Abstract
Poly(ethylene imine) (PEI) has abundant amino groups in a macromolecular chain and can be used as a graft source for metal nanocomposites, which shows excellent ability to form stable complexes with heavy metal ions. In this work, a simple and convenient method was used to make PEI into a stable hydrogel with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide-N-hydroxysuccinimide and subsequently coprecipitate with silver nitrate solution or palladium chloride solution to form metal-loaded composite hydrogels. In addition, the characterizations of composite hydrogels were investigated by scanning electron microscopy, specific surface area tests (Brunauer–Emmett–Teller), X-ray photoelectron spectroscopy, and ultraviolet spectroscopy. The properties of composite hydrogels on the catalytic reduction of 4-nitrophenol were studied. The results showed that the composite hydrogels could be easily separated from the water environment, which indicated the large-scale potential application in organic catalytic degradation and wastewater treatment.
1. Introduction
As an important source of human survival, water is not only vital to life but also plays a pivotal role in industrial and agricultural production.1,2 However, rapid population growth and industrial activity have led to a crisis of water availability. In addition, wastewater from untreated industrial and agricultural activities is uncontrolled, leading to serious environmental problems. Dyes and heavy metals are harmful substances, and 4-nitrophenol (4-NP) causes cancer in animals and leads to redox-related toxicity in various tissues.3,4 4-NP is a vital material for chemical processes to produce dyes, drugs, explosives, and pesticides. Wastewater discharged by chemical companies usually contains 4-NP. Therefore, the removal of 4-NP from aqueous solutions has become more and more important. Due to the slower effects of traditional methods, it is urgent to develop new catalysts. Many studies have shown the catalytic degradation of 4-NP. For example, Garole et al. did a lot of research on biosynthesized palladium nanocatalyst (Pd-NanoCata) using a plant extract from a medicinally important plant, and the separated palladium nanocatalyst is used for catalytic research and has good application for the reduction of 4-NP.5 Rasaki et al. successfully prepared Ag/ZnO nanorods by a facile and cost-saving liquid precipitation-citrate reduction process, which shows a synergetic catalytic performance for the 4-NP reduction reaction.6 Compared with Ag nanoparticles, the catalytic performance and stability are improved.
Hydrogels are a three-dimensional polymer network with high water content, chemically or physically cross-linked.7−11 Because of their good biocompatibility and stimulus response, hydrogels have many applications of wound healing, artificial organization, drug delivery, and antifouling.12−14 However, the disadvantages of traditional chemically cross-linked hydrogels are poor toughness, limited expandability, low mechanical strength, and limited recyclability, which limit the application of hydrogels.7 In this context, poly(ethylene imine) (PEI) molecules with three different types of amines are very attractive and have perfect water solubility.15−22 In addition, there are more important reports on PEI ionic liquid colloidal microgels. For example, Demirci et al. prepared PEI ionic liquid colloidal microgels and then used anion-exchanged PEI-based ionic liquid colloids (ILCs) for in situ preparation of metal nanoparticles templates of particles (such as Co, Ni, and Cu) to generate ILC composites (PEI-M) (M: Co, Ni, and Cu). Finally, PEI-M composites were used for the hydrolysis of NaBH4 to generate H2.23 Simple polymer networks such as PEI, polyamide, and their derivatives as catalysts could remove inorganic/organic components in water or wastewater. Thus, some researchers continue to explore the PEI hydrogel compound with carbon materials and metal nanoparticles for catalytic and adsorption experiments. Tang et al. found products extracted from plant cellulose, such as CNF, can be easily converted into aerogels with controlled density. A cross-linked three-dimensional network with high porosity is very effective for the adsorption of methyl orange and copper ions.24 In addition, Guo et al. successfully prepared GO/PEI hydrogels and used for dye adsorption.1 On this basis, the prepared RGO/PEI/Ag hydrogels showed good photocatalytic performance for both RhB and MB.25 Sahiner et al. prepared PEI-based hydrogels with different morphologies as bulk gel, microgel, and cryogel forms and used as templates for in situ Co, Ni, and Cu metal nanoparticle preparation. These composites were used as catalysts for hydrolysis of NaBH4 for production in the reduction reaction of 4- and 2- nitrophenols (4-NP and 2-NP, respectively) as well as the decolorization reaction of methylene blue (MB) and Eosin Y (EY) dyes.26
In this work, we used a simple and convenient method to synthesize the PEI hydrogels, while the initiator agent EDC-HCl can make the hydrogels more stable. Coprecipitation of palladium chloride solution and silver nitrate solution formed PEI-Pd or PEI-Ag composite hydrogels. We explored the effects of different times on the final products and of the finally formed complexes on the catalytic reduction of 4-NP.
2. Results and Discussion
2.1. Characterization of PEI Hydrogel and Nanocomposites
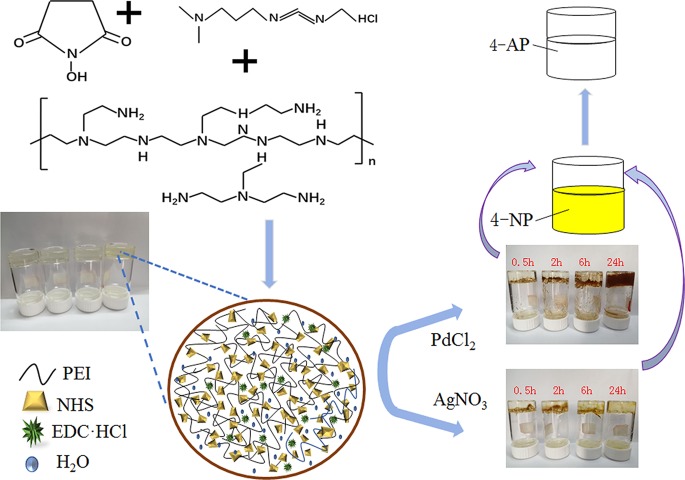
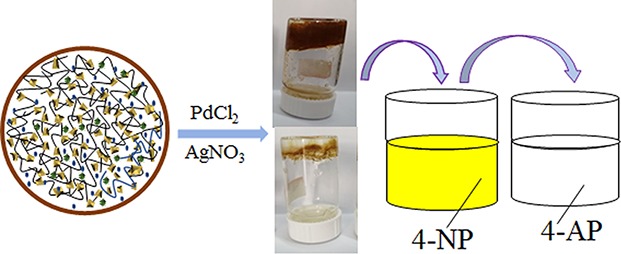
Figure 1 shows the mechanism of hydrogel formation. Abundant amine groups easily form hydrogel bonding with hydroxyl and carbonyl of N-hydroxysuccinimide (NHS). Due to a lot of acyl groups, in the presence of an initiator, they are easy to dehydrate and condense with an amine group to form a stable valence bond, thereby promoting the formation of a PEI hydrogel. After the hydrogels are formed, the excess initiator is filtered off by a hydrogel dialysis process for 7 days. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC-HCl) acts as an initiator and promotes changes in the molecular interactions. As the main substance in the hydrogels, PEI provides high adhesion and adsorption between Ag/Pd nanocomposites and substrate hydrogel. In addition, PEI acts as a polymeric cation in water to neutralize and adsorb all anionic species and chelate heavy metal ions.
Figure 1.
Synthesis and catalytic process of PEI hydrogel and PEI-Ag/PEI-Pd composites. Photograph courtesy of “Yao Feng”. Copyright 2019/2020.
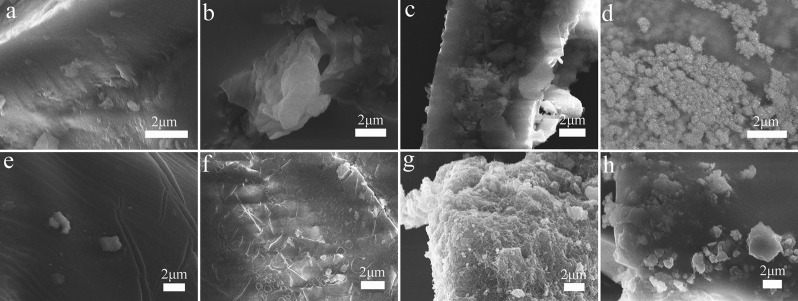
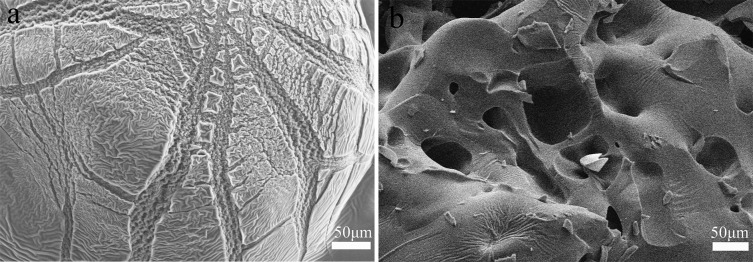
The photos of PEI-Ag or PEI-Pd composite hydrogels are shown in Figure 2. Different reaction times of the PEI hydrogel, AgNO3, or PdCl2 produced different Ag or Pd nanoparticles, which were complexed with the PEI hydrogel. It is not difficult to find that there are more and more nanocomposites of Ag and Pd that are attached to the surface of the hydrogel as the time increases. Especially, the product with 0.5 h (PEI-Ag0.5) reaction time has a small amount of Ag nanoparticles, but after 2 h (PEI-Ag2), significant changes have taken place. After 24 h (PEI-Ag24), large areas of Ag nanoparticles were discovered and tested, and the final catalytic effect was obvious. Similarly, Pd nanoparticles are reduced and attached to the surface of the PEI hydrogel. From Figure 2e–h, a large number of metallic nanocomposites were attached to the surface of PEI-Pd6 (reaction product for 6 h) and PEI-Pd24 (reaction product for 24 h). Different levels of metal substances directly affect the final catalytic effect. In addition, in the absence of composite metal silver and palladium nanoparticles, the morphologies of the blank PEI hydrogel are shown in Figure 3a,b. It was clear from the morphologies that the formed PEI hydrogel had a lot of wrinkles on the surface before dialysis. After the dialysis process, there were some mesoporous structures. Because dialysis removed some PEI molecules and excess EDC-HCL, some mesoporous structures appeared on the surface of the hydrogel. The results are consistent with the BET test results (Table 1). Due to the load of the metal nanoparticles, the pore volume and pore diameter become smaller.
Figure 2.
Representative SEM images of the (a) PEI-Ag0.5, (b) PEI-Ag2, (c) PEI-Ag6, and (d) PEI-Ag24; SEM images of (e) PEI-Pd0.5, (f) PEI-Pd2, (g) PEI-Pd6, and (h) PEI-Pd24.
Figure 3.
(a) Representative SEM images of blank PEI hydrogel before dialysis; (b) blank PEI hydrogel after dialysis.
Table 1. Data of Obtained PEI-Ag0.5/24 and PEI-Pd0.5/24.
| sample | specific surface area (m2/g) | average pore diameter (nm) | pore volume (cm3/g) |
|---|---|---|---|
| PEI-Ag0.5 | 38.889 | 6.083 | 0.059 |
| PEI-Ag24 | 13.879 | 7.737 | 0.027 |
| PEI-Pd0.5 | 23.044 | 7.093 | 0.409 |
| PEI-Pd24 | 11.221 | 7.268 | 0.020 |
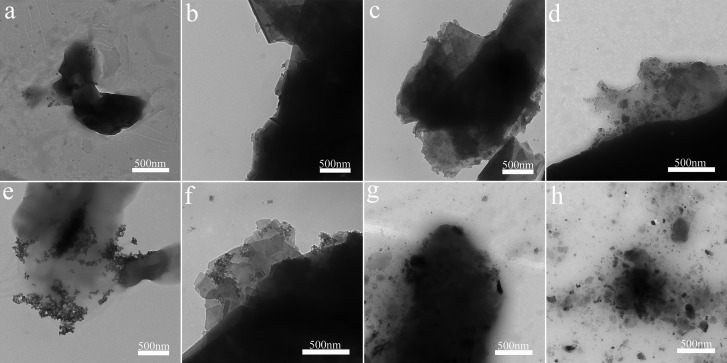
Figure 4 shows that the TEM of different morphologies of different products corresponds to Figure 2. Figure 4a–d respectively represents PEI-Ag0.5, PEI-Ag2, PEI-Ag6 (reaction product for 6 h), and PEI-Ag24. It can be clearly observed that as the reaction time increases, more and more metal particles are loaded. Figure 4e–h represents PEI-Pd0.5 (reaction product for 0.5 h), PEI-Pd2 (reaction product for 2 h), PEI-Pd6, and PEI-Pd24, respectively. Therefore, as the reaction time becomes longer, the particles and agglomerates become larger.
Figure 4.
Representative TEM images of the (a) PEI-Ag0.5, (b) PEI-Ag2, (c) PEI-Ag6, and (d) PEI-Ag24 ; TEM images of (e) PEI-Pd0.5, (f) PEI-Pd2, (g) PEI-Pd6, and (h) PEI-Pd24.
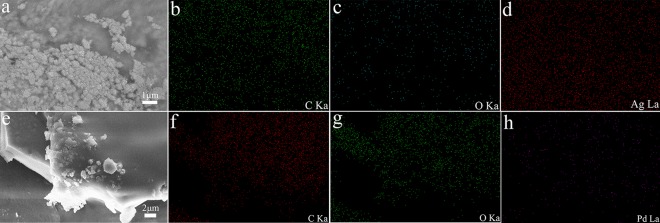
The elemental analysis of C, O, and Ag is shown in Figure 5a–d. Figure 5a shows a SEM image of PEI-Ag24. Ag nanoparticles can be observed and detected in large numbers (Figure 5d), which indicate that AgNO3 was reduced and successfully loaded onto the surface of the PEI hydrogel. Figure 5e shows the SEM image of PEI-Pd24, and elemental mappings of C, O, and Pd are tested. These results demonstrated that the metal nanoparticles and hydrogels are well compounded.
Figure 5.
SEM images of (a) PEI-Ag24 and (e) PEI-Pd24 composites, and elemental mappings of (b, f) C, (c, g) O, (d) Ag, and (h) Pd.
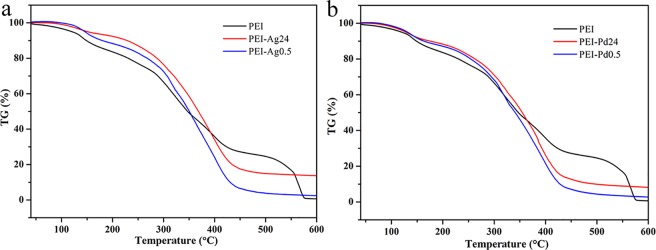
The thermogravimetric (TG) curves are demonstrated in Figure 6. The black line represents PEI hydrogel mass loss with a change of temperature from 40 to 600 °C. Due to the pyrolysis of functional groups in organic components, the mass changed significantly at 138, 289, and 551 °C. In Figure 6a, PEI-Ag0.5 composites show a higher thermal stability from 40 to 356 °C, and PEI-Ag24 is higher than PEI-Ag0.5, which could be attributed to the structural changes of intermolecular forces, but mass no longer changes when the temperature reaches 437 °C. From the final remaining mass, PEI-Ag24 is higher than PEI-Ag0.5, which is due to the long reaction time caused by sediment. In Figure 6b, the loss of PEI-Pd is faster than that of PEI-Ag, and the relative stability of PEI-Ag24 is better. The addition of the composite metal weakens the intermolecular forces between the original PEI hydrogels. When the temperature reaches 319 °C, the functional groups and components accelerate thermal decomposition.27,28 The metal contents of prepared PEI-Ag24 and PEI-Pd24 hydrogel composites were estimated as 132 and 94 mg/g, respectively. In addition, PEI-Ag0.5 and PEI-Pd0.5 were estimated as 23 and 30 mg/g, respectively. In this work, the nanoparticles formed in the composite hydrogel improved the thermal stability of the material to a certain extent.
Figure 6.
TG curves of (a) PEI-Ag0.5/24 and (b) PEI-Pd0.5/24 composites.
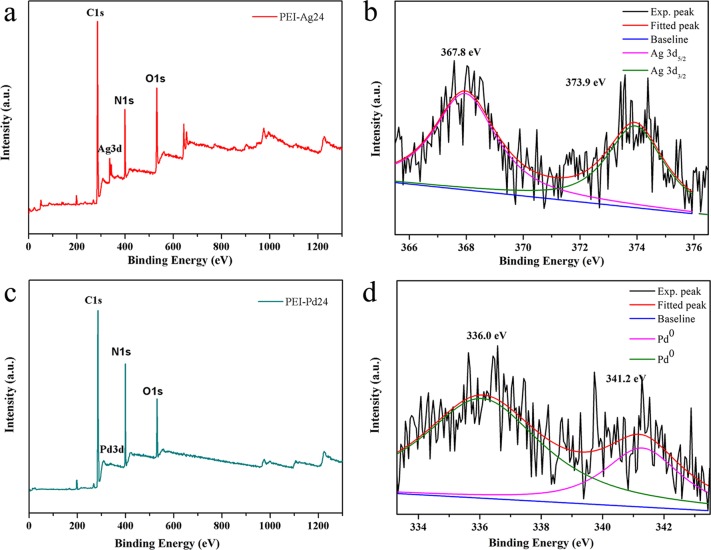
The X-ray photoelectron spectroscopy (XPS) is a commonly method used for analyzing the composition and valence of compounds. To further explore the composite of the PEI hydrogel and nanocomposites of Ag or Pd, PEI-Ag24 and PEI-Pd24 are selected for research. Figure 7a,c shows the complete measured spectra of PEI-Ag24 and PEI-Pd24 composites, respectively. The elements of C, N, O, Ag, and Pd are shown in the entire spectral range of the observable composite, which further demonstrates that the Ag or Pd coexist in the composite. In Figure 7b, there are two peaks at 367.8 eV (Ag 3d5/2) and 373.9 eV (3d3/2) corresponding to Ag0,25−27 and Figure 7c shows the characteristic peaks of Pd 3d, C 1s, N 1s, and O 1s in the curve of the PEI-Pd24 composite. The binding energies of Pd 3d with the detailed spectrum in Figure 7d, as shown in the peaks at 336.0 and 341.2 eV, correspond to metallic palladium Pd (0) (339.8 eV) and Pd (0) (341.6 eV), respectively.28,29 Therefore, the XPS results indicate the presence of metallic Pd nanoparticles in the product.
Figure 7.
(a, c) XPS survey spectra of PEI-Ag24 and PEI-Pd24 composites; (b, d) high-resolution scan of PEI-Ag24 and PEI-Pd24 composites.
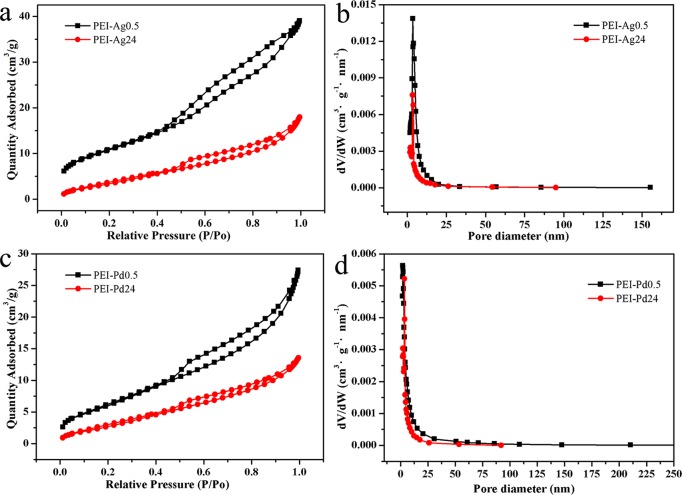
The pore size distribution and specific surface area of PEI-Ag0.5/24 and PEI-Pd0.5/24 were analyzed using BET. As shown in Figure 8a,c are the nitrogen adsorption–desorption isotherms of PEI-Ag0.5/24 and PEI-Pd0.5/24, and it can be ascribed as type II hysteresis loops at the P/P0 = 0.4–0.9, proving that there exist mesoporous structures in the PEI hydrogels.30,31 Due to the long-term reaction of PEI-Ag24 and PEI-Pd24, there is more sediment in these composites, so the adsorption capacity is greatly reduced compared with PEI-Ag0.5 and PEI-Pd0.5. Figure 8b,d shows the change of pore size distribution of PEI-Ag or PEI-Pd hydrogels. The PEI-Ag0.5 and PEI-Ag24 are located in the mesoporous region with average pore diameters of 6.08 and 7.73 nm (Table 1) and pore volumes of 0.059 and 0.026 cm3/g, respectively. This is because of the effect of the sediments on the pore volume. The same as PEI-Pd0.5 and PEI-Pd24, the average pore diameters are 7.09 and 7.26 nm and the pore volume are 0.040 and 0.02 cm3/g, respectively. These results proved that the larger the pore volume, the greater the impact on metal composites.
Figure 8.
(a, c) Nitrogen adsorption–desorption isotherms and (b, d) pore size distributions of the PEI-Ag0.5/24 and PEI-Pd0.5/24.
2.2. Catalytic Performances of PEI-Ag24 and PEI-Pd24 Nanocomposites
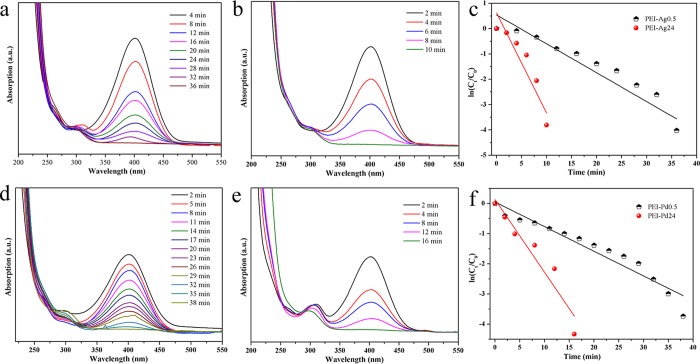
Catalytic tests were performed on pure PEI hydrogels and different products of PEI that react with AgNO3 or PdCl2 at different times. Pure PEI hydrogels have no catalytic reduction, and the color of 4-NP does not change. This conformed that 4-NP and 4-AP could seldom be adsorbed into the hydrogel. Figure 9 shows the catalytic reduction of 4-NP solution by PEI-Ag0.2, PEI-Ag24, PEI-Pd0.5, and PEI-Pd24. 4-NP (0.005 mol/L) and NaBH4 (0.01 mol/L) solutions were prepared for catalytic experiments. The peak of 4-NP is at 315.8 nm, NaBH4 is added into the 4-NP solution, and the peak is translation to 401 nm. Different hydrogels (0.02 g) of PEI-Ag0.5 and PEI-Ag24 were added into the reaction solution, as shown in Figure 9a,b; after 36 min, 10 mL of 4-NP and NaBH4 solution was degraded completely by PEI-Ag0.5, and a new peak appeared at 303 nm (Figure 9a), which represents the formation of 4-AP. The PEI-Ag24 needs 10 min to achieve complete catalysis (Figure 9b). According to the processing of the data at the maximum absorption wavelength, a linear fit was performed to obtain the relationship between the catalytic time t and ln(Ct/C0) in Figure 9c. Following the rate equation as ln(Ct/C0) = ln(At/A0) = −Kt, the experimental data of 4-NP reduction were evaluated.5,32−35 Also from the linear fitting (Figure 9c), it was calculated that the linear fitting constants of PEI-Ag0.5 and PEI-Ag24 are 0.11 and 0.39 min–1. Figure 9d,e shows the results of catalytic tests by PEI-Pd0.5 and PEI-Pd24, respectively. PEI-Pd0.5 takes 38 min, but PEI-Pd24 needs 16 min, so the catalytic effect of PEI-Pd24 is more obvious. The linear fitting constants of PEI-Pd0.5 and PEI-Pd24 are 0.08 and 0.24 min–1. According the Arrhenius equation (ln(K) = ln(A) – (Ea/RT)), activation energy (Ea) values could be used to evaluate the catalytic properties.23,26,36,37 The calculated activation energy (Ea) values of the obtained composite hydrogels are 25.29, 23.12, 36.10, and 35.31 kJ/mol for PEI-Ag0.5, PEI-Ag24, PEI-Pd0.5, and PEI-Pd24, respectively. The larger the linear fit constant, the better the catalytic effect. The fitting curves also proved that the fitting constant of PEI-Pd24 was larger than that of PEI-Pd0.5, as shown in Figure 9c,f.
Figure 9.
Catalytic reduction of 4-NP via present synthesized (a) PEI-Ag0.5, (b) PEI-Ag24, (d) PEI-Pd0.5, and (e) PEI-Pd24 composites. (c, f) Plot of ln(Ct/C0) versus time for 4-NP.
The stability of the catalyst is also one of the factors for measuring quality of the catalyst. Figure 10 shows the results of PEI-Ag24 and PEI-Pd24 for repeated experiments. Hydrogel pieces (0.02 g) were put into the 4-NP solution. The catalytic efficiency of PEI-Ag24 reduced to 92% and that of PEI-Pd24 reduced to 94% after eight repeated catalytic processes. After the catalytic experiment, the hydrogel sheet could be completely removed and easily cleaned, which proved the good mechanical properties and stable mechanical properties of the hydrogel. Compared to other nanoparticle catalysts reported, the results show that the PEI-Ag or PEI-Pd hydrogels can be well recovered from the solution. The Ag or Pd nanoparticle composite with the hydrogel is easy to recycle and separate. This has made a huge contribution to improving cycle utilization.38−45
Figure 10.
Relative regeneration studies of as-prepared (a) PEI-Ag24 and (b) PEI-Pd24 at room temperature.
3. Conclusions
In conclusion, we have successfully synthesized PEI-Ag and PEI-Pd composite hydrogels by a simple and convenient method for catalyzing 4-NP to form 4-AP in sewage. In addition, TEM, TG, BET, XPS, and other characterization methods were used to verify the deposition of metallic Ag and Pd nanoparticles in obtained hydrogels. The gelation process and optimal prepared conditions of the obtained polymer hydrogels were characterized, and different reaction times on the formation of complexes at 0.5, 2, 6, and 24 h were studied on the effects of corresponding reactants for catalytic reaction of 4-NP. The results show that the PEI-Ag24 and PEI-Pd24 composites have a large amount of metal Ag or Pd deposition with the best catalytic effect. Therefore, the large surface area and pore structure in the hydrogel not only provide the function of the template substrate but also improve the reuse stability of the composite hydrogel. The results show that the obtained product hydrogels used after eight repeated cycles have still showed high performance for catalytic reduction of 4-NP, demonstrating potential applications in wastewater treatment and composite materials.
4. Experimental Section
4.1. Materials
The starting materials, poly(ethylene imine) (PEI, Mw = 600 g·mol–1), palladium chloride (PbCl2, 59–60%), and silver nitrate (AgNO3, 99.8%) were purchased from Aladdin Reagent, Shanghai, China.1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC-HCl) and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich. Sodium borohydride (NaBH4) and p-nitrophenol (4-NP) were purchased from Shanghai Hushi Reagent. The deionized water (DI water) was made by a two-stage Millipore Milli-Q water purification system. All reagents were not further purified.
4.2. Preparation Procedure of PEI Hydrogels
PEI was dissolved into DI water at room temperature and ultrasonic treatment in a water bath. Then, 0.15 g of NHS and 0.5 g of EDC-HCl were added into the prepared PEI solution, and a moderate amount of DI water was mixed into the precursor fluid. After 15 min ultrasonic treatment, samples were treated by standing. Different concentrations of PEI hydrogels were formed for different times, the higher the concentration, the faster the molding.
There were four concentrations applied into PEI hydrogels, they are shown in Table 2. The prepared samples were designated as PEI#1, PEI#2, PEI#3, and PEI #4. The high concentrations are more suitable for further study, so the PEI#4 hydrogel was selected to reduce the metal ion.
Table 2. Gelation Behaviors at Room Temperature.
| samples | PEI (mg/mL) | product |
|---|---|---|
| PEI#1 | 150 | solution |
| PEI#2 | 200 | gel |
| PEI#3 | 250 | gel |
| PEI#4 | 300 | gel |
4.3. Reduce Metal Ion
PEI has moderate and weak reducibility, so PEI#4 was used in the experiment of the reducing metal ion. In this part, 0.355 g of PdCl2 and 0.339 g of AgNO3 were dissolved in 40 mL of DI water to prepare 0.05 mol/L solution. There were four contrast samples treated with PdCl2 and AgNO3 solutions for 0.5 h (PEI-Pd/Ag 0.5), 2 h (PEI-Pd/Ag2), 6 h (PEI-Pd/Ag6), and 24 h (PEI-Pd/Ag24). As shown in Figures 1 and 2, they demonstrate the different surface topographies of different reaction times. Then, these different productions were treated by dialysis and lyophilization for 7 days, and after that, the dry hydrogels were further tested and analyzed.
4.4. Characterization
The hydrogels were obtained at −50 °C, using a Beijing Boyikang Experimental Instrument Co., Ltd. FD-1C-50 lyophilizer, completely removing water after 5–7 days.46−54 A field-emission scanning electron microscope (SEM) (S-4800II, Hitachi, Japan) with 15 kV accelerating voltage and transmission electron microscope (TEM, HT7700, Hitachi High-Technologies Corporation) were used to analyze the nanomicroscopic surface of composite hydrogels. Elemental composition and lattice structure of complexes were determined by X-ray diffraction (XRD) with a copper Kα X-ray radiation source and Bragg diffraction X-ray radiation source settings (Smart Lab, Rigaku, Japan). The valence status and surface elements were analyzed by X-ray photoelectron spectroscopy (XPS, Kratos AXIS 165, Kratos Analytical, Kyoto, Japan). Thermogravimetry (TG) was tested by using a NETZSCH STA 409 PC Luxxsi multaneous thermal analyzer (Netzsch Instruments Manufacturing Co, Ltd., Germany) in argon gas. To research the pore size distribution and specific surface area of hydrogels, Brunauer–Emmett–Teller measurement (BET) (ASAP 2460) was adopted for analysis.
4.5. Catalytic Test of 4-NP
4-NP (0.005 mol/L) and NaBH4 (0.01 mol/L) solutions were prepared for catalytic experiments. First, 24 mL stock solution of 4-NP was put into a beaker and transferred a little to a quartz cuvette for testing the absorbance of ultraviolet wavelength from 200 to 550 nm at room temperature, Then, 5 mL of fresh NaBH4 was added to the 4-NP solution, and a small amount of mixed solution was put into a quartz cuvette for testing the absorbance of ultraviolet wavelength as in the above method. Finally, 0.02 g of different hydrogels with PdCl2 or AgNO3 was added to the prepared solution of NaBH4 and 4-NP. The color of the mixed solution changed from bright yellow to colorless, indicating that the catalysis was completed.
Acknowledgments
We greatly appreciate the financial support of the National Natural Science Foundation of China (no. 21872119), the Talent Engineering Training Funding Project of Hebei Province (no. A201905004), and the Research Program of the College Science and Technology of Hebei Province (no. ZD2018091).
Author Contributions
# Y.F. and J.Y. contributed equally to this work.
The authors declare no competing financial interest.
References
- Guo H.; Jiao T.; Zhang Q.; Guo W.; Peng Q.; Yan X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. 10.1186/s11671-015-0931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp K. C.; Seema H.; Saleh M.; Le N. H.; Mahesh K.; Chandra V.; Kim K. S. Environmental applications using graphene composites: water remediation and gas adsorption. Nanoscale 2013, 5, 3149–3171. 10.1039/c3nr33708a. [DOI] [PubMed] [Google Scholar]
- Feng X.; Jiang K.; Fan S.; Kanan M. W. A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Cent. Sci. 2016, 2, 169–174. 10.1021/acscentsci.6b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Li L.; Chen B.; Shi S.; Nie J.; Ma G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer 2019, 163, 74–85. 10.1016/j.polymer.2018.12.046. [DOI] [Google Scholar]
- Garole V. J.; Choudhary B. C.; Tetgure S. R.; Garole D. J.; Borse A. U. Palladium nanocatalyst: green synthesis, characterization, and catalytic application. Int. J. Environ. Sci. Technol. 2019, 16, 7885–7892. 10.1007/s13762-018-2173-1. [DOI] [Google Scholar]
- Rasaki S. A.; Zhao C.; Wang R.; Wang J.; Jiang H.; Yang M. Facile synthesis approach for preparation of robust and recyclable Ag/ZnO nanorods with high catalytic activity for 4-nitrophenol reduction. Mater. Res. Bull. 2019, 119, 110536. 10.1016/j.materresbull.2019.110536. [DOI] [Google Scholar]
- Jiang D.; Liu Z.; Han J.; Wu X. A tough nanocomposite hydrogel for antifouling application with quaternized hyperbranched PEI nanoparticles crosslinking. RSC Adv. 2016, 6, 60530–60536. 10.1039/C6RA07335B. [DOI] [Google Scholar]
- Peppas N. A.; Huang Y.; Torres-Lugo M.; Ward J. H.; Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- Hennink W. E.; van Nostrum C. F. Novel crosslinking methods to design hydrogels. Adv. Drug Delivery Rev. 2012, 64, 223–236. 10.1016/j.addr.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Hamley I. W. PEG-peptide conjugates. Biomacromolecules 2014, 15, 1543–1559. 10.1021/bm500246w. [DOI] [PubMed] [Google Scholar]
- Hirschberg J. H. K. K.; Brunsveld L.; Ramzi A.; Vekemans J. A. J. M.; Sijbesma R. P.; Meijer E. W. Helical self-assembled polymers from cooperative stacking of hydrogen-bonded pairs. Nature 2000, 407, 167. 10.1038/35025027. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Mooney D. J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Dong L.; Agarwal A. K.; Beebe D. J.; Jiang H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 2006, 442, 551. 10.1038/nature05024. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Mynar J. L.; Yoshida M.; Lee E.; Lee M.; Okuro K.; Kinbara K.; Aida T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339. 10.1038/nature08693. [DOI] [PubMed] [Google Scholar]
- Huang Z.-W.; Li Z.-J.; Wu Q.-Y.; Zheng L.-R.; Zhou L.-M.; Chai Z.-F.; Wang X.-L.; Shi W.-Q. Simultaneous elimination of cationic uranium(vi) and anionic rhenium(vii) by graphene oxide–poly(ethyleneimine) macrostructures: a batch, XPS, EXAFS, and DFT combined study. Environ. Sci.: Nano 2018, 5, 2077–2087. 10.1039/C8EN00677F. [DOI] [Google Scholar]
- Bucatariu F.; Ghiorghita C.-A.; Schwarz D.; Boita T.; Mihai M. Layer-by-layer polyelectrolyte architectures with ultra-fast and high loading/release properties for copper ions. Colloids Surf., A 2019, 579, 123704. 10.1016/j.colsurfa.2019.123704. [DOI] [Google Scholar]
- Chatterjee S.; Chatterjee T.; Woo S. H. Influence of the polyethyleneimine grafting on the adsorption capacity of chitosan beads for Reactive Black 5 from aqueous solutions. Chem. Eng. J. 2011, 166, 168–175. 10.1016/j.cej.2010.10.047. [DOI] [Google Scholar]
- Wan Ngah W. S.; Teong L. C.; Hanafiah M. A. K. M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. 10.1016/j.carbpol.2010.11.004. [DOI] [Google Scholar]
- Dragan E. S.; Cocarta A. I.; Dinu M. V. Facile fabrication of chitosan/poly (vinyl amine) composite beads with enhanced sorption of Cu2+. Equilibrium, kinetics, and thermodynamics. Chem. Eng. J. 2014, 255, 659–669. 10.1016/j.cej.2014.06.098. [DOI] [Google Scholar]
- Mihai M.; Bunia I.; Doroftei F.; Varganici C.-D.; Simionescu B. C. Highly Efficient Copper (II) Ion Sorbents Obtained by Calcium Carbonate Mineralization on Functionalized Cross-Linked Copolymers. Chem. – Eur. J. 2015, 21, 5220–5230. 10.1002/chem.201406011. [DOI] [PubMed] [Google Scholar]
- Tan Y. Z.; Wu D.; Lee H. T.; Wang H.; Honciuc A.; Chew J. W. Synthesis of ligand-carrying polymeric nanoparticles for use in extraction and recovery of metal ions. Colloids Surf., A 2017, 533, 179–186. 10.1016/j.colsurfa.2017.08.036. [DOI] [Google Scholar]
- Dinu M. V.; Dinu I. A.; Lazar M. M.; Dragan E. S. Chitosan-based ion-imprinted cryo-composites with excellent selectivity for copper ions. Carbohydr. Polym. 2018, 186, 140–149. 10.1016/j.carbpol.2018.01.033. [DOI] [PubMed] [Google Scholar]
- Demirci S.; Sel K.; Sahiner N. Ionic liquid colloids based on PEI for versatile use. Sep. Purif. Technol. 2015, 155, 66–74. 10.1016/j.seppur.2015.05.038. [DOI] [Google Scholar]
- Tang J.; Song Y.; Zhao F.; Spinney S.; da Silva Bernardes J.; Tam K. C. Compressible cellulose nanofibril (CNF) based aerogels produced via a bio-inspired strategy for heavy metal ion and dye removal. Carbohydr. Polym. 2019, 208, 404–412. 10.1016/j.carbpol.2018.12.079. [DOI] [PubMed] [Google Scholar]
- Jiao T.; Guo H.; Zhang Q.; Peng Q.; Tang Y.; Yan X.; Li B. Reduced graphene oxide-based silver nanoparticle-containing composite hydrogel as highly efficient dye catalysts for wastewater treatment. Sci. Rep. 2015, 5, 11873. 10.1038/srep11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahiner N.; Demirci S. PEI-based hydrogels with different morphology and sizes: Bulkgel, microgel, and cryogel for catalytic energy and environmental catalytic applications. Eur. Polym. J. 2016, 76, 156–169. 10.1016/j.eurpolymj.2016.01.046. [DOI] [Google Scholar]
- Wang R.; Liu Q.; Jiao T.; Li J.; Rao Y.; Su J.; Bai Z.; Peng Q. Facile Preparation and Enhanced Catalytic Properties of Self-Assembled Pd Nanoparticle-Loaded Nanocomposite Films Synthesized via the Electrospun Approach. ACS Omega 2019, 4, 8480–8486. 10.1021/acsomega.9b01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambriz-Peláez O.; Durón S.; Olivas A.; Valdez R.; Arriaga L. G.; Álvarez-Contreras L.; Guerra-Balcázar M.; Arjona N. Effect of molybdenum content on the morphology and electronic characteristics of Pd–MoO nanomaterials and activity evaluation for ethylene glycol electro–oxidation. Appl. Surf. Sci. 2019, 498, 143842. 10.1016/j.apsusc.2019.143842. [DOI] [Google Scholar]
- Wang Q.; Sang H.; Chen L.; Wu Y.; Wei Y. Selective separation of Pd (II) through ion exchange and oxidation-reduction with hexacyanoferrates from high-level liquid waste. Sep. Purif. Technol. 2020, 231, 115932. 10.1016/j.seppur.2019.115932. [DOI] [Google Scholar]
- Chen Y.; Xie X.; Si Y.; Wang P.; Yan Q. Constructing a novel hierarchical β-Ag2MoO4/BiVO4 photocatalyst with Z-scheme heterojunction utilizing Ag as an electron mediator. Appl. Surf. Sci. 2019, 498, 143860. 10.1016/j.apsusc.2019.143860. [DOI] [Google Scholar]
- Gao Y.; Guo R.; Feng Y.; Zhang L.; Wang C.; Song J.; Jiao T.; Zhou J.; Peng Q. Self-assembled hydrogels based on poly-cyclodextrin and poly-azobenzene compounds and applications for highly efficient removal of bisphenol A and methylene blue. ACS Omega 2018, 3, 11663–11672. 10.1021/acsomega.8b01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Sun S.; Zhang L.; Yin J.; Jiao T.; Zhang L.; Xu Y.; Zhou J.; Peng Q. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloids Surf., A 2019, 561, 283–291. 10.1016/j.colsurfa.2018.11.002. [DOI] [Google Scholar]
- Li K.; Jiao T.; Xing R.; Zou G.; Zhou J.; Zhang L.; Peng Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. 10.1007/s40843-017-9196-8. [DOI] [Google Scholar]
- Zhao X.; Jiao T.; Xing R.; Huang H.; Hu J.; Qu Y.; Zhou J.; Zhang L.; Peng Q. Preparation of diamond-based AuNP-modified nanocomposites with elevated catalytic performances. RSC Adv. 2017, 7, 49923–49930. 10.1039/C7RA10770F. [DOI] [Google Scholar]
- Feng Y.; Jiao T.; Yin J.; Zhang L.; Zhang L.; Zhou J.; Peng Q. Facile preparation of carbon nanotube-Cu2O nanocomposites as new catalyst materials for reduction of p-nitrophenol. Nanoscale Res. Lett. 2019, 14, 78. 10.1186/s11671-019-2914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chairam S.; Somsook E. Facile, versatile and green synthesis of silver nanoparticles by mung bean starch and their catalytic activity in the reduction of 4-nitrophenol. Chiang Mai J. Sci. 2016, 43, 609–619. [Google Scholar]
- Wi-Afedzi T.; Kwon E.; Tuan D. D.; Lin K.-Y. A.; Ghanbari F. Copper hexacyanoferrate nanocrystal as a highly efficient non-noble metal catalyst for reduction of 4-nitrophenol in water. Sci. Total Environ. 2020, 703, 134781. 10.1016/j.scitotenv.2019.134781. [DOI] [PubMed] [Google Scholar]
- He Y.; Wang R.; Jiao T.; Yan X.; Wang M.; Zhang L.; Bai Z.; Zhang Q.; Peng Q. Facile Preparation of Self-Assembled Layered Double Hydroxide-Based Composite Dye Films as New Chemical Gas Sensors. ACS Sustainable Chem. Eng. 2019, 7, 10888–10899. 10.1021/acssuschemeng.9b01780. [DOI] [Google Scholar]
- Cai C.; Wang R.; Liu S.; Yan X.; Zhang L.; Wang M.; Tong Q.; Jiao T. Synthesis of Self-Assembled Phytic Acid-MXene Nanocomposites via a Facile Hydrothermal Approach with Elevated Dye Adsorption Capacities. Colloids Surf., A 2020, 589, 124468. 10.1016/j.colsurfa.2020.124468. [DOI] [Google Scholar]
- Xu Y.; Wang R.; Zheng Y.; Zhang L.; Jiao T.; Peng Q.; Liu Z. Facile Preparation of Self-Assembled Ni/Co Phosphates Composite Spheres with Highly Efficient HER Electrocatalytic Performances. Appl. Surf. Sci. 2020, 509, 145383. 10.1016/j.apsusc.2020.145383. [DOI] [Google Scholar]
- Geng R.; Yin J.; Zhou J.; Jiao T.; Feng Y.; Zhang L.; Chen Y.; Bai Z.; Peng Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 1. 10.3390/nano10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F.; Yin J.; Zhou J.; Jiao T.; Zhang L.; Xia M.; Bai Z.; Peng Q. Facile preparation and highly efficient catalytic performances of Pd-Cu bimetallic catalyst synthesized via Seed-mediated method. Nanomaterials 2020, 10, 6. 10.3390/nano10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; Wang R.; Yin J.; Zhan F.; Chen K.; Jiao T.; Zhou J.; Zhang L.; Peng Q. Facile Synthesis of Cu2O nanoparticle-loaded Carbon Nanotubes Composite Catalysts for Reduction of 4-Nitrophenol. Curr. Nanosci. 2020, 10.2174/1573413715666191206161555. [DOI] [Google Scholar]
- Li H.; Yin J.; Meng Y.; Liu S.; Jiao T. Nickel/Cobalt-Containing Polypyrrole Hydrogel-Derived Approach for Efficient ORR Electrocatalyst. Colloids Surf., A 2020, 586, 124221. 10.1016/j.colsurfa.2019.124221. [DOI] [Google Scholar]
- Zhao J.; Yin J.; Zhong J.; Jiao T.; Bai Z.; Wang S.; Zhang L.; Peng Q. Facile preparation of a self-assembled artemia cyst shell–TiO2–MoS2 porous composite structure with highly efficient catalytic reduction of nitro compounds for wastewater treatment. Nanotechnology 2020, 31, 085603 10.1088/1361-6528/ab53c1. [DOI] [PubMed] [Google Scholar]
- Meng Y.; Yin J.; Jiao T.; Bai J.; Zhang L.; Su J.; Liu S.; Bai Z.; Cao M.; Peng Q. Self-assembled copper/cobalt-containing polypyrrole hydrogels for highly efficient ORR electrocatalysts. J. Mol. Liq. 2020, 298, 112010. 10.1016/j.molliq.2019.112010. [DOI] [Google Scholar]
- Yin J.; Zhan F.; Jiao T.; Deng H.; Zou G.; Bai Z.; Zhang Q.; Peng Q. Highly efficient catalytic performances of nitro compounds via hierarchical PdNPs-loaded MXene/polymer nanocomposites synthesized through electrospinning strategy for wastewater treatment. Chin. Chem. Lett. 2020, 10.1016/j.cclet.2019.08.047. [DOI] [Google Scholar]
- Ma K.; Wang R.; Jiao T.; Zhou J.; Zhang L.; Li J.; Bai Z.; Peng Q. Preparation and aggregate state regulation of co-assembly graphene oxide-porphyrin composite Langmuir films via surface-modified graphene oxide sheets. Colloids Surf., A 2020, 584, 124023. 10.1016/j.colsurfa.2019.124023. [DOI] [Google Scholar]
- Zhu J.; Wang R.; Geng R.; Zhang X.; Wang F.; Jiao T.; Yang J.; Bai Z.; Peng Q. A facile preparation method for new two-component supramolecular hydrogels and their performances in adsorption, catalysis, and stimuli-response. RSC Adv. 2019, 9, 22551–22558. 10.1039/C9RA03827B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou N.; Wang R.; Geng R.; Wang F.; Jiao T.; Zhang L.; Zhou J.; Bai Z.; Peng Q. Facile preparation of self-assembled hydrogels constructed from poly-cyclodextrin and poly-adamantane as highly selective adsorbents for wastewater treatment. Soft Matter 2019, 15, 6097–6106. 10.1039/C9SM00978G. [DOI] [PubMed] [Google Scholar]
- Wang C.; Yin J.; Han S.; Jiao T.; Bai Z.; Zhou J.; Zhang L.; Peng Q. Preparation of Palladium Nanoparticles Decorated Polyethyleneimine/Polycaprolactone Composite Fibers Constructed by Electrospinning with Highly Efficient and Recyclable Catalytic Performances. Catalysts 2019, 9, 559. 10.3390/catal9060559. [DOI] [Google Scholar]
- Ma K.; Chen W.; Jiao T.; Jin X.; Sang Y.; Yang D.; Zhou J.; Liu M.; Duan P. Boosting Circularly Polarized Luminescence of Small Organic Molecules via Multi-dimensional Morphology Control. Chem. Sci. 2019, 10, 6821–6827. 10.1039/C9SC01577A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Liu R.; Chen H.; Li H.; Guo P. Synthesis of self-assembled nickel cobaltite microspheres and their electrocapacitive behavior in aqueous electrolytes. Colloids Surf., A 2020, 587, 124329. 10.1016/j.colsurfa.2019.124329. [DOI] [Google Scholar]
- Sun J.; Yang M.; Gong Y.; Li H.; Guo P. Synthesis of Pd3Pb colloidal nanocrystal assembly and their electrocatalytic activity toward ethanol oxidation. Colloids Surf., A 2020, 586, 124224. 10.1016/j.colsurfa.2019.124224. [DOI] [Google Scholar]