Abstract
Antibiotic resistance is a massive and serious threat to human welfare and healthcare. Apart from being genetically resistant to antibiotics, the other important mechanism by which bacteria can evade antibiotics is multidrug tolerance. Here cells enter into a transiently nongrowing phase, and as a result, latent infection remains inside the host, causing disease recurrence. Biofilm-derived antibiotic tolerance and persister formation of the pathogenic bacteria inside the host remain a serious issue of treatment failure and recurrent chronic infection in the case of all major pathogens. As a result, new chemotherapeutic agents are sought that specifically inhibit biofilm formation or maturation as well as cause the dispersion of mature biofilms, thus allowing the conventional drugs to kill sensitive cells residing inside. This mini-review attempts to analyze different small-molecule-based chemical approaches that have been used to enable bacterial biofilm inhibition at different steps of maturation.
Introduction
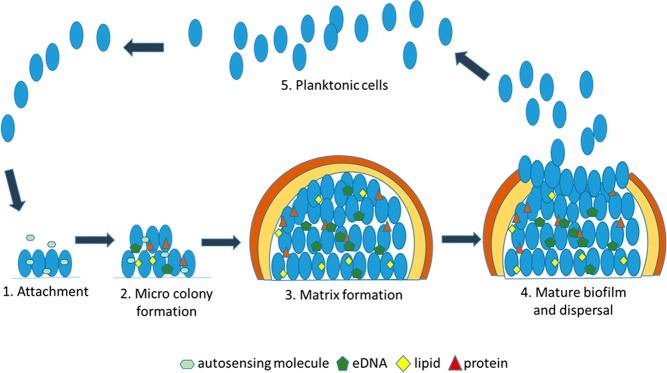
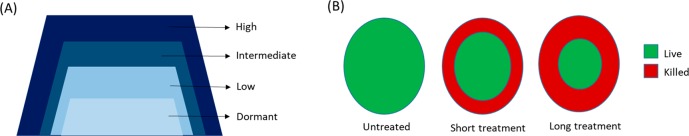
Bacterial biofilms, which are a self-synthesized, matrix-enclosed, surface-attached cell mass, harbor a large proportion of drug-tolerant population (Figure 1). Pathogenic bacteria residing inside the biofilm survive antibiotic treatment by three different, yet inter-related, mechanisms: (i) The outer thick layer of the biofilm extracellular matrix which consists of polysaccharide, proteins, extracellular DNA, lipids, complex sugars, etc.1 that physically blocks the penetration of the antibiotics, (ii) biofilm components sequester antibiotics, and (iii) the inner hostile environment of the biofilm which lacks nutrients and oxygen promotes bacteria to become metabolically inactive and thus survive the low dose of antibiotics. As most of the antibiotics are designed to target pathways such as DNA replication, cell wall biogenesis, and protein synthesis, these processes are either partly or completely absent in the biofilm-grown bacterial population (Figure 2). Unlike antibiotics, most designed antibiofilm agents should target auxiliary pathways without affecting bacterial survival and hence exert less selection pressure for the development of resistant mutants. In many instances, a combination of such compounds with front line drugs has proved to possess a superior therapeutic effect.
Figure 1.
Different steps of bacterial biofilm formation.
Figure 2.
(A) Metabolic activity of cells within biofilm is a function of depth, and (B) the biofilm enables partial penetration and killing by antibiotics.
Bacterial Biofilm: Clinical Relevance
Bacteria can form biofilms on living surfaces such as host tissue and nonliving surfaces such as medical devices and implants which are of the utmost clinical relevance. In humans, 80% of all the bacterial infections can be related to biofilm-derived pathogens. The most common biofilm-forming bacteria include Staphylococcus epidermidis, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Enterococcus faecalis, Streptococcus viridans, Escherichia coli, and Proteus mirabilis. Bacterial cells within the biofilm have proved to be a thousand times more resistant against standard antibiotics compared to the planktonic population and are resistant to host immune response, making them extremely difficult to eliminate. S. epidermidis is considered to be one of the most common bacteria associated with medical-device-related biofilm and leads to the spread of antibiotic resistance and treatment failure. Nosocomial pathogen P. aeruginosa is an efficient biofilm former and thus becomes difficult to treat in patients suffering from cystic fibrosis and ventilator-associated pneumonia (VAP). S. aureus and S. epidermidis biofilms are known to be responsible for 40–50% of prosthetic heart valve infections and 50–70% of catheter infections. In the case of K. pneumoniae, it has been reported that 50% of the time carbapenem-resistant strains have strong biofilm phenotype. Similarly, the opportunistic pathogen E. faecalis forms a biofilm on different medical devices such as catheters and heart valves, thereby promoting pathogenicity and antibiotic resistance. Clinical isolates of endocarditis-causing S. viridans strains have been shown to form dense biofilm in vitro and able to tolerate high concentration, as much as 128 times the minimum inhibition concentration of drugs compared to planktonic cells. P. mirabilis strains readily form biofilms in different abiotic surfaces such as glass, polystyrene, and silicon and are often associated with urinary tract infection. A recent study involving A. baumanii demonstrates the resistance profile of a bacterial population linked directly to the complex evolutionary dynamics of its biofilm lifestyle, hence impacting the treatment outcome. Uropathogenic strains of E. coli (UPEC) are reported to be involved in biofilm formation and the severity of catheter-based urinary tract infections in hospital. Thus, it is evident from a clinical point of view that biofilm plays a huge role in several important infectious diseases.
Biofilm Inhibition
Understanding the necessity of the development of antibiofilm compounds, many research groups around the world focus on various domain-specific approaches. Biofilm inhibition can be potentially targeted by either natural products or synthetic analogues. There are advantages in both of these approaches, which can be classified broadly as (i) inhibition of biofilm formation and (ii) disruption of preformed biofilm. There are different therapeutic applications for both, as the former approach could be useful to prevent biofilm formation after surgery or on medical devices, whereas the latter approach could be used in combination with standard drugs, specifically targeting the biofilm-forming population within infection sites.
Biofilm inhibition can be achieved in different steps: (i) the inhibition of bacterial surface adhesion or the initiation step; (ii) interference with the quorum-sensing system; (iii) modulation with the second nucleotide messenger signaling molecules; (iv) chemical inhibition of biofilm maturation; and (v) disruption of mature biofilms. Small organic compounds can inhibit the bacterial surface adhesion and interfere with the quorum-sensing system. On the other hand, modulation with the second nucleotide messenger-signaling molecules, chemical inhibition of biofilm maturation, and disruption of mature biofilms can be achieved with specific pathway inhibitors.
I. Inhibition of Bacterial Surface Adhesion
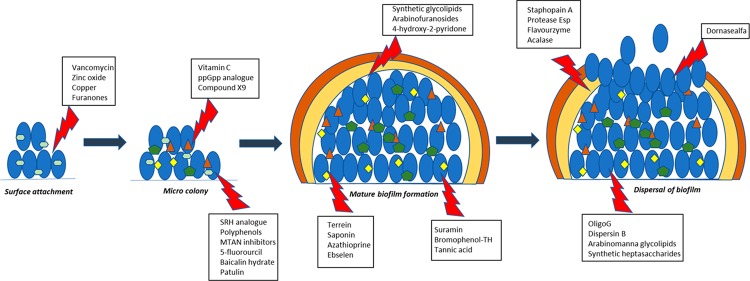
The first step of successful biofilm formation is attachment of the bacterial cells to the surface or substratum. This line of research is considered to be the nonspecific approach where surface modifications of the biomaterials are necessary with the help of antibiotics, metal ions, and other synthetic compounds. Bactericidal antibiotics are commonly used to coat medical devices in order to kill any pathogenic bacteria which come in contact with the outer surface. Vancomycin has been shown to prevent S. epidermidis biofilm formation when it is covalently bound to titanium alloy. Other bactericidal compounds that are in practice are zinc oxide nanoparticle coating on glass and iodine-coated titanium implants. Copper ion implantation is shown to have antibacterial property against S. aureus.2 Furanones are used widely as coating on medical devices in order to prevent biofilm formation by different pathogenic bacteria. Covalently coupled 3-(trimethoxysilyl)-propyldimethyloctadecyl ammonium chloride (QAS) is also shown to prevent biofilm formation due to its antimicrobial property. Antiadhesion surface coating is a similar approach to prevent initial bacterial attachment with host and device surfaces. Here, chemical compounds are used, in order to change hydrophobicity and hydrophilicity, surface roughness, and texture of the surface.
ii. Interference with the Quorum-Sensing System
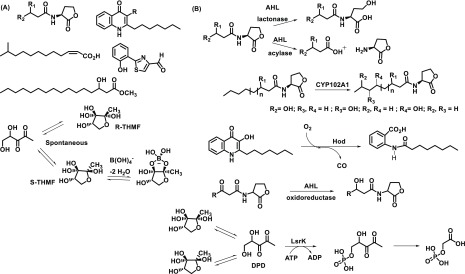
Quorum sensing (QS) refers to the bacterial communication system at the molecular level by which bacteria responds to different environmental signals and translates the message by up-regulating and/or down-regulating a set of genes involved in stress tolerance, virulence, and biofilm formation. QS is composed of density-dependent synthesis and sensing of certain extracellular small molecules called “autoinducers” which can vary across different bacterial species. The main class of autoinducers (AIs) studied in many Gram (-ve) bacteria such as Pseudomonas spp, Burkholderia spp, and Acinetobacter spp is acyl-homoserine lactones (AHLs) (Figure 3). AHLs have been broadly classified as traditional and noncanonical signal types in both Gram (+ve) and Gram (-ve) bacteria. Traditional AHLs such as C4–C8 and 3OC4–3OC18 are shown to be associated with biofilm formation, virulence, and swarming motility, whereas very few of the noncanonical AHLs could be linked to a distinct phenotype. p-Coumaroyl-HSL from Rhodopseudomonas palustris and N-carboxyl-acyl-HSL from Methanothrix harundinacea has been discovered to be involved in global gene expression and filamentous growth, respectively.3 Other types of autoinducers reported to date are quinolones in the case of Pseudomonas aeruginosa, ketone-based autoinducer-1 (CAI-1) for Vibrio spp and Legionella spp; fatty acids in Xanthomonas spp and Burkholderia spp known as diffusible signal factors (DSF); epinephrine; and AI-3. Similarly, indole is shown to be involved in intercellular signaling and modulate certain pathogenic bacterial phenotypes such as biofilm formation and virulence.4 In Gram (+ve) bacteria, species-specific autoinducer peptides (AIPs) play the crucial role of QS activation. In the case of Staphylococcus aureus, a broad range of AIPs from AIP-1 to AIP-4 have been described. Similarly, AIP Se-1,2,3 has been reported in a clinically relevant biofilm of Staphylococcus epidermidis. AI-2 acts as a QS stimulator in both Gram (+ve) and Gram (-ve) bacteria, and some bacteria are equipped to respond to multiple QS stimulators simultaneously in a definite order of a complex regulatory network.
Figure 3.
(A) Chemical structures of few Gram (-ve) bacterial quorum-sensing molecules. (B) Different reactions catalyzed by quorum-sensing molecule-degrading enzymes.
The molecular mechanism to hamper QS is called quorum quenching (QQ), which was first discovered in Erwinia carotovara. There are several naturally occurring and synthetic quorum-sensing inhibitors (QSIs) by which bacteria block these chemical messengers in different ways. Extracellular enzymatic hydrolysis of AIs by lactonases, acylases, and oxidoreductase enzymes have been reported by Fetzner and co-workers (Figure 3),5 whereas synthetic small molecules have shown to interfere with the production of AIs. Since almost all AI-2s are synthesized by LuxS enzyme, LuxS inhibition was targeted by different substrate and intermediate analogues. One such notable inhibitor is the SRH analogue 3,5,6-trideoxy 6-fluorohex-5-enofuranose, where the C3 hydroxyl group is absent, which is necessary for conversion.6 The phage display method was used to find a peptide inhibitor of LuxS, and one peptide, TNRHNPHHLHH, has shown promising activity with ∼25% reduction in catalytic activity of the LuxS enzyme. Another attracting target to inhibit QS in E. coli, S. pneumoniae, and V.cholerae is MTAN (methylthioadenosine nucleosidase), which is directly involved in AHL and AI-2 production. Though some MTAN inhibitors have shown antimicrobial activity, largely they are very specific to inhibit QS-derived biofilm formation without any effect on the bacterial growth profile, highlighting the importance in terms of preventing selection of resistant mutants.7 In E. faecalis and other Gram (+ve) bacteria, amburic acid has been shown to inhibit the production of signaling peptides with an unknown mechanism. The scavenging mechanism of the AIs by cyclodextrins and antibodies has been described as an alternative approach. QS antagonistic peptides have shown promising results to inhibit AI-2 and other QS molecules in both Gram (+ve) and Gram (-ve) bacteria. Several natural compounds have been studied to possess anti-QS properties like eugenol from clove, polyphenols from tea or honey, and ajoene from garlic. Synthetic small molecules like azithromycin and 5-fluorouracil (5-FU) were also studied extensively to demonstrate the QS control mechanism. Combination therapy of different QQs with standard antibiotics has been explored and shown to have synergistic effects in most occasions. Aminoglycosides and quinolones have been shown to have greater antimicrobial activity against Pseudomonas aeruginosa biofilm. Similarly, cephalosporin, glycopeptide, and polypetide antibiotics have been shown to have enhanced potency in the presence of different QSIs against P. aeruginosa. Brackman and co-workers have shown that the QSI compound baicalin hydrate and hamamelitannin has a definite synergistic effect with tobramycin against P. aeruginosa and with vancomycin against S. aureus both in vitro and in vivo (Figure 4).8 A benzimide–benzimidazole compound having a strong inhibitory effect on the QS regulator MvrF in P.aeruginosa thus plays a prominent role in reduced biofilm formation and increased susceptibility. Penicillic acid, patulin, and furanone C30 are known to have a synergistic effect against P. aeruginosa when combined with antibiotics or immune system-based killing.9 In general, coadministration of QSI along with antibiotics to disrupt biofilm formation and reduce virulence of the pathogenic bacteria seems to be an exciting approach as the biofilm harbors a large amount of drug-tolerant population. Thus, QSI helps to lower the dose of antibiotics with shorter treatment period and reduced toxicity as well as enhanced activity of the host innate immunity.
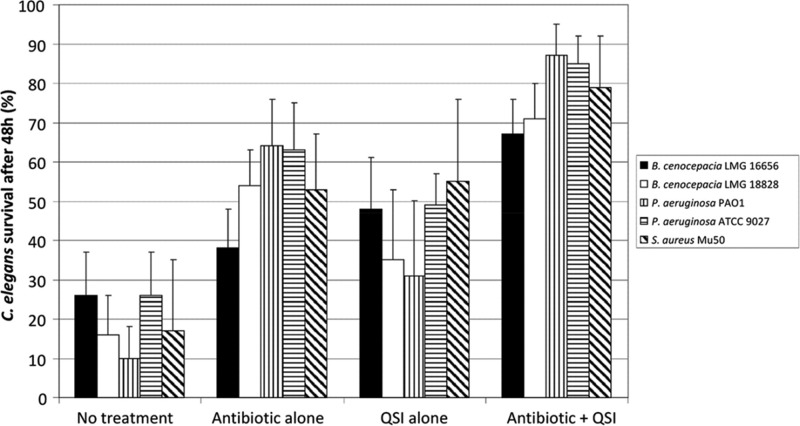
Figure 4.
Quorum-sensing inhibitors increase the antibiotic susceptibility of P. aeruginosa, S.aureus, and B. cenocepacia biofilms (reproduced with permission from Brackman and co-workers, 2011).
iii. Modulation with the Second Nucleotide Messenger Signaling
Accumulation of the nucleotide second messenger molecules under certain nonoptimal growth conditions remodels cellular metabolism and often translates into visible phenotypic changes. One of the most well-studied second messenger molecules, guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) (known collectively as (p)ppGpp), plays a critical role in a large number of biological processes to ensure survival under nutrient-limiting conditions. Intracellular (p)ppGpp levels appear to have a definite link with quorum sensing and biofilm formation and biofilm dispersion in many bacteria such as Vibrio cholarae, Enterococcus faecalis, and Bordetella pertussis, where the absence of ppGpp results in reduced biofilm formation. On the other hand, the ppGpp null mutant forms more biofilm than wild type in Francisella novicida and Actinobacillus pleuropneumoniae. In the case of E. coli, a relA-spoT deletion mutant forms more biofilm in the LB medium and less biofilm in minimal medium. Some recent reports by Liu et al.10 and Ge and co-workers suggested (p)ppGpp-driven biofilm regulation in P. putida and H. pylori, respectively.
Another important second messenger bis(3′,5′)-cyclic diguanylic acid (c-di-GMP) is known for regulating different physiological processes in response to environmental stimuli. c-di-GMP has been reported to contribute a “lifestyle transition” in many bacteria including Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica serovar Typhimurium, where generally low and high c-di-GMP concentration favors the motile and sessile states, respectively. In Mycobacterium smegmatis c-di-GMP has been shown to play a key role in quorum sensing and biofilm formation. This nucleotide messenger is synthesized by enzymes called diguanylate cyclases (DGCs) which convert 2 GTP molecules to c-di-GMP and are degraded by phosphodiesterase (PDE) enzymes into 5′-phosphoguanylyl-(3′-5′)-guanosine (pGpG) and/or GMP. The rate of synthesis and degradation dictates the intracellular c-di-GMP level which contributes to slow growth, biofilm formation, and drug tolerance.
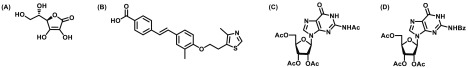
Both (p)ppGpp and c-di-GMP signaling pathways directly contribute to antimicrobial resistance development and hence can act as a promising therapeutic approach to reduce biofilm population, leading to increased susceptibility to standard drugs. The two second messenger synthetase enzymes, namely, Rel and DGC, are considered to be the most rational drug targets in order to reduce the intracellular concentration of (p)ppGpp and c-di-GMP, respectively. Syal and co-workers previously reported that vitamin C possesses structural similarity with Rel enzyme substrate guanosine diphosphate (GDP) and inhibits ppGpp biosynthesis by directly binding to Rel enzyme in M. smegmatis and subsequently prevents biofilm formation. Another study from the same group has shown that synthetic (p)ppGpp analogues directly inhibit ppGpp biosynthesis and can act as a potential biofilm inhibitor in mycobacteria.11 Recently, Dutta and co-workers described the compound screening approach and identified a lead compound X9 specifically targeting RelMtb and hence eliminating nutrient-starved persisters (Figure 5).12
Figure 5.
Structures of (p)ppGpp inhibitors: (A) vitamin C; (B) GSK-X9; and (C and D) acetylated and benzoylated nucleoside compounds.
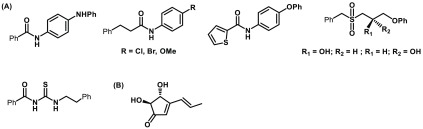
c-di-GMP is considered to be one of the master regulators of biofilm phenotype and has been shown to contribute biofilm formation in different bacteria. An organic synthetic molecule terrain (Figure 6) has been reported to reduce both QS and c-di-GMP concentration in P. aeruginosa.13 Glycosylated triterpenoid saponin has been reported as a DGC enzyme inhibitor, and more recently Sambanthamoorthy and co-workers described a high-throughput screening approach to identify several small molecules (Figure 6) inhibiting the DGC enzyme in V. cholerae and P. aeruginosa and subsequent reduction in biofilm formation.14 The immunosuppressive drug azathioprine has been reported to reduce biofilm formation in E. coli cells harboring P. aeruginosa DGC, WspR (wrinkly spreader phenotype regulator), by interfering with the nucleotide pool availability.15 Other notable approaches include the development of catechol-containing sulfonohydrazide compounds to inhibit DGC PleD from Caulobacter crescentus and repurposing of antioxidant drug ebselen to inhibit WspR and check the intracellular c-di-GMP level. The designed neutral small molecule, which selectively targets DGC, is one of the novel chemical approaches to block c-di-GMP synthesis, hence preventing bacterial biofilm formation.
Figure 6.
Chemical structures of (A) novel small molecule inhibiting DGC and (B) natural product terrain.
The emerging bacterial second messenger cyclic-di-AMP (c-di-AMP) also plays a role in biofilm formation in certain bacteria. Peng and co-workers showed that c-di-AMP binds to its receptor CabPA and promotes the biofilm formation in S. mutans.16 DAC (diadenylate cyclase) enzymes convert two ATP molecules into c-di-AMP and are considered to be one of the obvious targets to reduce c-di-AMP concentration. Bromophenol-TH was the first reported DisA synthetase (c-di-AMP synthesizing enzyme) inhibitor with limited therapeutic value.17 Temeng and co-workers have shown that the antiparasitic drug suramin could act as a potent inhibitor of DisA with IC50 1.1 μM. A similar work also identified polyphenols, such as tannic acid and theaflavin-3,3′-gallate as a DisA inhibitor in B. subtilis.
iv. Interference with Biofilm Maturation
Several natural and synthetic antibiofilm agents have been studied to control the biofilm formation and maturation primarily by interfering with the surface properties in both Gram (+ve) and Gram (-ve) bacteria. Deacylated lipopolysaccharide (dLPS) has been studied to show antibiofilm activity in the early stage of biofilm development in several Gram (-ve) bacteria by competing with the naturally occurring LPS in the cell wall, resulting in poor adherence and stability. Naturally occurring group II exopolysaccharide from E. coli uropathogenic strain CFT073 has been found to be a potent inhibitor of different bacterial biofilms including P. aeruginosa, E. coli, S. aureus, S. epidermidis, and K. pneumoniae. Rendueles and co-workers described the source of different exopolysccharides in terms of their broad application as an antibiofilm agent. Arabinomannan-containing glycolipids have been shown to possess potent antibiofilm activity against M. smegmatis biofilm and potentiate isoniazid killing (Figure 7).18 Similar studies with synthetic glycolipids and arabinofuranosides were found to interfere with biofilm formation and maturation in M. smegmatis (Figure 7).19 The synthetic compound 4-hydroxy-2-pyridone is effective against M. smegmatis in biofilm formation. Different antibiotics and bacteriocins are also potent agents to reduce biofilm formation and maturation against S. aureus, L. monocytogenes, P. aeruginosa, S. mutans, and E. faecalis.
Figure 7.
Molecular structures of (A) mannanglycolipids (1–3) and arabinomannan glycolipid (4) and (B) synthetic oligoarabinan glycolipids.
v. Disruption of Mature Biofilms
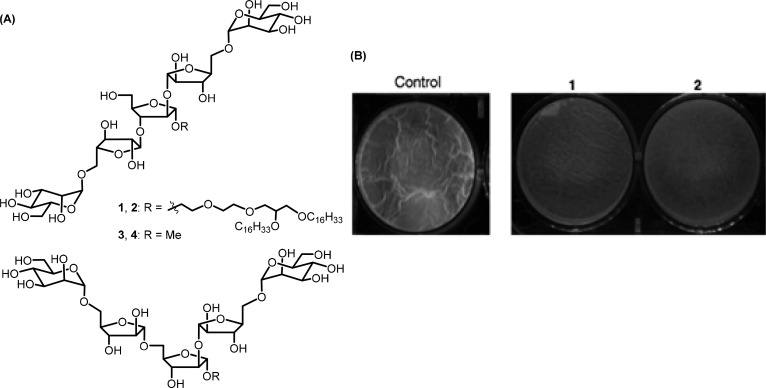
A large number of bacterial infections are associated with biofilm formation which serves as a reservoir of drug-tolerant population protected from antimicrobials and host defense. In order to overcome this physical obstacle, compounds need to be specifically designed to target mature biofilms. Year-long research has identified and elucidated the structural complexity of a biofilm and identification of key components responsible for its maintenance and robustness. There have been multiple approaches taken to disrupt preformed biofilms both in vitro and in vivo. Exopolysaccharides being an important component of the clinically relevant P. aeruginosa biofilm, Ray and co-workers have shown that human monoclonal antibodies (mAbs) targeting the biofilm exopolysaccharide Psl (a pentasaccharide composed of d-glucose, d-mannose, and l-rhamnose) within infected tissue could be a promising approach in combination therapy.20 Alginate oligosaccharide OligoG (CF-5/20) isolated from the marine algae Laminaria hyperborea has been shown to be active against established mucoid P. aeruginosa biofilms.21 Dispersin B is effective in hydrolyzing biofilm exopolysaccharide poly-b-1,6-N-acetyl-d-glucosamine (PNAG/PIA) of wound bacteria S. epidermidis, Acinetobacter baumanii, and Methicillin-resistant Staphylococcus aureus (MRSA).22P. aeruginosa exopolysaccharide processing enzymes PelAh and PslGh have been used as a successful tool to disrupt the established biofilm by Howell and co-workers. These two glycoside hydrolase enzymes are noncytotoxic and are known to potentiate antibiotics and neutrophil-mediated killing. Synthetic arabinomannan glycolipids (Figure 8) were found to be effective against a mature biofilm of Mycobacterium smegmatis as well as sliding motility possibly by interfering with the cell wall components.23 Synthetic Araf-Manp-containing heptasaccharide glycolipids were also found to be active against a preformed biofilm of M. smegmatis, resulting in significant dispersal particularly in the presence of isoniazid (synergistic effect) and hence effectively bringing down minimum biofilm inhibitory concentration (MBIC) of the drug.24
Figure 8.
(A) Chemical structures of arabinomannan glycolipids (1, 2) and the corresponding oligosaccharides (3, 4). (B) Disruption of preformed mature biofilm with compounds 1 and 2 (reproduced with permission from Syal and co-workers, 2016).
Surface-associated and secreted proteins also play a crucial role in biofilm development. Extracellular released cysteine proteases Staphopain A and Staphopain B of S. aureus are known to have a detrimental effect on the integrity of established biofilm. A similar study by Park and co-workers showed that protease released from Streptomyces sp. and Kribbella sp. exhibits antibiofilm activity against the preformed biofilm of S. aureus. Sugimoto and co-workers have shown that the extracellular serine protease Esp from S. epidermidis can degrade specific proteins in S. aureus biofilm, resulting in structural disintegration both in vitro and in vivo.25 Similarly, lysostaphin from S. simulans, endopeptidase from S. marcescens, and extracellular protease released from B. bacteriovorus have been reported to have good biofilm dispersion potential. Commercially available proteases such as neutrase, flavourzyme, and alcalase have shown antibiofilm activity against S. aureus and S. epidermidis.
Extracellular DNA (eDNA) is an essential matrix component of most biofilms and therefore serves as an attractive target for crumbling of biofilm. DNase treatment can disperse an established biofilm up to a threshold limit in both Gram (+ve) and Gram (-ve) bacteria, and after that, it becomes inaccessible for the enzyme. Dnase Dornasealfa is one of the most common enzymes in clinical use to disrupt P. aeruginosa biofilms. Other alternate approach to target eDNA could be to weaken the interaction with other matrix components, such as polysaccharide. Understanding such interactions in molecular detail will help researchers to design potential inhibitors.
Conclusion
Understanding the complex biology of biofilms, selected approaches have shown promising results in terms of getting better eradication of the pathogen, especially in combination with conventional drugs. The major shortcoming remaining is the lack of in vivo data, and in many cases, the mechanisms of action of the inhibitors are not clear and hence prevent precise structure–activity studies. However, in a clinical scenario, by the time any biofilm-related infection is diagnosed, the pathogen must have had progressed at a very advanced stage of biofilm formation or might have already formed a mature biofilm. In that case, knowing the organism-specific biology and molecular mechanism behind biofilm formation would not help much in order to control that. In addition, the fact that there are multiple genes and pathways involved in biofilm formation in a single organism makes it extremely difficult to inhibit biofilm formation by targeting one such protein in the first place. Rather, a precise small-molecule-based chemical approach should be taken in order to disperse in vivo biofilms irrespective of which bacteria forms. Future research should be carefully directed to gain greater insights about such interesting compounds that possess “drug”-like chemical property and the least probability of developing resistant mutants. A nanoparticle-based approach enhancing better penetration of drugs into the biofilm matrix and new technologies such as “on-demand activation” and “smart release” of bioactive compounds would significantly change the treatment outcome. The multidisciplinary approach of developing such antibiofilm compounds to the clinical studies would be a significant step forward in the fight against antimicrobial resistance.
Acknowledgments
A.G. thanks the Department of Biotechnology, Government of India, New Delhi, and N.J. thanks the Science and Engineering Research Board, Department of Science and Technology, New Delhi, for generous financial support.
Biographies
Dr. Anirban Ghosh completed his Master’s degree (Msc.) in Microbiology and worked as a research scientist in the AstraZeneca India Research and Development (R&D) center, Bangalore, primarily working on mycobacterial genetics. There he worked on developing different infection models of M. tuberculosis (M. tb) and compound efficacy testing in a BSL-3 facility with an aim to develop novel drugs for M. tb and remains a significant contributor for the pre-clinical development of the anti-TB compound AZD5847 which eventually progressed to human clinical trials. Later he went on to pursue doctoral studies at the University of Leuven, Belgium, where he exclusively worked on elucidating novel stress response pathways in E. coli and subsequently received his Ph.D. in December 2014. In January 2015, he moved to Sweden and started his Post-Doctoral research career in the Department of Cell and Molecular Biology, Uppsala University. There he worked on a novel inter-bacterial communication system called Contact Dependent Inhibition (CDI) and deciphered the role of CDI in the formation of drug-tolerant persister cells, in a clonal population of bacteria. Later in 2019, He joined the Molecular Biophysics Unit, Indian Institute of Science (IISc), Bangalore, as a Ramalingaswamy faculty fellow and established his own research group. His primary research interest at the moment is investigating the role of a c-di-AMP second messenger, in several clinically relevant phenotypes in mycobacteria such as antibiotic tolerance and biofilm formation. Other than that, his group also works on biophysical characterization enzymes responsible for maintaining intracellular c-di-AMP levels, as well as enzymatic regulation. Currently, Dr. Ghosh has more than 15 publications in International peer-reviewed journals. He is also the recipient of several prestigious International and National awards like the Erasmus-Mundus fellowship (for Ph.D.), Wenner-Gren fellowship (for Post-Doctoral training), and Ramalingaswamy re-entry fellowship by Govt. of India to start his career as a Principal Investigator in India.
Prof. Narayansaswamy Jayaraman joined in the faculty roll of the Department of Organic Chemistry in December 1999 and is a Professor and Chairman of the department currently. He completed his early studies, B.Sc. (University of Madras) and M.Sc. (Annamalai University), and conducted his doctoral research at the Indian Institute of Technology, Kanpur, under the supervision of Professor S. Ranganathan. He was a postdoctoral fellow in the group of Professor Sir J. F. Stoddart at the University of Birmingham, UK, and at the University of California Los Angeles, USA. His research group is working in the areas of carbohydrates and dendrimers. He was honored with a Shanti Swarup Bhatnagar Prize in 2009 and is an elected Fellow of the Indian Academy of Sciences.
Prof. Dipankar Chatterji started his career as a biophysical chemist and received his Ph.D. from the Indian Institute of Science, Bangalore, during 1977. Later he served as an Assistant Professor at the University of Hyderabad and then went to USA for postdoctoral research. After returning to India during 1983, he established a group at CCMB, Hyderabad, to work on the regulation of gene expression in bacteria. Apart from mapping the active site of the enzyme, which controls gene expression, his group was one of the first to show how intradomain interaction in different proteins regulates the maturation of this enzyme RNA polymerase. Later, Prof. Chatterji shifted his attention to the survival strategies of a bacterium, E. coli, under nutritional stress, called “stringent response”, and showed through a series of publications that RNA polymerase is the target for the stringent factor. Upon moving to the Indian Institute of Science, Bangalore, at the beginning of 1999 and here again, he and his group established the stringent response pathway in M. smegmatis. Recently, he has started working on another phenomenon connected with the persistence infection by microorganisms, called biofilm formation and quorum sensing. Certain unique leads that he has on the inhibitors of biofilms will go a long way. Prof. Chatterji has to his credit more than 180 original publications and several reviews and has guided around 31 students for a Ph.D. degree. He has served as an Honorary Professor at the National Institute of Genetics, Mishima, Japan, and a Visiting Scientist at the Johns Hopkins University, USA. He is a member of the editorial board of many international journals. Prof. Chatterji has received several awards like the S S Bhatnagar award, Ranbaxy award, UGC Hari Om Ashram award, and Homi Bhabha Fellowship amongst others. He is currently an Honorary Professor of the Molecular Biophysics Unit of the Indian Institute of Science and Honorary Professor of Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), at Bangalore. He is a fellow of all major science academies of India and a fellow of the World Academy of Sciences (TWAS).
The authors declare no competing financial interest.
References
- Flemming H. C.; Wingender J. Relevance of microbial extracellular polymeric substances (EPSs)--Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. 10.2166/wst.2001.0326. [DOI] [PubMed] [Google Scholar]
- Wan Y. Z.; Xiong G. Y.; Liang H.; Raman S.; He F.; Huang Y. Modification of medical metals by ion implantation of copper. Appl. Surf. Sci. 2007, 253, 9426–9429. 10.1016/j.apsusc.2007.06.031. [DOI] [Google Scholar]
- Tang K.; Zhang X. H. Quorum quenching agents: resources for antivirulence therapy. Mar. Drugs 2014, 12, 3245–3282. 10.3390/md12063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H.; Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- Fetzner S. Quorum quenching enzymes. J. Biotechnol. 2015, 201, 2–14. 10.1016/j.jbiotec.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Wnuk S. F.; Lalama J.; Garmendia C. A.; Robert J.; Zhu J.; Pei D. S-Ribosylhomocysteine analogues with the carbon-5 and sulfur atoms replaced by a vinyl or (fluoro)vinyl unit. Bioorg. Med. Chem. 2008, 16, 5090–5102. 10.1016/j.bmc.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J. A.; Crowder T.; Rinaldo-Matthis A.; Ho M. C.; Almo S. C.; Schramm V. L. Transition state analogs of 5′-methylthioadenosine nucleosidase disrupt quorum sensing. Nat. Chem. Biol. 2009, 5, 251–257. 10.1038/nchembio.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman G.; Cos P.; Maes L.; Nelis H. J.; Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M.; Wu H.; Andersen J. B.; Riedel K.; Rasmussen T. B.; Bagge N.; Kumar N.; Schembri M. A.; Song Z.; Kristoffersen P.; Manefield M.; Costerton J. W.; Molin S.; Eberl L.; Steinberg P.; Kjelleberg S.; Høiby N.; Givskov M. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Xiao Y.; Nie H.; Huang Q.; Chen W. Influence of (p)ppGpp on biofilm regulation in Pseudomonas putida KT2440. Microbiol. Res. 2017, 204, 1–8. 10.1016/j.micres.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Syal K.; Flentie K.; Bhardwaj N.; Maiti K.; Jayaraman N.; Stallings C. L.; Chatterji D. Synthetic (p)ppGpp Analogue Is an Inhibitor of Stringent Response in Mycobacteria. Antimicrob. Agents Chemother. 2017, 61, e00443–17 10.1128/AAC.00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N. K.; Klinkenberg L. G.; Vazquez M. J.; Segura-Carro D.; Colmenarejo G.; Ramon F.; Rodriguez-Miquel B.; Mata-Cantero L.; Porras-De Francisco E.; Chuang Y. M.; Rubin H.; Lee J. J.; Eoh H.; Bader J. S.; Perez-Herran E.; Mendoza-Losana A.; Karakousis P. C. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Science Advances 2019, 5, eaav2104 10.1126/sciadv.aav2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.; Park J.; Choi H.; Yoon S.; Kim W. Terrein is an inhibitor of quorum sensing and c-di-GMP in Pseudomonas aeruginosa: a connection between quorum sensing and c-di-GMP. Sci. Rep. 2018, 8, 8617. 10.1038/s41598-018-26974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambanthamoorthy K.; Sloup R.; Parashar V.; Smith J.; Kim E.; Semmelhack M.; Neiditch M.; Waters C. Identification of small molecules that antagonize diguanylatecyclase enzymes to inhibit biofilm formation. Antimicrob. Agents Chemother. 2012, 56, 5202–5211. 10.1128/AAC.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniani D.; Rossi E.; Rinaldo S.; Bocci P.; Lolicato M.; Paiardini A.; Raffaelli N.; Cutruzzolà F.; Landini P. The immunosuppressive drug azathioprine inhibits biosynthesis of the bacterial signal molecule cyclic-di-GMP by interfering with intracellular nucleotide pool availability. Appl. Microbiol. Biotechnol. 2013, 97, 7325–7336. 10.1007/s00253-013-4875-0. [DOI] [PubMed] [Google Scholar]
- Peng X.; Zhang Y.; Bai G.; Zhou X.; Wu H. Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 2016, 99, 945–959. 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Zhou J.; Sayre D.; Sintim H. Identification of bromophenolthiohydantoin as an inhibitor of DisA, a c-di-AMP synthase, from a 1000 compound library, using the coralyne assay. Chem. Commun. (Cambridge, U. K.) 2014, 50, 11234–11237. 10.1039/C4CC02916J. [DOI] [PubMed] [Google Scholar]
- Mahapa A.; Samanta G.; Maiti K.; Chatterji D.; Jayaraman N. Mannopyranoside Glycolipids Inhibit Mycobacterial and Biofilm Growth and Potentiate Isoniazid Inhibition Activities in M. smegmatis. ChemBioChem 2019, 20, 1966–1976. 10.1002/cbic.201900040. [DOI] [PubMed] [Google Scholar]
- Naresh K.; Bharati B.; Avaji P.; Chatterji D.; Jayaraman N. Synthesis, biological studies of linear and branched arabinofuranoside-containing glycolipids and their interaction with surfactant protein A. Glycobiology 2011, 21, 1237–1254. 10.1093/glycob/cwr068. [DOI] [PubMed] [Google Scholar]
- Mathur H.; Field D.; Rea M.; Cotter P.; Hill C.; Ross R. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ. Biofilms Microbiomes 2018, 10.1038/s41522-018-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. C.; Pritchard M. F.; Ferguson E. L.; Powell K. A.; Patel S. U.; Rye P. D.; Sakellakou S. M.; Buurma N. J.; Brilliant C. D.; Copping J. M.; Menzies G. E.; Lewis P. D.; Hill K. E.; Thomas D. W. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ. BiofilmsMicrobiomes. 2018, 4, 13. 10.1038/s41522-018-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawande P.; Leung K.; Madhyastha S. Antibiofilm and antimicrobial efficacy of DispersinB®-KSL-W peptide-based wound gel against chronic wound infection associated bacteria. Curr. Microbiol. 2014, 68, 635–641. 10.1007/s00284-014-0519-6. [DOI] [PubMed] [Google Scholar]
- Syal K.; Maiti K.; Naresh K.; Avaji P.; Chatterji D.; Jayaraman N. Synthetic arabinomannan glycolipids impede mycobacterial growth, sliding motility and biofilm structure. Glycoconjugate J. 2016, 33, 763–77. 10.1007/s10719-016-9670-6. [DOI] [PubMed] [Google Scholar]
- Maiti K.; Syal K.; Chatterji D.; Jayaraman N. Synthetic ArabinomannanHeptasaccharide Glycolipids Inhibit Biofilm Growth and Augment Isoniazid Effects in Mycobacterium smegmatis. ChemBioChem 2017, 18, 1959–1970. 10.1002/cbic.201700247. [DOI] [PubMed] [Google Scholar]
- Sugimoto S.; Iwamoto T.; Takada K.; Okuda K.; Tajima A.; Iwase T.; Mizunoe Y. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol. 2013, 195, 1645–1655. 10.1128/JB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]