Abstract
To identify associations between immunostimulated cytokine production and disease characteristics, peripheral blood lymphocytes were collected from 155 adult patients with rheumatoid arthritis (RA) before and after a 5-year interval. The lymphocytes were activated in vitro with T-cell stimulants, cytosine-phosphate-guanine (CpG) oligonucleotide, and medium alone (negative control). Expression of 17 cytokines was evaluated with immunoassays, and factor analysis was used to reduce data complexity and identify cytokine combinations indicative of cell types preferentially activated by each immunostimulant. The findings showed that the highest numbers of correlations were between cytokine levels and rheumatoid factor (RF) positivity and between cytokine levels and disease duration. Scores for cytokines driven by CpG and medium alone were negatively associated with RF positivity and disease duration at baseline but positively associated with both at 5 years. Our findings suggest that RF expression sustained over time increases activation of B cells and monocytes without requirements for T- cell functions.
Keywords: chemokines, cytokines, immunostimulation, rheumatoid arthritis, rheumatoid factor
Introduction
Rheumatoid arthritis (RA) exacts a costly toll on patients, with debilitating effects ranging from pain and disability to death, associated comorbidities, decreased quality of life, and increased financial costs. The autoimmune nature of RA has been clearly established, with involvement of both innate and adaptive immune systems. The resultant articular and systemic responses involve multiple lymphoid cell types with multiple effector functions. Accordingly, it is difficult to assign specific RA symptoms to specific cell types or to identify the most deleterious autoimmune mechanisms.
The assessment of disease progression and therapeutic efficacy in patients with RA is dependent on a combination of 1) laboratory tests for acute-phase proteins, 2) clinical assessment of joint inflammation and damage and the extent and severity of pain and disability, and 3) patient selfassessment of pain and disability. Despite the effort expended on developing informative assessments, the present approaches do not appear to be sufficiently sensitive to detect the low levels of inflammation that are suspected of driving continued joint damage in patients classified as having low disease activity [1, 2]. Biomarkers that can be objectively quantitated at relatively high-resolution levels and whose levels correlate with disease severity should provide an important adjunct to present clinical assessments.
Previously we reported our development of an experimental platform that was designed to probe the multiple lymphoid cell types involved in innate and adaptive responses in patients with RA. A panel of immunostimulants was chosen to activate a wide range of lymphoid cell types in vitro, with activation quantitated by expression of a diverse set of cytokines and chemokines that can be used to identify cell types that respond to individual stimulants. We have used this approach to develop immune signatures of cytokine and chemokine expression that distinguish patients with RA who differ by 1) duration of disease [3], 2) risk of infection [4], 3) severity of RA-associated left ventricular diastolic dysfunction [5], 4) probability of adequate response to initial disease-modifying antirheumatic drug therapy [6], and 5) severity of radiographic joint damage [7].
In the present study we aimed to evaluate changes in cytokine and chemokine expression after 5 years of follow-up in order to assess our immune signature platform for predicting future disease outcomes. We used our immune signature platform to assess the capacity of the immune system of patients with RA to express cytokines and chemokines before and after a 5-year interval during the course of the disease to compare levels of expression with disease characteristics. Factor analysis was used to reduce the complexity of data by identifying groups of associated cytokines and chemokines, to identify the responding lymphoid cell types, and to correlate changes in these cell types with different characteristics of the disease over the 5-year study period.
Materials and Methods
Study Design and Participants
We conducted a cross-sectional analysis of baseline and 5-year follow-up data from a prospective study of patients with RA in a community, population-based, incidence cohort as previously described [5]. This study used resources of the Rochester Epidemiology Project, a medical records linkage system providing access to complete medical records for residents of Olmsted County, Minnesota, who receive medical attention [8].
We identified Olmsted County residents who were 18 years or older and who first fulfilled the American College of Rheumatology classification criteria for RA between January 1, 1980, and December 31, 2007. From this cohort, 324 of475 eligible patients with RA were recruited for the initial study visit (visit 1). After 5 years, 155 patients returned to participate in the second study visit (visit 2). Of the 169 patients who did not return for the second visit, 42 had died or entered nursing homes and 127 had not responded to requests to participate. The Mayo Clinic and Olmsted Medical Center institutional review boards approved this study, which was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Clinical Data Collection
Study participants completed questionnaires consisting of visual analog scales for pain and patient global assessment of disease activity: the Health Assessment Questionnaire (HAQ) disability index and the Routine Assessment of Patient Index Data 3 (RAPID3). Medication use, including conventional and biologic disease-modifying antirheumatic drugs and glucocorticoids, was ascertained by patient interview and verified in the medical records. Laboratory testing of patient sera included measurements of rheumatoid factor (RF) (Nephelometer II; CSL Behring, King of Prussia, Pennsylvania), C-reactive protein (CRPL3 Reagent; Roche Diagnostics, Indianapolis, Indiana), and anti-citrullinated protein antibody (Quanta Lite CCP3; Inova Diagnostics, Inc, San Diego, California).
Peripheral Blood Mononuclear Cell Isolation, Cell Culture, and Panel of Immunostimulants
Venous blood samples were collected in heparinized tubes and maintained at room temperature. Within 1 to 2 hours, peripheral blood mononuclear cells (PBMCs) were enriched by Ficoll density gradient centrifugation, suspended in RPMI 1640 medium with 10% heat-inactivated fetal calf serum (FCS)/1% penicillin-streptomycin-glutamine, and stimulated in vitro under 6 separate conditions with a panel of immunostimulants as we previously described [3, 5]. Each of the 6 conditions involved culturing 4×105 PBMCs in 200 μL of the RPMI 1640 medium (with 10% FCS/1% penicillin-streptomycin-glutamine) with 1 of 5 immunostimulants or the control medium in quadruplicate wells. The 5 immunostimulants, selected to collectively activate a broad range of immune cell types, were 1) monoclonal antibodies to the CD3 receptor and the CD28 costimulatory molecule (anti-CD3/anti-CD28 Dynabeads; Invitrogen, Carlsbad, California) at 5×105 beads per well, 2) phytohemagglutinin (PHA) (Sigma-Aldrich Co, St Louis, Missouri) at 5 μ g/mL [9], 3) staphylococcal enterotoxins A (SEA) and B (SEB) (Toxin Technology Inc, Sarasota, Florida) (10 ng/mL for each) [10], 4) phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) (1 mg/mL) in combination with ionomycin (700 ng/mL) [9], and 5) synthetic cytosine- phosphate-guanine (CpG) oligonucleotide (ODN2006 with phosphorothioate backbone; Invitrogen). The PBMC cultures were incubated at 37°C in 5% carbon dioxide for 48 hours to capture the expression of cytokines that were produced by both primarily and secondarily activated cells. After supernatants were collected, they were stored at −80°C for later analysis.
Multiplexed Cytokine Immunoassays
Seventeen cytokines and chemokines were analyzed with a Meso Scale Discovery 96-well Multi-Spot Human Cytokine Assay Tissue Culture Kit (Meso Scale Discovery, Rockville, Maryland). The cytokines were interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 (also known as CXCL8), IL-10, IL-12, IL-13, IL-17A, interferon-γ, tumor necrosis factor-α, monocyte chemoattractant protein (MCP)-1 (also known as CCL2), macrophage inflammatory protein (MIP)-1β (also known as CCL4), granulocyte colony-stimulating factor (G-CSF), and granulocyte- macrophage colony-stimulating factor (GM-CSF). The 17-cytokine kits used for the visit 1 analysis included IL-7, but the kits were modified by the manufacturer to substitute IL-23 for IL-7 before visit 2. Immunoassays of the PBMC culture supematants followed the manufacturer’s protocol, and antibody-binding levels were estimated with the Sector Imager 2400 (Meso Scale Discovery). Cytokine concentrations were estimated from a standard curve generated for each plate with the manufacturer-supplied reagents and Discovery Workbench 2.0 software (Meso Scale Discovery). The intra-assay and interassay reproducibilities were favorable in comparison with the high level of informative biologic variation assessed with this approach [11].
Statistical Analysis
Baseline characteristics were analyzed with descriptive statistics, including mean (SD) or median (range) as appropriate. All statistical tests were 2-sided; the significance level was set at P<.05 for all analyses. Paired t tests were used to assess changes in characteristics between visits 1 and 2. Cytokine data were normalized and adjusted for age and sex with mixed models [3]. Because of the exploratory nature of this study, we focused our attention on P values as indicators of significance rather than attempt to make adjustments of P values for multiple comparisons.
For each PBMC sample, 102 data points were collected because the PBMCs from each participant were incubated under 6 conditions (5 stimulants and the control medium) and then tested for 17 cytokines. For data reduction, factor analysis was applied separately to the results for each stimulant, and the top 3 factors were retained, giving a more manageable summary of 18 values per participant. Each factor represented an underlying construct composed of similar cytokine measures. With the use of factor “loadings,” which depicted the strength of the relationship between each cytokine measure and the factor, individual cytokine measures were combined into a composite score for each factor. Factor scores were rescaled (0–100) for interpretation. The scores were created from data from the first visit, so as to match prior reports [5], and were then calculated for second visits. Spearman rank correlation and rank sum tests were used to assess differences between participants who did or did not return for a second visit and to associate cytokine changes from visit 1 to visit 2 with clinical disease characteristics (ie, the presence of disease or clinical changes).
Results
Patient Characteristics
A total of 324 patients with RA participated in visit 1 (median age, 60.7 years; median disease duration, 8.6 years) (Table 1). After 5 years, 155 patients returned for visit 2. Comparison of the characteristics of patients who did return and patients who did not return for visit 2 showed that those who returned were younger and had less disability (ie, lower HAQ scores) at visit 1 (some patients died before visit 2). Among those who returned, the HAQ disability index score significantly increased during the 5 years, but the increase was minimal (median change, 0.0; interquartile range: −0.1, 0.4). Patient assessments did not change greatly over 5 years. The distributions of treatment modalities were not significantly different between those who did and those who did not return for visit 2; there were also no significant differences in distributions of treatments at visits 1 and 2 among those who returned (Table 1). Only 2 patients with negative RF were positive for anti-citrullinated protein antibody, so RF positivity alone was examined in further analyses.
Table 1.
Patient Characteristics at Visit 1 and Visit 2
| Patients at Visit 1a | Patients at Visit 2a | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | All Patients (N=324) | Did Not Return for Visit 2 (n=169) | Did Return for Visit 2 (n=155) | P Valueb | All Patients (n=155) | Change From Visit 1 | P Valuec |
| Age, y | 60.7 (50.9, 70.5) | 63.7 (51.2, 73.0) | 57.7 (50.7, 67.5) | .01 | 62.7 (55.6, 72.4) | 5.0 (5.0, 5.1) | … |
| Disease duration, y | 8.6 (4.6, 14.3) | 7.6 (4.3, 13.5) | 9.7 (4.7, 15.2) | .12 | 14.7 (9.7, 20.3) | 5.0 (5.0, 5.1) | … |
| C-reactive protein, mg/L | 2.1 (0.8, 5.0) | 2.1 (0.8, 5.3) | 2.2 (0.8, 4.6) | .61 | 2.2 (0.9, 5.0) | 0.1 (−1.7, 1.6) | .78 |
| HAQ disability index | 0.4 (0.0, 0.9) | 0.5 (0.0, 1.0) | 0.2 (0.0, 0.8) | .02 | 0.5 (0.0, 1.1) | 0.0 (−0.1, 0.4) | <.001 |
| Pain VAS (0–100 points) | 22 (9, 47) | 24 (9, 49) | 20 (9, 45) | .21 | 22 (8, 47) | 0 (−11, 9) | .92 |
| Patient global disease activity VAS (0–100 points) | 16 (5, 39) | 17 (6, 42) | 13 (4, 35) | .15 | 17 (6, 39) | 1 (−10, 14) | .36 |
| RAPID3 (0–10 points) | 1.7 (0.8, 3.8) | 2.1 (1.0, 3.9) | 1.4 (0.7, 3.6) | .04 | 2.0 (0.7, 3.6) | 0.0 (−0.6, 1.1) | .20 |
| RF positive | 226 (70) | 119 (70) | 107 (69) | .79 | … | … | … |
| ACPA positive | 154 (48) | 82 (48) | 72 (46) | .71 | … | … | … |
| Positive for RF or ACPA (or both) Treatment | 230 (71) | 121 (72) | 109 (70) | .80 | … | … | … |
| Treatment | |||||||
| Methotrexate | 172 (53) | 88 (52) | 84 (54) | .70 | 86 (55) | … | .69 |
| Hydroxychloroquine | 103 (32) | 49 (29) | 54 (35) | .26 | 45 (29) | … | .06 |
| Other DMARD | 31(10) | 15 (9) | 16 (10) | .66 | 22 (14) | … | .16 |
| Biologics | 49 (15) | 23 (14) | 26 (17) | .43 | 29 (19) | … | .47 |
| Glucocorticoids | 84 (26) | 46 (27) | 38 (25) | .58 | 37 (24) | … | 85 |
Abbreviations: ACPA, anti-citrullinated protein antibody; DMARD, disease-modifying antirheumatic drug; HAQ, Health Assessment Questionnaire; RAPID3, Routine Assessment of Patient Index Data 3; RF, rheumatoid factor; VAS, visual analog scale.
Values are median (25th percentile, 75th percentile) or No. (%).
Comparison of patients at visit 1 with patients who did return and patients who did not return for visit 2.
Comparison of patients at visit 1 with patients who did return for visit 2.
Cytokine Response Profiles
PBMCs collected at visit 1 were activated by each stimulant for 48 hours in vitro, after which supernatants were analyzed for expression of the 17 cytokines and chemokines. Since individual cell types can produce multiple cytokines upon activation with a single stimulant, we reasoned that groupings of coordinately expressed cytokines should represent activated cell types. To find these groupings, we reduced the complexity of our data set with factor analysis to identify a smaller number of underlying constructs or factors that would most effectively explain the variation in a larger set of experimental variables. Such analysis results in ranked factors that are variably loaded by levels of expression of cytokines.
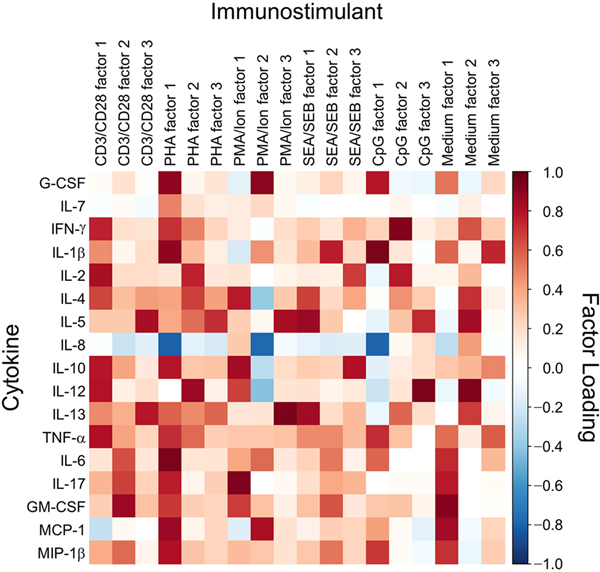
Using the levels of cytokines produced by PBMCs collected from all patients at visit 1, we identified the 3 highest ranking factors for each stimulant and the cytokines that most contributed to each factor as indicated by contributory values greater than 0.5 (Figure 1). We used those contributing cytokines to deduce responding cell types by considering multiple elements: 1) the principal cell type(s) predicted to be activated by each immunostimulant with documented effects on cytokine expression, 2) reported activation of secondary cell types, and 3) reported cytokine production by the secondarily activated cell types (Table 2). The rationale for the cell type assignments is described more fully in the Appendix.
Figure 1.
Loadings of Factors With Cytokine Expression After Activation of Peripheral Blood Mononuclear Cells With the Panel of Immunostimulants. Loading was scaled from high (red) to low (blue). CD3/CD28 indicates anti- CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; Medium, RPMI 1640 medium with 10% fetal calf serum; MIP, macrophage inflammatory protein; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B; TNF, tumor necrosis factor.
Table 2.
Inferred Lymphoid Sources of Cytokines With Loadings Greater Than 0.5 for Factors 1, 2, and 3a
| CD3/CD28 | PHA | PMA/Ion | SEA/SEB | CpG | Medium | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| G-CSF | T>APC | T>apc | B>APC | APC | ||||||||||||||
| IL-7 | ||||||||||||||||||
| IFN-γ | TH1/CD8 | TH1/CD8 | B cells | TH1/CD8 | ||||||||||||||
| IL-1β | T>APC | T>APC | B>APC | APC | APC | |||||||||||||
| IL-2 | TH1/CD8 | TH1/CD8 | TH1/CD8 | B cells | ||||||||||||||
| IL-4 | TH2/CD8 | TH2 | TH2 | TH2 | TH2 | |||||||||||||
| IL-5 | TH2 | TH2 | TH2 | TH2 | TH2 | B>TH2 | TH2 | |||||||||||
| IL-8 | ||||||||||||||||||
| IL-10 | TH1 | TH1/TH17 | TH17 | TH1/TH17 | ||||||||||||||
| IL-12 | T>APC | T>APC | T>APC | B cells | APC | |||||||||||||
| IL-13 | TH2 | TH2 | TH2 | TH2 | TH2 | B cells | TH2 | |||||||||||
| TNF-α | TH1/CD8 | TH1/CD8 | TH1/CD8 | B cells | Multiple | Multiple | ||||||||||||
| IL-6 | TH17>APC | T>APC | T>APC | T>APC | B cells | |||||||||||||
| IL-17 | TH17 | TH17 | TH17 | TH17 | ||||||||||||||
| GM-CSF | TH17 | T cells | TH17 | T cells | Multiple | |||||||||||||
| MCP-1 | T>APC | T>APC | Multiple | |||||||||||||||
| MIP-1β | TH17 | T cells | T cells | B cells | Multiple | |||||||||||||
Abbreviations: APC, antigen-presenting cell; B, B cell; CD3/CD28, anti-CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; Medium, RPMI 1640 medium with 10% fetal calf serum; MIP, macrophage inflammatory protein; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B; T, T cell; TH, helper T cell; TNF, tumor necrosis factor.
Inequality sign (>) indicates secondary activation.
The inferred associations between factors and responding cell types are exemplified by the cytokines that contributed to the factors for CD3/CD28 stimulation (Table 2). Factor 1 included cytokines that are predicted to be produced by helper T cell (TH)1, TH2, and CD8+ T cells. Factor 2 included cytokines that are expected to be produced by TH17 cells, and factor 3 consisted of cytokines predicted to be expressed by TH2 cells. In addition, 2 cytokines (IL-6 and IL-12) that contributed to factors 1 and 2 are expected to be expressed by secondarily activated antigen-presenting cells (APCs). Factors associated with the 4 T-cell stimulants (CD3/CD28, PHA, PMA-ionomycin, and SEA/SEB) varied in their cytokine associations, which is consistent with previously reported differences in cytokine expression after activation with these stimulants [12–15]. PMA-ionomycin and SEA/SEB can also directly stimulate expression of cytokines by APCs [16, 17]. According to previous studies, the CpG-ODN2006 oligonucleotide principally activates B cells in PBMCs with secondary activation of monocytes [18]. Interestingly, various cytokines were expressed after incubation in medium alone, which suggests that the range of responding cytokine-expressing cell types is relatively broad.
IL-8 was the only cytokine that was negatively associated with multiple factors for multiple stimulants (Figure 1). Considering the fundamentals of factor analysis, these negative associations suggested that the cell type(s) producing IL-8 was not the lymphoid cell type(s) that produced the cytokines that most contributed to the respective factors. Plausible sources of IL-8 are circulating endothelial cells and endothelial progenitors that are prominent producers of IL-8 and are present in PBMCs and at increased levels in patients with RA [19, 20]. The 3 strongest negative associations of IL-8 correlated with the strongest contributions of G-CSF to 3 factors, raising the possibility that lymphocyte-derived G-CSF may have driven expression of IL-8 by endothelial cells. This relationship between G- CSF and IL-8 is consistent with increased IL-8 levels in serum from human participants treated with G-CSF [21].
Baseline Comparisons
Factor scores were not significantly different between the 155 patients who did return for a second visit and those who did not, with the exception of factor 2 for PMA-ionomycin (Table 3). Factor scores at the first visit for the 155 patients who returned for visit 2 were compared with the following patient characteristics: age, disease duration, smoking, RF positivity, glucocorticoid use, methotrexate use, and use of biologic treatments. It was apparent that expression of RF and disease duration, as single characteristics, most consistently correlated with the largest numbers of stimulant factor scores. Surprisingly, correlations of factor scores with treatments (ie, methotrexate, glucocorticoid, and biologicals) were relatively limited.
Table 3.
Factor Scores for Cytokine Expression Driven bylmmunostimulants
| Patients at Visit 1a | Patients at Visit 2a | |||||||
|---|---|---|---|---|---|---|---|---|
| Stimulant | Factor | All Patients (N=324) | Did Not Return for Visit 2 (n=169) | Did Return for Visit 2 (n=155) | P Valueb | All Patients (n=155) | Change From Visit 1 | P Valuec |
| CD3/CD28 | 1 | 72.6 (58.6, 80.6) | 70.4 (57.4, 78.9) | 74.1 (60.9, 82.5) | .06 | 43.9 (30.2, 59.3) | −24.2 (−43.5, −6.3) | <.001 |
| 2 | 55.8 (44.9, 65.8) | 54.6(44.9, 64.1) | 57.2 (44.8, 68.0) | .22 | 66.2 (48.5, 77.6) | 6.8 (−14.0, 31.6) | .008 | |
| 3 | 70.1 (57.9, 80.9) | 68.7 (58.7, 77.7) | 70.7 (56.8, 83.6) | .35 | 68.6 (53.2, 80.7) | −1.2 (−22.7, 19.2) | .47 | |
| PHA | 1 | 65.8 (25.9, 80.3) | 63.4 (21.6, 78.6) | 67.6 (41.2, 80.8) | .09 | 40.4 (26.6, 56.9) | −22.8 (−43.3, −0.5) | <.001 |
| 2 | 46.7 (21.3, 70.2) | 39.2(18.9, 71.0) | 48.9 (25.8, 70.1) | .12 | 41.6 (26.0, 56.0) | −4.0 (−28.1, 17.8) | .04 | |
| 3 | 56.9 (19.5, 78.1) | 51.1 (18.1, 78.2) | 58.5 (30.3, 78.0) | .10 | 26.4(11.4, 41.5) | −29.7 (−49.6, 1.5) | <.001 | |
| PMA/Ion | 1 | 59.8 (41.2, 76.7) | 59.0(38.6, 75.8) | 61.2 (45.0, 77.7) | .10 | 37.2 (24.7, 52.7) | −17.7 (−44.2, 3.6) | <.001 |
| 2 | 37.9 (26.2, 56.3) | 39.8 (30.7, 57.4) | 33.6(21.4, 54.1) | .002 | 56.7 (43.7, 67.5) | 19.7 (−9.0, 38.8) | <.001 | |
| 3 | 52.3 (40.2, 63.9) | 52.3 (40.4, 64.8) | 52.2 (39.0, 63.0) | .74 | 51.6 (38.0, 59.5) | −3.9 (−17.1, 15.4) | .44 | |
| SEA/SEB | 1 | 45.5(31.0, 61.4) | 46.4 (33.0, 61.9) | 44.6 (28.8, 60.6) | .39 | 46.7 (30.0, 66.2) | 3.0 (−19.3, 23.0) | .31 |
| 2 | 38.8 (22.7, 58.7) | 40.8 (24.0, 59.9) | 36.4 (21.4, 58.4) | .33 | 43.7(31.4, 56.0) | 6.4 (−20.8, 26.9) | .09 | |
| 3 | 48.2 (35.8, 65.8) | 50.0(36.6, 65.7) | 47.2 (34.1, 66.0) | .43 | 55.2 (37.6, 71.6) | 5.6 (−15.8, 26.5) | .03 | |
| CpG | 1 | 44.4 (28.2, 63.2) | 42.9 (29.0, 63.0) | 45.3 (27.2, 64.2) | .85 | 24.9 (16.3, 41.0) | −12.8 (−39.4, 7.2) | <.001 |
| 2 | 30.4 (20.6, 36.0) | 29.5 (22.2, 35.6) | 30.9 (19.6, 38.3) | .86 | 57.5 (46.7, 76.8) | 27.3 (9.1, 46.8) | <.001 | |
| 3 | 40.9 (27.9, 55.6) | 40.9 (28.3, 55.6) | 40.9 (27.8, 54.2) | .70 | 38.7 (24.6, 57.0) | −2.1 (−20.6, 20.4) | .91 | |
| Medium | 1 | 56.2 (35.0, 69.1) | 55.7(33.0, 69.1) | 56.9 (35.2, 69.3) | .88 | 50.9 (33.4, 62.9) | −4.5 (−22.0, 17.7) | .16 |
| 2 | 68.3 (56.0, 79.6) | 68.5 (56.5, 78.4) | 68.1 (55.8, 80.1) | .98 | 57.1 (44.1, 72.5) | −6.8 (−27.7, 7.9) | <.001 | |
| 3 | 47.5 (34.1, 60.6) | 47.6(34.1, 60.7) | 47.5 (33.8, 60.3) | .77 | 51.5 (37.6, 67.7) | 6.3 (−11.3, 25.0) | .02 | |
Abbreviations: CD3/CD28, anti-CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; Medium, RPMI 1640 medium with 10% fetal calf serum; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B.
Values are median (25th percentile, 75th percentile).
Comparison of patients at visit 1 with patients who did return and patients who did not return for visit 2.
Comparison of patients at visit 1 with patients who did return for visit 2.
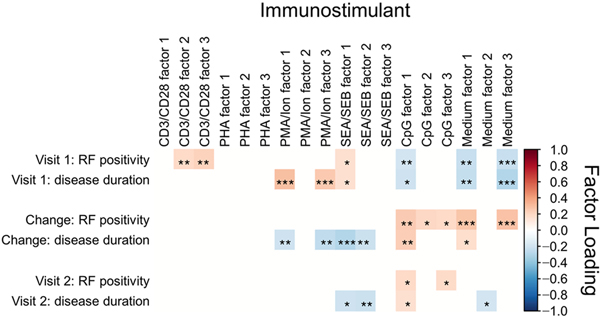
The scores for the 3 factors for each stimulant and for each cytokine-stimulant combination were tested for significant correlations (P<.05) with the presence of RF (Figure 2 and Table 4). At visit 1, the factor 2 and factor 3 scores for CD3/CD28 and the factor 1 score for SEA/SEB correlated positively with RF positivity. On the contrary, the factor 1 score for CpG and the factor 1 and factor 3 scores for incubation in medium alone correlated negatively with RF positivity (Figure 2). The correlations of RF positivity and factor scores for different stimuli were reflected in correlations between RF and scores from individual cytokine-stimulant combinations (Table 4). All the cytokines that were stimulated by T-cell activators and were significantly correlated with RF correlated positively with RF positivity, with CD3/CD28 and SEA/SEB being most prominent. Multiple cytokines that were produced by cells activated by CD3/CD28 and SEA/SEB were positively associated with RF positivity. Interestingly, most of these cytokines are predicted to be produced by TH2 and TH17 cells (Table 2). However, the majority of the cytokines that loaded factor 1 for CpG and factors 1 and 3 for medium alone correlated negatively with RF positivity. Similar trends were observed for associations between disease duration and factor scores and specific cytokine-stimulant combinations (Figure 2 and Table 5). As in associations with RF positivity, levels of cytokines stimulated by T-cell stimulants were positively associated with disease duration at visit 1. Virtually all these individual cytokines were driven by activation with PMA-ionomycin and SEA/SEB. Conversely, cytokines stimulated by incubation in CpG and medium alone correlated negatively with disease duration at visit 1, with the exception of IL-12 and IL-8, which were stimulated by CpG but had not contributed to any of the 3 factors.
Figure 2.
Associations Between Rheumatoid Factor (RF) and Disease Duration and Scores From Cytokine-Loaded Factors Associated With Experimental Activation. Associations ranged from positive (red) to negative (blue) for visit 1, visit 2, and the change between visits. * indicates P<.05; **, P<.01; ***, P<.001; CD3/CD28, anti-CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; Medium, RPMI 1640 medium with 10% fetal calf serum; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B.
Table 4.
Associations Between Rheumatoid Factor Positivity and Cytokine Expression After Activation of PBMCs With Immunostimulants
| Association | |||
|---|---|---|---|
| Time | Stimulant | Positive | Negative |
| Visit | CD3/CD28 | Factor 1* . factor 2**, factor 3**, IL-4* IL-5**. IL-17I*, GM-CSD* | … |
| PHA | … | … | |
| PMA/Ion | IL-6* | … | |
| SEA/SEB | Factor 1*,IL-4* IL-5*, IL-13*, GM-CSF* | … | |
| CpG | … | Factor 1**. IL-lβ**, TNF-α**, MIP-lβ** | |
| Medium | … | Factor 1**, factor 3***, G-CSF*, IL-1B*, TNF-α**, IL-6**, IL-17***, GM-cCSF**, MCP-1** | |
| Change between visits | CD3/CD28 | … | IL-5*, IL-17*, GM-CSF* |
| PHA | … | … | |
| PMA/Ion | … | IL-6*, GM-CSF* | |
| SEA/SEB | … | GM-CSDF* | |
| CpG | Factor 1**, factor 2*, factor 3*, G-CSF**, IFN-γ*, IL-lβ**, TNF-α**, MIP-lβ*** GM-CSF*a | … | |
| Medium | Factor 1***, factor 3***, G-CSF*, IFN-γ**, IL-lβ***, TNF-α. IL-6**, IL-17**, GM-CSF**, MCP-1**, MIP-lβ**, IL-2*a, IL-10**a | … | |
| Visit 2 | CD3/CD28 | … | … |
| PHA | … | … | |
| PMA/Ion | … | … | |
| SEA/SEB | … | … | |
| CpG | Factor 1*. factor 3*, G-CSF*, TNF-α* | … | |
| Medium | IFN-γ*, IL-lβ***, TNF-α*, MCP-1*, IL-2*, IL.−10*a | … | |
Abbreviations: CD3/CD28, anti-CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; Medium, RPMI 1640 medium with 10% fetal calf serum; MIP, macrophage inflammatory protein; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B; TNF, tumor necrosis factor.
Cytokine that was not loaded into a factor at 0.5 or more.
P<.05.
P≤.01.
P≤.001.
Table 5.
Associations Between Disease Duration and Cytokine Expression After Activation of PBMCs With Immunostimulants
| Association | |||
|---|---|---|---|
| Time | Stimulant | Positive | Negative |
| Visit 1 | CD3/CD28 | IL-5** | … |
| PHA | … | … | |
| PMA/Ion | Factor 1**, factor 3***, IL-10**, IL-12**, IL-6***, IL-17***, GM-CSF**, IL-4**,IL-5**, MIP-1β***a | ||
| SEA/SEB | Factor 1*, IL-5***, IL-17*, GM-CSF*, MCP-1*a | … | |
| CpG | IL-12*a, IL-8*a | Factor 1*, G-CSF**, IL-1β*, MIP-1β*, IL-17**a | |
| Medium | … | Factor 1**, factor 3***, G-CSF**, IFN-γ*, IL-1β**, IL-5***, TNF-α**, IL-6***, IL-17***, GM-CSF*, MCP-1*, IL-2*a, IL-10**a | |
| Change between visits | CD3/CD28 | … | IL-5**, GM-CSF** |
| PHA | IL-17* | … | |
| PMA/Ion | … | Factor 1**, factor 3**, IL-10*, IL-6***, IL-17***, GM-CSF***, IFN-γ*a, MIP-1β**a,IL-4** | |
| SEA/SEB | Factor 1***, factor 2**, IL-1β**, IL-17*, TNF-α**, IL-5*, GM- CSF***, MIP-1β*, IFN-Y**a, MCP-1*a | ||
| CpG | Factor 1**, G-CSF*, MIP-1β*, IL-17*a | … | |
| Medium | Factor 1*, G-CSF*, IL-1β***, IL-5*, TNF-α*, IL-6***, IL-17***, GM-CSF*, MCP-1**, MIP-1β***, IL-10*a | … | |
| Visit 2 | CD3/CD28 | … | GM-CSF* |
| PHA | … | IFN-γ**, IL-13* | |
| PMA/Ion | … | IL-6***, GM-CSF*, MIP-1β*a | |
| SEA/SEB | … | Factor 1*, factor 2**, IL-1β**, GM-CSF***, LFN-γ***a | |
| CpG | Factor 1* | IFN-γ***, IL-5* | |
| Medium | IL-1β*, IL-6**, IL-17*, GM-CSF**, MIP-1β** | Factor 2* | |
Abbreviations: CD3/CD28, anti-CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; Medium, RPMI 1640 medium with 10% fetal calf serum; MIP, macrophage inflammatory protein; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B; TNF, tumor necrosis factor.
Cytokine that was not loaded into a factor at 0.5 or more.
P<.05.
P≤.01.
P≤.001.
Changes in Cytokine Expression Over 5 Years
Comparison of the starting and ending values over the 5-year interval showed significant (P<.05) changes in 12 of the 18 factors. These changes fell into 2 general groups: changes of 4 to 18 units (7 factors) and shifts of 19 or more units (5 factors) (Table 3). Identification of the 3 highest factors for each immunostimulant facilitated the identification of significant shifts in expression of different sets of cytokines. The greatest downward changes were observed with factor 1 for 4 of the 5 immunostimulants: CD3/CD28, PHA, PMA-ionomycin, and CpG. As shown in Figure 1 and Table 2, factor 1, which was involved with the greatest variance, was associated with subsets of cytokines produced by multiple subsets of T cells and B cells. The greatest increases in scores were observed with factor 2 for PMA-ionomycin and CpG that were associated with activated APCs and B cells. The other changes with factors 2 and 3 were low-percentage changes. As a group, the median scores for factors involved in stimulation of T-cell subsets decreased significantly over the 5-year interval (Table 3). The 1 exception was factor 2 for CD3/CD28 activation that appeared to be associated with cytokine expression by TH17 cells (Table 2); the score for this factor significantly increased over 5 years in opposition to the greater decrease of the factor 1 score, which represented TH1/CD8 cytokine expression (Table 2). These results suggest that changes of cytokine expression were dependent both on responding cell types and on specific immunostimulants.
Correlations With Disease Characteristics Over 5 Years
When the relationships between presence of RF and disease duration and levels of cytokine expression over the 5 years between visits were investigated, no significant changes in factor scores for T-cell stimulants correlated with RF positivity (Figure 2 and Table 4). The changes in scores for the 6 cytokine-stimulant combinations that were significantly correlated with RF were negative correlations. However, the changes in scores with 5 of the 6 factors for CpG and medium alone were positively associated with RF positivity, and these positive correlations were also seen at the level of individual cytokine-stimulant combinations (Figure 2 and Table 4). In addition to cytokine-stimulant combinations that contributed most strongly to factors, changes in IL-2 and IL-10 expression with CpG stimulation and incubation in medium alone positively correlated with RF positivity. The dichotomy between changes in cytokines stimulated by T-cell stimulants compared with CpG and medium alone is exemplified by changes in GM-CSF expression between the visits. Changes in GM-CSF expression driven by T-cell stimulants negatively associated with RF positivity, but changes in GM-CSF expression driven by CpG and medium alone positively assoc iated with RF.
The opposing associations of RF with changes in expression of cytokines resulting from T-cell stimulants compared with CpG and medium alone were corroborated by associations between RF status and expression of cytokines at visit 2 (Figure 2 and Table 4). There were no significant associations between scores of cytokines driven by any of the T-cell stimulants and RF, which follows the negative associations between RF positivity and changes between visits in T-cell-stimulated cytokine expression (Table 4). Scores for 6 cytokine-stimulant combinations in CpG stimulation and medium alone incubation were significantly associated with RF, and, in each case, they were positively associated. This trend was consistent with the positive associations between RF and scores for changes between visits in expression of cytokines that were driven by these 2 incubation conditions. In addition, expression of IL-2 and IL-10 from incubation in medium alone was positively associated with RF at visit 2, which is consistent with their changes over the 5 years between visits. In summary, significant associations of RF positivity and expression of cytokines stimulated by T-cell activators compared with CpG stimulation and incubation in medium alone changed in opposing directions through the 5-year interval between visits.
Similar trends were observed for associations between disease duration and specific cytokine-stimulant combinations (Figure 2 and Table 5). Changes over 5 years in T-cell-stimulant-driven cytokines negatively correlated with disease duration, and cytokines stimulated by CpG and medium alone positively correlated with disease duration. These directions of association were also apparent for the cytokines at visit 2 with the exception that the only 2 cytokines driven by CpG were negatively associated with disease duration (Figure 2 and Table 5). As was the case with RF, the association of GM-CSF with disease duration was determined by the stimuli and activated cells and not the cytokine.
The concordant associations of multiple cytokine-stimulant combinations in positive or negative relationships with RF positivity and disease duration were not consistently observed with other patient characteristics. For immunosuppressive treatment regimens, relatively few associations existed between expression of cytokines and use of glucocorticoids and biologic treatments at either visit. However, there were increased numbers of associations with methotrexate use, with the majority of those associations limited to factors and individual cytokines driven by CD3/CD28 stimulation (Table 6). The associations between methotrexate use and factors and cytokines driven by T-cell stimulants were all negative at visit 1, suggesting that methotrexate had suppressed responses to these stimulants. However, during the 5 years between visits, these associations were reduced in number and were generally positive. Associations between history of smoking and cytokine levels were focused on those expressed by PBMCs collected at visit 2 and stimulated with PHA and medium alone. However, there were virtually no associations between smoking and cytokine expression at visit 1 or changes in expression between visits. Likewise, there were even fewer cytokine associations with scores of patient self-assessments of disease activity.
Table 6.
Associations Between Methotrexate Use and Cytokine Expression After Activation of PBMCs With Immunostimulants
| Association | |||
|---|---|---|---|
| Time | Stimulant | Positive | Negative |
| Visit 1 | CD3/CD28 | … | Factor 1*, factor 2*, factor 3*, IL-5**, IL-10*, IL-13*, IL-6**, GM-CSF*, MCP-1*a, MIP-1β* |
| PHA | … | … | |
| PMA/Ion | … | Factor 3**, IL-5***, IL-13** | |
| SEA/SEB | … | IL-6** | |
| CpG | … | IL-10*a | |
| Medium | IL-6*a | … | |
| Change between visits | CD3/CD28 | Factor 1*, factor 2**, Factor 3*, IL-8*a IL-2* | IL-8*a |
| PHA | … | … | |
| PMA/Ion | Factor 3* | ||
| SEA/SEB | … | … | |
| CpG | Factor 2*, factor 3*, IFN-γ*, IL-5*, MCP-1*a | … | |
| Medium | … | … | |
| Visit 2 | CD3/CD28 | Factor 1*, factor 2*, factor 3*, IL-1β***, IL-2* | IL-5*, IL-8*a |
| PHA | … | … | |
| PMA/Ion | … | … | |
| SEA/SEB | … | … | |
| CpG | Factor 2*, IFN-γ*. | ||
| Medium | … | … | |
Abbreviations: CD3/CD28, anti-CD3 and anti-CD28 microbeads; CpG, cytosine-phosphate-guanine oligonucleotide ODN2006; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; Medium, RPMI 1640 medium with 10% fetal calf serum; MIP, macrophage inflammatory protein; PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin; PMA/Ion, phorbol 12-myristate 13-acetate–ionomycin; SEA/SEB, staphylococcal enterotoxins A and B; TNF, tumor necrosis factor.
Cytokine that was not loaded into a factor at 0.5 or more.
P<.05.
P≤.01.
P≤.001.
Discussion
The experiments presented here monitored a panel of cytokines expressed by stimulated PBMCs from patients with RA before and after a 5-year interval to identify changes in cytokine expression that could be associated with disease characteristics. Factor analysis was incorporated into this study to identify the 3 highest scoring factors for each stimulant and the groups of cytokines that most significantly loaded each factor, thereby representing the cell types that responded to each stimulant. Scores for the majority of factors (12 of 18) significantly changed during the study period, and scores from factors for a single stimulant did not concordantly change, suggesting that the cell types underlying single factors increased or decreased their activation capacities with some level of independence. Further complexity was revealed by divergent changes in scores for factors that appear to be associated with single lymphoid cell types but are activated with different immunostimulants. One explanation is that changes in cytokine expression may be based partly on changes in numbers of responsive cells. Further investigations are necessary to assess the contribution of changes in sizes of lymphoid subpopulations to changes in cytokine expression over time in patients with RA.
The focus of this analysis was on the function of cytokine production rather than cell numbers since such numbers do not necessarily predict function. A meaningful analysis incorporating cell numbers would have required intracellular staining and flow cytometry, which would have been difficult with 17 cytokines. However, future investigations could be feasible for a study of limited numbers of cytokines, stimulants, and patients to assess the contribution of changes in sizes of lymphoid subpopulations to changes in cytokine expression in patients with RA.
Factor scores and levels of individual cytokines expressed by activated PBMCs were analyzed to identify associations with disease variables. These comparisons were exploratory, so the investigative team did not believe that adjustment of P values for multiple comparisons was warranted in this study. According to a Bonferroni adjustment, P less than .0024 (ie, .05/21=0024) would be significant for 7 stimulants and 3 factors each, so it was reasoned that such an adjustment would be overly conservative, considering the correlated nature of cytokine expression and the hypothesis-generating nature of this study. The largest numbers of associations with disease variables were observed with RF, disease duration, and methotrexate use. Negative associations of methotrexate with levels of cytokines following activation with T-cell stimulants, most notably CD3/CD28, at visit 1 are consistent with the immunosuppressant effects of methotrexate on cytokine expression by T cells [22].
Factor scores and levels of individual cytokines expressed by activated PBMCs showed 2 groups of associations with RF positivity and disease duration: those involving cytokines expressed after activation with T-cell stimulants and those expressed after CpG activation and incubation in medium alone. Positive associations for T-cell-stimulant-driven cytokines and RF and disease duration were observed at visit 1, but those associations were lost during the 5-year study period. Conversely, cytokines driven by CpG and medium alone started with negative associations at visit 1 and evolved to positive associations at visit 2. The concordant trends of cytokine expression between PBMCs incubated in CpG and medium alone suggested that either 1) the 2 incubation conditions stimulated similar or identical lymphoid populations in vitro or 2) the in vivo conditions before PBMC harvests drove activation states similar to those driven by CpG and did not require further stimulation in cultures with medium alone.
Although medium with FCS was used as a negative control under the assumption that it would support only the expression of cytokines that were expressed by PBMCs at the time of their collection without further activation, the FCS may be an immunostimulant and not just a nutrient source. FCS has long been known to increase in vitro responses of human PBMCs in relation to human AB serum [23]. Since both AB serum and heat- inactivated FCS contain nutrients, growth factors, and cytokines [24], it is likely that the superiority of heat-inactivated FCS may reflect other components, including 1) heat-aggregated immunoglobulins that activate cells through Fc receptor binding [25–27]; 2) lipopolysaccharide that activates cells by binding toll-like receptor (TLR)4 [28, 29]; 3) Mycoplasma bovis DNA, which signals through TLR9, and lipoprotein, which signals through TLR2 [30]; and 4) damage-associated molecules, such as heparan sulfate and hyaluronan fragments, that bind to multiple TLRs [31, 32]. The inability to identify specific immunostimulants in medium with FCS and their “target” cell types complicated cell type assignments for this incubation condition. However, the immunostimulatory potential of FCS and the concordant transitions of cytokines expressed after incubation in CpG and medium alone from visit 1 to visit 2 suggest that these 2 incubation conditions may have primarily activated similar cell types through congruent signaling pathways.
CpG and medium alone may stimulate through comparable mechanisms, but that does not answer why RF positivity was associated with 1) increased cytokine expression driven by medium alone and CpG and 2) decreased expression driven by T-cell stimulants over the 5-year study period. The answer may lie in the specificity and functional activity of RF, which is an anti-immunoglobulin (Ig)G autoantibody that, like other antibodies, starts as IgM with low affinity both in people who are healthy and in people with RA. However, unlike RF in healthy people, RF in patients with RA undergoes class switching and affinity maturation [33–35]. RF expression precedes the diagnosis of RA and may be present for 10 years before diagnosis, but the timing of changes in affinity in relation to the time of diagnosis is unclear [36, 37]. Several lines of evidence have implicated T cells in the generation and maturation of RF: 1) Class switching and somatic mutation at the IgG binding site of an RF antibody suggest T-cell help [38, 39]. 2) The range of potential T-cell help for RF production is expanded by the binding of immune complexes containing IgG and their previously bound antigens to RF-expressing B cells for antigen presentation to T cells that are specific for the bound antigens [40].
RF-producing B-cell populations are dynamic with continual turnover and net increases in affinity. Increases in RF affinity should increase the concentrations of immune complexes that contain RF and IgG since RF molecules in immune complexes have higher avidity than those that are free in autologous serum [35]. The increases in immune complexes should increase activation of B cells and monocytes through binding to Fc receptors [41, 42]. Such increased activation states have been observed in patients with RA who have increases in monocyte-derived cytokines, in particular tumor necrosis factor-α [42–44]. The increased activation states in these patients are accompanied by increases in expression of TLRs. At least in monocyte and macrophage populations, increased expression is observed for TLR2, TLR3, TLR4, and TLR9 [45–47]. The interplay between TLRs and Fc receptors is further shown by the upregulation of Fc receptors by TLR engagement [48] and alteration of TLR-stimulated cytokine expression by the binding of heat-aggregated Ig by monocytes [49]. We hypothesize that continued exposure to RF-containing immune complexes results in increased responsiveness to TLR ligands that is consistent with the increased cytokine production observed with CpG and medium alone and PBMCs from RF-positive patients at visit 2 compared with visit 1. In addition, this proposed heightening of B-cell activation could result in increased production of antibodies, including RF that is available for formation of immune complexes to continue the cycle of B-cell activation in a feedback loop.
We propose the following scenario based on the role of T-helper cells in the initial stages of RF selection and class switching and the increased responsiveness of monocytes and B cells during our 5-year study period. Even at the stage of disease when the patients with RA were enrolled (median, 9.7 years), T-cell responsiveness to the group of T-cell immunostimulants was associated with RF positivity because of the role of T cells in helping anti-IgG RF production. However, over the 5 years, when the majority of patients received immunosuppressive disease-modifying drugs, there was a trend of dissociation of T-cell reactivity from the RF status, suggesting that T cells were no longer required for RF production. The increased association of RF and cytokines produced by B cells and monocytes in response to CpG and FCS over 5 years suggests that B cells, including those producing RF, may have lost their dependence on T cells. TLR agonists can maintain polyclonal memory B-cell populations [50], and complexes between a patient’s own IgG and DNA effectively activate B cells without T-cell help [51], which could account for an increased association between RF positivity and B-cell and monocyte responses vis-à-vis the decreased association between T-cell responses and RF positivity.
The potential importance of innate immune responses in perpetuating RA should provide the impetus for more refined analysis of the relationships between cytokine responses ofB cells and monocytes and disease duration and characteristics of the disease. Analyses should involve greater resolution with purified lymphoid populations and expanded panels of immunostimulants and cytokines. Support for such a targeted approach for B cells is provided by results with use of an antibody specific for CD20+ B cells (rituximab) that has increased the efficacy of immunosuppression with improvement in RA symptoms compared with treatment with methotrexate (with or without glucocorticoids) alone [52–54]. Further, rituximab has been shown to effectively deplete activated B cells, which could be the products of sustained stimulation by RF-containing immune complexes [55]. Therapeutic success with rituximab may be related in part to these mechanisms. Two points of interest are 1) how other available synthetic and biologic disease-modifying therapies may regulate this nexus and affect disease activity and the disease process at the level of the synovium and other affected tissues, such as the lung, and 2) the therapeutic action that directly interferes with B-cell stimulation.
Conclusions
Co-associations of immune responses to CpG and medium alone and RF expression suggest that CpG and FCS in medium similarly drive innate immune responses in patients with RA. Our findings suggest that sustained RF expression over time increases the activation of B cells and monocytes without requirements for T-cell functions, with important implications for the role of B-cell-targeted therapy. The results of this study may be important in the development of functional assays for CpG-mediated and other TLR-mediated immune responses as predictors of response to rituximab in patients with RA.
Highlights.
Cytokines were associated with rheumatoid factor and rheumatoid arthritis duration.
Cytokine profiles switched from T-cell to B-cell cytokines over 5 years.
Cytosine-phosphate-guanine response profiles suggested an innate immune response.
Sustained rheumatoid factor expression likely activates B cells and monocytes.
Acknowledgments
Financial Support
This work was supported by a grant from the National Institutes of Health, NIAMS (R01 AR46849), and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Role of the Funding Source
The sponsor had no role in the design or conduct of this study, the analysis or interpretation of the results, or the decision to submit this article for publication.
Abbreviations
- APC
antigen-presenting cell
- CpG
cytosine-phosphate-guanine
- FCS
fetal calf serum
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HAQ
Health Assessment Questionnaire
- Ig
immunoglobulin
- IL
interleukin
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- PBMC
peripheral blood mononuclear cell
- PHA
phytohemagglutinin
- PMA
phorbol 12-myristate 13-acetate
- RA
rheumatoid arthritis
- RAPID3
Routine Assessment of Patient Index Data 3
- RF
rheumatoid factor
- SEA
staphylococcal enterotoxin A
- SEB
staphylococcal enterotoxin B
- TH
helper T cell
- TLR
toll-like receptor
Appendix.
Assignment of Lymphoid Cell Types That Produced Combinations of Cytokines That Loaded the Top 3 Factors for Each Immuno stimulant
The objective of factor analysis of complex data sets is the identification of a limited number of underlying factors that most effectively explain the variation in experimental variables. For the cytokine expression levels reported in the present study, these factors should represent the lymphoid cell types that responded to individual immunostimulants and produced the cytokines that were most strongly associated with the respective factors. We attempted to identify cell types that correlated with the 3 highest factors for each stimulant by considering the immuno stimulant and the cell types that have been reported to produce that cytokine when presented with the specific stimulant. We also considered that our 48-hour cultures could show cytokine production by both primarily and secondarily activated cells. Our approach to deducing responding cell types aimed at selecting the minimal number of cell types that could account for the cytokine grouping associated with each factor.
CD3/CD28 microbeads stimulate T cells by cross-linking T-cell receptors and CD28 coreceptors. Factor 1 for CD3/CD28 stimulation appears to represent helper T cell (TH)1 and CD8+ T cells according to expression of interferon-γ, interleukin (IL)-2, and tumor necrosis factor a [1]. The inclusion of IL-4 in this factor may reflect the expression of IL-4 by a subset of CD8+ T cells that are increased in elderly patients [2], and IL-10 can be produced by TH1 cells in addition to other cell types [3]. IL-12 expression in antigen-presenting cells can be regulated in a feedback loop by interferon-γ [4, 5]. Factor 2 loadings are consistent with cytokines produced by TH17 cells or cells that are activated by IL-17. Activated TH17 cells produce IL-17 that subsequently stimulates production of a wide range of cytokines, including IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage inflammatory protein 1β, that are strongly associated with factor 2 [6-8]. Factor 3 for CD3/CD28 includes only IL-5 and IL-13, which are produced by TH2 cells [9]. Therefore, the 3 factors for CD3/CD28 activation appear to be distinguished by activation of TH1/CD8+, which stimulates TH17, which stimulates TH2 cells.
There are important differences between the functions of T-cell subsets that are activated by CD3/CD28 microbeads and polyhydroxyalkanoate [10, ii]; these differences may be affected largely by the variation in CD28 expression in memory T-cell subsets with age-related increases in CD28− T cells that would not be coactivated by anti-CD28 [12, 13]. The factors for polyhydroxyalkanoate stimulation were loaded with T- cell cytokines, but the 3 highest factors did not appear to be distinguished by different T-cell subsets. Factor i included cytokines produced by several T - cell subsets, including TH1, TH2, TH17, and CD8+ T cells. In addition to T-cell-derived cytokines, factor 1 was associated with cytokines, including granulocyte-macrophage colony-stimulating factor (G-CSF), IL-1β, and monocyte chemoattractant protein (MCP)-1, produced by antigen-presenting cells (APCs) that can be stimulated by primary T-cell activation [14-16]. Likewise, factor 2 included cytokines that can be produced by activated TH1, TH2, and CD8+ T cells. As with CD3/CD28 activation, factor 3 included IL-5 and IL-13 that are produced by TH2 cells.
Phorbol 12-myristate 13-acetate (PMA)-ionomycin stimulates T cells and monocytes through induction of protein kinases in mitogenic pathways. PMA-ionomycin-activated T cells express cytokines that overlap with those produced by T cells activated by CD3/CD28 [17], and monocytes activated by PMA-ionomycin can express diverse cytokines [18, 19]. Factor 1 was principally associated with cytokines produced by TH17 and TH2 cells, and factor 3 was associated with TH2 cytokines. Factor 2 included cytokines predicted to be expressed by activated APCs whether they be secondarily stimulated by T cells or directly stimulated by PMA-ionomycin.
Finally, staphylococcal enterotoxins A (SEA) and B (SEB) superantigens stimulate CD4+ T cells by cross-linking major histocompatibility complex class II molecules on APCs to specific T-cell receptor β chains [20] and APCs through toll-like receptor (TLR) activation [21]. Factor 1 for SEA and SEB was strongly associated with TH2 cytokines, and factor 2 included multiple cytokines that can be produced by activated T cells and APCs. Factor 3 was less clear in its association with IL-2 and IL-10, which can be produced by multiple T-cell subsets. Therefore, the activation of T cells by the 4 T-cell-specific stimulants is mediated by different mechanisms, resulting in the expression of partially overlapping groups of T-cell–associated cytokines and fewer cytokines expressed by secondarily activated APCs.
In addition to the stimulants for T-cell activation, cytosine-phosphate-guanine (CpG) was selected to activate B cells and other innate immune cells in peripheral blood mononuclear cells (PBMCs). The CpG-B 2006 oligonucleotide directly activates B cells that express TLR9; activated B cells can subsequently activate monocytes that are, by themselves, nonresponsive to CpG-B 2006 [22, 23]. We have interpreted the associations between cytokines and the 3 factors associated with CpG stimulation under the assumption that B cells comprise the primary cell type that responded to CpG. With factor 1, CpG-stimulated B cells have been shown to express tumor necrosis factor α, IL-6, and macrophage inflammatory protein 1β [24–26], and, consequently, secondarily activated monocytes can produce G-CSF and IL-1β [15, 27]. Although the cytokines that are strongly included in factor 2 and factor 3 are generally associated with TH1 and TH2 cells, all of them can be produced by polyclonally stimulated effector B cells that have been polarized in previous encounters with the respective TH cell subsets [28, 29].
Although PBMCs produced cytokines when incubated in medium alone, the lack of identified stimulants or the potentially continued production of cytokines by PBMCs from their in vivo states complicated cell type assignments for the cytokines that loaded the top 3 factors. Therefore, we made assignments that were generally broad and focused on the cell types that are conventionally associated with the respective cytokines.
References
- [1].Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR, Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells, J. Virol, 81 (2007) 8468–8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schwaiger S, Wolf AM, Robatscher P, Jenewein B, Grubeck-Loebenstein B, IL-4-producing CD8+ T cells with a CD62L++(bright) phenotype accumulate in a subgroup of older adults and are associated with the maintenance of intact humoral immunity in old age, J. Immunol, 170 (2003) 613–619. [DOI] [PubMed] [Google Scholar]
- [3].Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A, Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose, Immunity, 31 (2009) 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML, High-level IL-12 production by human dendritic cells requires two signals, Int. Immunol, 10 (1998) 1593–1598. [DOI] [PubMed] [Google Scholar]
- [5].Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G, The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells, J. Exp. Med, 183 (1996) 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T, Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction, Immunity, 29 (2008) 628–636. [DOI] [PubMed] [Google Scholar]
- [7].Cheung PF, Wong CK, Lam CW, Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation, J. Immunol, 180 (2008) 5625–5635. [DOI] [PubMed] [Google Scholar]
- [8].Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS, Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids, J. Exp. Med, 211 (2014) 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wynn TA, Type 2 cytokines: mechanisms and therapeutic strategies, Nat. Rev. Immunol, 15 (2015) 271–282. [DOI] [PubMed] [Google Scholar]
- [10].Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U, Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function, J. Immunol. Methods, 293 (2004) 127–142. [DOI] [PubMed] [Google Scholar]
- [11].Duarte RF, Chen FE, Lowdell MW, Potter MN, Lamana ML, Prentice HG, Madrigal JA, Functional impairment of human T- lymphocytes following PHA-induced expansion and retroviral transduction: implications for gene therapy, Gene Ther, 9 (2002) 1359–1368. [DOI] [PubMed] [Google Scholar]
- [12].Zhang X, Nakajima T, Goronzy JJ, Weyand CM, Tissue trafficking patterns of effector memory CD4+ T cells in rheumatoid arthritis, Arthritis Rheum, 52 (2005) 3839–3849. [DOI] [PubMed] [Google Scholar]
- [13].Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, Di Pede P, Passeri G, Pedrazzoni M, Passeri M, Franceschi C, Sansoni P, Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence, Exp. Gerontol, 38 (2003) 981–987. [DOI] [PubMed] [Google Scholar]
- [14].Gruaz L, Delucinge-Vivier C, Descombes P, Dayer JM, Burger D, Blockade of T cell contact-activation of human monocytes by high-density lipoproteins reveals a new pattern of cytokine and inflammatory genes, PLoS One, 5 (2010) e9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Demetri GD, Griffin JD, Granulocyte colony-stimulating factor and its receptor, Blood, 78 (1991) 2791–2808. [PubMed] [Google Scholar]
- [16].Deshmane SL, Kremlev S, Amini S, Sawaya BE, Monocyte chemoattractant protein-1 (MCP-1): an overview, J. Interferon Cytokine Res, 29 (2009) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Olsen I, Sollid LM, Pitfalls in determining the cytokine profile of human T cells, J. Immunol. Methods, 390 (2013) 106–112. [DOI] [PubMed] [Google Scholar]
- [18].Foey AD, Brennan FM, Conventional protein kinase C and atypical protein kinase Czeta differentially regulate macrophage production of tumour necrosis factor-alpha and interleukin-10, Immunology, 112 (2004) 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang C, Ting AT, Seed B, PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines, Nature, 391 (1998) 82–86. [DOI] [PubMed] [Google Scholar]
- [20].Li H, Llera A, Malchiodi EL, Mariuzza RA, The structural basis of T cell activation by superantigens, Annu. Rev. Immunol, 17 (1999) 435–466. [DOI] [PubMed] [Google Scholar]
- [21].Kissner TL, Ruthel G, Alam S, Ulrich RG, Fernandez S, Saikh KU, Activation of MyD88 signaling upon staphylococcal enterotoxin binding to MHC class II molecules, PLoS One, 6 (2011) e15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krieg AM, CpG motifs in bacterial DNA and their immune effects, Annu. Rev. Immunol, 20 (2002) 709–760. [DOI] [PubMed] [Google Scholar]
- [23].Agrawal S, Gupta S, TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors, J. Clin. Immunol, 31 (2011) 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wagner M, Poeck H, Jahrsdoerfer B, Rothenfusser S, Prell D, Bohle B, Tuma E, Giese T, Ellwart JW, Endres S, Hartmann G, IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA, J. Immunol, 172 (2004) 954–963. [DOI] [PubMed] [Google Scholar]
- [25].Tasker L, Marshall-Clarke S, Functional responses of human neonatal B lymphocytes to antigen receptor cross-linking and CpG DNA, Clin. Exp. Immunol, 134 (2003) 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, Birmachu W, Comparison of human B cell activation by TLR7 and TLR9 agonists, BMC Immunol, 9 (2008) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Danis VA, Kulesz AJ, Nelson DS, Brooks PM, Cytokine regulation of human monocyte interleukin-1 (IL-1) production in vitro. Enhancement of IL-1 production by interferon (IFN) gamma, tumour necrosis factor-alpha, IL-2 and IL-1, and inhibition by IFN-alpha, Clin. Exp. Immunol, 80 (1990) 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE, Reciprocal regulation of polarized cytokine production by effector B and T cells, Nat. Immunol, 1 (2000) 475–482. [DOI] [PubMed] [Google Scholar]
- [29].Hajoui O, Janani R, Tulic M, Joubert P, Ronis T, Hamid Q, Zheng H, Mazer BD, Synthesis of IL-13 by human B lymphocytes: regulation and role in IgE production, J. Allergy Clin. Immunol, 114 (2004) 657–663. [DOI] [PubMed] [Google Scholar]
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Ethics Approval
The Mayo Clinic and Olmsted Medical Center institutional review boards approved this study, which was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Publisher: To expedite proof approval, send proof via email to scipubs @mayo.edu.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John M. Davis, III, Division of Rheumatology, Mayo Clinic, Rochester, Minnesota.
Cynthia S. Crowson, Division of Rheumatology, Mayo Clinic, Rochester, Minnesota; Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
Keith L. Knutson, Department of Immunology, Mayo Clinic, Jacksonville, Forida..
Sara J. Achenbach, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota
Michael A. Strausbauch, Immunochemical Core Laboratory, Mayo Clinic, Rochester, Minnesota
Terry M. Therneau, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
Eric L. Matteson, Division of Rheumatology, Mayo Clinic, Rochester, Minnesota.
Sherine E. Gabriel, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota.
Peter J. Wettstein, Department of Surgery, Mayo Clinic, Rochester, Minnesota.
References
- [1].Wolfe F, Rasker JJ, Boers M, Wells GA, Michaud K, Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis, Arthritis Rheum, 57 (2007) 935–942. [DOI] [PubMed] [Google Scholar]
- [2].Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, Hensor E, Wakefield RJ, O’Connor PJ, Emery P, An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis, Arthritis Rheum, 58 (2008) 2958–2967. [DOI] [PubMed] [Google Scholar]
- [3].Davis JM 3rd, Knutson KL, Strausbauch MA, Crowson CS, Therneau TM, Wettstein PJ, Matteson EL, Gabriel SE, Analysis of complex biomarkers for human immune-mediated disorders based on cytokine responsiveness of peripheral blood cells, J. Immunol, 184 (2010) 7297–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Davis JM, Knutson KL, Strausbauch MA, Green AB, Crowson CS, Therneau TM, Matteson EL, Gabriel SE, Immune response profiling in early rheumatoid arthritis: discovery of a novel interaction of treatment response with viral immunity, Arthritis Res. Ther., 15 (2013) R199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Davis III JM, Knutson KL, Strausbauch MA, Crowson CS, Myasoedova E, Therneau TM, Gabriel SE, Cytokine Response Profiling Identifies An Immunologic Signature of Myocardial Dysfunction in Rheumatoid Arthritis, Arthritis Rheum, 60 (2009) 592–593.19180474 [Google Scholar]
- [6].Ceuppens JL, Baroja ML, Lorre K, Van Damme J, Billiau A, Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal, J. Immunol, 141 (1988) 3868–3874. [PubMed] [Google Scholar]
- [7].Davis JM 3rd, Knutson KL, Skinner JA, Strausbauch MA, Crowson CS, Therneau TM, Wettstein PJ, Matteson EL, Gabriel SE, A profile of immune response to herpesvirus is associated with radiographic joint damage in rheumatoid arthritis, Arthritis Res. Ther, 14 (2012) R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maradit Kremers H, Crowson CS, Gabriel SE, Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases, Rheum. Dis. Clin. North Am, 30 (2004) 819–834, vii. [DOI] [PubMed] [Google Scholar]
- [9].Kruisbeek AM, Shevach E, Thornton AM, Proliferative assays for T cell function, Curr. Protoc. Immunol., Chapter 3 (2004) Unit 3 12. [DOI] [PubMed] [Google Scholar]
- [10].Godoy-Ramirez K, Franck K, Mahdavifar S, Andersson L, Gaines H, Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry, J. Immunol. Methods, 292 (2004) 1–15. [DOI] [PubMed] [Google Scholar]
- [11].Davis III JM, Knutson KL, Strausbauch MA, Crowson CS, Therneau TM, Matteson EL, Gabriel SE, An Immune Signature Based on Ex Vivo Responsiveness of Peripheral Blood Cells Is Associated with Radiographic Joint Damage in Rheumatoid Arthritis, Arthritis Rheum, 62 (2010) S657. [Google Scholar]
- [12].Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U, Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function, J. Immunol. Methods, 293 (2004) 127–142. [DOI] [PubMed] [Google Scholar]
- [13].Duarte RF, Chen FE, Lowdell MW, Potter MN, Lamana ML, Prentice HG, Madrigal JA, Functional impairment of human T-lymphocytes following PHA-induced expansion and retroviral transduction: implications for gene therapy, Gene Ther, 9 (2002) 1359–1368. [DOI] [PubMed] [Google Scholar]
- [14].Olsen I, Sollid LM, Pitfalls in determining the cytokine profile of human T cells, J. Immunol. Methods, 390 (2013) 106–112. [DOI] [PubMed] [Google Scholar]
- [15].Holzer U, Orlikowsky T, Zehrer C, Bethge W, Dohlsten M, Kalland T, Niethammer D, Dannecker GE, T-cell stimulation and cytokine release induced by staphylococcal enterotoxin A (SEA) and the SEAD227A mutant, Immunology, 90 (1997) 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gruaz L, Delucinge-Vivier C, Descombes P, Dayer JM, Burger D, Blockade of T cell contact-activation of human monocytes by high-density lipoproteins reveals a new pattern of cytokine and inflammatory genes, PLoS One, 5 (2010) e9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kissner TL, Ruthel G, Alam S, Ulrich RG, Fernandez S, Saikh KU, Activation of MyD88 signaling upon staphylococcal enterotoxin binding to MHC class II molecules, PLoS One, 6 (2011) e15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krieg AM, CpG motifs in bacterial DNA and their immune effects, Annu. Rev. Immunol, 20 (2002) 709–760. [DOI] [PubMed] [Google Scholar]
- [19].Schomig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, Schomig A, Ott I, Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction, Eur. Heart J, 27 (2006) 1032–1037. [DOI] [PubMed] [Google Scholar]
- [20].Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A, Levels of circulating endothelial progenitor cells in systemic sclerosis, Clin. Exp. Rheumatol, 25 (2007) 60–66. [PubMed] [Google Scholar]
- [21].Watanabe T, Kawano Y, Kanamaru S, Onishi T, Kaneko S, Wakata Y, Nakagawa R, Makimoto A, Kuroda Y, Takaue Y, Talmadge JE, Endogenous interleukin-8 (IL-8) surge in granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization, Blood, 93 (1999) 1157–1163. [PubMed] [Google Scholar]
- [22].Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA, Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis, Rheumatology (Oxford), 42 (2003) 1189–1196. [DOI] [PubMed] [Google Scholar]
- [23].Zielske JV, Golub SH, Fetal calf serum-induced blastogenic and cytotoxic responses of human lymphocytes, Cancer Res, 36 (1976) 3842–3846. [PubMed] [Google Scholar]
- [24].Ayache S, Panelli MC, Byrne KM, Slezak S, Leitman SF, Marincola FM, Stroncek DF, Comparison of proteomic profiles of serum, plasma, and modified media supplements used for cell culture and expansion, J. Transl. Med., 4 (2006) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soltis RD, Hasz D, Morris MJ, Wilson ID, The effect of heat inactivation of serum on aggregation of immunoglobulins, Immunology, 36 (1979) 37–45. [PMC free article] [PubMed] [Google Scholar]
- [26].Schreiber S, Stenson WF, MacDermott RP, Chappel JC, Teitelbaum SL, Perkins SL, Aggregated bovine IgG inhibits mannose receptor expression of murine bone marrow-derived macrophages via activation, J. Immunol, 147 (1991) 1377–1382. [PubMed] [Google Scholar]
- [27].Henney CS, Stanworth DR, The reactivity of rheumatoid factor with human gamma G globulin, Immunology, 9 (1965) 139–150. [PMC free article] [PubMed] [Google Scholar]
- [28].Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B, Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene, Science, 282 (1998) 2085–2088. [DOI] [PubMed] [Google Scholar]
- [29].De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I, et al. , Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation, Cytokine, 4 (1992) 239–248. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Liu S, Li Y, Wang Q, Shao J, Chen Y, Xin J, Mycoplasma bovis-derived lipid-associated membrane proteins activate IL-1beta production through the NF-kappaB pathway via toll-like receptor 2 and MyD88, Dev. Comp. Immunol, 55 (2016) 111–118. [DOI] [PubMed] [Google Scholar]
- [31].Jiang D, Liang J, Noble PW, Hyaluronan as an immune regulator in human diseases, Physiol. Rev, 91 (2011) 221–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Johnson GB, Brunn GJ, Kodaira Y, Platt JL, Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4, J. Immunol, 168 (2002) 5233–5239. [DOI] [PubMed] [Google Scholar]
- [33].Ingegnoli F, Castelli R, Gualtierotti R, Rheumatoid factors: clinical applications, Dis. Markers, 35 (2013) 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Song YW, Kang EH, The pathogenic role of rheumatoid factor in rheumatoid arthritis, Int. J. Clin. Rheumatol, 5 (2010) 651–658. [Google Scholar]
- [35].Newkirk MM, Fournier MJ, Shiroky J, Rheumatoid factor avidity in patients with rheumatoid arthritis: identification of pathogenic RFs which correlate with disease parameters and with the gal(0) glycoform of IgG, J. Clin. Immunol, 15 (1995) 250–257. [DOI] [PubMed] [Google Scholar]
- [36].Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA, Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors, Arthritis Rheum, 50 (2004) 380–386. [DOI] [PubMed] [Google Scholar]
- [37].Kolfenbach JR, Deane KD, Derber LA, O’Donnell C, Weisman MH, Buckner JH, Gersuk VH, Wei S, Mikuls TR, O’Dell J, Gregersen PK, Keating RM, Norris JM, Holers VM, A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA, Arthritis Rheum, 61 (2009) 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H, The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation, Immunity, 1 (1994) 167–178. [DOI] [PubMed] [Google Scholar]
- [39].Corper AL, Sohi MK, Bonagura VR, Steinitz M, Jefferis R, Feinstein A, Beale D, Taussig MJ, Sutton BJ, Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody-antigen interaction, Nat. Struct. Biol, 4 (1997) [DOI] [PubMed] [Google Scholar]
- [40].Roosnek E, Lanzavecchia A, Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells, J. Exp. Med, 173 (1991) 487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang L, Hakoda M, Iwabuchi K, Takeda T, Koike T, Kamatani N, Takada K, Rheumatoid factors induce signaling from B cells, leading to Epstein-Barr virus and B-cell activation, J. Virol, 78 (2004) 9918–9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cassatella MA, Pereira-da-Silva G, Tinazzi I, Facchetti F, Scapini P, Calzetti F, Tamassia N, Wei P, Nardelli B, Roschke V, Vecchi A, Mantovani A, Bambara LM, Edwards SW, Carletto A , Soluble TNF-like cytokine (TL1A) production by immune complexes stimulated monocytes in rheumatoid arthritis, J. Immunol, 178 (2007) 7325–7333. [DOI] [PubMed] [Google Scholar]
- [43].Mathsson L, Lampa J, Mullazehi M, Ronnelid J, Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells, Arthritis Res. Ther., 8 (2006) R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, Serre G, Induction of macrophage secretion of tumor necrosis factor alpha through F cgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen, Arthritis Rheum, 58 (2008) 678–688. [DOI] [PubMed] [Google Scholar]
- [45].Lacerte P, Brunet A, Egarnes B, Duchene B, Brown JP, Gosselin J, Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists, Arthritis Res. Ther., 18 (2016) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, Akashi S, Miyake K, Godowski PJ, Makino H, Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis, Arthritis Rheum, 50 (2004) 1457–1467. [DOI] [PubMed] [Google Scholar]
- [47].Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, Gay RE, Gay S, Kyburz D, Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis, Arthritis Rheum, 58 (2008) 3684–3692. [DOI] [PubMed] [Google Scholar]
- [48].Shah P, Fatehchand K, Patel H, Fang H, Justiniano SE, Mo X, Jarjoura D, Tridandapani S, Butchar JP, Toll-like receptor 2 ligands regulate monocyte Fcgamma receptor expression and function, J. Biol. Chem, 288 (2013) 12345–12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ambarus CA, Santegoets KC, van Bon L, Wenink MH, Tak PP, Radstake TR, Baeten DL, Soluble immune complexes shift the TLR-induced cytokine production of distinct polarized human macrophage subsets towards IL-10, PLoS One, 7 (2012) e35994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bernasconi NL, Traggiai E, Lanzavecchia A, Maintenance of serological memory by polyclonal activation of human memory B cells, Science, 298 (2002) 2199–2202. [DOI] [PubMed] [Google Scholar]
- [51].Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A, Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors, Nature, 416 (2002) 603–607. [DOI] [PubMed] [Google Scholar]
- [52].Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC, Group RT, Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks, Arthritis Rheum, 54 (2006) 2793–2806. [DOI] [PubMed] [Google Scholar]
- [53].Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM, Group DS, The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial, Arthritis Rheum, 54 (2006) 1390–1400. [DOI] [PubMed] [Google Scholar]
- [54].Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T, Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis, N. Engl. J. Med, 350 (2004) 2572–2581. [DOI] [PubMed] [Google Scholar]
- [55].Nakou M, Katsikas G, Sidiropoulos P, Bertsias G, Papadimitraki E, Raptopoulou A, Koutala H, Papadaki HA, Kritikos H, Boumpas DT, Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response, Arthritis Res. Ther., 11 (2009) R131. [DOI] [PMC free article] [PubMed] [Google Scholar]