Abstract
Korean herbal medicine treatment (KHMT) involves treating with a combination of natural products, which have been used for thousands of years. Recently, it has been reported to be effective and safe in cancer patients. This case report demonstrates the efficacy of KHMT in a 49-year-old man with malignant pleural mesothelioma (MPM), a rare and highly aggressive cancer. The patient showed recurrent pleural effusion and was diagnosed with epithelioid MPM at cT3NxM0 stage III in December 2017. The multidisciplinary care team recommended multimodal treatment based on an extrapleural pneumonectomy, but he refused this because the treatment was aggressive and the effectiveness was unclear. He decided to undergo pemetrexed plus cisplatin chemotherapy if his condition worsened. He visited the Korean Medicine Cancer Center for alternative treatment options. A KHMT regimen, consisting of twice-daily Gunchil-dan and thrice-daily Bangam-tang, was initiated in December 2017. Since commencement of KHMT, computed tomography and X-ray imaging scans have shown no significant interval changes and progression. At 21 months into treatment (September 2019), no significant adverse events have occurred. Given that the median overall survival of patients with MPM is approximately 1 year, the ongoing progression-free survival of this patient for 21 months is relatively long. This case, therefore, suggests that KHMT is a potential treatment option for MPM patients.
Keywords: malignant pleural mesothelioma, rare cancer, Korean medicine, herbal medicine, complementary and alternative medicine
Introduction
Malignant pleural mesothelioma (MPM) is a rare and highly aggressive cancer with poor prognosis.1,2 Surgery-based multimodal therapy is regarded as a curative approach, but it is difficult to complete and not indicated for most patients. The majority of patients undergo chemotherapy, but typically the response of MPM patients is poor and the available chemotherapeutic agents are limited.3 Hence, complementary and alternative options for MPM are needed.
Korean herbal medicine treatment (KHMT) is the traditional system of medicine in the Republic of Korea and involves the use of medicinal herbs and other plants. Recent scientific studies, both preliminary and clinical, have reported the efficacy of KHMT in cancer—anticancer effects, alleviation of adverse effects, and protective and sensitizing effects in chemotherapy and radiotherapy.4
Here, we report the case of an MPM patient, who exhibited relatively long progression-free survival (PFS) following KHMT alone.
Case Report
This case study was approved by the institutional review board of the Kyung Hee University Hospital at Gangdong (Institutional Review Board No. KHNMC-OH 2019-09-014).
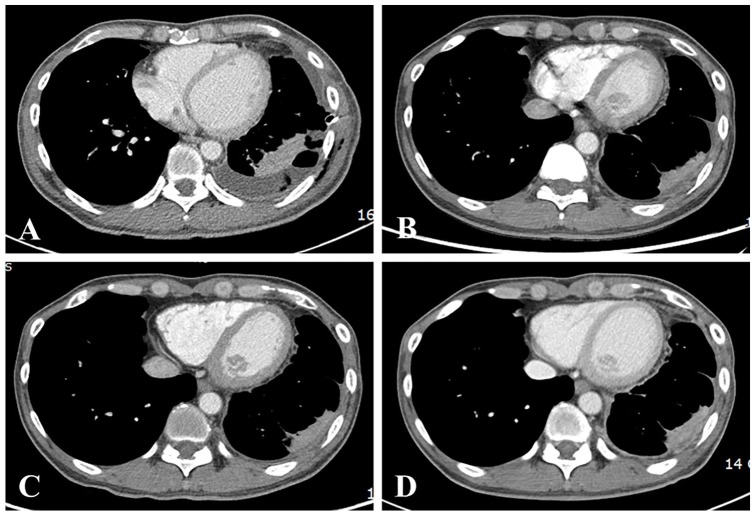
A 49-year-old man, who worked at an automobile factory and was exposed to chemicals on an intermittent basis, presented with left chest pain and dyspnea. A computed tomography (CT) scan in June 2017 showed diffuse thickening of the left pleura with a pleural effusion. The patient was diagnosed with tuberculous pleuritis and received drug treatment for 6 months, without effect. In December 2017, thoracoscopy was performed for recurrent pleural effusion; small lumps in the lung, mediastinal surface, and diaphragm were found. Subsequent pleural biopsy, baseline CT (Figure 1A), and positron emission tomography/CT (Figure 2) demonstrated epithelioid MPM (cT3NxM0) stage III.
Figure 1.
Computed tomography scans of the patient. (A) Baseline taken in December 2017 showing diffuse thickening of the left pleura with pleural effusion. (B) Taken in September 2018. (C) Taken in January 2019. (D) Taken in June 2019. No significant interval changes are visible.
Figure 2.
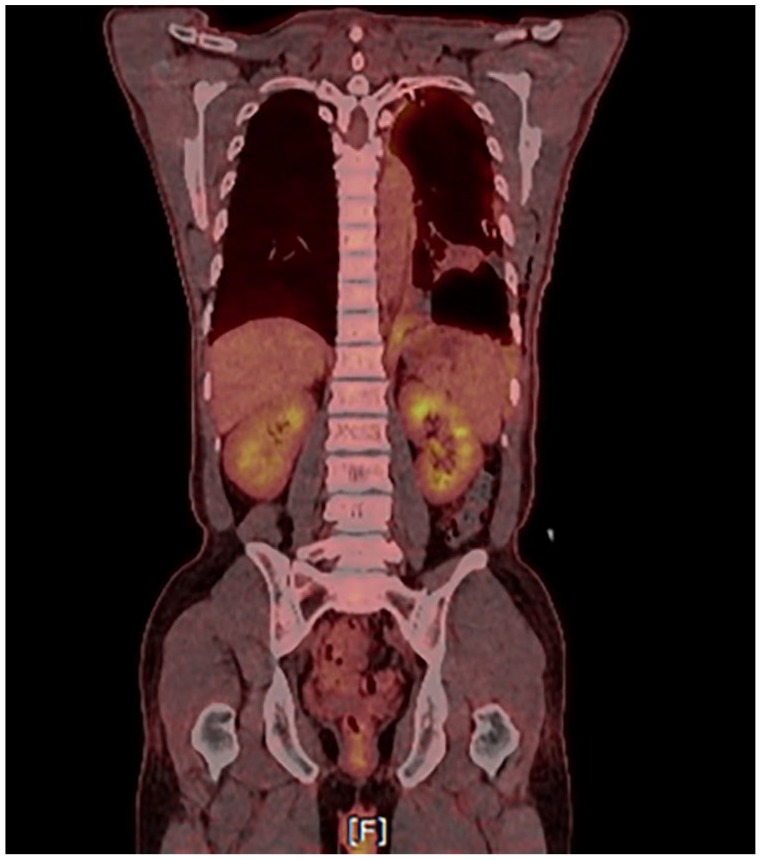
Positron emission tomography/computed tomography scan of the patient with increased FDG uptake showing thickening of the left pleura with pleural effusion in December 2017.
As the patient’s performance status was adequate with an Eastern Cooperative Oncology Group Performance Status score of 1, his multidisciplinary care team recommended surgery-based multimodal treatment involving an extrapleural pneumonectomy, left pericardial resection, diaphragmatic resection, and reconstruction. However, the treatment being highly aggressive and the expected benefit of the surgery unclear, he rejected it in favor of close observation. Chemotherapy effects are severe and chemotherapeutic agents for patients with MPM are limited, but following observation of chemotherapy, he agreed to consider pemetrexed plus cisplatin chemotherapy if his pleural effusion worsened. He approached the Korean Medicine Cancer Center to find alternatives.
KHMT of twice-daily Gunchil-dan and thrice-daily Bangam-tang was initiated in December 2017 and has been administered for 21 months at the time of writing, September 2019. There was no major change in lifestyle except quitting the job. Follow-up blood tests (Table 1) and imaging by CT and X-ray were performed. No significant adverse events were observed, defined as events of Grade 3 or higher according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.5 Carcinoembryonic antigen and carbohydrate antigen 19-9 were examined as experimental markers, but both were in the normal range. CT scans of the patient showed no significant interval changes in September 2018, January 2019, and June 2019 (Figure 1B, C, and D). According to the Response Evaluation Criteria in Solid Tumors version 1.1,6 the patient has stable disease. The PFS of the patient is now at 21 months, and he is still alive at the time of writing.
Table 1.
Blood Test Results of the Patient.
| December 22, 2017 | September 3, 2018 | November 12, 2018 | June 19, 2019 | |
|---|---|---|---|---|
| WBC (×103/µL) | No result | 5.47 | 5.27 | 5.89 |
| Hb (g/dL) | No result | 13.4 | 14.1 | 13.0 |
| PLT (×103/µL) | No result | 323 | 335 | 337 |
| CRP (mg/dL) | 0.5 | 0.8 | 0.8 | 0.9 |
| AST (U/L) | 24 | 15 | 21 | 18 |
| ALT (U/L) | 17 | 8 | 12 | 10 |
| BUN (mg/dL) | 15 | 14 | 12 | 15 |
| Cr (mg/dL) | 0.92 | 0.79 | 0.72 | 0.74 |
| LD (U/L) | 292 | No result | 267 | 255 |
| CEA (ng/mL) | 0.7 | No result | 0.9 | No result |
| CA19-9 (U/mL) | 4.6 | No result | No result | No result |
Abbreviations: WBC, white blood cell; Hb, hemoglobin; PLT, platelet; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; Cr, creatinine.
Discussion
Malignant pleural mesothelioma is a rare cancer originating in the cells of the mesothelial surfaces of the pleura, which accounts for most cases (81%) of mesothelioma.1 It is highly aggressive. Median overall survival (mOS) is approximately 1 year from the time of diagnosis,2 and 5-year relative survival is about 10% because most patients have advanced disease by the time of diagnosis.1 Improved survival has been reported following surgery-based multimodal therapy involving chemotherapy and radiotherapy. A few studies have reported survival benefits resulting from trimodal therapy with a mOS ranging from 15 to 29 months.7-9 Nelson et al found that multimodal therapy is frequently not completed due to mortality, dose constraints, postoperative morbidity or delayed recovery, patient refusal, or loss to follow-up.3 Among their 20 561 patients, only 4028 (20%) underwent cancer-directed surgery and 533 (2.6%) received trimodal therapy. Even after aggressive multimodal therapy, MPM frequently recurs.10 Surgical benefit has become unclear, following publication of the Mesothelioma and Radical Surgery(MARS) I trial.11 Consequently, most patients undergo systemic chemotherapy.
The National Comprehensive Cancer Network guideline for MPM (version 2.2019) recommends pemetrexed plus cisplatin, with or without bevacizumab, as a Category 1 first-line chemotherapy regimen.12 The combination of pemetrexed and cisplatin has been established since 2003, and the mOS and time to progression were found to be 12.1 months and 5.7 months, respectively.2 Bevacizumab is only used in selected patients.13 Due to the rarity and high lethality of the disease, there is a lack of clinical trials; treatment options are thus limited compared with those for other common malignancies. Complementary and alternative approaches for MPM are therefore needed.
Gunchil-dan is a capsule containing 350 mg Rhus verniciflua Stokes (RVS) extract from which the allergen urushiol has been removed. RVS is used as a traditional herbal therapy for the treatment of abdominal masses.14 Its efficacy and safety has been reported for various cancer types, including colon,15 gastric,16 hepatobiliary,17-19 renal,20 pancreatic,21 and pulmonary.22-24 In these, it has shown improved OS and PFS compared with standard treatments, without significant adverse events. The antitumor mechanism of RVS is likely to be anti-angiogenesis: it inhibits the proliferation and migratory activity of cells normally recruited via vascular endothelial growth factor. It also reduces the ability of cancer cells to invade healthy tissue by inhibiting the secretion of MMP-2 and MMP-9.25
Bangam-tang is a herbal decoction consisting of the following: Astragalus membranaceus Bunge 24 g/d, Atractylodes macrocephala Koidzumi 12 g/d, Poria cocos Wolf 12 g/d, Pinellia ternata Breitenbach 12 g/d, Citrus unshiu Markovich 12 g/d, Agastache rugosa O. Kuntze 4 g/d, Alisma orientale Juzepczuk 12 g/d, Plantago asiatica Linné 8 g/d, Spatholobus suberectus Dunn 8 g/d, Zizyphus jujuba Miller var. inermis Rehder 12 g/d, Glycine max Merrill 8 g/d, Crataegus pinnatifida Bunge 6 g/d, Hordeum vulgare Linné var. hexastichon Aschers 6 g/d, Prunus mume Siebold et Zuccarini 6 g/d, and Glycyrrhiza uralensis Fischer 6 g/d. This mixture has been used to improve gastrointestinal function and to modulate the immune system. Its efficacy and safety has been reported for anorexia associated with advanced cancer, producing improved appetite and increased body weight.26 The main component, Astragalus membranaceus Bunge, has several effects, including growth inhibition and reduction of tumor size; promotion of apoptosis; inhibition of cell proliferation, angiogenesis, cell invasiveness, and metastasis; attenuation of chemotherapeutic drug toxicity; and increase the sensitivity to chemotherapeutic drugs.27-31
Treatment with Gunchil-dan and Bangam-tang has been used in patients for whom standard treatments are not feasible, or who refuse standard treatments. No significant adverse events have been reported in either type of patient. Several traditional medicines including KHMT are attracting attention as alternatives to standard cancer treatments for reasons of efficacy and safety.4,32,33
The patient was misdiagnosed with tuberculous pleuritis at first and took tuberculous pleuritis drugs for 6 months, but pleural effusion recurred. In this context, the possibility of prolonged PFS by the drugs was low and the above KHMT might contribute to the PFS of patient.
This case has some limitations. First, the possibility of spontaneous regression (SR) cannot be excluded. Three case reports for SR of MPM have been reported,34-36 even though the SR of MPM is very rare.37 Second, pathologic findings with hematoxylin and eosin staining and immunohistochemistry were not supplemented, because there were no tissue samples left at the time of writing the case.
A patient with epithelioid MPM at cT3NxM0 stage III survives progression-free at the time of writing, 21 months after commencement of KHMT, and, given his original misdiagnosis, 26 months to date in total. This case suggests that KHMT could be a beneficial and safe alternative for patients with MPM. Further investigation, such as a randomized controlled trial, is needed to evaluate this possibility.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Traditional Korean Medicine R&D program funded by the Republic of Korea Ministry of Health and Welfare through the Korea Health Industry Development Institute (KHIDI; Grant No. HB16C0067).
ORCID iDs: Sung Soo Yoon  https://orcid.org/0000-0001-7970-215X
https://orcid.org/0000-0001-7970-215X
Jee Young Lee  https://orcid.org/0000-0002-1080-1915
https://orcid.org/0000-0002-1080-1915
References
- 1. American Cancer Society. Cancer facts & figures 2017: special section—rare cancers in adults. https://tinyurl.com/yb4joe3c. Accessed September 10, 2019.
- 2. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644. [DOI] [PubMed] [Google Scholar]
- 3. Nelson DB, Rice DC, Mitchell KG, et al. Return to intended oncologic treatment after surgery for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2019;158:924-929. [DOI] [PubMed] [Google Scholar]
- 4. Yoon SW, Jeong JS, Kim JH, Aggarwal BB. Cancer prevention and therapy: integrating traditional Korean medicine into modern cancer care. Integr Cancer Ther. 2014;13:310-331. [DOI] [PubMed] [Google Scholar]
- 5. US Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published November 27, 2017. Accessed September 10, 2019.
- 6. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [DOI] [PubMed] [Google Scholar]
- 7. Rea F, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer. 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Schil PE, Baas P, Gaafar R, et al. ; European Organisation for Research and Treatment of Cancer (EORTC) Lung Cancer Group. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J. 2010;36:1362-1369. [DOI] [PubMed] [Google Scholar]
- 9. Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:3007-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson DB, Rice DC, Niu J, et al. Predictors of trimodality therapy and trends in therapy for malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2018;53:960-966. [DOI] [PubMed] [Google Scholar]
- 11. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomized feasibility study. Lancet Oncol. 2011;12:763-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network. Malignant pleural mesothelioma, Version 2, 2019. https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf. Accessed September 10, 2019.
- 13. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405-1414. [DOI] [PubMed] [Google Scholar]
- 14. Yoo HT, Roh JR. Compendium of Prescriptions From the Countryside (Hyangyakjipseongbang). Vol 1433 Seoul, Republic of Korea: Hangrimchulpansa; 1977. [Google Scholar]
- 15. Lee SH, Choi WC, Yoon SW. Impact of standardized Rhus verniciflua stokes extract as complementary therapy on metastatic colorectal cancer: a Korean single-center experience. Integr Cancer Ther. 2009;8:148-152. [DOI] [PubMed] [Google Scholar]
- 16. Lee SH, Choi WC, Kim KS, Park JW, Lee SH, Yoon SW. Shrinkage of gastric cancer in an elderly patient who received Rhus verniciflua Stokes extract. J Altern Complement Med. 2010;16:497-500. [DOI] [PubMed] [Google Scholar]
- 17. Chae J, Lee S, Lee S. Potential efficacy of allergen removed Rhus verniciflua Stokes extract to maintain progression-free survival of patients with advanced hepatobiliary cancer. Explore (NY). 2018;14:300-304. [DOI] [PubMed] [Google Scholar]
- 18. Choi W, An S, Kwon E, Eo W, Lee S. Impact of standardized allergen-removed Rhus verniciflua stokes extract on advanced adenocarcinoma of the ampulla of Vater: a case series. Evid Based Complement Alternat Med. 2013;2013:203168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim KS, Jung HS, Choi WC, Eo WK, Cheon SH. A case of recurred hepatocellular carcinoma refractory to doxorubicin after liver transplantation showing response to herbal medicine product, Rhus verniciflua Stokes extract. Integr Cancer Ther. 2010;9:100-104. [DOI] [PubMed] [Google Scholar]
- 20. Lee SK, Jung HS, Eo WK, Lee SY, Kim SH, Shim BS. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: report of two cases. Ann Oncol. 2010;21:1383-1385. [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Kim K, Jung H, et al. Efficacy and safety of standardized allergen-removed Rhus verniciflua Stokes extract in patients with advanced or metastatic pancreatic cancer: a Korean single-center experience. Oncology. 2011;81:312-318. [DOI] [PubMed] [Google Scholar]
- 22. Lee J, Chae J, Lee S, et al. The efficacy and safety of standardized allergen-removed Rhus verniciflua extract as maintenance therapy after first-line chemotherapy in patients with advanced non-small cell lung cancer. Am J Chin Med. 2013;41:773-787. [DOI] [PubMed] [Google Scholar]
- 23. Cheon SH, Kim KS, Kim S, Jung HS, Choi WC, Eo WK. Efficacy and safety of Rhus verniciflua stokes extracts in patients with previously treated advanced non-small cell lung cancer. Forsch Komplementmed. 2011;18:77-83. [DOI] [PubMed] [Google Scholar]
- 24. Lee SH, Kim KS, Choi WC, Yoon SW. Successful outcome of advanced pulmonary adenocarcinoma with malignant pleural effusion by the standardized Rhus verniciflua Stokes extract: a case study. Explore (NY). 2009;5:242-244. [DOI] [PubMed] [Google Scholar]
- 25. Choi W, Jung H, Kim K, et al. Rhus vernicifluas stokes against advanced cancer: a perspective from the Korean Integrative Cancer Center. J Biomed Biotechnol. 2012;2012:874276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JJ, Lee JJ. A phase II study of an herbal decoction that includes Astragali radix for cancer-associated anorexia in patients with advanced cancer. Integr Cancer Ther. 2010;9:24-31. [DOI] [PubMed] [Google Scholar]
- 27. Auyeung KK, Law PC, Ko JK. Combined therapeutic effects of vinblastine and Astragalus saponins in human colon cancer cells and tumor xenograft via inhibition of tumor growth and proangiogenic factors. Nutr Cancer. 2014;66:662-674. [DOI] [PubMed] [Google Scholar]
- 28. Auyeung KK, Woo PK, Law PC, Ko JK. Astragalus saponins modulate cell invasiveness and angiogenesis in human gastric adenocarcinoma cells. J Ethnopharmacol. 2012;141:635-641. [DOI] [PubMed] [Google Scholar]
- 29. Auyeung KK, Law PC, Ko JK. Astragalus saponins induce apoptosis via an ERK-independent NF-kappaB signaling pathway in the human hepatocellular HepG2 cell line. Int J Mol Med. 2009;23:189-196. [PubMed] [Google Scholar]
- 30. Auyeung KK, Cho CH, Ko JK. A novel anticancer effect of Astragalus saponins: transcriptional activation of NSAID-activated gene. Int J Cancer. 2009;125:1082-1091. [DOI] [PubMed] [Google Scholar]
- 31. Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28:1347-1355. [DOI] [PubMed] [Google Scholar]
- 32. Aggarwal BB, Ichikawa H, Garodia P, et al. From traditional ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Ther Targets. 2006;10:87-118. [DOI] [PubMed] [Google Scholar]
- 33. Hsiao WLW, Liu L. The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights. Planta Med. 2010;76:1118-1131. [DOI] [PubMed] [Google Scholar]
- 34. Moser JC, Peikert T, Roden AC, Midthun DE, Mansfield AS. Spontaneous regression of malignant pleural mesothelioma in a patient with new-onset inflammatory arthropathy. Ann Am Thorac Soc. 2015;12:1416-1417. [DOI] [PubMed] [Google Scholar]
- 35. Allen RK. Apparent spontaneous complete regression of a multifocal malignant mesothelioma of the pleura. Med J Aust. 2007;187:413-415. [DOI] [PubMed] [Google Scholar]
- 36. Kawanishi R, Ueoka H, Tabata M, et al. Spontaneous regression of malignant pleural mesothelioma. Int J Clin Oncol. 1997;2:118-120. [Google Scholar]
- 37. Kumar T, Patel N, Talwar A. Spontaneous regression of thoracic malignancies. Respir Med. 2010;104:1543-1550. [DOI] [PubMed] [Google Scholar]