Abstract
Background
Endometriosis is the presence of endometrial glands or stroma in sites other than the uterine cavity and appears to be an oestrogen‐dependent condition. This dependency has prompted the therapeutic use of ovulation suppression agents in an effort to improve subsequent fertility.
Objectives
To assess the effectiveness of ovulation suppression agents, including danazol, progestins and oral contraceptives, in the treatment of endometriosis‐associated subfertility in improving pregnancy outcomes including live births.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register of trials (February 2009), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2009), MEDLINE (1966 to February 2009), EMBASE (1980 to February 2009), and reference lists of articles.
Selection criteria
Randomised trials comparing an ovulation suppression agent with placebo or no treatment, a suppressive agent with danazol, or a gonadotropin‐releasing hormone analogue (GnRHa) with oral contraception in women with endometriosis.
Data collection and analysis
Two review authors independently extracted data and assessed quality. We contacted study authors for additional information.
Main results
Twenty‐five trials were included. Only two studies reported live births. The odds ratios (OR) for pregnancy following ovulation suppression versus placebo or no treatment was 0.97 (95% confidence interval (CI) 0.68 to 1.34, P = 0.8) for all women randomised, and 1.02 (95% CI 0.70 to 1.52, P = 0.82) for subfertile couples only despite the use of a variety of suppression agents. There was no evidence of benefit from the treatment. The common OR for pregnancy following all agents versus danazol was 1.38 (95% CI 1.05 to 1.82, P = 0.02) for all women randomised, and 1.37 (95% CI 0.94 to 1.99, P = 0.10) for subfertile couples only. When GnRHa and danazol were directly compared, the OR was 1.45 (95% CI 1.08 to 1.95, P = 0.01) for all women randomised, and 1.63 (95% CI 1.12 to 2.37, P = 0.01) for subfertile couples only, in favour of GnRHa. No effect was observed for GnRHa compared with oral contraception (OR 0.93, 95% CI 0.41 to 2.12, P = 0.86 for all women randomised; OR 0.83, 95% CI 0.34 to 2.05, P = 0.69 for subfertile couples only).
Authors' conclusions
There is no evidence of benefit in the use of ovulation suppression in subfertile women with endometriosis who wish to conceive.
Plain language summary
Ovulation suppression for endometriosis
This review of 23 trials involving 3043 women with endometriosis has shown that there no evidence of benefit with the use of ovulation suppression for women with endometriosis and infertility. Endometriosis is caused by the lining of the uterus (endometrium) spreading to a site outside the uterus. It is associated with subfertility and can cause pain during both sexual intercourse and menstruation. The hormone oestrogen stimulates the growth of endometriosis. For many years, the use of drugs such as danazol to stop ovulation and the production of oestrogen has been standard practice in the treatment of pain and subfertility caused by endometriosis. This works well for pain, but does not appear to improve fertility. In fact, as ovulation and periods are stopped for the time of treatment, fertility may be reduced by this approach.
Background
Description of the condition
Endometriosis is determined by the finding of endometrial glands or stroma in sites other than the uterine cavity. The lesions are extremely variable when visualised with the naked eye. They include non‐pigmented blebs or vesicles, flame‐like echymoses, and classical blue‐black powder burn spots. In mildly affected women, the pelvis may initially appear healthy but in severe cases it may be totally disorganised by dense scar tissue formation. The most widely accepted classification of disease severity comes from the American Fertility Society (Am Fertil Soc 1985; Schenken, 1997). Numerical scores are assigned based on visual findings. Interestingly, this is a relatively insensitive tool for prediction but remains useful in describing the extent of visible pathology (Schenken, 1997).
Although the reason why women develop endometriosis is unknown, several theories exist. The most plausible theory is that endometrial fragments expelled into the pelvis by retrograde menstruation (when menstrual blood passes from the uterus through the tubes and into the pelvis) implant in its peritoneal surface (Sampson 1927). Other possibilities include metaplasia of celomic cells (Meyer 1919) spread by the blood or lymph systems, or both (Sampson 1927). More recently the development of endometriosis has been linked to a defect in the immune system (Gleicher 1987). Neither the incidence (annual occurrence) nor the prevalence (proportion of the population affected) of endometriosis is known. Estimates have ranged from 1% to 50% (Schweppe 1988). The minimal standard for diagnosis is the direct visualisation of lesions at the time of laparoscopy or laparotomy (Yuzpe 1986). The invasive nature of the diagnostic test makes population‐based incidence and prevalence studies impossible.
It is widely accepted that endometriosis of sufficient severity to cause pelvic adhesions (AFS Stages III and IV) impairs fertility by interfering with oocyte pick‐up and transport. The association is less clear in Stage I (minimal) and Stage II (mild) endometriosis. Although it has been recognised that endometriosis is more prevalent in nulliparous women, it is unclear if mild endometriosis causes infertility or if it is simply a marker for an underlying pathology which itself reduces fertility.
Description of the intervention
Visible disease generally regresses in response to medical or surgical oophorectomy (removal of the ovary or ovaries). It may recur with oestrogen replacement. Most endometriosis becomes inactive at the time of menopause. This hormonal dependency has prompted widespread use of agents which suppress ovarian activity and therefore stop ovulation. In the 1970s, danazol was identified as such an agent and quickly became a standard treatment for pain and subfertility associated with endometriosis. Other agents such as progestins or oral contraceptives, or both, have also been used.
How the intervention might work
Endometriosis appears to be an oestrogen‐dependent condition. Suppression of ovarian activity may result in the inactivation of endometriosis.
Why it is important to do this review
The current systematic review evaluates the best available data from randomised controlled trials and builds on a previously published overview, which included data from non‐randomised comparative studies (Hughes 1993).
Objectives
To assess the effectiveness of ovulation suppression agents, including danazol, progestins and oral contraceptives, in the treatment of endometriosis‐associated subfertility in improving pregnancy outcomes including live births.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing randomised controlled trials (RCTs) were included if they made the following comparisons for the treatment of endometriosis‐associated subfertility. 1) An ovulation suppression agent with placebo or no treatment. 2) Danazol with another ovulatory suppressive agent; where danazol was prospectively singled out for comparison with other agents because it has been considered the primary choice for medical suppression before the advent of gonadotropin‐releasing hormone analogues (GnRHa). If newer agents were more effective than danazol, this comparison would demonstrate the extent of the improvement. 3) GnRH versus oral contraception. Quasi‐randomised trials were excluded. If crossover design was used, only the first phase or stage would be extracted for analysis.
Types of participants
Women with visually diagnosed endometriosis, either by laparoscopy or laparotomy, who had failed to conceive after 12 or more months of unprotected intercourse. Trials where medical treatment was administered after surgical treatment for endometriosis were included.
Types of interventions
Interventions included danazol, medroxyprogesterone acetate (MPA), gestrinone, combined oral contraceptive pills (COC), GnRH analogues (GnRHa), and placebo. No dose ranges were specified.
Types of outcome measures
Primary outcomes
Live birth per woman randomised (defined as delivery of a live foetus after 20 completed weeks of gestation).
Secondary outcomes
Clinical pregnancy per woman randomised (presence of gestational sac with foetal heart motion confirmed by ultrasound).
Adverse events (including miscarriage, ectopic pregnancy, fetal abnormalities, drug side effects).
Search methods for identification of studies
Electronic searches
We searched for all publications which described (or might describe) randomised controlled trials of ovarian suppression for endometriosis. The original search was performed in 1995 and was updated in February 2009.
1) We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register of trials (February 2009).
2) The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 1) was searched in all fields.
3) MEDLINE (1966 to February 2009) and EMBASE (1980 to February 2009) were searched using Ovid software and the search string set out in Appendix 1.
Searching other resources
We also searched the citation lists of relevant publications, review articles, and included studies.
Data collection and analysis
Selection of studies
Three review authors (EH, DF, JC) undertook the original study selection. The titles and abstracts of articles found in the search were screened by EH and DF, who discarded studies that were clearly ineligible. Three authors (EH, DF, JC) then independently assessed whether the studies met the inclusion criteria, with disagreements resolved by discussion. Further information was sought from the authors if papers contained insufficient information to make a decision about eligibility. An updated search and data identification were conducted by JB in February 2009 and study inclusion and data extraction were duplicated by CF.
Data extraction and management
Two review authors (EH, DF in the original review; JB, CF for updated review) independently performed data extraction. Discrepancies were resolved by discussion. For each included trial, information was collected regarding the location of the study, methods of the study, the participants (age range, eligibility criteria), the nature of the interventions, and data relating to the outcomes specified above. If possible, we sought missing data from the authors.
Assessment of risk of bias in included studies
Two review authors (EH, DF in the original review; JB, CF for updated review) independently assessed the risk of bias of all studies that were deemed eligible for the review. Any discrepancies were resolved by discussion. The included studies were assessed for risk of bias to assess: sequence generation; allocation concealment; blinding of participants, providers, and outcome assessors; completeness of outcome data; selective outcome reporting; and other sources of potential bias (see Risk of bias tables).
Measures of treatment effect
We performed statistical analysis in accordance with the guidelines developed by The Cochrane Collaboration.
For dichotomous data, the number of events in the control and intervention groups of each study were used to calculate Peto odds ratios (OR) and 95% confidence intervals (CI). Heterogeneity (variations) between the results of different studies was examined by inspecting the scatter in the data points on the graphs and the overlap in their CIs and, more formally, by checking the results of the Chi2 test. The I2 statistic for heterogeneity between groups was computed and used. If possible, the outcomes were pooled statistically.
One study compared both danazol and MPA with a control (Telimaa 1988(a); Telimaa 1988(b)). For the purpose of this overview, each treatment arm has been included as a separate 'trial'. However, in order to avoid double counting of control patients in the analysis, the control participants have been divided evenly between the interventions. The design of the study is detailed in the table 'Characteristics of included studies'.
Data were described per woman randomised in the study. However, in some of the studies not all of the women were 'infertile'. Data were therefore reported, if available, for subfertile couples or couples wanting to conceive only, as well as for the whole study population of which a large proportion of women would have been subfertile. Outcome data was only reported prior to additional treatment regimens commencing as this additional intervention may have affected the primary outcome.
Unit of analysis issues
There were no anticipated unit of analysis issues. Data were reported as 'per woman randomised'.
Dealing with missing data
Data were analysed on an intention‐to‐treat basis as far as possible. Where data were missing or unable to be extracted from the paper the primary authors were contacted for clarification.
Assessment of heterogeneity
Where the clinical and methodological characteristics of the included studies were sufficiently similar, meta‐analysis was conducted. Statistical heterogeneity was assessed using the I2 statistic. Where the I2 statistic exceeded 50%, subgroup and sensitivity analyses were conducted to explain the heterogeneity.
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert to duplication of data. Data were available from a number of sources including published and unpublished data. Where 10 or more studies were identified, a funnel plot was used to explore the possibility of small study effects.
Data synthesis
For the following comparisons, the data from primary studies were combined using a fixed‐effect model.
Ovulation suppression versus placebo.
Ovulation suppression versus danazol.
GnRHa versus danazol.
GnRHa versus oral contraceptive pill.
Changes in the odds of live birth and clinical pregnancy were to be displayed graphically in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Where data were available, subgroup analyses were conducted to determine the separate evidence within the subgroups:
1. total population with endometriosis;
2. women with subfertility wanting a pregnancy;
3. agents other than danazol.
Sensitivity analysis
If heterogeneity was high sensitivity analyses were conducted by looking at the quality of studies, where eligibility was restricted to studies without high risk of bias.
Results
Description of studies
Results of the search
Fifty‐six potential studies were identified following the search.
Included studies
Twenty‐five studies were included. These compared ovulatory suppression with placebo or no treatment, or compared two or more suppression agents with each other (ANZ Zoladex 1996; Bayer 1988; Bergqvist 1998; Bianchi 1999; Bromham 1995; Burry 1989; Busacca 2001; Cosson 2002; Dmowski 1989; Fedele 1989a; Fedele 1989b; Fedele 1992; Fraser 1991; Harrison 2000; Henzl 1988; NEET 1992; Noble 1979;Loverro 2008; Parrazzini 1994; Shaw 1992;Shawki 2002; Telimaa 1988(a); Telimaa 1988(b); Thomas 1987; Vercellini 1999) (see the table Characteristics of included studies).
Excluded studies
Thirty‐one potentially relevant studies were excluded because treatment allocation was non‐random or duplicate data from other publications were identified during the data extraction process (see Characteristics of excluded studies table).
Risk of bias in included studies
For further details refer to the Risk of bias tables and Figure 1 and Figure 2.
1.
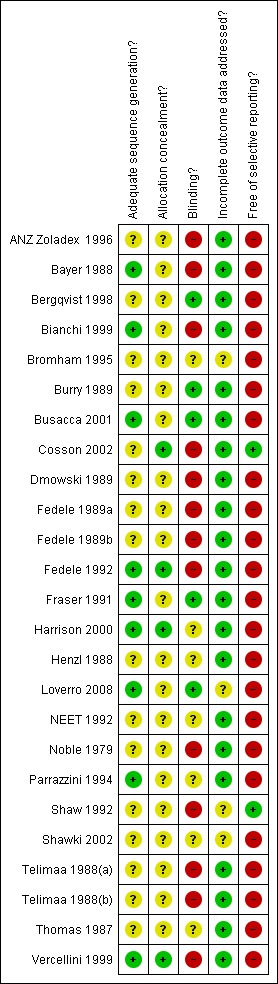
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
2.
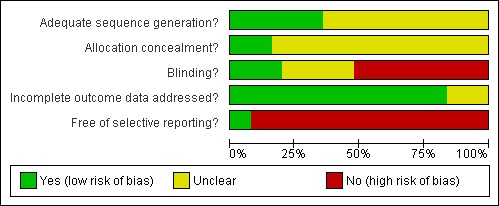
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
Allocation
RCTs comparing danazol, MPA, gestrinone, oral contraceptive (OC), or GNRHa with placebo or no treatment
Twelve RCTs were identified comparing an ovulation suppression agent (danazol, MPA, or gestrinone) with either placebo or no treatment (Bayer 1988; Bergqvist 1998; Bianchi 1999; Busacca 2001; Fedele 1992; Harrison 2000; Loverro 2008; Parrazzini 1994; Telimaa 1988(a); Telimaa 1988(b); Thomas 1987; Vercellini 1999). Of six randomised studies comparing ovulation suppression with expectant management (Bayer 1988; Bianchi 1999; Busacca 2001; Fedele 1992; Loverro 2008; Vercellini 1999) four described the method of randomisation (Bayer 1988; Bianchi 1999; Fedele 1992; Loverro 2008; Vercellini 1999;). Only two trials described the method of concealment, using a centralised third‐party method (Fedele 1992; Vercellini 1999). None of the trials used a crossover design.
RCTs comparing MPA, OC, gestrinone, or GNRHa with danazol
Live birth was reported in only one study (Shaw 1992). Twelve trials compared various suppressive agents with danazol (ANZ Zoladex 1996; Bromham 1995; Burry 1989; Dmowski 1989; Fedele 1989a; Fedele 1989b; Fraser 1991; Henzl 1988; NEET 1992; Noble 1979; Shaw 1992; Telimaa 1988(a)): eight studied GnRHa (ANZ Zoladex 1996; Burry 1989; Dmowski 1989; Fedele 1989a; Fedele 1989b; Fraser 1991; Henzl 1988; NEET 1992; Shaw 1992); two a nor‐testosterone derivative (gestrinone) (Bromham 1995; Fedele 1989a); and one each, medroxyprogesterone (Telimaa 1988(a)); and a high‐dose oral contraceptive (Noble 1979). None of these trials explained the methods of allocation concealment.
RCTs comparing GnRHa versus OC
One study which compared GnRH with OC reported live birth (Cosson 2002). Dienogest was compared with triptorelin in a trial with no details of concealment.
Blinding
RCTs comparing danazol, MPA, gestrinone, OC, or GNRHa with placebo or no treatment
Three trials reported details on the method of blinding (Bergqvist 1998; Busacca 2001; Loverro 2008). There were no details reported in any of the remaining studies.
RCTs comparing MPA, OC, gestrinone, or GNRHa with danazol
Of the 12 randomised trials which compared various methods of ovulation suppression with danazol as the control, only three used a secure double‐blind design (Bromham 1995; Fraser 1991; Henzl 1988).
RCTs comparing GnRHa versus OC
One study which compared GnRH with OC reported live birth (Cosson 2002). Dienogest was compared with triptorelin in a centrally randomised open‐label study.
Incomplete outcome data
RCTs comparing danazol, MPA, gestrinone, OC, or GNRHa with placebo or no treatment
Studies frequently included data on symptoms other than subfertility and reported pregnancies for the subgroup of women attempting to conceive post‐treatment. These women appear to have been identified as such after completion of suppression rather than at the time of randomisation. It is thus possible that some women who originally set out to conceive but were unsuccessful did not enter the denominator for subfertile women attempting to conceive. There is no way to determine whether this actually occurred and, if it did, whether it was more or less common following treatment rather than observation. Assuming that incomplete follow up was a feature shared equally by both groups, the relationship between pregnancy rates and the ORs that were calculated would not be affected. However, loss of women attempting but failing to conceive would artificially increase pregnancy rates in both groups. Data has therefore been analysed in this review, if possible, for all women randomised into the study and for those women specifically identified as having subfertility and trying to conceive, or both.
Selective reporting
Only two studies (Cosson 2002; Shaw 1992) reported on live birth. The remaining studies reported only on pregnancies.
Other potential sources of bias
RCTs comparing danazol, MPA, gestrinone, OC, or GNRHa with placebo or no treatment
Two studies comprised women who had undergone ablation of visible endometriosis at their diagnostic laparoscopy (Bianchi 1999; Vercellini 1999). In another trial, half the included women had conservative surgery for endometriosis prior to receiving medical treatment (Telimaa 1988(a)). The effect of this potentially effective co‐intervention was therefore balanced between study arms. However, follow up in the second of these trials varied between six and 36 months (Bianchi 1999). Since the mean duration of follow up was not reported for each study arm, there remains a potential for bias in results due to unequal follow up with danazol versus observation (Bianchi 1999). Uncertainty around completeness of follow up was a concern in all studies.
"Correction of other fertility factors" in addition to endometriosis was reported in one study. Since this was done for both active and control patients, its effect should not have biased the study comparison (Bayer 1988). Four studies explicitly excluded other infertility diagnoses from their patient sample (Bianchi 1999; Fedele 1992; Thomas 1987; Telimaa 1988(a)). This selective approach might be expected to improve the response of treated women since only those with endometriosis as their exclusive fertility problem received the treatment aimed at this disease process. If the treatment was indeed effective, it should appear more so in the 'endometriosis only' group than in any other group.
RCTs comparing MPA, OC, gestrinone, or GNRHa with danazol
Fraser 1991 included women with all stages of disease. All underwent conservative surgery for ablation or excision of residual disease upon completion of medical therapy. Although this may have confounded results if applied to only one study arm, participants in both the danazol and nafarelin arms received this potentially effective treatment, balancing its impact on conception. Bromham et al excluded women with severe disease requiring surgical excision and those with a previous failed response to danazol (Bromham 1995). While this limits the potential for generalisation of results to such patients, again this pre‐randomisation manoeuvre should not have influenced the comparison of gestrinone versus danazol. ANZ Zoladex 1996 only reported pregnancy data for the subfertile women in their study. None of the studies used a crossover design. None explicitly excluded participants with other subfertility diagnoses. Co‐intervention with clomiphene and other subfertility treatments was a concern in three studies with less rigorous concealment of allocation than those described above (Dmowski 1989; Fedele 1989a). Again, since this adjunctive therapy appears to have been equally available to women in treatment and control arms, it should not have affected the relative likelihood of pregnancy. If it was explicitly detailed that pregnancies occurred after the addition of a co‐intervention these pregnancies were excluded from analysis. In two trials, > 40% of patients dropped out of one or more treatment arms (Noble 1979; Shaw 1992). Since pregnancy data were reported on an intention‐to‐treat basis in these trials, the high rate of withdrawal from active treatment may have led to an underestimate of the treatment's true potential. This raises the important issue of common side effects with danazol and other suppressive agents leading to poor compliance. If women who failed to comply with treatment were excluded, to provide an efficacy rather than effectiveness analysis, the value of treatment in a real‐world context might be overestimated.
Effects of interventions
Subgroup analysis was performed where it was explicit that the total or partial sample comprised subfertile women only, otherwise data were presented for all women randomised in the individual study.
Adverse events are described in Table 5.
1. Adverse events.
| Trial name | Adverse events |
| ANZ Zoladex 1996 | Menopausal symptoms including vaginal dryness were more often reported in the zoladex group. Acne, oily skin were more often reported in the danazol group. Danazol was also associated with ankle oedema, marked change in breast size, depression, voice changes, headache, flu‐like symptoms, muscle cramps, insomnia, memory and concentration loss, skin rash. |
| Bayer 1988 | Not reported. |
| Berqvist 1988 | Menopausal symptoms were experienced by 80% of women in the GnRH analogues and 33% in the placebo group. |
| Bianchi 1999 | Danazol associated with hyperandrogenism (16.7%), weight gain (8.3%). |
| Bromham 1995 | Both gestrinone (91%) and danazol (89%) were associated with hyper‐androgenic side effects including hirsutism, headaches, nausea, voice change, depression, liver function, central nervous system complaints, lethargy, headaches, weight gain, acne and seborrhoea. |
| Burry 1989 | Weight gain was reported more commonly in danazol. |
| Busacca 2001 | Menopausal symptoms experienced by all women on GnRH analogue. |
| Cosson 2001 | Similar experience of hot flushes in both groups of women. |
| Dmowski 1989 | Menopausal symptoms more often with GnRHa and weight gain and oily skin more likely with danazol. |
| Fedele 1989a | Weight gain and hot flushes, acne, decreased breast size, more common with danazol than gestrinone. |
| Fedele 1989b | Menopausal symptoms, myalgia, hirsutism, depression. |
| Fedele 1992 | Miscarriage rate. |
| Fraser 1991 | Menopausal symptoms more often with GnRHa and weight gain and oily skin more likely with danazol. |
| Harrison 2000 | Acne, pain in 10% of the MPA group only. |
| Henzl 1988 | Menopausal symptoms and reduced libido more often with GnRHa and weight gain more likely with danazol. |
| Loverro 2008 | Not reported. |
| NEET 1992 | Menopausal symptoms more often with GnRHa and weight gain and oily skin more likely with danazol. |
| Noble 1979 | Weight gain, hypertension, hoarse voice, breast pain, depression, breathlessness, thrombosis, acne, fatigue, persistent bleeding, cramps, hirsuitism, nausea, depressed libido. |
| Parazzini 1994 | Not reported. |
| Shawki 2002 | Not reported. |
| Shaw 1992 | Menopausal symptoms including hot flushes (98% in goserelin and 58% in danazol groups), pain, headache, nausea, breast pain, weight gain, muscle cramps, acne, oily hair and skin, reduced libido. |
| Telimaa 1998 | Not reported. |
| Thomas 1987 | Not reported. |
| Vercellini 1999 | Mentioned only in the discussion. |
1) Ovulation suppression versus placebo or no treatment ( Figure 3) From the 12 trials, there were 88 pregnancies in 420 women administered an ovarian suppression agent compared with 84 pregnancies in 413 women receiving no treatment or placebo. The common OR for pregnancy across trials was 0.97 (95% CI 0.68 to 1.37, P = 0.85) for all women randomised, and 1.02 (95% CI 0.69 to 1.50, P = 0.22) for women clearly identified as subfertile (80 pregnancies from 287 women for ovarian suppression and 73 pregnancies from 270 women receiving placebo or no treatment). There was no evidence of clinical heterogeneity between studies with an I2 statistic of 25% and 24%, respectively, for both the total population and subgroup analysis.
3.

Funnel plot of comparison: 1 Ovulation suppression versus placebo or no treatment, outcome: 1.1 Clinical pregnancy
The possibility that agents other than danazol may be more effective led to further post hoc subgroup analyses. All studies comparing ovarian suppression agents other than danazol with no treatment or placebo were combined. The common OR for all women randomised was 1.02 (95% CI 0.69 to 1.52, P = 0.91); and for women clearly identified as subfertile the OR was 1.10 (95% CI 0.70 to 1.73, P = 0.69). Heterogeneity as assessed by the I2 statistic was 44% and 42%, respectively. There was no evidence of effect with newer agents. Further analysis was done with the exclusion of Vercellini et al (Vercellini 1999). This was done because all women undergoing ovarian suppression had recently undergone laparoscopy with ablation of visible disease. If the ablation had 'cured' them, suppression may have proven less effective than observation because of the period of amenorrhoea induced by treatment. Again the pooled data suggested no evidence of effect from agents other than danazol versus placebo (common OR 1.20, 95% CI 0.77 to 1.88, P = 0.42, I2 = 41%).
2) Other ovulation suppression agents versus danazol (Figure 4 and Figure 5) One study reported on live birth with no difference identified between ovarian suppression and danazol (OR 1.15, 95% CI 0.57 to 2.32, P = 0.70). The trials reporting on clinical pregnancies yield a pooled OR for pregnancy of 1.38 (95% CI 1.05 to 1.82, P = 0.02, I2 = 0%) for all women randomised. This effect was not observed when those women identified as subfertile were analysed (OR 1.37, 95% CI 0.94 to 1.94, P = 0.10, I2 = 0%). Although these studies assessed different interventions there was no statistical evidence of heterogeneity, as indicated by the I2 statistic. There was no statistically significant difference between the effectiveness of danazol and other ovulation suppression agents for the treatment of endometriosis‐associated subfertility. When the most widely used drugs GnRHa and danazol were directly compared, the pooled OR for pregnancy was 1.45 (95% CI 1.08 to 1.95, P = 0.001, I2 = 0%) for all women randomised, and 1.63 (95% CI 1.12 to 2.37, P = 0.01, I2 = 0%) for women identified as subfertile. This suggests there is evidence of a benefit of GnRH over danazol for subfertile women. This effect was not observed for agents other than GnRH versus danazol: OR 1.35 (95% CI 0.84 to 2.17, P = 0.22, I2 = 0%) for all women randomised; OR 1.10 (95% CI 0.53 to 2.29, P = 0.80, I2 = 0%) for women identified as subfertile.
4.
Funnel plot of comparison: 2 Other ovulation suppression agents versus danazol, outcome: 2.2 Clinical pregnancy
5.
Funnel plot of comparison: 3 GnRHa versus danazol, outcome: 3.1 Clinical pregnancy
3) GnRH versus OC (Figure 6) One study comparing GnRH with OC reported live birth: OR 0.69 (95% CI 0.26 to 1.85, P = 0.46). For clinical pregnancies the OR was 0.93 (95% CI 0.41 to 2.12, P = 0.86) for all women randomised; and OR 0.83 (95% CI 0.34 to 2.05, P = 0.69) for women identified with subfertility, indicating no evidence of benefit of one intervention over the other in this single study.
6.

Funnel plot of comparison: 4 GnRH versus oral contraception, outcome: 4.2 Clinical pregnancies
Discussion
Summary of main results
This updated review comparing ovulation suppression versus placebo for endometriosis reports no evidence of benefit on pregnancy outcomes. This is a reasonable body of evidence with little inconsistency and minimal evidence of heterogeneity. Ovulation suppression for up to six months, a hitherto common approach to endometriosis‐associated subfertility, has significant costs and side effects. It is difficult to interpret the improved pregnancy results for GnRHa versus danazol given that there was no evidence of benefit in terms of pregnancy outcomes for ovulation suppression versus placebo in women with endometriosis and infertility.
Overall completeness and applicability of evidence
Only two of the studies reported live birth as an outcome, which limits the applicability of the results. The other aspects of ovulation suppression are the side effects, not reported in all trials (refer to Table 5). GnRH analogues may accelerate the rate of bone loss to as much as 1% per month. Hot flashes, vaginal dryness, headache and nasal congestion are also common adverse effects. Danazol increases low density lipoprotein (LDL) cholesterol and causes weight gain and oily skin. Progestins may depress mood and libido, and cause weight gain. High‐dose combined oral contraceptives increase thromboembolic risk. The 'mainstream' drugs GnRHa and danazol are also both expensive and cost several hundred dollars per month.
Quality of the evidence
This review consists of 25 trials. The limitations of this review in the most part relate to the poor reporting of many of the older studies, with many of the studies failing to report concealment of allocation. There were also considerable losses to follow up with few studies applying the intention‐to‐treat principle. For a detailed description refer to the Risk of bias tables and Figure 1 and Figure 2.
Potential biases in the review process
The review authors consider that the search and identification of studies was thorough and systematic. The lack of data on live births remains an issue of bias as does the lack of data on adverse events such as miscarriage. The review authors tried unsuccessfully to obtain data on live birth outcomes from the primary authors.
Agreements and disagreements with other studies or reviews
The findings of these studies generally concur with current evidence.
Authors' conclusions
Implications for practice.
In the treatment of endometriosis‐associated subfertility, the combined data from trials comparing danazol, gestrinone, or MPA with placebo or no treatment do not provide convincing evidence of benefit. Trials comparing gestrinone, medroxyprogesterone, or an oral contraceptive pill with an 'active control', with danazol, demonstrate no statistically significant difference in subsequent fecundity between groups. Although ovulation suppression with GnRH analogues did appear to show evidence of benefit over danazol, no recommendation for clinical care can be made as there is no evidence of benefit with any ovulation suppression agent versus placebo. The adverse event profile with any of the ovulation suppression agents makes treatment an unpopular choice over expectant treatment.
Implications for research.
The available evidence comparing suppression with no treatment or placebo provides 80% power to detect a benefit of 20% (two tailed alpha 0.05), and consistently fails to do so. Larger trials of this comparison do not appear warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2009 | New search has been performed | Title change to reflect content/outcomes of consideration within the review, minor edits to Objectives section in the review abstract. Addition of SOF and ROB tables. |
| 20 April 2009 | Review declared as stable | No new data is anticipated for this topic. The review is therefore closed and will no longer be updated. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 7 November 2008 | Amended | Converted to new review format. |
| 8 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
The original review was prepared in 1996. For the update of this review in 2003, three new RCTs were added to the review and all sections of the text were substantially revised. The review was updated again in 2009 with the addition of one further study, and the title of the review was changed to reflect the content and outcomes, that is subfertility.
Acknowledgements
The authors wish to acknowledge the advice and support of the Cochrane Menstrual Disorders and Subfertility Group.
Appendices
Appendix 1. Search string
MEDLINE: 1. endometriosis/ 2 (adenomyosis or endometrio$).tw. 3 or/1‐2 4 Ovulation Inhibition/ 5 (Ovulat$ adj5 (Inhibit$ or suppress$)).tw. 6 exp contraceptives, oral/ or exp contraceptives, oral, combined/ 7 oral contraceptive$.tw. 8 Danazol/ 9 danazol.tw. 10 exp Gonadotropin‐Releasing Hormone/ 11 (Gonadotropin‐Releasing Hormone or GnRH).tw. 12 exp Medroxyprogesterone/ 13 (Medroxyprogesterone or MPA).tw. 14 gestrinone.mp. or Gestrinone/ 15 or/4‐14 16 3 and 15 17 randomised controlled trial.pt. 18 controlled clinical trial.pt. 19 Randomized Controlled Trials/ 20 Random allocation/ 21 Double‐blind method/ 22 Single‐blind method/ 23 or/17‐22 24 clinical trial.pt. 25 exp clinical trials/ 26 (clin$ adj25 trial$).ti,ab,sh. 27 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. 28 Placebos/ 29 placebo$.ti,ab,sh. 30 random$.ti,ab,sh. 31 Research design/ 32 or/24‐31 33 animal/ not (human/ and animal/) 34 23 or 32 35 34 not 33 36 16 and 35
EMBASE 1. endometriosis/ 2 (adenomyosis or endometrio$).tw. 3 or/1‐2 4 Ovulation Inhibition/ 5 (Ovulat$ adj5 (Inhibit$ or suppress$)).tw. 6 oral contraceptive$.tw. 7 Danazol/ 8 danazol.tw. 9 (Gonadotropin‐Releasing Hormone or GnRH).tw. 10 exp Medroxyprogesterone/ 11 (Medroxyprogesterone or MPA).tw. 12 gestrinone.mp. or Gestrinone/ 13 exp Oral Contraceptive Agent/ 14 exp Gonadorelin/ 15 or/4‐14 16 3 and 15 17 Controlled study/ or randomised controlled trial/ 18 double blind procedure/ 19 single blind procedure/ 20 crossover procedure/ 21 drug comparison/ 22 placebo/ 23 random$.ti,ab,hw,tn,mf. 24 latin square.ti,ab,hw,tn,mf. 25 crossover.ti,ab,hw,tn,mf. 26 cross‐over.ti,ab,hw,tn,mf. 27 placebo$.ti,ab,hw,tn,mf. 28 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. 29 (comparative adj5 trial$).ti,ab,hw,tn,mf. 30 (clinical adj5 trial$).ti,ab,hw,tn,mf. 31 or/17‐30 32 nonhuman/ 33 animal/ not (human/ and animal/) 34 or/32‐33 35 31 not 34 36 16 and 35
Data and analyses
Comparison 1. Ovulation suppression versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 13 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 clinical pregnancies all women randomised | 12 | 833 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.68, 1.37] |
| 1.2 clinical pregnancy infertile couples/those desiring pregnancy only | 11 | 557 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.69, 1.50] |
| 2 Clinical pregnancy ‐ Ovulation agents other than Danazol vs placebo/no treatment | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Clinical pregnancy in all women randomised | 9 | 781 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.69, 1.52] |
| 2.2 Clinical pregnancy in infertile couples only | 8 | 436 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.70, 1.73] |
1.1. Analysis.

Comparison 1 Ovulation suppression versus placebo, Outcome 1 Clinical pregnancy.
1.2. Analysis.

Comparison 1 Ovulation suppression versus placebo, Outcome 2 Clinical pregnancy ‐ Ovulation agents other than Danazol vs placebo/no treatment.
Comparison 2. Other ovulation suppression agents versus danazol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 1 | 191 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.57, 2.32] |
| 2 Clinical Pregnancy | 12 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 clinical pregnancy for all women randomised | 11 | 1059 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [1.05, 1.82] |
| 2.2 clinical pregnancy for infertile couples/those desiring pregnancy only | 8 | 574 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.94, 1.99] |
2.1. Analysis.

Comparison 2 Other ovulation suppression agents versus danazol, Outcome 1 Live birth.
2.2. Analysis.

Comparison 2 Other ovulation suppression agents versus danazol, Outcome 2 Clinical Pregnancy.
Comparison 3. GnRHa versus danazol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Pregnancy | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 clinical pregnancies for women randomised | 8 | 944 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [1.08, 1.95] |
| 1.2 Clinical pregnancies infertile couples/those desiring pregnancy only | 8 | 585 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.12, 2.37] |
3.1. Analysis.

Comparison 3 GnRHa versus danazol, Outcome 1 Clinical Pregnancy.
Comparison 4. GnRH versus oral contraception.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live births | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.26, 1.85] |
| 2 Clinical pregnancies | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Clinical pregnancies per woman randomised | 1 | 142 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.41, 2.12] |
| 2.2 Clinical pregnancies infertile couples/those desiring pregnancy only | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.34, 2.05] |
4.1. Analysis.
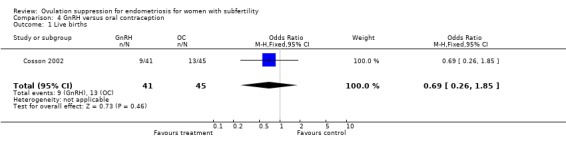
Comparison 4 GnRH versus oral contraception, Outcome 1 Live births.
4.2. Analysis.

Comparison 4 GnRH versus oral contraception, Outcome 2 Clinical pregnancies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ANZ Zoladex 1996.
| Methods | A multicentre (n=9), open, randomised comparison. Women were stratified into 2 groups, one of which included subfertile women who desired pregnancy and the other group included all other patients. No blinding due to different administration routes of medication. | |
| Participants | Study conducted in Australia and New Zealand. Women were eligible of they were aged between 18 and 40 years, diagnosed with endometriosis with rAFS score >2 or more confirmed laparoscopically within 2 months prior to study entry, normal length menstrual cycles (21‐42 days), normal cervical smear within previous 12 months. Exclusion: pregnancy or lactating, suffered significant medical problems, had used hormonal agents within last 2 months, or GnRH analogues or Danazol within previous 12 months, or had a history of trial drug hypersensitivity, showed signs of virilis ation or were taking anticoagulant therapy. Pre treatment surgical intervention was also an exclusion. Mean age of women was 29.7 years (range 24‐36) in the goserelin group and 29.9 years (range 21‐35) in the danazol group for subfertile women and 29.3 years (range 20‐40) in the goserelin group and 29.8 years (range 21‐40) for all other women. | |
| Interventions | Goserelin depot 3.6 mg SC at 4 weekly intervals for 6 injections (24 weeks) (n=35) versus danazol 200 mg TDS ( dose could be increased from 600 mg daily to 800 mg daily or decreased to 200 mg daily at doctor's discretion) taken orally for 24 weeks (n=36). Women seen monthly during treatment. Subfertile women followed for max of 48 weeks. | |
| Outcomes | Weight, BP, subjective symptom score, side effects, haematological and biochemical analysis, pregnancy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized' no details provided. |
| Allocation concealment? | Unclear risk | Unclear, no details provided. |
| Blinding? All outcomes | High risk | Open‐label study. |
| Incomplete outcome data addressed? All outcomes | Low risk | Of the 71 women entered into the study 48 remained until the end of active treatment. 2 danazol patients never commenced treatment, 8 were unwilling to continue with the therapy and 9 had a serious adverse drug reaction. In the goserelin group 4 withdrew because they were unwilling to continue. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Bayer 1988.
| Methods | Allocation by "randomly selected card". No details of concealment. No blinding. Nine danazol and 13 no treatment patients were lost to follow up although no details of reasons. | |
| Participants | USA study. Participants were subfertile couples. Mean age of danazol group 30.33±2.93 years; no treatment group 31.07±3.33 years. Inclusion: infertility > 12 months; ovulation confirmed by BBT and endometrial biopsy; tubal patency by HSG and/or laparoscopy; normal sperm quality; endometriosis at laparoscopy with no implants > 5mm, only avascular tubal adhesions and at least one ovary free of adhesions; Kistner stage I. No details of exclusions. | |
| Interventions | Danazol, 800 mg daily for 2 months followed by 600 mg daily for 2 months followed by 400 mg daily for 2 months (n=37) versus no treatment, (n=36). Women were followed up for 12 months immediately after laparoscopy in no treatment group or after completion of danazol. | |
| Outcomes | Clinical pregnancy by serum or urine BhCG. | |
| Notes | Other diagnoses included if "correctable", this presumably includes oligo‐ovulation; follow up for 12 months post‐treatment; life table analysis reported; ITT analysis done. No details of power calculation. No details of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by a randomly selected card. |
| Allocation concealment? | Unclear risk | Unclear, no details provided. |
| Blinding? All outcomes | High risk | Intervention versus no treatment, therefore no blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Total loss to follow up was 21/73: n= 9 from danazol group and n=13 from no treatment group. No details given for reason for loss to follow up. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Bergqvist 1998.
| Methods | Prospective, randomised double blind multicentre trial. Women and researchers blinded to treatment as identical kits provided for injections. No details of randomisation procedure. No details of allocation procedure. Attrition was 3/49, see quality table for details. | |
| Participants | Swedish study conducted in two departments of obstetrics and gynaecology and one general hospital. Age range 19‐44 years. All women were menstruating regularly 3 months before study (25‐35 day cycles). All had clinical symptoms. None had taken oral steroid therapy for 3 months nor long acting depot gestagens or GnRH a within 6 months. None were breast feeding and had not been pregnant within previous 3 months. There was no history of osteoporosis or coagulation disorders. Exclusion: Women with intraperitoneal adhesions making visual inspection and careful evaluation of the extension of endometriotic lesions difficult or impossible were excluded. All but one women with stage IV disease had a diagnosis of mild to moderate disease. | |
| Interventions | Triptorelin 3.75 mg depot every 4 weeks IM for 24 weeks (n=24) versus placebo every 4 weeks for 24 weeks IM (n=25). Follow up for 12 months. | |
| Outcomes | Signs and symptoms, pain, hormonal analysis, pelvic examination, self completed diary, pregnancy, menopausal symptoms. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Prospective, randomized.. ' No details provided. |
| Allocation concealment? | Unclear risk | Unclear, no details provided. |
| Blinding? All outcomes | Low risk | Identical kits for injection. Women and researchers blinded to treatment. |
| Incomplete outcome data addressed? All outcomes | Low risk | 3/49 withdrawn during treatment phase. One placebo subject became pregnant and one had insufficient effect. One woman on triptorelin experienced hypo estrogenic effects and depression. Only 8 women completed the entire 18 month study 5 triptorelin and 3 placebo. Reasons for dropping out of the triptorelin group were increased pain leading to hormonal treatment (n=4), pregnancy (n=5), initiation of fertility treatment (n=2), starting contraceptive pills (n=5) and reversible weight loss (n=1). Sixteen of the placebo group dropped out because of insufficient efficacy. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Bianchi 1999.
| Methods | Randomisation by computer‐generated list within 7 days of surgery but method of concealment not described. No blinding, no patient lost to follow up which ranged from 6‐36 months. | |
| Participants | Study conducted in Italy (n=77 women). Women aged less than or equal to 40 years with unexplained infertility, and or chronic pelvic pain, with laparoscopic surgery undertaken for moderate or severe endometriosis (disease stage III‐IV). Exclusions were previous medical or surgical treatment for endometriosis or having other diseases which might affect fertility or cause pelvic pain, women without pain symptoms and not desiring children and those with liver or endocrine disease. | |
| Interventions | After conservative laparoscopic surgery: danazol 600 mg daily for 3 months (total n=36, those attempting pregnancy n=11) versus no suppression (total n=41, those attempting pregnancy n=16). Assessed at 6 monthly intervals. | |
| Outcomes | Pelvic pain scores; rAFS staging; pregnancy, definition not given; adverse events; and disease recurrence. | |
| Notes | Follow up varied from 6‐36 months. Mean duration of follow up in each group was not compared. No evidence of power calculation, all women randomised were followed up and analysed. No details of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by computer‐generated list within 7 days of surgery. |
| Allocation concealment? | Unclear risk | Unclear, no details provided. |
| Blinding? All outcomes | High risk | Open label. |
| Incomplete outcome data addressed? All outcomes | Low risk | No patient lost to follow up. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Bromham 1995.
| Methods | Multicentre study (n=10), arm's length allocation, double blind, double dummy. No details of randomisation process. 109/269 patients withdrew or dropped out. See quality table for details. | |
| Participants | UK study of women aged 18‐45 years with endometriosis confirmed at laparoscopy or laparotomy. Those requiring surgical excision or long‐term treatment excluded. Previous "danazol failure" patients also excluded. No other hormonal treatment received in previous 2 months and an unwillingness to use mechanical contraception were also exclusions. | |
| Interventions | Gestrinone 2.5 mg capsule twice weekly (n=132) versus danazol 200 mg BD (n=137) for six months. All participants received two sets of capsules, one active and one dummy. Women were followed up for 12 months. | |
| Outcomes | AFS staging; abdominal pain; return of menstrual function; pregnancy, not reported by stage of disease; side effects; Ferriman‐Gallwey score; haematological and biochemical analysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Allocated at random' no further details. |
| Allocation concealment? | Unclear risk | Unclear, no details. |
| Blinding? All outcomes | Unclear risk | Study claimed to be double blind. All capsules were identical and patients blinded but no details as to who else was blinded. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 90/132 gestrinone and 83/137 danazol completed the 12 month follow up. 5 women (2 x gestrinone and 3 x danazol) withdrew because of pregnancy before commencing treatment. 54 women withdrew because of side effects (25 gestrinone and 29 danazol). 50 women withdrew (25 gestrinone and 25 danazol) or dropped out from the follow‐up phase. 22 in each group failed to return for follow up. One in each group for personal reasons, 3 (1 gestrinone, 2 Danazol ) for hysterectomy or other surgery and 1 gestrinone withdrew from infertility investigation. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Burry 1989.
| Methods | Randomised trial. Double‐blind, double‐dummy design. No details of randomisation. No details of allocation concealment. Attrition 2/53 details in quality table. | |
| Participants | American study of 53 women. Age 23‐38 years. All women complained of infertility, pain or both. Laparoscopic diagnosis was made within 3 months prior to study. Women were excluded if they had received medical therapy for endometriosis within preceding 6 months. 62% of the women had stage III and IV disease. | |
| Interventions | Danazol 800 mg daily (n=10) PO + placebo
versus
danazol 600 mg daily (n=8) PO + placebo
versus
nafarelin 800 µg daily (n=10) IN + placebo
versus
nafarelin 400 µg daily (n=25) IN + placebo. Women were followed up at 2, 4 and 6 months after treatment. |
|
| Outcomes | Weight, adverse events, compliances, change in symptoms, haematological analysis, pregnancy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly assigned' no further details. |
| Allocation concealment? | Unclear risk | Unclear, no further details. |
| Blinding? All outcomes | Low risk | Double‐blind double‐dummy design with both patients and investigators being blind to treatment. |
| Incomplete outcome data addressed? All outcomes | Low risk | One women from danazol 800 mg withdrew because of severe headaches, one woman from nafarelin 800 µg withdrew because of mood swings. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Busacca 2001.
| Methods | Randomised controlled trial. Randomisation according to computer‐generated list. Randomisation list unknown to physicians but unclear as to how allocation was concealed. Attrition n=1 due to unacceptable side effects. | |
| Participants | Italian study. 89/97 eligible women randomised who attended Dept of Obstetrics and Gynaecology to undergo laparoscopy. Median age leuprolide group 31 years (21‐38); median age expectant management group 28 years (20‐37). Inclusion: women were of reproductive age, not more than 40 years old with laparoscopic diagnosis of endometriosis stage III‐IV. Exclusion: previous medical therapy or surgical therapy for endometriosis, the presence of other diseases which might affect fertility or cause pelvic pain and diagnosis of liver, endocrine or neoplastic diseases. | |
| Interventions | Leuprolide acetate 3.75 mg IM every 4 weeks for 8 weeks (i.e. 3 injections). n=44 versus expectant management n=45. Women followed up for 6‐36 months. | |
| Outcomes | Pain, hormonal assays, pregnancy, menopausal symptoms. | |
| Notes | Post‐operative group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation according to computer‐generated list. |
| Allocation concealment? | Unclear risk | Unclear, randomisation list unknown to physicians but unclear as to how allocation was concealed. |
| Blinding? All outcomes | Low risk | Patients not blinded as intervention was injection versus no treatment. Physicians were blinded to intervention. |
| Incomplete outcome data addressed? All outcomes | Low risk | One patient withdrew from GnRH arm due to unacceptable side effects. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Cosson 2002.
| Methods | Randomisation was centralised with treatment units numbered 1‐8 for each centre. Treatment was only known when decision to enrol patient was made. Multicentre, open‐label study. No details of concealment. Attrition n=22 for major protocol violations. 12 withdrew prematurely (n=9 dienogest, n=3 decapeptyl). | |
| Participants | 142/144 eligible patients recruited in French study. Mean age dienogest 28.5± 4.9, decapeptly 30.3±5.1 years. Inclusion: Grade II‐IV endometriosis at laparoscopy, operative laparoscopy. Age 18‐40 years. No form of hormonal therapy for 3 months pre‐treatment. Exclusion: contraindications to laparoscopy or synthetic progestogens. Hormonal treatment within 3 months proceeding diagnostic laparoscopy. If using oral contraceptives required to have had at least 2 regular spontaneous cycles prior to initial laparoscopy. | |
| Interventions | Dienogest mg BD PO for 16 weeks (n=74) versus decaptyl (triptorelin) 3.75 mg IM every 4 weeks for 16 weeks. Follow up for 12 weeks post treatment for subfertile women. (n=68). | |
| Outcomes | Change in rAFS, pain, haematological assays, pregnancy, satisfaction with treatment, side effects. | |
| Notes | No details of intention to treat, no details of power calculation. Funding from Society Innothera and Schering (France) for financial and technical support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized, parallel group clinical trial' no further details. |
| Allocation concealment? | Low risk | Adequate. Randomisation was centralised, with the treatment units numbered from 1‐8 for each centre. The investigator and the patients were only aware of the type of treatment when the decision to enrol the patient was made. |
| Blinding? All outcomes | High risk | Open‐label study. |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up. |
| Free of selective reporting? | Low risk | Reported up to live birth. |
Dmowski 1989.
| Methods | Prospective randomised trial. Method not described. No evidence of concealment of allocation. Open label study; no blinding. 29/36 women completed study and were analysed. Only 26 reported in pregnancy data. | |
| Participants | USA study of 36 women with a mean age of 30.8±0.6 (SE) years (range 27‐38 years). 31 were subfertile. Endometriosis on laparoscopy, stage not specified; infertility duration and other relevant fertility factors unspecified. First diagnosed in 19 women and previously diagnosed and treated in 17 women. All women had regular menstrual cycles and had been diagnosed with endometriosis within 3 months of study enrolment. All women had been off hormonal treatment for at least 8 months before initial laparoscopic staging. No details of exclusions. | |
| Interventions | Buserelin subcutaneous (SC) 0.2 mg daily (n=9) or Buserelin intranasal (IN) 1.2 mg daily (n=10). versus danazol 800 mg daily (n=10) for 6 months. Women were followed up monthly for 12 months. | |
| Outcomes | Clinical pregnancy, symptoms, pain, hormonal and haematologic analysis, side effects, and ultrasonography. | |
| Notes | 12 months post‐treatment follow up; did not exclude other causes of infertility; no hormonal treatment for at least 8 months before enrolment; ITT analysis not possible. No power calculation. Funding by Hoechst‐Roussel Pharmaceuticals Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Prospective randomized study' no further details. |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details provided. |
| Blinding? All outcomes | High risk | Open‐label study. |
| Incomplete outcome data addressed? All outcomes | Low risk | 29/36 completed and were analysed, 5 in buserelin group and 2 in danazol group. Two patients reported family reasons, three were non‐compliant, one had severe emotional side effects on IN buserelin and one was allergic to danazol. Only 26 women were reported in pregnancy data. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Fedele 1989a.
| Methods | Random allocation. Method not stated. No details of concealment of allocation. No blinding. Details in quality table for attrition. All women analysed in pregnancy outcome. | |
| Participants | Italian study of 39 subfertile women. Mean age 29.8 years (23‐35). Endometriosis at laparoscopy diagnosed in previous 3 months, all stages included; primary infertility; biphasic BBT; tubal patency on HSG and laparoscopy; normal sperm parameters; normal PCT. No therapeutic procedure conducted at laparoscopy. Women taking danazol or other sex steroids in preceding 6 months or with severe systemic or endocrine disease were excluded. | |
| Interventions | Gestrinone 2.5 mg PO twice weekly for 6 months, n=20 patients allocated versus danazol 600 mg PO daily for 6 months, n=19. Doses increased to 2.5 mg three times a week for gestrinone or 800 mg per day for danazol if amenorrhoea was not achieved after one month. Women were followed up monthly. | |
| Outcomes | Clinical pregnancy; this outcome not reported by stage. Symptoms, side effects, hormonal analysis, haematological and biochemical analysis every 2 months during and 2 months after for at least 12 months. | |
| Notes | 18 months post‐treatment follow up. No details of power calculation. No details of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Allocated randomly' no further details. |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details provided. |
| Blinding? All outcomes | High risk | Open‐label as tablets versus spray. |
| Incomplete outcome data addressed? All outcomes | Low risk | 1/62 women withdrawn from study. Woman was in buserelin group and withdrew due to severe pelvic pain. |
| Free of selective reporting? | High risk | Not followed to live birth. |
Fedele 1989b.
| Methods | Random allocation. Method not described. No details of allocation concealment. no blinding. One women from buserelin group withdrawn for severe pelvic pain. | |
| Participants | Italian study of subfertile women (n=62). Mean age of buserelin group 29.8±3.3 years and for danazol group 31.3±4.3 years. Endometriosis at all stages confirmed by laparoscopy in previous 3 months; no therapeutic intervention. Ovulation confirmed by BBT and serum progesterone; tubal patency by HSG and/or laparoscopy; sperm and PCT assessed; bilateral tubal occlusion and severe male factor excluded. Women having taken danazol or other sex steroid hormones in previous 6 months and those with severe systemic or endocrine diseases were also excluded. | |
| Interventions | Buserelin 400 µg IN TDS for 6 months, n=30 versus danazol 200 mg PO TDS for 6 months, n=32. Women were followed up monthly to the end of treatment and then for 12 months after treatment. | |
| Outcomes | Clinical pregnancy, diagnosis not defined; not reported by stage. Haematological and biochemical analysis, adverse events. | |
| Notes | No details of power calculation. All women included in pregnancy analysis. No details of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Allocated randomly' no details. |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details provided. |
| Blinding? All outcomes | High risk | No blinding, treatment regimens were different. |
| Incomplete outcome data addressed? All outcomes | Low risk | 1/39 women: one women from gestrinone group was withdrawn because of hepatitis B virus. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Fedele 1992.
| Methods | Randomised by computer‐generated assignment. Allocation by central telephone. No blinding. No attrition although 4 eligible women refused to participate. | |
| Participants | Italian study of 71 consecutive women attending Obstetrics and Gynaecology Dept. Laparoscopically diagnosed RAFS stage I and II endometriosis; age <38 years trying to conceive; unexplained infertility >2 years; normal HSG, endometrial biopsy, hormone profile and post‐coital test; two normal semen analyses. Uterine anomaly or previous treatment for endometriosis or infertility reasons for exclusion. Dyspermia of partners also an exclusion. Previous clinical, laparoscopic or diagnosis by laparotomy of endometriosis or any other disease which might affect fertility. | |
| Interventions | Buserelin IN 400 µg TDS for six months, n=35 versus expectant management, n=36 women. Women were followed up 4 monthly for a median of 18 months. | |
| Outcomes | Clinical pregnancy, diagnosis not defined; cumulative pregnancy rates at 12 and 24 months; spontaneous abortion. | |
| Notes | Co‐intervention with clomiphene or hCG in 29 women data is presented for 12 month follow up prior to administration of this co‐intervention; The 6 months of amenorrhoea associated with buserelin use was not taken into account in comparing 12 and 24 month conception rates; ITT analysis done. No details of power calculation. Study supported by the Ministero della Sanita (Rome, Italy). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised by computer generated assignment. |
| Allocation concealment? | Low risk | Adequate. Allocation by central telephone. |
| Blinding? All outcomes | High risk | No blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | No attrition although 4 eligible women refused to participate. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Fraser 1991.
| Methods | Randomised double‐blind double‐dummy placebo‐controlled trial, method of allocation computer‐generated random number table. Randomisation ratio 2:1 nafarelin: danazol. No details of concealment. 3 dropouts from nafarelin and none from danazol. | |
| Participants | Australian study of 49 women (n=36 subfertile). Endometriosis confirmed and scored at laparoscopy; 40 women with RAFS stage 1 or 2, 9 with stage 3. Women were symptomatic, regular menstrual cycles (24‐36 days), negative pregnancy test and negative PAP smear and agreeing to use barrier method of contraception. Exclusion: concurrent disease which may interfere or interact with study drugs; surgical (<6 months) or medical treatment (< 3 months) prior to study entry. | |
| Interventions | Nafarelin 200 µg BD with danazol placebo for six months, n=22 subfertile women analysed
versus
danazol 200 mg TDS with nafarelin placebo nasal spray for six months, n=14 analysed. All women with stage I and II endometriosis underwent operative laparoscopy at the end of medical treatment, with ablation of visible residual disease. Women with stage III and IV disease underwent laparotomy for excision of residual disease. Women followed up for 12 months. |
|
| Outcomes | Conception during 12 month follow‐up period, diagnosis not defined; pregnancy not reported by stage. Resumption of menstrual bleeding, surgery and or drug therapy, compliance, clinical and hormonal analysis, pain, side effects. | |
| Notes | Primary goal of study to assess pain and visible disease reduction; Number of subfertile women randomised to each group not stated; other causes of subfertility not excluded; 3 of 49 randomised patients dropped out; uncertain whether all randomised subfertile women were included in analysis. All women underwent conservative surgery at the end of their medical treatment, to destroy residual disease. No details of power calculation and ITT. Funding: drug supplied by Syntex Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation using computer‐generated list of random numbers. |
| Allocation concealment? | Unclear risk | Unclear, no details. |
| Blinding? All outcomes | Low risk | Patients and investigators were blinded to treatment using double‐dummy design. |
| Incomplete outcome data addressed? All outcomes | Low risk | 3 dropouts from nafarelin and none from the danazol group, no details provided as to reasons. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Harrison 2000.
| Methods | Prospective, double‐blind parallel‐group randomised trial. Allocation by centralised list supplied to pharmacy. Randomisation to each arm by block design. Attrition 10/100 details in quality table. | |
| Participants | Irish study of women attending an infertility unit. Age range 20‐39 years. Mean age MPA group 31.5±2.8 years, placebo group 31.7±3.0 years. Women had to have a history of infertility ≥2 years, undergoing diagnostic laparoscopy as part of infertility work‐up. Exclusion: ovarian, uterine, tubal defects other than those due to endometriosis, history of cancer, renal or hepatic impairment and diabetes mellitus. Patients with a history of treatment that might have influenced endometriosis within 4 weeks of baseline laparoscopy. | |
| Interventions | MPA 50 mg daily (n=50) versus placebo daily (n=50). Taken for 12 weeks followed up again at 24 weeks and 36 weeks after beginning treatment. | |
| Outcomes | rAFS, pregnancy, pain, clinical variables, physicians evaluation of well‐being, compliance, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation using block design. |
| Allocation concealment? | Low risk | A ‐ adequate. Centralised list was used for allocation which was supplied to the pharmacy. |
| Blinding? All outcomes | Unclear risk | Double‐blind study. Patients were blinded to allocation but no details as to who else was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | 10/100 failed to complete study. One MPA and two placebo women dropped out before beginning treatment. Other reason for withdrawal were depression (n=1 MPA), failure to return (n=1 MPA), dropped out (n= 2 placebo), pregnant (n=3 placebo). Women assessed at 24 weeks were 49 MPA and 48 placebo. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Henzl 1988.
| Methods | Multicentre, international, parallel, randomised study. No details of randomisation. No details of allocation. Double‐blind, double‐dummy design. 213/236 evaluated for efficacy (see quality table for details). Authors of paper state that ITT analysis run on best and worse case scenarios showed that 9% exclusion did not affect the results but they did not analyse all patient data. | |
| Participants | International multicentre study. Women aged 18‐45 years. Primary or secondary infertility. Endometriosis evident on laparoscopy or laparotomy within 3 months of study participation, all stages included. Infertility duration unspecified. No surgical intervention undertaken. Exclusion: hormonal treatment taken less than 6 months prior to study admission. No details given of the number of women who were complaining or being treated for infertility. | |
| Interventions | Naferelin 400 or 800 mg daily for 6 months + placebo PO, n=156 versus danazol 800 mg daily PO for 6 months + placebo IN, n=80. Women followed up for 6 months post‐treatment. Those women wishing to become pregnant were followed for 12 months. | |
| Outcomes | Clinical pregnancy, diagnosis not specified; not reported by stage of disease. Self and physician reported pain, menstrual patterns, adverse effects, hormonal and biochemical analysis. | |
| Notes | Did not exclude other causes of infertility. No hormonal treatment for 6 months before the study. No surgical intervention; unclear whether all randomised subfertile women were included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly assigned' no details provided. |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details provided. |
| Blinding? All outcomes | Unclear risk | Double‐blind double‐dummy design with both patients and investigators being blind to treatment. |
| Incomplete outcome data addressed? All outcomes | Low risk | 23/236 patients were excluded from efficacy analysis. Seven nafarelin 800 µg and four danazol withdrew because of hot flushes; three danazol had a rise in serum levels and one withdrew because of inefficacy. Nine patients withdrew for reasons unrelated to the drugs but no details given as to which groups they belonged to. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Loverro 2008.
| Methods | Randomisation via computer‐generated randomisation table. | |
| Participants | Italian study, n= 60 patients of reproductive age (mean age 28.6 years). Of these women 14 in the triptorelin group and 13 in the expectant management group were subfertile. Inclusion: stage III‐IV endometriosis in women of reproductive age associated with chronic pelvic pain, adnexal mass or infertility; complete laparoscopic excision; endometriosis score greater than 15 points (rAFS) and no previous hormonal treatment. Six patients lost at first follow up. |
|
| Interventions | 3 months of: yriptorelin depot 3.75mg administered im on day 20 of the menstrual cycle and thereafter every 28 days for 3 months (n=29) versus expectant management saline injections using same regimen as above (n=25). Follow up every 3 months in first year, six monthly in year 2 and then annually. |
|
| Outcomes | Pelvic pain, Ca‐125, pregnancy, endometrioma relapse. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation via computer‐generated randomisation table. |
| Allocation concealment? | Unclear risk | No details provided. |
| Blinding? All outcomes | Low risk | Patients blinded to treatment group. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No evidence of pre‐publication protocol. No loss to follow up for subfertile subgroup. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
NEET 1992.
| Methods | Multicentre, parallel, randomised, double‐blind, double‐dummy trial. Method of randomisation not described. Randomised on a ratio of 2:1 nafarelin: danazol. No details of concealment. Placebo tablet and spray, blinded patients and investigators. 315 were randomised and 263 qualified for efficacy analysis, see quality table for details of attrition. | |
| Participants | European study of 307 women. Endometriosis of all stages, diagnosed at laparoscopy or laparotomy; age 18‐45 years; cycles 24‐36 days during 4 months prior to study; 45‐110 kg weight; negative pregnancy test and PAP smear. Exclusion: amenorrhoea, concurrent diseases which could interfere with the study or could contraindicate the use of androgenic therapy, surgical endometriosis treatment at baseline laparoscopy or within 6 months before trial, or the use of danazol, androgenic hormones, oestrogens or progestogens within 3 months preceding the trial. | |
| Interventions | Nafarelin 400 µg intranasally daily (200 µg BD) with oral placebo TDS for 6 months (n=171; subfertile women included) versus danazol 600 mg daily (200 mg TDS) with placebo nasal spray BD (n=92 subfertile women included in analysis). Followed up at 2 weeks, 1 month, and monthly during treatment followed by 1,3,6,12‐month follow up post‐treatment. | |
| Outcomes | Clinical pregnancy, diagnosis not defined; outcome not reported by stage of disease, symptoms, menstruation, adverse events, concomitant medication, hormonal analysis, PAP smear. | |
| Notes | Primary goals were to assess reduction in visible disease, pain relief and side effects of nafarelin and danazol; unclear whether all randomised Feritol women were included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized' no details provided. |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details provided. |
| Blinding? All outcomes | Unclear risk | States study was 'double blind' but no details as to who was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | 315 randomised, 8 never took medication: 307 in safety analysis (206 nafareline and 101 danazol); 263 qualified for efficacy analysis (171 nafarelin and 92 danazol). At 12‐month follow up data included women who had completed treatment and 2 others who had completed treatment but missed 2nd look laparoscopy but were still followed in post‐treatment period (nafarelin n=172; danazol n= 93). |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Noble 1979.
| Methods | Random allocation. Method not described. No evidence of concealment. Attrition was 8/42 women. | |
| Participants | UK study. Inclusion: endometriosis of all stages on laparoscopy or laparotomy. Infertility duration unspecified. Women were complaining of infertility or were symptomatic. No details of exclusions. | |
| Interventions | Mestronol or norethynodrel 5 mg BD (n=17)
versus
danazol 1000 mg daily (n=25), Dose increased every 2 weeks until amenorrhoea achieved. |
|
| Outcomes | Clinical pregnancy, diagnosis not defined; pregnancy not reported by stage of disease. Side effects, symptom improvement, surgical interventions. | |
| Notes | Follow‐up duration not stated. Trial did not exclude other causes of infertility; number of participants actually randomised was not reported, so uncertain whether ITT analysis done. No power calculation. No details of funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly allocated' no details provided. |
| Allocation concealment? | Unclear risk | B ‐ unclear, no details provided. |
| Blinding? All outcomes | High risk | Open‐label study. |
| Incomplete outcome data addressed? All outcomes | Low risk | 1/25 danazol and 7/17 mestronal or norethynodrel failed to complete the treatment. |
| Free of selective reporting? | High risk | Not reported on live birth. |
Parrazzini 1994.
| Methods | Multicentre (n=8). Randomisation was by computer‐generated randomisation list. Insufficient details of allocation concealment. Women and investigators were blinded to regimen. No loss to follow up. | |
| Participants | Italian study of women (n=75) with unexplained or primary infertility. Women were <38, diagnosis of unexplained primary or secondary infertility of >=1 year with or without chronic pain with Stage III or IV disease (rAFS). Women had undergone laparotomy as first surgical treatment for debulking or radical surgery of endometriotic lesions. Normal results on standard medical and gynaecological examinations and hysterosalpingogram, luteal‐phase endometrial biopsy, hormone profile and post coital test. Exclusion: previous clinical or laparoscopic diagnosis of endometriosis or other disease which might affect fertility or be related with pelvic pain. Partners with dyspermia also resulted in exclusion. Previous therapy for infertility or endometriosis. | |
| Interventions | Nafarelin IN 400 µg per day (N=36) versus placebo IN (n=39) administered for 3 months. Women were followed up every 4 months for 1 year. | |
| Outcomes | Gynaecological examination, pregnancy, pelvic pain. | |
| Notes | No details of funding, no details of power calculation. All women randomised were analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer generated randomization list'. |
| Allocation concealment? | Unclear risk | B ‐ unclear, 'assigned by phone'. No details as to who or how this was performed. |
| Blinding? All outcomes | Unclear risk | 'Both patients and investigators were blinded' no details as to how this was done. |
| Incomplete outcome data addressed? All outcomes | Low risk | No loss to follow up. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Shaw 1992.
| Methods | Multicentre (n=18) RCT; each centre used own randomisation schedule, security uncertain. Randomised on ratio of 2:1 goserelin:danazol. No details of concealment. Patients not blinded, same clinician performed 1st and 2nd look laparoscopy. 286/307 women satisfied selection criteria precisely. 81 goserelin and 54 danazol participants withdrew leaving 131 goserelin and 60 danazol for analysis. | |
| Participants | UK study. 304 women aged 18‐40 with menstrual cycle 21‐42 days. Laparoscopically‐confirmed endometriosis of all stages; 167 of 307 participants randomised were subfertile at inception; other causes of infertility not identified. Women were in good health and not taking anticoagulants, willing to use barrier contraception. Exclusion: no hormonal agents including oral contraceptives allowed 8 weeks pre‐study. GNRH or danazol not allowed during 24 weeks before starting study. | |
| Interventions | Goserelin depot 3.6 mg SC every 28 days for 24 weeks, n=204 allocated (113 subfertile) versus danazol 200 mg TDS PO for 24 weeks, n=103 allocated (n=54 subfertile). Followed up monthly for 24 weeks and then 8 weekly for 48 weeks. After 8 weeks of treatment goserelin could be increased to two depot (7.2 mg) every 28 days and danazol to 200 mg QDS if amenorrhoea not achieved and then could then be reduced if adverse events. | |
| Outcomes | Pregnancy, diagnosis not defined; live birth rate reported per pregnancy. Pain, adverse effects, hormonal assays. | |
| Notes | Follow up in each group for 12 months after cessation of treatment; analysis appears to be on an ITT basis. Power calculation based on objective response rate for pain. Study supported by ICI Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized' no details provided. |
| Allocation concealment? | Unclear risk | B ‐ unclear. No details of allocation. |
| Blinding? All outcomes | High risk | Open‐label study. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 286/307 satisfied selection criteria precisely. 81 goserelin withdrawn (13 before completion of treatment); 54 danazol withdrawn (21 before completion of treatment). Lack of effect, adverse findings, pregnancy and administrative reasons. |
| Free of selective reporting? | Low risk | Live birth rate reported. |
Shawki 2002.
| Methods | Randomised double‐blind study. | |
| Participants | Cairo, Egypt. 68 subfertile women diagnosed with Stage I‐II endometriosis during routine diagnostic laparoscopy. Mean age 31±1 years. Inclusion: positive dye test, normal semen parameters in partner. |
|
| Interventions | Zoladex 3.6 mg every 28 days for 6 months (n=34) versus no further treatment (n=34). One year of follow up, if no pregnancy occurred after 3 months then clomiphene citrate was prescribed. |
|
| Outcomes | Pregnancy rate. | |
| Notes | No funding was provided for the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No details of randomisation. |
| Allocation concealment? | Unclear risk | Unclear. No details. |
| Blinding? All outcomes | Unclear risk | Stated double‐blind but no details provided. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Conference abstract only. No full publication available. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Telimaa 1988(a).
| Methods | Random allocation. Method not described. | |
| Participants | Endometriosis, all stages, at laparoscopy; infertility duration unspecified. | |
| Interventions | Provera 100 mg daily for 6 months, n=17 patients randomised and included versus placebo daily for 6 months, n=14 patients randomised and included. | |
| Outcomes | Clinical pregnancy, diagnosis not defined; data reported by stage of disease. | |
| Notes | Up to 30 months post‐treatment follow up; 3‐arm trial; approximately 1/3 patients in each arm received treatment or placebo after conservative surgery. Pregnancy data not separable for medical treatment alone. Co‐intervention with electrocautery at laparoscopy in 9 women and clomiphene in 11 women, but these treatments were evenly distributed across allocation groups. ITT analysis done. Funding: drugs supplied by Farmos Group Ltd Turku, Finland. Note that this is the same study as Telimaa 1988 (b). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly allocated' no further details provided. |
| Allocation concealment? | Unclear risk | Unclear; no details provided. |
| Blinding? All outcomes | High risk | No details of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Data reported on all women. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Telimaa 1988(b).
| Methods | Random allocation. Method not described | |
| Participants | Endometriosis on laparoscopy, infertility duration was unspecified. | |
| Interventions | Danazol 600 mg daily for 6 months, n=18 patients randomised and included versus placebo daily for 6 months, n=14 patients randomised and included. | |
| Outcomes | Clinical pregnancy as above. | |
| Notes | Up to 30 months post‐treatment, 3‐arm trial as above. Funding: drugs supplied by Farmos Group Ltd Turku, Finland.
No details of power calculation or ITT. Note that this is the same study as Telimaa 1988 (a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly allocated' no further details provided. |
| Allocation concealment? | Unclear risk | Unclear; no details provided. |
| Blinding? All outcomes | High risk | No details of blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Data reported on all patients. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Thomas 1987.
| Methods | Double‐blind RCT. Method of allocation not stated. Randomisation method not described. Attrition n=3, see quality table for details. | |
| Participants | UK study, 40/98 eligible women. All 40 women were subfertile. Age range 21‐36 years. Endometriosis of all stages; primary or secondary infertility of > 12 months; cycle 25‐35 days with biphasic BBT; normal tubal anatomy by HSG and laparoscopy; normal sperm by WHO criteria; normal thyroid function and prolactin. There had to be no impediment to the collection of oocytes by the tubal fimbria. | |
| Interventions | Gestrinone 2.5 mg twice weekly for 24 weeks, n=20
versus
placebo, one tablet twice weekly for 24 weeks, n=20. Follow up for one year. |
|
| Outcomes | Cumulative pregnancy rates at 6 and 12 months; AFS score at second‐look laparoscopy in 36 women; pregnancy not reported by stage of disease. | |
| Notes | Cumulative conception rates were reported on ITT basis. Other causes of infertility were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomly allocated but no details of randomisation procedure. |
| Allocation concealment? | Unclear risk | B ‐ unclear. No details of allocation concealment. |
| Blinding? All outcomes | Unclear risk | No details provided on who was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Three women from placebo group withdrew, no reasons provided. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
Vercellini 1999.
| Methods | Centralised randomisation, by telephone, stratified by study centre. Multicentre trial (n=19). Open‐label study. 59 women withdrawn from study, see risk of bias table for details. Power calculation conducted based on 1 year pain recurrence, no evidence of ITT. | |
| Participants | Italian study of 269 post‐surgical women. Mean age of goserelin group 30.1±5.4 years, mean age of expectant management group was 30±5.3 years. Women were pre‐menopausal with chronic pelvic pain undergoing conservative surgery at laparoscopy or laparotomy, with rAFS endometriosis staging scores of four or more and at least one or more moderate or severe symptom on Biberoglu and Berhman scale pre‐operatively. Exclusion: pregnant or breast feeding; other serious concomitant disease or treated with hormonal agents in previous 3 months. | |
| Interventions | Within one week of conservative surgery, women began depot goserelin 3.6 mg SC monthly for six months (n=133, women seeking pregnancy n=69) versus expectant management (n=134, women seeking pregnancy n=76). Women followed up monthly for 6 months post‐surgery, then bimonthly for six months, and then 6 monthly until end of 2 year follow‐up period. | |
| Outcomes | Conception, definition not provided; pain symptoms and symptom recurrence. | |
| Notes | Only reported pregnancies in subgroup of women wanting children. Study partly supported by Zeneca Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation sequence used. |
| Allocation concealment? | Low risk | A ‐ adequate. Allocation done centrally away from study centre. |
| Blinding? All outcomes | High risk | Open‐label study. |
| Incomplete outcome data addressed? All outcomes | Low risk | Two patients and one centre were withdrawn before randomisation because of incomplete CRFs. 26 goserelin treated women and 31 women in the expectant management group were withdrawn for reasons other than symptom recurrence or were excluded from the primary efficacy analysis due to major protocol violations. |
| Free of selective reporting? | High risk | Not followed up to live birth. |
BhCG`= beta human chorionic gonadotropin rAFS = revised American Fertility Society IM = intramuscular ITT = intention to treat SE = standard error BBT = basal body temperature AFS = American Fertility Society BTS = Beltsville thawing solution
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergquist 1990 | Data included elsewhere as part of a multicentre nafarelin study. |
| Biberoglu 1981 | Different doses of danazol compared. No treatment alternative. |
| Claesson 1989 | Data included elsewhere as part of a multicentre nafarelin study. |
| Dmowski 1982 | This was a dosing study and as such there was no comparison with other drugs. |
| Dodin 1991 | Pregnancy data not reported. |
| Fedele 1993 | Primary outcome pelvic pain. |
| Franssen 1992 | Pregnancy data not reported. |
| Golland 1990 | Pregnancy data not reported. |
| Henzl 1989 | Data included in more recently published report. |
| Hickock 1991 | Pregnancy not reported. |
| Hornstein 1990 | Randomised to different doses of gestrionone. No treatment alternative. |
| Jacobs 1991 | Data included elsewhere as part of a multicentre nafarelin trial. |
| Kennedy 1990 | Data included elsewhere as part of a multicentre nafarelin trial. |
| Kennedy 1990b | Data included elsewhere as part of a multicentre nafarelin trial. |
| Lemay 1991 | Pregnancy not reported. |
| Low 1984 | Different doses of danazol compared. No treatment alternative. |
| Moore 1981 | Various doses of danazol compared. No treatment alternative. |
| Nowroozi 1987 | Surgical trial. |
| Rickes 2002 | Patients had intrauterine insemination. |
| Rock 1993 | Pregnancy data not reported. |
| Rolland 1990 | Data included elsewhere as part of a multicentre nafarelin trial. |
| Shaw 1990 | Data included elsewhere as part of a multicentre nafarelin trial. |
| Siebel 1982 | Data included in more recently published report. |
| Telimaa 1987 | Data included in more recently published report. |
| Telimaa 1989 | Endocrine data only. Pregnancy data included in earlier report. |
| Telimaa 1990 | Endocrine data only. Pregnancy data included in earlier report. |
| Tsai 2004 | All patients had intrauterine insemination with clomiphene citrate. |
| Valimaki 1989 | Data included elsewhere as part of a multicentre nafarelin trial. |
| Wheeler 1992 | Pregnancy data not reported. |
| Wheeler 1993 | Pregnancy data not reported. |
| Worthington 1993 | Randomised to different doses of gestrinone. No treatment alternative. |
Differences between protocol and review
Original title: 'Ovulation suppression for endometriosis' did not accurately reflect the content and outcomes of the review. Title therefore changed to 'Ovulation suppression for endometriosis for women with subfertility'.
Contributions of authors
Ed Hughes: took the lead in writing the original review and updates. He was involved in all aspects of review design, search, inclusion, data extraction and summary.
Donna Fedorkow: began work on the review with John Collins and was co‐author of original paper published in Fertility and Sterility. This formed the basis of the Cochrane review. Donna began the design, search, inclusion and extraction processes.
John Collins: worked with Donna Ferdorkow on the original project, focusing on study inclusion and data summary.
Patrick Vandekerckhove: was the Co‐ordinating Editor in Leeds when the protocol and review were developed; helped with literature search, selected studies for inclusion and reviewed the manuscript.
Julie Brown: was involved in the latest update of this review. She ran the search and responded to the editors' comments, added the risk of bias table to bring the review up to current Cochrane standards, identified new papers and entered data as required.
Cindy Farquhar: checked the data for the latest update, rewrote the discussion and completed the final edits.
Sources of support
Internal sources
No sources of support supplied
External sources
Royal Commission on New Reproductive Technologies, Not specified.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
ANZ Zoladex 1996 {published data only}
- The Australian and New Zealand Zoladex Study Group. Goserelin depot versus danazol in the treatment of endometriosis the Australian/New Zealand experience. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1996;36(1):55‐60. [DOI] [PubMed] [Google Scholar]
Bayer 1988 {published data only}
- Bayer SR, Seibel MM, Saffan DS, Berger MJ, Taymor ML. Efficacy of danazol treatment for minimal endometriosis in infertile women: A prospective, randomized study. The Journal of Reproductive Medicine 1988;33:179‐83. [PubMed] [Google Scholar]
Bergqvist 1998 {published data only}
- Bergqvist A, Bergh T, Hogstrom L, Mattson S, Nordenskold F, Rasmussen C. Effects of triptorelin versus placebo on the symptoms of endometriosis. Fertility and Sterility 1998;69(4):702‐8. [DOI] [PubMed] [Google Scholar]
Bianchi 1999 {published data only}
- Bianchi S, Busaca M, Agnoli B, Candiani M, Calia C, Vignali M. Effects of 3 month therapy with danazol after laparoscopic surgery for stage III/IV endometriosis: a randomized study. Human Reproduction 1999;14(5):1335‐7. [DOI] [PubMed] [Google Scholar]
Bromham 1995 {published data only}
- Bromham DR, Rose GL, Wardle PG, Newton JR. A multicentre comparative study of gestrinone and danazol in the treatment of endometriosis. Journal of Obstetrics and Gynaecology 1995;15:188‐94. [Google Scholar]
Burry 1989 {published data only}
- Burry K, Patton P, Illingworth D. Metabolic changes during medical treatment of endometriosis: Nafarelin acetate versus danazol. American Journal of Obstetrics and Gynecology 1989;160(6):1454‐61. [DOI] [PubMed] [Google Scholar]
Busacca 2001 {published data only}
- Busacca M, Somigliana E, Bianchi S, Marinis S, Calia C, Candiani M, et al. Post operative GnRH analogue treatment after conservative surgery for symptomatic endometriosis stage III‐IV: a randomised controlled trial. Human Reproduction 2001;16(11):2399‐402. [DOI] [PubMed] [Google Scholar]
Cosson 2002 {published data only}
- Cosson M, Querleu D, Donnez J, Madelenat P, Koninckx P, Audebert A, et al. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: results of a prospective multicenter, randomized study. Fertility and Sterility 2002;77(4):684‐92. [DOI] [PubMed] [Google Scholar]
Dmowski 1989 {published data only}
- Dmowski WP, Tummon I, Pepping P, Radwanska E, Binor Z. Ovarian suppression induced with buserelin or danazol in the management of endometriosis: A randomized comparative study. Fertility and Sterility 1989;51:395‐400. [DOI] [PubMed] [Google Scholar]
Fedele 1989a {published data only}
- Fedele L, Bianchi S, Viezzoli T, Arcaini L, Candiani, GB. Gestrinone versus danazol in the treatment of endometriosis. Fertility and Sterility 1989;51:781‐5. [DOI] [PubMed] [Google Scholar]
Fedele 1989b {published data only}
- Fedele L, Bianchi S, Arcaini L, Vercellini P, Candiani GB. Buserelin versus danazol in the treatment of endometriosis‐associated infertility. American Journal of Obstetrics and Gynecology 1989;161:871‐6. [DOI] [PubMed] [Google Scholar]
Fedele 1992 {published data only}
- Fedele L, Parazzini F, Radici E, Bocciolone L, Bianchi S, Bianchi C, et al. Buserelin acetate versus expectant management in the treatment of infertility associated with minimal or mild endometriosis: A randomized clinical trial. American Journal of Obstetrics and Gynecology 1992;166:1345‐50. [DOI] [PubMed] [Google Scholar]
Fraser 1991 {published data only}
- Fraser IS, Shearman RP, Jansen RPS, Sutherland PD. A comparative treatment trial of endometriosis using the gonadotrophin‐releasing hormone agonist, nafarelin, and the synthetic steroid, danazol. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1991;31(2):158‐63. [DOI] [PubMed] [Google Scholar]
Harrison 2000 {published data only}
- Harrison RF, Barry‐Kinsella C. Efficacy of medoxyprogesterone treatment in infertile women with endometriosis: a prospective, randomized, placebo controlled study. Fertility and Sterility 2000;74(1):24‐30. [DOI] [PubMed] [Google Scholar]
Henzl 1988 {published data only}
- Henzl M, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal naferelin as compared with oral danazol for endometriosis. A multicenter double‐blind comparative clinical trial. New England Journal of Medicine 1988;318:485‐9. [DOI] [PubMed] [Google Scholar]
Loverro 2008 {published data only}
- Loverro G, Carriero C, Rossi A, Putignano G, Nicolardi V, Selvaggi L. A randomized study comparing triptorelin or expectant management following conservative laparoscopic surgery for symptomatic stage III‐IV endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;136:194‐8. [DOI] [PubMed] [Google Scholar]
NEET 1992 {published data only}
- Nafarelin European Endometriosis Trial Group (NEET). Nafarelin for endometriosis: A large‐scale, danazol‐controlled trial of efficacy and safety, with 1‐year follow‐up. Fertility and Sterility 1992;57(3):514‐22. [PubMed] [Google Scholar]
Noble 1979 {published data only}
- Noble AD, Letchworth AT. Medical treatment of endometriosis: A comparative trial. Postgraduate Medical Journal 1979;55:37‐9. [PubMed] [Google Scholar]
Parrazzini 1994 {published data only}
- Parazzini F, Fedele L, Busacca M, Falsetti L, Pellegrini S, Venturini PL, et al. Postsurgical medical treatment of advanced endometriosis: Results of a randomized clinical trial. American Journal of Obstetrics and Gynecology 1994;171(5):1205‐7. [DOI] [PubMed] [Google Scholar]
Shaw 1992 {published data only}
- Shaw RW. An open randomized comparative study of the effect of goserelin depot and danazol in the treatment of endometriosis. Zoladex Endometriosis Study Team. Fertility and Sterility 1992;58:265‐72. [DOI] [PubMed] [Google Scholar]
Shawki 2002 {published data only}
- Shawki O, Hamza H, Sattar M. Mild endometriosis, to treat or not to treat: randomised controlled trial comparing diagnostic laparoscopy with no further treatment versus post‐operative Zoladex in cases with infertility associated with Stage I‐II endometriosis. Fertility and Sterility 2002;13 Suppl:O36. [Google Scholar]
Telimaa 1988(a) {published data only}
- Telimaa S. Danazol and medroxprogesterone acetate inefficacious in the treatment of infertility in endometriosis. Fertility and Sterility 1988;50:872‐5. [DOI] [PubMed] [Google Scholar]
Telimaa 1988(b) {published data only}
- Telimaa S. Danazol and medroxprogesterone acetate inefficacious in the treatment of infertility in endometriosis. Fertility and Sterility 1988;50:872‐5. [DOI] [PubMed] [Google Scholar]
Thomas 1987 {published data only}
- Thomas E, Cooke I. Successful treatment of asymptomatic endometriosis: Does it benefit infertile women?. BMJ 1987;294:1117‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vercellini 1999 {published data only}
- Vercellini P, Crosignani P, Fadini R, Radici E, Belloni C, Sismondi P. A gonadotrophin‐releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. British Journal of Obstetrics and Gynaecology 1999;106:672‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bergquist 1990 {published data only}
- Bergquist C. Effects of nafarelin versus danazol on lipids and calcium metabolism. American Journal of Obstetrics and Gynecology 1990;162:589‐91. [DOI] [PubMed] [Google Scholar]
Biberoglu 1981 {published data only}
- Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: Short‐term and long‐term effectiveness. American Journal of Obstetrics and Gynecology 1981;139:645‐54. [DOI] [PubMed] [Google Scholar]
Claesson 1989 {published data only}
- Claesson B, Bergquist C. Clinical experience treating endometriosis with nafarelin. The Journal of Reproductive Medicine 1989;34 Suppl(12):1025‐8. [PubMed] [Google Scholar]
Dmowski 1982 {published data only}
- Dmowski WP, Kapetanakis E, Scommegna A. Variable effects of danazol on endometriosis at 4 low dose levels. Obstetrics and Gynecology 1982;59(4):408‐15. [PubMed] [Google Scholar]
Dodin 1991 {published data only}
- Dodin S, Lemay A, Maheux R, Dumont M, Turcot‐Lemay L. Bone mass in endometriosis patients treated with GnRH agonist implant or danazol. Obstetrics and Gynecology 1991;77:410‐5. [PubMed] [Google Scholar]
Fedele 1993 {published data only}
- Fedele L, Marchini M, Bianchi S, Baglioni A, Zanotti F. Vaginal patterns during danazol and buserelin acetate therapy for endometriosis: Structural and ultrastructural study. Fertility and Sterility 1993;59(6):1191‐5. [DOI] [PubMed] [Google Scholar]
Franssen 1992 {published data only}
- Franssen AMHW, Heijden PFM, Thomas CMG, Doesburg WH, Willemsen WNP, Rolland R. On the origin and significance of serum CA‐125 concentrations in 97 patients with endometriosis before, during, and after buserelin acetate, nafarelin, or danazol. Fertility and Sterility 1992;57:974‐9. [DOI] [PubMed] [Google Scholar]
Golland 1990 {published data only}
- Golland IM, Vaughan‐Williams CA, Shalet SM, Elstein M. Influence of danazol and goserelin on insulin and glucagon in non‐obese women with endometriosis. Acta Endocrinologica 1990;123(4):405‐10. [DOI] [PubMed] [Google Scholar]
Henzl 1989 {published data only}
- Henzl MR. Role of nafarelin in the management of endometriosis. The Journal of Reproductive Medicine 1989;34 Suppl(12):1021‐4. [PubMed] [Google Scholar]
Hickock 1991 {published data only}
- Hickock LR, Burry KA, Cohen NL, Moore DE, Dahl KD, Soules MR. Medical treatment of endometriosis: A comparison of the suppressive effects of danazol and nafarelin on reproductive hormones. Fertility and Sterility 1991;56:622‐7. [DOI] [PubMed] [Google Scholar]
Hornstein 1990 {published data only}
- Hornstein MD, Gleason RE, Barbieri RL. A randomized double‐blind prospective trial of two doses of gestrinone in the treatment of endometriosis. Fertility and Sterility 1990;53(2):237‐41. [DOI] [PubMed] [Google Scholar]
Jacobs 1991 {published data only}
- Jacobs LA, Field CS, Thie JL, Coulam CB. Treatment of endometriosis with the GnRH agonist nafarelin acetate. International Journal of Fertility 1991;36(1):30‐5. [PubMed] [Google Scholar]
Kennedy 1990 {published data only}
- Kennedy SH, Williams IA, Brodribb J, Barlow DH, Shaw RW. A comparison of nafarelin acetate and danazol in the treatment of endometriosis. Fertility and Sterility 1990;53:998‐1003. [DOI] [PubMed] [Google Scholar]
Kennedy 1990b {published data only}
- Kennedy SH, Starkey PM, Sargent IL, Hicks BR, Barlow DH. Antiendometrial antibodies in endometriosis measured by an enzyme‐linked immunosorbent assay before and after treatment with danazol and nafarelin. Obstetrics and Gynecology 1990;75:914‐8. [PubMed] [Google Scholar]
Lemay 1991 {published data only}
- Lemay A, Brideau N‐A, Forest J‐C, Dodin S, Maheux R. Cholesterol fractions and apolipoproteins during endometriosis treatment by a gonadotrophin releasing horomone (GnRH) agonist implant or by danazol. Clinical Endocrinology 1991;35:305‐10. [DOI] [PubMed] [Google Scholar]
Low 1984 {published data only}
- Low RAL, Roberts ADG, Lees DAR. A comparative study of various dosages of danazol in the treatment of endometriosis. British Journal of Obstetrics and Gynaecology 1984;91:167‐71. [DOI] [PubMed] [Google Scholar]
Moore 1981 {published data only}
- Moore EE, Harger JH, Rock JA, Archer DF. Management of pelvic endometriosis with low‐dose danazol. Fertility and Sterility 1981;36:15‐9. [PubMed] [Google Scholar]
Nowroozi 1987 {published data only}
- Nowroozi K, Chase JS, Check JH, Wu CH. The importance of laparoscopic coagulation of mild endometriosis in infertile women. International Journal of Fertility 1987;32:442‐4. [PubMed] [Google Scholar]
Rickes 2002 {published data only}
- Rickes D, Nickel I, Kleinstein J. Increased pregnancy rates following ultralong postoperative therapy with GnRH analogues in patients with endometriosis. Fertility and Sterility 2002;11 Suppl:O31. [DOI] [PubMed] [Google Scholar]
Rock 1993 {published data only}
- Rock JA, Truglia JA, Caplan RJ. Zoladex (goserelin acetate implant) in the treatment of endometriosis: a randomized comparison with danazol. The Zoladex Endometriosis Study Group. Obstetrics and Gynecology 1993;82(2):198‐205. [PubMed] [Google Scholar]
Rolland 1990 {published data only}
- Rolland R, Heijden PFM. Nafarelin versus danazol in the treatment of endometriosis. American Journal of Obstetrics and Gynecology 1990;162:586‐8. [DOI] [PubMed] [Google Scholar]
Shaw 1990 {published data only}
- Shaw RW. Nafarelin in the treatment of pelvic pain caused by endometriosis. American Journal of Obstetrics and Gynecology 1990;162:574‐6. [DOI] [PubMed] [Google Scholar]
Siebel 1982 {published data only}
- Siebel MM, Berger MJ, Weinstein FG, Taymor ML. The effectiveness of danazol on subsequent fertility in minimal endometriosis. Fertility and Sterility 1982;38:534‐7. [DOI] [PubMed] [Google Scholar]
Telimaa 1987 {published data only}
- Telimaa S, Puolakka J, Ronnberg L, Kauppila. Placebo‐controlled comparison of danazol and high‐dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecological Endocrinology 1987;1(1):13‐23. [DOI] [PubMed] [Google Scholar]
Telimaa 1989 {published data only}
- Telimaa S, Kauppila A, Ronnberg L, Suikkari A‐M, Seppala M. Elevated serum levels of endometrial secretory protein PP14 in patients with advanced endometriosis. American Journal of Obstetrics and Gynecology 1989;161:866‐71. [DOI] [PubMed] [Google Scholar]
Telimaa 1990 {published data only}
- Telimaa S, Apter D, Reinila M, Ronnberg L, Kauppila A. Placebo‐controlled comparison of hormonal and biochemical effects of danazol and high‐dose medroxyprogesterone acetate. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1990;36:97‐105. [DOI] [PubMed] [Google Scholar]
Tsai 2004 {published data only}
- Tsai Y‐L, Hwang J‐L, Loo T‐C, Cheng W‐C, Chuang J, Seow K‐M. Trials where medical treatment was administered after surgical treatment for endometriosis were included. The Journal of Reproductive Medicine 2004;49:955‐9. [PubMed] [Google Scholar]
Valimaki 1989 {published data only}
- Valimaki M, Nilsson CG, Roine R, Ylikorkala O. Comparison between the effects of nafarelin and danazol on serum lipids and lipoproteins in patients with endometriosis. The Journal of Clinical Endocrinology and Metabolism 1989;69(6):1097‐103. [DOI] [PubMed] [Google Scholar]
Wheeler 1992 {published data only}
- Wheeler JM, Knittle JD, Miller JD. Depot leuprolide versus danazol in treatment of women with systematic endometriosis. I. Efficacy results. American Journal of Obstetrics and Gynecology 1992;167:1367‐71. [DOI] [PubMed] [Google Scholar]
Wheeler 1993 {published data only}
- Wheeler JM, Knittle JD, Miller JD. Depot leuprolide acetate versus danazol in the treatment of women with symptomatic endometriosis: A multicenter, double‐blind randomized clinical trial. II. Assessment of safety. The Lupron Endometriosis Study Group. American Journal of Obstetrics and Gynecology 1993;169:26‐33. [DOI] [PubMed] [Google Scholar]
Worthington 1993 {published data only}
- Worthington M, Irvine LM, Crook D, Lees B, Shaw RW, Stevenson JC. A randomized comparative study of the metabolic effects of two regimens of gestrinone in the treatment of endometriosis. Fertility and Sterility 1993;59(3):522‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Am Fertil Soc 1985
- American Fertility Society. Revised American Fertility Society (RAFS) classification of endometriosis. Fertility and Sterility 1985;43:351‐2. [DOI] [PubMed] [Google Scholar]
Gleicher 1987
- Gleicher N, El‐Roeiy A, Confino E, Friberg J. Is endometriosis an autoimmune disease?. Obstetrics and Gynecology 1987;70:115‐21. [PubMed] [Google Scholar]
Hughes 1993
- Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis‐associated infertility. Fertility and Sterility 1993;59:963‐70. [PubMed] [Google Scholar]
Meyer 1919
- Meyer R. The status of adenomyosis and adenomyoma, with a discussion of seroepithelial adenomyosis and sarcomatose adenomyometritis [Ueber den Stand der Frage of Adenomyositis und Adenomyoma in Algemeinen und Insbesondere ueber Adenomyositis seroepithelialis und Adenomyometritis sarcomatosa]. Zel Gynaecol 1919;43:745‐50. [Google Scholar]
Sampson 1927
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. American Journal of Obstetrics and Gynecology 1927;14:422‐69. [Google Scholar]
Schenken, 1997
- Schenken RS, Guzick DS. Revised endometriosis classification: 1996 [Revised endometriosis classification: 1996]. Fertility and Sterility May 1997;67(5):815‐6. [DOI] [PubMed] [Google Scholar]
Schweppe 1988
- Schweppe KW. Etiology pathogenesis and natural history of endometriosis. In: Rock JA, Schweppe KW editor(s). Recent Advances in Management of Endometriosis. Lancashire: Parthenon Publishing Group, 1988:13‐30. [Google Scholar]
Yuzpe 1986
- Yuzpe AA, Taylor PJ. Endoscopy in the patient with endometriosis. In: Gomel V, Taylor PJ, Rioux AA, & Yuzpe AA editor(s). Laparoscopy and Hysteroscopy in Gynecologic Practice. Chicago: Yearbook Medical Publishers, 1986:122. [Google Scholar]
References to other published versions of this review
Hughes 2003
- Hughes E, Fedorkow D, Collins J, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]


