Abstract
Background:
Interference screw fixation using bioabsorbable implants has become the most common form of tibial-sided graft fixation in anterior cruciate ligament reconstruction (ACLR). Complications related to implant use in the pediatric and adolescent population have not been well studied.
Purpose/Hypothesis:
The purpose of this study was to retrospectively analyze the complications associated with tibial bioabsorbable interference screw use in adolescents after ACLR. We hypothesized that complication rates would be low (<5%) and that different screw types would have similar complication rates and clinical outcomes.
Study Design:
Case series; Level of evidence, 4.
Methods:
Included in this study were patients aged ≤18 years who underwent ACLR with a bioabsorbable tibial interference screw between 2000 and 2011 at a single institution. The subpopulation with screw-related symptoms or complications were identified through chart review. The following 2 outcomes were considered: screw-related symptoms and secondary surgery related to the screw. Multivariable logistic regression was used for adjusted analysis of any screw-related problem.
Results:
There were 925 ACLR procedures in 858 patients (mean age, 15.7 years; range, 10-18 years) who met inclusion criteria. The median follow-up period was 32.0 months. Of the 925 knees, 89 (9.6%) developed a screw-related problem. In 44 (4.8%) cases, no surgery was required; in 45 (4.9%) cases, surgery for a screw-related problem occurred at a median of 24 months postoperatively. The most common surgical indication was pain at the tibial screw site (42/45, 93%), followed by intra-articular screw issues (3/45, 7%). In adjusted analysis, ACLR procedure performed by a “low-volume” ACL surgeon was the only significant predictor identified. After screw removal surgery, 25 of 27 (93%) patients with at least 12 months of follow-up had complete resolution of screw site symptoms, 18 of 23 (78%) evaluable patients returned to sports, while 8 of 27 (30%) patients underwent additional surgeries, 7 of which were unrelated to the screw procedure.
Conclusion:
The rate of clinical sequelae from bioabsorbable tibial interference screws was surprisingly high, with symptoms arising after approximately 1 of 10 ACLRs in adolescents. Reoperation for these symptoms was performed in approximately 5% of the knees in the study, at a median 2 years postoperatively. Most patients were able to return to sports after screw removal surgery.
Keywords: knee, ACL, pediatric sports medicine, tibial screw
Anterior cruciate ligament (ACL) tear is a common sports injury in adolescent patients, for whom ACL reconstruction (ACLR) is generally pursued to restore knee stability and allow return to sports (RTS). The use of an interference screw to secure a graft in the tibial tunnel became popular because noninterference methods, such as metal staples or tying the graft over a cortical post, were shown to be associated with loosening, loss of fixation, and need for subsequent hardware removal.9,18,23 Material options for tibial interference screws include metal or bioabsorbable compounds. Complications related to metal screws such as pain, graft injury at time of insertion, magnetic resonance imaging (MRI) incompatibility, need for a second operation for screw removal, and technical challenges with revision surgery have been reported.11,25,29 These reports led to the development and popularization of bioabsorbable interference screws.
A 2016 population-based epidemiologic study31 on over 20,000 ACLRs indicated that bioabsorbable screw fixation had become the most common method of tibial graft fixation for all graft choices, the most common of which is hamstring or patellar tendon autograft. The outcomes have generally been equivalent after the use of bioabsorbable screws for tibial fixation versus metal interference screw fixation, based on multiple randomized trials and meta-analyses.3,16,19,26,30 Advantages of the bioabsorbable screws are that they are less likely to damage the graft upon placement and that there is less stress shielding because of their ability to gradually transfer the load as they degrade.28 Reported complications for bioabsorbable screws include screw breakage, inflammatory or foreign body reaction, tunnel enlargement, and delayed graft healing/integration. In addition, in younger patients, many of whom have ongoing growth and unique properties of bone biology, the the screws may be broken down differently compared with their adult counterparts. To date, no large studies have evaluated complications related to the use of bioabsorbable tibial screws in ACLR specifically in pediatric and adolescent patients.
The purpose of this study was therefore to retrospectively analyze the complications associated with tibial bioabsorbable interference screw use in pediatric and adolescent athletes after ACLR. We hypothesized that complications would be minimal and that different screw types would not affect complication rates or clinical outcomes.
Methods
After institutional review board approval, Current Procedural Terminology billing codes were cross-referenced with computerized medical records to identify patients at a single institution who were aged ≤18 years and who underwent ACLR surgery with a bioabsorbable interference screw from January 1, 2000, to December 31, 2011. All graft types and revision ACLR cases were included. Computerized medical records in these patients were then scrutinized to identify all patients with screw-related complaints, including all patients who subsequently needed a reoperation to treat symptoms related to the tibial interference screw. Exclusion criteria consisted of patients who did not undergo ACLR with a bioabsorbable screw for tibial fixation, such as pediatric patients who underwent the physeal-sparing iliotibial band reconstruction technique.17 Patients needed to have completed a clinic visit at a minimum of 1 year after ACLR to be included in the study.
Recorded patient data included age, sex, body mass index, mechanism of injury, primary sport, length of time from injury to presentation and from injury to surgery, duration of follow-up, and history of prior knee surgery (as documented in the computerized medical records from patient reporting at initial visit). Imaging data were collected from direct review of MRI or, in a minority of cases where outside imaging was unavailable, from formal radiologist MRI reports as well the treating orthopaedic surgeon’s review as documented in the medical record. Recorded data from imaging included the presence of inflammation or cystic fluid around the tibial interference screw or the presence of loose fragments of the interference screw, along with assessment of concurrent injuries such as medial collateral ligament, posterior cruciate ligament, lateral collateral ligament, and meniscal and chondral injuries.
Procedural data were collected from operative reports, with recorded variables including date of surgery, surgeon, screw type, screw size, and features of the reconstruction, such as graft type (categorized as autograft hamstring, autograft bone–patellar tendon–bone, or cadaveric allograft tendon), revision or primary reconstruction, and concurrent procedures performed, including meniscectomy, meniscal repair, or other ligament repairs. “Low volume surgeons” were classified as averaging equal to or less than 10 ACLR surgeries per year over the study period, while “high volume surgeons” were classified as averaging more than 10 ACLR surgeries per year over the study period.
The following 2 primary outcomes after ACLR surgery were considered: screw-related symptoms and secondary surgery related to the screw. Percentages of cases experiencing these outcomes were compared between various subgroups using the Fisher exact test for unadjusted analyses and multivariable logistic regression for adjusted analyses. Factors with P values <.10 in unadjusted analysis were considered as candidates for evaluation in the regression models. The distributions of time from ACLR to screw removal and from screw removal to a second reoperation were estimated with the Kaplan-Meier method.
For the subgroup of cases who underwent screw removal surgery, postoperative variables (after the screw removal surgery), including persistent pain, ability to RTS, and need for secondary reoperation (ie, a third operation on the knee), were considered for all patients with ≥12 months of follow-up from screw removal surgery. Because so many uninjured children and adolescents are, at baseline, in transitional phases of athletic participation during middle school, high school, or graduation from high school (educational phases corresponding to the ages of the study population), analysis of RTS was restricted to patients in whom the initial injury occurred while playing sports and in whose medical records there was adequate detail to understand their RTS status. The Fisher exact test was used to compare groups with respect to percentage achieving RTS and percentage requiring secondary reoperation after screw removal surgery. No factors were statistically significant in these unadjusted analyses, and regression models were therefore not pursued.
The statistical assumption of independent observations was partially violated in the analysis of clinical outcomes after ACLR, as there was a subgroup of patients (8%) contributing data from more than 1 ACLR. To evaluate the sensitivity of our analyses to the clustered nature of the data, P values from the Fisher exact test and from a logistic model with a random patient effect were compared. In another sensitivity analysis, we compared the results of the Fisher exact test with those of the log-rank test, which takes into account the time to screw surgery or, in those who did not undergo screw surgery, the amount of follow-up. The results were substantively unchanged in these alternative analyses, and we report only the Fisher exact test results. In the analysis of outcomes after screw removal surgery, there was very little clustering, as only 1 of 45 patients with screw removal surgery contributed more than 1 case.
P values are 2-sided and considered statistically significant at the .05 level. All statistical analyses were conducted with SAS 9 software (Version 9.4, SAS Institute Inc).
Results
There were 925 ACLR cases in 858 patients who met the inclusion criteria. For 793 patients, a single knee was studied. A total of 63 patients contributed 2 ACLR cases each and 2 patients contributed 3 cases each. Demographic and surgical characteristics of the 925 cases are summarized in Table 1. A total of 409 cases in 375 patients did not meet the minimum follow-up requirements (12 months) and were therefore included in the demographic analyses, but excluded from the outcomes analyses. For the remaining 550 cases, follow-up from ACLR to last clinic visit ranged from 12 months to 13.2 years (median, 32.0 months; interquartile range [IQR], 20.9-49.2).
Table 1.
Characteristics of All Patientsa
| Characteristic | Value |
|---|---|
| Demographics | |
| Year of ACLR | |
| 2000-2004 | 189 (20) |
| 2005-2008 | 377 (41) |
| 2009-2011 | 359 (39) |
| Side | |
| Left | 446 (48) |
| Right | 479 (52) |
| Sex | |
| Male | 319 (34) |
| Female | 606 (66) |
| Patient age at ACLR, y | |
| 10-13 | 135 (15) |
| 14-16 | 576 (62) |
| 17-18 | 214 (23) |
| Mean (±SD) | 15.7 (±1.6) |
| ACLR procedure details | |
| Surgeon | |
| A | 441 (48) |
| B | 219 (24) |
| C | 157 (17) |
| D | 51 (6) |
| Others (n = 3) | 57 (6) |
| Graft type | |
| Autograft hamstring | 671 (73) |
| Autograft BTB | 158 (17) |
| Cadaveric allograftb | 96 (10) |
| Revision surgery | 33 (4) |
| Screw type: name (composition) (N = 919) | |
| BIO-RCI (PLLA-HA) | 838 (91) |
| Delta tapered or rounded (PLLA) | 43 (5) |
| Calaxo (PDLG 65%, CC 35%) | 5 (<1) |
| Others (mostly PLLA) | 33 (4) |
| Associated procedures | |
| Meniscal procedures | |
| None | 396 (43) |
| Meniscectomy only | 200 (22) |
| Repair ± meniscectomy | 329 (36) |
| Chondroplasty | 53 (6) |
| Other ligamentc injury or repair | 14 (2) |
| Any other procedured | 27 (3) |
aData are expressed as No. of knees (%) unless otherwise specified. N = 925 except where noted. ACLR, anterior cruciate ligament reconstruction; BTB, bone–patellar tendon–bone; CC, calcium carbonate; HA, hydroxyapatite; PDLG, poly-d,l-lactide-co-glycolide; PLLA, poly-l-lactic acid.
bTibialis anterior (n = 65), Achilles (20), hamstring (6), posterior tibial tendon (4), and BTB (1).
cMedial collateral ligament (n = 4), posterior cruciate ligament (3), lateral collateral ligament (8), and posterolateral corner repair (6). Some patients had more than 1 ligament injury or repair.
dLateral release (n = 13), loose body (8), osteochondritis dissecans drilling (3), removal of hardware (2), and hemarthrosis drainage (1).
Screw-Related Symptoms
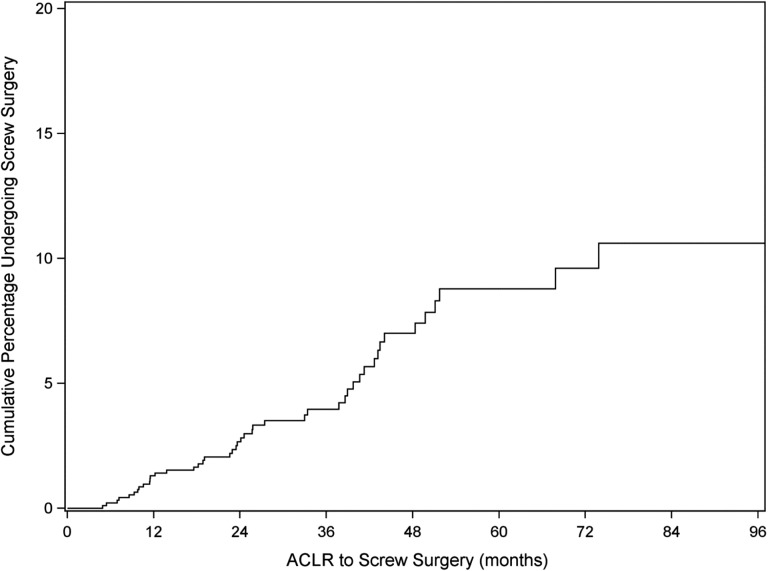
The outcomes assessed in the whole cohort were screw-related symptoms requiring or not requiring surgery. Of the 925 cases, 45 (4.9%) cases (in 44 patients) experienced screw-related symptoms requiring surgery. Figure 1 shows a Kaplan-Meier estimate of the cumulative probability of screw removal surgery over time. The 45 surgeries occurred 4.9 months to 6.2 years after ACLR (median, 24.1 months; IQR, 11.5-40.6). The time from onset of problem to date of surgery ranged from 0.1 to 14.1 months (median, 1.2 months; IQR, 0.7-2.5).
Figure 1.
Kaplan-Meier curve estimate of the cumulative probability of screw removal surgery over time. ACLR, anterior cruciate ligament reconstruction.
Another 44 (4.8%) cases (in 44 patients) experienced screw-related symptoms (screw site pain or prominence) that did not require surgery. In 13 of 41 (32%) patients for whom clear medical record–based information was available, the symptoms persisted at the last clinic visit. In the other 28 (68%) patients, the pain resolved after a period of observation. Therefore, a total of 45+44 or 89 (9.6%) patients had screw-related symptoms, with or without an additional screw-related surgery.
Table 2 summarizes the unadjusted analysis of predictors of screw-related symptoms requiring surgery and any screw-related symptoms. Right side, female sex, and revision surgery all had P values <.10 in unadjusted analysis of screw surgery and were considered simultaneously in a logistic model; all were statistically significant in the model (Table 3). In the unadjusted analysis of any screw problem, low-volume surgeon and right side were considered in logistic regression models. The final model retained only low-volume surgeon (Table 3). No particular screw type, of the 4 categories of screw makeup (see Table 1), was predictive of the need for secondary removal surgery, nor was any particular type predictive of screw-related symptoms.
Table 2.
Patient Characteristics for Screw-Related Symptoms Requiring Screw Removal Surgery and for Any Screw-Related Symptomsa
| Screw Removal | Any Screw-Related Symptoms | ||||
|---|---|---|---|---|---|
| Characteristic | Total | n (%) | P | n (%) | P |
| All | 925 | 45 (4.9) | 89 (9.6) | ||
| Demographics | |||||
| Year of ACLR | .81 | .61 | |||
| 2000-2004 | 189 | 11 (5.8) | 17 (9.0) | ||
| 2005-2008 | 377 | 17 (4.5) | 33 (8.8) | ||
| 2009-2011 | 359 | 17 (4.7) | 39 (10.9) | ||
| Side Left | 446 | 13 (2.9) | .009 | 35 (7.8) | .09 |
| Right | 479 | 32 (6.7) | 54 (11.3) | ||
| Sex | .04 | .29 | |||
| Male | 319 | 9 (2.8) | 26 (8.2) | ||
| Female | 606 | 36 (5.9) | 63 (10.4) | ||
| Patient age at ACLR, y | .44 | .53 | |||
| 10-13 | 135 | 4 (3.0) | 10 (7.4) | ||
| 14-16 | 576 | 28 (4.9) | 55 (9.5) | ||
| 17-18 | 214 | 13 (6.1) | 24 (11.2) | ||
| ACLR procedure details | |||||
| Surgeon volumeb | .23 | .01 | |||
| High | 817 | 37 (4.5) | 71 (8.7) | ||
| Low | 108 | 8 (7.4) | 18 (16.7) | ||
| Graft type | .94 | .68 | |||
| Autograft hamstring | 671 | 33 (4.9) | 65 (9.7) | ||
| Autograft BTB | 158 | 7 (4.4) | 13 (8.2) | ||
| Cadaver | 96 | 5 (5.2) | 11 (11.5) | ||
| Revision surgery | .07 | .24 | |||
| No | 892 | 41 (4.6) | 84 (9.4) | ||
| Yes | 33 | 4 (12.1) | 5 (15.2) | ||
| Screw type: name (composition) | .36 | .16 | |||
| BIO-RCI (PLLA-HA) | 838 | 40 (4.8) | 79 (9.4) | ||
| Delta tapered or rounded (PLLA) | 43 | 2 (4.7) | 7 (16.3) | ||
| Calaxo (PDLG 65%, CC 35%) | 5 | 1 (20.0) | 1 (20.0) | ||
| Others (mostly PLLA) | 33 | 1 (3.0) | 1 (3.0) | ||
| Associated procedures | |||||
| Meniscal procedures | .43 | .93 | |||
| None | 396 | 19 (4.8) | 39 (9.8) | ||
| Meniscectomy only | 200 | 13 (6.5) | 20 (10.0) | ||
| Repair ± meniscectomy | 329 | 13 (4.0) | 30 (9.1) | ||
| Chondroplasty | .17 | .15 | |||
| No | 872 | 40 (4.6) | 81 (9.3) | ||
| Yes | 53 | 5 (9.4) | 8 (15.1) | ||
| Other ligament injury or repairc | ≥.999 | ≥.999 | |||
| No | 911 | 45 (4.9) | 88 (9.7) | ||
| Yes | 14 | 0 (0.0) | 1 (7.1) | ||
| Any other procedure | .38 | .17 | |||
| No | 898 | 43 (4.8) | 84 (9.4) | ||
| Yes | 27 | 2 (7.4) | 5 (18.5) | ||
aACLR, anterior cruciate ligament reconstruction; BTB, bone–patellar tendon–bone; CC, calcium carbonate; HA, hydroxyapatite; PDLG, poly-d,l-lactide-co-glycolide; PLLA, poly-l-lactic acid.
bThree surgeons performed 157-441 surgeries each. Four surgeons performed 8-51 each.
cMedial collateral ligament, posterior cruciate ligament, lateral collateral ligament, and posterolateral corner repair.
Table 3.
Logistic Regression Models for Screw Surgery Outcome and Any Screw Problem Outcome
| Characteristic | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Screw surgery outcome | |||
| Right knee | 2.49 | (1.28-4.83) | .007 |
| Female sex | 2.25 | (1.07-4.76) | .03 |
| Revision surgery | 3.35 | (1.10-10.2) | .03 |
| Any screw problem outcome | |||
| Low-volume surgeon | 2.10 | (1.20-3.68) | .01 |
Outcomes Among Cases Requiring Screw Removal Surgery
Demographic, clinical, and operative characteristics of the 45 screw removal cases are demonstrated in Table 4. The most common surgical indication was pain at the tibial screw site (42/45, 93%). For these patients, the screws at the time of the ACLR surgery had measured between 8 and 11 mm in diameter and 25 and 35 mm in length (except for one 15-mm screw length). Screw “removal,” which frequently included debridement or lavage of partially reabsorbed, pastelike screw material, was performed through an open incision in all cases. Intraoperative screw site cultures were obtained in most cases but were positive in only 2 cases (Staphylococcus non-aureus), both of which were treated successfully with oral antibiotics alone.
Table 4.
Characteristics at Time of Screw Removal Surgerya
| Characteristic | Total | Value |
|---|---|---|
| Demographic and clinical | ||
| Sex | 45 | |
| Male | 9 (20) | |
| Female | 36 (80) | |
| Age at screw removal, y | 45 | |
| 14-16 | 12 (27) | |
| 17-18 | 14 (31) | |
| 19-24 | 19 (42) | |
| Mean (±SD) | 18.3 (±2.1) | |
| Injury to ACLR, d | 42 | |
| Mean (±SD) | 86.3 (±71.9) | |
| (range) | (10, 330) | |
| Time, ACLR to screw removal, y | 45 | |
| <2 | 22 (49) | |
| ≥2 | 23 (51) | |
| Weight, kg, mean (±SD) | 37 | 66.0 (±12.2) |
| BMI, kg/m2, mean (±SD) | 31 | 23.9 (±4.0) |
| Sport | 45 | |
| None | 1 (2) | |
| Soccer | 20 (44) | |
| Basketball | 7 (16) | |
| Lacrosse | 6 (13) | |
| Otherb | 11 (24) | |
| Duration of symptoms, mo | 42 | |
| 0-6 | 24 (57) | |
| 7-12 | 8 (19) | |
| >12 | 10 (24) | |
| Screw removal surgeonc | 45 | |
| A | 14 (31) | |
| B | 15 (33) | |
| C | 4 (9) | |
| D | 8 (18) | |
| Others (n = 3) | 4 (9) | |
| Surgical indications | 45 | |
| Painful screw | 42 (93) | |
| Arthrofibrosis | 1 (2) | |
| Locking | 1 (2) | |
| Partial graft tear | 1 (2) | |
| Procedures performed | ||
| Arthroscopyd | 45 | 21 (47) |
| Bone graft | 45 | 7 (16) |
| Intraoperative findings | ||
| Screw prominence with cystic inflammatory reaction | 45 | 44 (98) |
| Failure of fixation | 45 | 0 (0) |
| Screw appearance at removal | 45 | |
| Intact (did not resorb) | 22 (49) | |
| Degraded | 20 (44) | |
| Cracked | 3 (7) | |
| Intra-articular screw prominence | 45 | 3 (7) |
aData are expressed as No. of knees (%) unless otherwise specified. ACLR, anterior cruciate ligament reconstruction; BMI, body mass index.
bFootball (n = 3), field hockey (3), softball (2), baseball (1), hockey (1), and cross-country/track (1).
cSame surgeon as ACL repair in all but 4 cases.
d21 of 21 with arthroscopy performed showed ACL intact.
For the other 3 patients, the surgical indication was instability (associated with partial graft retear with the finding of prominent intra-articular screw, the proximal end of which underwent arthroscopic burring), stiffness (specifically loss of extension associated with a prominent intra-articular screw, for which arthroscopic lysis of adhesions and arthroscopic burring was performed), and a complaint of knee “locking” (associated with screw fragments seen as intra-articular loose bodies from presumed partial screw fragmentation that underwent arthroscopic removal of loose bodies).
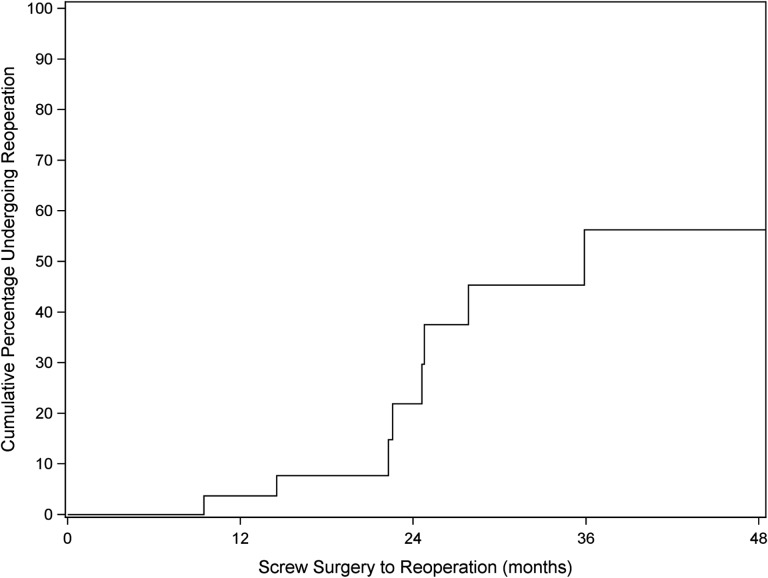
Not all of the 45 screw removal cases had sufficient follow-up data after this second surgery to assess additional outcomes, such as secondary reoperation, persistent pain, and RTS. Analysis of these additional outcomes was therefore restricted to the 27 patients with at least 12 months of follow-up after screw removal surgery (median follow-up, 22.8 months). Of these 27 patients, 25 (93%) had complete resolution of screw site symptoms. One of the 2 with persistent symptoms at the last visit demonstrated signs and symptoms of a meniscal tear and arthrofibrosis after burring of a prominent intra-articular screw, and the other had persistent saphenous nerve symptoms related to her tibial incision. Of the 27 patients, 8 (30%) underwent a reoperation after screw removal surgery. Indications for reoperation included chondral/meniscal issues (4), arthrofibrosis (2), partial ACL graft tear (1), and tibial tunnel cyst formation 2 years after removal of prominent tibial screw without a cystic component (1), the latter of which was felt to be the only reoperation directly related to the tibial screw removal procedure. Of the 8 reoperations, 4 patients had at least 12 months of additional follow-up after reoperation, and 1 of these required a second reoperation for a medial meniscal tear. Figure 2 shows a Kaplan-Meier estimate of the cumulative probability of reoperation after screw removal surgery over time.
Figure 2.
Kaplan-Meier curve showing estimation for all reoperations after tibial screw removal over time.
Of the 27 screw removal patients, 23 had at least 12 months of follow-up had RTS data; 18 of the 23 (78.3%) achieved RTS. There were no statistically significant associations between baseline characteristics and either RTS or secondary reoperation after tibial screw removal surgery.
Discussion
The rate of reoperation secondary to tibial screw issues in the current large series of pediatric and adolescent patients was approximately 5%, which occurred at a median time of 2 years after ACLR. Another approximately 5% of knees developed a screw-related problem that did not require surgery, yielding a bioabsorbable screw-related complication rate of 10%, which was higher than the study hypothesis of <5%. Regression analysis of potential predictive factors demonstrated that tibial screw–related complications were more common in low-volume surgeons. While most patients (78%) were able to RTS after screw removal surgery, the rate of requiring a third operation was high (30%), although mostly for non–screw related issues.
Bioabsorbable screw materials include biodegradable polymers such as poly(glycolic acid) (PGA), poly-l-lactic acid (PLLA), and poly-d,l-lactic acid. The rate of screw resorption depends on the screw design, size, shape, material, and environment of implantation. Pure PLLA implants have been used with good, long-term clinical results, but some studies16,20,24,33 have reported evidence of slow absorption, tunnel widening, and lack of bony ingrowth. Long-term MRI studies have been performed on PLLA screws to document absorption over time. Warden et al found that only 1 of 20 PLLA screws resorbed by 2-year follow-up,33 but at 10-year follow-up,32 all 6 patients who were available for study demonstrated complete screw resorption as well as intraosseous fluid collections at the tibial screw site, none of which were symptomatic. Although PLLA screws do resorb over time, there have been concerns about lack of bony ingrowth. Drogset et al,12 in an MRI study, reported complete resorption without bony ingrowth of PLLA screws on MRI in 16 patients at 7 years postsurgery.
Most bioabsorbable implants today combine a degradable polymer such as PLLA or PGA and an osteoconductive compound to promote bony ingrowth, such as calcium carbonate or trimethylene carbonate, or calcium phosphates, such as β-tricalcium phosphatate (β-TCP) and hydroxyapatite (HA).3,6 In an MRI study, Arama et al3 showed that PLLA-HA screws were associated with progressive screw resorption and gradual but partial re-ossification over 5 years and no adverse effects. Barber and Dockery4 have performed multiple computerized tomography studies on bioabsorbable screws to evaluate postimplantation bony ingrowth. For pure PLLA implants, they have shown that degradation is complete at 7 years postsurgery with very little bone ingrowth. For a composite 75% PLLA, 25% β-TCP screw, degradation was complete by 4 years with 75% incorporation of new bone,5 and for a screw composed of 70% PLLA/PGA copolymer with 30% β-TCP, osteoconductivity was observed in 80% of the screw sites and complete filling in 20% at 38 months after implantation.7
Previous case reports10,13,21,22 have documented cyst formation in relation to the use of tibial bioabsorbable screws, including those with composite osteoconductive agents. While most of these case reports describe sterile fluid collections in association with the screw, one case report27 identified a mycobacterium infection associated with an abscess around the bioabsorbable tibial screw. One case of transcutaneous extrusion was reported.1 Others have published case reports2,8,15 describing intra-articular migration of the bioabsorbable tibial interference screw or fracture of the screw causing locking symptoms and chondral damage after ACLR. Baums et al8 reviewed the medical literature from 1990 to 2005 and identified 6 cases of bioabsorbable screw failure associated with migration of screw into the knee joint. Potential factors identified included smaller diameter screws, poor bone quality, bone resorption, and screw divergence, although no specific risk factors were formally quantified.
Other than case reports or small–sample size (level 4) clinical series, there is a paucity of published literature on complications related to bioabsorbable tibial screws. In the largest previously published series, Ramsingh et al28 retrospectively reviewed 268 adult patients (mean age, 30 years) who underwent ACLR with the same PLLA-β-TCP biocomposite screw used for tibial fixation over a 4-year period. The authors identified 14 patients (5%) who developed pretibial pain and swelling over the tibial screw requiring surgical debridement at a mean time of 26 months postoperatively (range, 12-39 months). Gonzalez-Lomas et al14 reported histologic findings on 7 patients with pretibial cyst formation after ACLR with PLLA screws. The cohort included 140 ACLR procedures, suggesting a 5% incidence of pretibial cyst formation, given that no nonoperatively treated symptomatic patients were reported. The authors found that none of the cysts were infectious, the ACL grafts were well-incorporated, and histologically the cyst material contained fragments of PLLA surrounded by foamy histiocytes, consistent with foreign body/giant cell reaction as the mechanism for cyst formation.14
The complication rate in the current series is remarkably similar to the abovementioned studies, with the exception of a separate group of patients in the current series with similar symptoms who did not undergo surgical debridement (n = 44), of whom 68% improved over time with simple observation. In addition, the current series found that patients who underwent screw removal had a stable ACL (via either direct arthroscopic visualization or clinical examination under anesthesia) as well as successful RTS after screw removal surgery (only 1 patient went on to partial retear of ACL graft 3 years after screw removal surgery after a new injury). While tunnel size and the concept of enlargement or bony defects were not explored in the current study, the use of bone graft in association with screw removal was variable and may be somewhat surgeon dependent and unreliable as a proxy for tunnel enlargement. Importantly, however, the high rates of successful nonoperative treatment may indicate that cyst evolution may occur in some patients and may not necessitate surgical intervention if symptoms abate.
The single risk factor for tibial screw–related complications identified through regression analysis was a “low-volume” surgeon performing the initial ACLR, the reasons for which are unclear. It may be related to technical errors such as choice of screw size or screw position (too prominent intra-articularly or too prominent at the tibial side). Alternatively, low-volume surgeons may have been less familiar with this occasional phenomenon and therefore quicker to identify, document, and treat these complications, as they occurred. It can be argued that some of the complications reported, such as intra-articular screw prominence, are unrelated to screw material and may be related to surgeon skill (low volume vs high volume). There were 3 cases of intra-articular screw prominence, of which only 1 did not have extra-articular prominence with cystic inflammatory reaction. To explore whether the classification of intra-articular screw prominence as a screw-related complication contributed to the observed association between surgeon volume and complications, we reclassified these cases as not having any complications and repeated the analysis. The association became slightly weaker, but the P value only changed from .01 to .02. While no particular screw type, of the 4 categories of screw makeup studied, was predictive of the need for secondary removal surgery, or for the development of screw-related symptoms, this finding is limited by the infrequent use of some screw types in this retrospective study.
The strengths of the current study lie in the large sample size and the importance of establishing the rates and natural history of this phenomenon in the pediatric and adolescent subpopulation. Cultural trends in youth sports and epidemiologic trends in youth injuries found in this age group demonstrate it as the subgroup most affected by ACL tears; in addition, their high activity levels, active bone biology, and remaining growth are distinctly different from elderly populations. These factors may influence the frequency of this phenomenon secondary to more rapid breakdown of bioabsorbable material and may influence the success of the surgical treatment, or lack thereof. The limitations of the current study include its retrospective nature and the inherent limitations of clinical information obtained from existing medical records. While different surgeons and graft types were employed, therefore limiting the control of multiple variables, this variability may provide slightly more generalizability of the findings to multiple patients or surgeons. In addition, the study lacks comparison groups such as patients with metal screws; however, they were not utilized in our practice during the time period studied. Finally, while imaging review was performed on the patients who developed symptoms, there was a lack of available postoperative imaging in patients who did not develop symptoms, and detailed comparisons relating to tunnel size and implications of cyst formation could not be performed.
The results of the current study indicate that the complication rate related to tibial bioabsorbable screw fixation after ACLR in pediatric and adolescent patients is significant and higher than previously reported in adult series. These results reject our initial hypothesis and prior assumptions that complication rates related to these implants would be minimal in this age group. Patients and parents should be counseled appropriately before surgery with regard to this risk. Most complications were sterile cysts, although others were described earlier. Technical errors, specifically in cases of intra-articular screw prominence, made up a small minority of reported cases but may be unrelated to screw type or composition. Pediatric and adolescent patients with sterile cysts did well after reoperation for removal of screw material and cyst excision, similar to interventions reported in adult series, and a subset of nonoperatively treated patients reported resolution of symptoms over time. Factors that predispose to development of complications related to bioabsorbable tibial screw fixation remain difficult to elucidate. Low-volume surgeons may benefit from observing and discussing the details of screw selection and implantation technique with their more experienced colleagues. Neither specific screw properties, such as material or shape, nor patient-based factors appear to predispose certain patients to this phenomenon. However, given the rising rates of this surgery being performed, particularly on this age group, and the rising popularity of the category of implants, the topic benefits from continued research, particularly via prospective registry-type study models or randomized controlled trials utilizing different screw types (metal vs bioabsorbable) or comparison with complication rates related to other methods of tibial fixation after ACLR.
Footnotes
Final revision submitted November 5, 2019; accepted November 11, 2019.
One or more of the authors has declared the following potential conflict of interest or source of funding: D.E.K. has received educational support from Kairos Surgical. M.S.K. has received consulting fees from OrthoPediatrics, Smith & Nephew, Ossur, and Stryker; speaking fees from Smith & Nephew; royalties from OrthoPediatrics; and honoraria from Stryker. Y.-M.Y. has received consulting fees from Smith & Nephew and educational support from Kairos Surgical. B.E.H. has received educational support from Kairos Surgical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Boston Children’s Hospital Institutional Review Board (IRB-P00006536).
References
- 1. Anakwenze OA, Kancherla V, Kelly JD. Extrusion of tibial tunnel bioabsorbable screw 15 months after anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(12):1710–1713. [DOI] [PubMed] [Google Scholar]
- 2. Appelt A, Baier M. Recurrent locking of knee joint caused by intraarticular migration of bioabsorbable tibial interference screw after arthroscopic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):378–380. [DOI] [PubMed] [Google Scholar]
- 3. Arama Y, Salmon LJ, Sri-Ram K, Linklater J, Roe JP, Pinczewski LA. Bioabsorbable versus titanium screws in anterior cruciate ligament reconstruction using hamstring autograft: a prospective, blinded, randomized controlled trial with 5-year follow-up. Am J Sports Med. 2015;43(8):1893–1901. [DOI] [PubMed] [Google Scholar]
- 4. Barber FA, Dockery WD. Long-term absorption of poly-L-lactic acid interference screws. Arthroscopy. 2006;22(8):820–826. [DOI] [PubMed] [Google Scholar]
- 5. Barber FA, Dockery WD. Long-term absorption of beta-tricalcium phosphate poly-L-lactic acid interference screws. Arthroscopy. 2008;24(4):441–447. [DOI] [PubMed] [Google Scholar]
- 6. Barber FA, Dockery WD. Long-term degradation of self-reinforced polylevo (96%)/dextro (4%)-lactide/beta-tricalcium phosphate biocomposite interference screws. Arthroscopy. 2016;32(4):608–614. [DOI] [PubMed] [Google Scholar]
- 7. Barber FA, Dockery WD, Hrnack SA. Long-term degradation of a poly-lactide co-glycolide/beta-tricalcium phosphate biocomposite interference screw. Arthroscopy. 2011;27(5):637–643. [DOI] [PubMed] [Google Scholar]
- 8. Baums MH, Zelle BA, Schultz W, Ernstberger T, Klinger HM. Intraarticular migration of a broken biodegradable interference screw after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):865–868. [DOI] [PubMed] [Google Scholar]
- 9. Burks RT. Practical considerations in cruciate graft fixation. Oper Tech Orthop. 1992;2(2):71–75. [Google Scholar]
- 10. Busfield BT, Anderson LJ. Sterile pretibial abscess after anterior cruciate reconstruction from bioabsorbable interference screws: a report of 2 cases. Arthroscopy. 2007;23(8):911. [DOI] [PubMed] [Google Scholar]
- 11. Cook SD, Renz EA, Barrack RL, et al. Clinical and metallurgical analysis of retrieved internal fixation devices. Clin Orthop Rel Res. 1985;194:236–241. [PubMed] [Google Scholar]
- 12. Drogset JO, Straume LG, Bjorkmo I, Myhr G. A prospective randomized study of ACL-reconstructions using bone-patellar tendon-bone grafts fixed with bioabsorbable or metal interference screws. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dujardin J, Vandenneucker H, Bellemans J. Tibial cyst and intra-articular granuloma formation after anterior cruciate ligament reconstruction using polylactide carbonate osteoconductive interference screws. Arthroscopy. 2008;24(2):238–242. [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez-Lomas G, Cassilly RT, Remotti F, Levine WN. Is the etiology of pretibial cyst formation after absorbable interference screw use related to a foreign body reaction? Clin Orthop Relat Res. 2011;469(4):1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall MP, Hergan DM, Sherman OH. Early fracture of a bioabsorbable tibial interference screw after ACL reconstruction with subsequent chondral injury. Orthopedics. 2009;32(3):208. [PubMed] [Google Scholar]
- 16. Kaeding C, Farr J, Kavanaugh T, Pedroza A. A prospective randomized comparison of bioabsorbable and titanium anterior cruciate ligament interference screws. Arthroscopy. 2005;21(2):147–151. [DOI] [PubMed] [Google Scholar]
- 17. Kocher MS, Heyworth BE, Fabricant PD, Tepolt FA, Micheli LJ. Outcomes of physeal-sparing ACL reconstruction with iliotibial band autograft in skeletally immature prepubescent children. J Bone Joint Surg Am. 2018;100(13):1087–1094. [DOI] [PubMed] [Google Scholar]
- 18. Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15(3):225–229. [DOI] [PubMed] [Google Scholar]
- 19. Laupattarakasem P, Laopaiboon M, Kosuwon W, Laupattarakasem W. Meta-analysis comparing bioabsorbable versus metal interference screw for adverse and clinical outcomes in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):142–153. [DOI] [PubMed] [Google Scholar]
- 20. Ma CB, Francis K, Towers J, Irrgang J, Fu FH, Harner CH. Hamstring anterior cruciate ligament reconstruction: a comparison of bioabsorbable interference screw and endobutton-post fixation. Arthroscopy. 2004;20(2):122–128. [DOI] [PubMed] [Google Scholar]
- 21. Malhan K, Kumar A, Rees D. Tibial cyst formation after anterior cruciate ligament reconstruction using a new bioabsorbable screw. Knee. 2002;9(1):73–75. [DOI] [PubMed] [Google Scholar]
- 22. Martinek V, Friederich NF. Tibial and pretibial cyst formation after anterior cruciate ligament reconstruction with bioabsorbable interference screw fixation. Arthroscopy. 1999;15(3):317–320. [DOI] [PubMed] [Google Scholar]
- 23. Matthews LS, Soffer SR. Pitfalls in the use of interference screws for anterior cruciate ligament reconstruction: brief report. Arthroscopy. 1989;5(3):225–226. [DOI] [PubMed] [Google Scholar]
- 24. McGuire DA, Barber FA, Elrod BF, Paulos LE. Bioabsorbable interference screws for graft fixation in anterior cruciate ligament reconstruction. Arthroscopy. 1999;15(5):463–473. [DOI] [PubMed] [Google Scholar]
- 25. Miller MD. Revision cruciate ligament surgery with retention of femoral interference screws. Arthroscopy. 1998;14(1):111–114. [DOI] [PubMed] [Google Scholar]
- 26. Myers P, Logan M, Stokes A, Boyd K, Watts M. Bioabsorbable versus titanium interference screws with hamstring autograft in anterior cruciate ligament reconstruction: a prospective randomized trial with 2-year follow-up. Arthroscopy. 2008;24(7):817–823. [DOI] [PubMed] [Google Scholar]
- 27. Oh HL, Chen DB, Seeto BG, Macdessi SJ. Mycobacterium fortuitum infection after anterior cruciate ligament reconstruction using a polylactic acid bioabsorbable screw: case report. Knee. 2010;17(2):176–178. [DOI] [PubMed] [Google Scholar]
- 28. Ramsingh V, Prasad N, Lewis M. Pre-tibial reaction to biointerference screw in anterior cruciate ligament reconstruction. Knee. 2014;21(1):91–94. [DOI] [PubMed] [Google Scholar]
- 29. Shellock FG, Mink JH, Curtin S, Friedman MJ. MR imaging and metallic implants for anterior cruciate ligament reconstruction: assessment of ferromagnetism and artifact. J Magn Reson Imaging. 1992;2(2):225–228. [DOI] [PubMed] [Google Scholar]
- 30. Shen C, Jiang SD, Jiang LS, Dai LY. Bioabsorbable versus metallic interference screw fixation in anterior cruciate ligament reconstruction: a meta-analysis of randomized controlled trials. Arthroscopy. 2010;26(5):705–713. [DOI] [PubMed] [Google Scholar]
- 31. Tibor L, Chan PH, Funahashi TT, Wyatt R, Maletis GB, Inacio MC. Surgical technique trends in primary ACL reconstruction from 2007 to 2014. J Bone Joint Surg Am. 2016;98(13):1079–1089. [DOI] [PubMed] [Google Scholar]
- 32. Warden WH, Chooljian D, Jackson DW. Ten-year magnetic resonance imaging follow-up of bioabsorbable poly-L-lactic acid interference screws after anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):370. [DOI] [PubMed] [Google Scholar]
- 33. Warden WH, Friedman R, Teresi LM, Jackson DW. Magnetic resonance imaging of bioabsorbale polylactic acid interference screws during the first 2 years after anterior cruciate ligament reconstruction. Arthroscopy. 1999;15(5):474–480. [DOI] [PubMed] [Google Scholar]