Abstract
Purpose:
Adolescent and young adult (AYA) cancer survivors experience unique barriers that compromise receipt of survivorship care; therefore, development of innovative educational interventions to improve rates of AYA survivorship care are needed. The efficacy of text-messaging and peer navigation interventions was compared to standard-of-care survivorship educational materials to increase AYAs’ (1) late effects knowledge and (2) knowledge, attitudes, and self-efficacy towards seeking survivor-focused care.
Methods:
This was a three-armed, prospective, randomized controlled trial with one control group and two intervention groups. The control group received current standard-of-care educational materials. One intervention group participated in a text-messaging program, and the second participated in a peer navigator program. Participants completed pre- and post-intervention questionnaires. Study outcome variables were quantified using Fisher exact tests, two-sample t-tests, exact McNemar tests, conditional logistic regression models, and analysis of covariance.
Results:
Seventy-one survivors completed the study (control: n=24; text-messaging: n=23; peer navigation: n=24). Late effects knowledge was high at baseline for all groups. The text-messaging group had increased survivorship care knowledge compared to the control group (p<0.05); the peer navigation group had increased survivorship care self-efficacy compared to the control group; p<0.05. Both intervention groups showed increased attitudes towards seeking survivor-focused care compared to the control group (text-messaging: p<0.05; peer navigation: p<0.05).
Conclusions:
Each intervention demonstrated significant benefits compared to the control group.
Implications for Cancer Survivors:
Given the preliminary effectiveness of both interventions, each can potentially be used in the future by AYA cancer survivors to educate and empower them to obtain needed survivorship care.
Keywords: cancer survivors, adolescents and young adults, peer navigation, text-messaging, cancer survivorship care
Introduction
Due to continued advances in lifesaving treatments, an estimated 83% of childhood cancer patients are now surviving into adulthood.[1] This has produced a growing population of adolescent and young adult (AYA) childhood cancer survivors – a majority of whom will experience at least one chronic or late effect from their treatments, such as secondary malignancies and cardiotoxicity from chemotherapy and radiation.[2–9] As a result, the Institute of Medicine (IOM) recommended that childhood cancer survivors receive life-long, risk-based survivorship care for surveillance, prevention, and treatment of late effects.[10] Yet less than 50% of the childhood cancer survivor population currently receives the recommended survivorship care.[4, 5] In particular, AYA survivors face several healthcare barriers. These include patient-related barriers, such as survivors’ lack of education on their need for longitudinal survivorship care and their risk for late effects. These obstacles are unique when compared to survivors of adult malignancies, as AYA survivors face transition barriers moving into adult-centered healthcare.[11–14] Therefore, age-appropriate educational interventions to improve the receipt of survivorship care in AYA survivors is warranted.
Current standards of childhood cancer survivorship care have emphasized the use of the “Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers” published by the Children’s Oncology Group (COG). These guidelines include “Health Links”, a set of complementary, internet-based education materials written with the goal of enhancing patient follow-up visits and increasing survivorship guideline adherence.[15, 16] However, research has documented shortcomings of traditional, internet-based health materials similar in style to “Health Links” in educating AYA populations, such as they are too difficult to comprehend [17–19] and presented in an undesirable format.[18] Research to develop appealing, culturally- and age-appropriate educational materials targeting cancer survivorship care for the AYA population is needed.
One appropriate way to reach out to AYAs in order to disseminate health information is through mobile messaging technology. A Pew report found that the use of mobile phones cuts across sex, race/ethnicity, and household income: over 70% of AYAs over 14 years old have their own cell phone. Almost all of these phones have Short Message Service (SMS), or text-messaging, capabilities.[20, 21] Previous studies have shown mobile phone technology utilizing SMS is a cost-effective way to deliver important healthcare information and education.[22] It is also effective in sending health-related reminders and improving treatment plan compliance rates.[23–27] One previous study showed that an SMS-based tool can assist AYA survivors in coordinating late effect screening appointments, facilitating a partnership with their survivorship care team, and connecting them with relevant community resources.[28] Though utilizing mobile technology is seen as an emerging avenue for self-management of disease[29–32] and is a growing area within cancer survivorship research[33, 34], there have not been studies comparing the use of a text-messaging program to the traditional model of internet-based education for childhood cancer survivors.
The unique patient-related barriers, developmental and psychosocial factors, and transition-of-care challenges AYA cancer survivors face identify them as a high-risk group of patients.[11–14] Another education intervention that has been utilized within high-risk populations of cancer patients is the use of peer navigators to communicate important concepts in care and follow-up. The history of patient navigation programs dates back to 1990 at the Harlem Hospital Center, where significant disparities in cancer care and outcomes had been reported between different socioeconomic groups.[35] Patient navigation programs were created to target high-risk cancer patients and have been shown to improve their clinical and psychosocial outcomes.[35–39] However, the efficacy of patient navigation programs for high-risk cancer patients has not been well studied using prospective, randomized controlled trials.[40, 41] Given their success in other high-risk cancer populations, the use of a peer navigation model may be another innovative approach to address AYA-specific barriers and improve their knowledge and intent to seek survivorship care. Currently, there are no studies that have examined the efficacy of peer navigator programs compared to other models to educate AYA cancer survivors.
The goal of this study was to compare two innovative, affordable educational interventions – a text-messaging system and a peer navigator program – to traditional, standard-of-care online materials (“Health Links”) on their ability to inform AYAs on topics important to cancer survivorship care. The investigators assessed the ability of these three different approaches to improve AYA survivors’ (1) knowledge regarding late effects risks, and (2) knowledge, attitudes, and self-efficacy to seek survivor-focused care with continuous health insurance coverage. The research team hypothesized that the two intervention groups would have higher scores on late effects knowledge and cancer survivorship care knowledge, attitudes, and behaviors compared to the control group following study completion. These outcomes are important as improvement in AYA cancer survivors’ knowledge of, attitudes towards, and self-efficacy to seek survivor-focused care has the potential to improve long term morbidity and mortality outcomes through increasing early screening for late effects and improving health promotion behaviors.[10, 12]
Methods
Participants:
AYA survivors of childhood cancer from the greater Los Angeles area were recruited from the University of California Los Angeles (UCLA) Pediatric Hematology/Oncology Survivorship Database in person at clinic appointments, via email, and via conventional mailings. Inclusion criteria for this study were as follows: (1) 15–39 years old (the defined age limits for an AYA per the National Cancer Institute[42, 43]); (2) previously received surgery, chemotherapy, or radiation for their cancer treatment; (3) off cancer treatment (defined as no longer receiving surgery, and/or chemotherapy, and/or radiation) for more than one year; (4) possessed a personal cell phone with text-messaging capabilities; (5) English-speaking (due to text-message programming specifications and participants’ possible inclusion into this intervention group). Exclusion criteria included cognitive impairment (defined as the use of special education resources in school or documented cognitive delays as noted in school reintegration specialist’ documentation in potential participants’ medical records) as it is associated with lower intellectual abilities and reliance on parents for care needs/decision making, which could have impacted the outcomes of this study. It would have also required additional resources beyond program availability. Informed consent was obtained from all individual participants included in the study. Participants were assigned to the control arm and two intervention arms via simple randomization using sequentially numbered opaque sealed envelopes from a computer generated sequence.[44] Two research assistants worked together to enroll participants, generate the randomization sequence (with approval from the PI), and assign participants to their designated group. This study was approved by the UCLA Institutional Review Board (UCLA IRB#11–002228).
Study Design:
This was a three-armed, prospective, randomized controlled trial with one control group (“Health Links”) and two intervention groups (text-messaging and peer navigation). At the beginning of the study, all participants received educational materials based on their group assignment that were designed to educate the AYA cancer survivor on three key messages: (1) need for a treatment summary/survivorship care plan, (2) risk for medical and psychosocial late effects due to cancer treatment, and (3) need for continuous health insurance coverage. From these messages, participants developed their own unique set of personalized survivorship goals chosen from predefined categories, called their Adolescent and Young Adult Survivorship Action Plan (ASAP).
The first group, the control group, received the standard-of-care educational materials created by the Children’s Oncology Group (COG). Members of this group received paper copies of “Health Links” via conventional mailings after study enrollment. After receiving “Health Links”, a separate piece of paper included in the mailing asked participants to formulate their ASAP and develop strategies to help them achieve their ASAP goals. Participants were encouraged to seek answers to questions regarding the “Health Links” educational material, creation of their ASAP, and achieving their ASAP goals through direct discussion with their healthcare provider in a long-term follow-up visit during the eight-week study period, as this is also a standard-of-care practice.
The second group received the text-messaging intervention that was previously developed by this research group [28]. Members of this group received an educational booklet – created by the research team and entitled the “ASAP Book” – after study enrollment. Its content was AYA-focused and based on information from “Health Links” that addressed key survivorship messages. Appendix 1 includes selected excerpts from the “ASAP Book”. Participants could opt to receive a printed booklet via conventional mail or access an online version of the booklet via a password-protected link provided on the UCLA Jonsson Comprehensive Cancer Center (JCCC) website.[45] After reading the “ASAP Book”, they were asked to select their three ASAP goals and text them to a phone number provided with their initial study enrollment information. Once these goals were communicated, a two-way automated text-messaging system was initiated over an eight-week period to support survivor engagement in accessing community and cancer center resources to help them reach their individual ASAP goals. These text-messages were individualized based on the survivor’s demographics and ASAP goal selection. Examples of such messages can be found in Appendix 2.
The third group received the peer navigation intervention. Members of this group received the same “ASAP Book” as the text-messaging group. After reading the “ASAP Book”, they were asked to select three ASAP goals. Each participant was matched up with a peer navigator from a pool of undergraduate college students trained in utilization of the “Stages of Change” model and motivational interviewing.[46, 47] They performed an initial call to survivors, where they reviewed their ASAP goals and asked if they had any questions regarding potential community resources to help them achieve these goals. Subsequently, four weeks into the study, a booster call was made by the peer navigator to, again, review their ASAP goals and address successes and barriers in achieving these goals. Potential community and cancer center resources were then, again, discussed with survivors as solutions to helping them achieve their ASAP goals. Attempts were made to match the survivor with the same peer navigator for both the initial and booster calls; however, this could not be guaranteed since the call was based on their survivors’ availabilities. Communication occurred via telephone because it is cost effective, convenient, and preferred amongst the AYA population as a communication methods.[21, 48]
Study Evaluation:
All participants completed both pre- and post-intervention paper questionnaires to assess the primary outcome variables of (1) knowledge regarding risk of late effects, (2) knowledge, attitudes, and self-efficacy for survivorship care planning, and (3) knowledge, attitudes, and self-efficacy for health insurance planning. These were mailed to survivors and their families both pre- and post-intervention
Questionnaire items were modified from existing surveys used in previous work with this population by the UCLA JCCC [49]. These questionnaires also assessed demographics and medical/oncologic history. There was an eight-week period between administration of the pre- and post-intervention questionnaires.
Measures:
Survivorship care knowledge was first assessed by asking participants whether they understood the term “late effects”. Survivorship care knowledge was further assessed using three items that asked participants to rate reasons for receiving survivorship care on a five-point Likert scale. A knowledge scale was formed as the mean of these items.
Survivorship care attitude was assessed using four items rated as to their importance in a cancer survivor’s care, which included domains of receipt of survivorship care plan, access to medical care, health promotion, and health insurance coverage. A five-point Likert scale was used. A summary scale averaging these items had Cronbach alpha of 0.75/0.78 at baseline/follow-up (B/F).
Survivors’ self-efficacy assessments had three domains: late effects knowledge, survivorship care planning, and health insurance planning. Late effects self-efficacy was assessed using three items; the summary scale averaging these items had Cronbach alpha of 0.87/0.88 at B/F. Survivorship care planning self-efficacy was assessed using three items; the summary scale had Cronbach alpha of 0.93/0.93 at B/F. Self-efficacy for health insurance planning was assessed using five items; the summary scale had Cronbach alpha of 0.94/0.95 at B/F. All self-efficacy items and scales were assessed on a five-point Likert scale
Further details on the item measures are provided in Tables 2 and 3.
Table 2:
Study Completer Survivorship Care Knowledge Outcomes, Baseline vs. Follow-Up Questionnaire (n=71)
| Control (n=24) |
Peer Navigation (n=24) |
Text-messaging (n=23) |
Peer Navigation vs. Control | Text- messaging vs. Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pa | pa | pa | pb | pb | ||||||
| Know the term “late effects” | .69 | .50 | <.05 | .99 | .99 | |||||
| Pretest | ||||||||||
| Posttest | ||||||||||
| pa | pa | pa | pb | pb | ||||||
| The reason for survivorship care is to …d | ||||||||||
| Check for cancer recurrence | ||||||||||
| Pretest | .36 | .20 | .10 | .13 | <.05 | |||||
| Posttest | ||||||||||
| Obtain advice on how cancer treatment may affect health | ||||||||||
| Pretest | .50 | .38 | .06 | 35 | .05 | |||||
| Posttest | ||||||||||
| Obtain emotional/psychological support | ||||||||||
| Pretest | .70 | .89 | .09 | .33 | <.05 | |||||
| Posttest | ||||||||||
| Survivorship care knowledge scaled | ||||||||||
| Pretest | .67 | .38 | <.05 | .07 | <.05 | |||||
| Posttest | ||||||||||
p-values for change over time within each group were obtained using exact McNemar tests (know term “late effect”) and paired t-tests (other outcomes).
Difference in change over time for each intervention group compared to the control group was estimated using a time-by-group interaction in conditional logistic regression models (know term “late effect”) or analysis of covariance adjusting for baseline score and health insurance status (other outcomes).
Effect sizes were calculated as the difference in group means standardized by the pooled standard deviation (Cohen’s d).
Responses to knowledge scale items coded: 1=Not true, 2=Somewhat true, 3=True, 4=Very true, 5=Extremely true
Table 3:
Study Completer Survivorship Care Attitude and Self-Efficacy Outcomes, Baseline vs. Follow-Up Questionnaire (n=71)
| Control (n=24) |
Peer Navigation (n=24) |
Text-messaging (n=23) |
Peer Navigation vs. Control | Text- messaging vs. Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pa | pa | pa | pb | pb | ||||||
| SURVIVORSHIP CARE ATTITUDE MEASURESd | ||||||||||
| Importance of … | ||||||||||
| Having copy of survivorship care plan | ||||||||||
| Pretest | .99 | <.05 | .56 | <.05 | .29 | |||||
| Posttest | ||||||||||
| Having medical care related to cancer treatment and late effects | ||||||||||
| Pretest | .05 | .33 | <.05 | <.05 | <.05 | |||||
| Posttest | ||||||||||
| Taking better care of health compared to peers never treated for cancer | ||||||||||
| Pretest | .99 | .13 | .21 | .06 | <.05 | |||||
| Posttest | ||||||||||
| Having health insurance coverage as a cancer survivor | ||||||||||
| Pretest | .54 | <.05 | .06 | .06 | ||||||
| Posttest | ||||||||||
| Survivorship care attitude scale | ||||||||||
| Pretest | .35 | <.05 | .07 | <.05 | <.05 | |||||
| Posttest | ||||||||||
| LATE EFFECTS SELF-EFFICACYe | ||||||||||
| Confident know … | ||||||||||
| How long to continue screening for recurrence | ||||||||||
| Pretest | .81 | <.05 | .49 | .05 | .74 | |||||
| Posttest | 9 | |||||||||
| Steps to take if concerned about physical late effects | ||||||||||
| Pretest | .99 | <.05 | .86 | <.05 | .54 | |||||
| Posttest | ||||||||||
| Steps to take if concerned about psychological, emotional or social late effects | ||||||||||
| Pretest | .57 | <.05 | .99 | <.05 | .58 | |||||
| Posttest | ||||||||||
| Late effects self-efficacy scale | ||||||||||
| Pretest | .75 | <.05 | .70 | <.05 | .82 | |||||
| Posttest | ||||||||||
| SURVIVORSHIP CARE PLANNING SELF-EFFICACYe | ||||||||||
| Confident can obtain own copy of … | ||||||||||
| Medical records | ||||||||||
| Pretest | .40 | .18 | .83 | <.05 | .25 | |||||
| Posttest | ||||||||||
| Treatment summary | ||||||||||
| Pretest | .46 | .18 | .56 | <.05 | .15 | |||||
| Posttest | ||||||||||
| Survivorship care plan | ||||||||||
| Pretest | .25 | .15 | .35 | <.05 | .05 | |||||
| Posttest | ||||||||||
| Survivorship care planning self-efficacy scale | ||||||||||
| Pretest | .33 | .14 | .60 | <.05 | .10 | |||||
| Posttest | ||||||||||
| HEALTH INSURANCE SELF-EFFICACYe | ||||||||||
| Confident to … | ||||||||||
| Talk to insurance company about current coverage | ||||||||||
| Pretest | .65 | .37 | <.05 | .13 | .34 | |||||
| Posttest | ||||||||||
| Obtain a copy of health insurance plan | ||||||||||
| Pretest | .82 | .28 | .26 | .16 | .45 | |||||
| Posttest | ||||||||||
| Find out types of insurance plans accepted by oncologist | ||||||||||
| Pretest | .46 | .12 | .07 | .15 | .37 | |||||
| Posttest | ||||||||||
| Discuss insurance options with health care team | ||||||||||
| Pretest | .85 | <.05 | .07 | <.05 | .28 | |||||
| Posttest | ||||||||||
| Talk to billing department about medical bills | ||||||||||
| Pretest | .99 | .05 | <.05 | <.05 | .21 | |||||
| Posttest | ||||||||||
| Health insurance self-efficacy scale | ||||||||||
| Pretest | .82 | <.05 | .07 | <.05 | .24 | |||||
| Posttest | ||||||||||
p-values for change over time within each group were obtained using paired t-tests.
Difference in change over time for each intervention group compared to the control group was estimated using analysis of covariance adjusting for baseline score and health insurance status.
Effect sizes were calculated as the difference in group means standardized by the pooled standard deviation (Cohen’s d)
Responses to attitude items coded: 1=Not important, 2=Somewhat important, 3=Important, 4=Very important, 5=Extremely important
Responses to self-efficacy items coded: 1=Not confident, 2=Somewhat confident, 3=Confident, 4=Very confident, 5=Extremely confident
Data Analysis:
Outcome comparisons were made between AYA participants in the control group and AYA participants in each intervention group. Goal sample sizes of 25 in each arm were determined to provide 95% confidence intervals for mean differences between two groups with width of 1.13 standard deviation units, which were considered sufficient for estimating the expected effect sizes. Baseline differences between the control and each intervention group were assessed using Fisher exact and two-sample t-tests. Change over time was assessed within each group using exact McNemar tests (dichotomous outcomes) and paired t-tests (other outcomes). Difference in change over time for each intervention group compared to the control group was estimated using a time-by-group interaction in conditional logistic regression models (dichotomous outcomes) or analysis of covariance adjusting for baseline score and health insurance status (other outcomes). Effect sizes were calculated as the difference in group means standardized by the pooled standard deviation (Cohen’s d). Data management and analyses were conducted using Stata/SE® software (Version 15.1 for Windows; College Station, Texas, USA).
Results
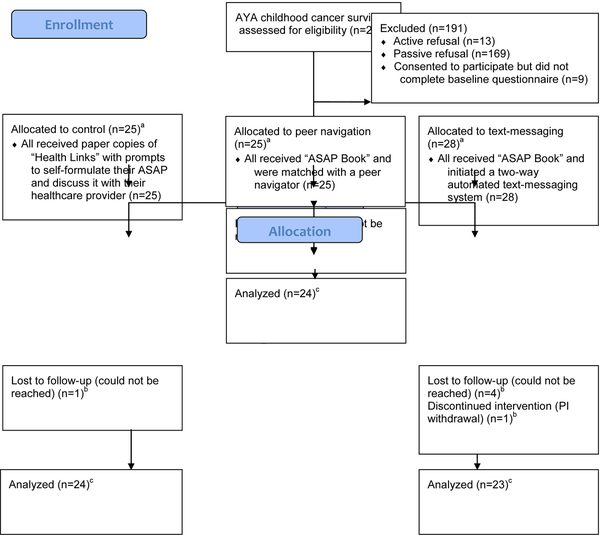
Figure 1 details participants’ recruitment and movement through the study. In total, 269 individuals met all inclusion criteria and were approached to participate in the study. Eighty-seven survivors consented to participate in this study; 13 actively refused to participate, and 169 passively refused (i.e. did not return/respond to phone calls). Of these, 78 survivors completed the baseline assessment and were randomized to one of the three study arms. Seventy-one survivors (91%) completed the entire study, which included the final follow-up assessment. Of the seven participants who were randomized, received their interventions, but did not complete the follow-up assessment, two stated they were “too busy” to complete the study, one was removed due to development of a secondary malignancy (transferred to active cancer care), and four could not be reached despite multiple attempts.
Figure 1:
Participant Flow Through the Study
Table 1 shows demographics, health statuses, and baseline survey items regarding survivorship identity for the 78 study participants (completed the baseline survey and were randomized to groups). The percentage of non-completers did not differ significantly among the three arms (p=0.21; Fisher exact test). The sample was ethnically diverse. Eighteen percent reported having no current health insurance, and survivors who did not complete the follow-up survey were more likely to lack health insurance than survivors who completed the full study (14% {10/71} for completers versus 57% {4/7} for non-completers, p<0.05). Otherwise, there were no statistically significant differences between study completers (n=71) versus non-completers (n=7) nor between the three arms. Most survivors described their current overall and emotional health status as very good or excellent. Survivorship identity responses were largely consistent with a positive survivorship identity.
Table 1:
Study Participant Demographics and Survivorship Identity Responses, Baseline Questionnaire (N=78)
| Control (n=25) |
Peer Navigation (n=25) |
Text-messaging (n=28) |
Overall (N=78) |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | na | (%) | na | (%) | na | (%) | na | (%) |
| Gender | ||||||||
| Male | 11 | (44) | 11 | (44) | 15 | (54) | 37 | (47) |
| Female | 14 | (56) | 14 | (56) | 13 | (46) | 41 | (53) |
| Age at survey, years (Mean ± SD) | 20 ± 5 | 21 ± 6 | 21 ± 5 | 21 ± 5 | ||||
| 15–19 | 15 | (60) | 13 | (52) | 11 | (39) | 5 | (50) |
| 20–29 | 9 | (36) | 10 | (40) | 15 | (54) | 34 | (44) |
| 30–39 | 1 | (4) | 2 | (8) | 2 | (7) | 39 | (6) |
| Race/ethnicity | ||||||||
| Non-Hispanic/Latino white | 8 | (32) | 10 | (40) | 11 | (39) | 29 | (37) |
| Black | 1 | (4) | 0 | (0) | 1 | (4) | 2 | (3) |
| Asian | 1 | (4) | 0 | (0) | 3 | (11) | 4 | (5) |
| Hispanic/Latino | 13 | (52) | 11 | (44) | 12 | (43) | 36 | (46) |
| Mixed race/ethnicity | 2 | (8) | 4 | (16) | 1 | (4) | 7 | (9) |
| Language spoken at home | ||||||||
| English only | 12 | (55) | 12 | (52) | 16 | (64) | 40 | (57) |
| Spanish only | 2 | (9) | 4 | (17) | 1 | (4) | 7 | (10) |
| English and Spanish | 8 | (36) | 6 | (26) | 7 | (28) | 21 | (30) |
| Other | 0 | (0) | 1 | (4) | 1 | (4) | 2 | (3) |
| US born | ||||||||
| Yes | 19 | (76) | 22 | (88) | 23 | (82) | 64 | (82) |
| No | 6 | (24) | 3 | (12) | 5 | (18) | 14 | (18) |
| Annual household income | ||||||||
| Under $20,000 | 2 | (8) | 6 | (24) | 5 | (18) | 13 | (17) |
| $20,000–$39,999 | 8 | (32) | 3 | (12) | 5 | (18) | 16 | (21) |
| $40,000 and above | 4 | (16) | 7 | (28) | 7 | (25) | 18 | (23) |
| Don’t know/not reported | 11 | (44) | 9 | (36) | 11 | (39) | 31 | (40) |
| Health insurance | ||||||||
| Yes | 22 | (88) | 16 | (64) | 24 | (86) | 62 | (79) |
| No | 2 | (8) | 8 | (32) | 4 | (14) | 14 | (18) |
| Don’t know | 1 | (4) | 1 | (4) | 0 | (0) | 2 | (3) |
| Cancer diagnosis | ||||||||
| Leukemia | 14 | (56) | 17 | (68) | 13 | (46) | 44 | (56) |
| Lymphoma (Hodgkin & non-Hodgkin) | 5 | (20) | 3 | (12) | 6 | (21) | 14 | (18) |
| Brain/central nervous system | 1 | (4) | 1 | (4) | 3 | (11) | 5 | (6) |
| Kidney | 1 | (4) | 0 | (0) | 0 | (0) | 1 | (1) |
| Neuroblastoma | 0 | (0) | 1 | (4) | 3 | (11) | 4 | (5) |
| Bone/soft-tissue sarcoma | 4 | (16) | 2 | (8) | 2 | (7) | 8 | (10) |
| Testicular | 0 | (0) | 0 | (0) | 1 | (4) | 1 | (1) |
| Liver | 0 | (0) | 1 | (4) | 0 | (0) | 1 | (1) |
| Cancer treatments received | ||||||||
| Chemotherapy only | 4 | (16) | 7 | (28) | 6 | (21) | 17 | (22) |
| Surgery only | 0 | (0) | 1 | (4) | 0 | (0) | 1 | (1) |
| Chemotherapy and surgery | 6 | (24) | 4 | (16) | 4 | (14) | 14 | (18) |
| Chemotherapy and radiation | 2 | (8) | 1 | (4) | 3 | (11) | 6 | (8) |
| Surgery and radiation | 1 | (4) | 1 | (4) | 1 | (4) | 3 | (4) |
| Chemotherapy, surgery and radiation | 4 | (16) | 4 | (16) | 2 | (7) | 10 | (13) |
| Bone marrow transplant (with or without other therapies) | 3 | (12) | 2 | (8) | 6 | (21) | 11 | (14) |
| Don’t know/not reported | 5 | (20) | 5 | (20) | 6 | (21) | 16 | (21) |
| Age at diagnosis, years (Mean ± SD) | 10 ± 5 | 9 ± 4 | 11 ± 5 | 10 ± 5 | ||||
| Less than 5 | 7 | (28) | 6 | (24) | 7 | (25) | 20 | (26) |
| 6–10 | 5 | (20) | 11 | (44) | 5 | (18) | 21 | (27) |
| 11–14 | 9 | (36) | 5 | (20) | 8 | (29) | 22 | (28) |
| 15–21 | 4 | (16) | 3 | (12) | 8 | (29) | 15 | (19) |
| Years since completing treatment (Mean ± SD) | 8 ± 7 | 9 ± 7 | 8 ± 5 | 8 ± 6 | ||||
| Less than 2 | 2 | (8) | 1 | (4) | 0 | (0) | 3 | (4) |
| 2–4 | 6 | (24) | 6 | (24) | 6 | (21) | 18 | (23) |
| 5–9 | 7 | (28) | 8 | (32) | 11 | (39) | 26 | (33) |
| 10 or more | 9 | (36) | 8 | (32) | 10 | (36) | 27 | (35) |
| Not reported | 1 | (4) | 2 | (8) | 1 | (4) | 4 | (5) |
| Current overall health statusb | ||||||||
| Excellent | 7 | (28) | 9 | (36) | 6 | (21) | 22 | (28) |
| Very good | 10 | (40) | 6 | (24) | 16 | (57) | 32 | (41) |
| Good | 7 | (28) | 8 | (32) | 4 | (14) | 19 | (24) |
| Fair | 1 | (4) | 2 | (8) | 2 | (7) | 5 | (6) |
| Poor | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Current overall emotional health statusb | ||||||||
| Excellent | 5 | (20) | 9 | (36) | 6 | (21) | 20 | (26) |
| Very good | 12 | (48) | 7 | (28) | 10 | (36) | 29 | (37) |
| Good | 5 | (20) | 7 | (28) | 11 | (39) | 23 | (29) |
| Fair | 3 | (12) | 2 | (8) | 1 | (4) | 6 | (8) |
| Poor | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Survivorship Identity Statementsb | ||||||||
| Being childhood cancer survivor important part of who I am | ||||||||
| Agree | 24 | (96) | 21 | (84) | 26 | (93) | 71 | (91) |
| Undecided | 1 | (4) | 2 | (8) | 2 | (7) | 5 | (6) |
| Disagree | 0 | (0) | 2 | (8) | 0 | (0) | 2 | (3) |
| I have no problem telling friends I am a childhood cancer survivor | ||||||||
| Agree | 23 | (92) | 17 | (68) | 26 | (93) | 66 | (85) |
| Undecided | 1 | (4) | 3 | (12) | 1 | (3.5) | 5 | (6) |
| Disagree | 1 | (4) | 5 | (20) | 1 | (3.5) | 7 | (9) |
| I am concerned how others may view me if knew I was childhood cancer survivor | ||||||||
| Agree | 8 | (32) | 3 | (12) | 6 | (21) | 17 | (22) |
| Undecided | 1 | (4) | 2 | (8) | 3 | (11) | 6 | (8) |
| Disagree | 16 | (64) | 20 | (80) | 19 | (68) | 55 | (70) |
| I feel like I did something to get cancer | ||||||||
| Agree | 1 | (4) | 1 | (4) | 1 | (4) | 3 | (4) |
| Undecided | 3 | (12) | 4 | (16) | 6 | (21) | 13 | (17) |
| Disagree | 21 | (84) | 20 | (80) | 21 | (75) | 62 | (79) |
| My cancer experience has impacted my life in a negative way | ||||||||
| Agree | 1 | (4) | 4 | (16) | 1 | (4) | 6 | (8) |
| Undecided | 5 | (20) | 3 | (12) | 7 | (25) | 15 | (19) |
| Disagree | 19 | (76) | 18 | (72) | 20 | (71) | 57 | (73) |
Some counts do not sum to the total due to blank survey responses
Self-reported
Table 2 summarizes late effects and survivorship care knowledge outcomes for study completers. Knowledge of the term “late effects” was high at baseline. The text-messaging group had a significant increase in late effects knowledge as well as survivorship care knowledge scale scores from pre- to posttest. The text-messaging group also had a significantly greater increase in the overall survivorship care knowledge scale score and two of three subscale items when compared to the control group. The peer navigation group showed no significant differences in knowledge items compared to the control group.
Table 3 summarizes survivorship care attitude and self-efficacy outcomes for study completers. Both intervention groups showed increases on the survivorship care attitude scale compared to the control group, with medium effect sizes of 0.40 for the peer navigation arm and 0.33 for the text-messaging arm.[50] The peer navigation group also had a significant increase in attitude scale scores from pre- to posttest. The peer navigation group had significant increases with medium to large effect sizes for the late effects, survivorship care planning, and health insurance self-efficacy scales in addition to most subscale items; this group also showed significant increases in late effects and health insurance self-efficacy scale scores from pre- to posttest. The text-messaging group showed no significant differences in self-efficacy items compared to the control group.
Discussion
AYA cancer survivors face distinct barriers when navigating the healthcare system compared to other groups of cancer survivors due to unique developmental barriers, such as transitioning to adult-centered healthcare models.[11–14] Therefore, they require receipt of innovative educational interventions in order to maximize their long-term follow-up care. This study presents two age- and culturally-appropriate methods to educate AYA survivors: a text-messaging system and a peer navigator program. The investigators aimed to compare the ability of these interventions to current standard-of-care educational materials to inform AYAs on topics important to cancer survivorship care. The investigators of this study hypothesized that the text-messaging and peer navigator groups would have increased post-intervention scores compared to the control “Health Links” group in their (1) knowledge regarding risk of late effects and (2) knowledge, attitudes, and self-efficacy to seek survivor-focused care with continuous health insurance coverage. The results show each intervention had positive outcomes, which were significant compared to the control group. The text-messaging group had a significant increase in posttest late effects knowledge as well as overall survivorship care knowledge compared to the control group, while the peer navigation group had significant increases in survivorship care planning and health insurance self-efficacy compared to the control group. Both intervention groups showed increased attitudes (responded with increased importance) towards seeking survivor-focused care and health insurance compared to the control group.
In regard to “late effects” knowledge, all groups had high pre-test scores, indicating an already informed group of AYA survivors. This was likely due to their recruitment from an established survivorship program. Despite the concern for a ceiling effect, the text-messaging group still showed significant increases in late effects knowledge as well as overall knowledge scale scores from pre- to posttest. This group also had a significantly greater increase in two of three subscale items when compared to the control group. Traditional education materials typically are written at a high reading comprehension level and are less desirable to read than more engaging forms of media.[17–19] Previous studies have documented AYAs’ use of both formal and informal language to display comprehension of complex healthcare topics.[51] Text-messaging utilizing a short text format of abbreviated educational material coupled with AYAs’ familiarity with mobile technology may explain the increased knowledge scores compared to traditional educational materials.
In contrast, the peer navigation group had significant increases in all self-efficacy measurements from pre- to posttest and when compared to the control group, meaning they had greater confidence in their ability to plan their survivorship care and seek continuous health insurance coverage post-intervention. Patient navigator programs were first designed to improve outcomes for high-risk cancer populations.[35] Navigators worked to present information in age-, socioeconomically-, and culturally-appropriate ways as well as motivate patients to engage fully in their care.[37–39] The peer navigators, through their training in Stages of Change and motivational interviewing models[45, 46], aimed to do the same. This culturally-appropriate delivery of content coupled with presenting information in a more desirable format than traditional education materials[18] may explain the increase in participants’ motivation to seek survivorship care and health insurance.
From the results, both intervention groups showed increased attitudes towards the importance of survivorship care planning compared to the control group. In addition, they each had their own strengths compared to the control group: the text-messaging group had increased knowledge scores and the peer navigator group had increased self-efficacy. The research team now hypothesizes that combining the two interventions into one complete intervention could result in further increases in AYA survivors’ knowledge, attitudes, and behaviors regarding survivorship care. Previous research has documented frequent physician reminders result in closer screening adherence behaviors in cancer survivors.[52] Cost-effective, novel educational approaches that target more vulnerable populations have also been called for in order to improve cancer survivors’ surveillance rates with the goal to ultimately improve their clinical outcomes.[53] A combination text-messaging and peer navigation education program could be an effective means of keeping track and following up with AYA survivors who may only see their health care provider annually for care. This type of program could help survivors reach and maintain their ASAP and other survivorship care goals between clinic visits through frequent reminders.
This study adds to the growing body of intervention studies in cancer survivorship literature, most importantly to the area of digital health interventions.[54–57] It builds upon a previous descriptive study that detailed the feasibility and acceptability of digital health interventions through demonstrating the effectiveness of an educational intervention that used digital health modalities to enhance patient-centered survivorship care.[54] Specifically, the interventions tested in this study included a text-messaging arm. Participants in this group showed improved knowledge regarding their late effects and need for survivorship care. There was also an option within both the text-messaging and peer navigator arms to use an online education booklet (the “ASAP Book”). Both of these intervention arms showed improved attitudes and self-efficacy towards seeking survivorship care. These collective findings have the potential to increase AYAs’ low rates of survivorship care screening and efficiently deliver needed survivorship health education through empowering survivors directly.
Importantly, in this intervention study, participants prioritized their own survivorship goals instead of their provider setting their survivorship care goals for them. These findings, thereby, add to the literature, which previously found that cancer survivors find it beneficial to have the ability to adjust content of healthcare interventions to their specific needs that can vary across the continuum of survivorship care.[55]
Recent research has also shown that even within a large, fully integrated health care system where cancer survivors have access to all required late effects testing based on their therapeutic exposures, AYA survivors still have low rates of late effects screening.[58] Given that both the peer navigator and the text-messaging intervention arms showed increased self-efficacy and knowledge regarding late effects risks, these interventions could be further tested (either individually or in a combined peer navigator and text-messaging intervention) to determine if they could improve rates of late effects screening.
There are several strengths to this study that should be highlighted. First, study participants were from an extremely diverse population. Two thirds of study participants were from racial/ethnic minority groups, and 43% primarily spoke a language other than English at home. The research team was able to effectively engage a culturally-diverse population of AYA survivors. It is critical for future intervention studies to reach broader populations of culturally diverse cancer survivors across the United States, given changing demographics.[59]
Another unique strength of the study is that the interventions were not delivered within a clinic setting, which allows for future research to explore applicability of the intervention to different communities. As discussed above, both the peer navigation and text-messaging interventions were delivered directly to the survivor. Most survivors have decreasing rates of returning to their oncology center as they age. Future research, therefore, can explore delivery of these interventions within larger AYA populations with limited access to survivorship clinics or oncology centers. This is timely, as there is a growing body of literature encouraging testing various modalities of technology to reach populations without access to care, including the use of mobile technologies to promote care adherence in a home-based setting.[60]
This study had some limitations. Of the initial 269 eligible survivors, there were 182 refusals (13 direct refusals and 169 passive refusals – meaning eligible participants did not respond to outreach for study involvement) and nine that did not complete the baseline questionnaires. Of the finalized 78 participants, 71 completed the study. Inclusion of more eligible survivors could have increased the overall impact of the intervention, given that a majority of survivors are unlikely to engage in comprehensive survivorship care. In addition, this study’s refusal and dropout rate is typical for large-scale cancer survivor studies of the AYA population.[61] Though research groups can overcome this through use of centralized recruiting offices[62], most survivorship programs (including this research group) do not possess this capability. Future research will explore partnerships with established survivorship consortiums, both nationally and regionally, to expand and improve recruitment efforts through centralized recruitment offices.[63] Also AYAs as a group have historically had difficulties following up with studies due to their busy and mobile lifestyles, lack of participation interest, and sporadic care compared to other groups of cancer survivors.[61, 64] Despite this, the final sample sizes were adequate to complete full data analyses for this study, including all logistic regressions. In addition, study participants identified themselves as knowledgeable regarding late effects at baseline. This, historically, has not been generalizable to the AYA cancer survivor population at large.[65, 66] However, there was still positive change in each intervention group from pre- to posttest, with the text-messaging group having significant increases posttest compared to the control group. This means the potential for knowledge increases in the general AYA survivor population could be higher than those experienced by study participants.
An ethnically diverse population was recruited with distribution of previous cancer diagnoses similar to national data,[1, 42] which aids in generalizability to the national population of AYA cancer survivors. Future directions for this research group include studying outcomes of a combined text-messaging and peer navigation educational intervention to improve AYA cancer survivors’ knowledge of late effects and rates of receipt of survivorship care.
In conclusion, this study offers two innovative, cost-effective, age- and culturally-appropriate interventions to educate AYA survivors on topics important to their health and care. Each intervention had significant strengths when compared to more traditional methods of educating AYA survivors. Future research will aim to combine and test the efficacy of these two innovative and affordable interventions to ultimately improve rates of survivorship care and clinical outcomes for diverse populations of AYA cancer survivors.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Andrew Shanholtzer and the organization Padres Contra El Cáncer for their contributions to this study. This research was supported by the Administrative Supplement NOT-CA-10–026 from the National Cancer Institute (Recipient: Dr. Jacqueline Casillas). Dr. Crespi was also supported by CA016042 from the National Cancer Institute.
Funding: This research was supported by the Administrative Supplement NOT-CA-10–026 from the National Cancer Institute (Recipient: Dr. Jacqueline Casillas). Dr. Crespi was also supported by CA016042 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: The authors declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2010 In April 2013 Edition. https://seer.cancer.gov/archive/csr/1975_2010/: National Cancer Institute; 2013. [Google Scholar]
- 2.Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin 2004; 54: 208–236. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2008; 100: 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Hudson MM et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med 2004; 2: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan PC, Greenberg ML, Ness KK et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 2008; 26: 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geenen MM, Cardous-Ubbink MC, Kremer LC et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 2007; 297: 2705–2715. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Mertens AC, Yasui Y et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA 2003; 290: 1583–1592. [DOI] [PubMed] [Google Scholar]
- 8.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008; 113: 2575–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariotto AB, Rowland JH, Yabroff KR et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 10.In Hewitt M, Weiner SL, Simone JV (eds): Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington (DC): 2003. [PubMed] [Google Scholar]
- 11.Kirchhoff AC, Lyles CR, Fluchel M et al. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer 2012; 118: 5964–5972. [DOI] [PubMed] [Google Scholar]
- 12.Io Medicine, Council NR. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press,2006. [Google Scholar]
- 13.Smits-Seemann RR, Kaul S, Zamora ER et al. Barriers to follow-up care among survivors of adolescent and young adult cancer. J Cancer Surviv 2017; 11: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zebrack BJ, Eshelman DA, Hudson MM et al. Health care for childhood cancer survivors: insights and perspectives from a Delphi panel of young adult survivors of childhood cancer. Cancer 2004; 100: 843–850. [DOI] [PubMed] [Google Scholar]
- 15.Group CsO. Establishing and Enhancing Services for Childhood Cancer Survivors: Long-Term Follow-Up Program Resource Guide. Monrovia, CA: Children’s Oncology Group,2007. [Google Scholar]
- 16.Landier W, Bhatia S, Eshelman DA et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 2004; 22: 4979–4990. [DOI] [PubMed] [Google Scholar]
- 17.Berland GK, Elliott MN, Morales LS et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA 2001; 285: 2612–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stinson JN, White M, Breakey V et al. Perspectives on quality and content of information on the internet for adolescents with cancer. Pediatr Blood Cancer 2011; 57: 97–104. [DOI] [PubMed] [Google Scholar]
- 19.Walsh TM, Volsko TA. Readability assessment of internet-based consumer health information. Respir Care 2008; 53: 1310–1315. [PubMed] [Google Scholar]
- 20.RapidSMS. In. https://www.rapidsms.org: 2013.
- 21.Lenhart A Teens and Mobile Phones Over the Past Five Years: Pew Internet Looks Back. In. http://www.pewinternet.org/2009/08/19/teens-and-mobile-phones-over-the-past-five-years-pew-internet-looks-back/: Pew Research Center; 2009. [Google Scholar]
- 22.Sharifi M, Dryden EM, Horan CM et al. Leveraging text messaging and mobile technology to support pediatric obesity-related behavior change: a qualitative study using parent focus groups and interviews. J Med Internet Res 2013; 15: e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester R, Karanja S. Mobile phones: exceptional tools for HIV/AIDS, health, and crisis management. Lancet Infect Dis 2008; 8: 738–739. [DOI] [PubMed] [Google Scholar]
- 24.Lester RT, Ritvo P, Mills EJ et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010; 376: 1838–1845. [DOI] [PubMed] [Google Scholar]
- 25.Mbuagbaw L, Bonono-Momnougui RC, Thabane L. Considerations in using text messages to improve adherence to highly active antiretroviral therapy: a qualitative study among clients in Yaounde, Cameroon. HIV AIDS (Auckl) 2012; 4: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niederhauser V, Johnson M, Tavakoli AS. Vaccines4Kids: Assessing the impact of text message reminders on immunization rates in infants. Vaccine 2015; 33: 2984–2989. [DOI] [PubMed] [Google Scholar]
- 27.Stockwell MS, Kharbanda EO, Martinez RA et al. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 28.Casillas J, Goyal A, Bryman J et al. Development of a text messaging system to improve receipt of survivorship care in adolescent and young adult survivors of childhood cancer. J Cancer Surviv 2017; 11: 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V et al. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev 2012; 12: CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med 2009; 36: 165–173. [DOI] [PubMed] [Google Scholar]
- 31.Klasnja P, Hartzler A, Powell C, Pratt W. Supporting cancer patients’ unanchored health information management with mobile technology. AMIA Annu Symp Proc 2011; 2011: 732–741. [PMC free article] [PubMed] [Google Scholar]
- 32.McCann L, Maguire R, Miller M, Kearney N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl) 2009; 18: 156–164. [DOI] [PubMed] [Google Scholar]
- 33.UCSF Health eHeart Study. In. https://www.health-eheartstudy.org: 2018.
- 34.Investigators CCSSCP. Principal Investigator Report In CCSS Investigator Meeting. Memphis, TN: CCSS; 2015. [Google Scholar]
- 35.Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer 2011; 117: 3539–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bush ML, Kaufman MR, Shackleford T. Adherence in the Cancer Care Setting: a Systematic Review of Patient Navigation to Traverse Barriers. J Cancer Educ 2018; 33: 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber DE, Hamann HA, Santini NO et al. Patient navigation for lung cancer screening in an urban safety-net system: Protocol for a pragmatic randomized clinical trial. Contemp Clin Trials 2017; 60: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Carlson E, Villarreal R et al. Cost-effectiveness of a patient navigation program to improve cervical cancer screening. Am J Manag Care 2017; 23: 429–434. [PubMed] [Google Scholar]
- 39.Vora S, Lau JD, Kim E et al. Patient Navigation Program for Colorectal Cancer Screening in Chinese Americans at an Urban Community Health Center: Lessons Learned. J Health Care Poor Underserved 2017; 28: 887–895. [DOI] [PubMed] [Google Scholar]
- 40.Berezowska A, Passchier E, Bleiker E. Evaluating a professional patient navigation intervention in a supportive care setting. Support Care Cancer 2019. [DOI] [PubMed] [Google Scholar]
- 41.Nickell A, Stewart SL, Burke NJ et al. Engaging limited English proficient and ethnically diverse low-income women in health research: A randomized trial of a patient navigator intervention. Patient Educ Couns 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleyer AOLM, Barr R, Ries LAG (eds). Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000. In Institute NC; (ed). Bethesda, MD: 2006. [Google Scholar]
- 43.Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: research and cancer care imperatives for adolescents and young adults with cancer (NIH Publication No. 06–6067). Department of Health and Human Services, National Institutes of Health, National Cancer Institute, LIVESTRONG Young Adult Alliance; Bethesda, MD; August 2006. Accessed May 6, 2019 https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf [Google Scholar]
- 44.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care 2005; 20: 187–191; discussion 191–183. [DOI] [PubMed] [Google Scholar]
- 45.UCLA Jonsson Comprehensive Cancer Center. In. https://cancer.ucla.edu/: 2014.
- 46.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983; 51: 390–395. [DOI] [PubMed] [Google Scholar]
- 47.Rollnick SMW. What is motivational interviewing? Behavioural and Cognitive Psychotherapy October 1995; 23: 325–334. [Google Scholar]
- 48.Cain SM, Moore R, Sturm L et al. Clinical assessment and management of general surgery patients via synchronous telehealth. J Telemed Telecare 2017; 23: 371–375. [DOI] [PubMed] [Google Scholar]
- 49.Casillas J, Syrjala KL, Ganz PA, et al. How confident are young adult cancer survivors in managing their survivorship care? A report from the LIVESTRONG™ Survivorship Center of Excellence Network. J Cancer Surviv. 2011;5(4):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates,1988. [Google Scholar]
- 51.Rempel GR, Ballantyne RT, Magill-Evans J et al. Texting teens in transition: the use of text messages in clinical intervention research. JMIR Mhealth Uhealth 2014; 2: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oeffinger KC, Ford JS, Moskowitz CS et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 2009; 301: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landier W, Skinner R, Wallace WH et al. Surveillance for Late Effects in Childhood Cancer Survivors. J Clin Oncol 2018; 36: 2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devine KA, Viola AS, Coups EJ, Wu YP. Digital Health Interventions for Adolescent and Young Adult Cancer Survivors. JCO Clin Cancer Inform. 2018. December;2:1–15. doi: 10.1200/CCI.17.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corbett T, Singh K, Payne L et al. Understanding acceptability of and engagement with Web-based interventions aiming to improve quality of life in cancer survivors: A synthesis of current research. Psychooncology. 2018. January;27(1):22–33. doi: 10.1002/pon.4566. Epub 2017 Nov 21. [DOI] [PubMed] [Google Scholar]
- 56.Kopp LM, Gastelum Z, Guerrero CH et al. Lifestyle behavior interventions delivered using technology in childhood, adolescent, and young adult cancer survivors: A systematic review. Pediatr Blood Cancer. 2017. January;64(1):13–17. doi: 10.1002/pbc.26166. Epub 2016 Jul 28. [DOI] [PubMed] [Google Scholar]
- 57.Bradford NK, Chan RJ. Health promotion and psychological interventions for adolescent and young adult cancer survivors: A systematic literature review. Cancer Treat Rev. 2017. April;55:57–70. doi: 10.1016/j.ctrv.2017.02.011. Epub 2017 Mar 6. [DOI] [PubMed] [Google Scholar]
- 58.Hahn EE, Wu YL, Munoz-Plaza CE et al. Use of recommended posttreatment services for adolescent and young adult survivors of Hodgkin lymphoma. Cancer. 2019. May 1;125(9):1558–1567. doi: 10.1002/cncr.31953. Epub 2019 Jan 8. [DOI] [PubMed] [Google Scholar]
- 59.Prochaska JJ, Coughlin SS, Lyons EJ. Social Media and Mobile Technology for Cancer Prevention and Treatment. Am Soc Clin Oncol Educ Book. 2017;37:128–137. doi: 10.14694/EDBK_173841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sirintrapun SJ, Lopez AM. Telemedicine in Cancer Care. Am Soc Clin Oncol Educ Book. 2018. May 23;38:540–545. doi: 10.1200/EDBK_200141. [DOI] [PubMed] [Google Scholar]
- 61.Tercyak KP, Donze JR, Prahlad S et al. Identifying, recruiting, and enrolling adolescent survivors of childhood cancer into a randomized controlled trial of health promotion: preliminary experiences in the Survivor Health and Resilience Education (SHARE) Program. J Pediatr Psychol 2006; 31: 252–261. [DOI] [PubMed] [Google Scholar]
- 62.Butterfield PG, Yates SM, Rogers B, Healow JM. Overcoming subject recruitment challenges: strategies for successful collaboration with novice research agencies. Appl Nurs Res 2003; 16: 46–52. [DOI] [PubMed] [Google Scholar]
- 63.Bhatia S, Gibson TM, Ness KK et al. Childhood cancer survivorship research in minority populations: A position paper from the Childhood Cancer Survivor Study. Cancer. 2016. August 1;122(15):2426–39. doi: 10.1002/cncr.30072. Epub 2016 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantrell MA, Conte T, Hudson M et al. Recruitment and retention of older adolescent and young adult female survivors of childhood cancer in longitudinal research. Oncol Nurs Forum 2012; 39: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadan-Lottick NS, Robison LL, Gurney JG et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002; 287: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 66.Syed IA, Klassen AF, Barr R et al. Factors associated with childhood cancer survivors’ knowledge about their diagnosis, treatment, and risk for late effects. J Cancer Surviv 2016; 10: 363–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.