Opinion Statement
Barrett’s esophagus (BE) is a well-established premalignant condition for esophageal adenocarcinoma (EAC); a cancer that is associated with a poor 5-year survival rate. Several strategies have been explored in the context of reducing the burden of EAC. Endoscopic eradication therapy (EET) is considered the standard of care for the management of patients with BE with dysplasia and early neoplasia; a practice that has been endorsed by all gastroenterology societal guidelines. The effectiveness of EET has been demonstrated in multiple studies and contemporary management includes a combination of endoscopic mucosal resection (EMR) of all visible lesions followed by eradication of the remaining BE using ablative techniques of which radiofrequency ablation (RFA) has the best evidence supporting effectiveness and safety. These techniques are being used increasingly at academic tertiary care centers and community practices. In this era of value-based health care, there is increased focus on the establishment, documentation, and reporting of quality indicators; indicators that are important to physicians, patients, and payers. The purpose of this review is to highlight the current status of quality indicators in EET for the management of patients with BE-related neoplasia and discuss the future steps required to ensure that these quality indicators are uniformly incorporated into practice.
Keywords: Quality indicators, Barrett’s esophagus, Endoscopic eradication therapy, Endoscopic ablation, Radiofrequency ablation
Introduction
Barrett’s esophagus (BE) is a condition characterized by the replacement of the normal stratified squamous epithelium of the distal esophagus with metaplastic intestinal-type columnar epithelium [1, 2]. BE is the only identifiable premalignant condition for EAC; a cancer that continues to increase in incidence and one that is associated with a dismal 5-year survival rate (<20%) [3]. Several strategies have been explored in the context of reducing the burden of EAC and include identification of individuals with BE (screening) and enrolling patients in surveillance programs with the aim of detecting EAC at an early and potentially curable stage. The probabilistic progression of BE to invasive EAC is believed to occur through the histologic stages of low-grade dysplasia (LGD), high-grade dysplasia (HGD), and intra-mucosal EAC and thus providing opportunities to prevent progression and decrease the incidence of EAC [4–8]. Endoscopic eradication therapy (EET) in patients with LGD, HGD, and intra-mucosal EAC is an effective and safe strategy in prevention of invasive EAC; a practice that has been endorsed by multiple recent gastroenterology (GI) society guidelines and consensus documents [1–2, 9]. Available data suggest that EET is used increasingly among academic, tertiary care centers, and community practices. With the increasing use of EET in patients with BE-related neoplasia, it is critical to define and implement quality indicators in EET. In this review, we discuss the current status of EET and the importance of establishing and implementing quality indicators in clinical practice especially in the field of EET. In addition, we review data from two recent expert consensus documents that defined quality indicators and set benchmarks for the treatment of BE patients in an effort to standardize healthcare delivery.
Current status of endoscopic eradication therapy
EET is now the standard of care for the management of patients with BE with dysplasia (LGD and HGD) and intra-mucosal EAC [1–2, 9]. The effectiveness and safety of EET in the eradication of BE-related neoplasia and maintaining remission has been demonstrated in randomized controlled trials and large observational studies [10••, 11••, 12•, 13, 14]. Contemporary management of patients with BE-related neoplasia includes endoscopic mucosal resection (EMR) of any visible lesions (no matter how subtle) followed by ablation of the remaining flat BE segment (see Figs. 1, 2, 3, and 4 for example of EMR). Of all the ablative techniques, radiofrequency ablation (RFA–see Figs. 5, 6, and 7 for example) is the most widely studied technique supporting the effectiveness and safety of this ablative modality [15, 16].
Fig. 1.
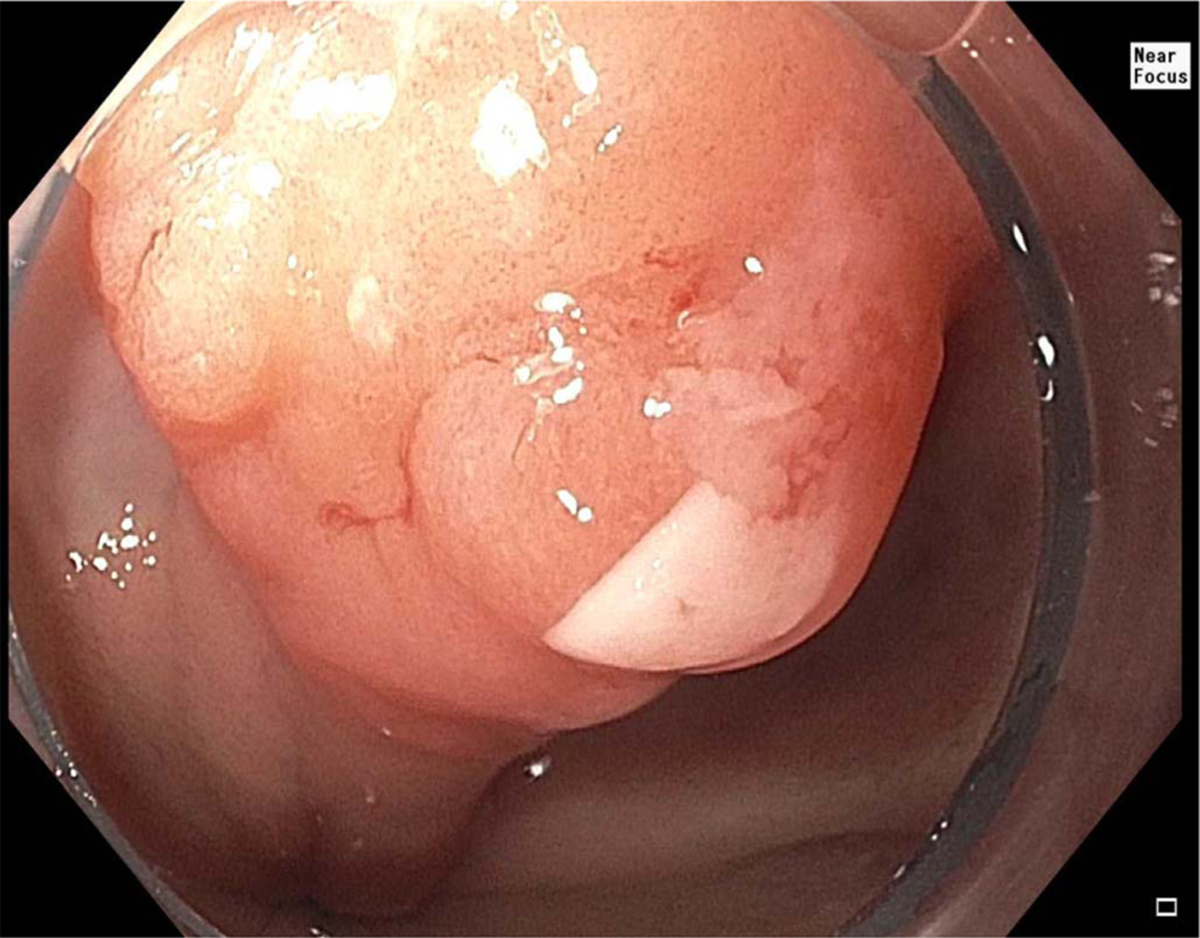
Example of nodular lesion with Barrett’s esophagus.
Fig. 2.
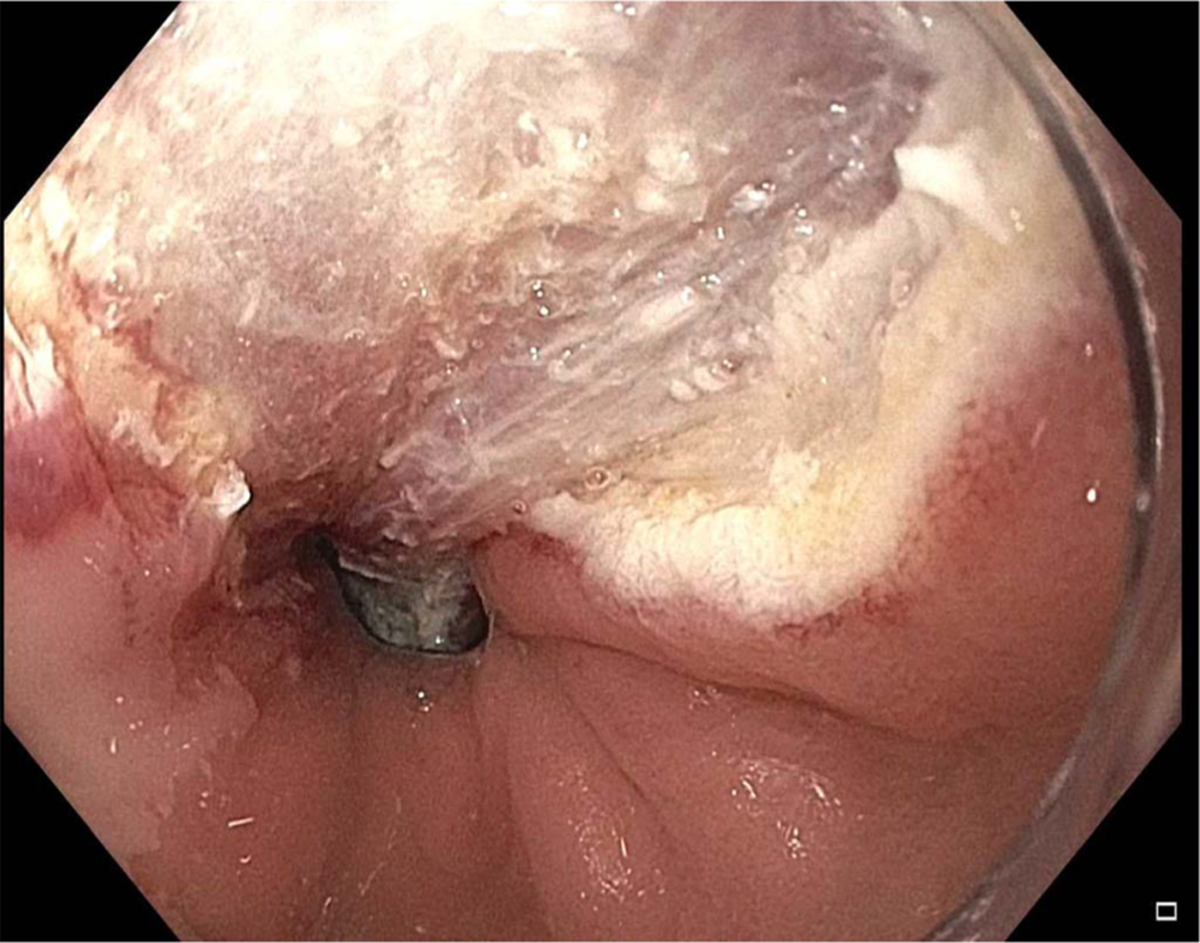
Lesion from Fig. 1 during endoscopic mucosal resection.
Fig. 3.
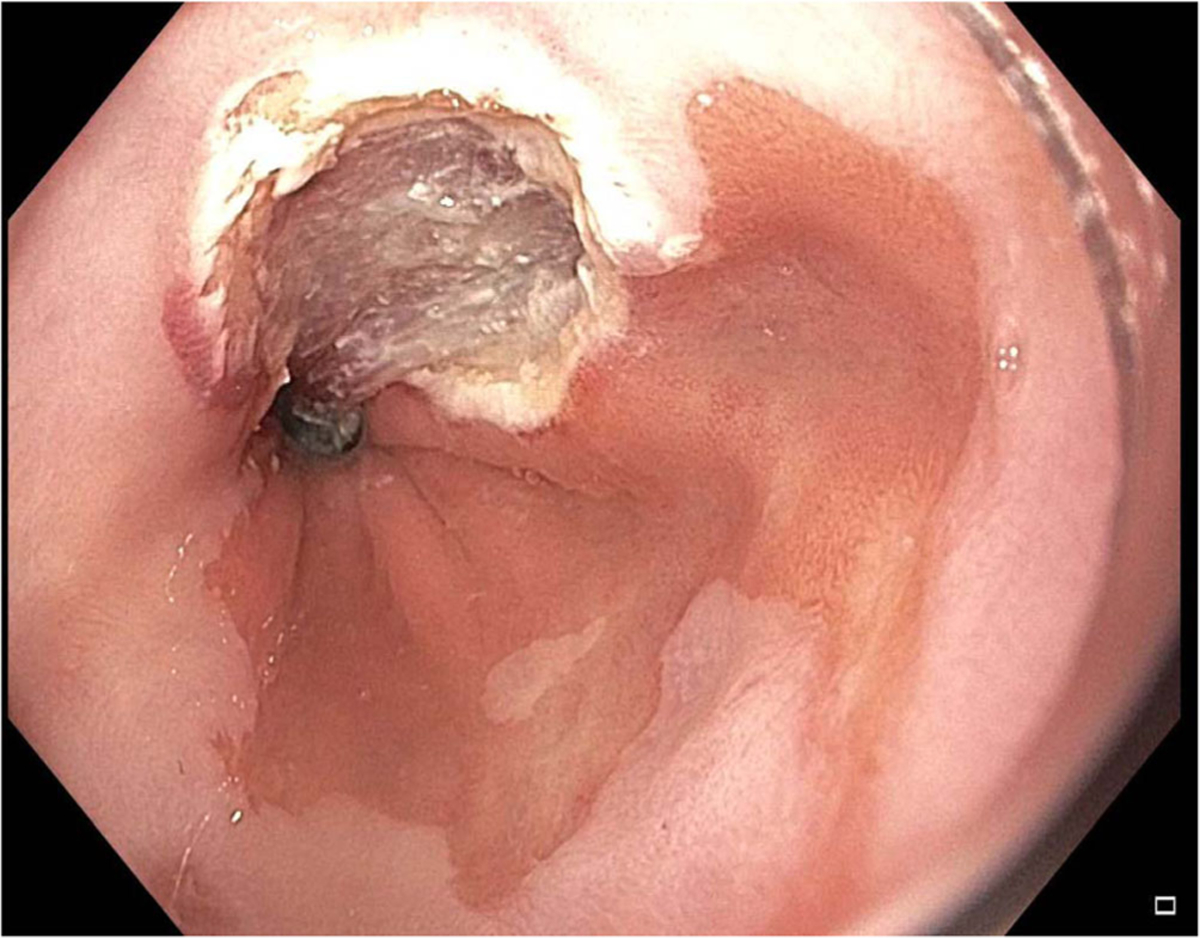
Image post-endoscopic mucosal resection of lesion from Fig. 1.
Fig. 4.
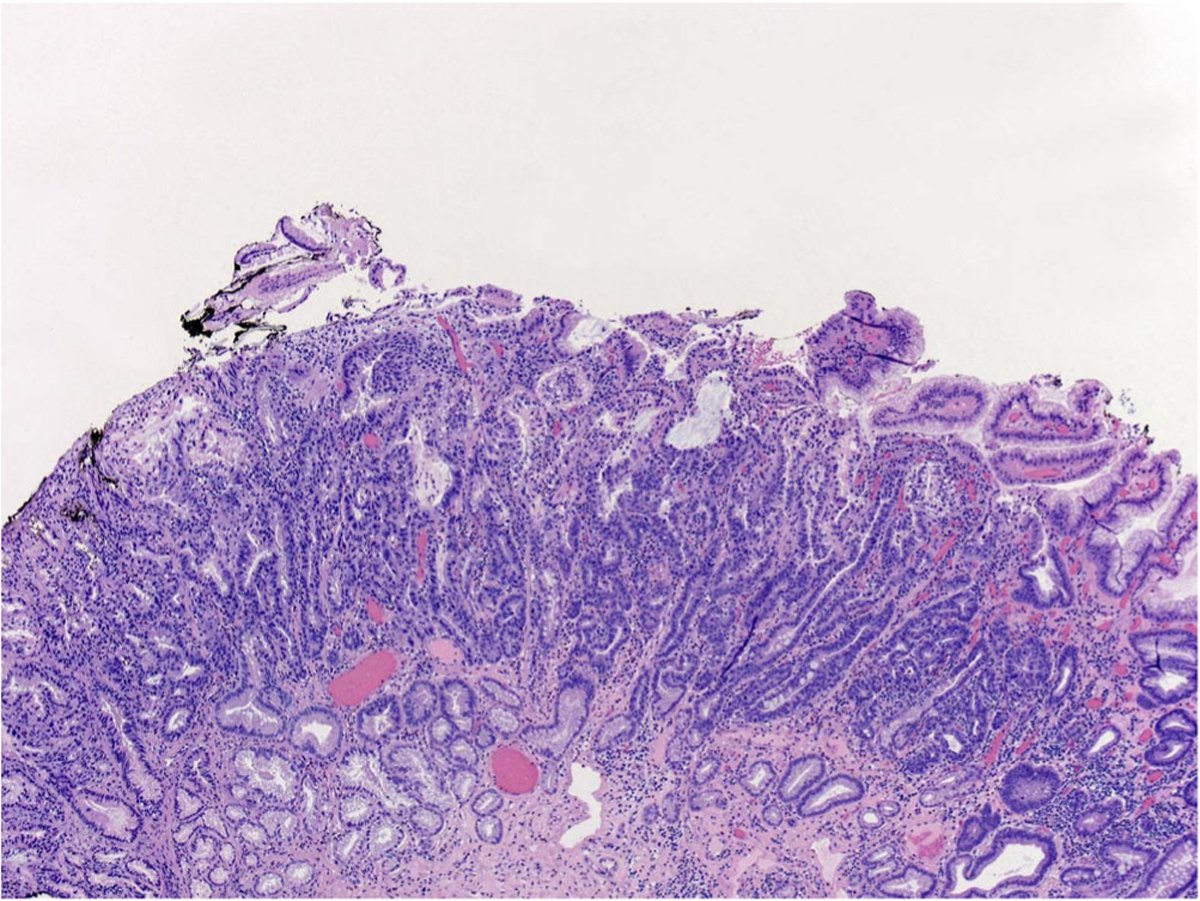
Histology of specimen from endoscopic mucosal resection from lesion in Fig. 1 demonstrating extensive high-grade dysplasia in Barrett’s esophagus.
Fig. 5.
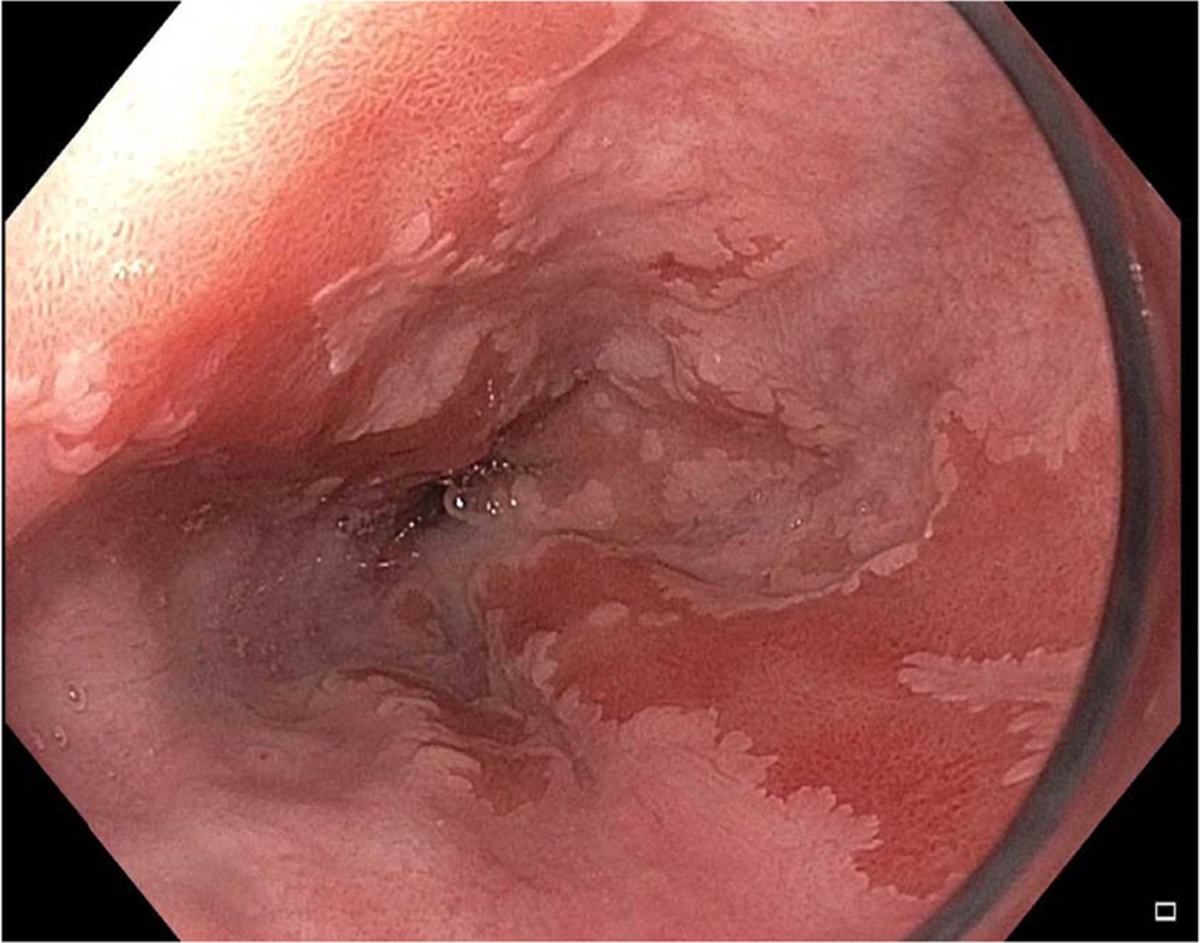
Example of flat Barrett’s esophagus using white light endoscopy.
Fig. 6.
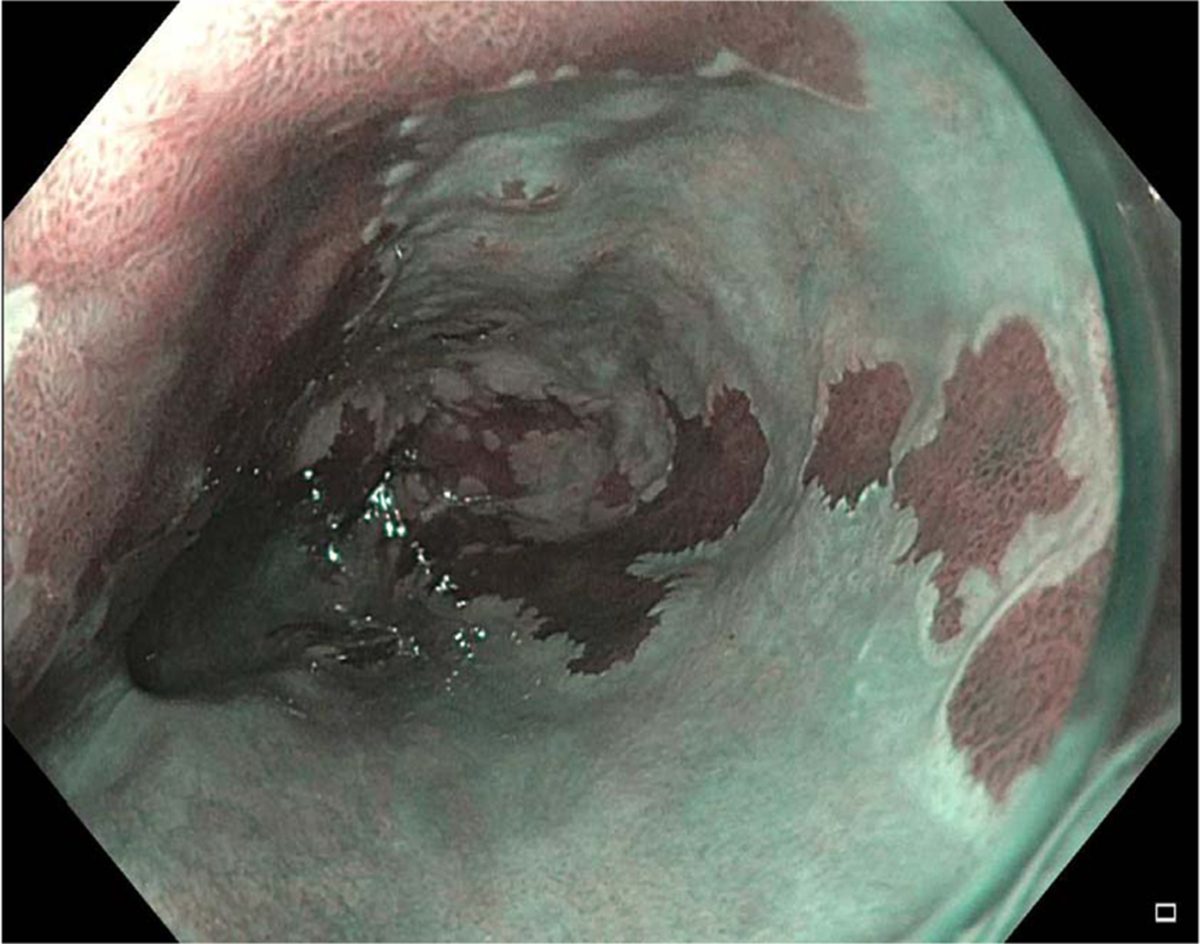
Image of flat Barrett’s esophagus using narrow-band imaging.
Fig. 7.
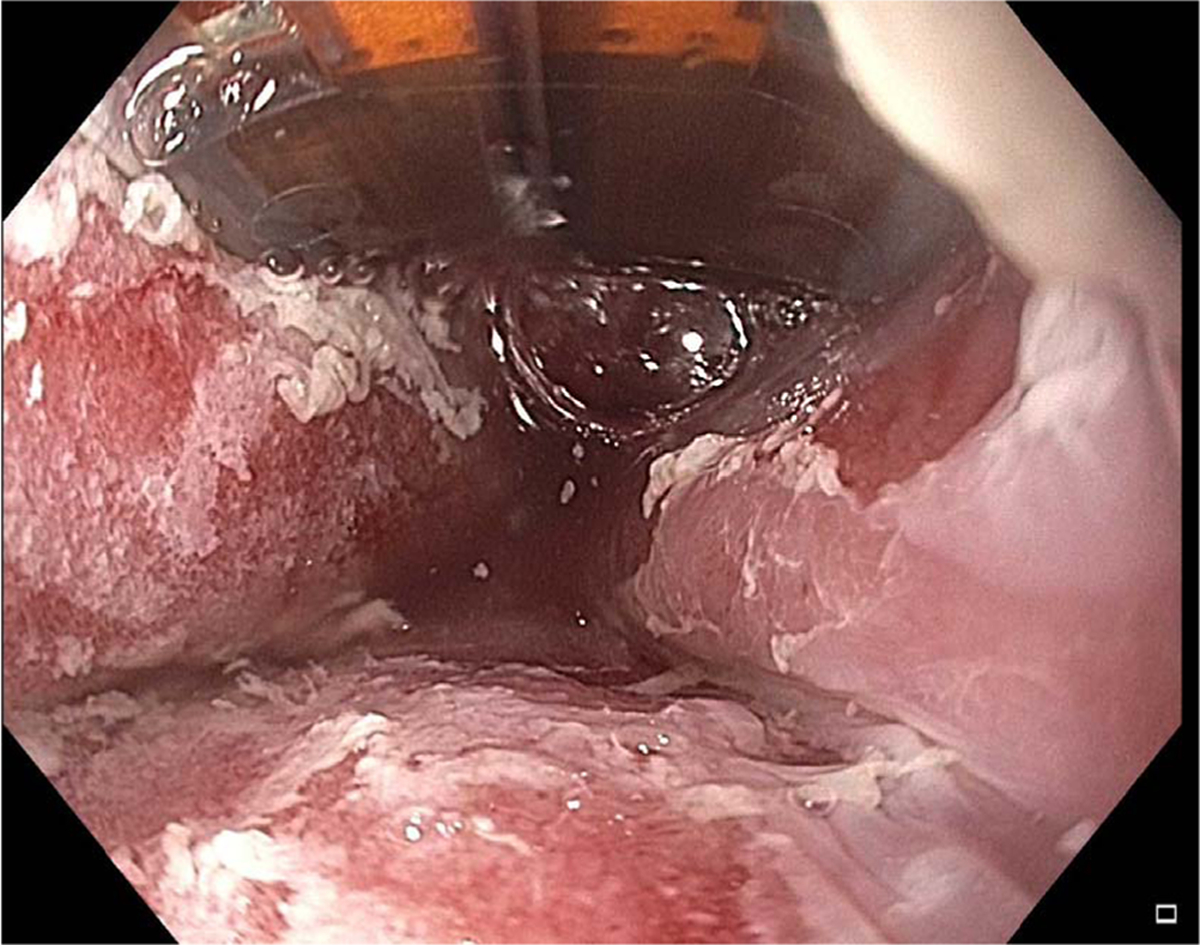
Image of Barrett’s esophagus during radiofrequency ablation.
Quality indicators
Quality of care and quality improvement in medicine and endoscopy in particular, is an evolving field and has garnered a great deal of interest in recent times. Quality measurement and improvement with the help of quality indicators has the potential to ensure the delivery of high-quality care. Defining quality as the “degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge,” a recent position paper recognized the need for objectively and precisely measuring quality through holistic quality indicators, which would be based on evidence and target the care of specific individuals as well as the entire system [17]. While this ideal is desirable for patients and physicians, quality indicators will also play an important role in the current changing health care environment as we transition from a fee-for-service to a pay for quality and value model. The Department of Health and Human Services aims to reform health care delivery through increased use of incentives to foster higher value care with the goal of linking 90% of all Medicare fee-for-service payments to outcomes and 50% to alternate payment models by 2018 [18]. Thus, in this era of value-based and quality-based healthcare, the development of quality indicators that benchmark performance is critical. Quality indicators are often reported as ratios between the incidence of correct performance and the opportunity for correct performance or as the proportion of interventions that achieve a predefined goal [19]. Quality can be measured by comparing the performance of an individual or a group of individuals with an ideal or benchmark [20]. It should be noted that quality indicators are held to a higher standard compared to published guidelines and non-adherence to a quality indicator reflect suboptimal care [21].
Quality indicators in endoscopic eradication therapy
This review will focus primarily on two recent articles that addressed quality indicators in EET for BE-related neoplasia patients [22••, 23••]. A recent consensus conference sponsored by the American Gastroenterological Association (AGA) proposed quality indicators for the management of BE, dysplasia, and EAC using a modified Delphi method [22••]. This all-encompassing document included quality indicators that addressed some indicators related to EET. This process involved inclusion of 25 international experts who first drafted potential quality indicators on different domains in BE, and a final approval of each statement was ultimately achieved through a series of electronic and in-person discussions and at least 80% agreement followed by grading of evidence. Of the eight quality indicators, two statements focused on the treatment and management of BE and early EAC. In addition, this expert group also identified statements that were based on low quality evidence but had ≥80% consensus agreement. Another recent document used a methodologically rigorous process to develop valid quality indicators specifically in EET for the management of BE-related neoplasia [23••]. Quality indicators were developed using the RAND/University of California, Los Angeles Appropriateness Methodology (RAM). RAM is a well-described methodology for the development of quality indicators and addresses the concept of appropriateness; the relative weight of the benefits and harms of an intervention. An appropriate indicator is one in which the expected health benefits exceed the expected negative consequences by a sufficiently wide margin exclusive of costs [24]. A total of 19 international experts participated in Round 1 and Round 2 and identified 14 “appropriate” quality indicators categorized into the pre-procedure, intra-procedure, and post-procedure domains. The strengths of this document include not only the development of appropriate and valid quality indicators in EET using a formal, well-described methodology, but also the development of indicators with well-defined inclusion and exclusion criteria for the numerator and denominator for each indicator, inclusion of outcomes measures tied to key outcomes of interest and defining the threshold benchmarks for each indicator for clinical practice and implementation. This document was recently endorsed by the American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG). Tables 1 and 2 describe the quality indicators selected by both consensus groups that are pertinent to this review. They are expounded upon below and have been divided into three categories: (1) pre-procedural quality indicators, (2) intra-procedural quality indicators, and (3) post-procedural quality indicators.
Table 1.
Quality indicators for endoscopic eradication therapy using the RAND/UCLA appropriateness methodology from Wanietal. [23••]
| Quality indicator | Threshold | Process or outcome measure | |
|---|---|---|---|
| Pre-procedure | For patients in whom a diagnosis of dysplasia has been made, the rate at which the reading is made by a GI pathologist or confirmed by a second pathologist before embarking on EET. | 90% [75, 100] | Process |
| If EET is performed, then centers should have high definition white light endoscopy (HDWLE), mucosal ablation, and EMR techniques available. | N/A | Process | |
| The rate at which documentation of a discussion of the risks, benefits, and alternatives to EET is obtained from the patient prior to embarking on a course of treatment. | 99% [85, 100] | Process | |
| Intra-procedure | The rate at which landmarks and length of BE is documented (e.g., Prague grading system) in patients with Barrett’s esophagus before EET. | 90% [75, 100] | Process |
| The rate at which presence or absence of visible lesions is reported (e.g., Paris classification) in patients with Barrett’s esophagus referred for EET. | 90% [60,100] | Process | |
| The rate at which the Barrett’s esophagus segment is inspected using high definition white light endoscopy (HDWLE). | 95% [0, 100] | Process | |
| Among patients undergoing EET who have not yet achieved CE-IM, the rate at which a defined interval for subsequent EET is documented. | 90% [0, 100] | Process | |
| The rate at which CE-IM is achieved by 18 months in patients with Barrett’s-related dysplasia and intra-mucosal cancer referred for EET. | 70% [50, 80] | Outcome | |
| Post-procedure | Among patients who achieve CE-IM, the rate at which a recommendation for endoscopic surveillance at a defined interval is documented. | 90% [50,100] | Process |
| During endoscopic surveillance after EET, the rate at which biopsies of any visible mucosal abnormalities are performed. | 95% [50, 100] | Process | |
| The rate at which an anti-reflux regimen is recommended after EET. | 90% [50, 100] | Process | |
| The rate at which adverse events are being tracked and xdocumented in individuals post EET | 90% [50, 100] | Process |
EET endoscopic eradication therapy, EMR endoscopic mucosal resection, BE Barrett’s esophagus, CE-IM complete eradication of intestinal metaplasia
Table 2.
Quality indicators for treatment and management of Barrett’s esophagus and early cancer using a modified Delphi method from Sharma etal. [22••]
| Quality indicator | Agreement | Grade of recommendation | |
|---|---|---|---|
| Screening, diagnosis, and staging | For patients in whom BE is being considered, the squamocolumnar junction, the gastroesophageal junction (GEJ), and the location of the diaphragmatic hiatus (if there is a hiatal hernia present) should be recorded on each upper endoscopy | 87% [35% strongly agree, 52% agree] | Weak |
| If BE is suspected on an endoscopy, the endoscopist should document the extent of suspected BE using Prague criteria | 82.6% [43.5% strongly agree, 39.1% agree] | Weak | |
| The normal-appearing and normally located squamocolumnar junction should not be biopsied | 86.3% [68.1% strongly agree, 18.2% agree] | Strong | |
| Surveillance | If systematic surveillance biopsies performed in a patient known to have BE show no evidence of dysplasia, follow-up surveillance endoscopy should be recommended no sooner than 3 to 5 years | 91.3% [17.3% strongly agree, 74% agree] | Weak |
| If a patient with known BE undergoes surveillance endoscopy, systematic biopsies should be taken from every 1 to 2 cm in 4 quadrants throughout the extent of the endoscopically involved segment. | 95.7% [52.2% strongly agree, 43.5% agree] | Strong | |
| If a patient with known BE undergoes surveillance endoscopy, biopsies from any visible raised or depressed lesions should be obtained and processed separately from the systematic biopsies | 95.7% [65.2% strongly agree, 30.5% agree] | Strong | |
| Treatment and management | In patients with dysplastic BE or early EAC, a diagnostic endoscopic resection should be performed on any raised or suspicious areas | 95.6% [65.2% strongly agree, 30.5% agree] | Strong |
| In patients with BE-associated neoplasia, the goal of endoscopic treatment should be complete eradication of the BE segment in addition to any dysplastic lesions | 100% [65.2% strongly agree, 34.8% agree] | Strong |
BE Barrett’s esophagus
Appropriate pre-procedure quality indicators:
-
For patients in whom a diagnosis of dysplasia has been made, the rate at which the reading is made by a GI pathologist or confirmed by a second pathologist before embarking on EET
This statement speaks to the lack of uniformity in the interpretation of grades of dysplasia between pathologists especially regarding the diagnosis of LGD [25–28]. In response to this inter-observer variability, the Vienna classification was developed, which consists of five categories: (1) negative for neoplasia/dysplasia, (2) indefinite for neoplasia/dysplasia, (3) non-invasive low-grade neoplasia (low-grade adenoma/dysplasia), 4) non-invasive high-grade neoplasia (high-grade adenoma/dysplasia, non-invasive carcinoma, and suspicion of invasive carcinoma), and (5) invasive neoplasia (intra-mucosal carcinoma, submucosa carcinoma, or beyond) [29]. Although several advances have been made in the field of biomarkers, the presence and the grade of dysplasia is the best available biomarker to predict progression in patients with BE [30]. Available data suggests that the number of pathologists who agree on the presence of dysplasia correlates with an increased risk of progression to cancer [26–27, 31]. The AGA recently defined an expert GI pathologist as a pathologist with a special interest in BE-related neoplasia who is recognized as an expert in this field by his/her peers [15]. Thus, this statement advocates for the use of a second, preferably GI-specialized pathologist, to confirm diagnoses of dysplasia.
-
Centers where EET is performed should have available high definition white light endoscopy (HDWLE), and expertise in mucosal ablation and endoscopic mucosal resection (EMR) techniques
This statement comments on the importance of having the appropriate resources to perform EET. As patients referred for ablation will frequently have focal lesions and nodular disease, it is important to also have the ability to perform EMR to best provide comprehensive care for each patient [1, 32]. A recent study by Schölvinck et al. demonstrated that community hospitals had a significantly lower detection rate of neoplastic lesions than expert centers, which may support the referral of ablation to expert centers [33]. This study also provided the requirements for an “expert center” regarding management of patients with BE-related neoplasia and include the following requirements: (i) minimum case load of ten new patients per year with dysplasia or EAC in BE to be treated at the expert center, (ii) the specialized care is delivered by one dedicated endoscopist and one or two pathologists, with documented training and expertise, (iii) availability of high resolution endoscopy and equipment for EMR and ablation for dysplasia or EAC in BE, (iv) multidisciplinary consultation with GIs, surgeons, oncologists, and pathologists regarding all patients with early EAC; and (v) expertise in treating adverse events and access to esophageal surgery.
-
The rate at which documentation of a discussion of the risks, benefits, and alternative to EET is obtained from the patient prior to embarking on a course of treatment
As with all procedures, proper informed consent should entail the description of risk of progression to cancer or dysplasia, the possible surveillance and treatment considerations, and risks and benefits of each option [34]. The informed consent should also include what follow-up would consist of, including its frequency and duration.
Appropriate intra-procedure quality indicators
-
The rate at which landmarks and length of BE is documented, (using Prague criteria) and the rate at which the presence or absence of visible lesions is reported (e.g., Paris classification) in patients with BE before EET
Supported by both consensus agreements [22••, 23••], this statement stresses the importance of systematically documenting the extent of BE as well as visible lesions using a standardized classification system as reporting can have poor inter-observer agreement. Both groups support the use of the Prague criteria, which consists of documenting the circumferential length (C) score, and the maximal length (M) score, which has a high inter-observer agreement between providers [35]. In addition, landmarks such as the diaphragmatic hiatal pinch, the squamocolumnar junction, and the proximal limit of the gastric folds should be documented in an effort to standardize the presence of BE in relation to the gastroesophageal junction, including when a hiatal hernia exists. In terms of visible lesions, Wani et al. support the use of the Paris classification [36], a standardized system for grading visible lesions in BE. Briefly, protruded lesions include: (1) 0–Ip (pedunculated) and (2) 0–Is (sessile), flat lesions include: (1) 0–IIa (superficially elevated), 0–IIb (flat), 0–IIc (superficially depressed), and 0–III (excavated). While the Paris classification does not offer prognostic value, lesions that are 0–Is, 0–IIc, and 0–III are likely to have invasive cancer, while 0–IIa and 0–IIb lesions are unlikely to have invasive cancer.
-
The rate at which the BE segment is inspected using high definition white light endoscopy (HDWLE)
HDWLE, as its name would suggest, offers a greater resolution and aspect ratio than standard definition, which provides greater visualization of the mucosal surface. Despite the lack of head-to-head randomized trials between standard and high definition endoscopy for BE, several studies have suggested that HDWLE is more sensitive in detecting BE-related neoplasia. The use of HDWLE is now considered the standard of care in the evaluation of BE patients referred for surveillance and EET [15, 37–40].
-
The rate at which endoscopic resection (defined as en bloc resection or piecemeal) is performed in patients with visible lesions
Performance of EMR for all visible lesions is supported by both quality indicator documents and published guidelines [1, 2, 9, 22••, 23••]. Several studies have shown that EMR results in a change in the histologic grade of dysplasia (upgrade or downgrade) in BE patients referred for EET [41–45]. In addition, studies have shown that provision of larger specimens obtained by EMR results in an improvement in the inter-observer agreement among pathologists compared to biopsy specimens [46, 47].
-
The rate at which complete eradication of neoplasia (CE-N) and complete eradication of intestinal metaplasia (CE-IM) is achieved by 18 months in patients with Barrett’s-related dysplasia or intra-mucosal cancer referred for EET
There is consensus among both documents that the goal of EET should be CE-IM and CE-N alone as the optimal endpoint for EET given the risk of metachronous neoplasia [22••, 23••]. The efficacy, effectiveness, and safety of EET has been demonstrated in several large randomized controlled trials, prospective, and retrospective studies [10••, 11••, 12•, 13, 14]. The proposed threshold for CE-IM by 18 months from initiation of EET for a patient with dysplastic BE was 70% and that for CE-N was 80% [23••]. These indicators were drafted after accounting for patient non-compliance and those referred for surgery. The decision to specify an 18-month time period to achieve CE-IM was primarily made to make these quality indicators more specific and also after accounting for the median number of sessions required to achieve CE-IM [23••].
Appropriate post-procedure quality indicators
-
Among patients who achieve CE-IM, the rate at which a recommendation for endoscopic surveillance at a defined interval is documented
While studies have demonstrated the efficacy of EET, recurrence of intestinal metaplasia and dysplasia represents a true concern with rates ranging from 5 to 39% and 0–15%, respectively. While no evidence suggests a specific timeline for surveillance after CE-IM, surveillance after CE-IM is essential [13, 48–58].
-
During endoscopic surveillance after EET, the rate at which biopsies of any visible mucosal abnormalities are performed
This statement speaks to the importance of obtaining tissue for histology to confirm persistent or recurrent metaplasia. There is currently no standardized technique for surveillance biopsies after EET. Obtaining biopsies from the gastric cardia and the neo-squamocolumnar junction along with Seattle protocol for sampling every 1–2 cm in a 4-quadrant fashion for the entire length of the pretreatment BE segment is based on expert opinion and not uniformly practiced. At the very least, surveillance biopsies should target any visible mucosal abnormalities during surveillance endoscopy post successful EET and CE-IM.
-
The rate at which an anti-reflux regimen is recommended after EET
This statement reflects several recent studies demonstrating that uncontrolled reflux was associated with persistence of intestinal metaplasia after RFA [59–61]. Krishnan et al. [59] found that patients with an incomplete response to RFA had more acidic and reflux events than those who had a complete response to RFA while Akiyama et al. [61] noted that effective intra-esophageal pH control in patients with BE was associated with improved outcomes from RFA. The data thus supports the necessity of maintaining adequate control of reflux, which is most easily accomplished with anti-reflux therapy.
-
The rate at which adverse events are being tracked and documented in individuals post EET
As with all procedures, this outcome measure is crucial in creating safety benchmarks for EET. A recent systematic review and meta-analysis found an adverse event rate of 8.8%, with the most common adverse event being stricture (6%), while perforation accounted for 0.6% of adverse events [16]. Given that endoscopic ablation is not a risk-free procedure, documentation of adverse events is critical in establishing quality indicators and allowing endoscopists to accurately relay these risks to their patients.
Conclusion
Quality indicators will continue to play an important role in the current healthcare system. This review highlights the quality indicators for EET driven by the need to promote best practice among individual practitioners and institutional EET programs and foster evidence-based care. Establishing and implementing quality indicators is in line with the current health care landscape as it transitions from a fee-for-service model to a pay for quality and value model. The benchmarks established will also be useful for training in EET and for understanding competency of individuals and EET programs. The future of quality indicators in endoscopy in general will focus on research specifically relating to the implementation of quality indicators. Studies will have to evaluate whether implementation of these quality indicators impacts patient outcomes. The feasibility of implementing quality indicators (process of measurement and evaluation) in routine clinical practice using data repositories such as the GI Quality Improvement Consortium, Ltd. (GIQuIC) will need to be addressed [62]. Furthermore, this will likely require changes in our electronic medical record systems which could potentially allow for effective measurement of adherence to quality indicators [63]. Adherence to these quality indicators has the potential to improve quality of care, reduce variability in healthcare, and ultimately improve patient outcomes.
Acknowledgements
This work was supported by the University of Colorado, Department of Medicine Outstanding Early Scholars Program (SW).
Abbreviations:
- BE
Barrett’s esophagus
- EAC
Esophageal adenocarcinoma
- EET
Endoscopic eradication therapy
- GI
Gastroenterology
- AGA
American Gastroenterological Association
- ASGE
American Society for Gastrointestinal Endoscopy
- ACG
American College of Gastroenterology
- RAM
RAND/University of California, Los Angeles Appropriateness Methodology
- HDWLE
High definition white light endoscopy
- CE-IM
Complete eradication of intestinal metaplasia
- CE-N
Complete eradication of neoplasia
Footnotes
Conflict of Interest
Samuel Han declares no conflict of interest.
Sachin Wani reports grants from University of Colorado, Department of Medicine Outstanding Early Scholars Program and consultancy fees from Boston Scientific and Medtronic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommented Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Gastroenterological Association, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett–s esophagus. Gastroenterology. 2011;140(3):1084–91. [DOI] [PubMed] [Google Scholar]
- 3.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179–86, 1186 e1. [DOI] [PubMed] [Google Scholar]
- 5.Wani S Population-based estimates of cancer and mortality in Barrett’s esophagus: implications for the future. Clin Gastroenterol Hepatol. 2011;9:723–4. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 7.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–83. [DOI] [PubMed] [Google Scholar]
- 8.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ASGE Standards of Practice Committee., Evans JA, Early JA, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012; 76(6): 108794. [DOI] [PubMed] [Google Scholar]
- 10.••.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311(12):1209–17. [DOI] [PubMed] [Google Scholar]; This randomized multicenter trial compares radiofrequency ablation to endoscopic surveillance alone in low grade dysplasia in Barrett’s demonstrating the superiority of the former in eradicating Barrett’s
- 11.••.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360(22):2277. [DOI] [PubMed] [Google Scholar]; This landmark trial demonstrated the efficacy of radiofrequency ablation in treating and eradicating dysplastic Barrett’s Esophagus.
- 12.•.Overholt BF, Lightdale CJ, Wang KA, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–98. [DOI] [PubMed] [Google Scholar]; This multicentered randomized trial compares photodynamic therapy to omeprazole alone, demonstrating the effectiveness of photodynamic therapy in treating high-grade dysplasia.
- 13.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. [DOI] [PubMed] [Google Scholar]
- 14.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630–5. [DOI] [PubMed] [Google Scholar]
- 15.Wani S, Rubenstein JH, Vieth M, et al. Diagnosis and management of low-grade dysplasia in Barrett’s esophagus: expert review from the clinical practice updates Committee of the American Gastroenterological Association. Gastroenterology. 2016;151(5):822–35. [DOI] [PubMed] [Google Scholar]
- 16.Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1086–95. [DOI] [PubMed] [Google Scholar]
- 17.Council of the Institute of Medicine America’s health in transition: protecting and improving quality. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services. Better, smarter, healthier: in historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. January 26, 2015. Available from: www.hhs.gov/about/news/2015/01/26/better-smarter-healthier-in-historic-announcement-hhs-set-clear-goals-and-timeline-for-shifting-medicare-reimbursements-from-volume-to-value.html. [Google Scholar]
- 19.Petersen BT. Quality assurance for endoscopists. Best Pract Res Clin Gastroenterol. 2011;25:349–60. [DOI] [PubMed] [Google Scholar]
- 20.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on health care quality. JAMA. 1998;280:1000–5. [DOI] [PubMed] [Google Scholar]
- 21.Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI endoscopic procedures. Gastrointest Endosc. 2015;81(1):3–16. [DOI] [PubMed] [Google Scholar]
- 22.••.Sharma P, Katzka DA, Gupta N. Quality indicators for the management of Barrett’s esophagus, dysplasia, and esophageal adenocarcinoma: international consensus recommendations from the American Gastroenterological Association symposium. Gastroenterology. 2015;149:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]; This expert panel position paper represents the consensus statement produced by the American Gastroenterological Association in regards to quality indicators for the management of Barrett’s Esophagus and Esophageal Adenocarcinoma.
- 23.••.Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: the TREAT-BE (treatment with resection and endoscopic ablation techniques for Barrett’s esophagus) consortium Gastrointest Endosc. 2017; In press. [DOI] [PubMed] [Google Scholar]; This study presents the findings by the TREAT-BE consortium relating to quality indicators in endoscopic techniques for the management of Barrett’s Esophagus.
- 24.Fitch K, Aguilar MD, Burnand B, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica: RAND; 2001. [Google Scholar]
- 25.McKenna BJ, Appelman HD. Dysplasia of the gut: the diagnosis is harder than it seems. J Clin Gastroenterol. 2002;34:111–6. [DOI] [PubMed] [Google Scholar]
- 26.Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–30. [DOI] [PubMed] [Google Scholar]
- 27.Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64:700–6. [DOI] [PubMed] [Google Scholar]
- 28.Sangle NA, Taylor SL, Emond MJ, et al. Overdiagnosis of high-grade dysplasia in Barrett’s esophagus: a multicenter, international study. Mod Pathol. 2015;28:758–65. [DOI] [PubMed] [Google Scholar]
- 29.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett’s esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379–88. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–780. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. [DOI] [PubMed] [Google Scholar]
- 33.Schölvinck DW, van der Meulen K, Bergman JJ, Weusten BL. Detection of lesions in dysplastic Barrett’s esophagus by community and expert endoscopists. Endoscopy. 2016. [DOI] [PubMed] [Google Scholar]
- 34.Wani S, Sharma P. Challenges with endoscopic therapy for Barrett’s esophagus. Gastroenterol Clin N Am. 2015;44:355–72. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–9. [DOI] [PubMed] [Google Scholar]
- 36.Anonymous. Paris workshop on columnar metaplasia in the esophagus and the Esophagogastric junction, Paris, France, December 11–12 2004. Endoscopy. 2005;37:879–920. [DOI] [PubMed] [Google Scholar]
- 37.Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology. 2008;135:24–31. [DOI] [PubMed] [Google Scholar]
- 38.Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy. 2005;37:929–36. [DOI] [PubMed] [Google Scholar]
- 39.Curvers W, Baak L, Kiesslich R, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology. 2008;134:670–9. [DOI] [PubMed] [Google Scholar]
- 40.Sami SS, Subramanian V, Butt WM, et al. High definition versus standard definition white light endoscopy for detecting dysplasia in patients with Barrett’s esophagus. Dis Esophagus. 2015;28:742–9. [DOI] [PubMed] [Google Scholar]
- 41.Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16–23. [DOI] [PubMed] [Google Scholar]
- 42.Peters FP, Brakenhoff KP, Curvers WL, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc. 2008;67:604–9. [DOI] [PubMed] [Google Scholar]
- 43.Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276–83. [DOI] [PubMed] [Google Scholar]
- 44.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684–92. [DOI] [PubMed] [Google Scholar]
- 45.Wani S, Abrams J, Edmundowicz SA, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett’s esophagus patients with visible and flat neoplasia: a multicenter cohort study. Digestive Diseases & Sciences. 2013;58:1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wani S, Mathur SC, Curvers WL, et al. Greater inter-observer agreement by endoscopic mucosal resection than biopsy samples in Barrett’s dysplasia. Clinical Gastroenterology & Hepatology. 2010;8:783–8. [DOI] [PubMed] [Google Scholar]
- 47.Mino-Kenudson M, Hull MJ, Brown I, et al. EMR for Barrett’s esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660–6. quiz 767, 769 [DOI] [PubMed] [Google Scholar]
- 48.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–73. [DOI] [PubMed] [Google Scholar]
- 50.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US multicenter consortium. Gastroenterology. 2013;145:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett’s neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370–9. [DOI] [PubMed] [Google Scholar]
- 52.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for Barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–9. [DOI] [PubMed] [Google Scholar]
- 53.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781–9. [DOI] [PubMed] [Google Scholar]
- 54.Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett’s esophagus with radiofrequency ablation. Dig Dis Sci. 2011;56:1996–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haidry RJ, Banks M, Gupta A, et al. Recurrence after successful radiofrequency ablation for Barrett’s related neoplasia is more likely in males: data from the United Kingdom patient registry. Gut. 2014;63:A113–4. [Google Scholar]
- 56.Cotton CC, Wolf WA, Pasricha S, et al. Recurrent intestinal metaplasia after radiofrequency ablation for Barrett’s esophagus: endoscopic findings and anatomic location. Gastrointest Endosc. 2015;81:1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orman ES, Kim HP, Bulsiewicz WJ, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett’s esophagus with radiofrequency ablation. Am J Gastroenterol. 2013;108:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad GA, Dunagan KT, Tian J, et al. Recurrence of intestinal metaplasia following radiofrequency ablation: rates and predictors. Gastrointest Endosc. 2011;73:AB145–6. [Google Scholar]
- 59.Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology. 2012;143:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuda K, Choi SE, Nishioka NS, et al. Incidence and predictors of adenocarcinoma following endoscopic ablation of Barrett’s esophagus. Digestive Diseases & Sciences. 2014;59:1560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci. 2012;57:2625–32. [DOI] [PubMed] [Google Scholar]
- 62.Leiman DA, Metz DV, Ginsberg GG, et al. A novel electronic medical record-based workflow to measure and report colonoscopy quality measures. Clin Gastroenterol Hepatol. 2016;14(3):333–7. [DOI] [PubMed] [Google Scholar]
- 63.Mehta SJ, Ahmad NA. Aligning quality with the academic mission: a quality improvement and delivery science Program in gastroenterology. Gastroenterolo gy. 2016;150(3):543–6. [DOI] [PubMed] [Google Scholar]


