Abstract
Objective
Hepatitis B virus (HBV) infection is a major public health problem worldwide. Several studies have reported that ABO blood groups may be associated with HBV infection. However, its association is still controversial. We performed a meta-analysis to investigate whether ABO blood groups were associated with HBV infection.
Design
Systematic review and meta-analysis.
Data sources
Relevant studies available before 1 December 2019 were identified by searching PubMed, EMBASE, Web of Science, ScienceDirect and the Cochrane Library.
Eligibility criteria
All cross-sectional or cohort studies from which the data of ABO blood group distribution and HBV infection could be extracted.
Data extraction and synthesis
Studies were identified and extracted by two reviewers independently. Risk ratios (RRs) and 95% CIs were pooled by random-effect models to quantify this association.
Results
Thirty-eight eligible articles including 241 868 HBV-infected subjects and 6 487 481 uninfected subjects were included. Overall, the risk of HBV infection had decreased by 8% in subjects with blood group B when compared with non-B blood group (RR=0.92, 95% CI 0.86 to 0.98). In the subgroup analyses, the inverse relationship between blood group B and HBV infection remained stable in higher endemic areas (HBV prevalence ≥5%), Asian people, larger sample size studies (≥2000), general population and blood donors, lower middle income group and studies published before the year 2010. Additionally, subjects with blood group O had a 12% increased risk of HBV infection (RR=1.12, 95% CI 1.01 to 1.24) in higher endemic areas. In the sensitivity analysis, the pooled risk estimates of blood group B and HBV infection were still stable.
Conclusions
Our data suggested that the blood group B was associated with a lower risk of HBV infection. More research is needed to clarify the precise role of the ABO blood group in HBV infection to address the global question of HBV infection.
Keywords: hepatitis B virus, ABO blood group, meta-analysis
Strengths and limitations of this study.
The breadth of the comprehensive systematic literature search is a strength of this study.
To our knowledge, this is the first meta-analysis of the association between ABO blood groups and HBV infection.
Although we performed subgroup analyses, the heterogeneity cannot be ignored because few published studies described the related risk factors of HBV infection in detail.
Introduction
Hepatitis B virus (HBV) infection is a major public health problem worldwide,1 especially in Africa and the Western Pacific region.2 According to the global hepatitis report in 2017, it is estimated that 257 million people, 3.5% of the general population, are living with HBV infection worldwide with about 0.88 million deaths caused by complications of chronic HBV infection every year.2 HBV infection has caused a high societal burden globally.1 2
The ABO blood group system, the most extensively investigated erythrocyte antigen system,3 is widely used in clinical practice, and influences the host susceptibility.4 5 As an easily accessible factor in an individual’s genetic makeup, ABO blood groups have been statistically and biologically associated with many chronic diseases such as vascular disease,6 coronary heart disease7 and tumorigenesis.3 4 8 For instance, by expressing on N-glycans of von Willebrand factor (VWF), ABH antigens (H antigen is the biosynthetic precursor to A and B antigens)5 impact the half-life of VWF, so VWF survival in O subjects is significantly shorter versus in non-O subjects.9–11 Therefore, because of the lower VWF levels, O subjects have lower risk of venous thromboembolism.10 Recently, a meta-analysis also found that patients with hepatocellular carcinoma (HCC) might have a lower proportion of O subjects than healthy subjects.12 Meanwhile, the association between ABO blood groups and host susceptibility to infectious diseases (such as H elicobacter pylori, P lasmodium falciparum, HIV, etc) has been shown in several studies.5 13 Previous studies have found that this association is because ABO antibodies are part of the innate immune system against some bacteria, parasites and enveloped viruses,5 and blood antigens are important as receptors for immune and inflammation responses,14 15 which means that a biological association between ABO blood groups and HBV infection probably exists.
Epidemiological studies have explored the relationship between blood group and HBV infection, however, the results have been contradictory. Lao et al 16 found that HBV prevalence was lower in blood group B (9.6%) and AB (9.1%), but higher in blood group O (10.2%). Liu et al 17 suggested that blood group O was associated with increased HBV infection. Mohammadali et al 18 found that the percentage of the hepatitis B surface antigen (HBsAg) was lower in donors with blood group O. However, Szmuness et al 19 20 and Behal et al 21 failed to find a link between blood group and HBV infection. Thus, controversy remains with regard to whether blood group is related to HBV infection and which antigen is a protective or a risk factor. We performed a systematic review and meta-analysis to elucidate the association between ABO blood groups and HBV infection risk to provide evidence on improving blood safety and preventing HBV infection, which can help to achieve the target of eliminating HBV as an international public health challenge.22
Materials and methods
Data sources and search strategy
Two reviewers (SZ and WJ) searched independently for articles, which were available online before 1 December 2019, from five databases including PubMed, EMBASE, Web of Science, ScienceDirect and Cochrane Central using the following keywords: ‘hepatitis B’ OR ‘hepatitis B virus’ OR ‘HBV’ OR ‘HBsAg’ and ‘blood type’ OR ‘blood group’ OR ‘ABO’ OR ‘Rh’ OR ‘rhesus’. Meanwhile, highly relevant reference articles were also searched by reviewing the list of references. There was no limitation of language or region. The full electronic search strategy for PubMed is shown in online additional file 1.
bmjopen-2019-034114supp001.pdf (60.4KB, pdf)
Inclusion and exclusion criteria
Articles were included in the meta-analysis if: (1) The article was a cross-sectional or cohort study. (2) The data of the ABO blood group distribution and HBV infection could be extracted to calculate the risk ratio (RR), which meant that the numbers of HBV-infected and uninfected subjects were reported in each blood group. The exclusion criteria were as follows: (1) The article was not relevant to the subject of the study (animal experiments, pathological researches, molecular researches). (2) Reviews. (3) Overlapped studies, where if studies overlapped, we only included the last published. (4) Duplicated studies, where if the same study was found in different databases, we only included the article once.
According to the inclusion and exclusion criteria, studies were identified by two reviewers (SZ and WJ) independently. Discrepancies were solved by consensus or decided by a third reviewer (JL).
Data extraction and quality assessment
According to the piloted forms, four main parts of the information were extracted independently by two reviewers (SZ and WJ) from the selected studies: (1) The basic information of the studies including first author, publication year, journal, survey time, study design. (2) The characteristics of the study population including country, income group, race, population type (eg, blood donors, patients, general population), sample size, the number of HBV-infected and uninfected subjects, age range, mean age, sex ratio. (3) The outcome measure: the number of HBV-infected and uninfected subjects in each ABO blood group. (4) The author’s general conclusions.
The quality of selected cohort studies was assessed using the Newcastle-Ottawa Scales (NOS) with a score ranging from 0 to 9.23 A score of 4–6 indicated moderate quality, and a score of 7–9 indicated high quality. The quality of the selected cross-sectional studies was assessed using an 11-item checklist recommended by the Agency for Healthcare Research and Quality (AHRQ)24 with a score ranging from 0 to 11. A score of 4–7 indicated moderate quality, and a score of 8–11 indicated high quality.
Statistical analysis
The main outcome was the prevalence of HBV infection (defined as HBsAg-positive) in our meta-analysis. The relationship between the ABO blood groups and HBV infection was quantified using RR values and the corresponding 95% CIs. RRs and 95% CIs (A vs non-A, B vs non-B, O vs non-O, AB vs non-AB) were pooled by using random-effect models with the estimate of heterogeneity being taken from the Mantel-Haenszel model, and a value of p<0.05 was deemed significant. Between-study heterogeneity was evaluated with the I2 statistic. When I2 ≤50%, the included studies were considered to have little heterogeneity; when I2 >50%, the included studies were considered to have substantial heterogeneity.25
Subgroup analyses were performed by HBV prevalence, race, sample size, population, income group, study type and publication year. The prevalence of HBV infection was calculated in each study based on the number of HBV-infected and uninfected subjects. Studies were divided into Caucasian, Asian and African subgroups depending on the major national race and divided into high, upper-middle, lower-middle and low income groups according to the World Bank list of economies.26 Sensitivity analyses were performed by excluding large sample sized studies orderly or at the same time, which dominated the results of the meta-analysis. Publication bias was evaluated by funnel plots and two-sided Egger’s tests, and a value of p<0.05 was deemed statistically significant. All statistical analyses were performed using STATA V.12.0.
Patient and public involvement
There was no direct patient or public involvement in this review.
Results
Study selection and study characteristics
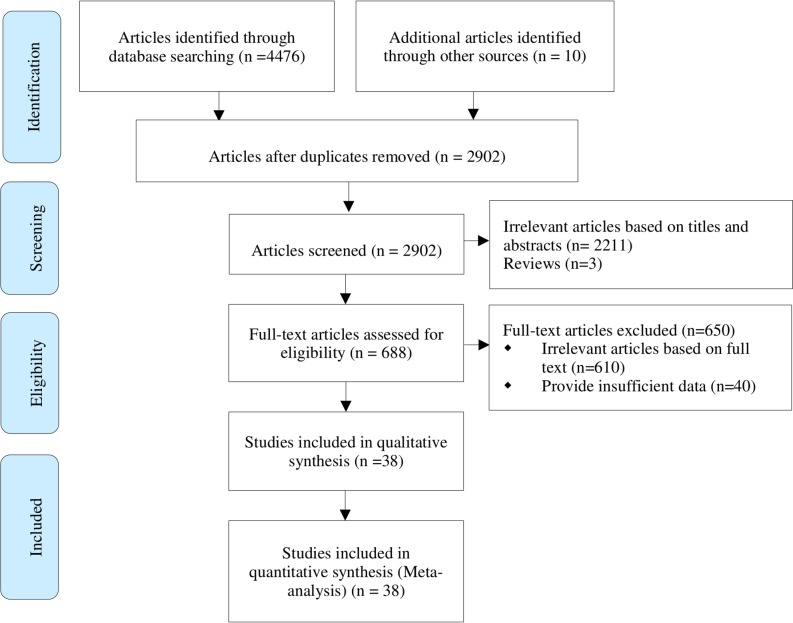
A total of 4486 articles (4476 from the database and 10 from other sources) was searched, of which 1584 were duplicate results. After reading the abstracts, 2211 were deemed irrelevant and 3 reviews were excluded. After reading the full text, 650 articles were excluded, of which 610 were irrelevant articles, and 40 studies provided insufficient information. Eventually, 38 eligible articles were included in the meta-analysis. A flow chart of study selection is shown in figure 1.
Figure 1.
The process of study selection for the meta-analysis.
The basic characteristics of the selected studies are shown in table 1. All selected articles were observational studies and published between 1970 and 2019. A total of 6487 481 subjects was included with 241 868 HBV-infected subjects and 6245 613 uninfected subjects. Among the Caucasian, Asian and African populations, there were 23, 7 and 8 studies, respectively. In addition, there were 7, 9, 18 and 4 studies in high income, upper middle income, lower middle income and low income groups, respectively. Furthermore, there were 14 studies in higher (HBV prevalence ≥5%) endemic and 24 studies in lower (HBV prevalence <5%) endemic areas. Meanwhile, there were 37 cross-sectional studies and 1 cohort study in the meta-analysis.
Table 1.
Characteristics of the included studies
| Author | Income group | Race | Population | Sample size | HBV infection (n/%) | ||||
| Total | A, non-A* | B, non-B* | AB, non-AB* | O, non-O* | |||||
| Terrier et al 29 | High | Caucasian | Blood donors | 5968 | 55/0.92 | 9/0.37, 46/1.31 | 4/0.66, 51/0.95 | 2/0.78, 53/0.93 | 40/1.51, 15/0.45 |
| Leski et al 30 | High | Caucasian | Patients | 155 | 34/21.94 | 16/23.19, 18/20.93 | 4/28.57, 30/21.28 | 0/0, 34/22.67 | 14/20.9, 20/22.73 |
| Szmuness et al 19 | High | Caucasian | Blood donors | 8096 | 177/2.19 | 61/2.06, 116/2.26 | 25/2.21, 152/2.18 | 13/3.57, 164/2.12 | 78/2.14, 99/2.23 |
| Zuberi and Lodi31 | Lower middle | Caucasian | Blood donors | 1111 | 38/3.42 | 9/3.36, 29/3.44 | 5/1.23, 33/4.69 | 2/3.64, 36/3.41 | 22/5.77, 16/2.19 |
| Vale et al 32 | Lower middle | African | General | 836 | 40/4.78 | 18/5.61, 22/4.27 | 6/4.11, 34/4.93 | 5/4.59, 35/4.81 | 11/4.23, 29/5.03 |
| Moore et al 33 | Low | Caucasian | Blood donors | 14 916 | 495/3.32 | 127/3.48, 368/3.27 | 103/3.21, 392/3.35 | 17/3.1, 478/3.33 | 248/3.3, 247/3.33 |
| Szmuness et al 20 | High | Caucasian | Blood donors | 51 019 | 58/0.11 | 22/0.11, 36/0.11 | 5/0.08, 53/0.12 | 4/0.16, 54/0.11 | 27/0.12, 31/0.11 |
| Lenka et al 34 | Lower middle | Caucasian | Blood donors | 500 | 24/4.8 | 12/9.3, 12/3.23 | 8/4.08, 16/5.26 | 0/0, 24/5.25 | 4/3.03, 20/5.43 |
| Nath et al 35 | High | Caucasian | Blood donors | 1585 | 68/4.29 | 22/4.03, 46/4.44 | 9/3.35, 59/4.48 | 3/4.17, 65/4.30 | 34/4.87, 34/3.83 |
| Kulkarni et al 36 | Lower middle | African | Blood donors | 1860 | 165/8.87 | 51/13.11, 114/7.85 | 17/3.11, 148/11.27 | 18/18.75, 147/8.33 | 79/9.54, 86/8.33 |
| Naidu and Rajyalakshmi37 | Lower middle | Caucasian | Blood donors | 1029 | 145/14.09 | 49/20.08, 96/12.40 | 42/12.39, 103/14.93 | 11/17.74, 134/13.86 | 43/11.20, 102/15.81 |
| Sebastian et al 38 | High | Asian | Blood donors | 3276 | 134/4.09 | 30/4.17, 104/4.08 | 30/3.50, 104/4.30 | 10/4.76, 124/4.04 | 64/4.30, 70/3.91 |
| Zhu et al 39 | Upper middle | Asian | Blood donors | 8683 | 153/1.76 | 44/1.62, 109/1.83 | 30/1.37, 123/1.89 | 18/2.59, 135/1.69 | 61/1.98, 92/1.64 |
| Joshi and Ghimire40 | Low | Asian | General | 613 | 17/2.77 | 4/2.09, 13/3.08 | 5/2.86, 12/2.74 | 1/2.13, 16/2.83 | 7/3.5, 10/2.42 |
| El-Gilany and El-Fedawy41 | Lower middle | Caucasian | Blood donors | 2157 | 93/4.31 | 27/3.42, 66/4.87 | 19/3.85, 74/4.45 | 12/5.88, 81/4.15 | 35/5.23, 58/3.90 |
| Behal et al 21 | Lower middle | Caucasian | Blood donors | 20 000 | 450/2.25 | 106/2.30, 344/2.24 | 174/2.34, 276/2.20 | 38/1.87, 412/2.29 | 132/2.23, 318/2.26 |
| Rifat-uz-Zaman42 | Lower middle | Caucasian | General | 1464 | 93/6.35 | 5/3.01, 88/6.90 | 35/6.63, 58/6.20 | 23/6.99, 70/6.17 | 30/6.80, 63/6.16 |
| Dirisu et al 43 | Lower middle | African | Blood donors | 427 | 200/46.84 | 32/45.71, 168/47.06 | 39/52, 161/45.74 | 1/33.33, 199/46.93 | 128/45.88, 72/48.65 |
| Saeed Anwar et al 44 | Lower middle | Caucasian | Blood donors | 16 695 | 467/2.80 | 103/2.60, 364/2.86 | 139/2.31, 328/3.07 | 17/2.64, 450/2.80 | 208/3.42, 259/2.44 |
| Omar et al 45 | Upper middle | Caucasian | Blood donors | 430 | 71/16.51 | 15/12.5, 56/18.06 | 21/21.43, 50/15.06 | 3/5.36, 68/18.18 | 32/20.51, 39/14.23 |
| Tyagi and Tyagi46 | Lower middle | Caucasian | Blood donors | 6000 | 95/1.58 | 27/1.87, 68/1.49 | 27/1.27, 68/1.75 | 9/1.98, 86/1.55 | 32/1.62, 63/1.57 |
| Sethi et al 47 | Lower middle | Caucasian | Blood donors | 7884 | 50/0.63 | 15/0.60, 35/0.65 | 10/0.41, 40/0.74 | 11/1.28, 39/0.56 | 14/0.68, 36/0.62 |
| Mohammadali and Pourfathollah18 | Upper middle | Caucasian | Blood donors | 2 028 068 | 7839/0.39 | 2553/0.40, 5286/0.38 | 1952/0.40, 5887/0.38 | 627/0.41, 7212/0.38 | 2707/0.36, 5132/0.40 |
| Nigam et al 48 | Lower middle | Caucasian | Blood donors | 4128 | 40/0.97 | 12/1.17, 28/0.90 | 11/0.75, 29/1.09 | 2/0.50, 38/1.02 | 15/1.22, 25/0.86 |
| Lao et al b16 | High | Asian | General | 78 705 | 7786/9.89 | 2038/9.90, 5748/9.97 | 1991/9.60, 5795/10.00 | 468/9.11, 7318/9.95 | 3289/10.20, 4497/9.68 |
| Zhao et al 49 | Upper middle | Asian | Patients | 500 | 66/13.20 | 17/11.18, 49/14.71 | 16/9.82, 50/14.84 | 15/16.67, 51/12.44 | 18/18.95, 48/11.85 |
| Siransy et al 50 | Upper middle | African | Blood donors | 59 514 | 4119/6.92 | 947/7.15, 3172/6.86 | 941/6.78, 3178/6.96 | 187/6.77, 3932/6.93 | 2044/6.9, 2075/6.94 |
| Navolan et al 51 | Upper middle | Caucasian | General | 1385 | 33/2.38 | 15/2.42, 18/2.37 | 7/3.11, 26/2.24 | 4/3.54, 29/2.28 | 7/1.64, 26/2.71 |
| Bisetegen et al 52 | Low | African | Blood donors | 390 | 37/9.49 | 7/6.73, 30/10.49 | 10/12.99, 27/8.63 | 2/22.22, 35/9.19 | 18/9, 19/10 |
| Abate and Wolde27 | Low | African | Blood donors | 6827 | 647/9.48 | 114/5.66, 533/11.10 | 54/5.45, 593/10.16 | 9/4.27, 638/9.64 | 470/13.02, 177/5.50 |
| Bharadva et al 53 | Lower middle | Caucasian | Blood donors | 41 909 | 237/0.57 | 62/0.63, 175/0.55 | 85/0.58, 152/0.56 | 22/0.55, 215/0.57 | 68/0.51, 169/0.59 |
| Naseri et al 54 | Upper middle | Caucasian | Blood donors | 228 409 | 640/0.28 | 208/0.29, 432/0.28 | 180/0.34, 460/0.26 | 42/0.24, 598/0.28 | 210/0.25, 430/0.30 |
| Memon et al 55 | Lower middle | Caucasian | Blood donors | 4683 | 66/1.41 | 15/1.37, 51/1.42 | 21/1.53, 45/1.36 | 9/2.94, 57/1.30 | 21/1.10, 45/1.63 |
| Liu et al 17 | Upper middle | Asian | General | 3 827 125 | 215455/5.63 | 64811/5.55, 150644/5.71 | 58286/5.18, 157169/5.82 | 18707/5.06, 196748/5.69 | 73651/6.34, 141804/5.32 |
| Batool et al 56 | Lower middle | Caucasian | Blood donors | 41 084 | 969/2.36 | 321/2.72, 648/2.22 | 289/2.21, 680/2.43 | 82/2.13, 887/2.38 | 277/2.24, 692/2.41 |
| Ngassaki-Y et al 57 | Upper middle | African | Blood donors | 4744 | 81/1.71 | – | – | – | 34/1.22, 47/2.41 |
| Fu et al 58 | Upper middle | Asian | Patients | 2000 | 389/19.45 | 105/21.43, 284/18.81 | 89/18.94, 300/19.61 | 59/21.85, 330/19.08 | 136/17.66, 253/20.57 |
| Nkansah et al 59 | Lower middle | African | Blood donors | 3306 | 342/10.34 | 48/11.76, 294/10.21 | 63/9.35, 279/10.67 | 1/3.33, 341/10.47 | 230/10.48, 112/10.07 |
*The number of HBV infected people in the X blood group/HBV prevalence (%) in the X blood group; the number of HBV-infected people in the non-X blood group/HBV prevalence (%) in the non-X blood group.
HBV, Hepatitis B virus.
The HBV infection prevalence in the 38 eligible articles ranged from 0.11% to 46.84%, and the HBV infection prevalence of blood groups A, B, AB, O ranged from 0.11% to 45.71%, 0.08% to 52.00%, 0.00% to 33.33% and 0.12% to 45.88%, respectively. The results of the quality assessment are shown in online additional file 2, with 15 high quality studies and 23 moderate quality studies. The score of the 37 articles assessed by AHRQ ranged from 3 to 9, while 14 of them were of high quality with a score from 8 to 9, and 23 of them were of moderate quality with a score from 4 to 7 (online supplementary table S1–1). The article assessed by the NOS scored 7 and was of high quality (online supplementary table S1–2).
Main, subgroup and sensitivity analyses
Overall, the risk of HBV infection had decreased by 8% in subjects with blood group B when compared with the non-B blood group (RR=0.92, 95% CI 0.86 to 0.98). However, blood groups A, O and AB were not significantly associated with an HBV infection risk (table 2). The results of the subgroup analyses were shown in table 2. In the subgroup analyses, the relationship between blood group B and HBV infection remained stable. The inverse relationship between blood group B and HBV infection was still observed in higher endemic areas (HBV prevalence ≥5%), Asian people, studies with larger sample sizes (≥2000), the general population and blood donors, the lower middle income group and articles published before the year 2010 (table 2).
Table 2.
The main, subgroup and sensitivity analyses
| Subgroup | No. of studies | Sample size | B versus non-B | O versus non-O | A versus non-A | AB versus non-AB | ||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |||
| All studies | 38 | 6 487 481 | 0.92 (0.86 to 0.98) | 0.007 | 1.07 (0.99 to 1.15) | 0.082 | 1.01 (0.96 to 1.07) | 0.728 | 1.04 (0.95 to 1.13) | 0.419 |
| HBV prevalence | ||||||||||
| Higher endemic areas (≥5%) | 14 | 3 983 732 | 0.90 (0.83 to 0.98) | 0.013 | 1.12 (1.01 to 1.24) | 0.025 | 0.99 (0.91 to 1.08) | 0.82 | 1.00 (0.89 to 1.14) | 0.962 |
| Lower endemic areas (<5%) | 24 | 2 503 749 | 0.93 (0.85 to 1.02) | 0.126 | 1.03 (0.93 to 1.15) | 0.566 | 1.03 (0.95 to 1.11) | 0.471 | 1.06 (0.95 to 1.18) | 0.292 |
| Race | ||||||||||
| Caucasian | 23 | 2 488 675 | 0.96 (0.87 to 1.05) | 0.386 | 1.04 (0.94 to 1.16) | 0.465 | 1.03 (0.94 to 1.13) | 0.472 | 1.05 (0.93 to 1.18) | 0.461 |
| Asian | 7 | 3 920 902 | 0.91 (0.86 to 0.97) | 0.003 | 1.10 (0.99 to 1.22) | 0.075 | 0.98 (0.97 to 0.99) | <0.001 | 0.96 (0.87 to 1.06) | 0.451 |
| African | 8 | 77 904 | 0.78 (0.58 to 1.05) | 0.099 | 1.04 (0.77 to 1.40) | 0.803 | 0.99 (0.73 to 1.33) | 0.919 | 1.02 (0.62 to 1.67) | 0.953 |
| Sample size | ||||||||||
| ≥2000 | 24 | 6 475 196 | 0.93 (0.87 to 0.99) | 0.018 | 1.07 (0.98 to 1.16) | 0.135 | 0.99 (0.94 to 1.05) | 0.795 | 1.00 (0.92 to 1.08) | 0.914 |
| <2000 | 14 | 12 285 | 0.85 (0.64 to 1.13) | 0.275 | 1.08 (0.90 to 1.29) | 0.398 | 1.07 (0.85 to 1.33) | 0.577 | 1.20 (0.89 to 1.61) | 0.238 |
| Population | ||||||||||
| General | 6 | 3 910 128 | 0.93 (0.87 to 0.99) | 0.016 | 1.07 (0.99 to 1.15) | 0.078 | 0.98 (0.96 to 1.00) | 0.035 | 0.89 (0.88 to 0.90) | <0.001 |
| Blood donors | 29 | 2 574 698 | 0.89 (0.81 to 0.97) | 0.011 | 1.08 (0.97 to 1.20) | 0.154 | 1.01 (0.92 to 1.10) | 0.885 | 1.08 (0.95 to 1.23) | 0.248 |
| Patients | 3 | 2655 | 0.92 (0.71 to 1.19) | 0.517 | 1.04 (0.71 to 1.54) | 0.828 | 1.09 (0.91 to 1.30) | 0.345 | 1.17 (0.94 to 1.46) | 0.169 |
| Income group | ||||||||||
| High | 7 | 148 804 | 0.96 (0.91 to 1.00) | 0.065 | 1.17 (0.95 to 1.44) | 0.135 | 0.91 (0.74 to 1.11) | 0.343 | 0.97 (0.84 to 1.13) | 0.712 |
| Upper middle | 9 | 6 101 344 | 1.01 (0.88 to 1.15) | 0.927 | 0.97 (0.82 to 1.15) | 0.756 | 1.00 (0.96 to 1.06) | 0.791 | 1.02 (0.88 to 1.17) | 0.814 |
| Lower middle | 18 | 214 587 | 0.86 (0.76 to 0.97) | 0.011 | 1.03 (0.93 to 1.13) | 0.582 | 1.13 (1.01 to 1.25) | 0.03 | 1.13 (0.95 to 1.34) | 0.173 |
| Low | 4 | 22 746 | 0.88 (0.56 to 1.38) | 0.572 | 1.34 0.72 to 2.48) | 0.353 | 0.71 (0.42 to 1.21) | 0.209 | 0.84 (0.43 to 1.64) | 0.613 |
| Study design | ||||||||||
| Cross-sectional | 37 | 6 408 776 | 0.91 (0.85 to 0.97) | 0.007 | 1.07 (0.98 to 1.17) | 0.111 | 1.01 (0.95 to 1.08) | 0.78 | 1.06 (0.96 to 1.17) | 0.244 |
| Cohort | 1 | 78 705 | 0.96 (0.92 to 1.01) | 0.098 | 1.05 (1.01 to 1.10) | 0.016 | 1.00 (0.95 to 1.05) | 0.957 | 0.92 (0.84 to 1.00) | 0.053 |
| Publication year | ||||||||||
| Before 2010 | 17 | 123 268 | 0.80 (0.67 to 0.96) | 0.015 | 1.12 (0.97 to 1.29) | 0.112 | 1.02 (0.85 to 1.22) | 0.83 | 1.22 (1.01 to 1.46) | 0.04 |
| After 2010 | 21 | 6 364 213 | 0.95 (0.88 to 1.01) | 0.106 | 1.05 (0.95 to 1.15) | 0.335 | 1.00 (0.94 to 1.06) | 0.91 | 0.98 (0.89 to 1.07) | 0.627 |
| Sensitive analyses | ||||||||||
| Removed Liu’s study17 | 37 | 2 660 356 | 0.91 (0.85 to 0.98) | 0.012 | 1.06 (0.98 to 1.15) | 0.138 | 1.01 (0.94 to 1.08) | 0.816 | 1.06 (0.97 to 1.17) | 0.213 |
| Removed Mohammedali’s study18 | 37 | 4 459 413 | 0.91 (0.85 to 0.97) | 0.002 | 1.08 (1.00 to 1.16) | 0.044 | 1.01 (0.94 to 1.07) | 0.857 | 1.04 (0.95 to 1.14) | 0.445 |
| Removed both Liu’s and Mohammedali’s study17 18 | 36 | 632 288 | 0.90 (0.83 to 0.97) | 0.007 | 1.07 (0.98 to 1.17) | 0.115 | 1.00 (0.92 to 1.09) | 0.946 | 1.08 (0.96 to 1.20) | 0.211 |
HBV, Hepatitis B virus; RR, risk ratio.
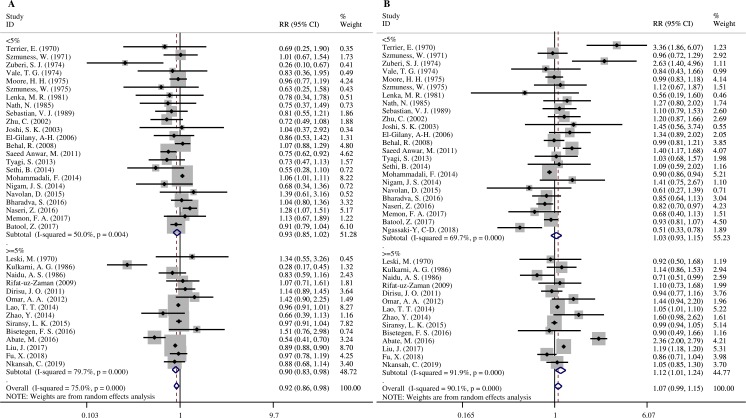
In higher endemic areas, subjects with blood group B had a significantly lower risk of HBV infection (RR=0.90, 95% CI 0.83 to 0.98) than the non-B blood group (figure 2A), while subjects with the blood group O had a significantly higher risk of HBV infection (RR=1.12, 95% CI 1.01 to 1.24) than the non-O blood group (figure 2B). According to the race of the subjects, blood groups A and B were linked with decreased risk of HBV infection in the Asian population when compared with non-A and non-B groups, respectively (RR=0.98, 95% CI 0.97 to 0.99; RR=0.91, 95% CI 0.86 to 0.97) (table 2). However, no association was found among the Caucasian or African populations. In the general population, blood group A, B and AB had a decreased risk of HBV infection compared with the non-A, non-B and non-AB groups, respectively (RR=0.98, 95% CI 0.96 to 1.00; RR=0.93, 95% CI 0.87 to 0.99 and RR=0.89, 95% CI 0.88 to 0.90, respectively) (table 2).
Figure 2.
Forest plots by prevalence: (A) B versus non-B. (B) O versus non-O.
In the sensitivity analysis, when the studies by Liu et al 17 and Mohammadali et al,18 which dominated the results of the meta-analysis, were orderly removed or both removed at the same time, the pooled risk estimates were still stable, showing that blood B was associated with a lower risk of HBV infection (table 2).
Publication bias
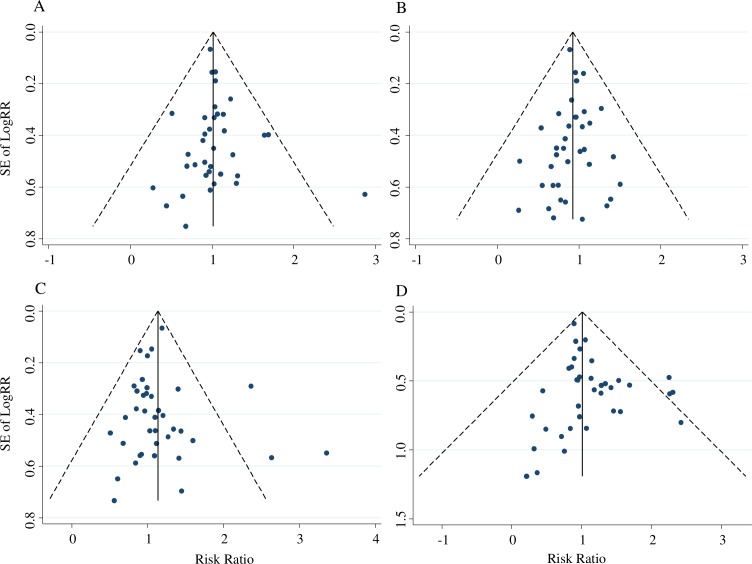
Funnel plots and Egger’s tests were performed to assess publication bias. No obvious evidence of publication bias was present for A versus non-A (figure 3A), B versus non-B (figure 3B) and O versus non-O (figure 3C) (p=0.148; p=0.223; p=0.364, respectively), while a publication bias of AB versus non-AB was observed (figure 3D) (p=0.002).
Figure 3.
Funnel plots: (A) A versus non-A. (B) B versus non-B. (C) O versus non-O. (D) AB versus non-AB.
Discussion
To our knowledge, this was the first meta-analysis of the association between ABO blood groups and HBV infection. Our meta-analysis results suggested that blood group B was associated with a lower risk of HBV infection, which was observed in the subgroups and was still stable in sensitive analyses, giving supportive evidence that statistical association and biological association between ABO blood groups and HBV infection probably exists.
As an infectious disease, aside from genetic susceptibility factors, there is the question of whether exposure to the source of infection is directly related to the risk of infection. People living in higher endemic areas are at higher risk of exposure to HBV infection than those living in lower endemic areas, which might be the reason why the association between the ABO blood group and HBV infection was only found in higher endemic areas but not in lower endemic areas. Additionally, this association might be partly attributed to the regional factors, due to the high relevance between HBV endemic and regional health and economic development.
The universal hepatitis B vaccination programme, proposed by WHO, was implemented for newborns from 1992. All the selected articles were published between 1970 and 2019, which meant that even in the same country, the prevalence of HBV infection had changed significantly due to the increasing coverage of hepatitis B vaccination. However, not enough information could be extracted from previous studies for comparing the pooled association of ABO blood groups and HBV infection between the vaccinated and unvaccinated groups. To partially examine the impact of hepatitis B vaccination on the results, we did subgroup analyses according to the publication year before and after 2010. Subjects in the selected articles were mainly over 18 years old. Thus, subjects in articles published after 2010 were more likely to be vaccinated at the time of birth, while subjects were mostly not vaccinated at birth in the articles published before 2010. We observed the association of blood group B and HBV infection in the articles published before 2010 rather than after 2010. The gradual establishment of an HBV immune barrier in the population may affect the occurrence of the relationship between ABO blood groups and HBV infection.
Our results found that subjects with blood group O were at higher risk of HBV infection than non-O blood group subjects in higher endemic areas, which was consistent with some previous studies by Lao et al,16 Liu et al 17 and Abate et al.27 That means more measures should be taken to ensure blood safety of the ‘universal’ blood group O population in high endemic areas because of the large unvaccinated population among the main blood donors in the current era and the window period for detection among the HBV-infected blood donors.17 However, this relationship was unobserved in other subgroup analyses, so whether this relationship is true remains to be further explored. Interestingly, our result that blood group B was associated with a lower risk of HBV infection compared with the non-B blood group was reported explicitly by few other studies, possibly because of the different analysis methods, such as the different reference of blood group in analysis.
However, the study by Mohammadali et al,18 with the second largest sample size, reported that HBV infection was lower in blood group O donors, in contrast to the study with the largest sample by Liu et al,17 probably due to the different HBV prevalence, geography and ethnicity. Our meta-analysis was inconsistent with a recent meta-analysis which found that patients with HCC might have a lower proportion of blood group O subjects than healthy subjects.12 The possible explanation for the inconsistence is the long-term and complicated process from HBV infection to the occurrence of HCC. To examine the reliability and stability of the results, we orderly removed the study by Liu et al 17 or Mohammadali et al,18 as well as removed both of them at the same time. In the sensitivity analysis, the relationship between blood group O and HBV infection might be unstable. However, the inverse relationship between blood group B and HBV infection was extremely stable. Therefore, we still think these findings are worthy of consideration due to the subgroup analyses, the sensitivity analyses and the relatively conservative random effects model.
Although the precise role that ABO blood groups play in host susceptibility and HBV infection has yet to be clarified,17 associations have been observed that are most likely related to the altered immune response16 and systemic inflammatory response,15 which are associated with different blood group phenotypes. A previous study has reported that the appearance of intestinal alkaline phosphatase in the plasma was associated with ABO blood group and secretor status, which might be due to genetically determined variations in the proportion of isoenzymes among the different blood types.28 Our study may indicate that a specific histo-blood group antigen may be a natural resistance factor for HBV infection, and that probably provides clues for correlative fundamental research of aetiologies and novel therapeutic targets for HBV. Further studies are warranted to elucidate the association between blood groups and HBV infection, and the way the blood type influences the process of HBV infection.
Meanwhile, several limitations need to be considered. First, although we performed subgroup analyses, analyses of previous studies have revealed that the heterogeneity cannot be ignored. Second, the analysed studies lacked basic information on the ethnicity data and the prevalence of different HBV genotypes. Third, few published studies on the association between HBV infection and blood group have controlled HBV infection related risk factors such as family history of HBV infection, age group, blood transfusion and acupuncture, thus we were not able to conduct the corresponding subgroup analyses.
In conclusion, blood group B is associated with a lower risk of HBV infection. In future, more research is needed to clarify the precise role of blood group ABO in HBV infection to address the global issue of HBV infection.
Supplementary Material
Footnotes
Contributors: All authors contributed to this work. ML and JL conceived and designed the study strategy; SZ and WJ independently completed the processes of the article search, article assessment, data extraction, quality assessment and data analysis; WJ wrote the manuscript. All authors read and approved the final manuscript.
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 71934002, No. 71874003 and No. 81703240).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053–63. 10.1016/S0140-6736(14)60220-8 [DOI] [PubMed] [Google Scholar]
- 2. WHO Global hepatitis report, 2017, 2017. Available: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/#
- 3. Li B, Tan B, Chen C, et al. Association between the ABO blood group and risk of common cancers. J Evid Based Med 2014;7:79–83. 10.1111/jebm.12098 [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Liu L, Wang Z, et al. ABO blood group and esophageal carcinoma risk: from a case-control study in Chinese population to meta-analysis. Cancer Causes Control 2014;25:1369–77. 10.1007/s10552-014-0442-y [DOI] [PubMed] [Google Scholar]
- 5. Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev 2015;28:801–70. 10.1128/CMR.00109-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alpoim PN, de Barros Pinheiro M, Junqueira DRG, et al. Preeclampsia and ABO blood groups: a systematic review and meta-analysis. Mol Biol Rep 2013;40:2253–61. 10.1007/s11033-012-2288-2 [DOI] [PubMed] [Google Scholar]
- 7. He M, Wolpin B, Rexrode K, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol 2012;32:2314–20. 10.1161/ATVBAHA.112.248757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miao S-Y, Zhou W, Chen L, et al. Influence of ABO blood group and rhesus factor on breast cancer risk: a meta-analysis of 9665 breast cancer patients and 244,768 controls. Asia Pac J Clin Oncol 2014;10:101–8. 10.1111/ajco.12083 [DOI] [PubMed] [Google Scholar]
- 9. Jenkins PV, O'Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion 2006;46:1836–44. 10.1111/j.1537-2995.2006.00975.x [DOI] [PubMed] [Google Scholar]
- 10. Gallinaro L, Cattini MG, Sztukowska M, et al. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood 2008;111:3540–5. 10.1182/blood-2007-11-122945 [DOI] [PubMed] [Google Scholar]
- 11. Anstee DJ. The relationship between blood groups and disease. Blood 2010;115:4635–43. 10.1182/blood-2010-01-261859 [DOI] [PubMed] [Google Scholar]
- 12. Liu F, Li C, Zhu J, et al. ABO blood type and risk of hepatocellular carcinoma: a meta-analysis. Expert Rev Gastroenterol Hepatol 2018;12:927–33. 10.1080/17474124.2018.1500174 [DOI] [PubMed] [Google Scholar]
- 13. Branch DR. Blood groups and susceptibility to virus infection: new developments. Curr Opin Hematol 2010;17:558–64. 10.1097/MOH.0b013e32833ece31 [DOI] [PubMed] [Google Scholar]
- 14. Paré G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet 2008;4:e1000118 10.1371/journal.pgen.1000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y, Zhou Q, Lin Q, et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer 2013;133:1867–75. 10.1002/ijc.28196 [DOI] [PubMed] [Google Scholar]
- 16. Lao TT, Sahota DS, Chung M-K, et al. Maternal ABO and rhesus blood group phenotypes and hepatitis B surface antigen carriage. J Viral Hepat 2014;21:818–23. 10.1111/jvh.12219 [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Zhang S, Liu M, et al. Distribution of ABO/Rh blood groups and their association with hepatitis B virus infection in 3.8 million Chinese adults: a population-based cross-sectional study. J Viral Hepat 2018;25:401–11. 10.1111/jvh.12829 [DOI] [PubMed] [Google Scholar]
- 18. Mohammadali F, Pourfathollah A. Association of ABO and Rh blood groups to blood-borne infections among blood donors in Tehran-Iran. Iran J Public Health 2014;43:981–9. [PMC free article] [PubMed] [Google Scholar]
- 19. Szmuness W, Prince AM, Cherubin CE. Serum hepatitis antigen (SH) carrier state: relation to ABO blood groups. Br Med J 1971;2:198–9. 10.1136/bmj.2.5755.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szmuness W, Hirsch RL, Prince AM, et al. Hepatitis B surface antigen in blood donors: further observations. J Infect Dis 1975;131:111–8. 10.1093/infdis/131.2.111 [DOI] [PubMed] [Google Scholar]
- 21. Behal R, Jain R, Behal KK, et al. Seroprevalence and risk factors for hepatitis B virus infection among general population in Northern India. Arq Gastroenterol 2008;45:137–40. 10.1590/S0004-28032008000200009 [DOI] [PubMed] [Google Scholar]
- 22. WHO Global health sector strategy on viral hepatitis 2016-2021, 2016. Available: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/
- 23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 24. Rostom A, Dube C, Cranney A, et al. Agency for healthcare research and quality (US), 2004. Available: https://www.ncbi.nlm.nih.gov/books/NBK35156/
- 25. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26. World Bank World bank list of economies, 2016. Available: http://siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS [Accessed Jul 2016].
- 27. Abate M, Wolde T. Seroprevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and syphilis among blood donors at Jigjiga blood bank, eastern Ethiopia. Ethiop J Health Sci 2016;26:153–62. 10.4314/ejhs.v26i2.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan X, Waterworth D, Perry JRB, et al. Population-Based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet 2008;83:520–8. 10.1016/j.ajhg.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terrier E, Simonneau M, Jaulmes B. Screening blood donors for Australia antigen carriers at the Broussais Hospital. Vox Sang 1970;19:352–6. 10.1159/000466012 [DOI] [PubMed] [Google Scholar]
- 30. Leski M, Grivaux C, Courouce-Pauty AM. Australia antigen in hemodialysis and renal transplantation units. Vox Sang 1970;19:359–68. 10.1159/000466014 [DOI] [PubMed] [Google Scholar]
- 31. Zuberi SJ, Lodi TZ. Hepatitis B antigen in blood donors in Karachi. 1974. J Pak Med Assoc 2004;54:S39–40. [PubMed] [Google Scholar]
- 32. Vale TG, Thomas HN, Hawkes RA, et al. ABO blood groups and hepatitis B antigen and antibody. Aust N Z J Med 1974;4:1–2. 10.1111/j.1445-5994.1974.tb03136.x [DOI] [PubMed] [Google Scholar]
- 33. Moore HH, Campling M, Hart GE. A comparison of hepatitis B antigen and blood groups in African donors. Cent Afr J Med 1975;21:175–6. [PubMed] [Google Scholar]
- 34. Lenka MR, Ghosh E, Bhattacharyya PK. ABO blood groups in relation to hepatitis-B surface antigen (Australia antigen). Trans R Soc Trop Med Hyg 1981;75:688–90. 10.1016/0035-9203(81)90149-8 [DOI] [PubMed] [Google Scholar]
- 35. Nath N, Mushahwar IK, Fang CT, et al. Antibodies to delta antigen in asymptomatic hepatitis B surface antigen-reactive blood donors in the United States and their association with other markers of hepatitis B virus. Am J Epidemiol 1985;122:218–25. 10.1093/oxfordjournals.aje.a114092 [DOI] [PubMed] [Google Scholar]
- 36. Kulkarni AG, Aloowooja FO, Wayo GB. Prevalence of hepatitis B surface antigen in northern Nigerian blood donors. Vox Sang 1986;50:151–3. 10.1159/000461414 [DOI] [PubMed] [Google Scholar]
- 37. Naidu AS, Rajyalakshmi K. Detection of hepatitis B surface antigen among professional blood donors in Hyderabad, India. J Commun Dis 1986;18:215–8. [PubMed] [Google Scholar]
- 38. Sebastian VJ, Bhattacharya S, Ray S, et al. Hepatitis-B surface antigen and VDRL in healthy blood donors of Brunei Darussalam. Singapore Med J 1989;30:568–70. [PubMed] [Google Scholar]
- 39. Zhu C, Zhang H, Xiong J, et al. Study on relationship between ABO Blood-Type and infection of hepatitis viruses. Acta Acad Med Jiangxi 2002;42:72–3. [Google Scholar]
- 40. Joshi SK, Ghimire GR. Serological prevalence of antibodies to human immunodeficiency virus (HIV) and hepatitis B virus (HBV) among healthy Nepalese males--a retrospective study. Kathmandu Univ Med J (KUMJ) 2003;1:251–5. [PubMed] [Google Scholar]
- 41. El-Gilany A-H, El-Fedawy S. Bloodborne infections among student voluntary blood donors in Mansoura University, Egypt. East Mediterr Health J 2006;12:742–8. [PubMed] [Google Scholar]
- 42. Rifat-uz-Zaman Relationship between HBV-markers prevalance and promotive factors among human urban population of Bahawalpur district, Pakistan: a cross-sectional study. Res J Pharmacol 2009;3:7–14. [Google Scholar]
- 43. Dirisu JO, Alli TO, Adegoke AO, et al. A survey of prevalence of serum antibodies to human immunodeficiency deficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) among blood donors. N Am J Med Sci 2011;3:35–8. 10.4297/najms.2011.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saeed Anwar M, Mujtaba Siddiqi G, Haq S, et al. Association of blood group types to hepatitis B and hepatitis C virus infection. Biomedica 2011;27:57–61. [Google Scholar]
- 45. Omar AA, Noor NA, Mahmood JM. The infection with HBV and HCV and their relationship to ABO blood group among blood donors. J Fac Med Baghdad 2012;54:52–6. [Google Scholar]
- 46. Tyagi S, Tyagi A. Possible correlation of transfusion transmitted diseases with Rh type and ABO blood group system. J Clin Diagn Res 2013;7:1930–1. 10.7860/JCDR/2013/6002.3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sethi B, Kumar S, Butola KS, et al. Seroprevalence pattern among blood donors in a tertiary health care center. Internet J Med Update 2014;9:10–15. [Google Scholar]
- 48. Nigam JS, Singh S, Kaur V, et al. The prevalence of transfusion transmitted infections in ABO blood groups and Rh type system. Hematol Rep 2014;6:5602 10.4081/hr.2014.5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao Y, Zhang Y, Huang J, et al. Clinical analysis of detection of markers related to infectious diseases. Chin J of Nosocomiol 2014;24:3635–7. [Google Scholar]
- 50. Siransy LK, Nanga ZY, Zaba FS, et al. ABO/Rh Blood Groups and Risk of HIV Infection and Hepatitis B among Blood Donors of Abidjan, Côte D’Ivoire. Eur J Microbiol Immunol 2015;5:205–9. 10.1556/1886.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Navolan D, Vladareanu S, Ciohat I, et al. Prevalence of HBsAg carrying pregnant women in Romania: demographic and behavioral features. Proceedings of the 49th Annual Scientific Meeting of the European Society for Clinical Investigation 2015:249–52. [Google Scholar]
- 52. Bisetegen FS, Bekele FB, Ageru TA, et al. Transfusion-Transmissible infections among voluntary blood donors at Wolaita Sodo university teaching referral Hospital, South Ethiopia. Can J Infect Dis Med Microbiol 2016;2016:1–6. 10.1155/2016/8254343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bharadva S, Vachhani J, Dholakiya S. ABO and Rh association to transfusion transmitted infections among healthy blood donors in Jamnagar, Gujarat, India. J Res Med Dent Sci 2016;4:58–62. 10.5455/jrmds.20164113 [DOI] [Google Scholar]
- 54. Naseri Z, Ghannad MS, Hosseini SM, et al. Evaluation of accompaniment of ABO blood groups system and rhesus blood group types with infection to hepatitis B virus and hepatitis C virus in Hamadan, Iran. Int J of Med Res Health Sci 2016;5:1–5. [Google Scholar]
- 55. Memon FA, Ujjan I, Memon AI, et al. Seroprevalence of transfusion transmitted infections among different blood group donors at blood bank LUMHS, Hyderabad. Pak J Med Sci 2017;33 10.12669/pjms.332.11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Batool Z, Durrani SH, Tariq S. Association of ABO and Rh blood group types to hepatitis B, hepatitis C, HIV and syphilis infection, a five year' experience in healthy blood donors in a tertiary care hospital. J Ayub Med Coll Abbottabad 2017;29:90–2. [PubMed] [Google Scholar]
- 57. Ngassaki-Yoka C-D, Ndong JMN, Bisseye C, et al. ABO, rhesus blood groups and transfusion-transmitted infections among blood donors in Gabon. SJMS 2018;13:12–21. 10.18502/sjms.v13i1.1685 [DOI] [Google Scholar]
- 58. Fu X, Xu C, Cai X, et al. Analysis of infection status and clinical significance of infectious diseases before transfusion. Chin J Nosocomiol 2018;28:2498–501. [Google Scholar]
- 59. Nkansah C, Serwaa D, Osei-Boakye F, et al. Seroprevalence and trend of hepatitides among blood donors in a district hospital in Ghana: a nine-year retrospective, descriptive cross-sectional study. J Immunoassay Immunochem 2020;41:71–83. 10.1080/15321819.2019.1682601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034114supp001.pdf (60.4KB, pdf)