Abstract
Vanillic acid, an oxidized form of vanilla, is a flavoring agent with a creamy odor. Several studies have reported the neuroprotective effects of vanillic acid, which are predominantly associated with anti-inflammatory and antioxidative properties. The anti-inflammatory and antioxidative properties may result from Akt or ERK signaling activation. The activation of the mammalian target of rapamycin (mTOR), a key downstream target of Akt and ERK signaling, is a crucial therapeutic target for treating depression. However, the antidepressant effects of vanillic acid remain unknown. The present study applied the forced swim test (FST) to investigate the antidepressant effects of vanillic acid and its association with Akt, ERK, and mTOR signaling and upstream α-amino-3-hydroxy-5-methyl-4-isoxazolepropionaic acid receptor (AMPAR) in the prefrontal cortex (PFC) of mice. Vanillic acid demonstrated antidepressant effects by significantly reducing behavioral despair in the FST. None of the treatments changed locomotor activity. Additionally, vanillic acid increased AMPAR throughput, Akt, and mTOR signaling but not ERK signaling in the PFC. NBQX (an AMPAR blocker), MK 2206 (an Akt blocker), and rapamycin (an mTOR blocker) used in pretreatment attenuated the antidepressant effects of vanillic acid, but SL327 (an ERK inhibitor) did not. The immunochemical results indicated that the antidepressant effects of vanillic acid depend on the AMPAR–Akt–mTOR signaling transduction pathway. Our findings reveal an Akt-dependent, but ERK-independent, the mechanism underlying the antidepressant effects of vanillic acid, which may be beneficial for some patients with depression.
Introduction
Vanillic acid with a creamy odor is an oxidized form of vanillin and is benzoic acid. Although vanillic acid is used as a flavoring and scenting agent in food,1 it also exhibits other properties and has been identified in a commonly used Chinese medicine herb: Dong quai (Angelica sinensis). Dong quai has been used for thousands of years in treating menstrual illness, cerebrovascular diseases, cardiovascular diseases, and cognitive impairments;2−5 recently, a beneficial effect of it against depression has also been noted in preclinical studies.6−9 Meanwhile, studies have reported that vanillic acid has neuroprotective effects in addition to analgesic, antioxidant, and anti-inflammatory benefits against nuclear factor-kappa B activation, proinflammatory cytokine production, oxidative stress, and acetylcholinesterase.10−13 In addition, the neuroprotective effect of vanillic acid has been suggested to be mediated by Akt or ERK signaling activation.14,15
Recent studies have reported that the N-methyl-d-aspartate receptor (NMDAR) antagonist ketamine, muscarinic acetylcholine receptor inhibitor scopolamine, the NMDAR co-agonists sarcosine or d-serine have antidepressant properties;16−19 accordingly, the stimulation of the Akt and ERK downstream mTOR signaling is a notable target in the development of new antidepressants.20 Because vanillic acid has Akt and ERK signaling activation characteristics,14,15 it has potential for use in the treatment of depression. However, clinical or preclinical studies about the effect of vanillic acid in depression treatment are lacking. Also, the molecular mechanisms of the possible antidepressant effects of vanillic acid remain unknown. To study the role of vanillic acid in treating depression, the present study first evaluated the antidepressant potential of vanillic acid by the forced swim test (FST), which is the most widely used rodent behavioral model for assessing the antidepressant-predictive effect.21,22 Several medications with antidepressant effects can restore the behavioral despair in the FST.21,23 Furthermore, we also hypothesized that the antidepressant-like effect of vanillic acid is mediated by the activation of mTOR via Akt or ERK signaling pathway. We investigated the association of Akt, ERK, and mTOR signaling with the antidepressant effects properties of vanillic acid by quantizing the levels of phosphorylation of mTOR, Akt, and ERK in the prefrontal cortex (PFC) of mice.
The effect of NMDAR is closely associated with another glutamate ionotropic receptor, namely, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionaic acid receptor (AMPAR); increased AMPAR insertion is also required for the antidepressant effects of the NMDAR antagonist ketamine and the NMDAR co-agonist d-serine.17,19,24 In addition to mTOR signaling activation, AMPAR-GluA1 receptor insertion is also important for the antidepressant effects, which may be a convergent mechanism of antidepressants.25 We also investigated the association of AMPAR insertion in the PFC with the antidepressant effects of vanillic acid.
Results and Discussion
Antidepressant Effects Following Vanillic Acid Treatment
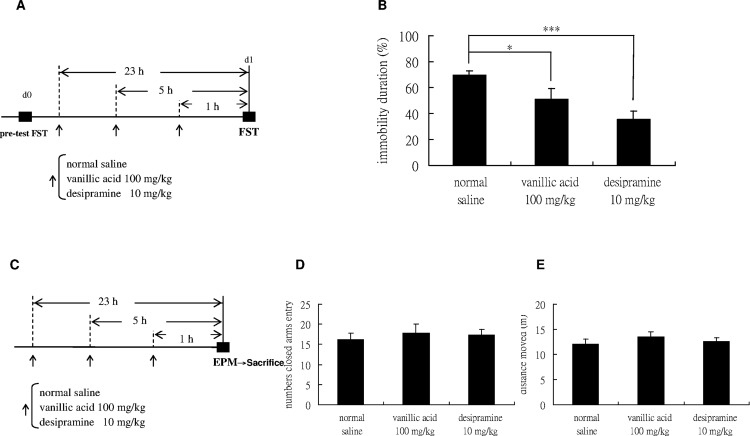
We examined the effects of vanillic acid using the FST, an antidepressant-predictive animal model; meanwhile, desipramine was also administered as a positive control (Figure 1A). The dose of vanillic acid was selected based on previous literature.26−31 Meanwhile, considering the consistency and the need to minimize variations in the drugs being administered, all drugs are by the intraperitoneal route (ip). In the FST, vanillic acid and desipramine significantly shortened the immobility period 1 h after the injection of three doses (Figure 1B). The EPM test is used for evaluating the possibility of a false-positive effect in the FST32,33 (Figure 1C). In the EPM test, vanillic acid and desipramine did not increase the number of entries into the closed arms (Figure 1D) or distance traveled (Figure 1E). These results indicate that vanillic acid exerts antidepressant-like effects.
Figure 1.
Behavioral actions of mice after three doses of saline, vanillic acid (100 mg/kg), or desipramine (10 mg/kg, a tricyclic antidepressant as a positive control) intraperitoneally injected in the forced swimming test (FST) and elevated plus-maze test (EPM). (A) All the mice were intraperitoneally injected with either normal saline (vehicle), vanillic acid 100 mg/kg, or desipramine 20 mg/kg (positive control group) three times 1 day, including at 1, 5, and 23 h before the forced swim test (FST). Twenty-four hours before the formal test, mice were forced to swim for 15 min (pretest) in a clear cylindrical tank with water. (B) Mice that received desipramine and vanillic acid prior to FST exhibited a significant reduction in the percentage of immobility time [F(2,27) = 14.334; p < 0.001]. (C) To evaluate the general locomotor activity, mice were intraperitoneally administered with saline, vanillic acid 100 mg/kg, or desipramine 10 mg/kg in 3 injections. The elevated plus-maze test (EPM) was conducted 1 h later. Immediately after EPM, four mice were sacrificed and then rapidly decapitated. The prefrontal cortex was removed for biochemical analysis later. The total closed arm entries (D) and distance moved (E) in EPM were measured to determine if vanillic acid could produce a general increase in general locomotor activity that could yield a false-positive result on the FST. None increased the locomotor activity [F(2,27) = 0.203; F(2,27) = 0.628; both p > 0.05; for total closed arm entries and distance moved, respectively]. n = 10 per group; *p < 0.05, ***p < 0.001 compared with the saline-treated group with the Tukey post-hoc analysis; values shown are mean ± SEM.
Involvement of AMPAR and mTOR Signaling in the Antidepressant Effects of Vanillic Acid
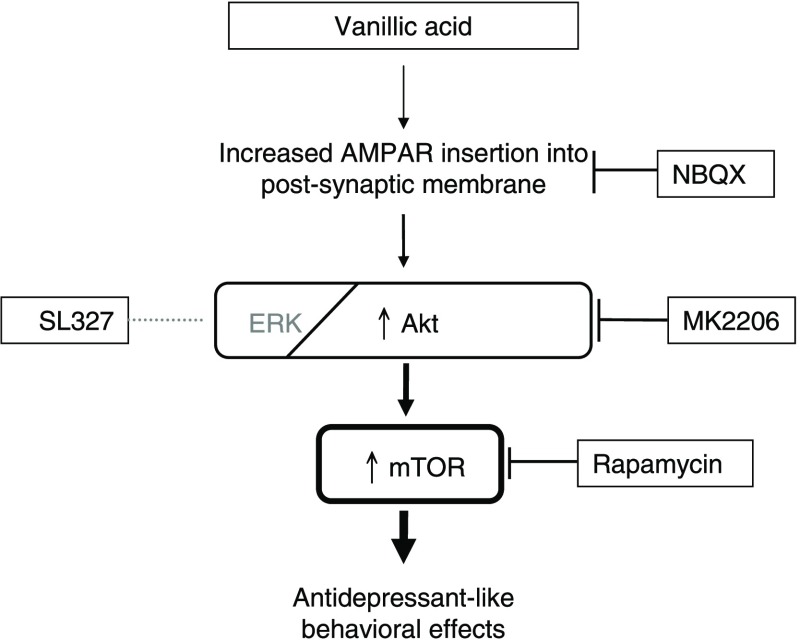
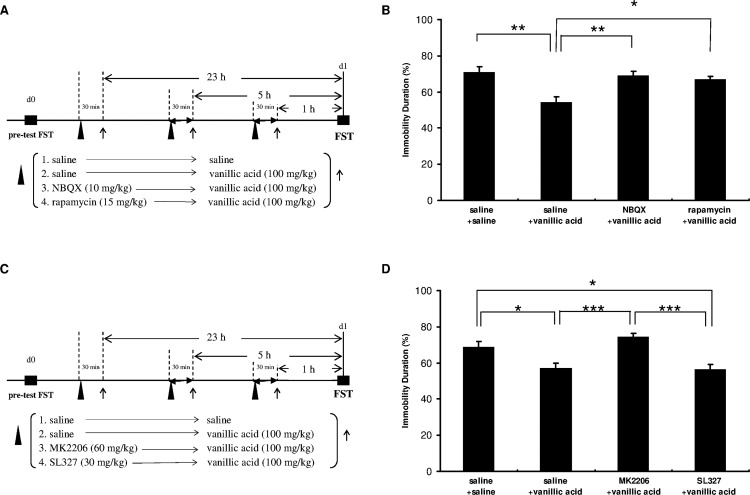
Using the AMPAR inhibitor NBQX and mTOR inhibitor rapamycin, we studied the roles of AMPAR and mTOR signaling in the antidepressant effects of vanillic acid. We conducted the FST to evaluate the behavior of mice that were ip pretreated with the AMPAR inhibitor NBQX or mTOR inhibitor rapamycin before the ip injection of vanillic acid (Figure 2A). As displayed in Figure 2B, both NBQX and rapamycin prevented the vanillic acid induced antidepressant effects, indicating that they are dependent on AMPAR and mTOR activation.
Figure 2.
Effect of NBQX (10 mg/kg), rapamycin (15 mg/kg), MK2206 (0 mg/kg), or SL327 (30 mg/kg) on the antidepressant-like effect of vanillic acid in FST. (A, C) Timelines represent the experimental procedure under the administration of drugs. (B) Note that the decreased immobility resulted from vanillic acid treatment is blocked when mice were pretreated with NBQX or rapamycin [F(3,36) = 7.168; p = 0.01]. (D) Similar effect is evidently observed when mice were pretreated with MK2206; SL327 did not affect the effect of vanillic acid [F(3,36) = 10.802; p < 0.001]. n = 10 per group; *p < 0.05, **p < 0.01, ***p < 0.001 compared between group with the Tukey post-hoc analysis; values shown are mean ± SEM.
Involvement of Akt and ERK Signaling in the Antidepressant Effects of Vanillic Acid
Using the Akt inhibitor MK2260 and ERK inhibitor SL327, we studied the roles of Akt and ERK in the antidepressant effects of vanillic acid. We ip pretreated the mice with MK2206 or SL327 before ip injection of vanillic acid (Figure 2C). As shown in Figure 2D, the anti-immobility effect of vanillic acid was prevented by treatment of mice with MK2206 in the FST, indicating a dependence on Akt signaling. By contrast, SL327 did not affect the antidepressant effects of vanillic acid, indicating that the antidepressant effects of vanillic acid are independent of ERK signaling.
Effect of Vanillic Acid on mTOR Signaling Pathway Alteration and AMPAR Throughput
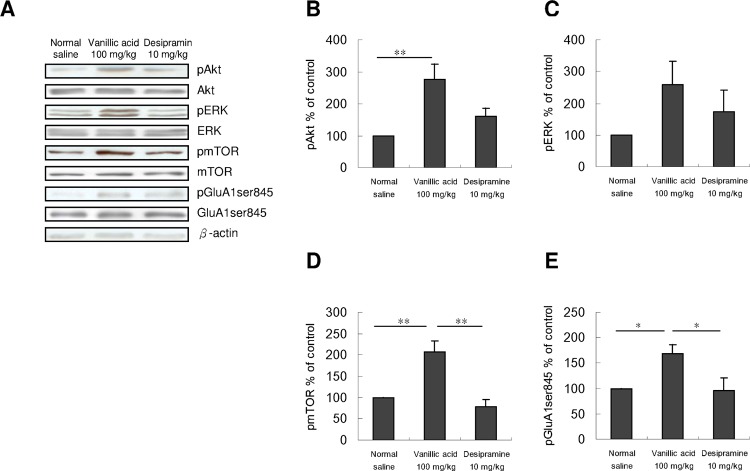
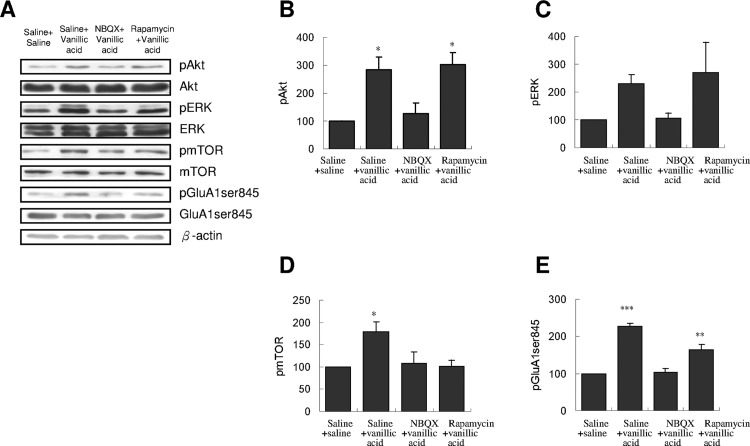
To study whether the decreased immobility in FST was accompanied by an increase in activated mTOR signaling, we examined the activated forms of mTOR and mTOR upstream regulatory proteins, namely, Akt and ERK, in the drug-treated mice. As illustrated in Figure 3, injecting vanillic acid at 100 mg/kg for three times significantly increased the levels of pAkt (Figure 3B) and pmTOR (Figure 3D) but not of pERK (Figure 3C) in the PFC. Subsequently, we determined whether vanillic acid functionally increased AMPAR throughput. We also found an increase in pGluA1ser845 (Figure 3E) in the PFC of vanillic acid treated mice. By contrast, desipramine did not affect these (Figure 3B–E). We did not observe changes in the levels of total Akt, ERK, mTOR, or GluA1ser845 (Figure 3A).
Figure 3.
Effect of three doses of saline, vanillic acid (100 mg/kg), or desipramine (10 mg/kg) intraperitoneally injected on the phosphorylation of Akt, ERK, mTOR, and GluA1ser845 in the PFC of mice. PFC samples were collected immediately after EPM. (A) Western blot analysis of pAkt, pERK, pmTOR, and pGluA1ser845 was performed. The densitometry analyses of the blot (normalized to β-actin) verify the enhanced activity of (B) pAkt, (D) pmTOR, and (E) pGluA1ser845, but not (C) of pERK in each group of the experiments. [pAkt, F(2,9) = 8.177, p < 0.01; pERK, F(2,9) = 1.967, p > 0.05; pmTOR, F(2,9) = 15.252, p = 0.01; pGluA1ser845, F(2,9) = 5.585, p < 0.05, n = 4 per group; *p < 0.05; **p < 0.01, comparison between group with the Tukey post-hoc analysis]. Total levels of Akt, ERK, mTOR, and GluA1ser845 were not different among the three groups. Values shown are mean ± SEM.
Role of Glutamatergic System in Antidepressant Effects of Vanillic Acid
To validate whether the antidepressant effects of vanillic acid are mediated by the activity of the AMPAR/mTOR signaling pathway, we studied pGluA1ser845, pmTOR, pERK, and pAkt activation by pretreating the mice with NBQX or rapamycin before vanillic acid treatment. As presented in Figure 4, NBQX administered before vanillic acid blocked the vanillic acid induced increase in pAkt (Figure 4B), pmTOR (Figure 4D), and pGluA1ser845 (Figure 4E) immunoreactions. Moreover, rapamycin completely abolished the increase in pmTOR immunoreactions engendered by vanillic acid (Figure 4D) but did not modulate the vanillic acid induced increases in pGluA1ser845 and pAkt expression (Figure 4B–E). These results confirm that the AMPAR–Akt–mTOR pathway mediated the antidepressant effects of vanillic acid. In addition, we did not observe changes in the levels of total Akt, ERK, mTOR, or GluA1ser845 (Figure 4A).
Figure 4.
Effect of NBQX (10 mg/kg, ip) and rapamycin (15 mg/kg, ip) on the phosphorylation of Akt, ERK, mTOR, and GluA1ser845 in the PFC of mice. PFC samples of 4 native mice were collected 1 h after last injection. (A) Western blot analysis of pAkt, pERK, pmTOR, and pGluA1ser845 was performed. Results showed the significantly increased expressions of (B) pAkt, (D) pmTOR, and (E) pGluA1ser845 in mice prefrontal cortex following vanillic acid treatment, but the change of (C) pERK did not achieve statically significant. Note that the increased expressions of (B) pAkt, (D) pmTOR, and (E) pGluA1ser845 resulted from vanillic acid treatment are blocked when mice were pretreated with NBQX. The increased expression of (D) pmTOR resulted from vanillic acid treatment is blocked when mice were pretreated with rapamycin and the increased expressions of (B) pAkt and (E) pGluA1ser845 resulted from vanillic treatment are not blocked. [pAkt, F(3,12) = 7.564, p < 0.01; pERK, F(3,12) = 2.399, p > 0.05; pmTOR, F(3,12) = 4.714, p < 0.05; pGluA1ser845, F(3,12) = 34.337, p < 0.001; n = 4 per group; *p < 0.05; **p < 0.01, ***p < 0.001, comparison to saline + saline group with the Tukey post-hoc analysis]. Total levels of Akt, ERK, mTOR, and GluA1ser845 were not different among the four groups. Values shown are mean ± SEM.
This study demonstrated that vanillic acid could produce antidepressant effects, which were mediated by AMPAR–Akt–mTOR signaling pathway activation. Another major finding of this study is that vanillic acid could not activate ERK signaling; in addition, the ERK inhibitor (SL327) did not block the antidepressant effects of vanillic acid. Our results suggest that Akt signaling was involved in the antidepressant effects of vanillic acid, but ERK signaling is not necessary.
Recently, preclinical and clinical researches have demonstrated that mTOR signaling plays a notable role in depression-related disorders.34,35 Moreover, research has suggested that rapid mTOR signaling activation is the mechanism underlying the rapid-acting antidepressant properties of ketamine and scopolamine.17,18 In addition to mTOR activation, the activation of the mTOR upstream target ERK constitutes the mechanism for antidepressant properties of ketamine; even a recent study found that the (R)-enantiomer of ketamine confers antidepressant effects by activating ERK signaling and mTOR is not necessary for the antidepressant effects of (R)-enantiomer of ketamine.36 Other studies have also demonstrated that ERK signaling is involved in the pathophysiology of depressive disorders.37,38 ERK signaling activation has been implicated in antidepressant responses.36,39−41 Furthermore, Akt, another upstream target of mTOR, has been found to be associated with the pathogenesis of depression42,43 and involved in antidepressant effects.44,45 In the present study, we observed Akt signaling activation to be a mechanism associated with the antidepressant effects of vanillic acid. Our findings are consistent with those of previous in vivo research,14 which demonstrated that vanillic acid can activate Akt signaling and confirmed the role of Akt in the antidepressant effects of the drug.
Although we found that vanillic acid treatment could induce ERK activation in the PFC, the results were not significant. An in vitro study reported that vanillic acid administered at dosages of 10–10–10–8 M could elicit ERK activation in osteoblast-like UMR 106 cells.15 However, in another in vitro study, vanillic acid administered at 4 mM was not involved in the effect of ERK phosphorylation on hepatocyte growth factor-induced migration of human lung cancer cells.46 No previous in vivo study has explored the effect of vanillic acid on ERK signaling. In this in vivo study, we found that ERK signaling was not significantly affected by vanillic acid. We also found that the ERK inhibitor SL327 did not alter the antidepressant effects of vanillic acid. These findings suggest that ERK signaling is not implicated in the antidepressant effects of vanillic acid.
In this study, the dose of vanillic acid was 100 mg/kg, which showed antioxidant, anti-inflammatory properties, and beneficial role in cerebrovascular insufficiency states in several animal models.26−31 We speculate that the dose in the current study did not engender significant changes. Additional detailed studies on the roles of Akt and ERK in the antidepressant actions of vanillic acid are warranted.
Based on the findings of Akt-dependent antidepressant effects of vanillic acid, to study the future upstream and downstream Akt signaling events, we conducted the FST to examine the behavior of mice treated with the AMPAR inhibitor NBQX and the mTOR inhibitor rapamycin after vanillic acid administration. Both NBQX and rapamycin could prevent the vanillic acid exerted behavioral responses in the FST, indicating the dependence on AMPAR and mTOR. To define the corresponding mechanisms, we investigated the intensities of phosphorylated proteins of Akt, ERK, mTOR, and AMPAR-GluA1 by pretreating the mice with AMPAR-GluA1 inhibitor NBQX or mTOR inhibitor rapamycin after vanillic acid treatment. Our results indicate that AMPAR–Akt–mTOR signal transduction activation is necessary for the antidepressant effects of vanillic acid. The antidepressant effects of vanillic acid on Akt signaling extend to upstream signaling AMPAR and downstream signaling mTOR at that molecular level and are consistent with other glutamate-based antidepressants.17,18,25
Based on the findings of ketamine with antidepressant properties, glutamate neurotransmission modulation is a novel strategy for treating depression. Several glutamatergic agents, including NMDAR inhibitors, glycine site agents, and allosteric modulators, as well as ketamine metabolites and stereoisomers exhibit antidepressant properties with common mechanisms; such mechanisms include activated synthesis of synaptic proteins, increased brain-derived neurotrophic factor, enhanced AMPAR-GluA1 receptor throughput, and, most notably, activated mTOR.25 Notably, although the critical roles of the mTOR signaling pathway in antidepressant behavioral responses have been suggested for several glutamate-based antidepressants, including ketamine, (S)-ketamine, (2R–6R)-hydroxynorketamine, and rapastinel, the roles of other signaling pathways, regardless of mTOR activation, have been reported. Yang et al. reported that ERK, not mTOR, activation plays a role in (R)-ketamine’s antidepressant effects.36 In contrast to (R)-ketamine, we found that vanillic acid exerts antidepressant effects through Akt signaling and that ERK signaling is not necessary. These findings reveal unique and differential effects of various antidepressants on diverse signaling pathways, which can be responsible for their distinct preclinical antidepressant profiles. The Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways regulate the activity of mTOR.47 Involvement in any component of these pathways modulates mTOR activation, possibly leading to the development of depression. Thus, vanillic acid may be a useful antidepressant for depressed patients with particular involvement in Akt signaling but not for patients with ERK-related depression.
Depressive disorders are highly heterogeneous diseases with a complex relationship of biological etiologies. There is no single convergent mechanism and there are many risk factors potentially responsible for the development of depression, which, in turn, results in individual differences in people’s response to the same antidepressant. For example, a study demonstrated that depressed patients carrying a Val/Val BDNF allele exhibited a superior antidepressant response to ketamine compared with Met carriers,48 suggesting that these patients may have a different endophenotype requiring an alternative pharmacological intervention. One can reasonably assume that the precise targeting of these pathways may be a critical therapeutic approach. Additional studies are still required to elucidate the unique molecular mechanisms underlying the effects of different antidepressants; the findings of such studies will be crucial for the development of next-generation individualized precision antidepressant pharmacotherapies to improve treatment efficacy.
Methods
Animals
C57BL/6 male mice weighing 15–20 g and aged 6–8 weeks were housed in the Animal Center of China Medical University under a 12 h light/12 h dark cycle at a temperature of 23 ± 1 °C, the humidity of 55 ± 5%; the mice had free access to food and water. All experimental steps were reviewed and approved by the institutional animal care and use committee at China Medical University, Taiwan (permit No. CMUIACUC-2017-318).
Drugs
All of the drugs were injected ip at a dose of 0.01 mL/g body weight. The concentrations were as follows: vanillic acid (Merck, St. Louis, MO), 100 mg/kg; desipramine (Merck, St. Louis, MO), 10 mg/kg; NBQX (Tocris, Bristol, U.K.), 10 mg/kg; rapamycin (Toku-E, Bellingham, WA), 15 mg/kg; MK2206 (MedChemExpress, Monmouth Junction NJ), 60 mg/kg; and SL327 (Merck, St. Louis, MO), 30 mg/kg. Vanillic acid and rapamycin were dissolved in 0.9% saline; the remaining substances were dissolved in 5% ethanol.
Study Design
Using the FST, we examined the antidepressant-like effects of vanillic acid. As illustrated in Figure 1A, 30 mice were randomly separated into three groups: control group, comprising mice ip injected with normal saline; experimental group, comprising mice ip injected with vanillic acid (100 mg/kg);26−31 and positive control group, comprising mice ip injected with desipramine (10 mg/kg)22 three times in 1 day, namely, at 1, 5, and 23 h before the FST. The mice were forced to swim for 15 min (pretest) in a clear cylindrical tank with water 24 h before the formal test. In addition, using the EPM, we measured the general locomotor activity in mice for evaluating the possibility of false-positive in the FST. Thirty additional mice were randomly separated into three groups: mice ip injected with normal saline; those ip injected with vanillic acid (100 mg/kg); and those ip injected with desipramine (10 mg/kg) for three times in 1 day, namely, at 1, 5, and 23 h before the EPM test. After the completion of all behavioral tests, all of the mice were sacrificed, and the tissues of four mice were collected for experimental analysis (Figure 1C).
The involvement of AMPAR and mTOR signaling in the antidepressant effects of vanillic acid was assessed using AMPAR inhibitor NBQX and mTOR inhibitor rapamycin. We conducted the FST to evaluate the behavior of mice that were ip pretreated with the AMPAR inhibitor NBQX (10 mg/kg)49 or mTOR inhibitor rapamycin (15 mg/kg)50 for three times before ip injecting vanillic acid (100 mg/kg) for three times (Figure 2A).
The involvement of Akt and ERK in the antidepressant effects of vanillic acid was assessed using the Akt inhibitor MK2260 and ERK inhibitor SL327. We ip pretreated the mice with MK2206 (60 mg/kg)51 or SL327 (30 mg/kg)50 for three times before ip injecting them with vanillic acid (100 mg/kg) for three times (Figure 2C).
FST Model
The FST is a preclinical behavioral test applied to evaluate the antidepressant effects of medications. We conducted a 15 min pretest and a 5 min formal FST according to a previously reported procedure.19 We use EthoVision Basic V3.1 program (Noldus, Wageningen, Netherlands) to record and evaluate the total immobility period during the 5 min testing period.
Elevated Plus-Maze Test
The elevated plus-maze (EPM) test is a behavioral test used to analyze anxiolytic effects and general locomotor activity in laboratory animals. The maze is an elevated plus-shaped apparatus with two open and two enclosed arms. The total number of closed arms entries (frequency) and distance traveled by mice during the EPM test are quantitatively assessed as an indicator of general activity to evaluate the possibility of false-positive data in the FST.32,33 In this study, a mouse was placed on the maze for a 5 min activity test. The test was recorded using EthoVision Basic V 3.1 program for automated analysis.
Animal Tissue Preparation
After the EPM test, the mice were sacrificed, and the tissues of the PFCs of four mice were collected and dried with nitrogen for Western blotting. The collected tissues were preserved at −80 °C until grinding. Before grinding, three centrifugal tubes were prepared for each sample: one for grinding (rough grinding and precise grinding), one for the supernatant, and one for testing. During grinding, 300 μL of buffer was added in the centrifugal tubes containing the tissue. Subsequently, we used a tissue grinder pestle for rough grinding and a tissue grinder for precise grinding. After grinding, the tubes were centrifuged at 15 000 rpm for 15 min at 4 °C. The supernatant was shifted to another new cylinder tube after centrifugation and heated for 7 min before being preserved at −80 °C until Western blotting.
Western Blotting
Western blotting was used to quantify specific protein levels. The analysis was performed in accordance with the procedure described in a previous study.19
Statistical Analysis
Each behavioral experimental group consisted of 10 mice. Each biochemical experimental group consisted of 4 mice. Our results were presented as mean ± SEM. The assumption of normality and homogeneity of variance, respectively, were evaluated by the Kolmogorov–Smirnov test and Levene’s test. The behavioral experimental data showed a parametric distribution. In addition, the expression of Western blot analysis and immunoprecipitation were quantified as fold changes in each band relative to saline-treated or saline + saline group. The level of saline-treated or saline + saline group set at 100%. The parameters of biochemical data were tested concerning normality (Kolmogorov–Smirnov test) and the data showed a parametric distribution. Finally, mice behavioral and biochemical experimental results were analyzed through a one-way analysis of variance, followed by the Tukey post-hoc tests using SPSS 12.0 software. All of the statistical tests were 2-tailed. A p value of less than 0.05 was regarded as statistically significant.
Conclusions
Our study is the first to report that vanillic acid would be a potential antidepressant whose effects are mediated by AMPAR–Akt–mTOR signaling pathway activation. Furthermore, these data reinforce the notion that Akt activation can be a relevant therapeutic target for depression.
Acknowledgments
This study was supported by the Ministry of Science and Technology, Taiwan (MOST 105-2314-B-039-027, MOST 105-2320-B-039-013-MY3, and MOST 106-2314-B-039-029-MY3) and the China Medical University Hospital, Taiwan (DMR-106-096 and DMR-107-094).
Glossary
Abbreviations
- mTOR
mammalian target of rapamycin
- ERK
extracellular signal-regulated protein kinase
- FST
forced swim test
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionaic acid receptors
- PFC
prefrontal cortex
- NMDAR
N-methyl-d-aspartate receptor
- EPM
elevated plus-maze test
- GluR1ser845
AMPA GluR1 serine845
- pmTOR
phospho-mTOR
- pERK
phospho-extracellular signal-regulated protein kinase
- pAkt
phospho-Akt
- pGluA1ser845
phospho-AMPA GluA1 serine845
- BDNF
brain-derived neurotrophic factor
Author Contributions
○ H.-W.C. and I.-H.W. contributed equally to this study.
The authors declare no competing financial interest.
References
- Sreedhar R. V.; Roohie K.; Venkatachalam L.; Narayan M. S.; Bhagyalakshmi N. Specific Pretreatments Reduce Curing Period of Vanilla (Vanilla planifolia) Beans. J. Agric. Food Chem. 2007, 55, 2947–2955. 10.1021/jf063523k. [DOI] [PubMed] [Google Scholar]
- Zhang S.; He B.; Ge J.; Li H.; Luo X.; Zhang H.; Li Y.; Zhai C.; Liu P.; Liu X.; Fei X. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia–reperfusion rats. Int. J. Biol. Macromol. 2010, 47, 546–550. 10.1016/j.ijbiomac.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Lau C. B.; Ho T. C.; Chan T. W.; Kim S. C. Use of dong quai (Angelica sinensis) to treat peri-or postmenopausal symptoms in women with breast cancer: is it appropriate?. Menopause 2005, 12, 734–740. 10.1097/01.gme.0000184419.65943.01. [DOI] [PubMed] [Google Scholar]
- Huang S. H.; Lin C. M.; Chiang B. H. Protective effects of Angelica sinensis extract on amyloid beta-peptide-induced neurotoxicity. Phytomedicine 2008, 15, 710–721. 10.1016/j.phymed.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Hou Y. Z.; Zhao G. R.; Yang J.; Yuan Y. J.; Zhu G. G.; Hiltunen R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004, 75, 1775–1786. 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Chen C.; Yin Q.; Tian J.; Gao X.; Qin X.; Du G.; Zhou Y. Studies on the potential link between antidepressant effect of Xiaoyao San and its pharmacological activity of hepatoprotection based on multi-platform metabolomics. J. Ethnopharmacol. 2019, 112432 10.1016/j.jep.2019.112432. [DOI] [PubMed] [Google Scholar]
- Chen H.; Huang Q.; Zhang S.; Hu K.; Xiong W.; Xiao L.; Cong R.; Liu Q.; Wang Z. The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice. Nutrients 2019, 11, 859. 10.3390/nu11040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zheng X.; Du G.; Li Z.; Qin X. Brain metabonomics study of the antidepressant-like effect of Xiaoyaosan on the CUMS-depression rats by (1)H NMR analysis. J. Ethnopharmacol. 2019, 235, 141–154. 10.1016/j.jep.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Shen J.; Zhang J.; Deng M.; Liu Y.; Hu Y.; Zhang L. The Antidepressant Effect of Angelica sinensis Extracts on Chronic Unpredictable Mild Stress-Induced Depression Is Mediated via the Upregulation of the BDNF Signaling Pathway in Rats. Evidence-Based Complementary Altern. Med. 2016, 2016, 7434692 10.1155/2016/7434692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai A.; Sawano T.; Ito H. Antioxidative properties of vanillic acid esters in multiple antioxidant assays. Biosci., Biotechnol., Biochem. 2012, 76, 314–318. 10.1271/bbb.110700. [DOI] [PubMed] [Google Scholar]
- Kim M. C.; Kim S. J.; Kim D. S.; Jeon Y. D.; Park S. J.; Lee H. S.; Um J. Y.; Hong S. H. Vanillic acid inhibits inflammatory mediators by suppressing NF-kappaB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. 10.3109/08923973.2010.547500. [DOI] [PubMed] [Google Scholar]
- Calixto-Campos C.; Carvalho T. T.; Hohmann M. S.; Pinho-Ribeiro F. A.; Fattori V.; Manchope M. F.; Zarpelon A. C.; Baracat M. M.; Georgetti S. R.; Casagrande R.; Verri W. A. Jr. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFkappaB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. 10.1021/acs.jnatprod.5b00246. [DOI] [PubMed] [Google Scholar]
- Singh J. C.; Kakalij R. M.; Kshirsagar R. P.; Kumar B. H.; Komakula S. S.; Diwan P. V. Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 2015, 53, 630–636. 10.3109/13880209.2014.935866. [DOI] [PubMed] [Google Scholar]
- Amin F. U.; Shah S. A.; Kim M. O. Vanillic acid attenuates Abeta1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753 10.1038/srep40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H. H.; Gao Q. G.; Zhang Y.; Wong K. C.; Dai Y.; Yao X. S.; Wong M. S. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J. Steroid Biochem. Mol. Biol. 2014, 144, 382–391. 10.1016/j.jsbmb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Chen K. T.; Tsai M. H.; Wu C. H.; Jou M. J.; Wei I. H.; Huang C. C. AMPA Receptor-mTOR Activation is Required for the Antidepressant-Like Effects of Sarcosine during the Forced Swim Test in Rats: Insertion of AMPA Receptor may Play a Role. Front. Behav. Neurosci. 2015, 9, 162. 10.3389/fnbeh.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.; Lee B.; Liu R. J.; Banasr M.; Dwyer J. M.; Iwata M.; Li X. Y.; Aghajanian G.; Duman R. S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti B.; Navarria A.; Liu R. J.; Banasr M.; Li N.; Terwilliger R.; Sanacora G.; Eid T.; Aghajanian G.; Duman R. S. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol. Psychiatry 2013, 74, 742–749. 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei I. H.; Chen K. T.; Tsai M. H.; Wu C. H.; Lane H. Y.; Huang C. C. Acute Amino Acid d-Serine Administration, Similar to Ketamine, Produces Antidepressant-like Effects through Identical Mechanisms. J. Agric. Food Chem. 2017, 65, 10792–10803. 10.1021/acs.jafc.7b04217. [DOI] [PubMed] [Google Scholar]
- Ignácio Z. M.; Reus G. Z.; Arent C. O.; Abelaira H. M.; Pitcher M. R.; Quevedo J. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br. J. Clin. Pharmacol. 2016, 82, 1280–1290. 10.1111/bcp.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt R. D.; Le Pichon M.; Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Lucki I.; Dalvi A.; Mayorga A. J. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 2001, 155, 315–322. 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Porsolt R. D.; Anton G.; Blavet N.; Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Maeng S.; Zarate C. A. Jr.; Du J.; Schloesser R. J.; McCammon J.; Chen G.; Manji H. K. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry 2008, 63, 349–352. 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Duman R. S.; Shinohara R.; Fogaca M. V.; Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol. Psychiatry 2019, 1816–1832. 10.1038/s41380-019-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavani P.; Subramanian P.; Kanimozhi S. Preventive Efficacy of Vanillic Acid on Regulation of Redox Homeostasis, Matrix Metalloproteinases and Cyclin D1 in Rats Bearing Endometrial Carcinoma. Indian J. Clin. Biochem. 2017, 32, 429–436. 10.1007/s12291-016-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu G.; Nishanthi E.; Sharmila R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: a biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. 10.1016/j.etap.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Prahalathan P.; Raja B. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in L-NAME-induced hypertensive rats: a dose-dependence study. Redox Rep. 2011, 16, 208–215. 10.1179/1351000211Y.0000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J.; Zhang C. Modulating Effects of Vanillic Acid on Sepsis-induced Oxidative Liver Injury in Rat Model. Pharmacology 2019, 15, 752–758. 10.3923/ijp.2019.752.758. [DOI] [Google Scholar]
- Wang J.; Guo Y.; Zhang S. Y. Vanillic Acid Improve Neural Function after Focal Cerebral Ischemia-reperfusion Rats. Int. J. Pharmacol. 2018, 14, 488–494. 10.3923/ijp.2018.488.494. [DOI] [Google Scholar]
- Khoshnam S. E.; Sarkaki A.; Khorsandi L.; Winlow W.; Badavi M.; Moghaddam H. F.; Farbood Y. Vanillic acid attenuates effects of transient bilateral common carotid occlusion and reperfusion in rats. Biomed. Pharmacother. 2017, 96, 667–674. 10.1016/j.biopha.2017.10.052. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol., Biochem. Behav. 1996, 54, 21–30. 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J.; Johnson N. J. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol., Biochem. Behav. 1995, 52, 297–303. 10.1016/0091-3057(95)00138-M. [DOI] [PubMed] [Google Scholar]
- Jernigan C. S.; Goswami D. B.; Austin M. C.; Iyo A. H.; Chandran A.; Stockmeier C. A.; Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1774–1779. 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K. T.; Liu R. J.; Voleti B.; Maldonado-Aviles J. G.; Duric V.; Iwata M.; Dutheil S.; Duman C.; Boikess S.; Lewis D. A.; Stockmeier C. A.; DiLeone R. J.; Rex C.; Aghajanian G. K.; Duman R. S. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014, 20, 531–535. 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; Ren Q.; Qu Y.; Zhang J. C.; Ma M.; Dong C.; Hashimoto K. Mechanistic Target of Rapamycin-Independent Antidepressant Effects of (R)-Ketamine in a Social Defeat Stress Model. Biol. Psychiatry 2018, 83, 18–28. 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Gourley S. L.; Wu F. J.; Kiraly D. D.; Ploski J. E.; Kedves A. T.; Duman R. S.; Taylor J. R. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry 2008, 63, 353–359. 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Wang Z.; Gao Z.; Xie K.; Zhang Q.; Jiang H.; Pang Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014, 271, 116–121. 10.1016/j.bbr.2014.05.068. [DOI] [PubMed] [Google Scholar]
- Reus G. Z.; Vieira F. G.; Abelaira H. M.; Michels M.; Tomaz D. B.; dos Santos M. A.; Carlessi A. S.; Neotti M. V.; Matias B. I.; Luz J. R.; Dal-Pizzol F.; Quevedo J. MAPK signaling correlates with the antidepressant effects of ketamine. J. Psychiatr. Res. 2014, 55, 15–21. 10.1016/j.jpsychires.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Duman C. H.; Schlesinger L.; Kodama M.; Russell D. S.; Duman R. S. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol. Psychiatry 2007, 61, 661–670. 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Wang C.; Xue Z.; Li C.; Zhang J.; Zhao X.; Liu A.; Wang Q.; Zhou W. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int. J. Neuropsychopharmacol. 2014, 18, pyu110 10.1093/ijnp/pyu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock C.; Ackermann T. F.; Hierlmeier M.; Lang F.; Borgwardt S.; Lang U. E. Akt2 deficiency is associated with anxiety and depressive behavior in mice. Cell. Physiol. Biochem. 2013, 32, 766–777. 10.1159/000354478. [DOI] [PubMed] [Google Scholar]
- Matsuda S.; Ikeda Y.; Murakami M.; Nakagawa Y.; Tsuji A.; Kitagishi Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. 10.3390/diseases7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil D. P.; Hemmings B. A. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 2001, 26, 657–664. 10.1016/S0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Marsden W. N. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 168–184. 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Lirdprapamongkol K.; Kramb J. P.; Suthiphongchai T.; Surarit R.; Srisomsap C.; Dannhardt G.; Svasti J. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J. Agric. Food Chem. 2009, 57, 3055–3063. 10.1021/jf803366f. [DOI] [PubMed] [Google Scholar]
- Steelman L. S.; Abrams S. L.; Whelan J.; Bertrand F. E.; Ludwig D. E.; Basecke J.; Libra M.; Stivala F.; Milella M.; Tafuri A.; Lunghi P.; Bonati A.; Martelli A. M.; McCubrey J. A. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008, 22, 686–707. 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- Laje G.; Lally N.; Mathews D.; Brutsche N.; Chemerinski A.; Akula N.; Kelmendi B.; Simen A.; McMahon F. J.; Sanacora G.; Zarate C. Jr. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry 2012, 72, e27–e28. 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska A.; Mlyniec K.; Siwek A.; Dybala M.; Opoka W.; Poleszak E.; Nowak G. Antidepressant-like effect of chromium chloride in the mouse forced swim test: involvement of glutamatergic and serotonergic receptors. Pharmacol. Rep. 2008, 60, 991–995. [PubMed] [Google Scholar]
- Moreno A.; Akcakanat A.; Munsell M. F.; Soni A.; Yao J. C.; Meric-Bernstam F. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr.-Relat. Cancer 2008, 15, 257–266. 10.1677/ERC-07-0202. [DOI] [PubMed] [Google Scholar]
- Leszczynska K. B.; Foskolou I. P.; Abraham A. G.; Anbalagan S.; Tellier C.; Haider S.; Span P. N.; O’Neill E. E.; Buffa F. M.; Hammond E. M. Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT. J. Clin. Invest. 2015, 125, 2385–2398. 10.1172/JCI80402. [DOI] [PMC free article] [PubMed] [Google Scholar]