Abstract
Background
Eclampsia, the occurrence of a seizure in association with pre‐eclampsia, remains a rare but serious complication of pregnancy. A number of different anticonvulsants are used to control eclamptic fits and to prevent further fits.
Objectives
The objective of this review was to assess the effects of magnesium sulphate compared with diazepam when used for the care of women with eclampsia. Magnesium sulphate is compared with phenytoin and with lytic cocktail in other Cochrane reviews.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2010) and CENTRAL (2010, Issue 3).
Selection criteria
Randomised trials comparing magnesium sulphate (intravenous or intramuscular administration) with diazepam for women with a clinical diagnosis of eclampsia.
Data collection and analysis
Two authors assessed and extracted data independently.
Main results
We have included seven trials, involving 1396 women. Three trials (1030 women) were good quality. Magnesium sulphate was associated with a reduction in maternal death (seven trials;1396 women; risk ratio (RR) 0.59, 95% confidence interval (CI) 0.38 to 0.92) and recurrence of seizures (seven trials;1390 women; RR 0.43, 95% CI 0.33 to 0.55) compared to diazepam. There were no clear differences in other measures of maternal morbidity.
There was no clear difference in perinatal mortality (four trials; 788 infants; RR 1.04, 95% CI 0.81 to 1.34) or neonatal mortality (four trials; 759 infants; RR 1.18, 95% CI 0.75 to 1.84). In the magnesium sulphate group, fewer liveborn babies had an Apgar score less than seven at one minute (two trials; 597 babies; RR 0.75, 95% CI 0.65 to 0.87) or at five minutes (RR 0.70, 95% CI 0.54 to 0.90), and fewer appeared to need intubation at the place of birth (two trials; 591 infants; RR 0.67, 95% CI 0.45 to 1.00). There was no difference in admission to a special care nursery (four trials; 834 infants; RR 0.91, 95% CI 0.79 to 1.05), but fewer babies in the magnesium sulphate group had a length of stay more than seven days (three trials 631 babies; RR 0.66, 95% CI 0.46 to 0.96).
Authors' conclusions
Magnesium sulphate for women with eclampsia reduces the risk ratio of maternal death and of recurrence of seizures, compared with diazepam.
Plain language summary
Magnesium sulphate versus diazepam for eclampsia
Magnesium sulphate leads to fewer maternal deaths and fewer further seizures than diazepam (Valium) when given for eclamptic seizures (fits).
Between two and eight in every 100 pregnant women develop pre‐eclampsia (toxaemia), which usually means they have high blood pressure and protein in the urine. A small number of women with pre‐eclampsia will also have a seizure (fit); this is called eclampsia. Eclampsia can occur in the second half of pregnancy, during labour, or after the birth. Women with eclampsia are given an anticonvulsant drug to control the eclamptic fit, and to prevent further fits. Eclampsia is an important condition because once women have an eclamptic fit they have a high risk of being seriously ill and dying. Worldwide, an estimated 358,000 women died in 2008 due to complications of pregnancy and childbirth, and 99% of these deaths are women in low‐ and middle‐income countries. Overall, 15% of maternal deaths are associated with eclampsia. Eclampsia is more common in low‐ and middle‐income countries than in high‐income countries.
Our review of seven randomised trials, involving 1396 women, found that intravenous or intramuscular magnesium sulphate was substantially better than intravenous diazepam in reducing the risk of maternal death and of having further seizures. Treatment was for 24 hours unless there was an indication to continue for longer. Diazepam infusion was titrated against the level of sedation, with the aim of keeping the woman drowsy but rousable. Use of magnesium sulphate requires monitoring of respiration rate, tendon reflexes and urine output to avoid adverse effects.
Fewer babies had low Apgar scores at birth with magnesium sulphate than with diazepam and, although admissions to a special care nursery were similar, fewer babies in the magnesium sulphate group had a length of stay of more than seven days.
In other Cochrane reviews, magnesium sulphate was also substantially better than other drugs (phenytoin and lytic cocktail).
Background
Description of the condition
Pre‐eclampsia ('toxaemia') is defined as raised blood pressure (hypertension) accompanied by proteinuria (protein in the urine) (NHBPEP 2000). Eclampsia is the occurrence of a seizure (fit) in association with pre‐eclampsia. When severe, pre‐eclampsia and eclampsia can involve the woman's liver, kidneys, clotting system, or brain. Rare but serious complications include stroke, HELLP syndrome (haemolysis, elevated liver enzymes and low platelets) and disseminated intravascular coagulation. These complications are associated with an increased risk of maternal death. As pre‐eclampsia and eclampsia can affect the placenta, risks for the baby are also increased. The most common problems are those related to poor intrauterine growth and premature birth, leading to an increase in perinatal mortality (Ananth 1995; Roberts 2005). Perinatal mortality is particularly high following eclampsia (Collab Trial 1995; Douglas 1994).
An estimated 358,000 maternal deaths occurred in 2008; 99% of these deaths occur in low‐ and middle‐income countries (WHO 2010). Maternal mortality in parts of Africa and Asia is 100 to 200 times greater than it is in Europe and North America. In Western countries, the average lifetime risk of dying from pregnancy‐related causes is between one in 4000 to one in 1000, whereas women in low‐income countries have a risk between one in 15 and one in 20. There is no other public health statistic for which the disparity between high‐ and low‐income countries is so wide. Eclampsia remains a major cause of maternal mortality and morbidity; 30% of maternal deaths in Africa are associated with pre‐eclampsia or eclampsia (Duley 1992; Khan 2006), as are 15% of direct obstetric deaths in the UK (Lewis 2007) and USA (MMWR 2003). In low‐ and middle‐income countries most maternal deaths due to hypertensive disorders of pregnancy are associated with eclampsia (Duley 1992), whilst in high‐income countries one‐third to one‐half are associated with eclampsia, the remainder being associated with pre‐eclampsia (Lewis 2007; Schutte 2009).
Pre‐eclampsia and eclampsia are part of a spectrum of conditions known as the hypertensive disorders of pregnancy: this includes women with pre‐eclampsia, eclampsia, pre‐existing hypertension, and pre‐existing hypertension with superimposed pre‐eclampsia or eclampsia. The definitions and classification of the hypertensive disorders of pregnancy are discussed more fully in the generic protocol, 'Interventions for treating pre‐eclampsia and its consequences' (Duley 2009).
The terminology of pre‐eclampsia and eclampsia is misleading, as it implies a progression from mild disease to more severe, that pre‐eclampsia precedes eclampsia, and that eclampsia is the most severe end of the spectrum. This is not the case, as some women have normal blood pressure at the time of their first fit, and some women become very sick and may even die without developing eclampsia. About one‐quarter of cases of eclampsia occur without signs or symptoms suggestive of imminent eclampsia, such as headache and proteinuria (Andersgaard 2006; Douglas 1994; Igberase 2006). Nevertheless, women with severe pre‐eclampsia are at particularly high risk of developing eclampsia.
Incidence of pre‐eclampsia and eclampsia
Pre‐eclampsia usually occurs during the second half of pregnancy and complicates 2% to 8% of all pregnancies (WHO 1988). Eclampsia is rare in Europe, with two to three cases reported per 10,000 births (Knight 2007; Kullberg 2002). In low‐ and middle‐income countries, eclampsia is more common, with the incidence estimated as 16 to 69 cases per 10,000 births (Frias 2003). An estimated 1.5 million to 8 million women develop pre‐eclampsia worldwide per year, of whom 150,000 may develop eclampsia (Villar 2003).
Eclampsia can occur in pregnancy, during labor, or after the birth. Where the incidence is high, a greater proportion of women with eclampsia have the onset before the birth. In high‐income countries, where incidence of eclampsia is lower, a greater proportion of women have postpartum onset (Andersgaard 2006; Douglas 1994; Igberase 2006; Onuh 2004). Gestation is also a factor, as women with eclampsia preterm are at least three times more likely to have their first seizure in the antepartum period than women who have eclampsia at term (Andersgaard 2006; Douglas 1994). Postpartum eclampsia is usually close to the time of birth, but may be days or even several weeks later (Collab Trial 1995; Sibai 2005).
Aetiology and pathophysiology
Despite a growing understanding of the pathophysiology of pre‐eclampsia, the underlying cause remains unclear. Factors that appear to have a role include maternal age, parity, obesity, maternal immune response, genetic predisposition, and maternal vascular disease (such as diabetes, chronic hypertension and autoimmune disease) (Duckitt 2005). Diet and nutrition may also have a role. Whether an individual woman will develop pre‐eclampsia probably depends on which of these factors she has, and how they interact.
Pre‐eclampsia is thought to occur as a result of inadequate blood supply to the placenta, related either to abnormal implantation, or to increased demand from the placenta (for example, in a multiple pregnancy). So, although pre‐eclampsia is usually diagnosed in the second half of pregnancy, the antecedents are present much earlier. Current thinking is that inadequate blood supply to the placenta leads to the release of unknown factors or materials into the maternal circulation which activate or injure the endothelial cells, resulting in endothelial dysfunction (abnormal functioning of cells lining blood vessels) (Roberts 2002a). Endothelial dysfunction results in widespread vasoconstriction and activation of platelets and the coagulation system. Injured endothelial cells allow leakage of fluid out of the blood vessels and into surrounding tissues, causing oedema and a reduction in the circulating blood volume. There is then inadequate blood flow to many of the woman's organs, especially the kidneys, liver, and brain. It is the vasoconstriction, micro clots, and reduced circulating blood volume that result in the clinical manifestations of pre‐eclampsia. For a more detailed review of the aetiology and pathophysiology of pre‐eclampsia, seeMeher 2005.
The aetiology and pathophysiology of eclampsia are also incompletely understood. Although there are similarities between eclampsia and hypertensive crisis, the two conditions are not identical (Redman 1984). Some women (around 6%) develop eclampsia with no apparent disturbance of blood pressure (Douglas 1994). Hence, although control of blood pressure is important it will not necessarily prevent or treat eclampsia. Eclampsia is associated with cerebral oedema and cerebral vasospasm; and women with eclampsia may have cerebral oedema or cerebral ischaemia (Belfort 1992; Katz 2000; Sibai 2005).
Risk factors for eclampsia include a family history, little or no antenatal care, being less than 20 years old, having had four or more previous pregnancies, and two or more signs and symptoms of imminent eclampsia (such as headache, epigastric pain, hyperreflexia, visual disturbances and severe hypertension). In low‐and middle‐income countries, the majority (around 90%) of women with eclampsia have had limited access to care (Igberase 2006; Onuh 2004).
Prevention of eclampsia
Primary prevention of eclampsia is preventing women from developing pre‐eclampsia. Once the woman has pre‐eclampsia, prevention is preventing progression to eclampsia. Screening for pre‐eclampsia is an important part of antenatal care, and is based on the clinical history and examination (Milne 2005; NICE 2008). Various diagnostic tests have been advocated to identify women at particularly high risk of developing pre‐eclampsia. So far none have proved to be of good predictive value, and so they are not recommended for clinical practice (Meads 2008).
Current strategies for prevention of pre‐eclampsia can be broadly classified as antenatal surveillance, modification of lifestyle, nutritional supplementation, and pharmacological therapy (seeMeher 2005). Cochrane reviews of strategies for preventing pre‐eclampsia include: lifestyle advice, such as altered dietary salt (Duley 2005) and exercise (Meher 2006); the use of nutritional supplementation, such as calcium (Hofmeyr 2007), magnesium (Makrides 2001), zinc (Mahomed 2007), marine oils (Makrides 2006), vitamins C and E (Rumbold 2008), and pharmacologic agents such as antiplatelet agents (Duley 2007) and nitric oxide (Meher 2007).
Once women have pre‐eclampsia, a Cochrane Review now provides robust evidence that magnesium sulphate halves the risk of eclampsia and probably reduces the risk of maternal death (Duley 2003b).
Description of the intervention
The only definitive treatment for pre‐eclampsia or eclampsia is to end the pregnancy. The aim of interventions for women with eclampsia is to improve outcome, for example by preventing further seizures, minimising and treating any complications and, if before delivery, to optimise the timing of birth and outcome for the baby. Other relevant Cochrane Reviews cover drug treatment for very high blood pressure (Duley 2006), plasma volume expansion (Duley 1999), and the timing of delivery for women before 34 weeks' gestation (Churchill 2002).
Currently, standard care for women with eclampsia is to use an anticonvulsant drug to control the immediate fit, and to continue maintenance treatment to prevent further seizures. This review compares a policy of using magnesium sulphate to a policy of using diazepam for the care of women with eclampsia.
Magnesium sulphate
Magnesium sulphate was introduced for care of women with eclampsia in the 1920s following reports of its use for control of convulsions due to tetanus (Duley 1996). Based on publication of several case series, it became standard care in several parts of the world, particularly in North America. Initially, magnesium sulphate was given in very low doses (Duley 1996), although it is now administered in relatively high doses. Common regimens are an initial intravenous loading dose of 4 grams (Dinsdale 1988; Pritchard 1955; Zuspan 1978): followed by maintenance intravenous infusions of 1 gram per hour (Dinsdale 1988; Zuspan 1978); or by 10 grams by intramuscular injection and then 5 grams intramuscularly every 6 hours (Eastman 1945).
Alternative regimens for magnesium sulphate are the topic of a separate Cochrane Review (Duley 2008).
Diazepam
Diazepam, one of the benzodiazepines, was first suggested for women with eclampsia in the 1960s (Lean 1968). It is commonly used for treating a wide range of conditions, including anxiety, insomnia, seizures, and muscle spasms. Diazepam is a core medicine in the World Health Organization's 'Essential Drugs List', which is a list of minimum medical needs for a basic healthcare system, and has been one of the most frequently prescribed medications in the world for the past 40 years. This wide availability probably contributed to its initial acceptance as a treatment for women with eclampsia.
Treatment with diazepam usually includes a loading dose of 40 mg by intravenous injection, followed by an infusion of 20 mg diluted in 500 ml. This infusion is titrated against the level of sedation, with the aim of keeping the woman drowsy but rousable.
How the intervention might work
Magnesium sulphate
The mode of action for magnesium sulphate in control of eclamptic seizures and prevention of recurrent convulsions is still not clearly understood. Magnesium sulphate is not a traditional anticonvulsant, but nevertheless is better than the traditional anticonvulsant drugs at control of eclamptic seizures. This anticonvulsant activity may be mediated by magnesium's role as an N‐methyl‐D‐aspartate (NMDA) antagonist (Euser 2009). Stimulation of NMDA receptors by neurotransmitters such as glutamate may lead to seizures when neuronal networks are over‐activated. Magnesium may prevent and control eclamptic seizures by inhibiting NMDA receptors. Other possible mechanisms are that magnesium sulphate may lead to cerebral vasodilatation with subsequent reduction of cerebral ischaemia (Belfort 1992), and it may block some of the neuronal damage associated with ischaemia (Goldman 1988; Sadeh 1989). The pathway for blocking neuronal damage may also be through NMDA inhibition.
Magnesium is also a calcium antagonist, and a smooth muscle relaxant. It may affect the cerebral endothelium which forms the blood‐brain barrier. Lowering intracellular calcium may limit paracellular transport of vascular contents, such as ions and proteins, effectively decreasing the factors which promote cerebral oedema and seizure activity (Euser 2009).
The calcium antagonist activity of magnesium sulphate has led to the belief that it also lowers systemic blood pressure, but this has not been supported by evidence from randomised trials (MAGPIE 2002). Magnesium sulphate does not appear to be an antihypertensive drug (Abalos 2007).
The adverse effects of magnesium sulphate come largely from its action as a smooth muscle relaxant. The most serious are respiratory depression, and respiratory and cardiac arrest. However, the adverse effects follow a dose response. Deep tendon reflexes are lost at a serum magnesium level of 10 mEq/L, with respiratory depression occurring at 15 mEq/L and cardiac arrest at more than 15 mEq/L. This dose response relationship means that clinical monitoring should ensure that toxicity and adverse effects are avoided. Provided deep tendon reflexes are present, toxicity and adverse effects will be avoided. Monitoring of serum magnesium levels is therefore not necessary, and clinical monitoring of tendon reflexes and respiration rate will ensure safe administration. As magnesium is excreted almost exclusively in the urine, women with impaired renal function will quickly have raised serum magnesium levels and be at risk of significant adverse effects if the dose is not reduced. Measuring hourly urine output should therefore, be included in the clinical monitoring. If toxicity does develop, calcium gluconate is an effective antidote.
Diazepam
Diazepam was introduced for treatment of eclampsia based on the belief that a traditional anticonvulsant would be the effective. Diazepam binds to a specific site of the ɣ‐aminobutyric acidA (GABAA) receptor, the major inhibitory neurotransmitter in the central nervous system. The GABAA receptor is an inhibitory channel which, when activated, decreases neuronal activity. The anticonvulsant properties of diazepam may be in part or entirely due to binding to voltage‐dependent sodium channels. Diazepam has near immediate onset of action, as it rapidly crosses the blood brain barrier, with a short duration of action (20 to 30 minutes) and half‐life time of elimination of 20 to 50 hours. Drug elimination is prolonged in neonates, the elderly and those with liver dysfunction.
Diazepam is primarily eliminated by the liver enzymes, hence its many potential drug interactions. While short‐term use of diazepam is generally well tolerated, common side effects are drowsiness, confusion and amnesia.
Why it is important to do this review
Eclampsia is a rare but serious complication of pregnancy, and a major cause of maternal mortality. Assessing which anticonvulsant drug is best to use for women with eclampsia is important if women are to receive optimal care.
Objectives
The aim was to compare the differential effects of magnesium sulphate, given either by the intramuscular or the intravenous route, rather than diazepam for the care of women with eclampsia. The comparison was in terms of maternal mortality, recurrence of convulsions, other serious maternal morbidity that could lead to death, and use of health service resources. For women who were entered into the trials before the birth, additional outcomes were those related to labour, delivery, and mortality and morbidity of the baby.
Methods
Criteria for considering studies for this review
Types of studies
All adequately randomised trials comparing magnesium sulphate with diazepam for treatment of women with eclampsia. This includes women who are antepartum, intrapartum and postpartum.
Cluster‐randomised study designs are unlikely to be relevant to interventions for treatment of women with eclampsia, and were therefore, unlikely to be identified. If such studies have been conducted, they were not automatically excluded; rather, the authors considered whether or not it was appropriate to include them.
We excluded studies with a quasi‐random design, such as allocation by alternation, day of week, or hospital numbers as they have a greater potential for bias (Higgins 2009). We also excluded studies with a crossover design.
Types of participants
Women with a clinical diagnosis of eclampsia at trial entry, irrespective of whether they were before or after delivery, had a singleton or multiple pregnancy, or whether an anticonvulsant had been given before trial entry. If women with pre‐eclampsia had also been entered into a trial, we included only data for women with eclampsia in this review.
Types of interventions
All randomised comparisons of magnesium sulphate (intravenous or intramuscular administration for the maintenance regimen) with diazepam for women with eclampsia.
Types of outcome measures
The most important outcome is maternal death but, as this is relatively rare even for women with eclampsia, we also included other measures of serious morbidity which could lead to death.
Primary outcomes
For the woman
Death: before discharge from hospital, up to six weeks postpartum, beyond six weeks postpartum.
Recurrence of seizures.
Stroke.
Any serious morbidity: defined as at least one of stroke, renal failure, liver failure, HELLP syndrome (haemolysis, elevated liver enzymes, low platelets) syndrome, disseminated intravascular coagulation, pulmonary oedema (fluid in the lungs), and cardiac arrest; or as reported in the trial.
For the child
Death: stillbirths (death in utero at or after 20 weeks' gestation), perinatal deaths (stillbirths plus deaths in first week of life), death before discharge, neonatal deaths (death in the first 28 days after birth), deaths after the 28 days.
Preterm birth: defined as birth before 37 completed weeks' gestation.
In a special care nursery for more than seven days.
Secondary outcomes
For the woman
Renal failure.
Liver failure.
HELLP syndrome.
Disseminated intravascular coagulation.
Pumonary oedema (fluid in the lungs).
Cardiac arrest.
Death or serious morbidity (any of 1 to 6, above).
Use of antihypertensive drugs.
Abruption of the placenta or antepartum haemorrhage.
Elective delivery: induction of labour or caesarean section.
Labour greater than eight hours.
Caesarean section.
Postpartum haemorrhage, defined as blood loss of 500 ml or more.
Serious adverse effects: such as respiratory depression, need for calcium gluconate.
Stopped treatment due to toxicity or adverse effects.
Side effects: such as flushing, skin reaction at site of injection, reduced respirations, absent tendon reflexes.
Use of hospital resources: intensive care (admission to intensive care unit, length of stay), need for ventilation, need for dialysis, transfer to another hospital for a higher level of care.
Postnatal depression.
Breastfeeding, at discharge and up to one year after delivery.
Women's experiences and views: childbirth experience, physical and psychological trauma, mother‐infant interaction and attachment.
For the child
Severity of preterm birth: very preterm birth (before 32 to 34 completed weeks) and extremely preterm birth (before 26 to 28 completed weeks).
Death before discharge from hospital or in a special care baby unit for more than seven days.
Respiratory distress syndrome.
Infection.
Necrotising enterocolitis.
Retinopathy of prematurity.
Intraventricular haemorrhage.
Apgar score at five minutes: low (less than seven), very low (less than four), or lowest reported.
Side effects associated with the intervention: respiratory depression shortly after birth.
Use of hospital resources: admission to special care nursery, length of stay, endotracheal intubation, use of mechanical ventilation.
Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy.
Economic outcomes
Costs to the health service resources: short term and long term for both mother and baby.
Costs to the woman, her family, and society.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (2010, Issue 3) using the search strategy detailed in Appendix 1.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Three review authors (L Duley (LD), D Henderson‐Smart (DHS), D Chou (DC)) independently assessed for inclusion all potentially eligible studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
For previous versions of this review two review authors (LD, DHS) extracted data from each report. For this update, during which the methods were also updated according to the generic protocol (Duley 2009), two review authors (DC, LD) extracted data from each report. We resolved all discrepancies by discussion. There was no blinding of authorship or results. We entered data into Review Manager software (RevMan 2008) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide clarification or further information.
Assessment of risk of bias in included studies
At least two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreement by discussion, or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear.
Studies with inadequate sequence generation were excluded.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses. We assessed methods as:
adequate;
inadequate;
unclear.
For outcomes up to the time of discharge from hospital, if data for more than 20% of participants were missing, we excluded that outcome or study from the analysis. For longer‐term follow up, attrition is likely to be greater, and we included studies provided there was reasonable reassurance about potential for bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias. For example, if the trial stopped early due to some data‐dependent process, or if there was an extreme baseline imbalance.
We will assess whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as a summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Although cluster‐randomised trials of interventions for treatment of pre‐eclampsia are unlikely, if we identified them and they met all other eligibility criteria, we included them along with individually randomised trials. If included, we adjusted their sample sizes or standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC.
If we identified both cluster‐randomised trials and individually‐randomised trials, we would plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We would also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Crossover trials
We excluded crossover trials, as for pre‐eclampsia they can only assess surrogate outcomes and markers. The important clinical outcomes are at birth, and beyond.
Dealing with missing data
For included studies, we have noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Where important data or information about the study design were missing, whenever possible we contacted trial authors to ask if they could provide it.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. When we identified substantial heterogeneity (above 50%), we explored it by prespecified subgroup analysis.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data could introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We performed statistical analyses using the Review Manager (RevMan 2008) software, with results presented as risk ratios (RR) and risk difference (RD). From 1/RD we calculated the number needed to treat for benefits, and for harmful or adverse effects. For each measure we have given the 95% confidence interval (CI). We used the fixed‐effect model for calculating RR. If there was clear heterogeneity between the studies in any one outcome, we used a random‐effects model. We explored possible factors in the heterogeneity, including quality of the concealment of allocation, clinical factors as determined by the prespecified subgroup analyses, and the play of chance.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses based on:
whether the woman had delivered before trial entry: not delivered at trial entry, postpartum at trial entry, unclear or mixed;
whether the woman received anticonvulsants before trial entry: anticonvulsant before trial entry, no anticonvulsant before trial entry, unclear or mixed;
gestation at trial entry: at least 32 to 34 weeks, less than 32 to 34 weeks, unclear or mixed.
We planned to use the primary outcomes in these subgroup analyses. Data were not presented by gestation at trial entry, and so this subgroup analysis was not possible.
We planned an additional subgroup analysis based on route for the maintenance regimen of magnesium sulphate:
4. allocated maintenance regimen: by intravenous route, intramuscular route, unclear or mixed.
We planned to use outcomes related to adverse effects and toxicity for this subgroup analysis.
For fixed‐effect meta‐analyses we planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. For random‐effects meta‐analyses we assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We carried out sensitivity analysis to explore the effect of trial quality, including only studies which have been assessed as having adequate control of the potential for bias.
Results
Description of studies
This review includes seven trials with data from 1396 women. The largest trial enrolled 905 women (Collab Trial 1995), the smallest enrolled 11 women (Malaysia 1994). One trial (Zimbabwe 1990; 51 women) enrolled only women prior to delivery, the six other studies included women with both antepartum and postpartum eclampsia (1345 women). Overall, about half the women in this review had received an anticonvulsant before trial entry. Two trials (Collab Trial 1995; Zimbabwe 1990) reported outcomes by whether or not women received an anticonvulsant before trial entry.
The treatment regimens all included a loading dose and maintenance therapy. For magnesium sulphate, the maintenance regimen could be either intravenous (Nigeria 2004) or intramuscular (Bangladesh 1998; India 2001; Zimbabwe 1990; Zimbabwe 1998). In the large Collaborative Eclampsia Trial, whether the intravenous or intermuscular maintenance regimen was used was decided by each of the 27 hospitals based on their clinical practice at that time. For one trial (Malaysia 1994) the treatment regimens were not described.
Most of the women who received magnesium sulphate in these trials had a 4 g intravenous loading dose. Those receiving the intramuscular maintenance regimen had this combined with 10 g by intramuscular injection. Maintenance therapy was either the intramuscular regimen of 5 g every four hours, or an infusion of 1 g/hour. For most women duration of treatment was 24 hours. Most studies reported that women were monitored using respiration rate, urine output and tendon reflexes. Serum monitoring was not reported to be used in any of these trials.
Five trials (Collab Trial 1995; Malaysia 1994; Nigeria 2004; Zimbabwe 1990; Zimbabwe 1998) reported perinatal mortality. Two trials which recruited women before delivery (Bangladesh 1998; India 2001) did not report outcome for the baby.
Risk of bias in included studies
Sequence generation was adequately described in four trials (Collab Trial 1995; Nigeria 2004; Zimbabwe 1990; Zimbabwe 1998). Information about sequence generation was not provided for the other three trials.
Three studies had adequate concealment of allocation (Collab Trial 1995; Zimbabwe 1990; Zimbabwe 1998). For three trials (Bangladesh 1998; India 2001; Malaysia 1994) it is unclear whether concealment of allocation was adequate. In one study (Nigeria 2004), allocation concealment was inadequate.
Once women were randomised, the allocated treatments could not be blinded in any of these studies, as this would not have been ethical. It is unlikely that any subsequent bias will have substantially influenced the results, however. The main outcomes assessed were objective, and the strength and consistency of the data indicate they represent true effects. In the large trial (Collab Trial 1995), assessment of outcome was by the attending clinicians. Although this was not discussed in most of the other studies, it is likely the same is true for them all. Follow up was more than 99% for all the trials.
The overall quality of a study was considered "good" if a trial adequately described sequence generation, allocation concealment, any loss of data and the study was assessed to be at low risk of bias. Three trials in this review (Collab Trial 1995; Zimbabwe 1990; Zimbabwe 1998) are of good quality.
One study is only available as an unpublished report (India 2001), but the author has provided clarification of the methods used.
Effects of interventions
This review includes seven trials (1396 women) comparing magnesium sulphate with diazepam for women with eclampsia.
Outcome for the women
Maternal death
1.1. Analysis.
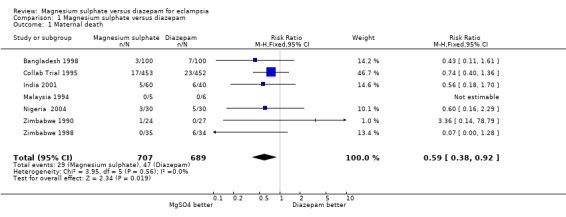
Comparison 1 Magnesium sulphate versus diazepam, Outcome 1 Maternal death.
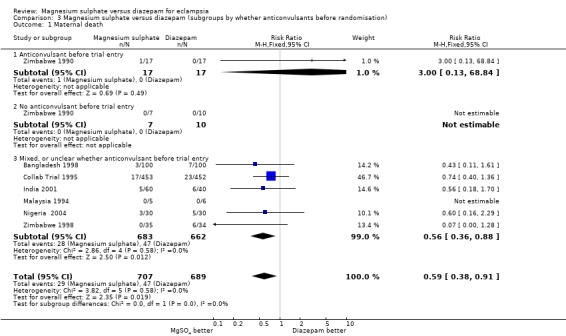
Using magnesium sulphate, rather than diazepam, reduces the RR of maternal death (seven trials;1396 women; RR 0.59, 95% CI 0.38 to 0.92). Most studies did not report maternal death by whether delivered at trial entry, nor by whether or not the woman had an anticonvulsant before trial entry. Where these data are reported, the subgroup results are consistent with the overall effect.
Recurrence of seizures
1.2. Analysis.
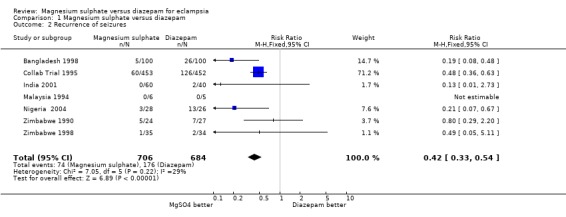
Comparison 1 Magnesium sulphate versus diazepam, Outcome 2 Recurrence of seizures.
Using magnesium sulphate, rather than diazepam, more than halves the RR of recurrence of seizures (seven trials; 1390 women; RR 0.43, 95% CI 0.33 to 0.55). This means that, on average, for every seven women treated with magnesium sulphate rather than diazepam one recurrence of seizures will be prevented (NNT 7; 95% CI 5 to 9 women).
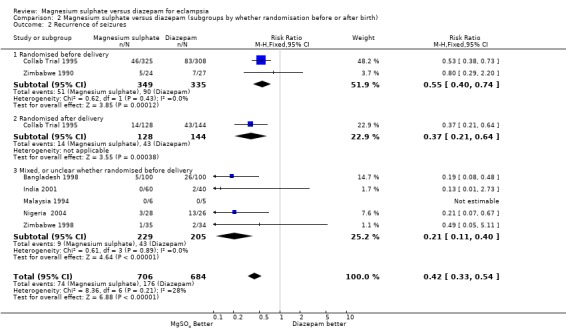
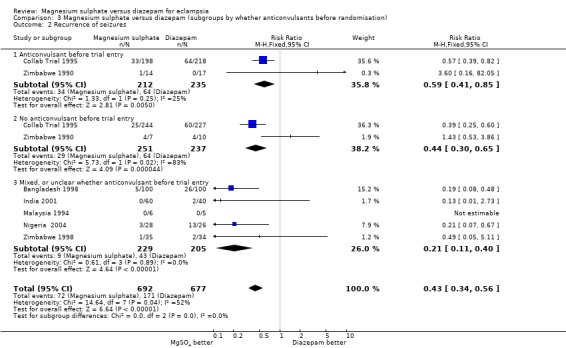
The effect on recurrence of seizures was consistent regardless of whether the woman was randomised before delivery (two trials; 684 women; RR 0.55, 95% CI 0.40 to 0.74) or after delivery (one trial; 272 women; RR 0.37, 95% CI 0.21 to 0.64); and irrespective of whether an anticonvulsant had been give before trial entry (anticonvulsant before entry: two trials; 447 women; RR 0.59, 95% CI 0.41 to 0.85. No anticonvulsant before entry: two trials; 488 women; RR 0.44, 95% CI 0.30 to 0.65).
Stroke
1.3. Analysis.
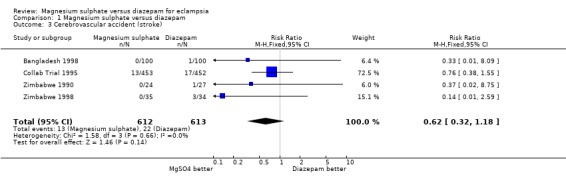
Comparison 1 Magnesium sulphate versus diazepam, Outcome 3 Cerebrovascular accident (stroke).
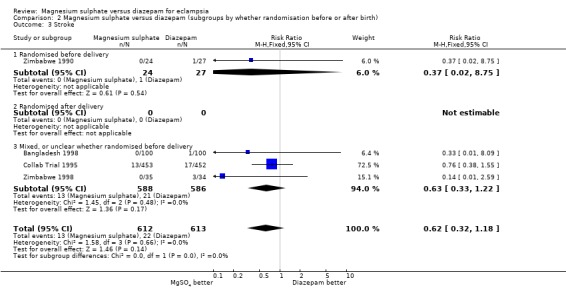
There was no significant difference between the two treatment groups in the risk ratio of stroke (four trials; 1225 women; RR 0.62, 95% CI 0.32 to 1.18).
Composite outcome: any serious maternal morbidity
1.4. Analysis.
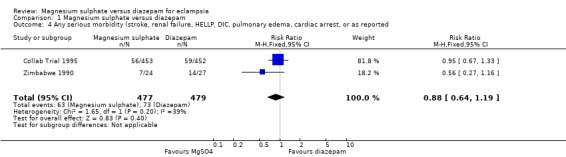
Comparison 1 Magnesium sulphate versus diazepam, Outcome 4 Any serious morbidity (stroke, renal failure, HELLP, DIC, pulmonary edema, cardiac arrest, or as reported.
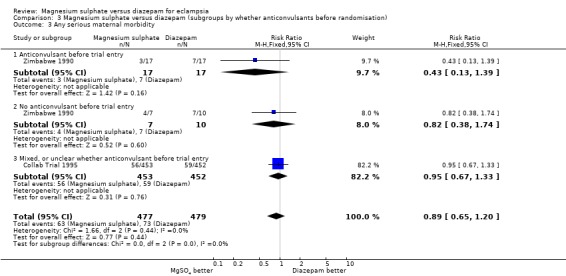
Two trials (956 women) reported a composite outcome of serious maternal morbidity. There was no significant difference between the groups in the RR of serious maternal morbidity (RR 0.88, 95% CI 0.64 to 1.19). This was consistent in the subgroup analysis by whether or not the women received anticonvulsants before trial entry.
Serious maternal morbidity
There were no statistically significant difference between women allocated magnesium sulphate and those allocated diazepam in the risk ratio of any single measure of serious maternal morbidity:
renal failure (1.5): five trials; 1164 women; RR 0.85, 95% CI 0.53 to 1.36;
liver failure: two trials; 974 women; RR 1.00, 95% CI 0.48 to 2.07;
coagulopathy/DIC: four trials; 1036 women; RR 0.89, 95% CI 0.56 to 1.41;
respiratory depression: three trials; 1025 women; RR 0.86, 95% CI 0.57 to 1.30;
pulmonary oedema: three trials; 1013 women; RR 0.86, 95% CI 0.35 to 2.07;
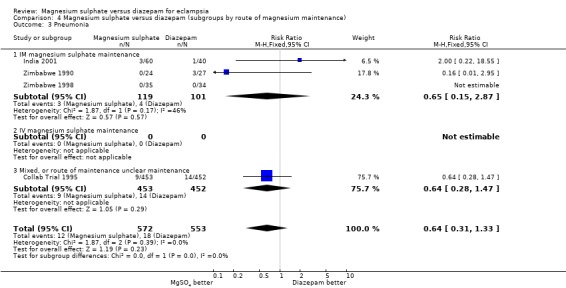
pneumonia: four trials; 1125 women; RR 0.64, 95% CI 0.31 to 1.33;
cardiac arrest: four trials; 1085 women; RR 0.80, 95% CI 0.41 to 1.54.
There was moderate heterogeneity for the three trials reporting respiratory depression. No trials reported HELLP syndrome or use of antihypertensive drugs.
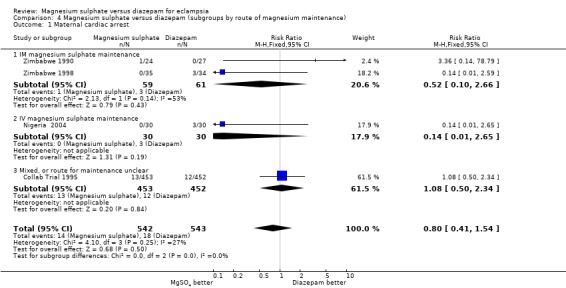
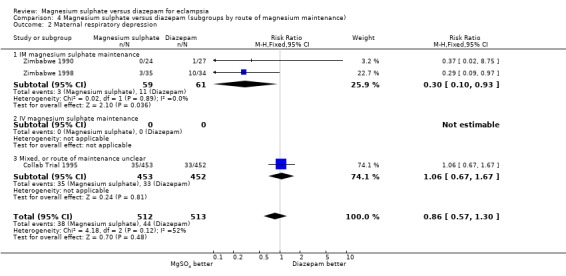
In the subgroup analysis by route of magnesium sulphate maintenance regimen, we included only outcomes potentially related to toxicity with either magnesium sulphate or diazepam. The results were consistent across the subgroups regardless of whether magnesium sulphate maintenance regimen was intramuscular or intravenous for cardiac arrest, pneumonia, and need for ventilation. For respiratory depression, there was modest heterogeneity. In the two trials (120 women) comparing intramuscular magnesium sulphate maintenance with diazepam the reduction in respiratory depression achieved statistical significance (RR 0.30, 95% CI 0.10 to 0.93). This should be interpreted with caution, however, in view of the small numbers with wide confidence intervals, and the heterogeneity.
Use of hospital resources
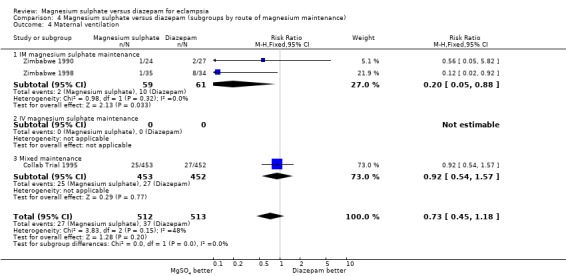
Three trials (1034 women) reported admission to an intensive care unit, there was no significant difference between the two groups (RR 0.80, 95% CI 0.59 to 1.07). The need for ventilation was also reported by three trials (1025 women), and there was no significant difference (RR 0.73, 95% CI 0.45 to 1.18). No trials reported the need for haemodialysis or maternal transfer to a higher level facility.
Additional outcomes for women randomised before delivery
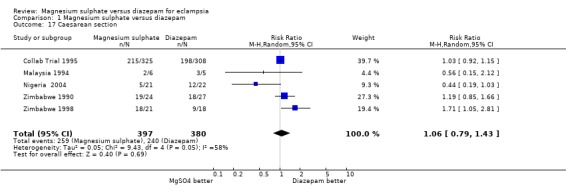
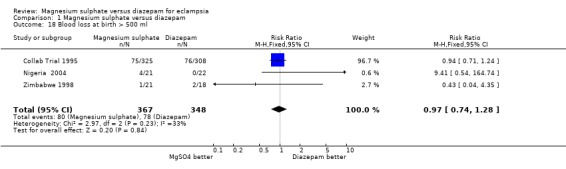
For women randomised before delivery, magnesium sulphate rather than diazepam was not associated with any significant difference in labour lasting more than eight hours (one trial; 633 women; RR 1.15, 95% CI 0.82 to 1.60), or blood loss greater than 500 ml (three trials; 715 women; RR 0.97, 95% CI 0.74 to 1.28). For caesarean section, there was heterogeneity (I2 58%) which was not explained by restricting the analysis to the three high quality studies (I2 55%). Subgroup analysis by whether or not the woman received anticonvulsants prior to delivery was not possible as the data were not available. We have therefore used random‐effects for this outcome (five trials, 777 women; RR 1.06, 95% CI 0.79 to 1.43), which gives the average for the range of treatment effects in these studies.
Outcome for the child
Outcome for the child is only relevant for women randomised before delivery.
Baby death
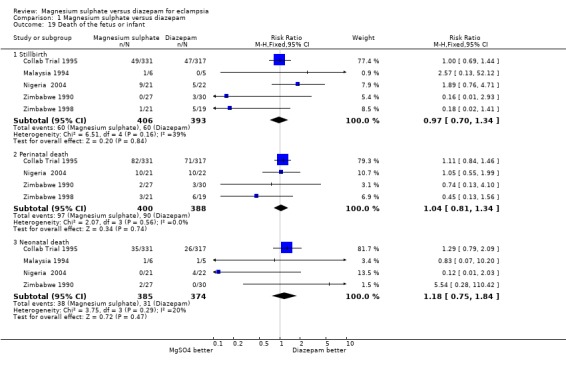
There were no significant differences between women allocated magnesium sulphate rather than diazepam in any of the measures of baby death:
stillbirth: five trials; 799 infants; RR 0.97, 95% CI 0.70 to 1.34;
perinatal mortality: four trials; 788 infants; RR 1.04, 95% CI 0.81 to 1.34;
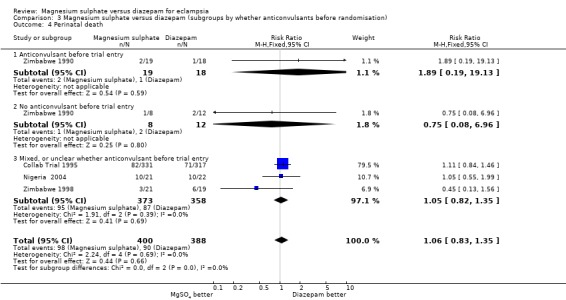
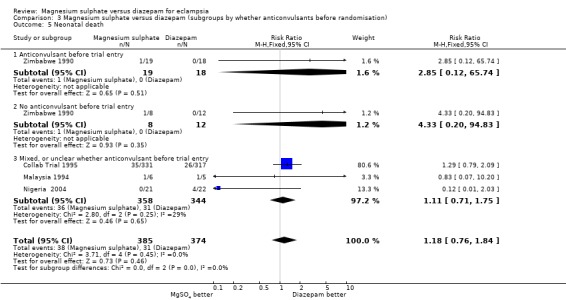
neonatal mortality: four trials; 759 infants; RR 1.18, 95% CI 0.75 to 1.84.
There were insufficient data for any reliable conclusions about whether perinatal or neonatal mortality was influenced by whether or not the woman received an anticonvulsant before trial entry.
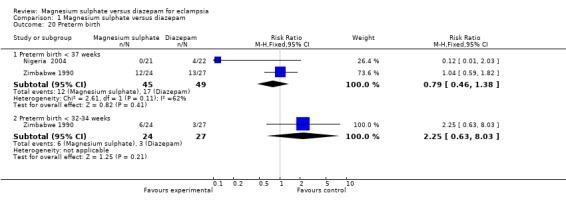
Preterm birth
There was no significant difference between the treatment groups in the RR of birth before 37 completed weeks (two trials; 94 infants; RR 0.79, 95% CI 0.46 to 1.38), nor on birth before 32 to 34 weeks (one trial; 51 infants; RR 2.25, 95% CI 0.63 to 8.03).
Apgar score and intubation at place of birth
Fewer liveborn infants of mothers allocated magnesium sulphate rather than diazepam had an Apgar score of less than seven at one minute (two trials; 597 infants; RR 0.75, 95% CI 0.65 to 0.87) and at five minutes (three trials; 643 infants; RR 0.70, 95% CI 0.54 to 0.90). Magnesium sulphate, rather than diazepam, was also associated with a reduction in the need for intubation at the place of birth that was borderline for statistical significance (two trials; 591 infants; RR 0.67, 95% CI 0.45 to 1.00).
Neonatal morbidity
No trials reported neonatal morbidity such as respiratory distress, intraventricular haemorrhage, necrotising enterocolitis, or infection.
Use of hospital resources
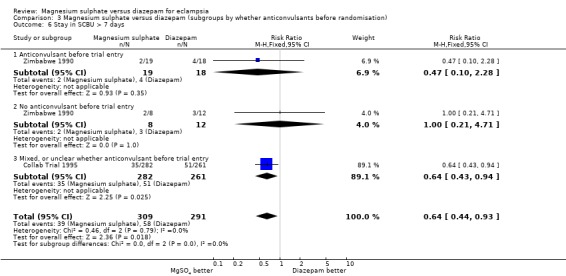
Length of stay in a special care baby unit of more than seven days was reduced for babies born to women allocated magnesium sulphate rather than diazepam, although confidence intervals are wide (three trials; 634 infants; RR 0.66, 95% CI 0.46 to 0.96). Admission to a special care baby unit was not significantly different between the two groups (three trials; 634 infants; RR 0.92, 95% CI 0.79 to 1.06).
Death or in a special care baby unit more than seven days
There was no significant difference in the composite outcome of death or in a special care baby unit for more than seven days (two trials; 688 infants; RR 0.95, 95% CI 0.77 to 1.16).
Long‐term outcome
No trials reported long‐term growth and developmental outcomes.
Sensitivity analysis
Only three studies (Collab Trial 1995; Zimbabwe 1990; Zimbabwe 1998) (1025 women) had no major methodological biases with regard to sequence generation, allocation concealment, and loss of follow‐up, and so met our definition of good quality. When the analyses were restricted to these good quality studies, the results are consistent with the overall results, although with slightly broader confidence intervals due to the reduced sample size: maternal death (RR 0.63, 95% CI 0.36 to 1.11), recurrence of convulsions (RR 0.50, 95% CI 0.38 to 0.66).
Discussion
Summary of main results
For women with eclampsia, using magnesium sulphate rather than diazepam reduces the RR of maternal death by 41% (95% CI 8% to 62%) and of recurrence of seizures by 57% (95% CI 45% to 67%). These effects appear consistent regardless of whether women were recruited before or after delivery, and irrespective of whether they had an anticonvulsant before trial entry. For the women, there is no clear effect on any other measure of maternal morbidity.
For the baby, although there is no clear effect on the risk ratio of perinatal or neonatal death, fewer liveborn babies allocated magnesium sulphate rather than diazepam had low Apgar scores at birth, and they appear to be less likely to need intubation at the place of birth. There is no clear effect on admission to a special care baby unit, but fewer babies allocated magnesium sulphate rather than diazepam stayed in the special care baby unit for more than seven days.
Overall completeness and applicability of evidence
All seven trials in this review reported maternal death and recurrence of seizures. Reporting of other measures of maternal morbidity varies, but missing data are largely from the small trials. Only two trials reported the composite outcome of any serious maternal morbidity, but reporting of individual types of morbidity was more complete.
Data on outcomes for the child are less complete, with two studies not reporting baby death and only three studies reporting any measures of neonatal morbidity.
Recruitment to all the trials in this review was from hospitals in low‐ and middle‐income countries, which is where 99% of maternal deaths occur. The evidence supporting magnesium sulphate, rather than diazepam, for women with eclampsia should also be applied to women in high‐income countries, however. The only data from developed countries are derived from uncontrolled case series, and so are prone to all the misleading biases associated with such studies. The low incidence of eclampsia makes it unlikely that trials of sufficient size could ever be conducted in high‐income countries. Also, the reduction in recurrence of seizures associated with magnesium sulphate, rather than diazepam or phenytoin, is large and consistent across the subgroups, and the settings included in this review. Although it may be less in other settings, it is unlikely to change direction.
Duration of treatment in these trials was 24 hours, unless there was an indication to continue for longer. In those using the intravenous route for maintenance therapy, the dose was 1 g/hour.
Most trials reported using clinical monitoring during administration of magnesium sulphate. No trial reported using serum monitoring. The benefits of magnesium sulphate, rather than diazepam, for women with eclampsia are based on administration with clinical monitoring alone. This is important, as magnesium sulphate is a relatively low‐cost drug but, if serum magnesium monitoring was necessary, this would make its use expensive, and also inappropriate as serum monitoring is not available in many of the places where women present with eclampsia. In high‐income countries, although 24‐hour access to serum magnesium monitoring may be more widely available, it is nevertheless costly.
The Collaborative Eclampsia Trial used sealed treatment packs for recruitment (Duley 1996). These 'eclampsia packs' were designed to make recruitment to the trial easier and quicker than usual clinical practice. Such packs are now widely used in high‐, middle‐ and low‐income countries. They include the loading dose of magnesium sulphate, maintenance therapy, and calcium gluconate for use in the event of toxicity, plus everything needed to give these (syringes and needles, intravenous canula, a giving set and a pack of 500 ml 0.9% intravenous saline) and instructions for clinical monitoring (Duley 1996). Administration and clinical monitoring of magnesium sulphate can be carried out by medical, midwifery or nursing staff, provided they are appropriately trained.
Women with eclampsia may present at primary care level. As these women are at high risk for adverse outcome, they are often transferred to secondary or tertiary care level. None of the trials in this review were conducted at primary care level, and none included evaluation of care during the transfer to a higher level of care.
Quality of the evidence
Although we have included seven trials in this review, most of the data (74%) come from three high‐quality studies (Collab Trial 1995; Zimbabwe 1990; Zimbabwe 1998), which recruited 1030 women. Of these three, the Collaborative Eclampsia Trial (910 women) contributed two‐thirds of the data to the review (910/1396). For the other four studies, study quality was unclear.
Potential biases in the review process
The search strategy for this review was extensive, and not restricted to studies published in English. One of the problems in identifying potentially eligible studies is that they are likely to have been conducted in low‐and middle‐income countries, where eclampsia is more common. If such studies are published in journals not easy to access, or are unpublished, they may not have been identified by our search strategy. Two studies potentially eligible for this review were identified through personal contact: one unpublished study (India 2001) is included, and one study published only as an abstract (Egypt 1993) was excluded. This suggests there may be other studies which have not been identified. We would welcome information about any potentially eligible studies for this review (please send information to l.duley@leeds.ac.uk).
As one of the review authors was a principal investigator for the Collaborative Eclampsia Trial (Collab Trial 1995), two other review authors performed data extraction for this study.
Agreements and disagreements with other studies or reviews
This review should be viewed in conjunction with the Cochrane reviews comparing magnesium sulphate with phenytoin (Duley 2003a) and with lytic cocktail (Duley 2000). Overall, there is now compelling evidence in favour of magnesium sulphate, rather than diazepam, phenytoin or lytic cocktail for the treatment of eclampsia. This has been endorsed by multiple editorials (Langer 2008; Neilson 1995; Roberts 2002b; Robson 1996; Sheth 2002) and by international guidelines (RCOG 2006; WHO 1988). Urgency to improve the routine and appropriate use of magnesium sulphate to treat women with eclampsia in middle‐ and low‐income countries has again been highlighted (Langer 2008).
The fifth Millennium Development Goal (MDG) calls for a reduction by three‐quarters between 1990 and 2015 in the maternal mortality ratio. Universal implementation of magnesium sulphate for treatment of women with eclampsia would be a substantial step towards achieving this goal (Langer 2008).
Ensuring more women with eclampsia receive appropriate treatment with magnesium sulphate will require dedicated, focused and co‐ordinated action at several levels. There is a need for governments, donors and international agencies concerned with women's health, such as the World Health Organization (WHO), UNFPA, the International Federation of Gynecology and Obstetrics (FIGO) and the International Confederation of Midwives (ICM) to, as a priority, increase support for effective care for women with eclampsia (Langer 2008; Lumbiganon 2007). This support should include advocacy and funding to help ensure magnesium sulphate is registered and available for care of women with eclampsia in all middle‐ and low‐income countries. In addition governments, together with nation associations of obstetricians and midwives, should ensure appropriate training of all relevant health professionals (including obstetricians, midwives, emergency‐room doctors, anaesthetists, nurses, medical officers and pharmacists) in the management of women with eclampsia and in the use of magnesium sulphate.
Authors' conclusions
Implications for practice.
This review presents strong evidence that magnesium sulphate is better than diazepam for women with eclampsia; other reviews present strong evidence that it is also better than either phenytoin or lytic cocktail (Duley 2000; Duley 2003a). Clearly, magnesium sulphate is the drug of choice for treatment of women with eclampsia, both whilst they have a seizure, and to prevent recurrence of further seizures. As it is inexpensive and easy to produce, it is especially suitable for use in low‐ and middle‐income countries. Duration of treatment should not normally exceed 24 hours. The intravenous or intramuscular route can be used for maintenance therapy. If the intravenous route is used, the dose should not exceed 1 g/hour. Clinical monitoring of respiration, urine output and tendon reflexes is essential. Serum monitoring should not be used.
It is unclear whether a loading dose of magnesium sulphate should be given to women at primary care level before they are transferred to hospital, but this would seem sensible to consider. Factors in this decision are likely to include how long it will take to get the woman to hospital, and whether support and clinical monitoring will be available during transfer.
Following publication of the Collaborative Eclampsia Study (Collab Trial 1995), practice in the UK changed within one year (Gülmezoglu 1998). Making magnesium sulphate available for women with eclampsia in low‐ and middle‐income countries has been more problematic, and access to magnesium sulphate is poor in many countries (Aaserud 2005; Sevene 2005). All low‐income countries should include magnesium sulphate on their national essential drug list for management of eclampsia. National guidelines for care of women with eclampsia should be available and widely disseminated in each country. Health workers should be trained in the appropriate use of magnesium sulphate.
The easy availability of ready‐to‐use eclampsia packs was a key issue in the success of the Collaborative Eclampsia Trial (Collab Trial 1995). Similar eclampsia packs (Duley 1996) should be available in all settings which provide care to women with eclampsia. Given that only an estimated 150,000 women develop eclampsia each year (Villar 2003) the provision of these kits would be a relatively modest cost in relation to global health assistance budgets.
Implications for research.
Magnesium sulphate is now the gold standard drug against which alternative anticonvulsant drug for women with eclampsia should be compared in properly designed large randomised trials.
The trials in this review recruited women in secondary and tertiary level hospitals. Questions which merit further research include whether a loading dose of magnesium sulphate should be administered to women at primary care level before transfer to hospital, the minimum effective dose, the optimal mode of administration, and duration of treatment.
Any future trials should be of adequate size, and report mortality, serious morbidity and use of health service resources for both the women and the child.
Eclampsia can be distinguished from other forms of seizures in that it is better controlled by magnesium sulphate than by either diazepam or phenytoin (both conventional anticonvulsants), which may offer opportunities to explore the pathogenesis of eclampsia.
Feedback
Dennis, 12 May 2008
Summary
This review reports a statistically significant difference for the outcome of maternal death (Analysis 1.1). As far as I am aware this analysis is the only one published that has found a difference in maternal mortality when magnesium sulphate is compared with other agents. This is an important finding. It would be useful to have more information about randomisation and allocation concealment in India 2001, as if this unpublished study is removed from the analysis the pooled statistic is no longer statistically significant for maternal death. (Summary of feedback from Alicia Dennis, May 2008)
Reply
Additional information has been provided by the trialists for this study. The randomisation list was generated by the Department of Statistics at Banaras Hindu University, who also prepared envelopes containing the allocation. Women were asked to choose an envelope, which determined the treatment allocation. The envelopes were not marked or numbered.
(Summary of response from Lelia Duley, June 2008)
Contributors
Lelia Duley, review author
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2010 | New search has been performed | Search updated. Nigeria 2004 included and Egypt 1993 now excluded. Dominican Republic 1990; India 1989; and India 2002 excluded. Prespecified subgroup analysis by timing of randomisation and whether or not the woman received anticonvulsants prior to trial entry incorporated. |
| 25 June 2010 | New citation required but conclusions have not changed | New authors prepared this update. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 8 August 2008 | Amended | Converted to new review format. |
| 8 August 2008 | Feedback has been incorporated | Feedback from Alicia Dennis added with a reply from Lelia Duley. |
| 1 July 2003 | New search has been performed | Two new included trials: India 2001 and Egypt 1993. |
| 22 September 1999 | New search has been performed | Two new trials have been added (Bangladesh 1998; Zimbabwe 1998) with a total of 269 women. |
Acknowledgements
Thanks to Meenu Rani for providing unpublished data for India 2001, and to Tarek Al‐Hussaini for providing unpublished data for Egypt 1993. Thanks also to Maria Tenorio for translation of Dominican Republic 1990.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Hypertension, Pregnancy‐Induced explode all trees #2 hypertens* near pregnan* #3 eclamp* #4 preeclamp* or pre next eclamp* #5 magnesium #6 diazepam #7 lytic next cocktail #8 phenytoin #9 chlorpromazine #10 meperidine or pethidine #11 MeSH descriptor Anticonvulsants explode all trees #12 anticonvulsant* or anti next convulsant* #13 (#1 OR #2 OR #3 OR #4) #14 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #15 (#13 AND #14)
Data and analyses
Comparison 1. Magnesium sulphate versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 7 | 1396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.92] |
| 2 Recurrence of seizures | 7 | 1390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 3 Cerebrovascular accident (stroke) | 4 | 1225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.18] |
| 4 Any serious morbidity (stroke, renal failure, HELLP, DIC, pulmonary edema, cardiac arrest, or as reported | 2 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.19] |
| 5 Renal failure | 5 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.36] |
| 6 Liver failure | 2 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.48, 2.07] |
| 7 Coagulopathy | 4 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.56, 1.41] |
| 8 Side effects: local skin reaction | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Pulmonary oedema | 3 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.07] |
| 10 Pneumonia | 4 | 1125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.31, 1.33] |
| 11 Respiratory depression | 3 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 12 Cardiac arrest | 4 | 1085 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.54] |
| 13 Death or any serious maternal morbidity | 1 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.80, 1.41] |
| 14 Woman admitted to intensive care unit | 3 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.07] |
| 15 Maternal ventilation | 3 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.18] |
| 16 Labour > 8 hours | 1 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.82, 1.60] |
| 17 Caesarean section | 5 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.79, 1.43] |
| 18 Blood loss at birth > 500 ml | 3 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.28] |
| 19 Death of the fetus or infant | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Stillbirth | 5 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.34] |
| 19.2 Perinatal death | 4 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.34] |
| 19.3 Neonatal death | 4 | 759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.75, 1.84] |
| 20 Preterm birth | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Preterm birth < 37 weeks | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.38] |
| 20.2 Preterm birth < 32‐34 weeks | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.63, 8.03] |
| 21 Apgar scores | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Apgar < 7 at 1 minute | 2 | 597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.65, 0.87] |
| 21.2 Apgar < 7 at 5 minutes | 3 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 22 Admitted to special care baby unit (SCBU) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Stay in SCBU > 7 days | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.96] |
| 22.2 Admission to SCBU | 3 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 23 Death or in SCBU > 7 days | 2 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.16] |
| 24 Intubation at place of birth | 2 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 1.00] |
1.5. Analysis.
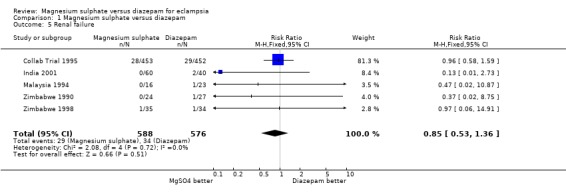
Comparison 1 Magnesium sulphate versus diazepam, Outcome 5 Renal failure.
1.6. Analysis.
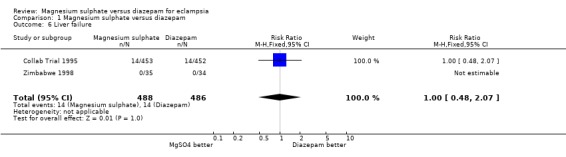
Comparison 1 Magnesium sulphate versus diazepam, Outcome 6 Liver failure.
1.7. Analysis.
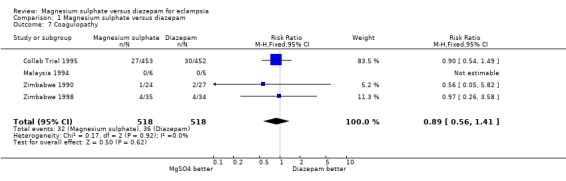
Comparison 1 Magnesium sulphate versus diazepam, Outcome 7 Coagulopathy.
1.8. Analysis.
Comparison 1 Magnesium sulphate versus diazepam, Outcome 8 Side effects: local skin reaction.
1.9. Analysis.
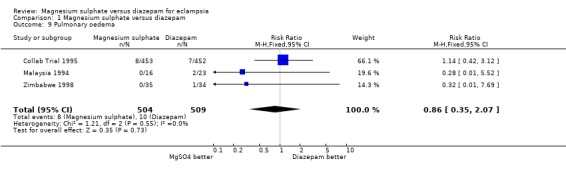
Comparison 1 Magnesium sulphate versus diazepam, Outcome 9 Pulmonary oedema.
1.10. Analysis.
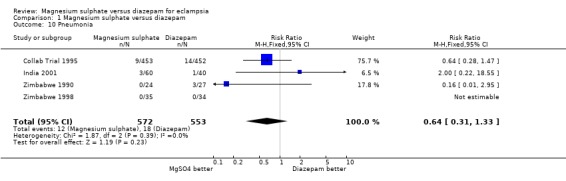
Comparison 1 Magnesium sulphate versus diazepam, Outcome 10 Pneumonia.
1.11. Analysis.
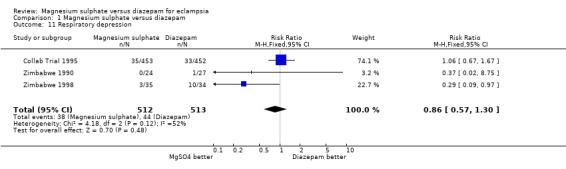
Comparison 1 Magnesium sulphate versus diazepam, Outcome 11 Respiratory depression.
1.12. Analysis.
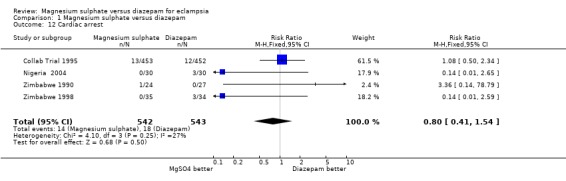
Comparison 1 Magnesium sulphate versus diazepam, Outcome 12 Cardiac arrest.
1.13. Analysis.
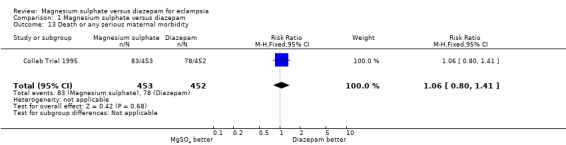
Comparison 1 Magnesium sulphate versus diazepam, Outcome 13 Death or any serious maternal morbidity.
1.14. Analysis.
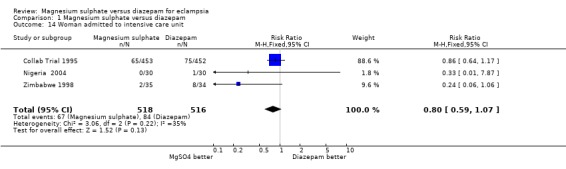
Comparison 1 Magnesium sulphate versus diazepam, Outcome 14 Woman admitted to intensive care unit.
1.15. Analysis.
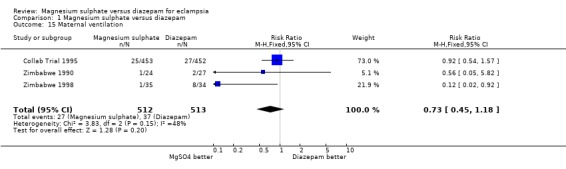
Comparison 1 Magnesium sulphate versus diazepam, Outcome 15 Maternal ventilation.
1.16. Analysis.
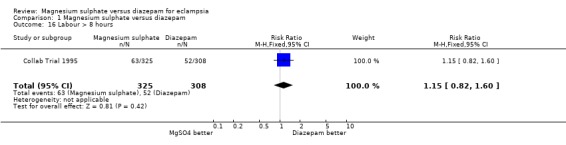
Comparison 1 Magnesium sulphate versus diazepam, Outcome 16 Labour > 8 hours.
1.17. Analysis.
Comparison 1 Magnesium sulphate versus diazepam, Outcome 17 Caesarean section.
1.18. Analysis.
Comparison 1 Magnesium sulphate versus diazepam, Outcome 18 Blood loss at birth > 500 ml.
1.19. Analysis.
Comparison 1 Magnesium sulphate versus diazepam, Outcome 19 Death of the fetus or infant.
1.20. Analysis.
Comparison 1 Magnesium sulphate versus diazepam, Outcome 20 Preterm birth.
1.21. Analysis.
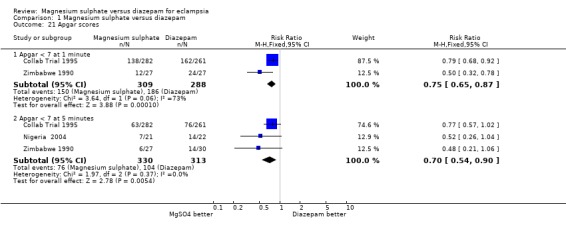
Comparison 1 Magnesium sulphate versus diazepam, Outcome 21 Apgar scores.
1.22. Analysis.
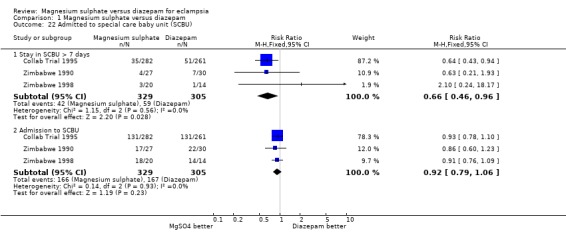
Comparison 1 Magnesium sulphate versus diazepam, Outcome 22 Admitted to special care baby unit (SCBU).
1.23. Analysis.
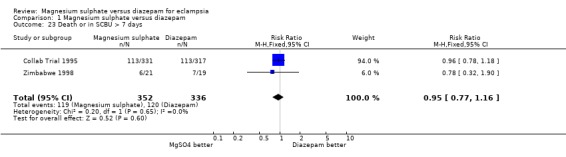
Comparison 1 Magnesium sulphate versus diazepam, Outcome 23 Death or in SCBU > 7 days.
1.24. Analysis.
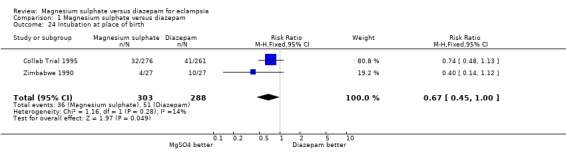
Comparison 1 Magnesium sulphate versus diazepam, Outcome 24 Intubation at place of birth.
Comparison 2. Magnesium sulphate versus diazepam (subgroups by whether randomisation before or after birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 7 | 1396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.92] |
| 1.1 Randomised before delivery | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.14, 78.79] |
| 1.2 Randomised after delivery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Mixed, or unclear whether randomised before delivery | 6 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.88] |
| 2 Recurrence of seizures | 7 | 1390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.1 Randomised before delivery | 2 | 684 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.40, 0.74] |
| 2.2 Randomised after delivery | 1 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.64] |
| 2.3 Mixed, or unclear whether randomised before delivery | 5 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.40] |
| 3 Stroke | 4 | 1225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.18] |
| 3.1 Randomised before delivery | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.75] |
| 3.2 Randomised after delivery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed, or unclear whether randomised before delivery | 3 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.22] |
2.1. Analysis.

Comparison 2 Magnesium sulphate versus diazepam (subgroups by whether randomisation before or after birth), Outcome 1 Death.
2.2. Analysis.
Comparison 2 Magnesium sulphate versus diazepam (subgroups by whether randomisation before or after birth), Outcome 2 Recurrence of seizures.
2.3. Analysis.
Comparison 2 Magnesium sulphate versus diazepam (subgroups by whether randomisation before or after birth), Outcome 3 Stroke.
Comparison 3. Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 7 | 1396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.91] |
| 1.1 Anticonvulsant before trial entry | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.84] |
| 1.2 No anticonvulsant before trial entry | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Mixed, or unclear whether anticonvulsant before trial entry | 6 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.88] |
| 2 Recurrence of seizures | 7 | 1369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.34, 0.56] |
| 2.1 Anticonvulsant before trial entry | 2 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.85] |
| 2.2 No anticonvulsant before trial entry | 2 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.65] |
| 2.3 Mixed, or unclear whether anticonvulsant before trial entry | 5 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.40] |
| 3 Any serious maternal morbidity | 2 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.20] |
| 3.1 Anticonvulsant before trial entry | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.13, 1.39] |
| 3.2 No anticonvulsant before trial entry | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.38, 1.74] |
| 3.3 Mixed, or unclear whether anticonvulsant before trial entry | 1 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.33] |
| 4 Perinatal death | 4 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.35] |
| 4.1 Anticonvulsant before trial entry | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.19, 19.13] |
| 4.2 No anticonvulsant before trial entry | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.08, 6.96] |
| 4.3 Mixed, or unclear whether anticonvulsant before trial entry | 3 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.35] |
| 5 Neonatal death | 4 | 759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.76, 1.84] |
| 5.1 Anticonvulsant before trial entry | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 65.74] |
| 5.2 No anticonvulsant before trial entry | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.20, 94.83] |
| 5.3 Mixed, or unclear whether anticonvulsant before trial entry | 3 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.71, 1.75] |
| 6 Stay in SCBU > 7 days | 2 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.93] |
| 6.1 Anticonvulsant before trial entry | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.28] |
| 6.2 No anticonvulsant before trial entry | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.71] |
| 6.3 Mixed, or unclear whether anticonvulsant before trial entry | 1 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.94] |
3.1. Analysis.
Comparison 3 Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation), Outcome 1 Maternal death.
3.2. Analysis.
Comparison 3 Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation), Outcome 2 Recurrence of seizures.
3.3. Analysis.
Comparison 3 Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation), Outcome 3 Any serious maternal morbidity.
3.4. Analysis.
Comparison 3 Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation), Outcome 4 Perinatal death.
3.5. Analysis.
Comparison 3 Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation), Outcome 5 Neonatal death.
3.6. Analysis.
Comparison 3 Magnesium sulphate versus diazepam (subgroups by whether anticonvulsants before randomisation), Outcome 6 Stay in SCBU > 7 days.
Comparison 4. Magnesium sulphate versus diazepam (subgroups by route of magnesium maintenance).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal cardiac arrest | 4 | 1085 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.54] |
| 1.1 IM magnesium sulphate maintenance | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.66] |
| 1.2 IV magnesium sulphate maintenance | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.65] |
| 1.3 Mixed, or route for maintenance unclear | 1 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.50, 2.34] |
| 2 Maternal respiratory depression | 3 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 2.1 IM magnesium sulphate maintenance | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.93] |
| 2.2 IV magnesium sulphate maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mixed, or route of maintenance unclear | 1 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.67, 1.67] |
| 3 Pneumonia | 4 | 1125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.31, 1.33] |
| 3.1 IM magnesium sulphate maintenance | 3 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.15, 2.87] |
| 3.2 IV magnesium sulphate maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed, or route of maintenance unclear maintenance | 1 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.47] |
| 4 Maternal ventilation | 3 | 1025 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.18] |
| 4.1 IM magnesium sulphate maintenance | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.88] |
| 4.2 IV magnesium sulphate maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Mixed maintenance | 1 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.54, 1.57] |
4.1. Analysis.
Comparison 4 Magnesium sulphate versus diazepam (subgroups by route of magnesium maintenance), Outcome 1 Maternal cardiac arrest.
4.2. Analysis.
Comparison 4 Magnesium sulphate versus diazepam (subgroups by route of magnesium maintenance), Outcome 2 Maternal respiratory depression.
4.3. Analysis.
Comparison 4 Magnesium sulphate versus diazepam (subgroups by route of magnesium maintenance), Outcome 3 Pneumonia.
4.4. Analysis.
Comparison 4 Magnesium sulphate versus diazepam (subgroups by route of magnesium maintenance), Outcome 4 Maternal ventilation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bangladesh 1998.
| Methods | See 'risk of bias' table below. | |
| Participants | 200 women with eclampsia. 14% postpartum. | |
| Interventions | MgSO4: 4 g iv + 6 g im loading dose. Then 2.5 g im 4 hrly, until 24 hr after delivery or last fit. If recurrence, 2 g iv. Diazepam: 10 mg loading dose, then 40 mg in 500 ml 5% dextrose for 24 hr after delivery or last fit. If recurrence, 10 mg slowly iv. |
|
| Outcomes | Women: death (see 'notes'), recurrence of seizures. Baby: no denominators reported. |
|
| Notes | Data are not presented separately for women randomised before delivery.
MgS04 was the unfamiliar treatment. Data on maternal death unclear: text states 4 deaths before delivery reported for the diazepam group, and it is unclear whether these are included in the 7 reported later. Hence although 7 diazepam deaths used in this review, total may be 11. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "consecutive patients" "randomly assigned", but no further information. |
| Allocation concealment? | Unclear risk | Allocation concealment not mentioned. |
| Blinding? All outcomes | Unclear risk | Blinding not mentioned. Would have been impossible to blind administration of the 2 treatment regimens. |
| Incomplete outcome data addressed? All outcomes | High risk | 200 women reported as recruited, and not stated whether any post‐randomisation exclusion. All outcome data reported as percentages or means, without denominators. Some women were recruited postpartum, but the numbers in each allocated group are not reported. Hence data on outcome after birth were not used in this review, as denominators not clear. |
Collab Trial 1995.
| Methods |
See 'risk of bias' table below. Recruitment from 23 hospitals in 8 countries in Africa, India, and South America. |
|
| Participants | 910 women with eclampsia. 54% allocated MgSO4had had an anticonvulsant before entry, as had 50% allocated diazepam. 30% were randomised after delivery. | |
| Interventions | Diazepam: 10 mg iv bolus. Then infusion of 40 mg/500 ml for 24 hr, rate titrated against conscious level. 20 mg/500 ml for a further 24 hr. For recurrent convulsions, 10 mg iv. MgS04: either (a) 4/5 g iv over 5 min and 10 g im. Then 5 g im every 4 hr, for 24 hr. Or (b) 4/5 iv over 5 min, then infusion of 1 g/hr for 24 hr. For both (a) and (b), if recurrent convulsions 2 g iv. No monitoring of serum MgSO4 levels. |
|
| Outcomes | All women: death, recurrent convulsions, pneumonia, respiratory depression, ventilation, cardiac arrest, arrhythmia, coagulopathy, renal failure, liver failure, cerebrovascular accident, admission intensive care, abscess. Women randomised before delivery: transfusion, induction, labour < 8 hr, caesarean section, blood loss. Baby: mortality, Apgar < 7 (1, 5 min), intubated, admitted SCBU, in SCBU > 7 days, death or in SCBU < 7 days. |
|
| Notes | For im MgS04 n = 229, for iv n = 224. In some centres MgS04 was the new treatment, in others it was the standard therapy. 99% compliance with the allocated anticonvulsant. The same study included a comparison of MgSO4 with phenytoin ‐ data for this comparison are in a separate review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated sequence, with balanced blocks of 2‐6 stratified by centre. |
| Allocation concealment? | Low risk | Consecutively numbered sealed treatment packs, identical in size, shape, weight and feel. In the study overall, 2.7% of packs were opened out of order: this seemed to be largely human error. |
| Blinding? All outcomes | High risk | Blinding of the 2 treatment regimens was not possible. Outcome assessment was by the attending clinician, but outcomes used were largely objective, reducing potential for bias. |
| Incomplete outcome data addressed? All outcomes | Low risk | 5 women recruited but excluded from analysis ‐ reasons presented. Exclusions are reported for both comparisons ‐ 7 in total (severe pre‐eclampsia n = 2; self‐discharge and took their own notes n = 2; no data available n = 2; hysteria n = 1). Where data are missing for individual outcomes, reason stated and denominators given. |
India 2001.
| Methods | See 'risk of bias' table below. | |
| Participants | 100 women with eclampsia. 70 in first pregnancy and 79 recruited before delivery. | |
| Interventions | Diazepam: 10 mg iv bolus. Then infusion of 40 mg/500 ml for 24 hr, rate titrated against conscious level. 20 mg/500 ml for a further 24 hr. Then 10 mg im, changed when possible to oral. For recurrent convulsions, 10 mg iv. MgSO4: 4 g in 25% MgSO4 over 10 min. The 5 g IM every 4 hr until 24 hr after delivery or, if postpartum at randomisation, for 24 hr. |
|
| Outcomes | Women: death, recurrence of convulsions, renal failure, pneumonia. Babies: denominators not reported. |
|
| Notes | Outcomes related to labour and delivery not reported separately for women randomised before delivery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Sequence generated by Department of Statistics at Banaras Hindu University, but method not stated. |
| Allocation concealment? | Unclear risk | Allocation was in envelopes, and patient was asked to choose one. No further information. |
| Blinding? All outcomes | Unclear risk | Blinding not mentioned. Blinding of the 2 treatment regimens would have been impossible. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Post‐randomisation exclusions not mentioned. No denominators reported for women randomised before birth. |
Malaysia 1994.
| Methods | See 'risk of bias' table below. | |
| Participants | 39 women; 11 with eclampsia, 28 with pre‐eclampsia. | |
| Interventions | Diazepam: not described. MgS04: "Pritchard's regime", not described further. |
|
| Outcomes | Women: death, recurrence of convulsions, caesarean section. Baby: stillbirth and neonatal death. |
|
| Notes | Published in abstract form only. Interim results of an ongoing trial. MgS04 was the unfamiliar treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Randomly allocated" no further information given. |
| Allocation concealment? | Unclear risk | Not described. |
| Blinding? All outcomes | High risk | Blinding not mentioned. Blinding of the 2 treatment regimens would have been impossible. |
| Incomplete outcome data addressed? All outcomes | Low risk | Data were complete. |
Nigeria 2004.
| Methods | See 'risk of bias' table below. | |
| Participants | 60 women with eclampsia, 25% delivered prior to entry. | |
| Interventions | Diazepam: loading dose of 10 mg iv over 2 minutes administered and repeated when convulsion recurred. Then 10 mg iv every 6 hours to keep patient sedated but arousable for next 24 hours. Subsequent doses were held if the patient was still sedated when the next dose due. MgSO4: "Zuspan's" method, no further details given. |
|
| Outcomes | Woman: recurrence of convulsions, death, life threatening events, including pulmonary edema, cardiac arrest, respiratory depression, aspiration pneumonitis, renal failure, disseminated intravascular coagulation, cerebrovascular accidents, liver failure, postpartum haemorrhage, Glasgow Coma Score on admission and 24 hours after commencement of treatment. For women randomised prior to delivery: mode of delivery. Baby: Apgar scores, need for neonatal unit for intensive care, and perinatal deaths. |
|
| Notes | MgSO4 was the unfamiliar treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Computer‐generated random numbers, but then this was used to prepare treatment packs labelled "A" or "B". |
| Allocation concealment? | High risk | Sealed treatment packs marked "A" or "B," identical in size, shape, weight, and feel. Not clear in what order these packs were opened. |
| Blinding? All outcomes | High risk | Blinding not mentioned. Blinding of the 2 treatment regimens would have been impossible. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | There is a discrepancy in some denominators, as the numbers for 'postpartum eclampsia' are not the same as randomised after delivery'. |
Zimbabwe 1990.
| Methods | See 'risk of bias' table below. | |
| Participants | 51 women with antepartum eclampsia, > 28 weeks' gestation, and a live fetus at admission. 67% had diazepam before entry; 71% of those allocated MgSO4and 63% diazepam. | |
| Interventions | Diazepam: 10 mg iv bolus. Then infusion of 80 mg/l for 24 hr, rate titrated against conscious level. 40 mg/l for a further 24 hr. For recurrent convulsions, 10 mg iv. MgS04: 4 g iv over 3‐5 min and 10 g im. Then 5 g im every 4 hr, until 24 hr after delivery. For recurrent convulsions, 2 g iv. |
|
| Outcomes | Woman: death, recurrence of convulsions, pneumonia, respiratory depression, ventilation, cardiac arrest, coagulopathy, acute renal failure, reduced urine output, caesarean section, abscess. Baby: mortality, Apgar < 7 (1, 5 min), intubated, admitted NICU, days on NICU (mean), in NICU > 7 days. |
|
| Notes | Subgroup analysis by whether anticonvulsants before trial entry, but numbers very small. MgS04 was the unfamiliar treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomly allocated, with 1:1 randomisation in blocks of 6, no stratification. |
| Allocation concealment? | Low risk | Consecutively numbered, sealed, opaque envelopes. Prepared by someone not involved in enrolment. |
| Blinding? All outcomes | High risk | Blinding not mentioned. Blinding of the 2 treatment regimens would have been impossible. |
| Incomplete outcome data addressed? All outcomes | Low risk | Data were complete. |
Zimbabwe 1998.
| Methods | See 'risk of bias' table below. | |
| Participants | 69 women with eclampsia. 40% had already had an anticonvulsant and 43% had delivered. | |
| Interventions | Diazepam: 10 mg iv bolus. Then infusion of 40 mg/500 ml for 24 hr, rate titrated against conscious level. 20 mg/500 ml for a further 24 hr. For recurrent convulsions, 10 mg iv. MgS04: either (a) 4/5 g iv over 5 min and 10 g im. Then 5 g im every 4 hr, for 24 hr. Or (b) 4/5 iv over 5 min, then infusion of 1 g/hr for 24 hr. For both (a) and (b), if recurrent convulsions 2 g iv. |
|
| Outcomes | Women: death, recurrence of convulsions, respiratory depression, cardiac arrest, renal failure, coagulopathy, stroke, caesarean section, blood loss > 500 ml. Baby: death, died or in SCBU > 7 days. |
|
| Notes | These data are from 1 hospital in the Collaborative Eclampsia Trial which continued recruitment and data collection after the end of that study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated sequence, with balanced blocks of 2‐6 stratified by centre. |
| Allocation concealment? | Low risk | Consecutively numbered sealed treatment packs, identical in size, shape, weight and feel. |
| Blinding? All outcomes | High risk | Blinding not mentioned. Blinding of the 2 treatment regimens would have been impossible. |
| Incomplete outcome data addressed? All outcomes | Low risk | Where data are not available for individual outcomes, reason stated and denominators given. |
g: gram(s) hr: hour(s) hrly: hourly im: intramuscular iv: intravenous MgS04: magnesium sulphate mg: milligram(s) min: minute(s) ml: millilitre(s) NICU: neonatal intensive care unit SCBU: special care baby unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dominican Republic 1990 | Randomised trial of 44 women with pre‐eclampsia or eclampsia. Only 1 woman with eclampsia recruited. Full English translation of the Spanish paper available. |
| Egypt 1993 | Randomised trial of 118 women with eclampsia or imminent eclampsia. Data for women with eclampsia were not presented separately. Available as an abstract and unpublished manuscript. |
| India 1989 | Although described as 'randomly allocated' it seems unlikely that this study was randomised. The groups are unbalanced (32 vs 20 vs 22) and the study continued over 4 years, plus there is no information about sequence generation or concealment of allocation. Participants: 74 women with eclampsia. Interventions: magnesium sulphate vs phenytoin vs diazepam plus pentazocine (opioid analgesic). |
| India 1997 | Not a randomised trial. this study began as a quasi‐random design with alternate allocation. It was then modified to be a case series. Participants: 100 women with eclampsia. Interventions: phenytoin (n = 40), lytic cocktail (n = 28), diazepam (n = 16), and magnesium sulphate (n = 16). |
| India 2002 | Not randomised trial. Before and after study. Participants: 150 women with eclampsia. Interventions: magnesium sulphate (n = 100) vs diazepam (n = 50). |
vs: versus
Contributions of authors
All review authors contributed to the design. Two authors (of Lelia Duley, David Henderson‐Smart and Doris Chou) conducted data extraction. Lelia Duley and Doris Chou entered data. Lelia Duley, Doris Chou and David Henderson‐Smart checked data. Lelia Duley and Doris Chou drafted the text for this update with comments and input from David Henderson‐Smart and Godfrey Walker.
Sources of support
Internal sources
Centre for Perinatal Health Services Research, Sydney, Australia.
Department for International Development, UK.
Medical Research Council, UK.
External sources
UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Reproductive Health and Research, WHO, Switzerland.
Declarations of interest
Lelia Duley was the Principal Investigator for the Collaborative Eclampsia Trial Collab Trial 1995. Godfrey Walker was involved in securing funding for the Collaborative Eclampsia Trial. Trial eligibility, quality assessment and data extraction for this trial was carried out by two other members of the review team who were not directly involved in the study.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bangladesh 1998 {published data only}
- Shamsuddin L, Rouf S, Hussain AZM, Khan JH. Magnesium sulphate vs diazepam in the management of eclampsia. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167:1):34. [Google Scholar]
- Shamsuddin L, Rouf S, Khan JH, Tamanna S, Hussain AZ, Samsuddin AK. Magnesium sulphate versus diazepam in the management of eclampsia. Bangladesh Medical Research Council Bulletin 1998;24:43‐8. [PubMed] [Google Scholar]
Collab Trial 1995 {published data only}
- Duley L. Magnesium and eclampsia. Lancet 1995;346:1365. [DOI] [PubMed] [Google Scholar]
- Duley L. Magnesium sulphate in eclampsia. Lancet 1998;352:67. [DOI] [PubMed] [Google Scholar]
- Duley L. Magnesium sulphate regimens for women with eclampsia: messages from the Collaborative Eclampsia Trial. British Journal of Obstetrics and Gynaecology 1996;103:103‐5. [DOI] [PubMed] [Google Scholar]
- Duley L. Magnesium sulphate should be used for eclamptic fits. BMJ 1996;312:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duley L. Magnesium sulphate, diazepam or phenytoin for women with eclampsia: results of the Collaborative Eclampsia Trial. Proceedings of the 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin. 1995:Abstract no: 32.
- Duley L, Carroli G, Moodley J, Korula G. Anticonvulsants for eclampsia. Lancet 1995;346:501‐2. [Google Scholar]
- The Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 1995;345:1455‐63. [PubMed] [Google Scholar]
India 2001 {unpublished data only}
- Rani M, Sharma D, Prakash A. Maternal and perinatal outcome in eclampsia: diazepam vs magnesium sulphate regimen (a prospective trial). Personal communication 2001.
Malaysia 1994 {published data only}
- Adeeb N, Hatta AZ, Shariff J. Comparing magnesium sulphate to diazepam in managing severe pre‐eclampsia and eclampsia. Proceedings of 10th World Congress International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, Washington, USA. 1996:246.
- Adeeb N, Ho CM. Comparing magnesium sulphate versus diazepam in the management of severe pre‐eclampsia and eclampsia. 9th Congress of the International Society for the study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994.
Nigeria 2004 {published data only}
- Ola RE, Odeneye OT, Abudu OO. Eclampsia: a randomized double blind trial of magnesium sulphate and diazepam in Lagos, Nigeria. Tropical Journal of Obstetrics and Gynaecology 2004; Vol. 21, issue 2:143‐7.
Zimbabwe 1990 {published data only}
- Crowther C. Magnesium sulphate versus diazepam in the management of eclampsia: a randomized controlled trial. British Journal of Obstetrics and Gynaecology 1990;97:110‐7. [DOI] [PubMed] [Google Scholar]
Zimbabwe 1998 {published and unpublished data}
- Duley L, Mahomed K. Magnesium sulphate in eclampsia. Lancet 1998;351:1061‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dominican Republic 1990 {published data only}
- Rodriquez Cruz CM, Suero Holguin HJ, Pena Acosta CA, Cots Martinez JM, Garcia CA, Camilo MA. Magnesium sulfate versus diazepam in the treatment of severe preeclampsia [Sulfato de magnesio versus diazepam en el manejo de la pre‐eclampsia severa]. Acta Medica Dominicana 1990;12(5):171‐5. [Google Scholar]
Egypt 1993 {published and unpublished data}
- Sayed EH, Sayed GH, Abdel Aal DM, Abdullah SA, Mostafa SA. A comparative trial of magnesium sulphate and diazepam in the management of eclampsia. Proceedings of the 10th Annual Scientific Conference of Assiut Faculty of Medicine; 1993 April. 1993.
- Sayed EH, Sayed GH, Awad HMA, Abdel Aal DM, Abdullah SA, Mostafa SA. A comparative trial of magnesium sulphate and diazepam in the management of eclampsia. Unpublished manuscript.
India 1989 {published data only}
- Maheshwari JR, Desai SV, Hansotla, Walvekar VR. Anti‐convulsant therapy in eclampsia. Journal of Postgraduate Medicine 1989;35(2):66‐9. [PubMed] [Google Scholar]
India 1997 {published and unpublished data}
- Chatterjee A, Mukheree J. Comparative study of different anticonvulsants in eclampsia. Journal of Obstetrics and Gynaecology Research 1997;23:289‐93. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Phadiker A. Comparative study of different anticonvulsants in eclampsia. International Journal of Obstetrics and Gynecology 1994;46:121. [DOI] [PubMed] [Google Scholar]
India 2002 {published data only}
- Datta MR, Pant L, Kabiraj M, Basu SB. Magnesium sulphate in eclampsia: a safe, efficient and cost‐effective approach. Journal of Obstetrics and Gynecology of India 2002;52(3):65‐8. [Google Scholar]
Additional references
Aaserud 2005
- Aaserud M, Lewin S, Innvaer S, Paulsen EJ, Dahlgren AT, Trommald M, et al. Translating research into policy and practice in developing countries: a case study of magnesium sulphate for pre‐eclampsia. BMC Health Services Research 2005;5:68. [PUBMED: 16262902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abalos 2007
- Abalos E, Duley L, Steyn DW, Henderson‐Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002252.pub2] [DOI] [PubMed] [Google Scholar]
Ananth 1995
- Ananth CV, Savitz DA, Bowes WA Jr. Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstetricia et Gynecologica Scandinavica 1995;74(10):788‐93. [DOI] [PubMed] [Google Scholar]
Andersgaard 2006
- Andersgaard AB, Herbst A, Johansen M, Ivarsson A, Ingemarsson I, Langhoff‐Roos J, et al. Eclampsia in Scandinavia: incidence, substandard care, and potentially preventable cases. Acta Obstetricia et Gynecologica Scandinavica 2006;85(8):929‐36. [DOI] [PubMed] [Google Scholar]
Belfort 1992
- Belfort MA, Moise KJ Jr. Effect of magnesium sulfate on maternal brain blood flow in preeclampsia: a randomized, placebo‐controlled study. American Journal of Obstetrics and Gynecology 1992;167(3):661‐6. [PUBMED: 1530019] [DOI] [PubMed] [Google Scholar]
Churchill 2002
- Churchill D, Duley L. Interventionist versus expectant care for severe pre‐eclampsia before term. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003106] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Dinsdale 1988
- Dinsdale HB. Does magnesium sulfate treat eclamptic seizures? Yes. Archives of Neurology 1988;45(12):1360‐1. [PUBMED: 3058096] [DOI] [PubMed] [Google Scholar]
Douglas 1994
- Douglas K, Redman C. Eclampsia in the United Kingdom. BMJ 1992;309:1395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duckitt 2005
- Duckitt K, Harrington D. Risk factors for pre‐eclampsia at antenatal booking: systematic review of controlled studies. BMJ 2005;220(7491):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 1992
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. British Journal of Obstetrics and Gynaecology 1992;99:547‐53. [DOI] [PubMed] [Google Scholar]
Duley 1996
- Duley L. The Collaborative Eclampsia Trial: which anticonvulsant for women with eclampsia [thesis]. Aberdeen: University of Aberdeen, 1996. [Google Scholar]
Duley 1999
- Duley L, Williams J, Henderson‐Smart DJ. Plasma volume expansion for treatment of women with pre‐eclampsia. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2000
- Duley L, Gulmezoglu AM. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002960] [DOI] [PubMed] [Google Scholar]
Duley 2003a
- Duley L, Henderson‐Smart DJ. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000128] [DOI] [PubMed] [Google Scholar]
Duley 2003b
- Duley L, Gülmezoglu AM, Henderson‐Smart DJ. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000025] [DOI] [PubMed] [Google Scholar]
Duley 2005
- Duley L, Henderson‐Smart D, Meher S. Altered dietary salt for preventing pre‐eclampsia, and its complications. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2006
- Duley L, Henderson‐Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001449.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2007
- Duley L, Henderson‐Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004659.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2008
- Duley L, Matar HE, Almerie MQ, Hall DR. Alternative magnesium sulphate regimens for women with pre‐eclampsia and eclampsia. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007388] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2009
- Duley L, Henderson‐Smart DJ, Walker GJA. Interventions for treating pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007756] [DOI] [Google Scholar]
Eastman 1945
- Eastman NJ, Stepoe PP. The management of pre‐eclampsia. Canadian Medical Association Journal 1945;52:562‐8. [PMC free article] [PubMed] [Google Scholar]
Euser 2009
- Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke 2009;40(4):1169‐75. [PUBMED: 19211496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frias 2003
- Frias AE Jr, Belfort MA. Post Magpie: how should we be managing severe preeclampsia?. Current Opinion in Obstetrics and Gynecology 2003;15(6):489‐95. [DOI] [PubMed] [Google Scholar]
Goldman 1988
- Goldman RS, Finkbeiner SM. Therapeutic use of magnesium sulfate in selected cases of cerebral ischemia and seizure. New England Journal of Medicine 1988;319(18):1224‐5. [PUBMED: 3173462] [DOI] [PubMed] [Google Scholar]
Gülmezoglu 1998
- Gülmezoglu AM, Duley L. Use of anticonvulsants for eclampsia and pre‐eclampsia: a survey of obstetricians in the United Kingdom and Republic of Ireland. BMJ 1998;316:975‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Hofmeyr 2007
- Hofmeyr GJ, Duley L, Atallah A. Dietary calcium supplementation for prevention of pre‐eclampsia and related problems: a systematic review and commentary. BJOG: an international journal of obstetrics and gynaecology 2007;114(8):933‐43. [PUBMED: 17565614] [DOI] [PubMed] [Google Scholar]
Igberase 2006
- Igberase GO, Ebeigbe PN. Eclampsia: ten years of experience in a rural tertiary hospital in the Niger delta, Nigeria. Journal of Obstetrics and Gynaecology 2006;26(5):414‐7. [DOI] [PubMed] [Google Scholar]
Katz 2000
- Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: toward a new paradigm. American Journal of Obstetrics and Gynecology 2000;182(6):1389‐96. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066‐74. [DOI] [PubMed] [Google Scholar]
Knight 2007
- Knight M. Eclampsia in the United Kingdom 2005. BJOG: an international journal of obstetrics and gynaecology 2007;114(9):1072‐8. [DOI] [PubMed] [Google Scholar]
Kullberg 2002
- Kullberg G, Lindeberg S, Hanson U. Eclampsia in Sweden. Hypertension in Pregnancy 2002;21(1):13‐21. [DOI] [PubMed] [Google Scholar]
Langer 2008
- Langer A, Villar J, Tell K, Kim T, Kennedy S. Reducing eclampsia‐related deaths‐‐a call to action. Lancet 2008;371(9614):705‐6. [PUBMED: 18313488] [DOI] [PubMed] [Google Scholar]
Lean 1968
- Lean TH, Ratnam SS, Sivasamboo R. Use of benzodiazepines in the management of eclampsia. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75(8):856‐62. [PUBMED: 5673330] [DOI] [PubMed] [Google Scholar]
Lewis 2007
- Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer ‐ 2003‐2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH, 2007. [Google Scholar]
Lumbiganon 2007
- Lumbiganon P, Gulmezoglu AM, Piaggio G, Langer A, Grimshaw J. Magnesium sulfate is not used for pre‐eclampsia and eclampsia in Mexico and Thailand as much as it should be. Bulletin of the World Health Organization 2007;85(10):763‐7. [PUBMED: 18038057] [DOI] [PMC free article] [PubMed] [Google Scholar]
MAGPIE 2002
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre‐eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo‐controlled trial. Lancet 2002;359(9321):1877‐90. [PUBMED: 12057549] [DOI] [PubMed] [Google Scholar]
Mahomed 2007
- Mahomed K, Bhutta ZA, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000230.pub3] [DOI] [PubMed] [Google Scholar]
Makrides 2001
- Makrides M, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000937] [DOI] [PubMed] [Google Scholar]
Makrides 2006
- Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre‐eclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003402.pub2] [DOI] [PubMed] [Google Scholar]
Meads 2008
- Meads CA, Cnossen JS, Meher S, Juarez‐Garcia A, Ter Riet G, Duley L, et al. Methods of prediction and prevention of pre‐eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2008;12(6):iii‐iv, 1‐270. [DOI] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Meher 2006
- Meher S, Duley L. Exercise or other physical activity for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005942] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2007
- Meher S, Duley L. Nitric oxide for preventing pre‐eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milne 2005
- Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C, et al. The pre‐eclampsia community guideline (PRECOG): how to screen for and detect onset of pre‐eclampsia in the community. BMJ 2005;330(7491):576‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
MMWR 2003
- Chang J, Elam‐Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, et al. Pregnancy‐related mortality surveillance‐‐United States, 1991‐1999. MMWR Surveillance Summaries 2003;52(2):1‐8. [PubMed] [Google Scholar]
Neilson 1995
- Neilson JP. Magnesium sulphate: the drug of choice in eclampsia. BMJ (Clinical research ed.) 1995;311(7007):702‐3. [PUBMED: 7549676] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHBPEP 2000
- Anonymous. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics and Gynecology 2000;183(1):S1‐S22. [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Antenatal Care. London: NICE, 2008. [Google Scholar]
Onuh 2004
- Onuh SO, Aisien AO. Maternal and fetal outcome in eclamptic patients in Benin City, Nigeria. Journal of Obstetrics and Gynaecology 2004;24(7):765‐8. [DOI] [PubMed] [Google Scholar]
Pritchard 1955
- Pritchard JA. The use of the magnesium ion in the management of eclamptogenic toxemias. Surgery, Gynecology & Obstetrics 1955;100(2):131‐40. [PUBMED: 13238166] [PubMed] [Google Scholar]
RCOG 2006
- Tuffnell DJ, Shennan AH, Waugh JJS, Walker JJ, on behalf of the Royal College of Obstetricians and Gynaecologists. The management of severe pre‐eclampsia/eclampsia (Green‐top guideline No.10A). London: RCOG, 2006. [Google Scholar]
Redman 1984
- Redman CWG. Hypertension in Pregnancy. In: Swiet M editor(s). Medical disorders in obstetric practice. Oxford: Blackwell Scientific, 1984:141‐191. [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roberts 2002a
- Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre‐eclampsia. Placenta 2002;23(5):359‐72. [DOI] [PubMed] [Google Scholar]
Roberts 2002b
- Roberts JM, Villar J, Arulkumaran S. Preventing and treating eclamptic seizures. BMJ (Clinical research ed.) 2002;325(7365):609‐10. [PUBMED: 12242159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2005
- Roberts CL, Algert CS, Morris JM, Ford JB, Henderson‐Smart DJ. Hypertensive disorders in pregnancy: a population‐based study. Medical Journal of Australia 2005;182(7):332‐5. [DOI] [PubMed] [Google Scholar]
Robson 1996
- Robson SC. Magnesium sulphate: the time of reckoning. British Journal of Obstetrics and Gynaecology 1996;103(2):99‐102. [PUBMED: 8616153] [DOI] [PubMed] [Google Scholar]
Rumbold 2008
- Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004227.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadeh 1989
- Sadeh M. Action of magnesium sulfate in the treatment of preeclampsia‐eclampsia. Stroke 1989;20(9):1273‐5. [PUBMED: 2672428] [DOI] [PubMed] [Google Scholar]
Schutte 2009
- Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, Boer K, Pel M, et al. Rise in maternal mortality in the Netherlands. BJOG: an international journal of obstetrics and gynaecology 2009;117(4):399‐406. [DOI] [PubMed] [Google Scholar]
Sevene 2005
- Sevene E, Lewin S, Mariano A, Woelk G, Oxman AD, Matinhure S, et al. System and market failures: the unavailability of magnesium sulphate for the treatment of eclampsia and pre‐eclampsia in Mozambique and Zimbabwe. BMJ 2005;331:765‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheth 2002
- Sheth SS, Chalmers I. Magnesium for preventing and treating eclampsia: time for international action. Lancet 2002;359(9321):1872‐3. [PUBMED: 12057545] [DOI] [PubMed] [Google Scholar]
Sibai 2005
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstetrics & Gynecology 2005;105(2):402‐10. [DOI] [PubMed] [Google Scholar]
Villar 2003
- Villar J, Say L, Gülmezoglu M, Merialdi M, Lindheimer MD, Bétran AP, et al. Eclampsia and pre‐eclampsia: a health problem for 2000 years. In: Critchley HOD, MacLean AB, Poston L, Walker JJ editor(s). Pre‐eclampsia. London: RCOG Press, 2003:189‐207. [Google Scholar]
WHO 1988
- World Health Organization. Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. American Journal of Obstetrics and Gynecology 1988;158(1):80‐3. [PubMed] [Google Scholar]
WHO 2010
- World Health Organization, UNICEF, ENFPA, The World Bank. Trends in maternal mortality: 1990 to 2008. Estimates developed by WHO, UNICEF, UNFPA and The World Bank. Geneva: World Health Organization, 2010. [Google Scholar]
Zuspan 1978
- Zuspan FP. Problems encountered in the treatment of pregnancy‐induced hypertension. A point of view. American Journal of Obstetrics and Gynecology 1978;131(6):591‐7. [PUBMED: 686045] [DOI] [PubMed] [Google Scholar]


