074 |
| CT | 1.028 | 1.012 | 1.044 | 0.006* | 1.049 | 1.010 | 1.090 | 0.013* | 1.078 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Other specialist MD costs | CONT | 1.105 | 1.056 | 1.156 | 0.000* | 1.075 | 1.011 | 1.143 | 0.022 | 1.188 |
| CT | 1.094 | 1.045 | 1.145 | 0.000* | 1.072 | 1.008 | 1.141 | 0.027 | 1.173 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Psychologist costs | CONT | 1.073 | 0.667 | 1.727 | 0.772 | 0.987 | 0.795 | 1.226 | 0.906 | 1.059 |
| CT | 0.972 | 0.588 | 1.607 | 0.912 | 1.053 | 0.848 | 1.309 | 0.640 | 1.024 | |
| REF | 1.000 | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Physiotherapist costs | CONT | 1.163 | 1.036 | 0.011* | 1.107 | 1.107 | 0.968 | 1.266 | 0.139 | 1.287 |
| CT | 1.170 | 1.042 | 0.008* | 1.032 | 1.032 | 0.902 | 1.181 | 0.642 | 1.208 | |
| REF | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | 1.000 | – | 1.000 | |
| Prescription drug costs | CONT | 1.036 | 1.014 | 0.001* | 1.063 | 1.063 | 0.967 | 1.167 | 0.205 | 1.101 |
| CT | 1.041 | 1.019 | 0.000* | 1.044 | 1.044 | 0.950 | 1.147 | 0.371 | 1.087 | |
| REF | 1.000 | 1.000 | – | 1.000 | 1.000 | 1.000 | 1.000 | – | 1.000 |
Cumulative effect=relative risk × factor. After adjustments for multiple testing using the method of Benjamini-Hochberg the level of statistical significance was asserted at 0.0202.
CONT, control group from DLCST; CT, CT group from DLCST; DLCST, Danish Randomised Controlled Lung Cancer Screening Trial; GP, general practitioner; REF, Reference group; RR, relative risk.
Figure 3.
Relative risk (incidence) and factor (quantity) of healthcare use in the DLCST groups compared with the reference group. DLCST, Danish Randomised Controlled Lung Cancer Screening Trial; GP, general practitioner.
The participants in the reference group (unexposed to any of the trial interventions) had a mean total annual healthcare cost of EUR 2348. If this reference group had been exposed to CT screening, the mean total annual healthcare costs would increase by 60%, from EUR 2348 to EUR 3756. If the reference group had been exposed to lung function tests and smoking counselling alone, the mean total annual healthcare costs would increase by 48% to EUR 3474.
Total costs excluding the direct costs of a lung CT screening programme
CT screening increased the mean total annual healthcare costs by 52% (table 3, figure 3). Lung function tests and smoking counselling alone increased the mean total annual healthcare costs by 48% (table 3, figure 3). As lung function tests and smoking counselling were part of the DLCST budget, the 52% and 48% increases in costs are attributed to altered healthcare use by participants after the invitation to attend screening.
Primary healthcare outcomes
Compared with the reference group, the participants in the CT group had significantly increased healthcare use in five out of six outcomes, and the participants in the control group in four out of six outcomes. With respect to quantity (part two of the model), only one outcome differed significantly between the CT group and the control group, respectively, compared with the reference group (table 3, figure 3).
Secondary healthcare outcomes
Compared with the reference group, the participants in both the CT group and the control group had significantly increased healthcare use in two out of five outcomes and, with respect to quantity, in one out of five outcomes (table 3, figure 3).
Discussion
Lung cancer CT screening increased mean total annual healthcare costs by 60% and a substantial part of these costs, 52%, were indirect costs. Lung function tests and smoking counselling alone accounted for a 48% increase in mean costs, and only 12% (60%–48%) of the indirect costs can be attributed to more lung-cancer cases in the CT group. The remaining 48% can be partly explained by finding a drop in lung function. As the results show, however, an increased use of emergency room contacts, for example, which cannot be attributed to more chronic obstructive pulmonary disease diagnosis, indicates that other potential explanations must be explored. Our findings underline the importance of including all relevant costs, including those from the primary care sector. In addition, these findings emphasise the need for using a blinded control group that is unaware of the ongoing screening trial. The unblinded control group in the DLCST was affected negatively after randomisation, which could cause a statistically non-significant difference in costs between the intervention and control groups.22
The cost-effectiveness of CT screening has been reported by other researchers.15 Only two studies included indirect costs: one included lost wages and another included losses due to travel time associated with screening.15 If primary care sector costs had been included in the analysis, total costs would most likely have been higher, as our data show that the increased costs are not limited to the secondary care sector.
If CT screening is effective, one would expect more lung-cancer cases to be found in the CT group than in the control group, and for that reason higher healthcare utilisation in the CT group than in the control group. Incidental findings described to a varying degree27 could also contribute to increased healthcare utilisation and costs in the CT group compared with the control group. Incidental findings such as coronary artery calcification and emphysema could cause less morbidity and mortality but also potentially overtreatment, complications and increased costs.15
In DLCST, a high degree of overdiagnosis has been reported (67.2%)28 which would also contribute to increased costs in the CT group. Our data show, however, that both the CT group and the control group had increased healthcare utilisation. Consequently, extra healthcare use induced by the CT screening programme cannot be attributed to an increase in lung cancer cases and incidental findings alone. Both trial groups were offered annual lung function tests and smoking counselling. Therefore, a part of the extra healthcare use in both groups might stem from finding a drop in lung function. Studies have shown that smokers who participated in lung cancer screening had a higher smoking cessation rate compared with smokers in the general population.29 30 Smoking counselling intervention increases the cost of a screening programme but might reduce morbidity and long-term health costs.21
Studies have shown that non-lung cancer outcomes such as mortality reductions or long-term improvements to quality of life for participants without lung cancer were drivers of the cost-effectiveness of lung cancer screening.20 31 In contrast to the mentioned findings of long-term improvements to quality of life, a study from DLCST demonstrated that the participants in the CT group and in the control group experienced more negative psychosocial consequences after randomisation compared with baseline.32 A systematic review of the psychological burden of lung cancer screening in different countries found variable results with large heterogeneity in outcome measures.33 The questionnaire used to measure the potential psychosocial consequences of lung cancer screening in DLCST had high content validity and adequate psychometric properties, fulfilling the COSMIN criteria for valid patient-reported outcome measures.34 This is in contrast to the questionnaires used in other trials.
Studies have shown that negative expectations for the future can change how a person perceives signs and symptoms in the body,35 36 and can, in addition, give rise to actual physical changes.37 38 If a person feels concerned or experiences more physiological symptoms, it would be natural to seek medical attention and thereby have a higher utilisation of healthcare. The increased negative psychosocial consequences experienced in the two groups of DLCST may have been because they were reminded of being at risk of serious illness. In short, the need for further tests or treatment for a drop in lung function, appointments to address anxiety-related issues and seeking immediate medical advice for things that might have resolved without any medical intervention are all possible explanations for increased healthcare costs and utilisation.
The present study has limitations. First, it was not an RCT, but we adjusted the analysis for a number of possible confounders. Second, the following costs have not been accounted for: costs in relation to days off work due to CT screening and follow-up; costs of psychiatric hospitalisation; costs of medication bought without prescription; costs of retirement and of sick days, and the fact that a cancer diagnosis could influence the insurance conditions of the participants. The latter is especially important when the high rate of overdiagnosis in DLCST is taken into account. Third, implementing lung cancer screening could affect smokers who are not the target of the screening programme, for example, smokers with a smoking history <20 pack-years; smokers aged <50, >70 or smokers with body weight >130 kg or FEV1 <30%. Costs are underestimated if smokers who are not invited for screening are psychosocially affected, as seen in the DLCST control group, assuming that some of the extra costs were caused by a change in illness perception. A study on mammography screening concluded that the absence of an invitation to breast cancer screening had a negative psychosocial impact.39 Fourth, the cost of a CT scan in a screening setting (EUR 238/scan) was higher than the cost of a CT scan in a diagnostic setting (EUR 186).22 If screening is implemented and becomes routine, the cost of a scan will probably drop and the same goes for the total costs. Fifth, as seen in figure 2, data collection extended beyond the screening period. This means that our estimates of ‘total costs including the costs of the CT-screening programme’ are probably underestimated.
We are assuming that the number of missing data is insignificant due to the economic incentive to report all services provided. Denmark has a publicly financed healthcare system. When doctors and other healthcare professionals and hospitals are providing a service, they must report it to the health authorities to be refunded. The reported services are registered in National registries.
It has been argued that patients are most likely to take the decision to visit their GP, and thus this decision reflects patient characteristics. Costs per user, on the other hand, are more related to the characteristics of the healthcare system.25 Our results on ‘total costs’ might not be generalisable to other countries because of presumed differences between healthcare systems. Healthcare utilisation figures and the percentage increase in ‘total costs’ induced by the screening programme might be generalisable.
The risk of contamination, defined as screening in the control group (or reference group), was most likely low.40
Future lung cancer screening trials should, where possible, be designed with a blinded control group, that does not take part in lung function testing or visits to a screening clinic AND is unaware that a screening programme is ongoing. In addition, future cost analyses should also include indirect costs.
Conclusion
CT screening for lung cancer increased mean total annual healthcare costs by 60% including the costs of the CT screening programme. This corresponds to an increase in the total annual healthcare costs per participant from EUR 2348 to EUR 3756. The increased costs were the result of increased use of healthcare in the primary as well as in the secondary sector.
Supplementary Material
Acknowledgments
Thanks to all the participants who volunteered in the study. Thanks to data manager Willy Karlslund for generating the database and thanks to the other members of the DLCST steering committee: Jesper Holst Petersen, Asger Dirksen, Karen Skjøldstrup Bach, Paul Frost Clementsen, Martin Døssing, Hanne Hansen, Klaus Fuglsang Kofoed, Klaus Richter Larsen, Jann Mortensen, Niels Seersholm, Birgit Guldhammer Skov, Hanne Thorsen, Philip Tønnesen, Haseem Ashraf and Zaigham Saghir.
Footnotes
Contributors: The study was devised by JFR, VS and JB. Data collection was done by MDJ and JFR. VS did the analysis. MDJ drafted the manuscript. VS and JB contributed to revisions of the manuscript and approved the final version. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The DLCST were funded by the Danish Ministry of Health and Prevention (grant number 0900814). MDJ was funded by PLU fonden (grant number R406-A28178-B22773).
Disclaimer: The funders had no role in study design, data collection, analysis, interpretation, writing process or the decision to submit the article.
Competing interests: All authors have completed the ICMJE uniform disclosure form at ‘http://www.icmje.org/coi_disclosure.pdf’.
Patient consent for publication: Not required.
Ethics approval: The DLCST was approved by the Danish Scientific Ethical Committee, number KA-02045.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. No additional data available. Data sharing is possible only if approved by the Danish data protection agency because participants may be identifiable in the dataset. On request, we will help applying for this approval. In the long term, when all data have been processed and anonymised, data will be accessible to the public.
References
- 1. Ott JJ, Ullrich A, Mascarenhas M, et al. Global cancer incidence and mortality caused by behavior and infection. J Public Health 2011;33:223–33. 10.1093/pubmed/fdq076 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 3. Field JK, van Klaveren R, Pedersen JH, et al. European randomized lung cancer screening trials: post NLST. J Surg Oncol 2013;108:280–6. 10.1002/jso.23383 [DOI] [PubMed] [Google Scholar]
- 4. Pinsky PF, Church TR, Izmirlian G, et al. The National lung screening trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013;119:3976–83. 10.1002/cncr.28326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kauczor H-U, Bonomo L, Gaga M, et al. ESR/ERS white paper on lung cancer screening. Eur Respir J 2015;46:28–39. 10.1183/09031936.00033015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. U.S. Preventive Services Task Force Lung cancer: screening. Available: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening
- 8. American Cancer Society Lung cancer screening guidelines. Available: http://www.cancer.org/healthy/informationforhealthcareprofessionals/acsguidelines/lungcancerscreeningguidelines/index
- 9. Manser R, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev 2013;160 10.1002/14651858.CD001991.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han D, Heuvelmans MA, Vliegenthart R, et al. An update on the European lung cancer screening trials and comparison of lung cancer screening recommendations in Europe. J Thorac Imaging 2019;34:65–71. 10.1097/RTI.0000000000000367 [DOI] [PubMed] [Google Scholar]
- 11. Wille MMW, Dirksen A, Ashraf H, et al. Results of the randomized Danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med 2016;193:542–51. 10.1164/rccm.201505-1040OC [DOI] [PubMed] [Google Scholar]
- 12. Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608–14. 10.1097/JTO.0b013e3181a0d98f [DOI] [PubMed] [Google Scholar]
- 13. Coureau G, Salmi LR, Etard C, et al. Low-dose computed tomography screening for lung cancer in populations highly exposed to tobacco: A systematic methodological appraisal of published randomised controlled trials. Eur J Cancer 2016;61:146–56. 10.1016/j.ejca.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 14. Snowsill T, Yang H, Griffin E, et al. Low-dose computed tomography for lung cancer screening in high-risk populations: a systematic review and economic evaluation : Health technology assessment, no. 22.69. 22 Southampton, UK: NIHR Journals Library, 2018: 1–276. 10.3310/hta22690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raymakers AJN, Mayo J, Lam S, et al. Cost-Effectiveness analyses of lung cancer screening strategies using low-dose computed tomography: a systematic review. Appl Health Econ Health Policy 2016;14:409–18. 10.1007/s40258-016-0226-5 [DOI] [PubMed] [Google Scholar]
- 16. Goulart BHL, Bensink ME, Mummy DG, et al. Lung cancer screening with low-dose computed tomography: costs, National expenditures, and cost-effectiveness. J Natl Compr Canc Netw 2012;10:267–75. 10.6004/jnccn.2012.0023 [DOI] [PubMed] [Google Scholar]
- 17. Black WC, Gareen IF, Soneji SS, et al. Cost-Effectiveness of CT screening in the National lung screening trial. N Engl J Med 2014;371:1793–802. 10.1056/NEJMoa1312547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pyenson BS, Sander MS, Jiang Y, et al. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff 2012;31:770–9. 10.1377/hlthaff.2011.0814 [DOI] [PubMed] [Google Scholar]
- 19. Pyenson BS, Henschke CI, Yankelevitz DF, et al. Offering lung cancer screening to high-risk Medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits 2014;7:272–82. [PMC free article] [PubMed] [Google Scholar]
- 20. Goffin JR, Flanagan WM, Miller AB, et al. Cost-Effectiveness of lung cancer screening in Canada. JAMA Oncol 2015;1:807–13. 10.1001/jamaoncol.2015.2472 [DOI] [PubMed] [Google Scholar]
- 21. Wade S, Weber M, Caruana M, et al. Estimating the cost-effectiveness of lung cancer screening with low-dose computed tomography for high-risk smokers in Australia. J Thorac Oncol 2018;13:1094–105. 10.1016/j.jtho.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen JF, Siersma V, Pedersen JH, et al. Healthcare costs in the Danish randomised controlled lung cancer CT-screening trial: a registry study. Lung Cancer 2014;83:347–55. 10.1016/j.lungcan.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen JF. Psychosocial consequences and healthcare costs in lung cancer CT screening: faculty of health and medical sciences University of Copenhagen PHD thesis Jakob F. Rasmussen psychosocial consequences and healthcare costs in lung cancer CT screening this thesis has been submitted to the graduate school at the faculty of health and medical sciences. University of Copenhagen, 2014. [Google Scholar]
- 24. Hestbech MS, Siersma V, Dirksen A, et al. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer 2011;73:325–31. 10.1016/j.lungcan.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 25. Diehr P, Yanez D, Ash A, et al. Methods for analyzing health care utilization and costs. Annu Rev Public Health 1999;20:125–44. 10.1146/annurev.publhealth.20.1.125 [DOI] [PubMed] [Google Scholar]
- 26. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 27. Tsai EB, Chiles C, Carter BW, et al. Incidental findings on lung cancer screening: significance and management. Semin Ultrasound CT MR 2018;39:273–81. 10.1053/j.sult.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 28. Heleno B, Siersma V, Brodersen J. Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening: a secondary analysis of the Danish lung cancer screening trial. JAMA Intern Med 2018;178:1420–2. 10.1001/jamainternmed.2018.3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084 10.1093/jnci/dju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomez MM, LoBiondo-Wood G. Lung cancer screening with low-dose CT: its effect on smoking behavior. J Adv Pract Oncol 2013;4:405–14. 10.6004/jadpro.2013.4.6.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cressman S, Peacock SJ, Tammemägi MC, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol 2017;12:1210–22. 10.1016/j.jtho.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 32. Aggestrup LM, Hestbech MS, Siersma V, et al. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open 2012;2:e000663 10.1136/bmjopen-2011-000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu GX, Raz DJ, Brown L, et al. Psychological burden associated with lung cancer screening: a systematic review. Clin Lung Cancer 2016;17:315–24. 10.1016/j.cllc.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mokkink LB, de Vet HCW, Prinsen CAC, et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1171–9. 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reventlow SD, Hvas L, Malterud K. Making the invisible body visible. bone scans, osteoporosis and women's bodily experiences. Soc Sci Med 2006;62:2720–31. 10.1016/j.socscimed.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 36. Hvas L, Reventlow S, Jensen HL, et al. Awareness of risk of osteoporosis may cause uncertainty and worry in menopausal women. Scand J Public Health 2005;33:203–7. 10.1080/14034940510005716 [DOI] [PubMed] [Google Scholar]
- 37. Reeves RR, Ladner ME, Hart RH, et al. Nocebo effects with antidepressant clinical drug trial placebos. Gen Hosp Psychiatry 2007;29:275–7. 10.1016/j.genhosppsych.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 38. Johansen O, Brox J, Flaten MA. Placebo and nocebo responses, cortisol, and circulating beta-endorphin. Psychosom Med 2003;65:786–90. 10.1097/01.PSY.0000082626.56217.CF [DOI] [PubMed] [Google Scholar]
- 39. Osterø J, Siersma V, Brodersen J. Breast cancer screening implementation and reassurance. Eur J Public Health 2014;24:258–63. 10.1093/eurpub/ckt074 [DOI] [PubMed] [Google Scholar]
- 40. Saghir Z, Ashraf H, Dirksen A, et al. Contamination during 4 years of annual CT screening in the Danish lung cancer screening trial (DLCST). Lung Cancer 2011;71:323–7. 10.1016/j.lungcan.2010.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.