Abstract
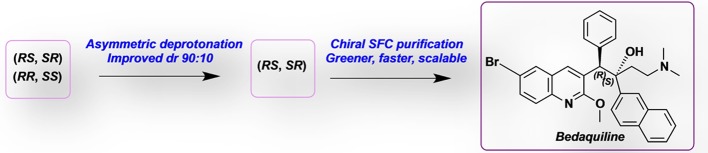
Bedaquiline (BDQ) is the most critical pharmaceutical in the world for treating multidrug-resistant Mycobacterium tuberculosis. Despite it being highly effective, BDQ asymmetric synthesis remains a challenge. Herein, the influence of chiral bases, namely, bis(1-phenylethyl)amine, bisoxazoline, and sparteine on the diastereoselective lithiation reaction to obtain BDQ was investigated. The highest diastereoselective ratio (dr) emerged as 90:10 from the (+)-bis[(R)-1-phenylethyl] lithium amide. This is a significant improvement from the 50:50 dr achieved from the commercial synthesis. Thereafter, the desired (90:10 RS, SR) diastereomeric mixture was easily isolated via a gravity column and subjected to chiral supercritical fluid chromatography (SFC) to access the desired enantiomer (1R, 2S)-BDQ. The advantages of this procedure are enhanced diastereoselection as well as a greener, faster way to achieve excellent enantioseparation (up to 1.0 g scale).
Introduction
Tuberculosis (TB) is a global endemic cause of disability and death.1 It is estimated that up to one-third of the world’s population has been infected with TB, more commonly in areas where crowded housing, poverty, poor general health, malnutrition, HIV coinfection, and other social disruptions are present.2 It is mainly caused by an infection with Mycobacterium tuberculosis (M. tuberculosis). M. tuberculosis has developed multidrug resistance (MDR) toward first-line TB treatment due to mutation of the pathogen upon patient discontinuation or an incorrect drug prescription.3 MDR-TB regimens are more intensive, prolonged, expensive, and often unsuccessful. Therefore, there is an urgent calling for development of new agents that may shorten the duration of therapy, have minimal interaction with antiretroviral drugs,4 have simple dosage schedules, improve adherence, decrease the risk of relapse while reducing mortality, and potentially control the MDR-TB epidemic.5
In 2005, Andries and co-workers reported the first MDR antituberculosis drug known as TMC 2076 at the time and now referred to as bedaquiline (BDQ) fumarate marketed under the trade name Sirturo.7 It was given fast approval by the Food and Drug Administration (FDA) in late 2012 under the accelerated approval program.8 It emerged as the first antituberculosis-specific drug in 40 years; it inhibits the proton pump for M. tuberculosis’s adenosine triphosphate (ATP) synthase representing a novel mechanism of action for pulmonary drug-resistant tuberculosis.9 BDQ has a bactericidal and sterilizing activity against M. tuberculosis by hindering the ATP generation whereby it targets the central region of a c subunit of the ATP synthase enzyme. It has proven to be a significant step toward providing a shorter and more tolerable regimen for patients infected with drug-resistant tuberculosis.10 BDQ appears on the World Health Organization’s List of Essential Medicines and is only commercially available in a handful of countries.11 The cost of BDQ (per 6-month treatment) ranges from US$30,000 to US$900, from high- to low-income countries, respectively.12
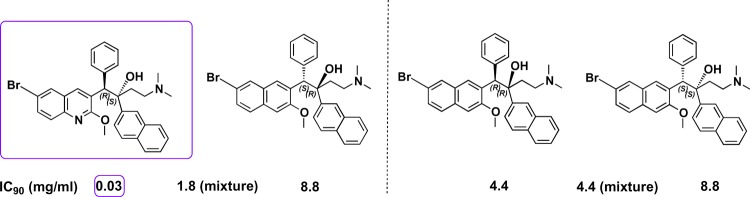
On a molecular level, BDQ (Figure 1) contains two stereogenic centers; therefore, in the absence of an external chiral medium, its chemical synthesis will lead to four isomers, which are distributed in a mixture of two pairs of diastereoisomers, namely, (RS, SR) and (RR, SS). The (1R, 2S) stereoisomer is the most active isomer against tuberculosis and is known as BDQ. Andries et al. performed the experiments to test the activity of all four stereoisomers against multidrug-resistant strains of M. tuberculosis. They reported the concentration that causes 90% inhibition to bacterial growth (IC90) for each isomer as well as that of the mixture of the two isomers. The (R,S) and (S,R) gave values of 0.03 and 8.8 μg/mL, respectively, and when combined, produced a value of 1.8 μg/mL, as shown in Figure 1. The (R,R) and (S,S) isomers gave IC90 values of 4.4 and 8.8 μg/mL, respectively, while the mixture yielded a value of 4.4 μg/mL.13 These results demonstrated the need to optimally isolate the (R,S) isomer to exclusively treat M. tuberculosis.
Figure 1.
Four isomers of bedaquiline with the most active isomer, as depicted with its antimycobacterial activity (IC90) against M. tuberculosis.13
The initial synthesis conducted by Johnson & Johnson reported the one-step deprotonation of the starting quinoline (1) with LDA. They accomplished the separation of the desired diastereomer from the mixture by taking advantage of the uniqueness of the diastereomer’s solubility in acetic acid/THF (tetrahydrofuran). Later, they separated the mixture of two enantiomers using a chiral resolution agent. However, after separation, three-quarters of the final products were discarded, half was the undesired diastereomer, and a quarter was the undesired enantiomer.14 The overall yield obtained was merely 1%. This method resulted in low yields, poor enantioselectivity, and low efficiency, which opened the door for possible improvement. To date, there are a few synthetic routes published for the preparation of BDQ.15 The asymmetric routes include the work done by Saga et al. that developed a convergent asymmetric synthesis. Apart from the complexity of their method and the use of expensive reagents, their synthesis involved 12 linear steps from commercially available compounds with an overall yield of 5%.16 Later, Chandrasekhar and co-workers reported the asymmetric synthesis of the desired isomer. This method took the linear tactic in 10 steps with an overall yield of 12%.17 The most likely (Janssen Pharmaceutical) industrial process relies on a one-step lithiation reaction (using LDA as the base) after which chiral resolution (with binaphthol phosphate) is being utilized to isolate the desired isomer for its large-scale production;18 however, drawbacks still include the loss of the desired diastereomer in the first step (due to the absence of chiral environment) and low overall yields. The diastereomer (RS, SR) is separated from the crude mixture of diastereomers (50:50) by recrystallization. Subsequently, the diastereomer (RS, SR) is then converted into its diastereomeric salt with a chiral resolution agent from which the desired stereoisomer (R, S) is isolated by recrystallization following neutralization of the salt to afford the free drug as a single enantiomer. The chiral resolution adds two more steps to the already low-yielding one-pot procedure.
Herein, we report the influence of replacing the nonchiral base LDA in the lithiation step to form BDQ with chiral bases, namely, bis-phenylethylamine,19−21 bisoxazoline,22,23 and sparteine24−26 with the intention of improving the yield of the desired diastereoisomer (RS, SR) without increasing the number of synthetic steps. Thereafter, we report the optimization and isolation of the desired enantiomer (R,S) from the mixture using chiral supercritical fluid chromatography (SFC).27
Nowadays, SFC has become a widely used technique on both analytical and preparative scales due to its advantages over traditional high-performance liquid chromatography.28 A remarkably faster separation speed, lower viscosity that allows higher flow rates, and high diffusivity, which result in an overall better efficiency and reduction of using organic solvents (as much as 90% less), are achieved with the use of inexpensive CO2. These “green” aspects coupled to the ease of sample collection make SFC an attractive option to be used to assist in accessing pharmaceutically important molecules27 such as BDQ, especially when the purification step could substitute some of the expensive steps within the synthetic route.
Results and Discussion
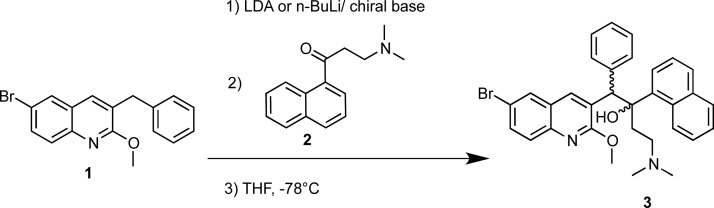
First, we conducted the synthesis of BDQ using lithium diisopropylamide (LDA) as a base as per the original procedure (Scheme 1).14 This route used 5:5 equiv of n-BuLi/diisopropylamine in THF, the deprotonation step took place for 30 min and the reaction with the electrophile for 3 h at −78 °C.
Scheme 1. Lithiation Reaction between the Appropriate 3-Benzylquinoline (1) and 3-(Dimethylamino)phenylpropan-1-one (2) To Obtain Bedaquiline (3).
This method formed the product mixture with a 50:50 diastereomeric ratio (dr) of (RS, SR) and (RR, SS), with approximately 10 and 17% yield, respectively, which was in keeping with the original report.14
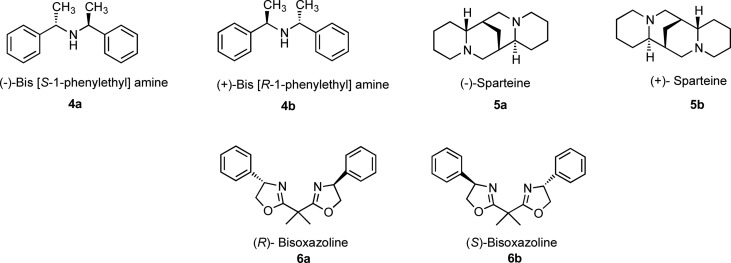
We aimed to substitute the diisopropylamine with a chiral base, that is, 4a-b, 5a-b, and 6a-b (typically reported to be used in asymmetric deprotonation lithiation) as represented in Figure 2 in hope of increasing the dr of the desired isomer.
Figure 2.
Chiral bases utilized in the lithiation step.
Following the conditions from the LDA reaction, the single enantiomer 4a(19) as the chiral base was evaluated using 5:5 equiv of n-BuLi/4a. After careful examination of the results obtained during the lithiation step, we discovered that the chiral complex behaved very differently to the nonchiral LDA complex. Also, further meticulous care had to be taken to prevent the complex from being quenched with regard to the introduction of moisture from the solvent, apparatus setup, glassware used, and the concentration of the n-BuLi that had to be consistently checked. Although the reaction took place, there was no desired product formation; instead, the abstraction of the bromine atom took place from the commercially available quinoline 1 as well as the product 3 (refer to Scheme 1), which could have been a result of the high reactivity of the n-BuLi complex. After further optimization, the n-BuLi/4a concentration of 3:2 provided a 5% conversion of 1 to 3. Further lowering the amount of the Li complex resulted in no reaction at all. (The following optimization results can be found in the Supporting Information.)
Next, we investigated the temperature of the deprotonation step (of quinoline 1) since the literature reports this step to play a crucial role in the reactivity of certain substrates.20,24 The temperature was increased within the range of −60 to 0 °C to determine the optimal energy required for the chiral complex to deprotonate the substrate (1). The highest conversion was observed at −20 °C, which was around 15% conversion. The temperature of 0 °C resulted in a quite messy reaction indicating several undesirable reactions taking place. At this point, we considered using solvents such as toluene, diethyl ether, cumene, and hexane as they have been reported as typical solvents to conduct lithiation.29,30 However, the limitation was the insolubility of the reagents irrespective of concentration or temperature. Thus, THF remained as the only option to conduct further experiments.
Thereafter, the deprotonation time was optimized (from 15 to 120 min) using the same chiral base 4a to investigate the possibility of obtaining a higher % conversion and/or a better dr. The deprotonation time of 60 min resulted in the highest % conversion of 33% even though the dr obtained was still 50:50. The dr was measured by integration of the chiral proton (H6) where 5.89 ppm (desired) and 5.73 ppm (undesired) were determined from the crude 1H NMR spectrum.
Previous studies have indicated that the selectivity of the chiral Li complex, when using base 4a-b for deprotonation, can be influenced by the formation of mixed aggregates between LiCl, formed by the reaction of the respective lithium complex with 1, and 4a-b, which is more reactive and selective than the monomer or dimer of 4a-b.31 Simpkins and Bunn reported a substantial improvement in the stereochemistry of chiral base-mediated deprotonation reaction products with the presence of added LiCl.32 There are several reports that generate the 4a-b.LiCl mixed heterodimeric Li-amide/LiCl complexes, that is, either from the direct addition of LiCl to 4a-b or the addition of BuLi to the 4a-b hydrochloride salt.21,31,33
Thus, in the next experiment, 4a was treated first with LiCl (2 equiv) to generate 4a·LiCl followed by the sequential addition of 1 and 2. In this case, the dr of the product changed to 45:55. Thereafter, the deprotonation of 1 was carried out with the in situ-generated 4a·LiCl that was obtained by treatment of the 4a hydrochloride salt with 3 equiv of n-BuLi. This reaction yielded a crude mixture with 30:70 dr in which 30% was the desired (RS, SR) and 70% was the (RR, SS) undesired diastereomer.
In the hope of reversing the dr to favor the formation of the desired diastereomer, we then conducted an experiment with the opposite enantiomer of 4a, that is, using n-BuLi/4b. To our delight, this led to 90% of the desired (RS, SR) and 10% of the undesired (RR, SS) diastereomer. At this point, we still tried to improve the conversion with longer reaction times. However, this increased the formation of the undesired diastereomer. A possible reason could be that the mixed 4b–LiCl complex is equilibrated toward a less active complex when the lithium alkoxide product is increasing in concentration, for example, by competing for LiCl from the Li-amide or forming less reactive mixed Li-amide/Li-alkoxide complexes.34 This proved to be a limitation in the reaction from going to completion. The summary of these modifications and dr obtained are shown in Table 1.
Table 1. Summary of Modifications Done with the Chiral Ligand 4a-b.
| entry | base | dr | % conversiona |
|---|---|---|---|
| 1 | LDA | 50:50 | 31 |
| 2 | n-BuLi/4a | 50:50 | 33 |
| 3 | n-BuLi/4a·LiClb | 45:55 | 31 |
| 4 | n-BuLi/4a·LiClc | 30:70 | 32 |
| 5 | n-BuLi/4b·LiCl | 90:10 | 33 |
% Conversion was determined by liquid chromatography–mass spectrometry using a YMC-Triart-C18 column unless stated otherwise, and the dr was determined by crude 1H NMR. The deprotonation step was conducted at −20 °C for 60 min; thereafter, the reaction proceeded at −78 °C. All optimization reactions were carried out on a 0.15 mmol scale unless stated otherwise.
Addition of LiCl to neutral 4a.
Addition of n-BuLi to 4a·HCl salt.
Subsequently, we focused our attempts on using the other chiral bases, that is, sparteine and bisoxazoline using our optimized conditions. With chiral complexes made from 5a and 5b, we again encountered the formation of 250 m/z during the deprotonation, which indicated abstraction of bromine taking place from 1. Further attempts to optimize the reaction conditions were endeavored such as modifications in temperatures, chiral base equivalents, and the use of s-BuLi instead of n-BuLi as well as the reaction times. However, the optimal deprotonation still did not occur. For 6a and 6b, no reaction took place due to the failure of the deprotonation step to occur, as only the starting material was observed.
With the optimal conditions in hand, with n-BuLi/4b·LiCl (Table 1, entry 5), the reaction was carried out on a 1 g scale. The mixture of the (RS, SR) and (RR, SS) diastereomers (39% yield, 330 mg, dr of 90:10) thus obtained was then separated by a gravity column of which the desired diastereomer (86%, 285 mg, (RS, SR)) and undesired diastereomer (10%, 35 mg, (RR, SS)) were obtained. The isolated desired diastereomer was then subjected to chromatographic separation using a compact Sepiatec Prep basic SFC system fitted with a chiral analytical column for separation of the enantiomers. The simplified handling of this instrument proved to be very convenient when using the stacked injection feature. The enantiomers were successfully eluted under isocratic conditions using stacked injections to afford the desired enantiomer (1R, 2S; 130 mg) and the undesired enantiomer (1S, 2R; 142 mg). The overall reaction yield was 13% and >99 ee. The BDQ obtained from this study was further used to investigate its central nervous system penetration in an animal model.35 During the preparation of this manuscript, we came across a 2017 patent written in Chinese having applied a similar concept to make BDQ. They made use of a chiral amino alcohol as the chiral inducer for the lithiation step in the presence of a mixture of both LDA and n-BuLi.36 The obtained ratio of the diastereomers (RS, SR) and (RR, SS) was 80:20; thereafter, they made use of a chiral resolution agent to obtain the desired enantiomer in a 15% overall yield. This makes the study herein only one of the two reports that have improved the ratio of the diastereomers for the synthesis of this important drug.
Conclusions
We have achieved a highly diastereoselective synthesis of BDQ using the optimized lithiation conditions with the chiral ligand bis(1-phenylethyl)amine 4b. The use of 3:2 equiv of n-BuLi/4b afforded the dr of 90:10 for (RS, SR) and (RR, SS) diastereomers, respectively, with 33% yield, compared to the dr of 50:50 obtained from syntheses in the absence of a chiral environment. This suggests that such a method can be utilized for the diastereoselective synthesis of BDQ. Thereafter, the desired diastereomer (RS, SR) was easily isolated via a gravity SiO2 column and subjected to chiral SFC separation to obtain the desired (1R, 2S) enantiomer with an overall reaction yield of 13%; this is similar to other attempts reported to advance the BDQ asymmetric synthesis. To this end, the reaction turnover requires improvement without compromising the diastereoselectivity, which poses a challenge. There is also a gap for industrial chemists to further address practical isolation procedures toward the larger-scale synthesis of BDQ. Nevertheless, the advantages of this technique are improved diastereoselection and a greener and faster way to achieve excellent enantioseparation (scalable) to obtain BDQ in the laboratory.
Experimental Section
Optimized Procedure Using 4b·HCl
The salt was made according to the reported procedure.31 To a solution of 4b·HCl salt (1.6 g, 2 equiv) in THF (20.0 mL), 5.61 mL of 1.6 M n-BuLi in hexane (9.2 mmol, 3 equiv) was added dropwise at −78 °C under the flow of argon. The solution was stirred for 10 min, warmed up to room temperature, and stirred further for 1 h. The clear yellow solution was recooled to −78 °C and again stirred for 1 h to form a pinkish solution of the chiral base complex.
Lithiation Step
A solution of 1 (1.0 g, 1 equiv) in THF (10.0 mL) was slowly added to an in situ-formed chiral base from above and stirred at −20 °C. After exactly 1 h, a solution of 2 (0.83 g, 1.2 equiv) in THF (10.0 mL) was added dropwise over 20 min via a cannula, and the resulting solution was stirred at −78 °C for 3 h. The reaction was warmed up to room temperature, quenched with aqueous NaCl (20.0 mL), and then extracted with EtOAc (5× 10.0 mL). The organic layer was dried over anhydrous MgSO4 and concentrated in vacuo to give a clear yellow oil. The crude product was further purified by flash column chromatography (1:1 EtOAc/hexane). The Rf values were 0.30 for the undesired (35 mg) and 0.44 for the desired (285 mg) diastereomer obtained as white solids. The (RS, SR) was then subjected to semipreparative SFC for separation, which provided the desired enantiomer (1R,2S) (130 mg).
Acknowledgments
The authors would like to thank the South African National Research Foundation, College of Health Sciences-UKZN, and the Medical Research Council for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04037.
Further experimental details, optimization tables, and full characterization data (PDF).
The authors declare no competing financial interest.
Supplementary Material
References
- Wallis R. S.; Maeurer M.; Mwaba P.; Chakaya J.; Rustomjee R.; Migliori G. B.; Marais B.; Schito M.; Churchyard G.; Swaminathan S.; Hoelscher M.; Zumla A. Tuberculosis—advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect. Dis. 2016, 16, e34–e46. 10.1016/S1473-3099(16)00070-0. [DOI] [PubMed] [Google Scholar]
- AlMatar M.; AlMandeal H.; Var I.; Kayar B.; Köksal F. New drugs for the treatment of Mycobacterium tuberculosis infection. Biomed. Pharmacother. 2017, 91, 546–558. 10.1016/j.biopha.2017.04.105. [DOI] [PubMed] [Google Scholar]
- Millard J.; Ugarte-Gil C.; Moore D. A. J. Multidrug resistant tuberculosis. BMJ 2015, 350, h882. 10.1136/bmj.h882. [DOI] [PubMed] [Google Scholar]
- Koul A.; Arnoult E.; Lounis N.; Guillemont J.; Andries K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483. 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- Chahine E. B.; Karaoui L. R.; Mansour H. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. Ann. Pharmacother. 2014, 48, 107–115. 10.1177/1060028013504087. [DOI] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Göhlmann H. W. H.; Neefs J.-M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science 2005, 307, 223. 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Jones D. Tuberculosis success. Nat. Rev. Drug Discovery 2013, 12, 175. 10.1038/nrd3957. [DOI] [PubMed] [Google Scholar]
- Sacksteder K. A.; Protopopova M.; Barry C. E.; Andries K.; Nacy C. A. Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action. Future Microbiol. 2012, 7, 823–837. 10.2217/fmb.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A. H.; Pym A.; Grobusch M.; Patientia R.; Rustomjee R.; Page-Shipp L.; Pistorius C.; Krause R.; Bogoshi M.; Churchyard G.; Venter A.; Allen J.; Palomino J. C.; de Marez T.; van Heeswijk R. P. G.; Lounis N.; Meyvisch P.; Verbeeck J.; Parys W.; de Beule K.; Andries K.; Mc Neeley D. F. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 2009, 360, 2397–2405. 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- Pontali E.; Sotgiu G.; D’Ambrosio L.; Centis R.; Migliori G. B. Bedaquiline and multidrug-resistant tuberculosis: a systematic and critical analysis of the evidence. Eur Respiratory J. 2016, 47, 394. 10.1183/13993003.01891-2015. [DOI] [PubMed] [Google Scholar]
- WHO Model List of Essential Medicines (19th List)″ (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- The selection and use of essential medicines: Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children), WHO technical report series ; no. 994,.
- Guillemont J.; Meyer C.; Poncelet A.; Bourdrez X.; Andries K. Diarylquinolines, synthesis pathways and quantitative structure–activity relationship studies leading to the discovery of TMC207. Future Med. Chem. 2011, 3, 1345–1360. 10.4155/fmc.11.79. [DOI] [PubMed] [Google Scholar]
- Van Gestel J. F. E.; Guillemont J. E. G.; Venet M. G.; Poignet H. J. J.; Decrane L. F. B.; Odds F. C.. Novel mycobacterial inhibitors In US 20,050,148,581 A1: USA, 2005.
- Ding H. X.; Leverett C. A.; Kyne R. E. Jr.; Liu K. K.-C.; Sakya S. M.; Flick A. C.; O’Donnell C. J. Synthetic approaches to the 2012 new drugs. Bioorg. Med. Chem. 2014, 22, 2005–2032. 10.1016/j.bmc.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Saga Y.; Motoki R.; Makino S.; Shimizu Y.; Kanai M.; Shibasaki M. Catalytic Asymmetric Synthesis of R207910. J. Am. Chem. Soc. 2010, 132, 7905–7907. 10.1021/ja103183r. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S.; Babu G. S. K.; Mohapatra D. K. Practical Syntheses of (2S)-R207910 and (2R)-R207910. Eur. J. Org. Chem. 2011, 2011, 2057–2061. 10.1002/ejoc.201001720. [DOI] [Google Scholar]
- Porstmann F. R.; Horns S.; Bader T.. Process for preparing (alpha S, beta R)-6-bromo-alpha-[2-(dimethylamino) ethyl]-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-3-quinolineethanol. In WO2006/125769; Janssen Pharmaceutica NV: USA, 2006.
- Simpkins N. S. Recent Advances in Asymmetric Synthesis Using Chiral Lithium Amide Bases. Quim. Nova 1995, 18, 295–297. 10.1351/pac199668030691. [DOI] [Google Scholar]
- Clayden J.; Menet C. J.; Mansfield D. J. Asymmetric deprotonation and dearomatising cyclisation of N-benzyl benzamides using chiral lithium amides: formal synthesis of (−)-kainic acid. Chem. Commun 2002, 38–39. 10.1039/b109188c. [DOI] [PubMed] [Google Scholar]
- Lutz V.; Park N.; Rothe C.; Krüger C.; Baro A.; Laschat S. Doing it twice: asymmetric deprotonation/alkylation of Weiss diketone derivatives as key steps in the functionalization of bicyclo [3.3. 0] octanes. Eur. J. Org. Chem. 2013, 2013, 761–771. 10.1002/ejoc.201201409. [DOI] [Google Scholar]
- Nakamura S.; Nakagawa R.; Watanabe Y.; Toru T. Highly Enantioselective Reactions of Configurationally Labile α-Thioorganolithiums Using Chiral Bis(oxazoline)s via Two Different Enantiodetermining Steps. J. Am. Chem. Soc. 2000, 122, 11340–11347. 10.1021/ja0025191. [DOI] [Google Scholar]
- Nakamura S.; Ito Y.; Wang L.; Toru T. Enantioselective Reaction of α-Lithiated Dithioacetals Using Chiral Bis(oxazoline)s: New Chiral Formyl Anion Equivalents. J. Org. Chem 2004, 69, 1581–1589. 10.1021/jo035558e. [DOI] [PubMed] [Google Scholar]
- Casimiro M.; Oña-Burgos P.; Meyer J.; Styra S.; Kuzu I.; Breher F.; Fernández I. On the Solution Behaviour of Benzyllithium·(−)-Sparteine Adducts and Related Lithium Organyls – A Case Study on Applying 7Li, 15N{1H} HMQC and Further NMR Methods, Including Some Investigation into Asymmetric Synthesis. Chem. – Eur. J. 2013, 19, 691–701. 10.1002/chem.201202346. [DOI] [PubMed] [Google Scholar]
- Gross K. M. B.; Jun Y. M.; Beak P. Asymmetric Deprotonations: Lithiation of N-(tert-Butoxycarbonyl)indoline with sec-Butyllithium/(−)-Sparteine. J. Org. Chem 1997, 62, 7679–7689. 10.1021/jo9708856. [DOI] [Google Scholar]
- Wu S.; Lee S.; Beak P. Asymmetric deprotonation by BuLi/(−)-sparteine: Convenient and highly enantioselective syntheses of (S)-2-aryl-Boc-pyrrolidines. J. Am. Chem. Soc. 1996, 118, 715–721. 10.1021/ja9524661. [DOI] [Google Scholar]
- Phinney K. W. Peer Reviewed: SFC of Drug Enantiomers. Anal. Chem. 2000, 72, 204 A–211 A. 10.1021/ac0027542. [DOI] [PubMed] [Google Scholar]
- Barhate C. L.; Wahab M. F.; Tognarelli D. J.; Berger T. A.; Armstrong D. W. Instrumental idiosyncrasies affecting the performance of ultrafast chiral and achiral sub/supercritical fluid chromatography. Anal. Chem. 2016, 88, 8664–8672. 10.1021/acs.analchem.6b01898. [DOI] [PubMed] [Google Scholar]
- Bailey W. F.; Luderer M. R.; Jordan K. P. Effect of Solvent on the Lithium– Bromine Exchange of Aryl Bromides: Reactions of n-Butyllithium and tert-Butyllithium with 1-Bromo-4-tert-butylbenzene at 0° C. J. Org. Chem. 2006, 71, 2825–2828. 10.1021/jo060026u. [DOI] [PubMed] [Google Scholar]
- Tai O.; Hopson R.; Williard P. G. Aggregation and Solvation of n-Butyllithium. Org. Lett. 2017, 19, 3966–3969. 10.1021/acs.orglett.7b01644. [DOI] [PubMed] [Google Scholar]
- Vaulont I.; Gais H.-J.; Reuter N.; Schmitz E.; Ossenkamp R. K. L. Asymmetric Synthesis of 3-Oxacarbacyclin and 3-Oxaisocarbacyclin by a Common Enantioselective Deprotonation Based Route. Eur. J. Org. Chem. 1998, 1998, 805–826. . [DOI] [Google Scholar]
- Bunn B. J.; Simpkins N. S. An enhancement of enantioselectivity in chiral lithium amide deprotonations due to lithium chloride. J. Org. Chem 1993, 58, 533–534. 10.1021/jo00055a001. [DOI] [Google Scholar]
- Lutz V.; Baro A.; Fischer P.; Laschat S. Synthesis of Functionalized Hydropentalenes by an Asymmetric Deprotonation/Alkylation Strategy. Eur. J. Org. Chem. 2010, 2010, 1149–1157. 10.1002/ejoc.200901154. [DOI] [Google Scholar]
- Majewski M.; Gleave D. M. Enantioselective formation of cis-3,5-dimethylcyclohexanone lithium enolate and stereoselective aldol reaction with benzaldehyde. J. Org. Chem 1992, 57, 3599–3605. 10.1021/jo00039a018. [DOI] [Google Scholar]
- Pamreddy A.; Baijnath S.; Naicker T.; Ntshangase S.; Mdanda S.; Lubanyana H.; Kruger H. G.; Govender T. Bedaquiline has potential for targeting tuberculosis reservoirs in the central nervous system. RSC Adv. 2018, 8, 11902–11907. 10.1039/C8RA00984H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingjun W.; Zhongyu W.: Chiral inducer for synthesizing (1R,2S)-Bedaquiline. In CN106866525A: China, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.