Abstract
Smoking has become a major cause of chronic obstructive pulmonary disease through weakening of the respiratory mucus-ciliary transport system, impairing cough reflex sensitivity, and inducing inflammation. Recent researches have indicated that hydrogen sulfide is essential in the development of various lung diseases. However, the effect and mechanism of hydrogen sulfide on cigarette smoke-induced chronic obstructive pulmonary disease have not been reported. In this study, rats were treated with cigarette smoke to create a chronic obstructive pulmonary disease model followed by treatment with a low concentration of hydrogen sulfide. Pulmonary function, histopathological appearance, lung edema, permeability, airway remodeling indicators, oxidative products/antioxidases levels, inflammatory factors in lung, cell classification in bronchoalveolar lavage fluid were measured to examine the effect of hydrogen sulfide on chronic obstructive pulmonary disease model. The results showed that hydrogen sulfide effectively improved pulmonary function and reduced histopathological changes, lung edema, and permeability. Airway remodeling, oxidative stress, and inflammation were also reduced by hydrogen sulfide treatment. To understand the mechanisms, we measured the expression of TGF-β1, TGF-βIand TGF-βII receptors and Smad7 and phosphorylation of Smad2/Smad3. The results indicated that the TGF-β1 and Smad were activated in cigarette smoke-induced chronic obstructive pulmonary disease model, but inhibited by hydrogen sulfide. In conclusion, this study showed that hydrogen sulfide treatment alleviated cigarette smoke-induced chronic obstructive pulmonary disease through inhibition of the TGF-β1/Smad pathway.
Impact statement
COPD has become a severe public health issue in the world and smoking has become a major cause of COPD. As a result, it is a demandingly needed to explore new potential therapy for cigarette smoke-associated COPD. The present study suggested that H2S treatment improved pulmonary function and reduced histopathological changes, lung edema, permeability, inflammation, airway remodeling and oxidative injury in a COPD model induced by cigarette smoke. Although additional studies are required to elucidate the pharmacodynamics, pharmacokinetics, and pharmacology of H2S in the cigarette smoke-associated COPD, our findings provide an experimental basis for the potential clinical application of H2S in COPD treatment.
Keywords: Hydrogen sulfide, cigarette smoke, chronic obstructive pulmonary disease, TGF-β1, Smad
Introduction
Chronic obstructive pulmonary disease (COPD) has been identified as incomplete reversible airflow limitation that can progress to respiratory failure and pulmonary heart disease.1 The pathological process of COPD mainly includes progressively developed airflow limitation and lung chronic inflammatory response.2 Smoking is the chief pathogenic factor of COPD.3 Over 90% of deaths of COPD patients are related to smoking.3 Previous experiments have shown that stimulation by cigarette smoke (CS) makes the respiratory system more prone to inflammatory reactions.4
A variety of signal transduction pathways participate in the development of COPD. The transforming growth factor-β1 (TGF-β1) and Smads proteins are most closely related to airway remodeling in COPD. TGF-β1 belongs to the transforming growth factor super family,5 which can induce the proliferation and transformation of fibroblasts and airway smooth muscle cells6 and plays an essential role in the lung remodeling.7 Microarray studies in patients with COPD have shown that TGF-β1 is associated with activation of thrombospondin.8 CS can transiently increase TGF-β1 expression in mice, causing sustained phosphorylation of the downstream signaling protein Smad2, and increasing collagen deposition.9 TGF-β receptors (TGF-βRs) have three forms according to structural and functional characteristics.10 TGF-βRI/II are glycoproteins belonging to the transmembrane receptor serine/threonine kinase family.10 TGF-β1 reacts with both TGF-βRI and TGF-βRII, which activates downstream effector molecules and signal transduction, leading to chemotaxis, proliferation, and immune cell activation.11 TGF-βRIII is not directly involved in signal transduction as its intracellular region does not contain a serine/threonine protein kinase active region.12
As downstream signal proteins of TGF-β1, Smad proteins regulate many cellular functions, including cell proliferation and protein synthesis.13 After they are activated by TGF-β1, Smad2 and Smad3 form a Smad complex (Smad4) and regulate target gene expression,14 resulting in a series of airway remodeling changes, such as cell phenotypic transformation and airway wall extracellular matrix deposition.15 Inhibitory Smads, including Smad7, bind activated TGF-βRI and inhibit signal transduction of the TGF-β family.16 Smad7 blocks the signal transduction process by specifically binding to TGF-βRI.16
Hydrogen sulfide (H2S) is involved in multiple lung diseases, including pulmonary hypertension, diffuse obstructive pulmonary disease, and acute lung injury.17–20 It can inhibit the release of inflammatory factors and lung cell apoptosis, and affect the expression of alveolar surfactants.20,21 NaHS (an exogenous H2S donor) inhibits chemotaxis of leukocytes, reduces lung damage, and improves the circulation state of sepsis and the survival rate of rats.20,22 However, the effect and underlying mechanism of H2S on CS-associated COPD has not yet been explored. Therefore, the present study examined the effect of H2S inhalation on CS-associated COPD and identified the underlying mechanism.
Methods and materials
Animals
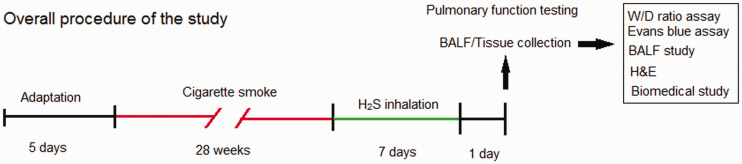
Sprague–Dawley rats (male, 250–270 g) were purchased from Hebei Chest Hospital Animal Center. All rats were allowed to get food and water ad libitum and tested in the specific pathogen-free barrier laboratory of Hebei Chest Hospital Animal Center. The laboratory was on a 12-h light:dark cycle under 23°C and 35% humidity. The investigators took all efforts to minimize pain or distress to the animals. Rats were kept in a holding room for five days after arrival at the laboratory for adaptation. The whole protocol has been authorized by the Animal Ethics Committee of the Hebei Chest Hospital Animal Center and completed complying with the Guide of the Care and Use of Laboratory Animals published by NIH. The overall procedure is demonstrated in Figure 1.
Figure 1.
The flow chart to demonstrate the overall procedure of the study. (A color version of this figure is available in the online journal.)
CS-induced COPD model
Rats were designated into the following groups in a random manner (N = 10), including Control, Sham, CS, CS + H2S, and H2S groups. The COPD model of rats was created according to the report of Ke et al.23 Rats in the CS and CS + H2S groups inhaled CS for 28 weeks using the BUXCO system (Data Sciences International, New Brighton, NM, USA). The smoke was produced by Derby cigarettes (Wuhu Cigarettes, China). Each cigarette contains 10 mg tar, 0.9 mg cotinine, and 12 mg carbon monoxide. Rats were treated with inhalation of the equivalent of 20 cigarettes for 2 h, and then allowed to rest for 4 h, which was repeated again on the same day. Rats inhaled CS for six days/week. Sham rats breathed air using the BUXCO animal CS-exposure system for 28 weeks, then breathed air in a 20-L plastic chamber for seven days.
H2S inhalation
For H2S inhalation, after the CS-induced COPD model was established, rats were put in a 20-L plastic chamber and allowed to breathe air mixed with H2S for 8 h daily for seven days. H2S passed through a regulator and flowmeter and mixed with air. The flow of H2S and air was adjusted to maintain the concentration of H2S at 40 ppm. Rats in the H2S group also inhaled 40 ppm H2S for 8 h daily for seven days, without the CS-induced COPD model. H2S was purchased from Shijiazhuang Specialty Gas Company (Shijiazhuang, Hebei, China).
Histopathology evaluation
One day after the H2S inhalation period, rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and pancuronium bromide (6 mg/kg, i.p.). They were sacrificed by cervical dislocation and the lungs were exposed by thoracotomy. The lungs were perfused with sterile phosphate-buffered saline (PBS). The left lobes of lungs were harvested and fixed with 4% formalin for 48 h at 25°C. Afterwards, the lung was cut into 4 μm sections, which were stained with H&E method. These slides were evaluated with a microscope by a pathologist to evaluate the histopathological appearance (three slides and four fields of view per sample, magnification ×200). The lung injury score was graded on a 5-point scale. The changes in airway remodeling indicators, including smooth muscle thickness, collagen thickness, the ratio of wall thickness/bronchiole diameter, and the ratio of wall area/total bronchiole area, were measured with the Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, USA) on H&E-stained histological sections, with the method described by Fei et al.24
Lung wet/dry ratio and Evans blue assay
Lung edema was evaluated with the W/D weight ratio method. Briefly, one day after H2S inhalation was finished, the lung tissue was collected and weighed (wet weight, about 400 mg) and kept in a 70°C oven for 60 h, after which the dry weight was measured to calculate the lung W/D ratio.
For the Evans blue assay, one day after H2S inhalation was finished, rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and pancuronium bromide (6 mg/kg, i.p.); 1% Evans blue dye in PBS was then injected via the tails (30 mg/kg). Half an hour later, the lungs were perfused with PBS and homogenized in PBS with formamide and incubated overnight at 60°C. Next day, it was centrifuged (10,000 × g, 30 min). The absorbance of the lung homogenate at 620 nm was obtained, and the permeability index was calculated as described previously.25
Pulmonary function measurement
The pulmonary function was measured with an AniRes 2005 system (SYNOL High-Tech, Beijing, China). Briefly, one day after H2S inhalation was finished, rats were anesthetized. Afterwards, endotracheal intubation was performed on the rats, and the tube was connected to a ventilator (Voltek Enterprises, Toronto, ON, Canada). The peak expiratory flow (PEF) and maximal mid-expiratory flow curve (MMF) were obtained from 30 respiratory cycles.
Evaluation of oxidative products/antioxidases
The levels of 8-Hydroxydeoxyguanosine (8-OHdG), malondialdehyde (MDA), and protein carbonyl in the lung were examined using commercial kits. The 8-OHdG was measured with the method of Ji et al.26 A MDA detection kit (Beyotime, Shanghai, China) was used to measure MDA levels in lung tissue. MDA reacts with thiobarbituric acid and forms a red product. Briefly, the lung tissue was extracted with radio-immunoprecipitation Assay (RIPA) lysis buffer. The lung tissue lysate was incubated with 200 µL of testing solution for half an hour. The absorbance values (532 nm) were quantified with a microplate reader (BioTek Instruments, Winooski, USA) to calculate the concentration (µmol/mg protein). The protein carbonyl in the supernatant was measured with the method of Levine et al.27
The activities of antioxidases, including catalase, superoxide dismutase, and glutathione peroxidase were investigated using commercial kits (Beyotime, Shanghai, China). The lung tissue was homogenized and centrifuged at 5000 × g for 10 min. Afterwards, the supernatant was collected and incubated with testing solution for 20 min, and the absorbances at 520, 560, or 340 nm were quantified by a microplate reader. The catalase, superoxide dismutase, and glutathione peroxidase activities (U/mg protein) were calculated based on the absorbance values.
Cell classification in BALF
Following the CS-induced COPD model and H2S inhalation, rats were sacrificed and the lung was exposed. BALF was collected from the left lung and centrifuged at 110 × g (10 min, 4°C), and the cells were re-suspended in Hank’s solution (Beyotime, Shanghai, China). An aliquot of the cell suspension (100 µL) was analyzed using a blood cell counter to determine the total number of cells. Another aliquot (200 µL) was subjected to Diff-Quick staining (Baxter Diagnostics, Deerfield, IL, USA).28 Approximately 200 cells were counted in each sample and classified as neutrophils, lymphocytes, and macrophages according to morphological characteristics.29
Measurement of inflammatory factors
BALF was collected as previously described. The supernatant of lung homogenate was collected after it was centrifuged (12,000 × g, 20 min). The levels of inflammatory factors were measured using ELISA kits (Beyotime, Shanghai, China). Briefly, the monoclonal capture antibody was pre-coated on an ELISA plate. The absorbance of the reaction solution was quantified by a microplate reader (450 nm). The data of absorbance were used to calculate the concentrations of the inflammatory factors.
Western blotting
The lung tissue was homogenized with a lysis buffer (Beyotime, Shanghai, China) and centrifuged (12,000 × g, 10 min, 4°C) to collect the proteins, which were separated by SDS-PAGE membranes (Millipore, Burlington, MA, USA) and transferred to PVDF membranes (Millipore, Burlington, MA, USA). The blotted membranes were blocked with 5% nonfat dry milk (Beyotime, Shanghai, China). After 2 h, they were rinsed with TBST solution and incubated with primary antibodies, then with goat anti-rabbit IgG. Finally, the blots were washed with TBST and imaged with enhanced chemiluminescence reagent (Thermo Scientific, Rockford, USA).
Statistical tests
Statistical tests were completed using SPSS 17.0 by one-way analysis of variance with Tukey–Kramer multiple comparisons tests. P < 0.05 was considered to be significant.
Results
Histopathological changes, lung edema, permeability, and pulmonary function
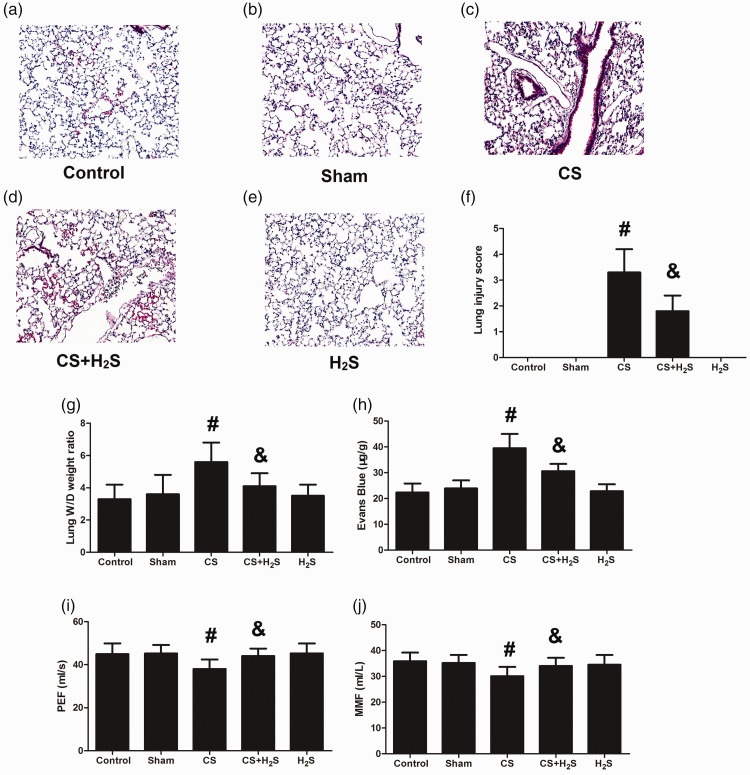
CS treatment induced alveolar septa thickening and inflammatory cells infiltration, which was reduced significantly by H2S (Figure 2(a) to (e)). The lung injury score, lung edema, and permeability were also significantly increased by CS, but decreased by H2S (Figure 2(f) to (h)). Figure 2(i) and (j) shows the changes of pulmonary function as tested by PEF and MMF. PEF and MMF were both significantly decreased by CS treatment, but recovered by H2S. These results indicated that H2S could not only attenuate the structural damage caused by CS, but could also recover pulmonary function.
Figure 2.
Effect of H2S inhalation on histopathological appearance, lung edema, permeability, and pulmonary function in rats. Values shown are mean±S.E.M (N = 10). a-e, H&E staining of lung; f, lung injury score; g, lung W/D weight ratio; h, Evans blue leakage amounts; i, peak expiratory flow (PEF); j, maximal mid-expiratory flow (MMF). #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS + H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide. (A color version of this figure is available in the online journal.)
Changes in airway remodeling indicators and proteins
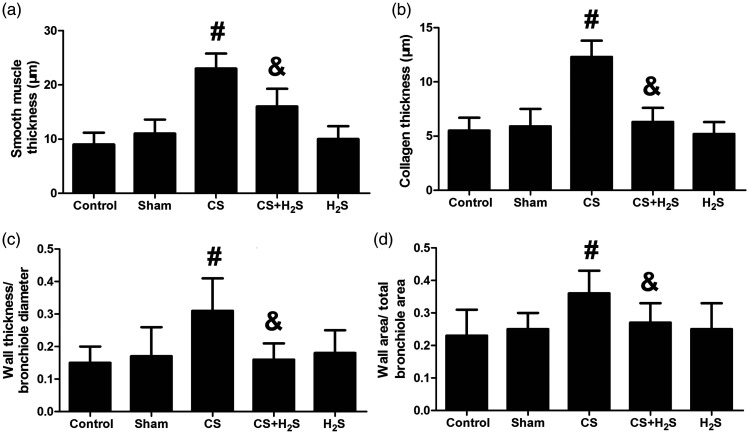
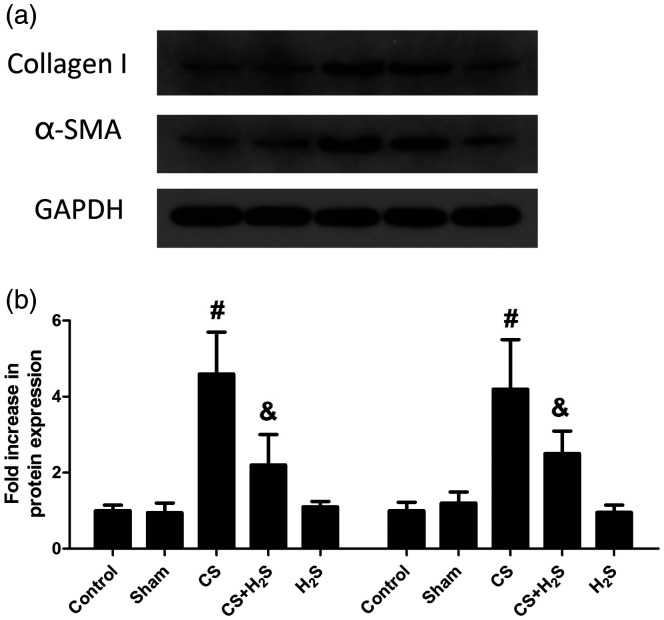
Figure 3 shows the changes in airway remodeling indicators, including smooth muscle thickness, collagen thickness, the ratio of wall thickness/bronchiole diameter, and the ratio of wall area/total bronchiole area. These indicators were significantly increased in the CS group, but were reversed by H2S (P < 0.05). As demonstrated in Figure 4, the expression of Collagen I and α-SMA was significantly increased in the CS group compared to the Control or Sham groups (P < 0.05), but were significantly inhibited by H2S treatment (P < 0.05).
Figure 3.
Effect of H2S inhalation on airway remodeling indicators in rats. Smooth muscle thickness (a), collagen thickness (b), ratio of wall thickness/bronchiole diameter (c), and the ratio of wall area/total bronchiole area (d) are shown. Values shown are mean±S.E.M (N = 10). #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS+H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
Figure 4.
Effect of H2S inhalation on airway remodeling proteins (Collagen I and α-SMA) in rats. The representative protein bands are shown in (a); The quantitative results are shown in (b). Values shown are mean±S.E.M (N = 10). #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS+H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
The levels of oxidative products and antioxidases in the lung
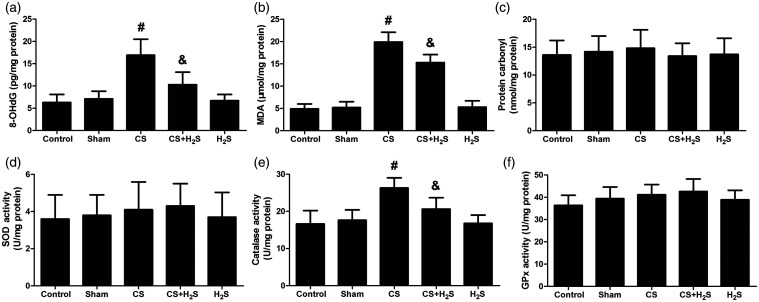
The levels of 8-OHdG and MDA were both significantly increased by CS, but significantly inhibited by H2S (Figure 5). The level of carbonyl protein has no change among the groups. Oppositely, the level of CAT was significantly decreased by CS, but significantly recovered by H2S. In Sham or H2S-alone groups, it was not significantly changed. The levels of SOD and GPx did not change among groups.
Figure 5.
Effect of H2S inhalation on oxidative products (8-OHdG, MDA and protein carbonyl) and antioxidases (SOD, catalase and GPx) in the lung. Values shown are mean±S.E.M (N = 10). a, 8-OHdG levels; b, MDA levels; c, protein carbonyl levels; d, SOD levels; e, catalase levels; f, GPx levels. #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS+H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
Changes in inflammatory factors and cell classification in BALF
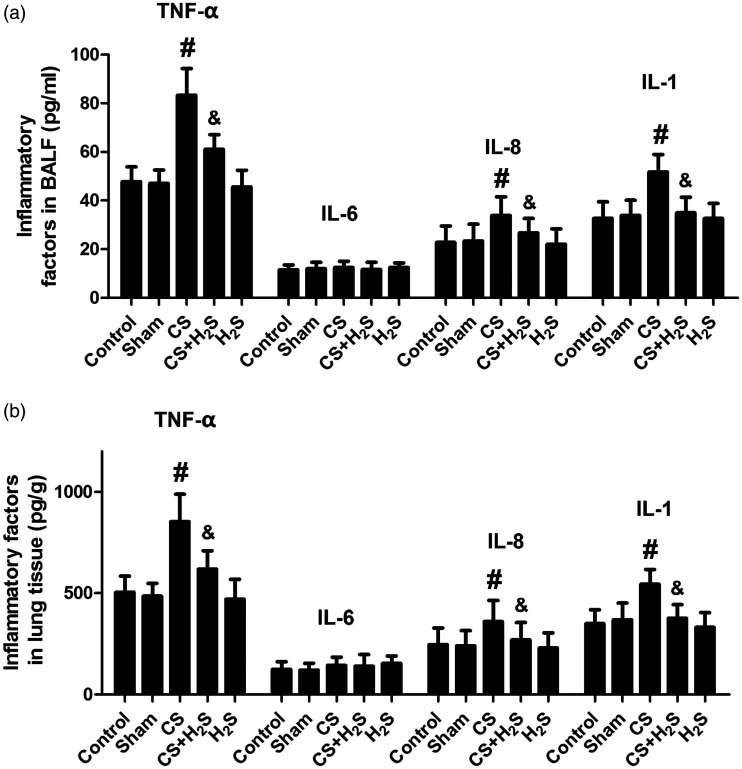
As shown in Figure 6, the levels of TNF-α, IL-8, and IL-1β in BALF and lung tissue were all significantly increased by CS group, but significantly decreased by H2S. In Sham or H2S-alone groups, they were not significantly changed. The level of IL-6 was not changed between groups.
Figure 6.
Effect of H2S inhalation on inflammatory factors (TNF-α, IL-8, IL-1β and IL-6) in BALF and lung tissue. Values shown are mean±S.E.M (N = 10). a, levels of TNF-α, IL-6, IL-8, and IL-1β in BALF; b, levels of TNF-α, IL-6, IL-8, and IL-1β in lung tissue. #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS+H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
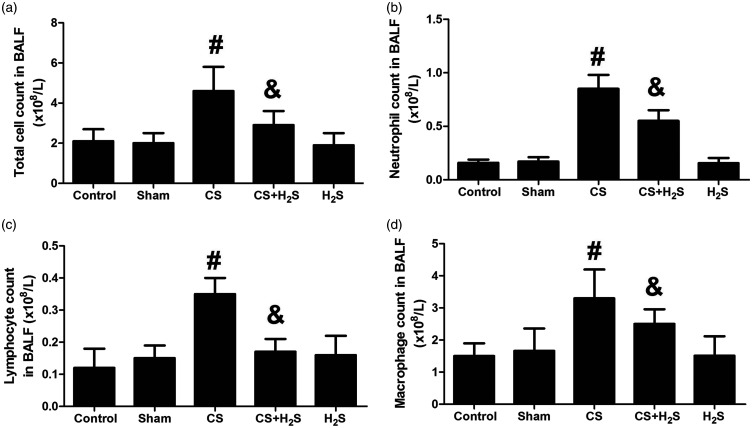
Figure 7 shows the changes in cell classification in BALF. The numbers of total cells, neutrophils, lymphocytes, and macrophages were all significantly increased by CS (P < 0.05 compared to Control), which were significantly decreased by H2S treatment (P < 0.05). In Sham or H2S-alone groups, the cell classification did not significantly change.
Figure 7.
Effect of H2S inhalation on cell classification (total cells, neutrophils, lymphocytes, and macrophages) in BALF. Values shown are mean ± S.E.M (N = 10). a, total cell count in BALF; b, neutrophil count in BALF; c, lymphocyte count in BALF; d, macrophage count in BALF. #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS + H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
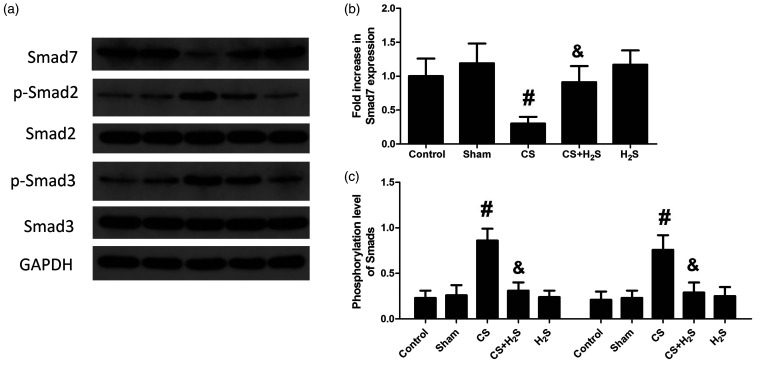
Expression of TGF-β1, TGF-β receptors and Smad7 and phosphorylation levels of Smad2/Smad3
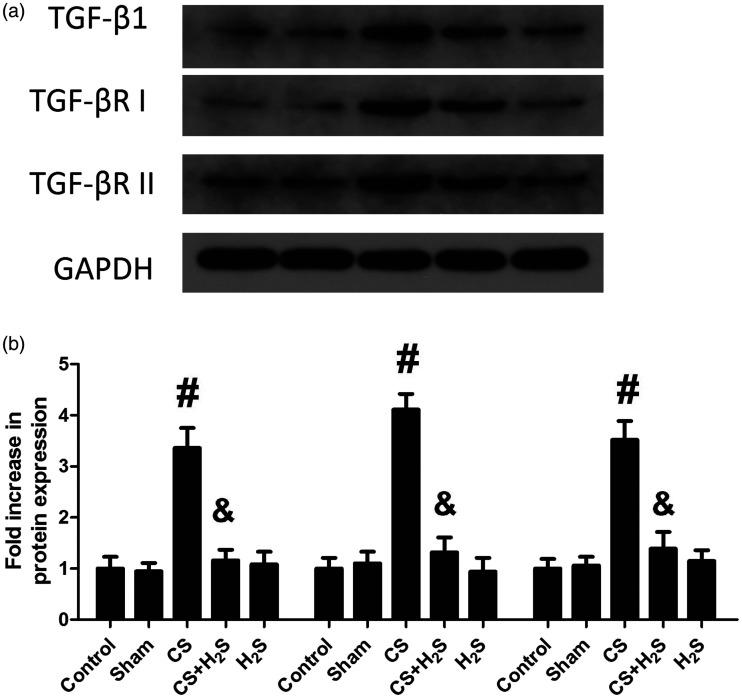
Figure 8 shows the expression levels of TGF-β1 and TGF-β receptors in lung tissue. They were increased significantly in the CS group, but were significantly reduced by H2S. In Sham or H2S-alone groups, their protein levels had no change. Figure 9 shows the protein levels of Smad7 and phosphorylation levels of Smad2 and Smad3. The phosphorylation levels of Smad2 and Smad3 were significantly increased by CS but decreased by H2S treatment. By contrast, the expression of Smad7 was decreased by CS, while significantly increased by H2S. In Sham or H2S-alone groups, the expression of Smad7 or phosphorylation of Smad2/Smad3 did not significantly change.
Figure 8.
Effect of H2S inhalation on expression of TGF-β1 and TGF-β receptors (TGF-βRI and TGF-βRII) in the lung. Values shown are mean ± S.E.M (N = 10). a, representative blots of TGF-β1 and TGF-β receptors; b, expression levels of TGF-β1 and TGF-β receptors. #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS + H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
Figure 9.
Effect of H2S inhalation on expression of Smad7 and phosphorylation of Smad2/Smad3 in the lung. Values shown are mean±S.E.M (N = 10). a, representative blots of Smad7 and phosphorylated Smad2 and Smad3; b, expression levels of Smad7; c, phosphorylation levels of Smad2 and Smad3. #P < 0.05 compared to Control; &P < 0.05 compared to the CS group. CS: cigarette smoke; CS+H2S: cigarette smoke and hydrogen sulfide; H2S: hydrogen sulfide.
Discussion
Inflammation, oxidative stress, protease/anti-protease imbalance, and autonomic nervous system dysfunction are the main mechanisms of COPD pathogenesis.30 Airway remodeling involves tissue repair, epithelial metaplasia, increased mucosal epithelial hyperplasia, submucosal gland hyperplasia, bronchial wall fibrosis, increased collagen deposition, and airway smooth muscle hyperplasia.31 The present study showed that after treatment with CS, the lung injury score, lung W/D weight ratio, and permeability were all increased in rats. Pulmonary function, indicated by PEF and MMF, was significantly decreased by treatment with CS. Airway remodeling indicators, including smooth muscle thickness, collagen thickness, the ratio of wall thickness/bronchiole diameter, the ratio of wall area/total bronchiole area, and airway remodeling proteins (Collagen I and α-SMA), were all significantly increased by CS. These results suggest that CS caused lung structural damage and pulmonary function impairment, indicating the successful establishment of the CS-induced COPD model.
Studies have confirmed that H2S functions in inflammatory response regulation,32 anti-oxidative stress,33 vasodilation,34 and anti-fibrosis.35 Intravenous administration of sodium hydrosulfide, which provides exogenous H2S, has anti-inflammatory, anti-oxidative, anti-apoptotic, and pulmonary protective effects.33,36 The present study confirmed that inhalation of H2S significantly improved the histopathological appearance, lung edema, permeability, and pulmonary function of rats in the COPD model induced by CS. H2S diminished the injury score, lung W/D weight ratio, and Evans blue leakage induced by CS. PEF and MMF values were also significantly increased by H2S. Airway remodeling indicators, including smooth muscle thickness, collagen thickness, the ratio of wall thickness/bronchiole diameter, the ratio of wall area/total bronchiole area, and airway remodeling proteins (Collagen I and α-SMA) were all significantly decreased by H2S treatment. These results suggest that H2S might be effective in protection against CS-induced COPD and can attenuate airway remodeling.
Inflammatory cells, such as neutrophils, macrophages, eosinophils, and lymphocytes, are closely related to airway obstruction in COPD patients.37 Our results showed that the numbers of inflammatory cells in BALF were significantly enhanced in the COPD model, but were decreased by treatment with H2S. These cells secrete inflammatory cytokines, chemokines and proteases, which lead to the development of COPD.38,39 In the early stages of COPD, macrophages and neutrophils cause the release of a series of inflammatory mediators. On the other hand, elastase and reactive oxygen species (ROS) secreted by inflammatory cells can degrade extracellular matrix components of the alveolar wall, leading to emphysema.40 Next, we measured oxidative products and antioxidases and inflammatory factors in BALF and lung tissue. The levels of 8-OHdG and MDA in the lung were significantly increased by CS, but this increase was inhibited by H2S. The level of protein carbonyl in the lung, however, was not significantly changed by H2S, indicating that H2S did not attenuate the oxidative injury of proteins. By contrast, the level of CAT in the lung was significantly decreased by CS but was increased by H2S. The SOD and GPx activities were not significantly changed by H2S, indicating that rather than restoring all the antioxidases, H2S selectively affected the activity of CAT. These results suggest that H2S can reduce oxidative stress and selectively restore some antioxidases in a CS-induced COPD model, which may contribute to its protective effects against COPD.
In the present study, the amounts of TNF-α, IL-8 and IL-1β were significantly increased by CS, but were significantly decreased by H2S. TNF-α, IL-8, and IL-1β majorly contribute to CS-induced COPD. CS induced the release of IL-1β and IL-8 from the bronchial epithelium through oxidative stress, causing neutrophil and monocyte chemotaxis.41 TNF-α is essential in the airway inflammatory response in COPD.42 It is involved in remodeling of extracellular matrices by enhancing the release of elastase, as well as MMP-2 and MMP-9.43 A TNF-α receptor knockout mouse had less CS-induced connective tissue damage, suggesting a role of TNF-α in tissue remodeling.44 IL-8, secreted by macrophages and neutrophils, plays an important role in the development of COPD. IL-8 is a key cytokine in neutrophil recruitment and activation in the airway.45 IL-8 levels were positively correlated with the degree of airflow obstruction in COPD patients.4 IL-1β is essential for neutrophil airway inflammation in COPD patients and is a major inducer of IL-8 in bronchial epithelial cells.45 The decrease of TNF-α, IL-8, and IL-1β induced by H2S indicates that H2S could effectively inhibit the inflammatory reaction in the COPD model. However, the level of IL-6 was not significantly changed by H2S, indicating that rather than inhibiting all the inflammatory factors, H2S selectively inhibited the levels of TNF-α, IL-8, and IL-1β.
To further explore the underlying mechanisms of H2S, we inspected the participation of the TGF-β1/Smad pathway, a key pathway in airway remodeling. It has been shown that progesterone could inhibit TGF-β1/Smad signaling and airway remodeling in bronchopulmonary dysplasia (BPD), and attenuates its development.46 Our results showed that the protein levels of TGF-β1, TGF-βRI, and TGF-βRII were escalated by CS, but the escalation was inhibited by H2S. These results suggest that TGF-β1 and its receptors were activated in the CS-induced COPD model, but H2S treatment could inhibit their activation. Smads are important downstream proteins regulated by TGF-β1. Previous studies have shown that Smad3-specific inhibitors inhibit fibroblast-type collagen expression in normal fibroblasts and scleroderma induced by TGF-β1.47 Le et al.16 showed that in Smad3 gene knockout mice, airway remodeling pathological changes such as collagen deposition and decreased smooth muscle proliferation were alleviated. Our results indicated that the phosphorylation levels of Smad2 and Smad3 were significantly boosted in COPD rats, indicating that these AR-Smads were activated by CS. On the other hand, protein levels of Smad7, an inhibitory Smad, were decreased in the CS-induced COPD model. Taken together, it is suggested that smoking can activate the TGF-β1/Smad signaling pathway. Previous studies have shown that smoking induced airway inflammation and oxidative stress, and activated MMP-9 and TGF-β1/Smad pathway.48 TGF-β1 is expressed in CS extract-stimulated lung fibroblasts in vitro.49 ROS can enhance the phosphorylation of Smad2/3 and promote the conversion of TGF-β into an active form that activates TGF-β/Smad signaling.50,51 Activated TGF-β/ROS signaling promotes airway remodeling by inducing smooth muscle cell proliferation, fibroblastic fibrosis, and extracellular matrix synthesis and deposition.50,51 Our results further demonstrated that H2S increased the expression of Smad7 and inhibited the activation of Smad2 and Smad3, indicating that H2S treatment can inhibit the TGF-β/Smad signaling pathway. Previously, Cheng et al.52 revealed that H2S inhibited inflammation and the TGF-β/Smad pathway in peritoneal mesothelial cells. It was also revealed that H2S inhibited airway remodeling caused by CS by attenuating oxidative injury and epithelial-mesenchymal transition.53 Perry et al.54 reported that H2S inhibited the proliferation of smooth muscle and cytokine release in airway. These studies suggest that the mechanism of H2S involves many signal pathways and proteins. Our study showed that in a CS-induced COPD model, H2S treatment inhibited TGF-β/Smad signaling. Given the participation of TGF-β/Smad signaling in COPD, inhibition of this pathway may be associated with its protective effect on COPD induced by CS.
In conclusion, this study demonstrated that H2S improved pulmonary function and reduced lung injury in a CS-induced COPD model. The underlying mechanism may be associated with its inhibition of the TGF-β1/Smad pathway. The clinical implications of our findings include the role of H2S in the development of COPD and the potential use of H2S in the treatment of COPD. Medicine that targets TGF-β1/Smad pathway may also be developed to treat COPD. Although further studies are required to elucidate the pharmacodynamics, pharmacokinetics, and pharmacology of H2S in the CS-induced COPD model, our findings provide an experimental basis for the potential clinical application of H2S in COPD treatment.
Authors’ contributions
Liang Wang conducted the pulmonary function experiments and prepared the manuscript; Jing Meng conducted the lung injury experiments; Caicai Wang measured the airway remodeling indicators, and protein levels; Chao Yang measured the inflammatory factors and the cell classification; Yuan Wang did the statistical analysis; Yamei Li funded and designed the study; Yujing Li funded the study and revised the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was personally funded by Yamei Li and Yujing Li.
ORCID iD
References
- 1.Montserrat-Capdevila J, Godoy P, Marsal JR, Barbe F, Pifarre J, Alseda M, Ortega M. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung 2017; 195:77–85 [DOI] [PubMed] [Google Scholar]
- 2.Berg K, Wright JL. The pathology of chronic obstructive pulmonary disease: progress in the 20th and 21st centuries. Arch Pathol Lab Med 2016; 140:1423–8 [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP. Smoking cessation in chronic obstructive pulmonary disease. Semin Respir Crit Care Med 2015; 36:491–507 [DOI] [PubMed] [Google Scholar]
- 4.Chung KF. Inflammatory mediators in chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 2005; 4:619–25 [DOI] [PubMed] [Google Scholar]
- 5.Islam MS, Greco S, Janjusevic M, Ciavattini A, Giannubilo SR, D’Adderio A, Biagini A, Fiorini R, Castellucci M, Ciarmela P. Growth factors and pathogenesis. Best Pract Res Clin Obstet Gynaecol 2016; 34:25–36 [DOI] [PubMed] [Google Scholar]
- 6.van der Velden JL, Wagner DE, Lahue KG, Abdalla ST, Lam YW, Weiss DJ, Janssen-Heininger Y. TGF-beta1-induced deposition of provisional extracellular matrix by tracheal basal cells promotes epithelial-to-mesenchymal transition in a c-Jun NH2-terminal kinase-1-dependent manner. Am J Physiol Lung Cell Mol Physiol 2018; 314:L984–L97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrielson AT, Spitz JT, Hellstrom W. Collagenase clostridium histolyticum in the treatment of urologic disease: current and future impact. Sex Med Rev 2018; 6:143–56 [DOI] [PubMed] [Google Scholar]
- 8.Wang IM, Stepaniants S, Boie Y, Mortimer JR, Kennedy B, Elliott M, Hayashi S, Loy L, Coulter S, Cervino S, Harris J, Thornton M, Raubertas R, Roberts C, Hogg JC, Crackower M, O’Neill G, Pare PD. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med 2008; 177:402–11 [DOI] [PubMed] [Google Scholar]
- 9.Churg A, Tai H, Coulthard T, Wang R, Wright JL. Cigarette smoke drives small airway remodeling by induction of growth factors in the airway wall. Am J Respir Crit Care Med 2006; 174:1327–34 [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Chen JM, Xiao TG, Shu XB, Xu HC, Yang LL, Xing LJ, Zheng PY, Ji G. Qinggan huoxue recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-beta1/smad signaling pathway. World J Gastroenterol 2016; 22:4695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu PC, Hsu WL, Chen CL, Lam CF, Huang YB, Huang CC, Lin MH, Lin MW. Morphine induces fibroblast activation through up-regulation of connexin 43 expression: implication of fibrosis in wound healing. Int J Med Sci 2018; 15:875–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujio K, Komai T, Inoue M, Morita K, Okamura T, Yamamoto K. Revisiting the regulatory roles of the TGF-beta family of cytokines. Autoimmun Rev 2016; 15:917–22 [DOI] [PubMed] [Google Scholar]
- 13.Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ, Ran N. Inhibition airway remodeling and transforming growth factor-beta1/smad signaling pathway by astragalus extract in asthmatic mice. Int J Mol Med 2012; 29:564–8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Lan HY, Chung AC. TGF-β/smad signaling in kidney disease. Semin Nephrol 2012; 32:236–43 [DOI] [PubMed] [Google Scholar]
- 15.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major smad pathways in TGF-beta superfamily signalling. Genes Cells 2002; 7:1191–204 [DOI] [PubMed] [Google Scholar]
- 16.Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, Broide DH. Inhibition of allergen-induced airway remodeling in smad 3-deficient mice. J Immunol 2007; 178:7310–6 [DOI] [PubMed] [Google Scholar]
- 17.Scharstuhl A, Diepens R, Lensen J, Vitters E, van Beuningen H, van der Kraan P, van den Berg W. Adenoviral overexpression of smad-7 and smad-6 differentially regulates TGF-beta-mediated chondrocyte proliferation and proteoglycan synthesis. Osteoarthr Cartil 2003; 11:773–82 [DOI] [PubMed] [Google Scholar]
- 18.Cao H, Zhou X, Zhang J, Huang X, Zhai Y, Zhang X, Chu L. Hydrogen sulfide protects against bleomycin-induced pulmonary fibrosis in rats by inhibiting NF-kappaB expression and regulating Th1/Th2 balance. Toxicol Lett 2014; 224:387–94 [DOI] [PubMed] [Google Scholar]
- 19.Brampton J, Aaronson PI. Role of hydrogen sulfide in systemic and pulmonary hypertension: cellular mechanisms and therapeutic implications. Cardiovasc Hematol Agents Med Chem 2016; 14:4–22 [DOI] [PubMed] [Google Scholar]
- 20.Ding HB, Liu KX, Huang JF, Wu DW, Chen JY, Chen QS. Protective effect of exogenous hydrogen sulfide on pulmonary artery endothelial cells by suppressing endoplasmic reticulum stress in a rat model of chronic obstructive pulmonary disease. Biomed Pharmacother 2018; 105:734–41 [DOI] [PubMed] [Google Scholar]
- 21.Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med 2008; 233:1081–7 [DOI] [PubMed] [Google Scholar]
- 22.Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci 2008; 82:1196–202 [DOI] [PubMed] [Google Scholar]
- 23.Ke Q, Yang L, Cui Q, Diao W, Zhang Y, Xu M, He B. Ciprofibrate attenuates airway remodeling in CIGARETTE SMOKE-exposed rats. Respir Physiol Neurobiol 2019; 271:103290. [DOI] [PubMed] [Google Scholar]
- 24.Fei X, Zhang X, Zhang GQ, Bao WP, Zhang YY, Zhang M, Zhou X. Cordycepin inhibits airway remodeling in a rat model of chronic asthma. Biomed Pharmacother 2017; 88:335–41 [DOI] [PubMed] [Google Scholar]
- 25.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002; 110:1703–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji K, Xue L, Cheng J, Bai Y. Preconditioning of H2S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/akt/Nrf2 pathway. Brain Res Bull 2016; 121:68–74 [DOI] [PubMed] [Google Scholar]
- 27.Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol Biol 2000; 99:15–24 [DOI] [PubMed] [Google Scholar]
- 28.Ogata-Suetsugu S, Yanagihara T, Hamada N, Ikeda-Harada C, Yokoyama T, Suzuki K, Kawaguchi T, Maeyama T, Kuwano K, Nakanishi Y. Amphiregulin suppresses epithelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Biochem Biophys Res Commun 2017; 484:422–8 [DOI] [PubMed] [Google Scholar]
- 29.Aleksoniene R, Zeleckiene I, Mataciunas M, Puronaite R, Jurgauskiene L, Malickaite R, Strumiliene E, Gruslys V, Zablockis R, Danila E. Relationship between radiologic patterns, pulmonary function values and bronchoalveolar lavage fluid cells in newly diagnosed sarcoidosis. J Thorac Dis 2017; 9:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005; 142:233–9 [DOI] [PubMed] [Google Scholar]
- 31.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 2013; 144:1026–32 [DOI] [PubMed] [Google Scholar]
- 32.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem 2010; 113:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li PC, Chen WC, Chang LC, Lin SC. Substance P acts via the neurokinin receptor 1 to elicit bronchoconstriction, oxidative stress, and upregulated ICAM-1 expression after oil smoke exposure. Am J Physiol Lung Cell Mol Physiol 2008; 294:L912–20 [DOI] [PubMed] [Google Scholar]
- 34.Esechie A, Kiss L, Olah G, Horvath EM, Hawkins H, Szabo C, Traber DL. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci 2008; 115:91–7 [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Li H, Tang G, Wu L, Zhao K, Cao Q, Xu C, Wang R. Increased neointimal formation in cystathionine gamma-lyase deficient mice: role of hydrogen sulfide in alpha5beta1-integrin and matrix metalloproteinase-2 expression in smooth muscle cells. J Mol Cell Cardiol 2012; 52:677–88 [DOI] [PubMed] [Google Scholar]
- 36.Long J, Liu M, Liu S, Tang F, Tan W, Xiao T, Chu C, Yang J. H2S attenuates the myocardial fibrosis in diabetic rats through modulating PKC-ERK1/2MAPK signaling pathway. Technol Health Care 2019; 27:307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Sun J, Cao Y, Liu B, Li L, Mohammadtursun N, Zhang H, Dong J, Wu J. Bu-Shen-Fang-Chuan formula attenuates T-lymphocytes recruitment in the lung of rats with COPD through suppressing CXCL9/CXCL10/CXCL11-CXCR3 axis. Biomed Pharmacother 2019; 123:109735. [DOI] [PubMed] [Google Scholar]
- 38.Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology 2010; 113:104–15 [DOI] [PubMed] [Google Scholar]
- 39.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:2645–53 [DOI] [PubMed] [Google Scholar]
- 40.Cosio MG, Majo J. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest 2002; 121:160S–5S [DOI] [PubMed] [Google Scholar]
- 41.Sethi S, Mahler DA, Marcus P, Owen CA, Yawn B, Rennard S. Inflammation in COPD: implications for management. Am J Med 2012; 125:1162–70 [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Guan WJ, Huang RQ, Xie YQ, Zheng JP, Zhu SX, Chen M, Zhong NS. Carbocisteine attenuates TNF-α-induced inflammation in human alveolar epithelial cells in vitro through suppressing NF-κB and ERK1/2 MAPK signaling pathways. Acta Pharmacol Sin 2016; 37:629–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kothapalli CR, Ramamurthi A. Induced elastin regeneration by chronically activated smooth muscle cells for targeted aneurysm repair. Acta Biomater 2010; 6:170–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sproul EP, Argraves WS. A cytokine axis regulates elastin formation and degradation. Matrix Biol 2013; 32:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan YM, Kirkham P, Barnes PJ, Adcock IM. Brd4 is essential for IL-1beta-induced inflammation in human airway epithelial cells. PLoS One 2014; 9:e95051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunzmann S, Ottensmeier B, Speer CP, Fehrholz M. Effect of progesterone on smad signaling and TGF-beta/smad-regulated genes in lung epithelial cells. PLoS One 2018; 13:e0200661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol 2006; 69:597–607 [DOI] [PubMed] [Google Scholar]
- 48.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 2008; 294:L612–31 [DOI] [PubMed] [Google Scholar]
- 49.Guan S, Liu Q, Han F, Gu W, Song L, Zhang Y, Guo X, Xu W. Ginsenoside Rg1 ameliorates cigarette smoke-induced airway fibrosis by suppressing the TGF-beta1/smad pathway in vivo and in vitro. Biomed Res Int 2017; 2017:6510198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang F, Liu GS, Dusting GJ, Chan EC. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol 2014; 2:267–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan EC, Peshavariya HM, Liu GS, Jiang F, Lim SY, Dusting GJ. Nox4 modulates collagen production stimulated by transforming growth factor beta1 in vivo and in vitro. Biochem Biophys Res Commun 2013; 430:918–25 [DOI] [PubMed] [Google Scholar]
- 52.Cheng S, Lu Y, Li Y, Gao L, Shen H, Song K. Hydrogen sulfide inhibits epithelial-mesenchymal transition in peritoneal mesothelial cells. Sci Rep 2018; 8:5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guan R, Wang J, Cai Z, Li Z, Wang L, Li Y, Xu J, Li D, Yao H, Liu W, Deng B, Lu W. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol 2020; 28:101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry MM, Tildy B, Papi A, Casolari P, Caramori G, Rempel KL, Halayko AJ, Adcock I, Chung KF. The anti-proliferative and anti-inflammatory response of COPD airway smooth muscle cells to hydrogen sulfide. Respir Res 2018; 19:85. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]