Abstract
Background
Some breastfed infants with atopic eczema benefit from elimination of cow milk, egg, or other antigens from their mother's diet. Maternal dietary antigens are also known to cross the placenta.
Objectives
To assess the effects of prescribing an antigen avoidance diet during pregnancy or lactation, or both, on maternal and infant nutrition and on the prevention or treatment of atopic disease in the child.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (6 July 2012).
Selection criteria
All randomized or quasi‐randomized comparisons of maternal dietary antigen avoidance prescribed to pregnant or lactating women. We excluded trials of multimodal interventions that included manipulation of the infant's diet other than breast milk or of non‐dietary aspects of the infant's environment.
Data collection and analysis
We extracted data from published reports, supplemented by additional information received from the trialists we contacted.
Main results
The evidence from five trials, involving 952 participants, does not suggest a protective effect of maternal dietary antigen avoidance during pregnancy on the incidence of atopic eczema during the first 18 months of life. Data on allergic rhinitis or conjunctivitis, or both, and urticaria are limited to a single trial each and are insufficient to draw meaningful inferences. Longer‐term atopic outcomes have not been reported. The restricted diet during pregnancy was associated with a slightly but statistically significantly lower mean gestational weight gain, a non‐significantly higher risk of preterm birth, and a non‐significant reduction in mean birthweight.
The evidence from two trials, involving 523 participants, did not observe a significant protective effect of maternal antigen avoidance during lactation on the incidence of atopic eczema during the first 18 months or on positive skin‐prick tests to cow milk, egg, or peanut antigen at one, two, or seven years.
One crossover trial involving 17 lactating mothers of infants with established atopic eczema found that maternal dietary antigen avoidance was associated with a non‐significant reduction in eczema severity.
Authors' conclusions
Prescription of an antigen avoidance diet to a high‐risk woman during pregnancy is unlikely to reduce substantially her child's risk of atopic diseases, and such a diet may adversely affect maternal or fetal nutrition, or both. Prescription of an antigen avoidance diet to a high‐risk woman during lactation may reduce her child's risk of developing atopic eczema, but better trials are needed.
Dietary antigen avoidance by lactating mothers of infants with atopic eczema may reduce the severity of the eczema, but larger trials are needed.
Keywords: Female; Humans; Infant; Infant, Newborn; Pregnancy; Diet, Protein‐Restricted; Lactation; Allergens; Allergens/adverse effects; Dermatitis, Atopic; Dermatitis, Atopic/prevention & control; Dietary Proteins; Dietary Proteins/adverse effects; Food Hypersensitivity; Hypersensitivity, Immediate; Hypersensitivity, Immediate/prevention & control; Randomized Controlled Trials as Topic; Risk Factors
Plain language summary
Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child
Evidence is inadequate to advise women to avoid specific foods during pregnancy or breastfeeding to protect their children from allergic diseases like eczema and asthma.
We included five trials, involving 952 participants. Trials of mothers' avoidance of milk, eggs, and other potentially 'antigenic' foods during pregnancy or breastfeeding, or both, provide inadequate evidence about whether such avoidance helps prevent atopic eczema or asthma in the child. Women who avoided eating these foods gained significantly less weight during pregnancy in the one trial reporting on this outcome, raising the possibility of adverse nutritional effects on the mother or fetus. Finally, one small trial reported an inconclusive response of breastfed infants with atopic eczema when their mothers avoided consumption of cow milk and egg.
Background
Atopic (allergic) diseases such as eczema, asthma, allergic rhinitis or conjunctivitis, or both, (hay fever), and urticaria (hives) are known to have a strong genetic component, but the increased incidence and severity of asthma and perhaps of other atopic diseases over the last few decades (Upton 2000; Williams 1992; Woolcock 1997) underlines the etiologic importance of environmental factors. Maternal dietary antigens are known to cross the placenta (Loibichler 2002) and pass into breast milk (Cant 1985; Jakobsson 1985; Shannon 1921; Stuart 1984; Troncone 1987; Vadas 2001). A recent study, however, found that inhalant and food antigen‐specific IgE in cord blood reflected maternal IgE but was no longer present in the infant's blood at six months of age, suggesting that the infant is not sensitized to those antigens through in utero exposure (Bonnelykke 2008). Some cases of atopic eczema in breastfed infants have been reported to improve following elimination of offending antigens ingested by the mother during lactation (Gerrard 1979; O'Keefe 1920; Talbot 1918). The diagnosis of allergic diseases is somewhat subjective, and the clinical course of these diseases is highly variable. (Although positive skin prick tests to specific antigens indicate that the subject is allergic to those antigens and suggest a general atopic diathesis, persons with allergic diseases do not always react with positive skin tests, and many persons with positive skin tests do not exhibit signs or symptoms of these diseases.) Methodologically rigorous trials are therefore necessary to establish the efficacy of maternal dietary antigen avoidance during pregnancy or lactation, or both (Zeiger 2003).
Objectives
To assess the effects of prescribing an antigen avoidance diet during pregnancy or lactation on the nutritional status of the mother and newborn and on the development of atopic disease in the child; positive skin prick tests to dietary antigens; and cord blood levels of IgE (a predictor of subsequent atopic disease). The main focus is on women at high risk for giving birth to an atopic child, based on a history of atopic disease in the mother, father, or a previous child.
The review also assesses the effects of prescribing an antigen avoidance diet to lactating mothers of infants with established atopic eczema on the severity of the eczema.
Methods
Criteria for considering studies for this review
Types of studies
All acceptably controlled (randomized or quasi‐randomized) comparisons of maternal dietary antigen avoidance prescribed to pregnant (at any time during pregnancy) or lactating women at high risk, regardless of degree (number of foods eliminated from the diet) or duration. We have included trials comparing groups of women randomized to diets containing different levels of potential antigens, even if the experimental treatment involved only a reduction in antigen exposure, rather than total avoidance. We have also included data on breastfed infants in trials in which the maternal antigen avoidance began during pregnancy and continued into the lactation period. We have excluded, however, trials of multimodal interventions that include, in addition to maternal dietary antigen avoidance, manipulation of the infant's diet other than breast milk or of other non‐dietary aspects of the infant's environment (i.e., exposure to inhaled allergens).
Finally, we have also included all acceptably controlled (randomized or quasi‐randomized) comparisons of maternal antigen avoidance prescribed to lactating mothers of infants with atopic eczema, regardless of degree or duration.
Types of participants
Pregnant or lactating women at high risk for giving birth to an atopic child, based on a history of atopic disease (eczema, asthma, or hay fever) in the mother, father, or a previous child.
Lactating mothers of infants with established atopic eczema.
Types of interventions
Prescription of diet with exclusion (or reduced quantity) of potentially antigenic foods such as cow milk, egg, peanuts, fish, and chocolate.
Types of outcome measures
Primary outcomes
Occurrence and severity of atopic disease in the child.
Secondary outcomes
Nutritional status of mother (gestational weight gain) and fetus (birthweight); other pregnancy outcomes (e.g., preterm birth); positive skin prick tests to ingested antigen (especially egg and milk); and cord blood IgE levels.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (6 July 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 1
For this update we used the following methods when assessing the trials identified by the updated search:
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we would have consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we would have consulted a third person. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or we would have involved a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we would re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it was likely to impact on the findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Dealing with missing data
In future updates, for included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We would have regarded heterogeneity as substantial if I2 had been greater than 30% and either T2 was greater than zero, or there was a low P‐value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there had been 10 or more studies in the meta‐analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually, and used formal tests for funnel plot asymmetry. For continuous outcomes we would have used the test proposed by Egger 1997, and for dichotomous outcomes we would have used the test proposed by Harbord 2006. If asymmetry had been detected in any of these tests or was suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we would have used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would be treated as the average range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we would not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity in the future, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
Sensitivity analysis
None undertaken.
Results
Description of studies
We identified 12 trials.
Included studies
We included five trials (Appelt 2004; Cant 1986; Falth‐Magnusson 1987; Lilja 1988; Lovegrove 1994), involving 925 participants. For details, seeCharacteristics of included studies.
Excluded studies
We excluded seven trials (Arshad 1992; Arshad 2007; Chandra 1986; Chandra 1989; Hattevig 1989; Vance 2004; Zeiger 1989). For details, seeCharacteristics of excluded studies.
We included two of the excluded studies in the original version of this review (Chandra 1986; Chandra 1989) but subsequently excluded them because investigations by Chandra's university (Memorial University of Newfoundland) and by the Canadian Broadcasting Corporation strongly suggest that the published data may have been fabricated. The exclusion of these trials does not change the conclusions of the review.
Risk of bias in included studies
The two included trials on antigen avoidance diet during pregnancy are of uneven methodological quality. Only Falth‐Magnusson 1987 provided details of the randomization procedure, and neither based its primary analysis on 'intention to treat', i.e., mothers who failed to comply with the antigen avoidance diet were not analyzed as part of their allocated group. In both trials, examinations of children at age 18 months were performed by physicians blind to treatment allocation. We excluded the five‐year follow up of the trial by Falth‐Magnusson 1992 from analysis because the results are based on the diets actually followed, rather than those allocated by randomization.
Of the two trials of dietary antigen avoidance during lactation, Lovegrove 1994 has methodologic shortcomings: details of the randomization procedure were not provided, and analysis was not based on 'intention to treat' (i.e., mothers who failed to comply with the antigen avoidance diet were not analyzed as part of their allocated group). Data were provided, however, on compliance with the experimental or control diet, and the physicians who examined study children for evidence for atopic disease were masked, although mothers may have unblinded them. Risk of bias in Appelt 2004 is difficult to judge, because the information available is based solely on a published abstract that provides no details on the method of randomization or on losses to follow‐up between randomization and one‐year follow‐up.
Although two separate trials on antigen avoidance diet for lactating mothers of infants with established atopic eczema are included in Cant 1986, only the first was sufficiently well controlled to warrant inclusion in this review; it used a double‐blind, randomized crossover design and is of acceptable quality. (The second trial was an unblinded, non‐randomized crossover trial.) For the first (included) trial, the results were analyzed appropriately in the original publication using a paired t‐test, but the data presented in the tables in this review are group means at the end of the exclusion (intervention) diet and common standard deviations (i.e., for all study subjects) at the end of the trial after four weeks back on the pre‐trial diet.
Effects of interventions
Two trials, involving 334 pregnant women, were included in the review of dietary antigen avoidance during pregnancy (Falth‐Magnusson 1987; Lilja 1988). The combined evidence from the trials does not suggest a strong protective effect of maternal antigen avoidance on the incidence of atopic eczema (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.57 to 1.79) or asthma (RR 2.22, 95% CI 0.39 to 12.67) during the first 18 months of life. Data on allergic rhinitis or conjunctivitis, or both, and urticaria are limited to a single trial each and are insufficient to draw meaningful inferences. Both trials suggest a lower incidence of positive skin prick tests to egg antigen at six months of age, but the effect was no longer evident at 18 months, nor was any benefit apparent at either age for skin prick tests to milk antigen. In the Lilja 1988 trial, cord blood IgE levels were, if anything, higher in the experimental (antigen avoidance) group. Longer‐term atopic outcomes have not been reported. Based on the single trial (Falth‐Magnusson 1987) providing pertinent data, the restricted diet during pregnancy was associated with a small but statistically significant lower mean gestational weight gain (mean difference (MD) ‐3.00, 95% CI ‐5.21 to ‐0.79) percentage of pre‐pregnancy weight, i.e., 1.8 kg for a 60‐kg woman). The pooled results of two trials (Falth‐Magnusson 1987; Lovegrove 1994, the latter including an intervention that continued into lactation) suggest that maternal antigen avoidance may be associated with a higher (but statistically highly unstable) risk of preterm birth (RR 10.06, 95% CI 0.53 to 192.26) and a possible adverse effect on mean birthweight (MD ‐83.45, 95% CI ‐221.87 to 54.97).
One trial of antigen avoidance during lactation reporting on only 26 lactating women (Lovegrove 1994) found no significant protective effect of maternal antigen avoidance on the incidence of atopic eczema during the child's first 18 months of life (RR 0.73, 95% CI 0.32 to 1.64). The two excluded Chandra trials (Chandra 1986; Chandra 1989) both contributed data to this comparison in the original versions of this review. A larger included trial (Appelt 2004) did not report on atopic eczema or other allergic disease outcomes, but found no evidence of sensitization to milk, egg, or peanut antigen on skin‐prick testing at one, two, or seven years of age.
One small crossover trial (Cant 1986) involving 17 lactating mothers found that dietary antigen avoidance by mothers of infants with established atopic eczema was associated with a non‐significant reduction in eczema severity.
Discussion
Prescription of an antigen avoidance diet to a high‐risk woman during pregnancy or lactation is unlikely to reduce substantially her risk of giving birth to an atopic child. More data are necessary on potential adverse effects of maternal antigen avoidance during pregnancy on gestational weight gain, fetal growth, and preterm birth.
The unimpressive results of the single trial assessing restricted diet for lactating mothers of infants with eczema should be interpreted with caution because of its small size (n = 17) and because the trial compared exposure to cow milk and egg with exposure to soya milk (soya can itself be allergenic). Future trials should be larger and should ensure adequate antigen restriction of the maternal diet.
Authors' conclusions
Implications for practice.
Prescription of an antigen avoidance diet to a high‐risk woman during pregnancy or lactation is unlikely to reduce substantially her risk of giving birth to an atopic child and may have an adverse effect on maternal or fetal nutritional status, or both. The original version of this review suggested a possible benefit of antigen avoidance during lactation, but after exclusion of the two trials with suspected data fabrication, the evidence no longer suggests a beneficial effect.
As stated by the authors of the single trial of antigen avoidance in mothers of infants with atopic eczema, "most breastfed babies with eczema do not respond to maternal dietary manipulation." Even if this is so, maternal reports of changes in the severity of their breastfed infants' eczema following ingestion of certain foods should not be summarily dismissed. Rather, such reports should be pursued by performing multiple (preferably double‐blinded) challenges and dechallenges with the suspected foods.
Implications for research.
Future trials on the effect of maternal dietary antigen avoidance during pregnancy will require much larger sample sizes to detect more modest reductions in risk of atopic disease in infants and children, and longer follow‐up will be necessary to detect potential benefits on other atopic outcomes (i.e., allergic rhinitis and conjunctivitis and asthma). More data are required on potential adverse effects of maternal antigen avoidance on gestational weight gain, fetal growth, and preterm birth.
As to the effects of maternal dietary antigen avoidance during lactation, better and larger trials are required, specifically with respect to allergic disease outcomes.
Finally, since adherence to an antigen avoidance diet during pregnancy and/or lactation requires considerable effort, more information is necessary on women's experience and compliance with such diets.
Future trials on the effects of antigen avoidance diet for lactating mothers of infants with established eczema should be larger and should ensure adequate antigen restriction of the maternal diet.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2012 | New citation required but conclusions have not changed | Review updated with a new search. |
| 6 July 2012 | New search has been performed | Search updated. No new trial reports identified. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 30 April 2011 | New search has been performed | Search updated. Three new trials identified; one included (Appelt 2004) and two excluded (Arshad 2007; Vance 2004). |
| 17 September 2008 | Amended | Converted to new review format. |
| 29 April 2006 | New search has been performed | Search updated. Two new trial reports added to 'Studies awaiting assessment.' One new report added to Falth‐Magnusson 1987 (included study) and another added to Arshad 1992 (excluded). |
| 29 April 2006 | New citation required but conclusions have not changed | We have excluded two trials by Chandra et al (Chandra 1986; Chandra 1989) included in previous versions of this review because of evidence that the published data may have been fabricated. The exclusion of these trials does not change the conclusions of the review. However, the earlier version of this review suggested that there may be a possibility of benefit with antigen avoidance during lactation. In this update, with the exclusion of the two trials reporting data of uncertain veracity, the evidence is insufficient to infer that antigen avoidance during lactation is beneficial or harmful. |
| 1 October 2002 | New search has been performed | This updated review combines and replaces three previously published Cochrane reviews: (1) 'Maternal antigen avoidance during pregnancy and/or lactation for preventing or treating atopic disease in the child' (CDSR 2003a); (2) 'Maternal antigen avoidance during lactation for preventing atopic eczema in infants' (Kramer 1996a); (3) 'Maternal antigen avoidance during lactation for preventing atopic disease in infants of women at high risk' (Kramer 1996b). This combination was suggested by colleagues in the field, by the PCG editors, and by the Cochrane Pregnancy and Childbirth Group's Consumer Panel. |
Acknowledgements
None.
Appendices
Appendix 1. Methods used in previous version
Two review authors independently evaluated trials under consideration for methodological quality and appropriateness for inclusion, without consideration of their results. We processed included trial data as described in the Cochrane Reviewers' Handbook (Clarke 2003).
We have not included Appelt 2004 and Lovegrove 1994 in the comparison of atopic outcomes for dietary antigen avoidance during pregnancy, because the intervention continued into lactation.
We have excluded from this update two trials by Chandra et al (Chandra 1986; Chandra 1989) included in the original versions of this review because of evidence that the published data may have been fabricated.
Because of the small sample sizes of trials and large differences in the control rates of atopic outcomes, all pooled effect estimates are derived from random‐effects models even in the absence of statistically significant heterogeneity in the results of the pooled trials.
Data and analyses
Comparison 1. Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Atopic eczema in first 12‐18 months | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.79] |
| 2 Asthma in first 18 months | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [0.39, 12.67] |
| 3 Allergic rhinitis/conjunctivitis in first 18 months | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Allergic urticaria in first 18 months | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.21, 4.87] |
| 5 Any atopic condition in first 18 months | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.38] |
| 6 Positive skin prick test to egg at 6 months | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.32, 1.05] |
| 7 Positive skin prick test to egg at 18 months | 2 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.74] |
| 8 Positive skin prick test to milk at 6 months | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.29, 4.51] |
| 9 Positive skin prick test to milk at 18 months | 2 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.16, 4.59] |
| 10 Detectable cord blood IgE (>= .125 U/ml) | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.94, 1.44] |
| 11 Cord blood IgE > 1 U/ml | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.00, 7.90] |
| 12 Preterm birth | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 10.06 [0.53, 192.26] |
| 13 Gestational weight gain (as % of prepregnancy weight) | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐5.21, ‐0.79] |
| 14 Birthweight (g) | 2 | 236 | Mean Difference (IV, Random, 95% CI) | ‐83.45 [‐221.87, 54.97] |
1.1. Analysis.
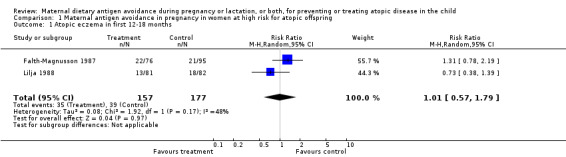
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 1 Atopic eczema in first 12‐18 months.
1.2. Analysis.
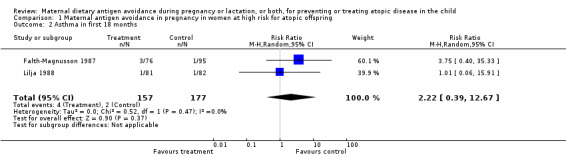
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 2 Asthma in first 18 months.
1.3. Analysis.
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 3 Allergic rhinitis/conjunctivitis in first 18 months.
1.4. Analysis.
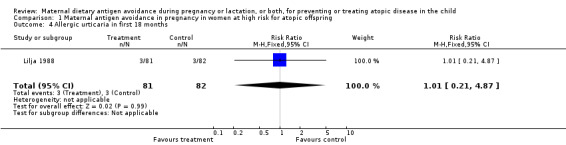
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 4 Allergic urticaria in first 18 months.
1.5. Analysis.
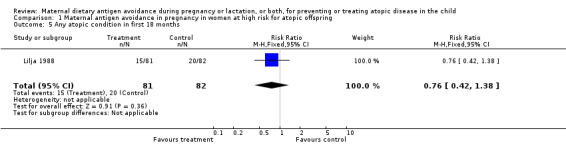
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 5 Any atopic condition in first 18 months.
1.6. Analysis.
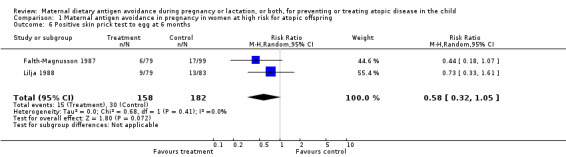
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 6 Positive skin prick test to egg at 6 months.
1.7. Analysis.
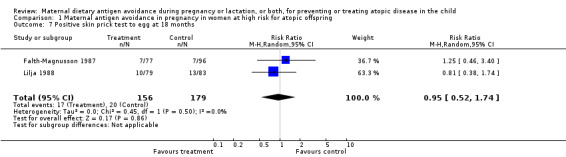
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 7 Positive skin prick test to egg at 18 months.
1.8. Analysis.
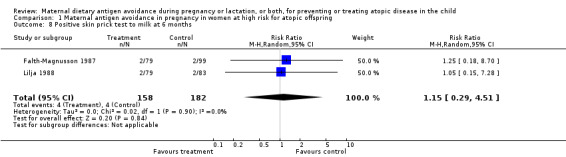
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 8 Positive skin prick test to milk at 6 months.
1.9. Analysis.
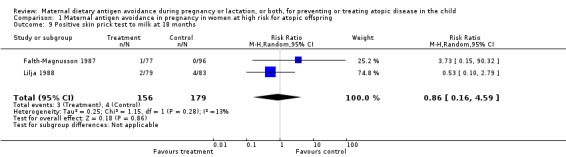
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 9 Positive skin prick test to milk at 18 months.
1.10. Analysis.
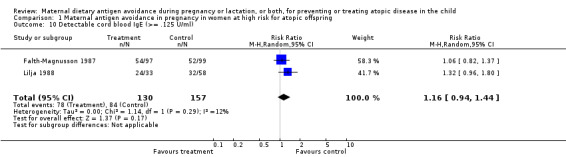
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 10 Detectable cord blood IgE (>= .125 U/ml).
1.11. Analysis.
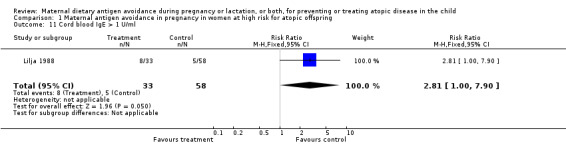
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 11 Cord blood IgE > 1 U/ml.
1.12. Analysis.

Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 12 Preterm birth.
1.13. Analysis.
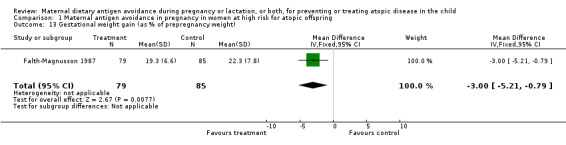
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 13 Gestational weight gain (as % of prepregnancy weight).
1.14. Analysis.
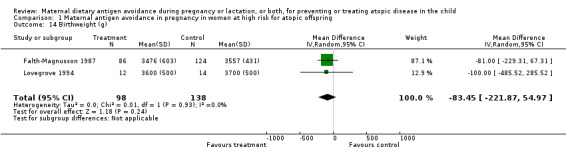
Comparison 1 Maternal antigen avoidance in pregnancy in women at high risk for atopic offspring, Outcome 14 Birthweight (g).
Comparison 2. Maternal antigen avoidance during lactation in women at high risk for atopic offspring.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of atopic eczema in first 18 months | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.32, 1.64] |
| 2 Positive skin prick test to milk at 1 year | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.68, 3.01] |
| 3 Positive skin prick test to egg at 1 year | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.88, 1.94] |
| 4 Positive skin prick test to peanut at 1 year | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.60] |
| 5 Positive skin prick test to milk at 2 years | 1 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.94, 19.67] |
| 6 Positive skin prick test to egg at 2 years | 1 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.03, 3.53] |
| 7 Positive skin prick test to peanut at 2 years | 1 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.80] |
| 8 Positive skin prick test to milk at 7 years | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.41] |
| 9 Positive skin prick test to egg at 7 years | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.25] |
| 10 Positive skin prick test to peanut at 7 years | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.81, 3.15] |
2.1. Analysis.
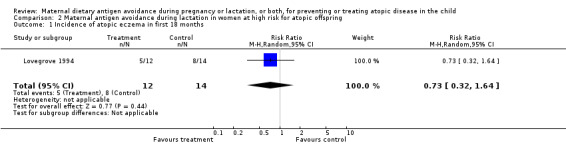
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 1 Incidence of atopic eczema in first 18 months.
2.2. Analysis.
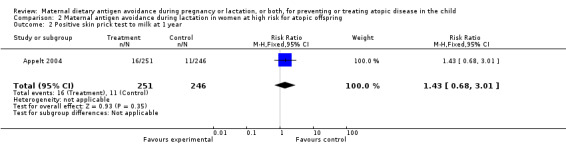
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 2 Positive skin prick test to milk at 1 year.
2.3. Analysis.
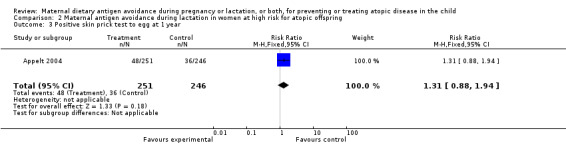
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 3 Positive skin prick test to egg at 1 year.
2.4. Analysis.
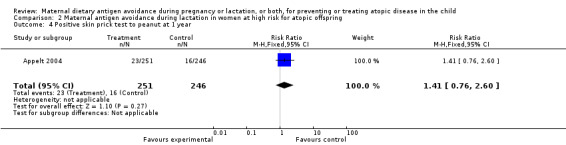
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 4 Positive skin prick test to peanut at 1 year.
2.5. Analysis.
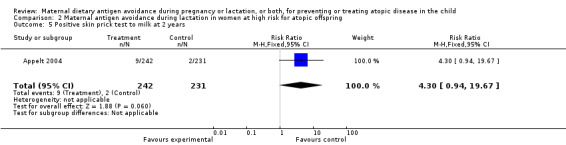
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 5 Positive skin prick test to milk at 2 years.
2.6. Analysis.
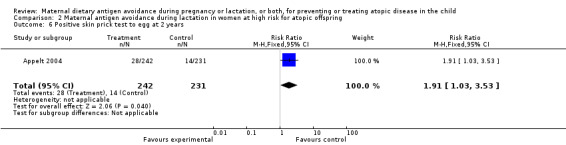
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 6 Positive skin prick test to egg at 2 years.
2.7. Analysis.
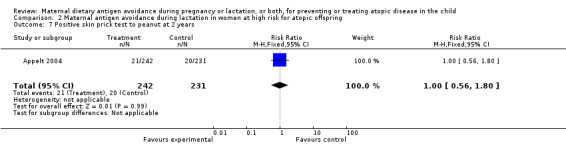
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 7 Positive skin prick test to peanut at 2 years.
2.8. Analysis.
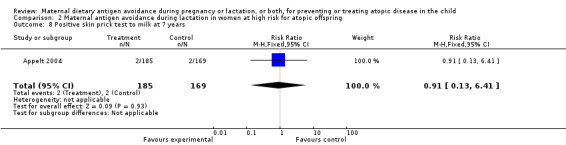
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 8 Positive skin prick test to milk at 7 years.
2.9. Analysis.
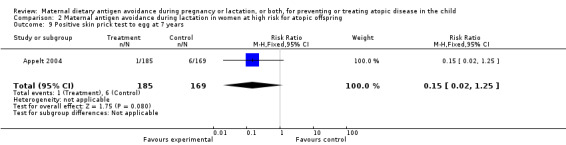
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 9 Positive skin prick test to egg at 7 years.
2.10. Analysis.
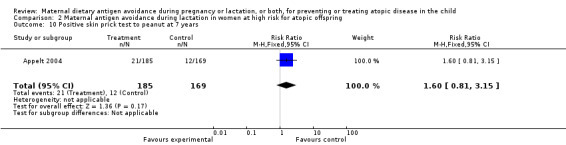
Comparison 2 Maternal antigen avoidance during lactation in women at high risk for atopic offspring, Outcome 10 Positive skin prick test to peanut at 7 years.
Comparison 3. Maternal antigen avoidance during lactation for treating atopic eczema in infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eczema area score | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐4.43, 2.83] |
| 2 Eczema activity score | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐7.18, 4.38] |
3.1. Analysis.
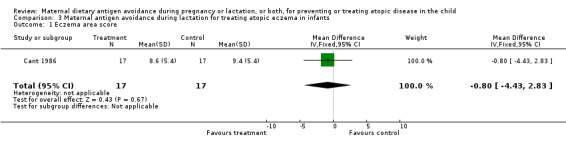
Comparison 3 Maternal antigen avoidance during lactation for treating atopic eczema in infants, Outcome 1 Eczema area score.
3.2. Analysis.
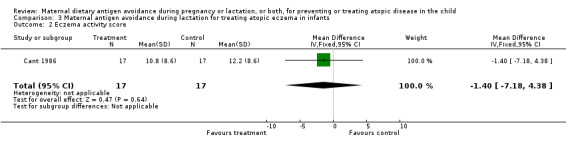
Comparison 3 Maternal antigen avoidance during lactation for treating atopic eczema in infants, Outcome 2 Eczema activity score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Appelt 2004.
| Methods | Randomization method not described. | |
| Participants | 497 Canadian mothers of infants from high‐risk families, defined as having at least 1 first‐degree relative with asthma or 2 with a history of other IgE‐mediated allergy. | |
| Interventions | Experimental: complete avoidance of peanut, nuts, and fish and decreased intake of milk and eggs during the third trimester of pregnancy and while breastfeeding for up to 1 year. A partially hydrolyzed formula supplement was provided for the first year, if required. Control: usual care and diet. |
|
| Outcomes | Skin‐prick testing for hypersensitivity to cow milk, egg, and peanut antigen at 1, 2, and 7 years. | |
| Notes | Based on published abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Published as abstract only; no details provided on randomization. |
Cant 1986.
| Methods | Randomized crossover trial based on random numbers table, but details of allocation procedure not reported. | |
| Participants | 19 US mothers of infants 6 weeks to 6 months of age with atopic eczema. | |
| Interventions | Experimental: maternal exclusion of cow milk, egg, chocolate, wheat, nuts, fish, beef, chicken, citrus fruits, colorings, and preservatives, with use of soya‐based milk substitute for 4 weeks. Control: same dietary exclusions for same duration (4 weeks), but substitute contained cow milk and egg. Each of the 2 interventions allocated to all subjects in randomized order. | |
| Outcomes | 2, continuous‐scale eczema severity scores evaluated after 4 weeks on each diet: area (number of involved body areas from 0 to 20) and "activity" (severity within each area on 0‐3 scale X number of involved areas). | |
| Notes | 1) 2 poor compliers excluded. 2) No data presented on compliance in the 17 women not excluded. 3) Results in original publication analyzed appropriately for crossover trial; data tables in this review contain group means after exclusion diet (intervention vs control) and common SD for each outcome at the end of the trial. 4) A second, non‐randomized crossover trial reported in the same publication has been excluded from this review, because the sequential treatments were used in the same order in all subjects (i.e., not randomized). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
Falth‐Magnusson 1987.
| Methods | Randomization based on random numbers assigned in advance, allocation contained in sealed envelope opened only after consent was obtained. | |
| Participants | 212 pregnant Swedish women with positive family history of allergy (herself, husband, or previous children). | |
| Interventions | Experimental: cow milk and egg avoidance diet from 28 weeks of gestation. Control: normal diet. | |
| Outcomes | Gestational weight gain, cord blood IgE, atopic eczema, asthma, birthweight, and preterm birth. | |
| Notes | 1) Birthweight, 5‐year atopic outcomes analyzed by compliance with diet, not by randomized allocation group. 2) SDs for cord blood and maternal IgEs do not add up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Lilja 1988.
| Methods | Cluster randomization used in Stockholm/Uppsala, but randomization method not described. | |
| Participants | 171 pregnant women in Stockholm/Uppsala with a history of respiratory allergy to pollen and/or dander. 84 pregnant women from Linkoping are also mentioned in the 1988 report, but these appear to be a subset of the women reported in Falth‐Magnusson 1987. | |
| Interventions | Experimental: low‐milk and low‐egg diet during third trimester. Control: high‐milk and high‐egg diet during third trimester. | |
| Outcomes | Cord blood IgE, total atopy, asthma, atopic eczema, allergic rhinitis, and urticaria. | |
| Notes | 1) Analysis based on individual subjects despite cluster randomization. 2) Small number of non‐compliers not analyzed. 3) Large differences at baseline in type of allergy in both trials raises question about randomization. 4) Cord blood IgE levels not tabulated; graph only. 5) Cord blood IgE sample sizes do not add up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. |
Lovegrove 1994.
| Methods | Randomization method not described. | |
| Participants | Pregnant English women with atopic histories in themselves or in partners, recruited at 30 weeks' gestation. | |
| Interventions | Experimental: milk‐free diet from 36 weeks' gestation and during lactation. Control: unrestricted diet. | |
| Outcomes | Atopic eczema at 6, 12, and 18 months, preterm birth, and birthweight. Atopic outcomes restricted to comparison of antigen avoidance during lactation, while birth outcomes (preterm birth and birthweight) restricted to comparison of antigen avoidance during pregnancy. | |
| Notes | 1) Number of women randomized not reported, but 44 original participants included non‐atopic women. 2) 6 of 44 total participants (including non‐atopic, non‐randomized) not included in analysis. 3) Non‐blind mothers may have unblinded examining physicians. 4) Funded by manufacturer of hypoallergenic formula. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. |
SD: standard deviation vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arshad 1992 | Experimental intervention included dietary antigen avoidance in infant's diet, as well as the mother's, and also included application of acaricidal powder to reduce exposure to inhaled dust mite antigen. |
| Arshad 2007 | Maternal antigen avoidance during lactation included, in the experimental group, a subgroup of breastfed that was combined with (and inseparable from) a subgroup of non‐breastfed infants fed hydrolyzed formula, and both subgroups also received interventions to reduce exposure to house dust mite: acaricide treatment of upholstery and carpets and plastic mattress covers. |
| Chandra 1986 | Included in previous versions of this review. Now excluded because investigations by Chandra's university (Memorial University of Newfoundland) and by the Canadian Broadcasting Corporation strongly suggest that the published data may have been fabricated. |
| Chandra 1989 | Included in previous versions of this review. Now excluded because investigations by Chandra's university (Memorial University of Newfoundland) and by the Canadian Broadcasting Corporation strongly suggest that the published data may have been fabricated. |
| Hattevig 1989 | Experimental and control interventions allocated by site (2 different hospital clinics), not randomized or quasi‐randomized. |
| Vance 2004 | Offspring atopic outcomes reported only in those with cord serum IgG levels obtained (136 of the 229 randomized), and numbers in each treatment arm not provided. |
| Zeiger 1989 | Experimental intervention included dietary antigen avoidance in infant's diet, as well as the mother's. In addition, the trial suffered from large and grossly unequal losses to follow up due to non‐compliance with dietary restriction. |
Contributions of authors
M Kramer developed the three original separate reviews that have been combined in this updated amalgamation. R Kakuma carried out independent quality rating and data extraction of all included studies and helped revise the Characteristics of included studies table. M Kramer updated the review in April 2011.
Sources of support
Internal sources
McGill University, Canada.
External sources
Canadian Institutes of Health Research, Canada.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Appelt 2004 {published data only}
- Appelt GK, Chan‐Yeung M, Watson WTA, Dimich‐Ward H, Ferguson A, Manfreda J, et al. Breastfeeding and food avoidance are ineffective in preventing sensitization in high risk children. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S99. [Google Scholar]
Cant 1986 {published and unpublished data}
- Cant AJ, Bailes JA, Marsden RA, Hewitt D. Effect of maternal dietary exclusion on breast fed infants with eczema: two controlled studies. BMJ 1986;293:231‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falth‐Magnusson 1987 {published and unpublished data}
- Falth‐Magnusson K, Kjellman NIM. Allergy prevention by maternal elimination diet during late pregnancy ‐ a 5‐year follow‐up of a randomized study. Journal of Allergy and Clinical Immunology 1992;89:709‐13. [DOI] [PubMed] [Google Scholar]
- Falth‐Magnusson K, Kjellman NIM. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy ‐ a randomized study. Journal of Allergy and Clinical Immunology 1987;80:868‐75. [DOI] [PubMed] [Google Scholar]
- Falth‐Magnusson K, Oman H, Kjellman NIM. Maternal abstention from cow milk and egg in allergy risk pregnancies. Allergy 1987;42:64‐73. [DOI] [PubMed] [Google Scholar]
- Kjellman NIM, Bjorksten B, Hattevig G, Falth‐Magnusson K. Natural history of food allergy. Annals of Allergy 1988;61:83‐7. [PubMed] [Google Scholar]
Lilja 1988 {published and unpublished data}
- Lilja G, Dannaeus A, Falth‐Magnusson K, Graff‐Lonnevig V, Johansson SGO, Kjellman NIM, et al. Immune response of the atopic woman and fetus: effects of high‐ and low‐dose food allergen intake during late pregnancy. Clinical Allergy 1988;8:131‐42. [DOI] [PubMed] [Google Scholar]
- Lilja G, Dannaeus A, Foucard T, Graff‐Lonnevig V, Johansson SGO, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of IgE and egg‐ and milk‐specific IgE and IgG antibodies in infants. Clinical and Experimental Allergy 1991;21:195‐202. [DOI] [PubMed] [Google Scholar]
- Lilja G, Dannaeus A, Foucard T, Graff‐Lonnevig V, Johansson SGO, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of atopic disease in infants up to 18 months of age ‐ in‐vivo results. Clinical and Experimental Allergy 1989;19:473‐9. [DOI] [PubMed] [Google Scholar]
Lovegrove 1994 {published data only}
- Lovegrove JA, Hampton SM, Morgan JB. The immunological and long‐term atopic outcome of infants born to women following a milk‐free diet during late pregnancy and lactation: a pilot study. British Journal of Nutrition 1994;71:223‐38. [DOI] [PubMed] [Google Scholar]
- Lovegrove JA, Morgan JB, Hampton SM. Dietary factors influencing levels of food antibodies and antigens in breast milk. Acta Paediatrica 1996;85:778‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arshad 1992 {published data only}
- Arshad SH, Mathews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992;339:1493‐7. [DOI] [PubMed] [Google Scholar]
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51(2):89‐93. [PubMed] [Google Scholar]
Arshad 2007 {published data only}
- Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. Journal of Allergy and Clinical Immunology 2007;119(2):307‐13. [DOI] [PubMed] [Google Scholar]
Chandra 1986 {published data only}
- Chandra RK, Puri S, Suraiya C, Cheema PS. Influence of maternal food antigen avoidance during pregnancy and lactation on incidence of atopic eczema in infants. Clinical Allergy 1986;16:563‐9. [DOI] [PubMed] [Google Scholar]
Chandra 1989 {published data only}
- Chandra RK, Puri S, Hamed A. Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. BMJ 1989;299:228‐30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Hattevig 1989 {published data only}
- Hattevig G, Kjellman B, Sigurs N, Bjorksten B, Kjellman NIM. Effect of maternal avoidance of egg, cow's milk and fish during lactation upon allergic manifestations in infants. Clinical and Experimental Allergy 1989;19:27‐32. [DOI] [PubMed] [Google Scholar]
Vance 2004 {published data only}
- Vance GH, Grimshaw KE, Briggs R, Lewis SA, Mullee MA, Thornton CA, et al. Serum ovalbumin‐specific immunoglobulin g responses during pregnancy reflect maternal intake of dietary egg and relate to the development of allergy in early infancy. Clinical and Experimental Allergy 2004;34:1855‐61. [DOI] [PubMed] [Google Scholar]
- Vance GH, Lewis SA, Grimshaw KE, Wood PJ, Briggs RA, Thornton CA, et al. Exposure of the fetus and infant to hens' egg ovalbumin via the placenta and breast milk in relation to maternal intake of dietary egg. Clinical and Experimental Allergy 2005;35(10):1318‐26. [DOI] [PubMed] [Google Scholar]
Zeiger 1989 {published data only}
- Zeiger RS, Heller S. The development and prediction of atopy in high‐risk children: follow‐up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. Journal of Allergy and Clinical Immunology 1995;95:1179‐90. [DOI] [PubMed] [Google Scholar]
- Zeiger RS, Heller S, Mellon M, O'Connor R, Hamburger RN. Effectiveness of dietary manipulation in the prevention of food allergy in infants. Journal of Allergy and Clinical Immunology 1986;78:224‐38. [DOI] [PubMed] [Google Scholar]
- Zeiger RS, Heller S, Mellon MH, Forsythe AB, O'Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food‐allergen avoidance on development of atopy in early infancy: a randomized study. Journal of Allergy and Clinical Immunology 1989;84:72‐89. [DOI] [PubMed] [Google Scholar]
- Zeiger RS, Heller S, Mellon MH, Halsey JF, Hamburger RN, Sampson HA. Genetic and environmental factors affecting the development of atopy through age 4 in children of atopic parents: a prospective randomized study of food allergen avoidance. Pediatric Allergy and Immunology 1992;3:110‐27. [Google Scholar]
Additional references
Bonnelykke 2008
- Bonnelykke K, Pipper CB, Bisgaard H. Sensitization does not develop in utero. Journal of Allergy and Clinical Immunology 2008;121:646‐51. [DOI] [PubMed] [Google Scholar]
Cant 1985
- Cant AJ, Marsden RA, Kilshaw PJ. Egg and cow's milk hypersensitivity in exclusively breast fed infants with eczema, and detection of egg protein in breast milk. BMJ 1985;291:932‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2003
- Clarke MJ, Oxman AD, editors. Cochrane Reviewers’ Handbook 4.2.0 [updated March 2003]. In: The Cochrane Library, Issue 2, 2003. Oxford: Update Software. Updated quarterly.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falth‐Magnusson 1992
- Falth‐Magnusson K, Kjellman NIM. Allergy prevention by maternal elimination diet during late pregnancy ‐ a 5‐year follow‐up of a randomized study. Journal of Allergy and Clinical Immunology 1992;89:709‐13. [DOI] [PubMed] [Google Scholar]
Gerrard 1979
- Gerrard JW. Allergy in breast fed babies to ingredients of breast milk. Annals of Allergy 1979;42:69‐72. [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jakobsson 1985
- Jakobsson I, Lindberg T, Benediktsson B, Hansson BG. Dietary bovine beta‐lactoglobulin is transferred to human milk. Acta Paediatrica Scandinavica 1985;74:342‐5. [DOI] [PubMed] [Google Scholar]
Loibichler 2002
- Loibichler C, Pichler J, Gerstmayr M, Bohle B, Urbanek R, Szepfalusi Z. Materno‐fetal passage of nutritive and inhalant allergens across placentas of term and pre‐term deliveries perfused in vitro. Clinical and Experimental Allergy 2002;32:1546‐51. [DOI] [PubMed] [Google Scholar]
O'Keefe 1920
- O'Keefe ES. The relation of food to infantile eczema. Boston Medical and Surgical Journal 1920;183:569‐73. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Shannon 1921
- Shannon WR. Demonstration of food proteins in human breast milk by anaphylactic experiments on guinea pigs. American Journal of Diseases of Children 1921;22:223‐31. [Google Scholar]
Stuart 1984
- Stuart CA, Twiselton R, Nicholas MK, Hide DW. Passage of cows' milk protein in breast milk. Clinical Allergy 1984;14(6):533‐5. [PUBMED: 6509768] [DOI] [PubMed] [Google Scholar]
Talbot 1918
- Talbot FB. Eczema in childhood. Medical Clinics of North America 1918;1:985‐96. [Google Scholar]
Troncone 1987
- Troncone R, Scarcella A, Donatiello A, Cannataro P, Tarabuso A, Auricchio S. Passage of gliadin into human breast milk. Acta Paediatrica Scandinavica 1987;76:453‐6. [DOI] [PubMed] [Google Scholar]
Upton 2000
- Upton MN, McConnachie A, McSharry C, Hart CL, Davey Smith D, Gillis CR, et al. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ 2000;321:88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vadas 2001
- Vadas P, Wai Y, Burks W, Perelman B. Detection of peanut antigens in breast milk of lactating women. JAMA 2001;285:1746‐8. [DOI] [PubMed] [Google Scholar]
Williams 1992
- Williams HC. Is the prevalence of atopic dermatitis increasing?. Clinical and Experimental Dermatology 1992;17:385‐91. [DOI] [PubMed] [Google Scholar]
Woolcock 1997
- Woolcock AJ, Peat JK. Evidence for the increase in asthma worldwide. CIBA Foundation Symposium 1997;206:122‐34. [DOI] [PubMed] [Google Scholar]
Zeiger 2003
- Zeiger RS. Food allergen avoidance in the prevention of food allergy in infants and children. Pediatrics 2003;111:1662‐71. [PubMed] [Google Scholar]
References to other published versions of this review
Kramer 1996a
- Kramer MS. Maternal antigen avoidance during lactation for preventing atopic disease in infants of women at high risk. Cochrane Database of Systematic Reviews 1996, Issue 3. [DOI: 10.1002/14651858.CD000132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 1996b
- Kramer MS. Maternal antigen avoidance during lactation for preventing atopic eczema in infants. Cochrane Database of Systematic Reviews 1996, Issue 3. [DOI: 10.1002/14651858.CD000131] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2003
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy and/or lactation for preventing or treating atopic disease in the child. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000133] [DOI] [PubMed] [Google Scholar]


