Abstract
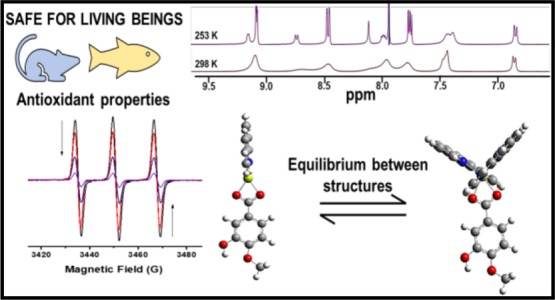
The beneficial effect of polyphenols and magnesium(II) against oxidative stress motivated our research group to explore the antioxidant activity of phenMgIso, an aqueous soluble magnesium(II) complex containing 1,10-phenanthroline (phen) and isovanillic acid (Iso) as ligands. Combined electrospray ionization–mass spectrometry and DOSY-NMR techniques identified two complexes in methanolic solution: hexacoordinated [Mg(phen)2(Iso)]+ and tetracoordinated [Mg(phen)(Iso)]+. The cyclic voltammogram of phenMgIso in the anodic region showed a cyclic process that interrupts the isovanillic acid degradation, probably by stabilization of the corresponding phenoxyl radical via complexation with Mg(II), which is interesting for antioxidant applications. phenMgIso competes with 2,2,6,6-tetramethylpiperidine by 1O2 with IC50(1O2) = 15 μg m–1 and with nitrotetrazolium blue chloride by superoxide ions (IC50(O2•–) = 3.6 μg mL–1). Exposure of both zebrafish (2 mg L–1) and wistar male rats (3 mg kg–1 day–1 dose for 21 days) to phenMgIso does not cause mortality or visual changes compared with the respective control groups, thus phenMgIso could be considered safe under the conditions of this study. Moreover, no significant changes in comparison to both control groups were observed in the biochemical parameters on the brain-acetylcholinesterase activity, digestive tract enzyme catalase, and glutathione-S-transferase. Conversely, the performance of superoxide dismutase activity in wistar male rats increased in the presence of a complex, resulting in enhanced capacity of rats for superoxide radical enzymatic scavenging. The synergistic action of phenMgIso may be explained by the strong electrostatic interaction between Mg(II) and the O,O(phenolate) group, which makes the Iso ligand easier to oxidize and deprotonate, generating a cyclic stable species under oxidative conditions.
1. Introduction
The therapeutic effects of polyphenols associated with cytoprotection during inflammation and with prevention and treatment of cardiovascular and neurodegenerative diseases1 have aroused considerable interest in the protective mechanisms of these compounds against the oxidative stress chemistry and biochemistry of polyphenols2 and their delivery mechanisms. A fundamental issue that should be considered in planning a delivery system of polyphenols is their low bioavailability and susceptibility to generate harmful radical species.
In this regard, considerable attention has turned to the reactivity of complexes formed by chelation of polyphenols with essential metals, in particular those involved in antioxidant enzymes such as Cu/Zn superoxide dismutase (SOD) and Fe(II/III) metabolism.4−6 The interest in understanding the role of these metals in natural processes is justified, in part, because they represent a guideline to exemplify the mechanisms of oxidative stress control by these polyphenols and provide clues to the development of new pharmacological antioxidant compounds. For example, coordination of a deprotonate polyphenol to Fe(III) produces a neutral quinone and free Fe(II), which at low pH may participate in Fenton reactions to produce an OH• radical. Consequently, Fe(II/III)–O,O(phenolate) complexes present both antioxidant and pro-oxidant properties. Redox-inactive metal ions such as Zn(II) may also control the oxidative stress in the biological medium. Zn(II) protection occurs indirectly either by chelation of specific protein sites or by competing with pro-oxidant metals (Cu and Fe).3−8 A recent study on Zn(II)–kaempferol has shown interesting antioxidant properties.9
Similar to zinc(II), magnesium(II) is essential in a remarkable number of biological processes, in which it plays a structural and/or catalytic role, and its deficiency also affects the antioxidant enzyme system; however, the mechanism behind these effects is still not fully understood.10−19 Mg(II) is a hard acid and forms, preferentially, hexacoordinated complexes with oxygen atoms,20−23 and those containing carboxylate groups have gained particular attention. For example, the interconversion from the monodentate (through hydroxyl) to the bidentate (through hydroxyl and carboxyl) coordination of the carboxylate group to Mg(II) in control of the reactivity of Mg(II) has been studied.24 Furthermore, increased attention has also been directed to the contribution of hydrogen bonds in the control of coordinated water acidity.25 Recent studies have explored this in detail in a series of magnesium carboxylate complexes containing intramolecular NH···O hydrogen bonds using theoretical calculations and X-ray analysis.26−29
Additionally, neutral chelating N,N-aromatic ligands can also be employed to control the water ligand exchange in hexacoordinated Mg(II) complexes. Among the many ligands investigated, 1,10-phenanthroline (phen) provides a selection of Mg–phen complexes with a varying number of coordinated water molecules, such as [Mg(phen)2(H2O)2]2+,9 [Mg(SO4)(phen)(H2O)3],10 and [Mg(phen)3]2+.11 Mixed mono- and binuclear aquo-Mg(II) complexes containing the anionic tetrazole ligand have been prepared and investigated.30 Among them, Mg(II)-1,3-benzene-dicarboxilic (DBHC) [Mg(H2O)2(phen)(HBDC)2] shows chiral properties.31
Mg(II) complexes containing the flavonoid quercetin have also been investigated, which showed encouraging antioxidant properties compared to free quercetin for the free radical scavenging activity of 2,2-diphenyl-1-picrylhydrazyl.32
In this context, we have recently prepared the Mg–phen complex containing the flavonoid hesperidin (hesp). The [Mg(hesp)2(phen)] complex shows an interesting profile of activities with high hydrophilicity and superoxide radical scavenging activity (IC50 = 68.3 μM at pH 7.8) and uptake by Hela cancer cells with no apparent cytotoxicity while maintaining luminescence in vitro, which enables cellular and distribution localization investigations.33
These findings created the opportunity to investigate the effects of the coordination of a polyphenol in the Mg(II)-polypyridine moiety on the reactivity of the potential antioxidant complex. This study reports the synthesis and characterization of a Mg(II) complex containing 1,10-phenanthroline (phen) and isovanillic acid (Iso) as ligands. It also describes the antioxidant activity of this complex against the reactive oxygen species, the toxicity to zebrafish and wistar male rats, and the protective effect to SOD enzymes.
2. Results and Discussion
2.1. Synthesis and Structural Characterization of the Complex in Solution
The synthesis of the Mg complex using Mg(CH3COO)2, 1,10-phenanthroline, and isovanillic acid in 1:1:1 proportion in methanol with tetraethylammonium led to the formation of a mixture of hexacoordinated and tetracoordinated complexes, as illustrated in Figure 1. The complexes were characterized by mass spectrometry (MS) [electrospray ionization (ESI)–MS] and NMR spectroscopy, and the geometries were optimized using density functional theory (DFT).
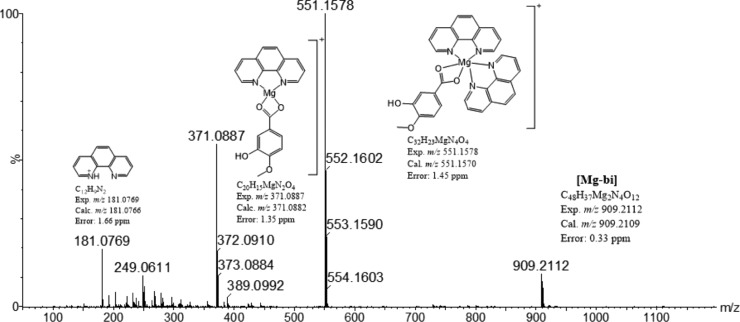
Figure 1.
ESI(+)-MS spectrum of a freshly prepared methanolic solution of the phenMgIso complex.
2.1.1. ESI–Mass Spectrometry
The mass spectrum of a freshly prepared methanolic solution shows the presence of three signals at m/z 551.1578 (hexacoordinated [Mg(phen)2(Iso)]+, Mg-hex), m/z 331.0887 (tetracoordinated [Mg(phen)(Iso)]+, Mg-tet), and at m/z 909.2112 (Mg-bi) (low-intensity peak) corresponding to two mononuclear complexes and one binuclear complex, respectively, and a signal corresponding to a free phenanthroline ligand at m/z 181.0769 (Figures 1 and S1). The fragmentation pattern of the ion of m/z 551 at 35 eV shows the loss of one phen ligand (Figure S2), giving rise to a tetradentate [Mg(phen)(Iso)]+ ion complex (m/z 371.08) and indicating that the interaction of Mg with Iso is stronger than that with phen in the gas phase. By increasing the collision energy to >50 eV, no further loss of intact ligand occurs; instead, formation of fragments from the cleavage of covalent bonds was observed (m/z 356.06, 327.09, and 312.07).
The MS/MS spectrum of m/z 909.21 (Mg-bi) at 20 eV shows a base peak at m/z 729.14, corresponding to the loss of a phen ligand [(Mg-bi)-1phen]+, and two low abundant peaks at m/z 551.15 and 371.08, which were attributed to the [Mg-hex]+ and [Mg-tet]+ ions, respectively (Figure S2).
It should be noted that when the same solution is kept in the dark for 3 days, only two complexes are present, Mg-hex and Mg-tet, in addition to a free phen ligand, indicating the transient characteristic of the high mass species. In agreement, Mg(II) preferentially forms octahedral complexes, although tetrahedral complexes have also been reported depending on the ligand strength.34,35
2.1.2. NMR Studies
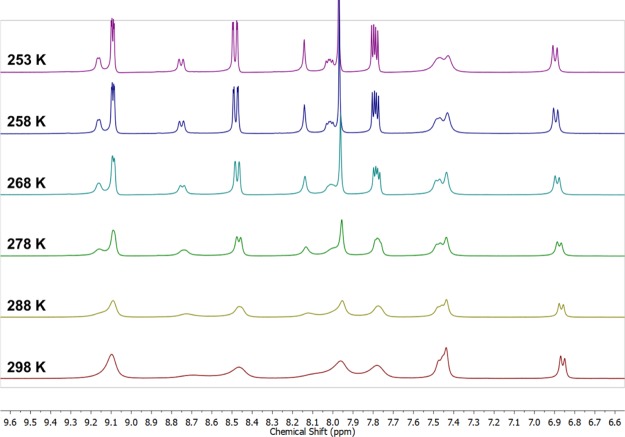
The 1H NMR spectra of the mixture of phenMgIso complexes at 298 K in MeOD (Figure 2, bottom spectrum) show a set of broad signals that correspond to the isovanillic acid (6.7–7.5 ppm) and phenanthroline (7.5–9.2 ppm) ligands. The broadening of the NMR signal can be associated with the low natural abundance (almost 10%) for the 25Mg isotope (spin 5/2) and the extension of the quadrupolar moment relaxation from these ions on the adjacent protons,36 but it may also be attributed to ligand-exchange reactions.37
Figure 2.
Variable-temperature 1H NMR spectra of the phenMgIso complex in MeOD.
In this context, an NMR experiment was performed under variable temperatures: 298–253 K (Figure 2). During the progressive decrease in temperature, the 1H NMR spectra showed a split of the phenanthroline signals into two different sets: one with sharp lines, which resembles a free-phen solution, and the other described by broad signals, which is expected for a coordinated phen ligand. To probe the mediated temperature dynamics of this complexation, a DOSY-NMR investigation was performed.
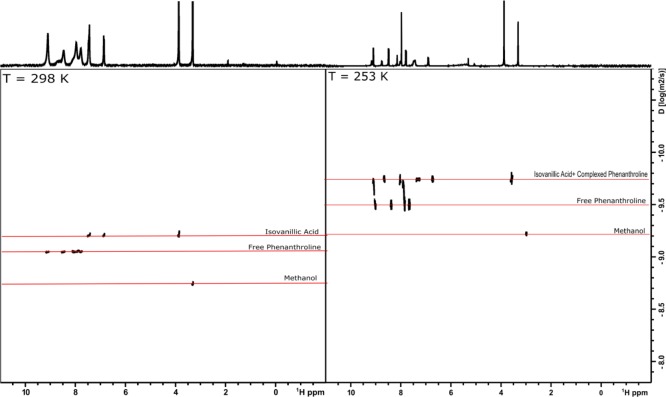
The NMR-DOSY map collected at 298 K shows two distinguishable diffusion coefficients (Figure 3, left-hand side), proving the existence of two individual components. The component with signals associated with the phenanthroline moiety (with D = 1.53 × 10–5 mm2/s) exhibits faster diffusion compared with the second component, which is attributed to the isovanillic acid moiety (D = 1.55 × 10–6 mm2/s). In contrast, in the map collected at 253 K (Figure 3, right-hand side), two distinguishable diffusions are still observed but with some differences. The first component, with faster diffusion (D = 1.07 × 10–5 mm2/s), remains associated with a free phenanthroline group, here with a smaller D because of the lower temperature, whereas the second component exhibits a diffusion coefficient associated with both broader phenanthroline and Iso signals (D = 1.22 × 10–6 mm2/s), proving that these two ligands are complexed into the same magnesium center. These results indicate that at low temperature, the chemical equilibrium shifts to the formation of only one complex, probably the tetracoordinated one, Mg-tet. Moreover, at room temperature, the fast equilibrium between the two structures might be responsible for the two different diffusions. At intermediate temperatures (278–258 K, Figure S3), the phenanthroline equilibrium dynamics is quite well observed, for example, where their resonances split into two sets of signals, both with two different diffusion coefficients.
Figure 3.
1H-DOSY-NMR map obtained for the phenMgIso complex in MeOD at 298 K (left-hand side) and 253 K (right-hand side).
This pattern is consistent with equilibrium between the Mg-hex and Mg-tet complexes and can be associated with a rapid phen ligand exchange process. Moreover, the presence of a free phenanthroline signal at m/z 181.0769 reinforces the hypothesis of a dynamic phen ligand-exchange process. Indeed, the molecular structures of the complexes were also confirmed by DFT calculations using B3LYP/6-31+G*. Both structures are in their true energy minima because no imaginary frequency was detected (Tables S1 and S2). Also, a previous report demonstrated that some Cu(I) complexes containing phen ligands exist in equilibrium between the Cu(I) structures.37
The small size of the Mg(II) ion facilitates the steric repulsion between the phen ligands, thus a steric control between two phen units may occur and promote the interconversion between the two structures identified in the mass spectra.
2.2. Cyclic Voltammetry
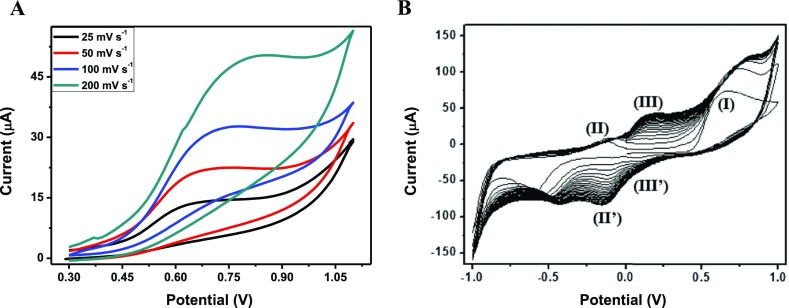
The cyclic voltammetry of isovanillic acid in aqueous solution, pH 7.4, in the first oxidative process showed only one single broad anodic wave at 0.6 V versus Ag/AgCl and no reverse cathodic response. As shown in Figure 4, dependence of the anodic current on the scan rate was indicative of a chemical reaction because of oxidation of the phenol group. These data confirm the findings of Simić for related polyphenols.38
Figure 4.
Cyclic voltammograms of isovanillic acid (A) and phenMgIso (B) in buffered solution at pH 7.4 with 0.1 M KCl in a screen-printed electrochemical array formed by three-electrochemical cell with glassy carbon as the working (d = 4 mm) and counter electrode and silver as the reference electrode. The reduction peak around −0.50 V is ascribed to blank solution, phosphate buffer, in the presence of KCl.
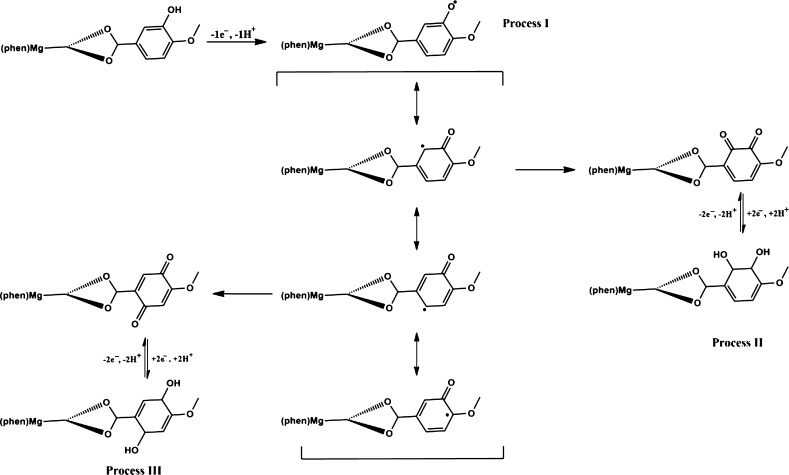
The coordination of Iso to the Mg(II) complex has a significant effect on the stability of phenoxyl radical generated. As illustrated in Figure 4, for phenMgIso, the first oxidative scan shows only one single anodic wave at +0.65 V. The second scan shows a new oxidation at −0.12 V (II) and −0.14 V (process II′), whereas the original wave shifted to a more positive potential with an increase in the current height. In subsequent scans, the peak currents for waves II′ and II decreased in intensity at the expense of two new responses at +0.17 V (III) and +0.10 V (III′), whereas the peak current for wave I kept increasing in current intensity and shifted to +0.8 V. After 10 scans, the species generated electrochemically are stabilized. As shown in Figure S4, the cyclic voltammetry process is highly dependent on solution pH; the reductive response III, III′ is facilitated at high pH, whereas the second couple is facilitated at low pH.
This type of behavior is characteristic of an electron-transfer process coupled to a proton-transfer process.38,39
In the phenMgIso complex, the role of Mg(II) is to stabilize the phenolate group of the Iso ligand through a strong electrostatic interaction that makes the Iso ligand easier to deprotonate and oxidize, generating a stable cyclic species under the electrochemical process. Based on the voltammetric data obtained and in the literature results,39 the entire electrochemical process is associated with the formation and stabilization of the phenoxyl radical, as shown in Scheme 1.
Scheme 1. Mechanism Proposed to the Electrochemical Process of phenMgIso.
Distribution of the Mulliken charges on phenMgIso is in agreement with the increased acidity of the Iso ligand with coordination to Mg(II) (Table S3). For the Mg–Iso bond, the negative charge on the oxygen of O–H(Iso) is decreased from −0.59 to −0.25 when the complex is formed so that the isovanillate ligand becomes less negatively charged (0.34 net charge) and easier to deprotonate, thus making the complex easier to oxidize.
2.3. In Vitro Antioxidant Activity
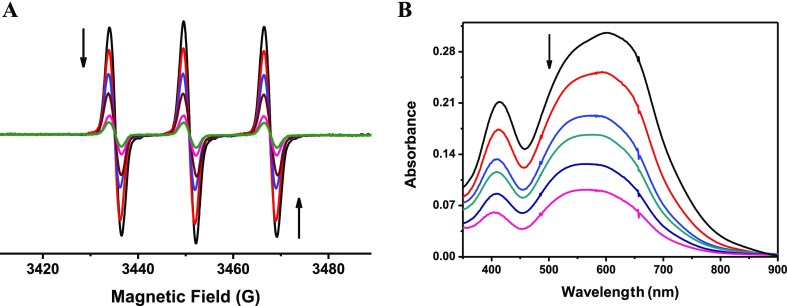
According to these results, it seems reasonable to consider the antioxidant activity of phenMgIso. As shown in Figure 5, in aqueous solution, the complex competes efficiently with 2,2,6,6-tetramethylpiperidine (TEMP) by 1O2. The electron paramagnetic resonance (EPR) assay shown in Figure 5 was based on the competition between TEMP and the sample in scavenging the 1O2 generated by the photoactivation of riboflavin.40 The IC50 value was 15 ± 0.3 μg mL–1 for phenMgIso. No inhibitory effect was found for free 1,10-phenanthroline.
Figure 5.
(A) EPR spectra of the Oxo-TEMPO adduct produced by the reaction of TEMP (100 mmol L–1) with 1O2 generated in situ by photosensitization of riboflavin (black line) and after addition of phenMgIso (control–—black line; 3.2 μg mL–1—red line; 6.4 μg mL–1—blue line; 12 μg mL–1—dark red line; 20 μg mL–1—pink line; 28 μg mL–1—green line). (B) Inhibition of the reduction of NBT (245 mmol L–1) as a function of phenMgIso concentration (control—black line; 1.6 μg mL–1—red line; 2.4 μg mL–1—blue line; 3.3 μg mL–1—green line; 8.4 μg mL–1—dark blue line; 12 μg mL–1—pink line).
The phenMgIso complex is also a good quencher for superoxide scavenging. In this experiment, the antioxidant activity of phenMgIso was assessed using the VitB2/Met/NBT assay.41 Accordingly, the results of nitrotetrazolium blue chloride (NBT) spectrophotometric analysis show that the IC50 value of the complex for superoxide scavenging is 3.7 μg L–1.
Based on the promising results from the antioxidant activity of phenMgIso, the toxicity profile in vivo using zebrafish and wistar male rat models was investigated.
2.4. In Vivo Toxicity against Zebra Fish and Wistar Male Rats
2.4.1. Zebrafish
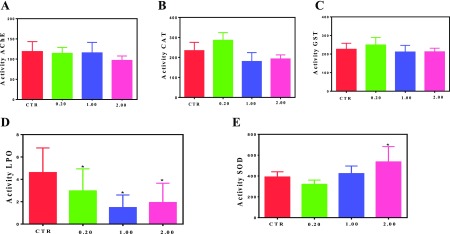
In the course of resistance test, regardless of exposure of zebrafish to complex concentrations up to 2.0 mg L–1, no mortality or visual alterations on the fishes were observed after 96 h of treatment. Figure 6 shows that exposure of zebrafish to phenMgIso does not affect neither the brain-AChE activity nor the digestive-tract-enzyme catalase (CAT) and glutathione-S-transferase (GST) in all concentrations investigated.
Figure 6.
Activity of (A) brain acetylcholinesterase (AChE), (B,C) digestive-tract CAT, and GST and levels of (D) lipid peroxidation and (E) SOD enzymes of zebrafish after 96 h of exposure to different concentrations of phenMgIso (0.20, 1.00, and 2.00 mg L–1)and control (CTR). Data are mean ± SE, n = 6–10. No significant difference in the mean value compared with the CTR (p < 0.05). Activity is expressed as μmol/min/mg protein.
2.4.2. Wistar Male Rats
No mortality or adverse signs of toxicity were observed in the laboratory-bred wistar male rats exposed to repeat treatment with phenMgIso at a concentration of 3.0 mg kg–1 per day for 21 days until a total dose of 62 mg kg–1 was reached.
Figure S5 shows that phenMgIso produces an increase in the spontaneous locomotor activity of rats and a reduction of climbing. Accordingly, there was a slight increase in the percentage of time spent in the open arm compartment as compared with control. The treatment used in this experiment is in accordance with the standard anxiolytic drugs delivered for mice when they were treated with diazepam (2 mg/kg), imipramine (20 mg/kg), and fluoxetine (20 mg/kg).42
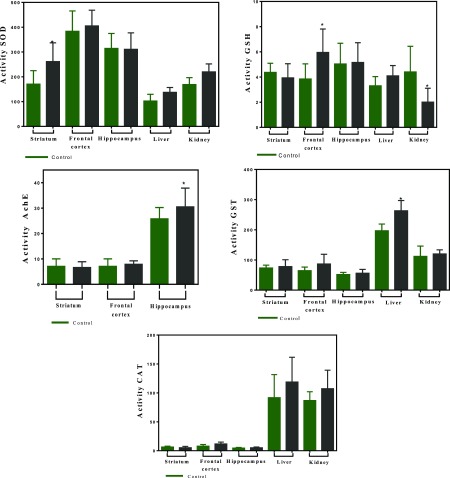
In agreement with these results, the levels of brain enzymes SOD in the striatum, glutathione reductase (GSH) in the frontal cortex, and acetylcholinesterase (AChE) in the hippocampus increased after phenMgIso treatment (Figure 7), indicating that the complex crosses the blood–brain barrier. Moreover, the biochemical analysis of kidney and liver showed increased activity of CAT and SOD enzymes (Figure 7), evincing that the complex does not induce toxic manifestations in these organs. The decrease in the activity of GSH in the kidney after treatment with the complex probably reflects the increased antioxidant activity of CAT and SOD because of phenMgIso presence.
Figure 7.
Activity of acetylcholinesterase (AChE), SOD, CAT, GST, and GSH in the regions of brain (hippocampus, striatum, and frontal cortex) as well as in the liver and kidney of wistar male rats after 21 days of exposure to 3.0 mg kg–1 per day of phenMgIso and CTR. Data are mean ± SE, n = 6–10. No significant difference in mean value compared with the CTR (p < 0.05). Activity is expressed as μmol/min/mg protein. Control results are shown in the dotted areas.
In light of these results, it is probable that the antioxidant activity of phenMgIso to aquatic zebrafish and terrestrial wistar male rats involves superoxide radical scavenging.
3. Conclusions
Two phenMgIso complexes coexist in methanolic solution, [Mg(phen)2(Iso)] and [Mg(phen)Iso], although the complexes seem to have preference for a hexacoordinated complex at room temperature over a tetracoordinated complex at low temperature. Electrochemical oxidation indicates a cyclic mechanism that stabilizes the phenoxyl radical and corroborates the high in vitro and probable in vivo antioxidant activity of phenMgIso. No mortality or evident toxic effect to zebrafish and wistar male rats exposed to phenMgIso was observed. Altogether, these results indicate the potential of phenMgIso as a novel antioxidant candidate.
4. Experimental Section
4.1. Materials and Methods
All NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer for hydrogen nucleus. Data acquisition and processing were performed with Bruker software TopSpin 3.5 pl 7. For NMR experiments, the samples were dissolved in tetradeuterated methanol (methanol-d4) and were analyzed at different temperatures from 298 lowering to 253 K. All chemical shifts (δ) are given in parts per million unit, with reference to the hydrogen signal of the methyl group of tetramethylsilane as the internal reference, and the coupling constants (J) are described in hertz.
Magnesium acetate tetrahydrate, 1,10′-phenanthroline (phen), isovanillic acid (iso), phosphoric acid, monobasic potassium phosphate, dibasic potassium phosphate, and tribasic potassium phosphate were purchased from Aldrich. The following reagents, purchased from Aldrich, were used to assess the antioxidant capacity of the complex: riboflavin (vitamin B2), TEMP, NBT, and methionine. Organic solvents were of high-performance liquid chromatography grade, and aqueous solutions were prepared with Milli-Q water.
4.2. Synthesis of Mg(II) Complexes
Solid Mg(CH3COO)24.H2O (0.86 g; 4.00 mmol), 1,10′-phenanthroline, (0,76 g; 4.20 mmol), and isovanillic acid (0.70 g; 4.20 mmol) were dissolved in solution containing methanol (20.0 mL) and triethylamine (580 μL, 4.20 mmol). The solution was stirred under the N2 atmosphere at 110 °C for 2 h. The reaction was filtered, the excess of solvent was removed by rotary evaporation, and 10.0 mL of cold water was added to the remaining solution. The gray precipitate was filtered, washed with water, and dried under vacuum.
The experimental procedures for the impact of the complex on terrestrial and aquatic life are described in the Supporting Information.
The effects of treatment with the complex on zebrafish and wistar rats regarding toxicity and on the behavior of wistar rats investigated through open field, forced swim, and elevated plus maze tests are described in the Supporting Information.
Also, all the description of all the other experiments described in the Supporting Information are listed as follows: diffusion ordered spectroscopy on nuclear magnetic resonance, DOSY-NMR, MS, DFT calculations, cyclic voltammetry, superoxide radical scavenging assay, and oxygen singlet assay.
Acknowledgments
The authors would like to acknowledge FAPESP (proc. nos. 2017/00839-1, 2013/05536-6, 2015/23146-6, 2018/09145-5, 2018/16040-5, and 2017/15455-4), CNPq (455630/2014-3, 166303/2017-8, and 306005/2017-4), and CAPES (Finance Code 001) for the grants and fellowships received.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03804.
Experimental section; references; structures observed in solution and the binuclear complex corresponding to Mg-bi (top) and the structures of the hexacoordinated (Mg-hex) and tetracoordinated (Mg-tet) complexes (bottom); ESI(+)-MS/MS of the m/z 551.15 (top) and of m/z 909.21 (bottom) in methanol; 1H-DOSY-NMR map obtained for the Mg complex at 288 K (top left-hand side), 278 K (top right-hand side), 268 K (bottom left-hand side), and 258 K (bottom right-hand side); optimized structure of tetracoordinated [Mg(phen)(iso)]+ in the gas-phase B3LYP/6-31+G* basis set; optimized structure of hexacoordinated [Mg(phen)2(iso)]+ in the gas-phase B3LYP/6-31+G* basis set; cyclic voltammograms of 5 mg of complex in pure water, buffered solution at pH 4.5 and pH 10.0, with 0.1 M KCl and v = 100 mV s–1; distribution of Mulliken charges over the atoms present at the complexes; gas-phase B3LYP/6-31+G* determined xyz coordinates (in Å) for the complex [Mg(phen)(iso)]+ in the GS; and locomotion and anxiety test in male wistar rats after 21 days after exposure of 3.0 mg kg–1/day of the complex and control (green bar) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Potì F.; Santi D.; Spaggiari G.; Zimetti F.; Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 351. 10.3390/ijms20020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider R. C.; Liu Z. D.; Khodr H. H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. 10.1016/s0076-6879(01)35243-6. [DOI] [PubMed] [Google Scholar]
- Scalbert A.; Mila I.; Expert D.; Marmolle F.; Albrecht A. M.; Hurrell R.; Huneau J. F.; Tomé D.. Polyphenols, metal ion complexation and biological consequences. Plant Polyphenols; Springer: Boston, MA, 1999; Vol. 2, pp 545–554. [DOI] [PubMed] [Google Scholar]
- Perron N. R.; Brumaghim J. L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- Lee S. R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longevity 2018, 2018, 1. 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray T. M.; Bettger W. J. The physiological role of zinc as an antioxidant. Free Radicals Biol. Med. 1990, 8, 281–291. 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- McCall K. A.; Huang C.-c.; Fierke C. A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. 10.1093/jn/130.5.1437s. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Qian L.-L.; Yang J.; Han R.-M.; Zhang J.-P.; Skibsted L. H. Kaempferol Binding to Zinc (II), Efficient Radical Scavenging through Increased Phenol Acidity. J. Phys. Chem. B 2018, 122, 10108–10117. 10.1021/acs.jpcb.8b08284. [DOI] [PubMed] [Google Scholar]
- Shahi A.; Aslani S.; Ataollahi M.; Mahmoudi M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649. 10.1007/s10787-019-00603-7. [DOI] [PubMed] [Google Scholar]
- Osawa T.; Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. N.Y. Acad. Sci. 2005, 1043, 440–451. 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- Wegner M.; Araszkiewicz A.; Zozulińska-Ziółkiewicz D.; Wierusz-Wysocka B.; Pioruńska-Mikołajczak A.; Pioruńska-Stolzmann M. The relationship between concentrations of magnesium and oxidized low density lipoprotein and the activity of platelet activating factor acetylhydrolase in the serum of patients with type 1 diabetes. Magnesium Res. 2010, 23, 97–104. 10.1684/mrh.2010.0207. [DOI] [PubMed] [Google Scholar]
- Cernak I.; Savic V.; Kotur J.; Prokic V.; Kuljic B.; Grbovic D.; Veljovic M. Alterations in magnesium and oxidative status during chronic emotional stress. Magnesium Res. 2000, 13, 29–36. [PubMed] [Google Scholar]
- Bae Y.-J.; Choi M.-K. Magnesium intake and its relevance with antioxidant capacity in Korean adults. Biol. Trace Elem. Res. 2011, 143, 213–225. 10.1007/s12011-010-8883-y. [DOI] [PubMed] [Google Scholar]
- Hans C. P.; Chaudhary D. P.; Bansal D. D. Magnesium deficiency increases oxidative stress in rats. Indian J. Exp. Biol. 2002, 40, 1275–1279. [PubMed] [Google Scholar]
- Blache D.; Devaux S.; Joubert O.; Loreau N.; Schneider M.; Durand P.; Prost M.; Gaume V.; Adrian M.; Laurant P.; Berthelot A. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radicals Biol. Med. 2006, 41, 277–284. 10.1016/j.freeradbiomed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Wolf F. I.; Trapani V.; Simonacci M.; Ferré S.; Maier J. A. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved?. Magnesium Res. 2008, 21, 58–64. 10.1684/mrh.2008.0129. [DOI] [PubMed] [Google Scholar]
- Dickens B. F.; Weglicki W. B.; Li Y.-S.; Mak I. T. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992, 311, 187–191. 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- Zheltova A. A.; Kharitonova M. V.; Iezhitsa I. N.; Spaso A. A. Magnesium deficiency and oxidative stress: an update. Biomedicine 2016, 6, 8–14. 10.7603/s40681-016-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudev T.; Cowan J. A.; Lim C. Competitive Binding in Magnesium Coordination Chemistry: Water versus Ligands of Biological Interest. J. Am. Chem. Soc. 1999, 121, 7665–7673. 10.1021/ja984470t. [DOI] [Google Scholar]
- Bock C. W.; Katz A. K.; Markham G. D.; Glusker J. P. Manganese as a replacement for magnesium and zinc: functional comparison of the divalent ions. J. Am. Chem. Soc. 1999, 121, 7360–7372. 10.1021/ja9906960. [DOI] [Google Scholar]
- Dudev T.; Cowan J. A.; Lim C. Competitive binding in magnesium coordination chemistry: water versus ligands of biological interest. J. Am. Chem. Soc. 1999, 121, 7665–7673. 10.1021/ja984470t. [DOI] [Google Scholar]
- Jernigan R.; Raghunathan G.; Bahar I. Characterization of interactions and metal ion binding sites in proteins. Curr. Opin. Struct. Biol. 1994, 4, 256–263. 10.1016/s0959-440x(94)90317-4. [DOI] [Google Scholar]
- Dudev T.; Lim C. Monodentate versus Bidentate Carboxylate Binding in Magnesium and Calcium Proteins: What Are the Basic Principles?. J. Phys. Chem. B 2004, 108, 4546–4557. 10.1021/jp0310347. [DOI] [Google Scholar]
- Cowan J. A. Metallobiochemistry of magnesium. Coordination complexes with biological substrates: site specificity, kinetics and thermodynamics of binding, and implications for activity. Inorg. Chem. 1991, 30, 2740–2747. 10.1021/ic00013a008. [DOI] [Google Scholar]
- Okamura T.-a.; Furuya R.; Onitsuka K. Synthesis and structures of soluble magnesium and zinc carboxylates containing intramolecular NH···O hydrogen bonds in nonpolar solvents. Dalton Trans. 2015, 44, 7512–7523. 10.1039/c5dt00053j. [DOI] [PubMed] [Google Scholar]
- Okamura T.-a.; Furuya R.; Onitsuka K. Regulation of the Hydrolytic Activity of Mg2+-Dependent Phosphatase Models by Intramolecular NH···O Hydrogen Bonds. J. Am. Chem. Soc. 2014, 136, 14639–14641. 10.1021/ja509006x. [DOI] [PubMed] [Google Scholar]
- Kavitha S.; Deepa P.; Karthika M.; Kanakaraju R. Hybrid DFT study on non-covalent interactions and their influence on pKa’s of magnesium-carboxylate complexes. J. Mol. Graphics Modell. 2018, 85, 13–24. 10.1016/j.jmgm.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Carta V.; Mehr S. H. M.; MacLachlan M. J. Controlling Ligand Exchange through Macrocyclization. Inorg. Chem. 2018, 57, 3243–3253. 10.1021/acs.inorgchem.8b00031. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/s2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.-x.; Liu G.; Li H.; Wang J.-d.; Yue F. Synthesis, Crystal Structure and Luminescence Property of a Chiral Magnesium (II) Mixed-ligand Complex [Mg(H2O) (phen)(HBDC)2] (phen). J. Chem. Crystallogr. 2011, 41, 143–146. 10.1007/s10870-010-9853-0. [DOI] [Google Scholar]
- Ghosh N.; Chakraborty T.; Mallick S.; Mana S.; Singha D.; Ghosh B.; Roy S. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta, Part A 2015, 151, 807–813. 10.1016/j.saa.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Oliveira R. M. M.; de Souza Daniel J. F.; de Aguiar I.; das Graças Fernandes Silva M. F.; Batista Fernandes J.; Carlos R. M. Structural effects on the hesperidin properties obtained by chelation to magnesium complexes. J. Inorg. Biochem. 2013, 129, 35. 10.1016/j.jinorgbio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Grabowski S. J. Magnesium Bonds: From Divalent Mg Centres to Trigonal and Tetrahedral Coordination. ChemistrySelect 2018, 3, 3147–3154. 10.1002/slct.201703137. [DOI] [Google Scholar]
- Bruyere J.-C.; Gourlaouen C.; Karmazin L.; Bailly C.; Boudon C.; Ruhlmann L.; de Frémont P.; Dagorne S. Synthesis and Characterization of Neutral and Cationic Magnesium Complexes Supported by NHC Ligands. Organomet 2019, 38, 2748–2757. 10.1021/acs.organomet.9b00304. [DOI] [Google Scholar]
- Drevenšek P.; Košmrlj J.; Giester G.; Skauge T.; Sletten E.; Sepčić K.; Turel I. X-Ray crystallographic, NMR and antimicrobial activity studies of magnesium complexes of fluoroquinolones – racemic ofloxacin and its S-form, levofloxacin. J. Inorg. Biochem. 2006, 100, 1755–1763. 10.1016/j.jinorgbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Mitrofanov A.; Manowong M.; Rousselin Y.; Brandès S.; Guilard R.; Bessmertnykh-Lemeune A.; Chen P.; Kadish K. M.; Goulioukina N.; Beletskaya I. Structural and Electrochemical Studies of Copper (I) Complexes with Diethoxyphosphoryl -1, 10-phenanthrolines. Eur. J. Inorg. Chem. 2014, 2014, 3370–3386. 10.1002/ejic.201402161. [DOI] [Google Scholar]
- Simić A.; Manojlović D.; Šegan D.; Todorović M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007, 12, 2327–2340. 10.3390/12102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil E. S.; Couto R. O. Flavonoid electrochemistry: a review on the electroanalytical applications. Rev. Bras. Farmacogn. 2013, 23, 542–558. 10.1590/s0102-695x2013005000031. [DOI] [Google Scholar]
- Leonhardt M.; Gebert S.; Wenk C. Stability of ?-Tocopherol, Thiamin, Riboflavin and Retinol in Pork Muscle and Liver during Heating as Affected by Dietary Supplementation. J. Food Sci. 1996, 61, 1048–1052. 10.1111/j.1365-2621.1996.tb10930.x. [DOI] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem. J. 1979, 182, 625–628. 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari S.; Minda M.; Tonpay S. D. Anxiolytic and antidepressant activities of methanol extract of Aegle marmelos leaves in mice. Indian J. Physiol. Pharmacol. 2010, 54, 318–328. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.