Abstract
Quinoid-based ligands constitute the most common class of redox-active ligands used to construct electrically conductive and magnetic metal–organic frameworks (MOFs). Whereas this chemistry is intensively explored for transition-metal and lanthanide ions, any related actinide compound has not received attention. In particular, the MOF chemistry of actinide ions in the lower oxidation states is underexplored. We herein report the synthesis, and structural and physical property characterization of a uranium(IV) quinoid-based MOF, [U(Cl2dhbq)2(H2O)2]·4H2O (1, Cl2dhbq2– = deprotonated 2,5-dichloro-3,6-dihydroxybenzoquinone). 1 is a rare example of a U(IV)-based coordination solid and the first material to incorporate bona fide reducible bridging ligands. Despite the anticipated thermodynamic driving force, no indications of valence tautomerism are evident from magnetometry, near-IR spectroscopy, and X-band electron paramagnetic resonance measurements. These initial results suggest that reduction potentials alone are insufficient as guidelines for the prediction of the occurrence of electron transfer in uranium–quinoid-based materials.
Introduction
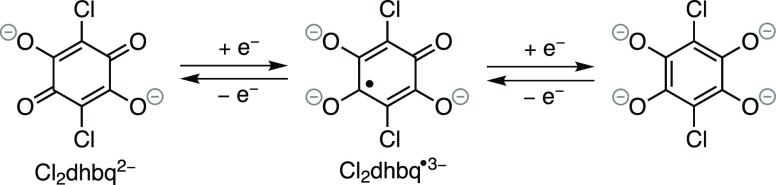
Metal–organic frameworks (MOFs), and coordination solids in general, receive ever-increasing attention and numerous examples of transition-metal-based MOFs have been reported to exhibit interesting properties relevant for gas separation and storage, and catalysis.1−3 Recent studies have highlighted the possibilities to design MOFs with high electrical conductivity applicable in supercapacitors and chemical sensors, owing to the significant orbital overlap between the metal ions and the bridging ligands.4−6 On the contrary, the f-element-based MOFs are not as widely explored,7 and actinide-based MOFs are relatively rare.8−11 Whereas the majority of the existing uranium MOFs encompass UO22+ units,8,12 the reducing nature of the lower oxidation states U(IV) and U(III) make these metal ion units interesting components for MOFs comprising redox-active ligand scaffolds. Notably, the few, previously reported uranium(IV) MOFs are all based on redox-inactive carboxylate ligands.13−17 The introduction of U(IV)–L ↔ U(V)–L• valence tautomerism in MOFs would parallel the current methodology employed in transition-metal chemistry to reveal strong magnetic interaction and electronic conductivity through ligand-based mixed-valency.18 Despite the growing interest in uranium coordination chemistry of redox-active ligands,19,20 no noninnocent systems have been reported to extend beyond the dimensionality of an isolated molecule. In transition-metal-based coordination solids, an often-used redox-active ligand is 2,5-dichloro-3,6-dihydroxybenzoquinone (“chloranilic acid”; Cl2dhbqH2), which may be reduced by up to two electrons, owing to the energetically low-lying π* orbitals (Figure 1).21,22
Figure 1.
Schematic overview of the accessible redox states of Cl2dhbqn–.
Several examples of iron-based dihydroxybenzoquinone coordination solids have been shown to display strong magnetic interactions and high electrical conductivity attributed to mixed-valency in the dihydroxybenzoquinone ligand scaffold.23−27 Whereas many lanthanide systems incorporating closed-shell dihydroxybenzoquinone ligands have been reported,28 the only few examples of actinide ion systems incorporate nonoxidizable Th(IV) and U(VI).28−31 In aqueous solution, U(IV) is moderately reducing and comparable in reduction potential to the Fe(III)/Fe(II) redox-couple.32 It could thus be hypothesized that U(IV) should be capable of reducing Cl2dhbq2– to the monoradical Cl2dhbq3–, thereby creating a pathway for strong 2p–5f magnetic superexchange interactions and facilitating hopping conduction between Cl2dhbq2–/•3– units. Following this hypothesis we, herein, report on a unique example of a uranium(IV) coordination solid constructed using quinoid-based bridging struts.
Results and Discussion
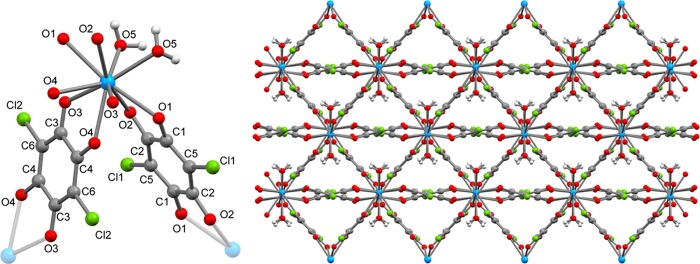
The slow diffusion of an aqueous solution of Cl2dhbqH2 into an aqueous suspension of uranium(IV) sulfate tetrahydrate yielded a black, crystalline material after several weeks. Single-crystal X-ray diffraction analysis revealed the solid to be a three-dimensional polymeric structure, isostructural to the Th(IV) analogue reported by Robson and co-workers,28 with the stoichiometry of [U(Cl2dhbq)2(H2O)2]·4H2O (1). X-ray powder diffractometry (see Figure S1) and elemental analysis confirm the homogeneity of the material and the absence of any traces of unreacted starting materials. The local coordination environment around the crystallographically unique uranium center is shown in Figure 2 (left) and selected structural metrics are provided in the figure caption. The structure comprises a ten-coordinate uranium center bridged to adjacent uranium centers through four Cl2dhbqn– ligands, which coordinate to each uranium center in a bidentate fashion (Figure 2). The coordination sphere is completed by two water molecules which are hydrogen-bonded to cocrystallized water molecules (Figure S2). The C2/c crystallographic space group generates triangular pores perpendicular to the [1, 0, −1] crystallographic plane as depicted in Figure 2 (right). Heating of polycrystalline 1 initially leads to loss of six water molecules as determined by thermogravimetric analysis (Figure S3) and a concurrent loss of crystallinity. Likewise, N2-sorption measurements of polycrystalline 1 activated in vacuo at 80 °C reveal no permanent porosity. The structure features two crystallographically distinct Cl2dhbqn– ligands, as shown in Figure 2 (left). The C1–C2 (1.552(2) Å) and C3–C4 (1.531(4) Å) bond lengths are in the normal range for Cl2dhbq2– and significantly longer than the values previously found for Cl2dhbq•3– (e.g,. 1.456(3) Å in [(TPyA)CoIII(Cl2dhbq•3–)CoIII(TPyA)]3+; TPyA = tris(2-pyridylmethyl)-amine).33 Similarly, the C–O bonds are all in the range of 1.258(3) to 1.267(4) Å, which are far from the 1.312(2) Å found in [(TPyA)CoIII(Cl2dhbq•3–)CoIII(TPyA)]3+. Further comparison to the bond lengths of the Th(IV) analogue reveals only little differences with an average Th–O bond length of 2.50 Å versus 2.47 Å in 1, and an average O–C bond length in the compound of 1.26 Å versus 1.26 Å in 1.28 Analysis of a crystal at T = 285 K reveals only a small thermal expansion of the unit cell (coefficient of thermal expansion = 1.6 × 10–4 K–1, Table S1) as compared to the 120 K data set. In conclusion, the structural analysis of 1 suggests the absence of any valence tautomerism and a formulation of 1 based on U(IV) and closed-shell Cl2dhbq2–. Mixed-valency systems commonly exhibit intense intervalence charge transfer (IVCT) transitions in the near-infrared energy regime. For Cl2dhbqn–-based coordination solids, these transitions are attributed to the interconversion of Cl2dhbq2– and Cl2dhbq•3–.23 The near-infrared absorption spectra of 1 reveal the absence of any IVCT bands between 4000 and 10 000 cm–1 (see Figure S4), suggesting an absence of any organic mixed-valency in 1. Likewise, the pressed pellet electrical resistance of 1 exceeds several megaohm at room temperature, reflecting its highly insulating nature. To corroborate the oxidation state assignment, the magnetic moment of 1 was measured as a function of temperature and magnetic field (Figures 3 and S5). The room temperature value of the magnetic susceptibility-temperature product (χT) amounts to 0.62 cm3 K mol–1. This value is significantly lower than the one calculated for the Russell-Saunders 3H4 (5f2, gJ = 4/5) term of 1.60 cm3 K mol–1. The magnetic moment of uranium-based complexes has been shown to be diagnostic of the oxidation state.34 However, the room temperature magnetic moments are broadly distributed around 0.96 cm3 K mol–1 and show overlap with the corresponding values for both U(III) and U(V). Upon descending temperature, the χT product gradually decreases to withdraw (∼6 × 10–3 cm3 K mol–1) at 2 K. The vanishing χT product suggests the presence of a nonmagnetic ground state, which is commonly encountered in U(IV), and is thus not expected to arise from antiferromagnetic U(IV)–U(IV) interactions. Recently, Arnold and co-workers reported the first examples of dinuclear U(IV) complexes bridged by quinoid ligands.35 Their experimental and theoretical investigations revealed a complete irrelevance of superexchange interactions across closed-shell quinoids bridging two U(IV) centers. The low-temperature magnetic field dependence of the magnetization of 1 is shown in Figure 3 (right panel). The approximately linear behavior of M versus μ0H is reminiscent of a nonmagnetic ground state with a significant contribution from temperature-independent paramagnetism. This conclusion is supported by X-band electron paramagnetic resonance spectroscopy where no resonances could be observed between room temperature and liquid N2-temperature, suggesting a complete absence of any Cl2dhbq•3– radical species in 1 (Figure S6).
Figure 2.
Left: Local coordination environment of the uranium center in 1 (T = 120 K). Cocrystallized water molecules are omitted for clarity. Selected bond lengths (Å): U–O1 2.502(2), U–O2 2.477(2), U–O3 2.401(2), U–O4 2.476(2), C1–O1 1.258(3), C2–O2 1.267(4), C1–C2 1.522(4), C3–O3 1.265(3), C4–O4 1.258(3), C3–C4 1.531(4). Right: View of the extended coordination polymer structure shown along the triangular pores corresponding to viewed perpendicular to the crystallographic [1, 0, −1] plane. Pore-filling cocrystallized water molecules have been omitted for clarity. Color code: U, blue; Cl, green; O, red; C, gray; H, white.
Figure 3.
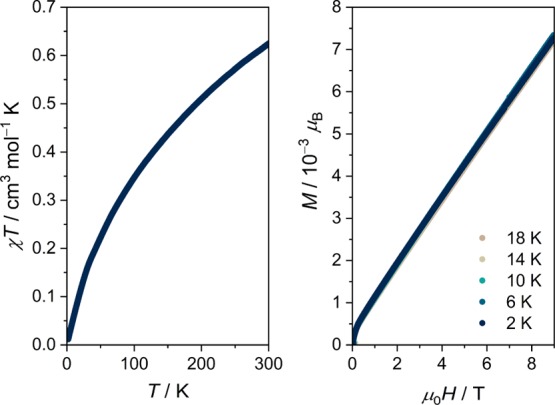
Left: Temperature dependence of the χT (χ ≡ M/μ0H) product of a polycrystalline specimen of 1 obtained in a magnetic field of μ0H = 1 T. Right: Magnetic field dependence of the magnetization at selected low temperatures.
Conclusions
We have reported the synthesis and structural characterization of a rare example of a uranium-based MOF not built on uranyl(VI)-nodes. Analysis of the structural, spectroscopic, and magnetic data suggest the presence of uranium in oxidation state +IV, despite the company of reducible Cl2dhbq2– bridging ligands. It is tempting to speculate that, irrespective of the favorable redox potentials of U(IV), the absence of strong uranium–ligand covalency may impede the metal–ligand electron-transfer mechanism found in a plethora of 3d transition metal ion analogues. These initial results may thus encourage further interest in the synthesis and characterization of actinide coordination solids based on redox-active ligands, with the ultimate goal of authenticating strong magnetic superexchange interactions and electronic conductivity.
Experimental Section
Materials and Instrumentation
Warning: Depleted uranium (primarily 238U, t1/2 = 4.5 × 109 y) is a weak α-emitter and its chemical compounds are toxic. All manipulations were done in ventilated fume hoods at ambient conditions. U(SO4)2·xH2O was synthesized following the procedure reported by Peres et al.36 The x = 4 formulation was determined by powder X-ray diffraction analysis (see Figure S7). 2,5-Dichloro-3,5-dihydroxybenzoquinone was purchased from Sigma-Aldrich and used as received. Elemental analyses were performed on a Thermo Scientific FlashSmart CHNS/O Elemental Analyzer. The magnetization and electrical conductivity data were obtained on a QuantumDesign DynaCool Physical Property Measurement System equipped with a vibrating sample magnetometer and a 9 T dc magnet. The sample was immobilized in a standard QuantumDesign powder capsule attached to a brass sample holder. The magnetization was measured as a function of temperature (1.8–300 K) and field (0–9 T) and the data were corrected for diamagnetic contributions from the sample holder and the sample. The MIR and NIR attenuated-total-reflectance (ATR) spectra were collected by a Bruker VERTEX80v Fourier transform vacuum spectrometer employing a single-reflection diamond ATR accessory. The apparatus was configured with KBr (MIR)/CaF2 (NIR) beam splitters, liquid-nitrogen-cooled HgCdTe (MIR)/InSb (NIR) detectors, and globar (MIR)/tungsten (NIR) radiation sources. Extended ATR corrections were applied to account for the wavelength-dependent penetration depth of the probe beams. The complementary MIR Fourier transform Raman spectrum was collected by a Bruker RAM II module employing a 1000 mW near-infrared Nd:YAG excitation laser (1064 nm) and a liquid-nitrogen-cooled Ge detector. A Mettler-Toledo thermogravimetric analyzer equipped with a built-in calibration weight was used to obtain data in the temperature range 25–465 °C with a ramping speed of 0.5 °C min–1.
Synthesis
An aqueous solution of 2,5-dichloro-3,6-dihydroxybenzoquinone (16 mg, 0.076 mmol) was carefully layered on top of an aqueous suspension (6.5 mL) of uranium(IV) sulfate tetrahydrate (32 mg, 0.064 mmol) in a borosilicate vial (⌀ 20 mm). The vial was sealed and left undisturbed for 2 weeks at room temperature. The highly crystalline, black material was isolated by filtration. Yield: ∼50%. Anal. Calcd (found) for C12H12Cl4O14U: C, 19.0% (18.9%); H, 1.6% (1.81%). Characteristic Raman shifts (cm–1): 292, 389, 738, 1217, 1300, 1382 (cf. Figure S8). Characteristic IR frequencies (cm–1): 444, 571, 609, 855, 1002, 1250, 1356, 1471 (cf. Figure S8).
X-ray Diffraction
Single crystals of 1 were selected and mounted on nylon loops on a SuperNova dual source CCD-diffractometer. The crystal was kept at 120 K during data collection. Using Olex2,37 the structure was solved by direct methods using the SIR200438 structure solution program and refined by Least Squares using version 2014/7 of ShelXL (Table 1).39 All nonhydrogen atoms were refined anisotropically. Hydrogen atom positions were calculated geometrically and refined using the riding model. The X-ray powder patterns were measured on a Huber G670 powder diffractometer using Cu Kα1 (λ = 1.5406 Å, quartz monochromator) radiation .
Table 1. Crystallographic and Refinement Parameters of 1 (120 K).
| CCDC number | 1897474 |
| empirical formula | C12H12Cl4O14U |
| formula weight | 760.05 |
| crystal system | monoclinic |
| space group | C2/c |
| a/Å | 13.7293(4) |
| b/Å | 11.3630(4) |
| c/Å | 12.5175(4) |
| α/deg | 90 |
| β/deg | 95.847(3) |
| γ/deg | 90 |
| V/Å3 | 1942.6(1) |
| Z | 4 |
| ρcalc/g cm–3 | 2.599 |
| μ/mm–1 | 8.980 |
| F(000) | 1424.0 |
| radiation | Mo Kα (λ = 0.71073 Å) |
| 2θ range for data collection/deg | 5.52–58.988 |
| index ranges | –13 ≤ h ≤ 18 |
| –14 ≤ k ≤ 13 | |
| –17 ≤ l ≤ 17 | |
| –14 ≤ k ≤ 13 | |
| –17 ≤ l ≤ 17 | |
| reflections collected | 9176 |
| independent reflections | 2437 [Rint = 0.0282, Rsigma = 0.0249] |
| data/restrains/parameters | 2437/0/148 |
| goodness of fit on F2 | 1.042 |
| final R indexes [I ≥ 2σ(I)] | R1 = 0.0183, wR2 = 0.0435 |
| final R indexes [all data] | R1 = 0.0201, wR2 = 0.0442 |
| largest diff. peak/hole/e Å–3 | 1.53/–0.66 |
Acknowledgments
K.S.P. thanks the VILLUM Foundation for a VILLUM Young Investigator grant (15374), and the Carlsberg Foundation (CF-17-0637), the Brdr. Hartmanns foundation, and the Torkil Holm foundation for research infrastructure grants. Dr. J. J. Mielby, L. Voigt, and B. F. Holten are thanked for experimental assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03727.
The authors declare no competing financial interest.
Supplementary Material
References
- Li J.-R.; Sculley J.; Zhou H.-C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Liu X.-Q.; Jiang H.-L.; Sun L.-B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. 10.1021/acs.chemrev.7b00091. [DOI] [PubMed] [Google Scholar]
- Sun L.; Campbell M. G.; Dincă M. Electrically Conductive Porous Metal-Organic Frameworks. Angew. Chem., Int. Ed. 2016, 55, 3566–3579. 10.1002/anie.201506219. [DOI] [PubMed] [Google Scholar]
- Dou J.-H.; Sun L.; Ge Y.; Li W.; Hendon C. H.; Li J.; Gul S.; Yano J.; Stach E. A.; Dincă M. Signature of Metallic Behaviour in Metal-Organic Frameworks M3(hexaiminobenzene)2 (M = Ni, Cu). J. Am. Chem. Soc. 2017, 139, 13608–13611. 10.1021/jacs.7b07234. [DOI] [PubMed] [Google Scholar]
- Sheberla D.; Bachman J. C.; Elias J. S.; Sun C.-J.; Shao-Horn Y.; Dincă M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. 10.1038/nmat4766. [DOI] [PubMed] [Google Scholar]
- Aubrey M. L.; Kapelewski M. T.; Melville J. F.; Oktawiec J.; Presti D.; Gagliardi L.; Long J. R. Chemiresistive Detection of Gaseous Hydrocarbons and Interrogation of Charge Transport in Cu[Ni(2,3-pyrazinedithiolate)2] by Gas Adsorption. J. Am. Chem. Soc. 2019, 141, 5005–5013. 10.1021/jacs.9b00654. [DOI] [PubMed] [Google Scholar]
- Bosch M.; Chen B.; Chen J.; Chen L.; Cheng P.; Cimpoesu F.; Ferbinteanu M.; Fordham S.; Hong M.; Jiang F.; Li B.; Liu K.; Liu W.; Shi W.; Song S.-Y.; Song X.-Z.; Su J.; Tanase S.; Tang X.; Wang X.; Wu M.; Zhang H.-J.; Zhang Z.; Zheng Z.; Zhou H.-C.; Zhou K.. Lanthanide Metal–Organic Frameworks; Springer International Publishing Switzerland, 2015. [Google Scholar]
- Su J.; Chen J.. Lanthanide Metal–Organic Frameworks; Cheng P., Ed.; Springer International Publishing Switzerland, 2015; MOFs of Uranium and the Actinides, pp 265–296. [Google Scholar]
- Volkringer C.; Mihalcea I.; Vigier J.-F.; Beaurain A.; Visseaux M.; Loiseau T. Metal-Organic-Framework-Type 1D-Channel Open Network of a Tetravalent Uranium Trimesate. Inorg. Chem. 2011, 50, 11865–11867. 10.1021/ic2021963. [DOI] [PubMed] [Google Scholar]
- Falaise C.; Volkringer C.; Loiseau T. Mixed Formate-Dicarboxylate Coordination Polymers with Tetravalent Uranium: Occurrence of Tetranuclear {U4O4} and Hexanuclear {U6O4(OH)4} Motifs. Cryst. Growth Des. 2013, 13, 3225–3231. 10.1021/cg400643g. [DOI] [Google Scholar]
- Martin N. P.; März J.; Volkringer C.; Henry N.; Hennig C.; Ikeda-Ohno A.; Loiseau T. Synthesis of Coordination Polymers of Tetravalent Actinides (Uranium and Neptunium) with Phthalate or Mellitate Ligand in Aqueous Medium. Inorg. Chem. 2017, 56, 2902–2913. 10.1021/acs.inorgchem.6b02962. [DOI] [PubMed] [Google Scholar]
- Li P.; Vermeulen N. A.; Malliakas C. D.; Gómez-Gualdrón D. A.; Howarth A. J.; Mehdi B. L.; Dohnalkova A.; Browning N. D.; O’Keeffe M.; Farha O. K. Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science 2017, 356, 624–627. 10.1126/science.aam7851. [DOI] [PubMed] [Google Scholar]
- Imaz I.; Bravic G.; Sutter J.-P. Structural and zeolitic features of a series of heterometallic supramolecular porous architectures based on tetrahedral {M(C2O4)4}4– primary building units. Dalton Trans. 2005, 2681–2687. 10.1039/b503964a. [DOI] [PubMed] [Google Scholar]
- Imaz I.; Bravic G.; Sutter J.-P. Structural and zeolitic features of a 3D heterometallic porous architecture constructed from a {M(oxalate)4}4– building unit. Chem. Commun. 2005, 993–995. 10.1039/b414190c. [DOI] [PubMed] [Google Scholar]
- Yeon J.; Smith M. D.; Sefat A. S.; zur Loye H.-C. Crystal Growth, Structural Characterization, and Magnetic Properties of New Uranium(IV) Containing Mixed Metal Oxalates: Na2U2M(C2O4)6(H2O)4 (M = Mn2+, Fe2+, Co2+, and Zn2+). Inorg. Chem. 2013, 52, 2199–2207. 10.1021/ic3026733. [DOI] [PubMed] [Google Scholar]
- Wang C.-M.; Liao C.-H.; Chen P.-L.; Lii K.-H. UF3(H2O)(C2O4)0.5: A Fluorooxalate of Tetravalent Uranium with a Three-Dimensional Framework Structure. Inorg. Chem. 2006, 45, 1436–1438. 10.1021/ic051960v. [DOI] [PubMed] [Google Scholar]
- Mörtl K. P.; Sutter J.-P.; Golhen S.; Ouahab L.; Kahn O. Structure and Magnetic Characteristics of an Oxalate-Bridged U(IV)–Mn(II) Three-Dimensional Network. Inorg. Chem. 2000, 39, 1626–1627. 10.1021/ic9911825. [DOI] [PubMed] [Google Scholar]
- Murase R.; Leong C. F.; D’Alessandro D. M. Mixed Valency as a Strategy for Achieving Charge Delocalization in Semiconducting and Conducting Framework Materials. Inorg. Chem. 2017, 56, 14373–14382. 10.1021/acs.inorgchem.7b02090. [DOI] [PubMed] [Google Scholar]
- Cladis D. P.; Kiernicki J. J.; Fanwick P. E.; Bart S. C. Multi-electron reduction facilitated by a trianionic pyridine(diimine) ligand. Chem. Commun. 2013, 49, 4169–4171. 10.1039/c2cc37193f. [DOI] [PubMed] [Google Scholar]
- Kias F.; Talbi F.; Elkechai A.; Boucekkine A.; Hauchard D.; Berthet J.-C.; Ephritikhine M. Redox Properties of Monocyclooctatetraenyl Uranium(IV) and (V) Complexes: Experimental and Relativistic DFT Studies. Organometallics 2017, 36, 3841–3853. 10.1021/acs.organomet.7b00585. [DOI] [Google Scholar]
- Kitagawa S.; Katawa S. Coordination compounds of 1, 4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 2002, 224, 11–34. 10.1016/s0010-8545(01)00369-1. [DOI] [Google Scholar]
- Guo D.; McCusker J. K. Spin Exchange Effects on the Physicochemical Properties of Tetraoxolene-Bridged Bimetallic Complexes. Inorg. Chem. 2007, 46, 3257–3274. 10.1021/ic070005y. [DOI] [PubMed] [Google Scholar]
- Darago L. E.; Aubrey M. L.; Yu C. J.; Gonzalez M. I.; Long J. R. Electronic Conductivity, Ferrimagnetic Ordering, and Reductive Insertion Mediated by Organic Mixed-Valence in a Ferric Semiquinoid Metal–Organic Framework. J. Am. Chem. Soc. 2015, 137, 15703–15711. 10.1021/jacs.5b10385. [DOI] [PubMed] [Google Scholar]
- Jeon I.-R.; Negru B.; Van Duyne R. P.; Harris T. D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. 10.1021/jacs.5b10382. [DOI] [PubMed] [Google Scholar]
- DeGayner J. A.; Jeon I.-R.; Sun L.; Dincă M.; Harris T. D. 2D Conductive Iron-Quinoid Magnets Ordering up to Tc = 105 K via Heterogenous Redox Chemistry. J. Am. Chem. Soc. 2017, 139, 4175–4184. 10.1021/jacs.7b00705. [DOI] [PubMed] [Google Scholar]
- Murase R.; Abrahams B. F.; D’Alessandro D. M.; Davies C. G.; Hudson T. A.; Jameson G. N. L.; Moubaraki B.; Murray K. S.; Robson R.; Sutton A. L. Mixed Valency in a 3D Semiconducting Iron–Fluoranilate Coordination Polymer. Inorg. Chem. 2017, 56, 9025–9035. 10.1021/acs.inorgchem.7b01038. [DOI] [PubMed] [Google Scholar]
- Sahadevan S. A.; Abhervé A.; Monni N.; Sáenz de Pipaón C.; Galán-Mascarós J. R.; Waerenborgh J. C.; Vieira B. J. C.; Auban-Senzier P.; Pillet S.; Bendeif E.-E.; Alemany P.; Canadell E.; Mercuri M. L.; Avarvari N. Conducting Anilate-Based Mixed-Valence Fe(II)Fe(III) Coordination Polymer: Small-Polaron Hopping Model for Oxalate-Type Fe(II)Fe(III) 2D Networks. J. Am. Chem. Soc. 2018, 140, 12611–12621. 10.1021/jacs.8b08032. [DOI] [PubMed] [Google Scholar]
- Abrahams B. F.; Coleiro J.; Ha K.; Hoskins B. F.; Orchard S. D.; Robson R. Dihydroxybenzoquinone and chloranilic acid derivatives of rare earth metals. J. Chem. Soc., Dalton Trans. 2002, 1586–1594. 10.1039/b109296k. [DOI] [Google Scholar]
- Zucchi G.; Thuery P.; Ephritikhine M. CSD Private communication. Deposition-ID: CCDC 909452. [Google Scholar]
- Bram A.; Bruederl G.; Burzlaff H.; Lange J.; Rothammel W.; Spengler R.; Karayannis M. I.; Veltsistas P. G. Disodium bis(o-chloranilato)uranyl(VI) hexahydrate. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1994, 50, 178–180. 10.1107/s0108270193011680. [DOI] [Google Scholar]
- Spengler R.; Zimmermann H.; Burzlaff H.; Jansen J.; Peschar R.; Schenk H. Ab initio structure determination from powder data using direct methods. Acta Crystallogr., Sect. B: Struct. Sci. 1994, 50, 578–582. 10.1107/S010876819400306X. [DOI] [Google Scholar]
- Here we compare the potentials of Fe3+ + e– → Fe2+ (E° = +0.77 V vs NHE at 25 °C, Housecroft, C. E.; Sharpe, A. G. Inorganic Chemistry, 5th ed.; Pearson Education Limited, 2018; Reduction and Oxidation, pp 255–282) with the two half-cell reactions UO2+ + e– → U4+ and UO22+ + 2e– → U4+, which have E° values in the range +0.38 to +0.62 V, and +0.27 to +0.33 V (vs NHE at 25 °C), respectively (Kihara, S.; Yoshida, Z.; Aoyagi, H.; Maeda, K.; Shirai, O.; Kitatsuji, Y.; Yoshida, Y. A critical evaluation of the redox properties of uranium, neptunium and plutonium ions in acidic aqueous solutions (Technical Report). Pure Appl. Chem. 1999,71, 1771–1807.).
- Min K. S.; DiPasquale A. G.; Golen J. A.; Rheingold A. L.; Miller J. S. Structure, and Magnetic Properties of Valence Ambiguous Dinuclear Antiferromagnetically Coupled Cobalt and Ferromagnetically Coupled Iron Complexes Containing the Chloranilate(2−) and the Significantly Stronger Coupling Chloranilate(•3−) Radical Trianion. J. Am. Chem. Soc. 2007, 129, 2360–2368. 10.1021/ja067208q. [DOI] [PubMed] [Google Scholar]
- Kindra D. R.; Evans W. J. Magnetic Susceptibility of Uranium Complexes. Chem. Rev. 2014, 114, 8865–8882. 10.1021/cr500242w. [DOI] [PubMed] [Google Scholar]
- Hohloch S.; Pankhurst J. R.; Jaekel E. E.; Parker B. F.; Lussier D. J.; Garner M. E.; Booth C. H.; Love J. B.; Arnold J. Benzoquinonoid-bridged dinuclear actinide complexes. Dalton Trans. 2017, 46, 11615–11625. 10.1039/c7dt02728a. [DOI] [PubMed] [Google Scholar]
- Perez F. M.; Gil J. M.; Gil F. J. M. Preparation, Infrared and Visible Spectra of Sulfate Complexes of Uranium(IV). Z. Anorg. Allg. Chem. 1980, 462, 231–240. 10.1002/zaac.19804620127. [DOI] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. 10.1107/s0021889808042726. [DOI] [Google Scholar]
- Burla M. C.; Caliandro R.; Camalli M.; Carrozzini B.; Cascarano G. L.; De Caro L.; Giacovazzo C.; Polidori G.; Siliqi D.; Spagna R. IL MILIONE: a suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. 10.1107/s0021889807010941. [DOI] [Google Scholar]
- Sheldrick G. M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/s2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.