Abstract
Friedreich’s ataxia is an autosomal recessive disorder characterized by impaired mitochondrial function, resulting in oxidative stress. In this study, we aimed at evaluating whether tocotrienol, a phytonutrient that diffuses easily in tissues with saturated fatty layers, could complement the current treatment with idebenone, a quinone analogue with antioxidant properties. Five young Friedreich’s ataxia patients received a low-dose tocotrienol supplementation (5 mg/kg/day), while not discontinuing idebenone treatment. Several oxidative stress markers and biological parameters related to oxidative stress were evaluated at the time of initiation of treatment and 2 and 12 months post-treatment. Some oxidative stress-related parameters and some inflammation indices were altered in Friedreich’s ataxia patients taking idebenone alone and tended to be normal values following tocotrienol supplementation; likewise, a cardiac magnetic resonance study showed some improvement following one-year tocotrienol treatment. The pathway by which tocotrienol affects the Nrf2 modulation of hepcidin gene expression, a peptide involved in iron handling and in inflammatory responses, is viewed in the light of the disruption of the iron intracellular distribution and of the Nrf2 anergy characterizing Friedreich’s ataxia. This research provides a suitable model to analyze the efficacy of therapeutic strategies able to counteract the excess free radicals in Friedreich’s ataxia, and paves the way to long-term clinical studies.
Impact statement
Oxidative stress is involved in the pathogenesis of Friedreich's ataxia (FRDA), a genetic disorder causing neurodegeneration due to the dramatic reduction in the expression of frataxin. To date, no cure is available for FRDA patients. In some countries, FRDA patients assume idebenone in order to counteract the effects of frataxin deficiency. We demonstrate that idebenone treatment alone is not able to abrogate oxidative stress in FRDA patients, whereas the combined treatment with tocotrienols might be more efficient and perhaps produce clinical improvement. In fact, a decrease in oxidative stress and inflammation markers can be seen after two months and is more pronounced after one year of treatment. This is, in our opinion, valuable information for clinicians, since idebenone is the treatment of choice for FRDA patients in some countries.
Keywords: Friedreich’s ataxia, tocotrienol, oxidative stress markers, inflammation, lipidomics, hepcidin
Introduction
Friedreich’s ataxia (FRDA, OMIM #229300) is a hereditary ataxia, with a clinical onset around puberty. It is an autosomal recessive disease causing the degenerative atrophy of the posterior columns of the spinal cord and leading to progressive neurologic disability, sensory loss, and muscle weakness. Hypertrophic cardiomyopathy is rather common, affecting 45–63% of FRDA patients and can cause premature death. In some patients, diabetes mellitus, scoliosis, and foot deformity are also observed.1,2
FRDA is caused by the partial deficiency of frataxin, a 210-amino acid protein, which localizes in the mitochondria. Most patients are homozygous for an unstable GAA trinucleotide expansion in the first intron of the gene, FXN, located on chromosome 9q13.3 The expanded GAA repeats cause a decrease in transcription, owing to an abnormal chromatin conformation. Frataxin function is somewhat unclear, although there is general agreement that it is involved in cellular iron homeostasis; in particular, it participates in the mitochondrial synthesis of iron–sulfur-containing proteins and in heme synthesis.4 Its deficiency leads to multiple enzyme deficits, mitochondrial dysfunction, deficit of ATP production and oxidative damage.5–7 In addition, in vitro studies with Schwann cell lines which underwent FXN knockdown8 showed that frataxin deficiency causes a strong activation of inflammatory pathways. Numerous studies have pointed out a hypersensitivity to oxidative stress (OS) in cell models and FRDA patients and the presence of some oxidation biomarkers in urine and blood samples from FRDA patients.9–13 OS is a major cause of neuron degeneration and death, although it is unclear whether, in FRDA patients’ cells, OS is a consequence of the observed iron accumulation in mitochondria, coupled to an iron cytoplasmic depletion (for an in-depth discussion, see reviews14,15). Some authors have suggested that frataxin deficiency is linked to oxidative stress condition mainly because Nrf2-driven responses appear to be impaired.16 In addition, altered lipid metabolism is considered a hallmark of FRDA pathophysiology. The mechanisms by which the lipid metabolism is impaired in FRDA remain uncertain. It has been suggested that frataxin deficiency and the consequent dysfunction of the mitochondrial respiratory chain lead to the alteration of main pathways involved in fatty acid synthesis and degradation.17
Based upon these considerations, we suggest that FRDA patients might benefit of treatment with antioxidants. Italian FRDA patients are currently treated with idebenone, a short chain quinone analogue, which acts as a free-radical scavenger; the rationale for treatment is based on the consideration that mitochondrial energy shortage and oxidative stress are critical factors for FRDA in neuronal cells.18,19 Notwithstanding the therapeutic benefits of idebenone in improving neurological functions20 and in the prevention of cardiopathy,21 patients still experience a progressive worsening of their condition. The present study was devised in order to understand whether idebenone is effective in abating OS and whether patients might better benefit of a combined therapy with another antioxidant, tocotrienol.
Tocotrienols are natural forms of vitamin E, which are found in several natural sources, including coconut oil, palm oil, rice bran oil, cocoa butter, barley, and wheat germ.22 Tocotrienols, as compared to tocopherols, are characterized by three double bonds. Such small difference, involving six hydrogen atoms, confers to tocotrienols the ability to diffuse in tissues with high lipid content22,23 and improves their anticancer, antioxidant, cardioprotective and neuroprotective activities, both in vitro and in vivo.24,25 Tocotrienols have quite different behavior as compared to tocopherols: a specific metabolism and specific routes of tissue delivery and storage,26 and the “chain-breaking” ability to neutralize peroxyl radicals through the formation of phenoxyl radicals, which are relatively stable. Tocotrienols also decrease oxidative-nitrosative stress and the inflammatory cascade in experimental models of alcoholic and diabetic neuropathy.24 Noteworthy, tocotrienols lead to a specific and significant increase in FXN-3 expression. In FRDA patients, this protein is a minor, functionally active, FXN isoform.27 Tocotrienols increase, in vitro, transcription of IKBKAP gene, mutated in the neurodegenerative genetic disorder Familial dysautonomia, leading to an increase in the correctly spliced transcript and normal protein.28 Moreover, tocotrienols possess in vitro antifibrotic properties,29 and, recently, Yang et al. elucidated the mechanisms by which δ-Tocotrienol reduces inflammation through the inhibition of TNF-α-induced activation of NF-κB and the LPS-stimulated IL-630 expression. Finally, tocotrienols are able to modulate the sensitivity to ferroptosis by inhibiting lipid peroxidation.31 Ferroptosis is a form of regulated iron-dependent cell death, recently identified in FRDA fibroblasts.32
Recently, EPI-743 (alpha-tocotrienol quinone; vatiquinone), a last-generation tocotrienol variant, was tested in FRDA patients and found to improve neurological and disease progression parameters following a 24-month treatment.33
Here we report data from a pilot study carried out with five FRDA patients, taking idebenone and a tocotrienol mixture (5 mg/kg/day) for one year, where biomarkers related to OS were compared with data obtained from age- and sex-matched healthy controls. Patients’ blood samples were collected for the initiation of treatment and 2 and 12 months of post-treatment; however, due to a freezer failure, some biological evaluations could not be carried out for the 12-month treatment. Patients were also studied by conventional and advanced cardiac magnetic resonance (CMR) techniques, including a CMR cardiac study, completed by gadolinium perfusion late enhancement evaluation.34 Improvements observed in OS biomarkers and, to a lesser extent, in clinical outcomes suggested that adjunctive treatment with tocotrienols might be worthy of a study on a wider scale.
Materials and methods
Patients and treatment
Seven FRDA patients and seven healthy volunteers of similar age were enrolled by the Child Neurology and Psychiatry Unit of the IRCCS Istituto delle Scienze Neurologiche of Bologna. FRDA diagnosis was performed according to Harding diagnostic criteria35 and confirmed by genetic testing, which showed that all FRDA patients were homozygous for GAA triplet expansion in the FXN gene. The level of clinical disability was quantified according to ICARS scale.36 Data are summarized in Table 1.
Table 1.
Subject characteristics.
|
Healthy control subjects |
FRDA subjects |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Gender | Age at time of study | FXN mRNA expression (±std dev) | No. | Gender | Age at time of study | Age of disease onset | Number of GAA repeats in the less expanded allele | Total ICARS SCORE | FXN mRNA expression (±std dev) |
| 1 | F | 19 | 1 ± 0.11 | 1 | F | 13 | 9 | 830 | 16 | 0.1 ± 0.01 |
| 2 | M | 19 | 1.25 ± 0.23 | 2 | M | 29 | 16 | 360 | 29 | 0.16 ± 0.02 |
| 3 | M | 19 | 2.35 ± 0.49 | 3 | M | 24 | 11 | 625 | 59 | 0.17 ± 0.03 |
| 4 | F | 19 | 0.9 ± 0.15 | 4 | F | 18 | 8 | 621 | 10 | 0.26 ± 0.02 |
| 5 | F | 19 | 1.24 ± 0.37 | 5 | F | 27 | 3 | 560 | 39 | 0.17 ± 0.02 |
| 6 | F | 26 | 1.24 ± 0.06 | 6 | M | 23 | 8 | 760 | 67 | 0.17 ± 0.05 |
| 7 | F | 23 | 1.23 ± 0.06 | 7 | F | 16 | 12 | 600 | 19 | 0.3 ± 0.01 |
Note: FXN mRNA expression was arbitrarily set to 1 in control subject #1, then all FXN mRNA expression values were referred to that sample.
All patients were treated with idebenone (5 mg/kg body weight/day) throughout the study. FRDA patients 1–5 shown in Table 1 assumed tocotrienol (5 mg/kg body weight/day) for 12 months. A venous blood sample was obtained before the initiation of tocotrienol (FRDA), following two months (FRDA post-2 months) and following the full term of treatment (FRDA post-1 year). Biomarkers were compared in seven age-and-sex-matched healthy control subjects (CTR). Controls were devoid of any clinical sign of psychiatric or neurological diseases and did not take antioxidant and/or vitamin supplements or medications potentially affecting oxidative status. The Local Ethical Committee approved the study (1635– 08092011).
At the beginning of the study and following one year of treatment with tocotrienols, patients were evaluated according to the ICARS scale and assessed with conventional cerebral magnetic resonance imaging (MRI), MRI study of cerebral protons (1HMRS), muscle phosphorus spectroscopy study (31PMRS), diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), and CMR imaging. For details, please see Fortuna.34
Tocotrienol
The toxicity level of tocotrienols for humans is presently undetermined; however, they are deemed to be safe as for long-term use in nutrition. According to Nakamura et al.,37 the estimated no-observed-adverse-effect level (NOAEL) in rats is 120–130 mg/kg body weight/day, while Tasaki et al.38 report higher values (303 mg/kg/day for males, and 472 mg/kg body weight/day for females). No adverse effects were reported in humans administered with 240 mg/kg body weight/day tocotrienols for 18–24 months.39 The dose administered in the present study was 5 mg/kg body weight/day, i.e. well below that reported by Tomeo et al.39 Moreover, it was demonstrated by Patel et al.40 that 400 mg/day of tocotrienol oral supplementation, a dose similar to the one used in this study, was sufficient to detect the presence of tocotrienol isoforms in blood, heart, and brain after 80 days, 155 days (range 30–443), and 261 days (range 78–672 days), respectively.
The tocotrienol mixture was developed by Ambrosialab s.r.l., a spin-off company of the Ferrara University, Italy. The tocotrienol mixture, consisting of a Palm Oil (Elaeis Guineensis) phytocomplex, is formulated as soft gel capsule “OXI-3” (internal ref. name ALAB103). Its composition is reported in Table 2. Dose, composition of tocotrienol mix, time of treatment used in this study were in the same ranges as those reported in Patel et al.40
Table 2.
Composition of OXI-3 (*), containing tocotrienols and tocopherols in the enantiomerically pure natural form.
| Total tocotrienols and tocopherol minimum 250 mg/g (25%) |
Isomeric composition of tocotrienols | |||
|---|---|---|---|---|
| Composition | Range | Typical value | ||
| Total D-Tocotrienols in the mix | Min. 190 mg/g | 197.5 mg/g | D-α-Tocotrienol | 55–70 mg |
| Total D-Tocopherol in the mix | Min. 51 mg/g | 59 mg/g | D-β-Tocotrienol | >7.5 mg |
| D-γ-Tocotrienol | 102.5–120 mg | |||
| D-δ-Tocotrienol | 27.5–35 mg | |||
Note: (*) OXI-3 was developed by Ambrosialab, University of Ferrara, Italy.
Blood sample treatment
Fifteen milliliters of blood were drawn and collected in a test tube containing EDTA. An aliquot of whole blood (1 mL) was used for lipidomic analysis; the remaining part was used to obtain the blood components. Following centrifugation of blood at 1000 × g for 10 min, plasma was retrieved and stored at −20°C for subsequent use. Peripheral blood mononuclear cells (PBMCs) and erythrocytes were obtained by density gradient centrifugation with Ficoll (Histopaque 1077, Sigma-Aldrich, St.Louis, Mo) after dilution (1:1) with sterile phosphate-buffered saline (PBS). PBMCs were collected, lysed in 1 mL Trizol® Reagent (Invitrogen, Milan, Italy), and stored at −80°C for subsequent RNA extraction.
GSH and GSSG analysis
High-performance capillary electrophoresis was used to analyze the levels of glutathione (GSH) and glutathione disulphide (GSSG) in plasma samples according to Raggi et al.41 and Kong et al.42 An Agilent 3D CE Instrument (Palo Alto, Ca) electrophoresis system with a DAD detector read the samples at 200 nm.
Samples were deproteinized with acetonitrile, then analyzed with transient pseudo-isotachophoresis. An uncoated fused silica capillary (20 cm × 75 µm internal diameter) was used. Runs were carried out with 10 kV separation voltage, using 300 µM phosphate buffer (pH 7.4). Samples were pressure injected for 40 s, in order for the sample length to reach 25% of the effective capillary length, yielding a 15–20 fold increase in sensitivity.
Carbonyl group evaluation
Plasma sample preparation
Plasma albumin/IgG-depletion was carried out following the protocol described in Guidi et al.43 by using the Blue Albumin and IgG Depletion kit (Sigma-Aldrich, France) according to the manufacturer’s instructions. Samples were stored at −20°C before use. The Bradford assay (Bio-Rad Laboratories, Hercules, CA) was used to assess protein concentration of each sample.
Two-dimensional gel electrophoresis
Two-dimensional polyacrylamide gel electrophoresis was performed under the same conditions described previously.43 Briefly, protein plasma samples were diluted in 125 μL of the lysis buffer previously described,43 except that 0.2% of the appropriate IPG buffer (GE Healthcare, USA) was used. At least three samples were studied for each subject, in order to assess biological and analytical variation. Immobilized non-linear pH gradient strips (pH 4–7; 7 cm length IPG strips; GE Healthcare, USA) were rehydrated at 16°C with lysis buffer containing 0.2% carrier ampholyte.
Isoelectrofocusing (IEF) was performed using the Ettan™ IPGphor™ system (GE Healthcare, USA) at 16°C following electrical conditions described previously.43 Before the second-dimension run, the IPG strips were incubated with two different equilibrating buffer solutions43 for 12 and 15 min, respectively. The second-dimension was applied on 9–16% polyacrylamide linear gradient gels at 10°C. The applied current was 40 mA/gel and the gels were run until the dye reached their lower end. The Coomassie gels were stained with colloidal blue Coomassie G-250.
Derivatization of protein carbonyls and immunostaining for DNP
Protein carbonyl analysis was assessed according to the protocol described previously.43 In particular, after DNPH staining and removal of excess dye,43 IPG strips were used for the second-dimension separation. The plasma proteins were then blotted into a PVDF membrane as reported previously.44,45 According to Guidi et al.,43 the protein carbonyls were detected incubating the PVDF membrane with DNP/IgG primary antibody, and then with a specific secondary antibody. Spot detection was carried out using the Immobilon Western Chemiluminescent AP substrate (Millipore) according to the manufacturer’s instructions.
Image acquisition and analysis
Two-dimensional Coomassie-stained gels and oxyblots were scanned using an Epson expression 1680 PRO scanner; the acquired images were analyzed using ImageMaster 2D Platinum 6.0 software (GE Healthcare, USA). For each subject, a minimum of three technical replicates were performed and only the spots present in all the replicates were taken into consideration for subsequent analysis. Coomassie gels were used to normalize the intensity of carbonylated spots, as previously reported.43 The normalized oxyblots obtained from all patients before therapy were then compared with the oxyblots obtained from patients after therapy (two months and one year) and from healthy controls.
Oxygen radical absorbance capacity assay
Total antioxidant activity was evaluated by the ORAC assay according to a method modified by us (see Pessina et al.,46 and references quoted therein). In the final reaction mixture (0.2 mL total volume), plasma samples were initially mixed with fluorescein sodium salt (85 nM). The reaction was started by the addition of 2,2ʹ-azobis(2-amidino-propane) dihydrochloride (AAPH), a free radical initiator targeting the fluorescein. A calibration curve (10, 20, 30, 40, 50 μM) was made with a standard solution of Trolox, a derivative of vitamin E. The fluorescence intensity was monitored at 37°C, every 5 min for 30 min using Fluoroskan Ascent FL® (Thermo Fisher Scientific, Inc. Waltham, MA) (excitation at 485 nm, emission at 538 nm). The ORAC values were obtained by subtracting the areas under the quenching curve of fluorescein of the blank from that of each evaluation. ORAC values were referred as Trolox equivalents (TE), pH = 7.4. The experiments were carried out in triplicate.
Lipidomic analysis
Lipidomic analysis was performed as reported previously.47 Briefly, plasma was separated from the whole blood by centrifugation; erythrocytes were lysed and then centrifuged in order to obtain plasma membranes. The extraction of phospholipids from red blood cell membranes was carried out according to the method of Bligh and Dyer.48 The total phospholipid fraction was then incubated with KOH/MeOH solution (0.5 M) for 10 min at room temperature, and fatty acid methyl esters were subsequently extracted with n-hexane. All fatty acids and their isomers, separated by gas chromatography, were identified by comparing them with commercially available standard markers and with a custom-made library of geometrical trans MUFA and PUFA obtained by thiyl radical-catalyzed reaction of naturally occurring lipids.49 The amount of each fatty acid in red blood cell membranes was expressed as percentages of the total fatty acids identified; however, we reported only the percentage of the main saturated and unsaturated residues of phospholipids, along with some relevant measures, such as total saturated (SFA), total monounsaturated (MUFA), and total polyunsaturated (PUFA n-3 and PUFA n-6) residues, some indicative ratios, peroxidation Index (PI)50 and unsaturation index (UI).50
RNA separation and quality control
Total RNA was obtained from the Trizol® lysates according to the manufacturer’s data sheet.51 RNA quality was assessed as described previously.27 Briefly, the integrity of RNA was confirmed by visualization of the 28S and 18S band sharpness. For removal of genomic contaminating DNA from RNA samples, an RNase-free deoxyribonuclease I (DNase I) (Amplification Grade DNase I, Sigma, St. Louis, Mo) treatment was performed. After treatment, the absence of genomic DNA was confirmed by evaluating the expression of the HSP70 promoter using a couple of specifically designed primers (Table S1). RNA concentration was evaluated by the Ultrospec 3000 spectrophotometer (Pharmacia Biotech, Cambridge, England).
The RevertAid™ First Strand cDNA Synthesis Kit (Fermentas International Inc., Thermo Fisher, Burlington, Ontario) was used to reverse transcribe equal amounts of total RNA, according to the manufacturer’s data sheet.
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was carried out in triplicate in a BioRad CFX96 real-time thermal cycler using the SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories, Hercules, CA). Data were normalized to the housekeeping genes Beta-Actin and GADPH. The primer sequences are shown in Table S1. Primers were chosen with the help of the PRIMER3 and AMPLIFY free software; whenever possible, primers were designed so as to span an exon–exon junction and they were obtained from Sigma-Genosys (Sigma-Aldrich, St. Louis, Mo). Melting curve analysis followed by agarose gel electrophoresis confirmed primer specificity of PCR products. Data were analyzed with the 2−ΔΔCT method,52 taking into account the efficiency of the real-time PCR reaction53 which was between 95% and 105%. The software package CFX Manager™ (Bio-Rad Laboratories) and qbase plus (http://www.biogazelle.com/) were used for data calculation and statistical analysis. Data were expressed as fold change with respect to controls ± confidence interval.
Statistical analysis
The Shapiro–Wilk test was applied to assess normalcy of the data. Depending on data distribution, non-parametric (Kruskal–Wallis) or parametric (ANOVA) tests were used for assessing statistical significance of results. Benjamini and Hochberg false discovery rate (FDR) was applied to correct P-values thus obtained. In order to compare two sets of data, non-parametric (Wilcoxon-Mann-Whitney) or parametric (two-tailed unpaired Student’s t) tests were used. For all analysis, statistical significance was set at P ≤ 0.05.
Results
Patient’s outcomes
No adverse side effects were observed after tocotrienol treatment in the five patients with FRDA participating in the study. They constituted a group with heterogeneous clinical and genetic parameters. The neurological evaluation, represented by the total score on the ICARS scale and repeated after one year of treatment with tocotrienols, showed a slightly worsening trend, in accordance with the slow progressive trend generally observed for FRDA.
Among the instrumental examinations carried out, the only one to demonstrate slight variations after one year of treatment was the CMR study. In fact, the tests carried out after one year of treatment showed a trend to a slight reduction in the extent of the myocardial mass characterized by myocardial damage (positive to late enhancement), which seemed to vary in a directly proportional relationship with the cardiac mass, and more specifically with hypertrophy. However, this figure was not statistically significant, most likely due to the small number of patients studied (Table 3).
Table 3.
Cardiac magnetic resonance data of patients before and following one-year tocotrienol treatment (mean ± std dev) and relative statistical significance (Student’s t test).
| FE Vsx(56–79%) | Vol TD Vsx(47–92 mL/m2) | Vol TS Vsx(14–39 mL/m2) | SV Vsx(37–62 mL/m2) | Sp Td SIV(5–8 mm) | Sp Td PP(5–7 mm) | M Vsx(70–113 g/m2) | M Lenha(g/m2) | % M Lenha | |
|---|---|---|---|---|---|---|---|---|---|
| FRDA patients at time 0 | 65.56 ± 12.18 | 62.20 ± 16.08 | 22.60 ± 13.58 | 39.60 ± 4.93 | 12.0 ± 3.67 | 10.0 ± 3.46 | 65.86 ± 17.19 | 12.36 ± 9.50 | 14.78 ± 8.05 |
| FRDA patients post-1 year treatment | 61.66 ± 10.49 | 57.26 ± 29.93 | 29.02 ± 18.50 | 43.04 ± 13.90 | 12.20 ± 3.96 | 8.80 ± 2.17 | 60.54 ± 12.78 | 7.50 ± 5.82 | 13.62 ± 7.58 |
| P | 0.090 | 0.677 | 0.137 | 0.619 | 0.883 | 0.493 | 0.311 | 0.497 | 0.852 |
Note: Values in brackets are the normal range for the healthy subjects. Normal values for Lenh are not reported.
aData relating only to four of the five patients.
FE: ejection fraction, Vsx left ventricle; VolTd: end-diastolic volume; VolTs: end-systolic volume; SV: stroke volume; Sp: thickness; SIV: interventricular septum; PP: posterior wall; M: mass; Lenh: late enhancement (delayed wash out of intramyocardial gadolinium); % M: percentage of myocardial mass with positivity to late enhancement.
GSH and GSSG content of plasma
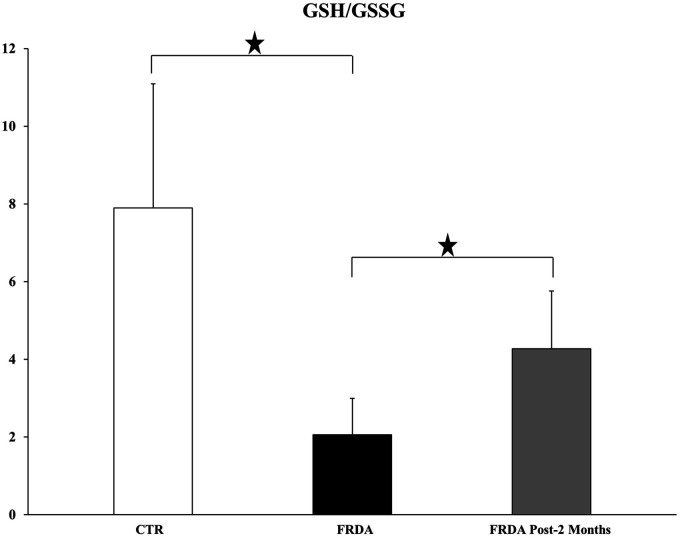
The mean GSH content of FRDA patients' plasma was 2.5 ± 0.97 μM, a value not significantly different from that of healthy controls. However, patients were found to have a much higher (almost 9-fold) plasma concentration of GSSG, and therefore their GSH/GSSG ratio was significantly lower than the controls (Figure 1). The two-month tocotrienol treatment caused a marked and significant increase of the GSH/GSSG ratio, due to both an increase in GSH and a decrease in GSSG content. It should be noted that GSH/GSSG ratio, a useful indicator of the plasma oxidative stress, is more sensible than the absolute amounts of GSH and GSSG.54
Figure 1.
GSH/GSSG ratio in plasma derived from healthy control subjects (CTR) and FRDA patients before (FRDA) and after two month (FRDA post-2 months) tocotrienol supplementation. Data are presented as Mean ± SD. ANOVA (P = 0.0089) and two-tailed Student’s t-test were applied. Significance was maintained after P value false discovery rate (FDR) correction. Significance: (*) P < 0.05.
Carbonyl groups
Under the denomination “Carbonyl groups” fall the aldehyde and ketone groups which are caused by metal-catalyzed oxidation of amino acidic residues under OS; therefore, carbonyl groups are useful markers of protein oxidation.55 For each patient, we obtained a pattern of total plasma proteins stained in the 2-DE gels with Coomassie and a pattern of oxidized proteins recognized by Western blot analysis. For each plasma protein sample, 2D-oxyblots were compared with Coomassie-stained 2D-gels for normalization purpose and for evaluation of the oxidation levels.
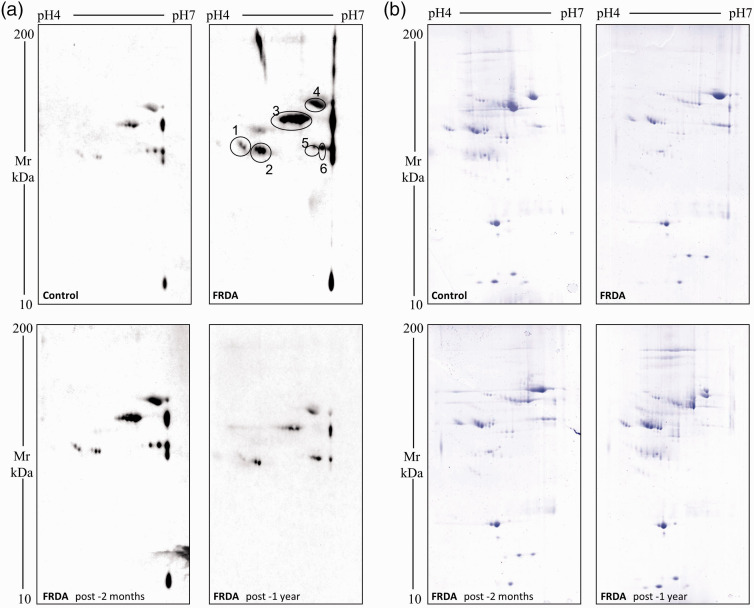
Figure 2(a) shows the representative images of the oxyblots obtained from the plasma; Figure 2(b) shows the corresponding Coomassie-stained gels indicating that the same amount of protein had been separated in the different gels. Experimental reproducibility was verified by performing several replicates of random samples. The profiles of the five FRDA patients were remarkably similar as for the total amount of oxidized proteins as well as for the presence of proteins with the same apparent molecular weight. About 400 protein spots were identified in the Coomassie-stained 2D gels, as shown in the representative gel (Figure 2(b)). Overall, the pattern of protein spots was similar in the plasma samples of normal subjects, of FRDA patients before therapy and of the same patients after two-month and one-year tocotrienol treatment. However, the comparison of the 2D electrophoresis gels from normal control subjects and from FRDA patients showed that excess carbonyl groups are clearly present in the patient samples; moreover, the decrease of carbonylated proteins upon two-month tocotrienol treatment and their almost complete clearance upon one-year therapy was evident.
Figure 2.
Representative images of two-dimensional electrophoresis gels and oxyblots of plasma from one healthy control subject (CTR) and one Friedreich ataxia patient before (FRDA) and after two month (FRDA post-2 months) or one-year (FRDA post-1 year) tocotrienol supplementation. The pH range was 4 to 7. Panel (a) Carbonylated proteins were detected by Western blot using a primary antibody directed against DNPH-derivatized carbonyl groups (see methods). Labeled spots represent the spots which showed a higher variation in carbonylation level. Panel (b) Total proteins were visualized by colloidal blue Coomassie G-250 staining. The Coomassie-stained gels correspond to the oxyblots shown in panel a. (A color version of this figure is available in the online journal.)
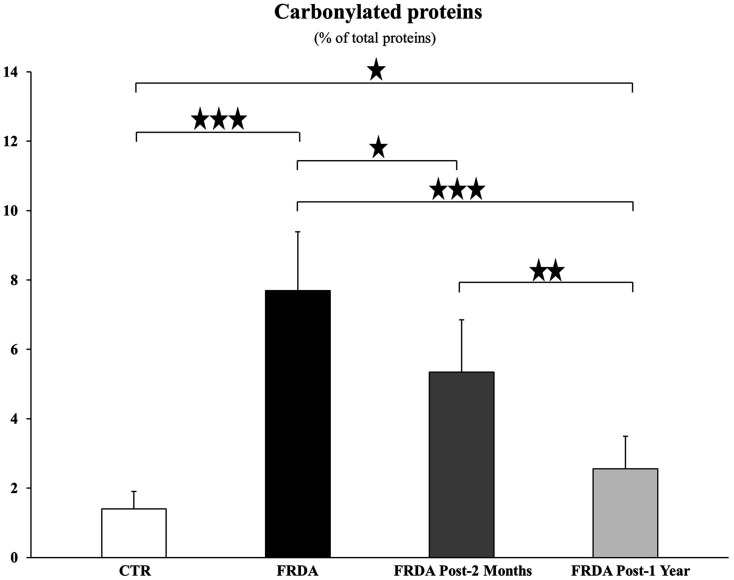
In Figure 2(a), six different spots in the oxyblot are marked, which greatly decrease in carbonylation level with tocotrienol treatment. The intensity of these carbonylated spots was expressed as the mean ± SD of normalized percentage of oxyblot spot volumes, calculated according to the formula reported in Guidi et al.43 and drawn in the histogram of Figure 3. The identification of the proteins that are differentially carbonylated is currently being performed by means of MALDI-TOF analysis.
Figure 3.
Levels of plasma protein carbonylation in healthy control subjects (CTR) and Friedreich ataxia patients before (FRDA) and following two-month (FRDA post-2 months) or one-year (FRDA post-1 year) add-on tocotrienol supplementation. Values are expressed as Means ± SD of percentage oxyblot spot volumes normalized to the Coomassie-stained spot volumes (%V, where V= integration of OD over the spot area and where % V= V single spot/V total spots). Significance was evaluated by ANOVA (P < 0.00001) and maintained after P value false discovery rate (FDR) correction. Stars show statistical significance for relevant two-tailed Student’s t test comparisons: (*) P < 0.05; (**) P <0.01; (***) P <0.001.
The decrease in anti-DNP reactive proteins was already significant following two-month tocotrienol treatment (P = 0.0498) and even more marked following one-year tocotrienol treatment (P = 0.0004). After one-year tocotrienol, the amount of carbonylated proteins, though highly reduced, was still higher in plasma from FRDA patients than in controls (P = 0.04324).
Oxygen radical absorbance capacity
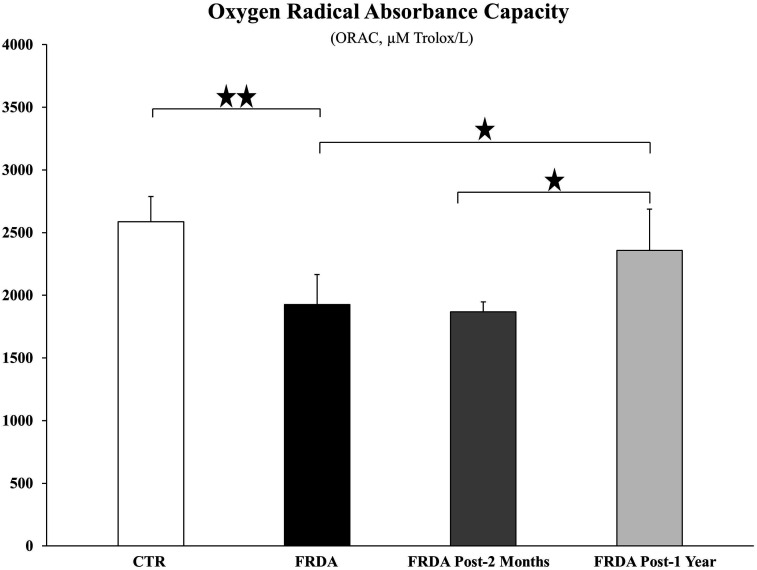
In Figure 4, the ORAC values of controls and of FRDA patients are compared. Tocotrienol treatment for two-months did not increase the plasma oxygen radical absorbance capacity of FRDA patients, whereas one-year-long supplementation was able to increase significantly (P = 0.0454) ORAC values.
Figure 4.
Oxygen radical absorbance capacity (ORAC) was analyzed in plasma derived from healthy control subjects (CTR) and FRDA patients before (FRDA) and after two-month (FRDA post-2 months) and one-year (FRDA post-1 year) tocotrienol supplementation. Data are expressed as μM Trolox/L (Mean ± SD). Significance was evaluated by ANOVA (P = 0.0007) and maintained after P value false discovery rate (FDR) correction. Stars show statistical significance for relevant Student’s two-tailed t test comparisons: (*) P < 0.05; (**) P < 0.01.
Lipidomics
Lipidomic analysis was carried out on only four out of five FRDA patients and on healthy controls; control values were comparable with those of healthy subjects with mean age (25 ± 10 years) or younger.56 Although membrane fatty acid composition is somehow tissue-specific, it was found that erythrocyte membranes are representative of the fatty acid composition of most body tissues.57,58
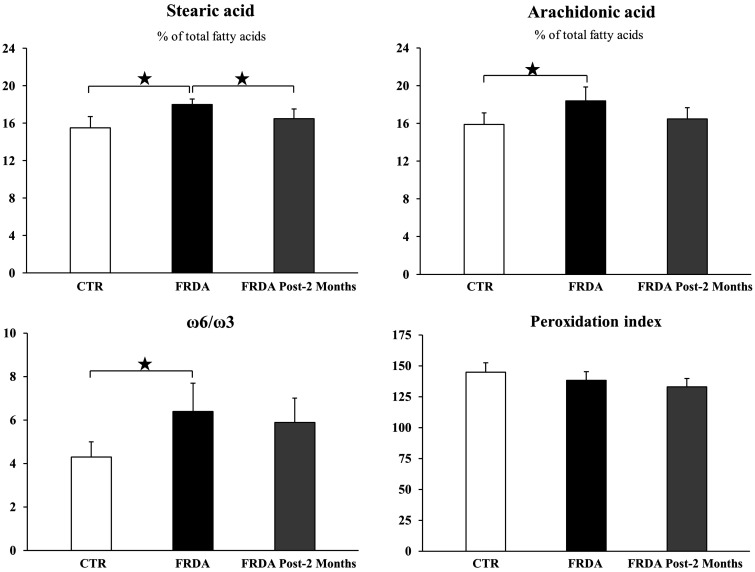
The complete analysis is reported in Table S2, while Figure 5 shows some relevant results. In particular, patients displayed an excess (P = 0.025) of the saturated lipid stearic acid (18:0), which, remarkably, decreased to normal levels as a consequence of the two-months tocotrienol treatment (P = 0.044). However, the statistical significance of ANOVA for stearic acid was not maintained after false discovery rate correction. The increase of stearic acid could be due to the de novo synthesis of saturated fatty acids, which is the first step in the regeneration of the lipid pools after OS. Two indices of inflammation were found to be increased in FRDA patients, namely arachidonic acid (20:4), a precursor of a number of inflammation mediators, which tends to decrease towards normal levels with tocotrienol treatment and the ω6/ω3 ratio, an index of the balance between the prevalently inflammatory omega-6 pathway and the anti-inflammatory omega-3 pathway.59 The arachidonic acid content can be correlated with the inflammatory status since, when released from the membranes, arachidonic acid initiates the eicosanoid cascade.59 Worth nothing, the peroxidation index was found to be similar in controls and in FRDA patients. This value depends on the amount of PUFA residues and reflects the propensity to peroxidation.
Figure 5.
Relevant lipidomic parameters (Mean ± SD) from healthy control volunteers (CTR) and from FRDA patients before (FRDA) and after two month (FRDA post-2 months) tocotrienol supplementation. The content of individual fatty acids in erythrocyte membranes is expressed as percentages of the total fatty acids identified. Significance was evaluated by ANOVA; significance was not maintained for stearic acid after P value false discovery rate (FDR) correction. Stars show statistical significance for relevant Student’s two-tailed t test comparisons: (*) P < 0.05; (**) P < 0.01.
Gene expression of some antioxidant enzymes and inflammation molecules
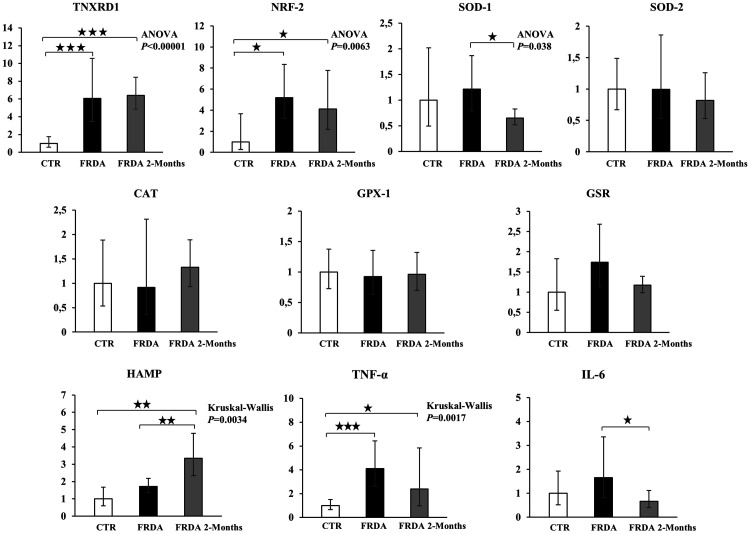
Gene expression was evaluated by qPCR in leukocyte extracts, in order to study the expression of a number of antioxidant enzymes not otherwise evaluable. The reaction efficiency was between 0.95 and 1.05 for all genes and both the melting curve and the gel electrophoresis showed that a single product was amplified in the PCR, thus demonstrating that the primers had been correctly chosen. Figure 6 shows the results of the gene expression study of the following genes: Thioredoxin Reductase 1, (TXNRD1); Nuclear Factor, Erythroid 2 like 2 (NRF-2); Superoxide Dismutase-1 (SOD-1), Superoxide Dismutase-2 (SOD-2), Catalase (CAT), Glutathione Peroxidase-1 (GPX-1), Glutathione Reductase (GSR), Hepcidin (HAMP), Tumor Necrosis Factor-alpha (TNF-α), Interleukin-6 (IL-6).
Figure 6.
Messenger RNA expression of antioxidant and inflammation genes in mononuclear white blood cell extracts from healthy control subjects (CTR) and FRDA patients before (FRDA) and after (FRDA Post-2 Months) two months of tocotrienol by qPCR. Thioredoxin Reductase 1, (TNXRD1); Nuclear Factor, Erythroid 2 like 2 (NRF-2); Superoxide Dismutase-1 (SOD-1); Superoxide Dismutase-2 (SOD-2); Catalase (CAT); Glutathione Peroxidase (GPX-1); Glutathione Reductase (GSR); Hepcidin (HAMP); Tumor Necrosis Factor-alpha (TNF-α); Interleukin-6 (IL-6). Data were normalized with two housekeeping genes, Beta-Actin and GAPDH. For each gene, the normalized expression value of CTR was set to 1, and the other gene expression data were reported to that sample. Data are expressed as fold change ± confidence interval (CI). Parametric analysis was applied for all genes except for GPX-1, HAMP, and TNF-α, which were analyzed by non-parametric methods. Significance was maintained after P value FDR correction, except for SOD-1. Stars show statistical significance for relevant comparisons: (*) P < 0.05; (**) P < 0.01; (***) P < 0.001.
The mRNA expression of TNXRD1, a selenoprotein thioredoxin reductase, and of Nrf2, the major regulator of cellular responses to OS, was significantly upregulated in FRDA subjects, independently of tocotrienol treatment. The gene expression analysis of the antioxidant enzymes examined does not show significant variations in the comparison between control and FRDA subjects, although a trend for an increased expression in SOD-1 and GSR can be perceived. Following tocotrienol supplementation, a trend of decreased mRNA expression was observed. Moreover, we evaluated the gene expression of GST (Glutathione S-Transferase), which may be expressed independently of Nrf2 activation. Data (not shown) do not show any significant difference between groups.
The expression of HAMP, a peptide hormone that regulates iron homeostasis, was increased in FRDA patients. Such increase was significantly higher after two month-tocotrienol supplementation. The expression of two inflammatory genes, TNF-α and IL-6, was increased in FRDA patients compared to controls; such increase was less enhanced in two-month tocotrienol supplemented patients.
Limitations
This study has several limitations, including that some evaluations were carried out only for the two-month intermediate time, since a freezer failure destroyed some samples obtained at time 12 months, and that this was a pilot study, involving only a small number of patients (N = 5). However, it is worth noting that other studies involving the evaluation of therapies in Friedreich’s ataxia had comparable sample size.60 Moreover, in accordance with other results, gene expression data support the involvement of oxidative stress and inflammation in FRDA patients and the positive effects of tocotrienol supplementation, but, due to the insufficient amount of the PBMCs we could recover, we were not able to confirm these results by protein expression analysis. In any case, the ensuing discussion is meant to show that the present study provided new information that can be valuable for the scientific community.
Discussion
Owing to the severe disruption of Fe-S cluster assembly in key mitochondrial enzymes caused by frataxin deficiency, there is little doubt that significant levels of ROS are generated in FRDA patient’s cells during mitochondrial activity, damaging key cellular structures and activities and causing apoptosis in the more metabolically active cells, such as neurons and cardiomyocytes. This undoubtedly qualifies FRDA as an OS-related disease9 and suggests that counteracting OS generation or OS-induced damages would rescue sensitive tissues.61
In order to evaluate the efficacy of antioxidant treatments, the appropriate OS markers should be assessed taking into account the peculiarities of FRDA pathobiology. For instance, it has been reported that FRDA patients exhibit increased levels of plasma malondialdehyde,9 a highly reactive compound generated by radical attack to polyunsaturated fatty acids, while other markers of radical damage, such as urinary 8-oxo-2′-deoxyguanosine (8-oxodG), were not consistently found.10 Similarly, since the Nrf2 system appears to be impaired in FRDA,16 it should not come as a surprise that OS failed to increase the expression of antioxidant genes regulated by Nrf2, whereas TNXRD1, which is upstream rather than downstream from Nrf2,62 and Nrf2 itself, is upregulated in FRDA patients, even if they are treated with idebenone.
Discussing into the detail the individual data,
The GSH/GSSG ratio, a relevant index of ROS detoxifying ability, was found to be markedly reduced in the plasma of FRDA patients, but tended to be normal values following the two-month treatment with tocotrienols.
Carbonyl groups, an index of OS-induced damage to cell proteins, were markedly increased in FRDA patients compared to healthy subjects, but displayed a reduction by about 30% after two-month tocotrienol treatment and by about 66% following one-year tocotrienol supplementation. Notably, oxidation did not appear to affect all proteins at the same extent, and it would be of interest to identify these through further analyses.
Plasmatic ORAC displayed a reduction in FRDA patients and was restored to normality only following a one-year tocotrienol supplementation, suggesting that this is not a very sensitive indicator of the OS affecting the whole organism.
Changes in membrane fatty acid composition, when examined by functional lipidomics tools,63 were suggestive of the presence of both OS and inflammation in FRDA patients, because of the combined increase in stearic acid, arachidonic acid, and the ω6/ω3 ratio; notably, stearic acid is related to the production of TNF-alpha, an inflammation marker.64 The two-month tocotrienol supplementation favored the decrease of these indices. It can also be hypothesized that the patients' lipid membrane profile would have displayed a further normalization, should it be evaluated following a longer anti-oxidant treatment. It is worth mentioning that no dietary treatment of patients regarding the essential fatty acid (EFA) intake was made during treatment, thus suggesting that the observed specific fatty acid changes can be likely ascribed to the antioxidant supplementation. Moreover, our results suggest further investigations on the association of tocotrienol supplementation with a diet containing a specific EFA balance.
Gene expression of antioxidant enzymes, as already pointed out, can be understood by taking into account the inability of chronically stressed cells to activate the Nrf2 driven response,16 which controls the level of SOD1, SOD2, catalase, GPX1, and GSR, among others. On the other hand, TXNRD1 and Nrf2 were upregulated in FRDA patients.
Notwithstanding the limited number of subjects in this study, the data examined so far clearly show that (i) FRDA patients are affected by OS, despite long-term idebenone treatment: a result which accounts for the limited efficacy of idebenone in counteracting the progression of the disease; (ii) add-on tocotrienol treatment considerably reduces OS biomarkers even when administered at very low doses and for a relatively short time (two months); moreover, data suggest that tocotrienol supplementation protracted for one year or longer may further decrease the OS biomarkers. Since we only evaluated the add-on treatment, we are unable to make any statement about the presence or absence of synergistic effects. On the clinical side, our results suggest that the combined treatment with idebenone and low tocotrienol doses may be beneficial. In effect, one should bear in mind that the efficacy of idebenone in delaying FRDA progression is supported by a relatively weak clinical evidence65 and has not been previously investigated using OS biomarker studies. Our data support the finding that FRDA patients show increased inflammatory biomarkers, in accordance with the observation by Nachum et al.,66 who described the transcriptional upregulation of inflammatory innate immune response genes. This conclusion is based on the functional lipidomic analysis and on the upregulation of two cytokine mRNAs, TNF-α and IL-6; these indices were less pronounced following the two-month tocotrienol supplementation. Although we unfortunately were unable to assess the data relative to the one-year tocotrienol treatment, this study does show clear relationship between OS and inflammation.
Finally, this study examined for the first time the expression of HAMP mRNA in FRDA PBMCs. Compared to controls, HAMP gene expression showed a trend towards increase, which became highly significant after the two month-tocotrienol supplementation. HAMP is a key regulator of iron homeostasis, both at the systemic and at the cell level67 and may be involved in the iron dysregulation occurring in cells of FRDA patients.68 Here, the increase of HAMP expression should be viewed at the light of its upregulation in inflammation, where HAMP plays a double role in preserving the macrophage iron pool and in downregulating the levels of TNF-α and IL-6 expression.69 An antioxidant response element has been identified in the promoter of the murine HAMP gene,70 suggesting that HAMP might be a pivot in the Nrf2-regulated battery of genes acting in OS responses, iron toxicity control, and anti-inflammatory activities. The increase of HAMP expression following the two-months tocotrienol administration can be tentatively explained by a role for tocotrienols as Nrf2 activators, in a manner similar to that of Vitamins A and C and small polyphenols71,72 or by their ability to decrease OS, with consequent reduction of the Nrf2 anergy.
Supplemental Material
Supplemental material, EBM890873 Supplemental Material1 for Effects of tocotrienol supplementation in Friedreich’s ataxia: A model of oxidative stress pathology by Alessandra Bolotta, Antonella Pini, Provvidenza M Abruzzo, Alessandro Ghezzo, Alessandra Modesti, Tania Gamberi, Carla Ferreri, Francesca Bugamelli, Filippo Fortuna, Silvia Vertuani, Stefano Manfredini, Cinzia Zucchini and Marina Marini in Experimental Biology and Medicine
Supplemental material, EBM890873 Supplemental Material2 for Effects of tocotrienol supplementation in Friedreich’s ataxia: A model of oxidative stress pathology by Alessandra Bolotta, Antonella Pini, Provvidenza M Abruzzo, Alessandro Ghezzo, Alessandra Modesti, Tania Gamberi, Carla Ferreri, Francesca Bugamelli, Filippo Fortuna, Silvia Vertuani, Stefano Manfredini, Cinzia Zucchini and Marina Marini in Experimental Biology and Medicine
ACKNOWLEDGMENTS
Tocotrienol was a kind gift of Mr. R. Baldini (ASAMSI). Formulation studies and ORAC assays were kindly supported by Ambrosialab, Ferrara, Italy. Authors are grateful to Dr. David Muhesam for his expert revision of the English text.
Authors’ contributions
FF, SM and MM participated in the design of the study; CZ, SM and MM acquired funds; AB, AP, PMA, AG, AM, TG, CF, FB, FF and SV performed experiments, interpreted and analyzed the data; AB, PMA, CZ and MM wrote and edited the original draft; all authors reviewed and approved the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by a grant from AISA (Italian Association of patients with Ataxic Syndrome), by Italian Ministry of University and Research [grant 20082L3NFT_003] to SM and by University of Bologna research funds awarded to MM and CZ.
ORCID iD
Provvidenza M Abruzzo https://orcid.org/0000-0001-5242-9448
Supplemental material
Supplemental material for this article is available online.
References
- 1.Pandolfo M. Friedreich ataxia. Semin Pediatr Neurol 2003; 10:63–2 [DOI] [PubMed] [Google Scholar]
- 2.Filla A, De Michele G, Coppola G, Federico A, Vita G, Toscano A, Uncini A, Pisanelli P, Barone P, Scarano V, Perretti A, Santoro L, Monticelli A, Cavalcanti F, Caruso G, Cocozza S. Accuracy of clinical diagnostic criteria for Friedreich’s ataxia. Mov Disord 2000; 15:1255–8 [DOI] [PubMed] [Google Scholar]
- 3.Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zar F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996; 271:1423–7 [DOI] [PubMed] [Google Scholar]
- 4.Gille G, Reichmann H. Iron-dependent functions of mitochondria-relation to neurodegeneration. J Neural Transm 2011; 118:349–59 [DOI] [PubMed] [Google Scholar]
- 5.Chiang S, Kovacevic Z, Sahni S, Lane DJ, Merlot AM, Kalinowski DS, Huang ML, Richardson DR. Frataxin and the molecular mechanism of mitochondrial iron-loading in Friedreich's ataxia. Clin Sci 2016; 130:853–70 [DOI] [PubMed] [Google Scholar]
- 6.Vaubel RA, Isaya G. Iron-sulfur cluster synthesis, iron homeostasis and oxidative stress in Friedreich ataxia. Mol Cell Neurosci 2013; 55:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jasoliya MJ, McMackin MZ, Henderson CK, Perlman SL, Cortopassi GA. Frataxin deficiency impairs mitochondrial biogenesis in cells, mice and humans. Hum Mol Genet 2017; 26:2627–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C, Schoenfeld R, Shan Y, Tsai HJ, Hammock B, Cortopassi G. Frataxin deficiency induces Schwann cell inflammation and death. Biochim Biophys Acta 2009; 1792:1052–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emond M, Lepage G, Vanasse M, Pandolfo M. Increased levels of plasma malondialdehyde in Friedreich ataxia. Neurology 2000; 55:1752–3 [DOI] [PubMed] [Google Scholar]
- 10.Schulz JB, Dehmer T, Schöls L, Mende H, Hardt C, Vorgerd M, Bürk K, Matson W, Dichgans J, Beal MF, Bogdanov MB. Oxidative stress in patients with Friedreich ataxia. Neurology 2000; 55:1719–21 [DOI] [PubMed] [Google Scholar]
- 11.Piemonte F, Pastore A, Tozzi G, Tagliacozzi D, Santorelli FM, Carrozzo R, Casali C, Damiano M, Federici G, Bertini E. Glutathione in blood of patients with Friedreich’s ataxia. Eur J Clin Invest 2001; 31:1007–11 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong JS, Khdour O, Hecht SM. Does oxidative stress contribute to the pathology of Friedreich's ataxia? A radical question. FASEB J 2010; 24:2152–63 [DOI] [PubMed] [Google Scholar]
- 13.Lupoli F, Vannocci T, Longo G, Niccolai N, Pastore A. The role of oxidative stress in Friedreich's ataxia. FEBS Lett 2018; 592:718–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos R, Lefevre S, Sliwa D, Seguin A, Camadro JM, Lesuisse E. Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Sign 2010; 13:651–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayot A, Santos R, Camadro JM, Rustin P. Friedreich's ataxia: the vicious circle hypothesis revisited. BMC Med 2011; 9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paupe V, Dassa EP, Goncalves S, Auchère F, Lönn M, Holmgren A, Rustin P. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS One 2009; 4:e4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamarit J, Obis È, Ros J. Oxidative stress and altered lipid metabolism in Friedreich ataxia. Free Radic Biol Med 2016; 100:138–46 [DOI] [PubMed] [Google Scholar]
- 18.Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AM, Butterfield DA. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci 2005; 233:145–62 [DOI] [PubMed] [Google Scholar]
- 19.Abeti R, Parkinso MH, Hargreaves IP, Angelova PR, Sandi C, Pook MA, Giunti P, Abramov AY. Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich's ataxia. Cell Death Dis 2016; 7:e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier T, Perlman SL, Rummey C, Coppard NJ, Lynch DR. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich's ataxia: data from a 6-month controlled study followed by a 12-month open-label extension study. J Neurol 2012; 59:284–91 [DOI] [PubMed] [Google Scholar]
- 21.Kearney M, Orrell RW, Fahey M, Pandolfo M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database Syst Rev 2012; 4:CD007791. [DOI] [PubMed] [Google Scholar]
- 22.Packer L, Weber SU, Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr 2001; 131:369S–73S [DOI] [PubMed] [Google Scholar]
- 23.Peh HY, Tan WS, Liao W, Wong WS. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther 2016; 162:152–69; PMID: 26706242. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol 2010; 80:1613–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab 2014; 11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comitato R, Ambra R, Virgili F. Tocotrienols: a family of molecules with specific biological activities. Antioxidants 2017; 6:pii: E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abruzzo PM, Marini M, Bolotta A, Malisardi G, Manfredini S, Ghezzo A, Pini A, Tasco G, Casadio R. Frataxin mRNA isoforms in FRDA patients and normal subjects: effect of tocotrienol supplementation. Biomed Res Int 2013; 2013:276808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson SL, Qiu J, Rubin BY. Tocotrienols induce IKBKAP expression: a possible therapy for familial dysautonomia. Biochem Biophys Res Commun 2003; 306:303–9 [DOI] [PubMed] [Google Scholar]
- 29.Tappeiner C, Meyenberg A, Goldblum D, Mojon D, Zingg JM, Nesaretnam K, Kilchenmann M, Frueh BE. Antifibrotic effects of tocotrienols on human tenon's fibroblasts. Graefes Arch Clin Exp Ophthalmol 2010; 248:65–71 [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Jiang Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J Nutr Biochem 2019; 64:101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017; 171:273–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotticelli MG, Xia S, Lin D, Lee T, Terrab L, Wipf P, Huryn DM, Wilson RB. Ferroptosis as a novel therapeutic target for Friedreich's ataxia. J Pharmacol Exp Ther 2019; 369:47–54 [DOI] [PubMed] [Google Scholar]
- 33.Zesiewicz T, Salemi JL, Perlman S, Sullivan KL, Shaw JD, Huang Y, Isaacs C, Gooch C, Lynch DR, Klein MB. Double-blind, randomized and controlled trial of EPI-743 in Friedreich's ataxia. Neurodegener Dis Manag 2018; 8:233–42 [DOI] [PubMed] [Google Scholar]
- 34.Fortuna F. Marcatori RM di neurodegenerazione nell’Atassia di Friedreich e loro applicazione in uno studio pilota con tocotrienolo Specialty Thesis in Clinical Pathology, University of Bologna Medical School, tutor prof. R. Lodi, Bologna, Italy, discussed November, 2009
- 35.Harding AE. Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981; 104:589–620 [DOI] [PubMed] [Google Scholar]
- 36.https://en.wikipedia.org/wiki/International_Cooperative_Ataxia_Rating_Scale (accessed 13 May 2019)
- 37.Nakamura H, Furukawa F, Nishikawa A, Miyauchi M, Son HY, Imazawa T, Hirose M. Oral toxicity of a tocotrienol preparation in rats. Food Chem Toxicol 2001; 39:799–805 [DOI] [PubMed] [Google Scholar]
- 38.Tasaki M, Umemura T, Inoue T, Okamura T, Kuroiwa Y, Ishii Y, Maeda M, Hirose M, Nishikawa A. Induction of characteristic hepatocyte proliferative lesion with dietary exposure of Wistar Hannover rats to tocotrienol for 1 year. Toxicology 2008; 250:143–50 [DOI] [PubMed] [Google Scholar]
- 39.Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids 1995; 30:1179–83 [DOI] [PubMed] [Google Scholar]
- 40.Patel V, Rink C, Gordillo GM, Khanna S, Gnyawali U, Roy S, Shneker B, Ganesh K, Phillips G, More JL, Sarkar A, Kirkpatrick R, Elkhammas EA, Klatte E, Miller M, Firstenberg MS, Chiocca EA, Nesaretnam K, Sen CK. Oral tocotrienols are transported to human tissues and delay the progression of the model for end-stage liver disease score in patients. J Nutr 2012; 142:513–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raggi MA, Mandrioli R, Sabbioni C, Mongiello F, Marini M, Fanali S. High-performance capillary electrophoretic determination of glutathione in human lymphocytes. J Micro Sep 1998; 10:503–9 [Google Scholar]
- 42.Kong Y, Zheng N, Zhang Z, Gao R. Optimization stacking by transient pseudo-isotachophoresis for capillary electrophoresis: example analysis of plasma glutathione. J Chromatography B 2003; 795:9–15 [DOI] [PubMed] [Google Scholar]
- 43.Guidi F, Magherini F, Gamberi T, Bini L, Puglia M, Marzocchini R, Ranaldi F, Modesti PA, Gulisano M, Modesti A. Plasma protein carbonylation and physical exercise. Mol Biosyst 2011; 7:640–50 [DOI] [PubMed] [Google Scholar]
- 44.Korolainen MA, Nyman TA, Nyyssönen P, Hartikainen ES, Pirttilä T. Multiplexed proteomic analysis of oxidation and concentrations of cerebrospinal fluid proteins in Alzheimer disease. Clin Chem 2007; 53:657–65 [DOI] [PubMed] [Google Scholar]
- 45.Reinheckel T, Grune T, Davies KJ. The measurement of protein degradation in response to oxidative stress. Methods Mol Biol 2000; 99:49–60 [DOI] [PubMed] [Google Scholar]
- 46.Pessina F, Marazova K, Ninfali P, Avanzi L, Manfredini S, Sgaragli G. In vitro neuroprotection by novel antioxidants in guinea-pig urinary bladder subjected to anoxia-glucopenia/reperfusion damage. Naunyn Schmiedebergs Arch Pharmacol 2004; 370:521–8 [DOI] [PubMed] [Google Scholar]
- 47.Ghezzo A, Visconti P, Abruzzo PM, Bolotta A, Ferreri C, Gobbi G, Malisardi G, Manfredini S, Marini M, Nanetti L, Pipitone E, Raffaelli F, Resca F, Vignini A, Mazzanti L. Oxidative stress and erythrocyte membrane alterations in children with autism: correlation with clinical features. PLoS One 2013; 8:e66418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37:911–7 [DOI] [PubMed] [Google Scholar]
- 49.Ferreri C, Kratzsch S, Brede O, Marciniak B, Chatgilialoglu C. Trans lipid formation induced by thiols in human monocytic leukemia cells. Free Radic Biol Med 2005; 38:1180–7 [DOI] [PubMed] [Google Scholar]
- 50.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 2007; 87:1175–213 [DOI] [PubMed] [Google Scholar]
- 51.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162:156–9 [DOI] [PubMed] [Google Scholar]
- 52.Livak JK, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 53.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones DP. Redefining oxidative stress. Antioxid Redox Sign 2006; 8:1865–79 [DOI] [PubMed] [Google Scholar]
- 55.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci 2000; 899:191–208 [DOI] [PubMed] [Google Scholar]
- 56.Decsi T, Koletzko B. Fatty acid composition of plasma lipid classes in healthy subjects from birth to young adulthood. Eur J Pediatr 1994; 153:520–5 [DOI] [PubMed] [Google Scholar]
- 57.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 1994; 60:189–94 [DOI] [PubMed] [Google Scholar]
- 58.Zambonin L, Ferreri C, Cabrini L, Prata C, Chatgilialoglu C, Landi L. Occurrence of trans fatty acids in rats fed a trans-free diet: a free radical-mediated formation? Free Radic Biol Med 2006; 40:549–56 [DOI] [PubMed] [Google Scholar]
- 59.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010; 268:280–9 [DOI] [PubMed] [Google Scholar]
- 60.Aranca TV, Jones TM, Shaw JD, Staffetti JS, Tetsu A, Kuo S-H, Fogel BL, Wilmot GR, Perlman SL, Onyike CU, Ying SH, Zesiewicz TA. Emerging therapies in Friedreich’s ataxia. Neurodegener Dis Manag 2016; 6:49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cotticelli MG, Crabbe AM, Wilson RB, Shchepinov MS. Insights into the role of oxidative stress in the pathology of Friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox Biol 2013; 1:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cebula M, Schmidt EE, Arnér ES. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal 2015; 23:823–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreri C, Chatgilialoglu C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev Mol Diagn 2012; 12:767–80 [DOI] [PubMed] [Google Scholar]
- 64.de Lima-Salgado TM, Alba-Loureiro TC, do Nascimento CS, Nunes MT, Curi R. Molecular mechanisms by which saturated fatty acids modulate TNF-alpha expression in mouse macrophage lineage. Cell Biochem Biophys 2011; 59:89–97 [DOI] [PubMed] [Google Scholar]
- 65.Lynch DR, Perlman SL, Meier T. A phase 3, double-bind, placebo-controlled trial of idebenone in Friedriech ataxia. Arch Neurol 2010; 67:941–7 [DOI] [PubMed] [Google Scholar]
- 66.Nachun D, Gao F, Isaacs C, Strawser C, Yang Z, Dokuru D, Van Berlo V, Sears R, Farmer J, Perlman S, Lynch DR, Coppola G. Peripheral blood gene expression reveals an inflammatory transcriptomic signature in Friedreich's ataxia patients. Hum Mol Genet 2018; 27:2965–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vela D. Balance of cardiac and systemic hepcidin and its role in heart physiology and pathology. Lab Invest 2018; 98:315–26 [DOI] [PubMed] [Google Scholar]
- 68.Bolotta A, Abruzzo PM, Baldassarro VA, Ghezzo A, Scotlandi K, Marini M, Zucchini C. New insights into the Hepcidin-Ferroportin axis and iron homeostasis in iPSC-derived cardiomyocytes from Friedreich's ataxia patient. Oxid Med Cell Longev 2019; 2019:7623023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, Daynes RA, Kushner JP, Li D, Ward DM, Kaplan J. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest 2010; 120:2395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayele HK, Balesaria S, Srai SK. Phytoestrogens modulate hepcidin expression by Nrf2: implications for dietary control of iron absorption. Free Radic Biol Med 2015; 89:1192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katsarou A, Pantopoulos K. Hepcidin therapeutics. Pharmaceuticals 2018; 11: pii: E127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imam MU, Zhang S, Ma J, Wang H, Wang F. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients 2017; 9:pii: E671 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM890873 Supplemental Material1 for Effects of tocotrienol supplementation in Friedreich’s ataxia: A model of oxidative stress pathology by Alessandra Bolotta, Antonella Pini, Provvidenza M Abruzzo, Alessandro Ghezzo, Alessandra Modesti, Tania Gamberi, Carla Ferreri, Francesca Bugamelli, Filippo Fortuna, Silvia Vertuani, Stefano Manfredini, Cinzia Zucchini and Marina Marini in Experimental Biology and Medicine
Supplemental material, EBM890873 Supplemental Material2 for Effects of tocotrienol supplementation in Friedreich’s ataxia: A model of oxidative stress pathology by Alessandra Bolotta, Antonella Pini, Provvidenza M Abruzzo, Alessandro Ghezzo, Alessandra Modesti, Tania Gamberi, Carla Ferreri, Francesca Bugamelli, Filippo Fortuna, Silvia Vertuani, Stefano Manfredini, Cinzia Zucchini and Marina Marini in Experimental Biology and Medicine