Abstract
Purpose:
Protease-activated receptors (PARs) are a family of G-protein-coupled receptors distributed in a number of tissues. PAR-2 is expressed on airway epithelium and smooth muscles and overexpressed under pathological conditions, such as asthma and chronic obstructive pulmonary disease. However, the role of PAR-2 in airways has not yet been defined. In this study, we investigated the role of PAR-2-activating peptide (SLIGRL) on histamine-induced bronchoconstriction and the mechanisms underlying the bronchoprotective effect both in vivo and in vitro.
Materials and Methods:
The effect of SLIGRL was tested in vivo using histamine-induced bronchoconstriction in the guinea pig and in vitro using isolated tracheal spiral strips.
Results:
In vivo pretreatment with SLIGRL significantly reduced the histamine-induced increased bronchoconstriction. Neither propranolol nor vagotomy abolished the inhibitory effect of SLIGRL. Furthermore, indomethacin or glibenclamide did not antagonize the inhibitory response to SLIGRL. In isolated tracheal spiral strips in vitro, SLIGRL did not affect the contractile response to acetylcholine or potassium chloride; however, histamine-induced contraction was inhibited in a dose-dependent manner.
Conclusion:
Our data demonstrate the protective effect of SLIGRL in airways; however, this effect appears to be mediated independently of prostanoids, nitric oxide, circulating adrenaline, ATP-sensitive K + channels, and vagal stimulation.
Keywords: Airway resistance, histamine, protease-activated receptors-2-activating peptide, SLIGRL
INTRODUCTION
Protease-activated receptors (PARs) are a family of G-proteincoupled receptors activated by site-specific proteolytic cleavage of a “tethered ligand” or PAR-activating peptide (PAR-AP).[1] Four members of this family have been identified to date, and expression has been detected in many different cell types including immune cells, platelets, endothelial cells, and smooth muscle cells.[2] PARs function in a variety of physiological and pathological processes such as hemostasis, thrombosis, embryonic development, wound healing, inflammation, and cancer progression.[3]
While PAR-1, 3, and 4 are activated by thrombin, PAR-2 is activated by serine proteases such as trypsin and tryptase,[4,5,6] an enzyme released after mast cell degranulation and considered to play an important role in airway inflammation and hyperresponsiveness.[7] Activation of PAR-1 and PAR-2 leads to an endothelium-dependent relaxation of a large array of arterial blood vessels[8] and contraction of gastric smooth muscle.[9] Under physiological conditions, PAR-2 is expressed on several human tissues such as the gastrointestinal tract, pancreas, kidney, liver, lung, ovary, and eye[10,11] and also under pathological conditions such as asthma and chronic obstructive pulmonary disease.[12] PAR-2 is also markedly upregulated after exposure to pro-inflammatory stimuli or cytokines,[13] which have been shown to play a critical role in chronic airway diseases. Moreover, human bronchial smooth muscle cells isolated from asthmatic patients express increased PAR-2 levels, which may contribute to airway hyperresponsiveness.[14]
Cicala et al.[15] showed that in vivo, inflammatory stimuli, such as bacterial lipopolysaccharide (LPS), upregulate PAR-2 expression on vascular endothelium, and smooth muscle cells, correlating with an increase in the hypotensive effect of the synthetic PAR-2-AP. These data suggest a pro-inflammatory effect of PAR-2 activation. In contrast, there is also evidence for a protective anti-inflammatory effect following activation of PAR-2. PAR-2 is expressed in the human lung[11,16] and in the airways, activation of PAR-2 causes an epithelium-dependent relaxation of mouse-isolated bronchi that correlates with PAR-2 immunoreactivity in the cytoplasmic regions of airway epithelial cells[17] and of mouse tracheal rings.[18] In vivo, PAR-2 has been shown to protect against 5HT-induced bronchoconstriction in the rats.[17] Furthermore, bronchi from LPS-treated rats showed an increased relaxant response to PAR-2-AP in vitro.[19] In contrast, PAR-2 activation was shown to induced to a sensory neuropeptide-dependent bronchoconstrictor response.[20] These conflicting data make the role of PAR-2-AP in airway resistance unclear; therefore, in this study, we investigated the role of PAR-2 in histamine-induced bronchoconstriction in the guinea pig as well as the signaling mechanism involved in the bronchoprotective effect of PAR-2 receptor activation in vivo and in vitro.
MATERIALS AND METHODS
Animals
Male guinea pigs (300–350 g) were obtained from the animal house of our University. Animal were maintained under standard conditions at a temperature of 25°C ± 2°C with free access to a standard laboratory diet and water.
Drugs
PAR-2-AP (SLIGRL) (MW: 656.83 g/mol) and a control peptide with a scrambled sequence (LSIGRL) (MW: 656.83) were kindly supplied as a white powder by the Immunopharmacology Department at Southampton General Hospital (UK). Histamine acid phosphate, indomethacin, L-NAME, propranolol, glibenclamide, acetylcholine (ACh), and potassium chloride (KCl) were purchased from Sigma Chemical Co (St. Louis, MO USA). Drugs were dissolved in phosphate-buffered saline (PBS), except ACh and KCl, which were dissolved in water.
In vivo experiments
The airway resistance of the anesthetized guinea pigs was measured according to the method described by Bertrand et al.[21] Male guinea pigs (weight, 300–400 g) were anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneally). The trachea was exposed, and animals were ventilated artificially through a tracheal cannula, which was connected to a respirator (Miniature Ideal pump Assembly 230 v., Bioscience, UK). The frequency of respiration was 60 breaths/min and the pump was adjusted to provide a volume of air at a frequency sufficient to abolish spontaneous respiration. The volume of the expired air was measured by connecting one end of a piece of airtight rubber tube to the tracheal cannula, while the other end was connected to a Harvard pressure module 275. The Harvard Chart Mover (mode L 480) was used to record changes in the air outflow, which is the index for the degree of airway resistance. The right femoral vein was cannulated for intravenous drug administration. Animals were allowed a 20-min stabilization period before each experiment.
Experimental protocol
The guinea pigs were randomly allocated to seven groups (G1–G7; n = 5 per group). On the basis of preliminary studies of the effects of different doses of histamine on airway responsiveness, we selected the submaximal dose of 10 μg/kg for evaluating the effects of PAR-2-AP. Five minutes after histamine treatment, scrambled peptide (LSIGRL, 1 mg/kg i.v) (G1) or the PAR-2-AP (SLIGRL, 1 mg/kg i.v) (G2) was administered. Histamine was readministered 1, 5, and 10 min later and the response compared to that obtained before administration of the peptides.[22] Intravenous injection (1 ml/kg) of (PBS) as a control was shown to have no effect on the baseline airway resistance. In Group G3, the guinea pigs were pretreated with propranolol (1 mg/kg i.v)[23] 15 min before the administration of histamine and the effect of PAR-2-AP was then evaluated to rule out the possibility that the effect of PAR-2-AP was due to the adrenergic system activation. In Group G4, the guinea pigs were vagotomized by excision of both the vagal nerves in the neck region[24] before the effect of PAR-2-AP on histamine-induced bronchoconstriction was investigated. In Group G5, the guinea pigs were pretreated with indomethacin (5 mg/kg i.p)[25] 30 min before the administration of histamine and the effect of PAR-2-AP was then evaluated to investigate the role of endogenous prostaglandins.
In Group G6, the guinea pigs were pretreated with L-NAME (30 mg/kg i.v)[22] 15 min before the administration of histamine and the effect of PAR-2-AP was then evaluated to investigate whether nitric was involved in PAR-2-AP effect. In Group G7, the guinea pigs were pretreated with glibenclamide (30 mg/kg i.v)[26] 35 min before the administration of histamine and the effect of PAR-2-AP was then evaluated to investigate the role of ATP-sensitive potassium channels.
In vitro experiments
Tracheal responsiveness experiments were carried out using isolated spiral strips of the guinea pig trachea using the method described by Patterson.[27] The animals were anesthetized by intraperitoneal injection of pentobarbital (100 mg/kg) before the trachea was dissected to remove adhering tissues and cut spirally. The strip was then mounted in 10-ml organ baths containing a modified Krebs–Henseleit solution (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl, 0.5 mM MgCl2, 25 mM NaHCO3, 1 mM NaHPO4, and 11.1 mM glucose) maintained at 37°C and oxygenated with a mixture of 95% O2 and 5% CO.2. The preparations were allowed to equilibrate for a period of 1 h, during which they were washed at 15 min intervals. An optimal tension of 1 g was applied to tissues fixed to the base of the organ bath. The responses of the tracheal strips to ACh, KCl, and histamine were recorded using an isotonic-sideway writing lever.
Experimental protocol
The responses of the tracheal strips to previously determined submaximal contraction responses to histamine (4 μg/ml), ACh (4 µg/ml), and KCl (0.2 mg/ml) were recorded in the presence of SLIGRL (10 µM).[20] The effects of different concentrations of SLIGRL (1, 5, and 10 µM) on histamine-induced contraction was also investigated.
Statistical analysis
All data represent the mean ± standard deviation. Differences between two groups were analyzed using Student's t-test. P < 0.05 was considered to indicate statistical significance.[28]
RESULTS
Effect of SLIGRL on airway resistance in vivo
Intravenous administration of histamine at a dose of 10 μg/kg increased baseline airway resistance by a mean of 33.49% ± 2.25%. The control peptide LISGRL had no effect on the histamine-induced increase in baseline resistance [Figure 1 and Supplement 1 (373.3KB, tif) ].
Figure 1.
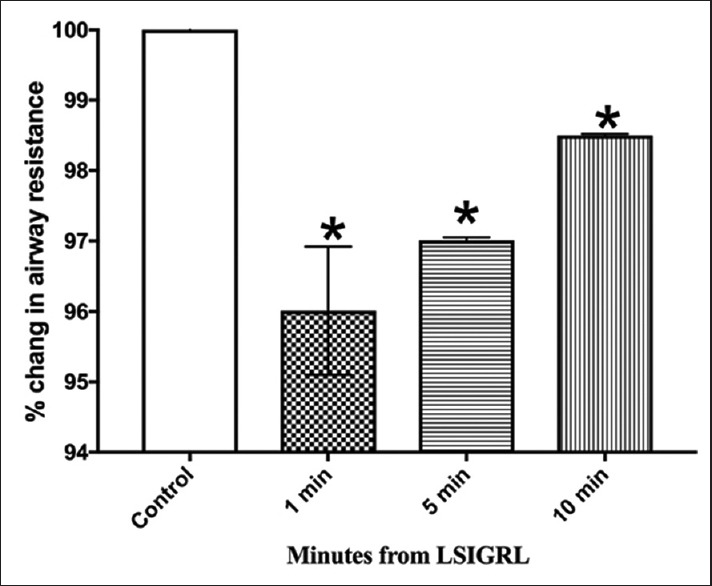
Effect of LS1GRL on histamine-induced bronchoconstriction
However, the pretreatment of animals with SLIGRL (1 mg/kg, i.v) 1 min before histamine challenge produced a significant reduction in histamine-induced airway resistance by 43.94% ± 1.12%) after 1 min (n = 5; P < 0.05) and by 28.55% ± 3.04% after 5 min (n = 5; P < 0.05), while a reduction of only 1.49% ± 0.02% was observed after 10 min [Figure 2 and Supplement 1 (373.3KB, tif) ].
Figure 2.
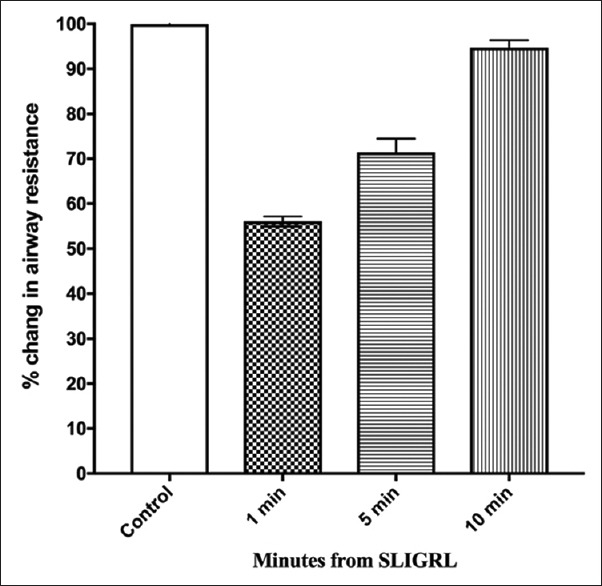
Effect of SL1GRL on histamine-induced bronchoconstriction
In animals pretreated with propranolol (1 mg/kg i.v, 15 min), the response to histamine was increased compared to the pretreatment values [Figure 3 and Supplement 1 (373.3KB, tif) ]. However, propranolol did not abolish the inhibitory effect of SLIGRL on histamine-induced bronchoconstriction.
Figure 3.
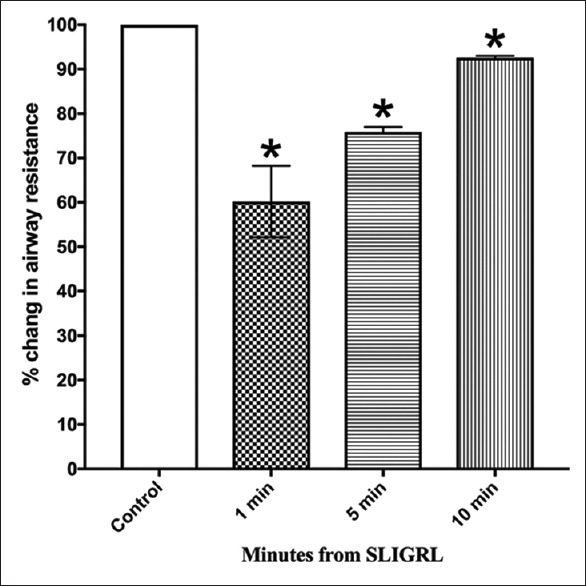
Effect of propranolol on bronchoprotection induced by SLIGRL
In vagotomized guinea pigs, the protective effect of SLIGRL against histamine-induced bronchoconstriction was still present [Figure 4 and Supplement 1 (373.3KB, tif) ].
Figure 4.
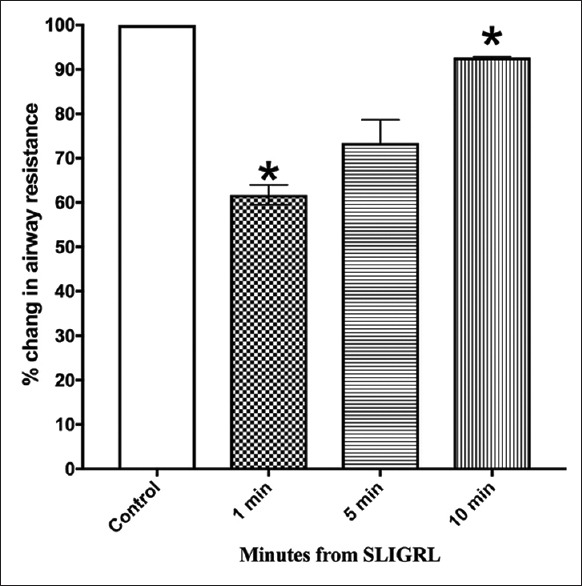
Effect of vagotomy on bronchoprotection induced by SLIGRL
In animals pretreated with indomethacin (5 mg/kg i.p, 30 min), the response to histamine was increased compared to the pretreatment values [Figure 5 and Supplement 1 (373.3KB, tif) ].
Figure 5.
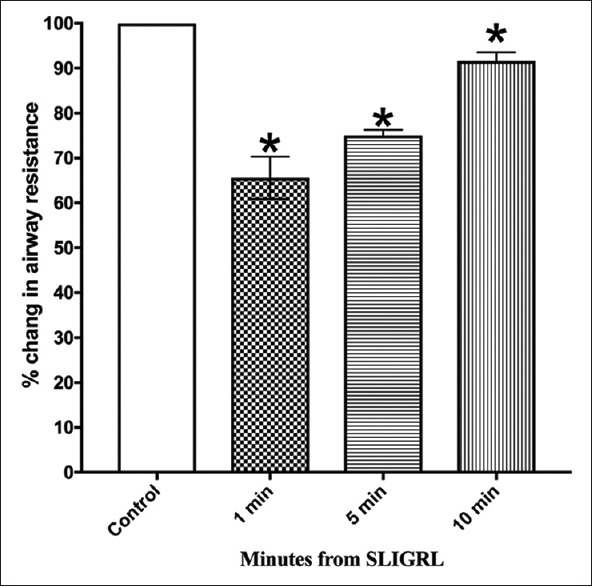
Effect of indomethacin on bronchoprotection induced by SLIGRL
However, indomethacin failed to antagonize the inhibitory response of SLIGRL on the histamine-induced increase in airway resistance. Pretreatment with LNAME (30 mg/kg i.v, 15 min) did not significantly change the response to histamine compared to the pretreatment values [Figure 6 and Supplement 1 (373.3KB, tif) ].
Figure 6.
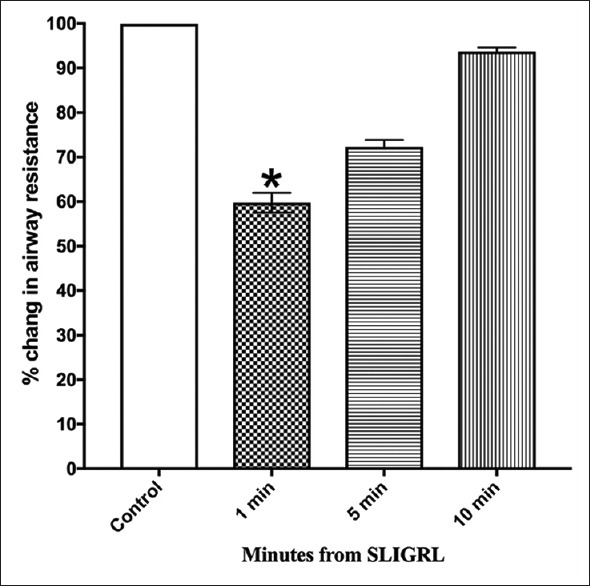
Effect of L-NAME on bronchoprotection induced by SLIGRL
Similarly, in animals pretreated with glibenclamide (30 mg/kg i.v, 35 min), the response to histamine was changed compared to the pretreatment values although this effect did not reach the level of statistical significance [Figure 7 and Supplement 1 (373.3KB, tif) ]. Animals, pretreated with either L-NAME or glibenclamide, did not show significant changes in the airway resistance compared to those induced by SLIGRL at 5 min and 10 min. However, the inhibitory effect of SLIGRL was prolonged by pretreatment with propranolol, vagotomy, and indomethacin.
Figure 7.
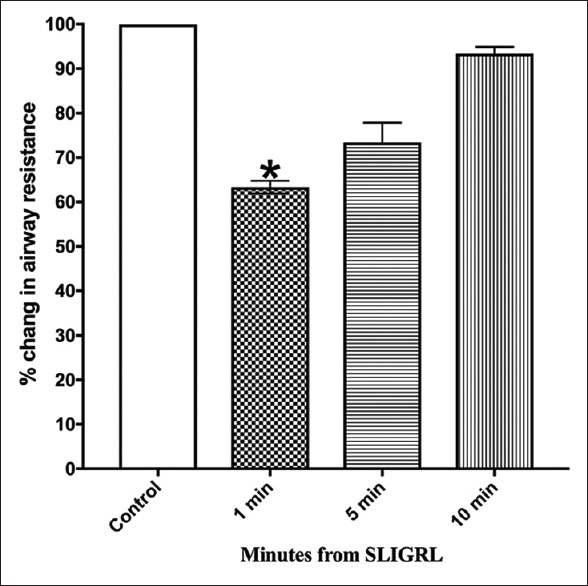
Effect of glibenclamide on bronchoprotection induced by SLIGRL
Effect of SLIGRL on isolated tracheal strips in vitro
Effect of SLIGRL on acetylcholine, potassium chloride, and histamine-induced contractions of isolated guinea pigs tracheal strips
SLIGRL had no effect on the contractile response to ACh (4 μg/ml) and KCl (0.2 mg/ml), while it decreased the histamine-induced contraction [Figure 8].
Figure 8.
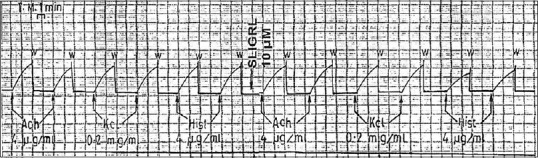
Effect of (SLIGRL) on acetylcholine, potassium chloride and histamine-induced contraction of isolated guinea pig tracheal strips
Effect of SLIGRL on histamine-induced contractions of isolated guinea pig tracheal strips
SLIGRL produced a significant reduction in histamineinduced contraction by 11.2%, 21.6%, and 42% at concentrations of 1 μM, 5 μM, and 10 μM, respectively [Table 1, Figure 9 and Supplement 2].
Table 1.
Effect of SLIGR on histamine-induced contraction of isolated guinea pig tracheal strips
| Drug concentration | Histamine- induced contraction (cm) | Change (%) |
|---|---|---|
| Histamine (4 g/ml) | 3.7±0.5 | |
| SLIGRL (1M) | 3.3±0.6 | -11.2 ± 4.2 |
| SLIGRL (5 µM) | 2.9±0.4* | -21.6 ± 0.2 |
| SLIGRL (10 µM) | 2.2±0.9* | -42 ± 16.6 |
Data represent the mean±SD of contraction and mean percent reduction of histamine-induced contraction. *P<0.05 versus the control (n=5). SD: Standard deviation
Figure 9.
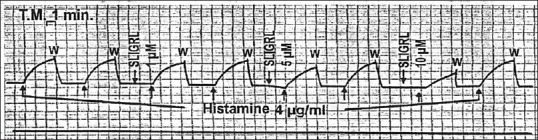
Effect of different doses of SLIGRL on histamine-induced contraction contraction of isolated guinea pig tracheal strips
Supplement 2.
Effect of propranolol, vagotomy, indomethacin, L-NAME and glibencalamide on bronchoprotection induced by PAR-2-AP
| Baseline (cm) | Control (histamine) (10 µg/kg) | LSIG (l mg/kg) | SLIGRL (l mg/kg) | Propranolol (l mg/kg) | Vagotomy | Indomethacin (5 mg/kg) | L-NAME (30 mg/kg) | Glibenclamide (30 mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| Change in airway resistance from baseline (%) | 4.06±0.48 | 6.l0±0.69 (cm) +33.49% ±2.25 | |||||||
| Change in airway resistance after drug administration compared to control (%) | +20.48±2.99 | +11.80±l. 82 | +25.82±8.08 | +7.55±0.29 | +9.94±1.79 | ||||
| Percentage decrease from 1 control min | −3.99*±0.91 | −43.94±1.12 | −39.83*±8.04 | −38.27*±2.22 | −34.41*±4.72 | −40.24*±2.2 | −36.67*±l. 44 | ||
| Percentage of airway resistance relative to control | 96.01 | 56.06 | 60.17 | 61.73 | 65.59 | 59.76 | 63.33 | ||
| Percentage decrease from 5 control min | −2.99*±0.04 | −28.55±3.04 | −21.04*±1.12 | −26.57±5.20 | −25*±1.27 | −27.7±1.54 | −26.63±4.45 | ||
| Percentage of airway resistance relative to control | 97.01 | 71.45 | 75.88 | 73.43 | 75 | 72.3 | 73.37 | ||
| Percentage decrease from 10 control mm | −1.49*±0.02 | −5.23±l. 60 | −7.42*±0.41 | −7.36*±0.22 | −8.37*±1.94 | −6.27±0.87 | −6.65±1.53 | ||
| Percentage of airway resistance relative to control | 98.5 | 94.77 | 92.58 | 92.64 | 91.63 | 93.73 | 93.35 | ||
Data represent mean±SD of percentage change of airway resistance. *Significant compared to the corresponding SLIGRL value P<0.05. Response to histamine (control) was evaluated 1, 5 and 10 min after SLIGRL administration. SD: Standard deviation
DISCUSSION
In the present study, we demonstrated the bronchodilator effect of PAR-2-AP (SLIGRL) against histamine-induced bronchospasm both in vivo and in isolated tissue preparations in vitro. Intravenous injection of SLIGRL produced a significant reduction in airway resistance induced by histamine in anesthetized guinea pigs. In addition, SLIGRL produced a dose-dependent reduction in histamine-induced contractions of isolated guinea pig tracheal strips. These findings are consistent with those reported by Cicala et al.[22] showing that SLIGRL protected against histamine-induced bronchoconstriction in a guinea pig model. Furthermore, Kawabata et al.[29] reported that SLIGRL-NH2 elicited tracheal relaxation. In contrast to the findings of the present study, Barrios et al.[30] reported that SLIGRL treatment increased the responsiveness to histamine in isolated guinea pig bronchi. Furthermore, Chambers et al.[31] demonstrated that PAR-2 activation induced human airway contraction and potentiated the effects of histamine, which may contribute to airway diseases such as asthma. Similarly, Schmidlin et al.[32] reported that PAR-2 activation mobilized intracellular calcium and increased human bronchial smooth muscles contraction. In 2002, Schmidlin et al.[33] used a mouse model of allergic airway inflammation to show that PAR-2 deletion reduced airway hyperresponsiveness, while PAR-2 overexpression had the opposite effect. Thus, these discrepancies suggest that PAR-2 activation can occur through different pathways.
The mechanism of the observed SLIGRL-induced bronchodilation is still not clear; therefore, in the present study, we investigated the role of indirect mechanisms underlying bronchoprotection, including the release of prostaglandins E2 (PGE2), nitric oxide (NO), activation of β-adrenergic receptors, and opening of ATP-sensitive K + channels. We used cyclooxygenase (COX) and NO synthase (NOS) inhibitors, β-adrenergic receptor antagonists, ATP-sensitive K + channel blockers, and vagotomy to investigate each of these pathways. Our results demonstrate the noncholinergic, nonadrenergic bronchodilator effects of SLIGRL in vitro. SLIGRL failed to antagonize ACh-induced contraction and the β-adrenoceptor blocker, propranolol, failed to antagonize the relaxant effect of SLIGRL on histamine-induced contraction. Moreover, vagotomy did not abolish the relaxant effect of SLIGRL. These results are in contrast to those reported by Ricciardolo et al.[20] showing that SLIGRL-NH2(0.1–10 μM) caused a concentration-dependent relaxation of isolated tracheal rings precontracted with carbachol (1 µM). Similarly, Lan et al. and De Campo and Henry reported that a PAR-2-AP has been shown to inhibit methacholine-induced bronchoconstriction in mice.[18,34]
Our data showed that intravenous administration of PAR-2-AP to guinea pigs inhibits the histamine-induced increase in the lung resistance through a mechanism independent of the release of prostaglandin and NO. Furthermore, this effect was not dependent on either circulating adrenaline or opening of ATP-sensitive K + channels. These findings are consistent with the report by Cicala et al.[22] that intravenous administration of PAR-2-AP to guinea pigs inhibited the histamine-induced increase in the lung resistance through a mechanism that was independent of the release of prostaglandin, NO, and the effects of circulating adrenaline.
Cocks et al.[17] proposed that the protective effect of PAR-2-AP on airway reactivity in vitro is dependent on the involvement of epithelial PGE2. The discrepancy between the in vivo data obtained in the present study and those obtained by others using in vitro airway preparations is likely to be due to the complex interactions present in an in vivo setting. A similar discrepancy between data obtained in vitro and in vivo has also been observed for hemodynamic changes mediated by PAR-2 activation.[22] In contrast to our findings, Emilsson et al.[35] and Moffatt and Cocks[36] showed that PAR-2-AP-induced relaxation of isolated vascular tissues through a mechanism that was clearly dependent upon NO release from endothelial cells in vitro.
In the present study, indomethacin treatment significantly augmented the histamine-induced increase in baseline resistance. The mechanism by which indomethacin induces an increase in airway responsiveness to histamine is still uncertain but is known to be dependent on vagal reflex pathways[37,38] and also to the inhibition of airway-derived PGE2, which is known to have a bronchoprotective effect.[39,40,41]
Morello et al.[19] found that PGE2 release by tissues was significantly increased following incubation with PAR-2-AP. In contrast to the present study, the bronchorelaxant effect of PAR-2-AP was inhibited by ibuprofen. In addition, a selective COX-2 inhibitor blocked the bronchorelaxant effect of PAR-2-AP, suggesting strongly that COX-2-derived PGE2 is involved in this effect. Furthermore, PGE2 synthesis by gastrointestinal myofibroblasts is induced by PAR-2 activation.[42]
In contrast to the present study, Lan et al.[18] demonstrated that the upregulation of PARs in the airways is coupled to increased COX activation and enhanced generation of bronchodilatory prostanoids. Similarly, Kawabata et al.[29] found that PAR-2-mediated relaxation in mouse tracheal and bronchial smooth muscle through a mechanism involving both COX-1 and COX-2. Kawao et al.[43] also showed that the PAR-2-AP SLIGRL increased PGE2 synthesis in human A549 alveolar epithelial cells through a mechanism that involved COX-2 upregulation.
L-NAME failed to reverse the bronchoprotection observed with SLIGRL, indicating that the bronchoprotective effect of SLIGRL is unlikely to be due to the release of NO. In accordance with our findings, Chow et al.[44] reported that the SLIGRL-induced airway relaxation was unaffected by L-NAME. In contrast, Cicala et al.[45] found that PAR-2 modulates vascular reactivity both in vitro and in vivo and that PAR-2-AP-induced vasorelaxation is modulated by basal NO. In contrast to the present study, Ricciardolo et al.[20] showed that injection of SLIGRL-NH2 caused a significant increase in airway resistance (increased bronchoconstriction). This effect was significantly increased by pretreatment with a NOS inhibitor, while indomethacin pretreatment caused a significant decrease in the effect of SLIGRL-NH2. Robin et al.[46] reported that PAR-2-mediated vasodilatation in humans in vivo was reduced by both NO and prostanoids, while Risse et al.[47] reported that PAR-2 activation in guinea pigs induced smooth muscle relaxation through epithelial release of prostanoids but not NO.
Relaxation of human isolated airway smooth muscle is mediated through activation of K + channels on the airway epithelium.[48] ATPsensitive K + channel activators may inhibit airway smooth contraction induced by chemical mediators.[49] McGuire et al.[50] also reported that glibenclamide and propranolol did not inhibit relaxation induced by SLIGRL in mouse mesenteric arterioles, whereas relaxation was partially reduced by L-NAME and indomethacin. They demonstrated that glibenclamide did not inhibit relaxation induced by PAR-2, whereas relaxation was inhibited by apamin/charybdotoxin, suggesting that endothelium-dependent hyperpolarization involves the activation of apamin/charybdotoxin-sensitive K + channels not ATP-sensitive K + channels.
In the present study, SLIGRL failed to inhibit KCl-induced contraction of isolated tracheal spiral strips. The findings of the present work were consistent with those of McGuire et al.[51] who demonstrated that the effects of the SLIGRL on membrane potential and tension were not observed in the blood vessels that were contracted with KCl. On the other hand, McGuire et al. found that PAR-2-induced relaxation was inhibited by KCl precontraction.
CONCLUSION
We have demonstrated the protective role of PAR-2 activation against histamine-induced contraction in the guinea pig airways in vivo; however, it appears that PAR-2-AP mediates this effect independently of prostanoids, NO, and circulating adrenaline. In vitro studies showed that SLIGRL did not affect the tracheal contraction induced by ACh and KCl but inhibited histamine-induced contraction in a dose-dependent manner. Furthermore, the effects of PAR-2 are species specific, thus demonstrating the necessity of clinical trials to evaluate the effects of PAR-2-AP in humans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplement Materials
Effect of SLIGRL on airway resistance in anesthetized guinea pigs. The response to histamine was evaluated 1, 5, and 10 min after SLIGRL administration
REFERENCES
- 1.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: Therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11:69–86. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- 3.Gieseler F, Ungefroren H, Settmacher U, Hollenberg MD, Kaufmann R. Proteinase-activated receptors (PARs) – Focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Commun Signal. 2013;11:86. doi: 10.1186/1478-811X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–73. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Déry O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: Novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–52. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 6.Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther. 2011;130:248–82. doi: 10.1016/j.pharmthera.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Abraham WM, Fishman CE, Forteza R, Ahmed A, Cortes A, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. Am J Respir Crit Care Med. 1995;152:2076–83. doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- 8.Hollenberg MD. Protease-mediated signalling: New paradigms for cell regulation and drug development. Trends Pharmacol Sci. 1996;17:3–6. doi: 10.1016/0165-6147(96)81562-8. [DOI] [PubMed] [Google Scholar]
- 9.Saifeddine M, al-Ani B, Cheng CH, Wang L, Hollenberg MD. Rat proteinase-activated receptor-2 (PAR-2): CDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br J Pharmacol. 1996;118:521–30. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–12. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, et al. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–64. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 12.Peters T, Henry PJ. Protease-activated receptors and prostaglandins in inflammatory lung disease. Br J Pharmacol. 2009;158:1017–33. doi: 10.1111/j.1476-5381.2009.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271:14910–5. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 14.Allard B, Bara I, Gilbert G, Carvalho G, Trian T, Ozier A, et al. Protease activated receptor-2 expression and function in asthmatic bronchial smooth muscle. PLoS One. 2014;9:e86945. doi: 10.1371/journal.pone.0086945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, et al. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99:2590–7. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- 16.Hauck RW, Schulz C, Schömig A, Hoffman RK, Panettieri RA., Jr Alpha-thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am J Physiol. 1999;277:L22–9. doi: 10.1152/ajplung.1999.277.1.L22. [DOI] [PubMed] [Google Scholar]
- 17.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–60. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 18.Lan RS, Stewart GA, Goldie RG, Henry PJ. Altered expression and in vivo lung function of protease-activated receptors during influenza A virus infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L388–98. doi: 10.1152/ajplung.00286.2003. [DOI] [PubMed] [Google Scholar]
- 19.Morello S, Vellecco V, Roviezzo F, Maffia P, Cuzzocrea S, Cirino G, et al. A protective role for proteinase activated receptor 2 in airways of lipopolysaccharide-treated rats. Biochem Pharmacol. 2005;71:223–30. doi: 10.1016/j.bcp.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med. 2000;161:1672–80. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand C, Nadel JA, Graf PD, Geppetti P. Capsaicin increases airflow resistance in guinea pigs in vivo by activating both NK2 and NK1 tachykinin receptors. Am Rev Respir Dis. 1993;148:909–14. doi: 10.1164/ajrccm/148.4_Pt_1.909. [DOI] [PubMed] [Google Scholar]
- 22.Cicala C, Spina D, Keir SD, Severino B, Meli R, Page CP, et al. Protective effect of a PAR2-activating peptide on histamine-induced bronchoconstriction in guinea-pig. Br J Pharmacol. 2001;132:1229–34. doi: 10.1038/sj.bjp.0703934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura M, Belvisi MG, Barnes PJ. Modulation of nonadrenergic noncholinergic neural bronchoconstriction by bradykinin in anesthetized guinea pigs in vivo. J Pharmacol Exp Ther. 1994;268:482–6. [PubMed] [Google Scholar]
- 24.Nayler RA, Mitchell HW. Airways hyperreactivity and bronchoconstriction induced by vanadate in the guinea-pig. Br J Pharmacol. 1987;92:173–80. doi: 10.1111/j.1476-5381.1987.tb11309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tohda Y, Muraki M, Kubo H, Itoh M, Haraguchi R, Nakajima S, et al. Role of chemical mediators in airway hyperresponsiveness in an asthmatic model. Respiration. 2001;68:73–7. doi: 10.1159/000050466. [DOI] [PubMed] [Google Scholar]
- 26.Buchheit KH, Hofmann A, Manley P, Pfannkuche HJ, Quast U. Atypical effect of minoxidil sulphate on guinea pig airways. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:418–24. doi: 10.1007/s002100000218. [DOI] [PubMed] [Google Scholar]
- 27.Patterson R. The tracheal strip: Observations on the response of tracheal muscle. J Allergy. 1958;29:8. [Google Scholar]
- 28.Broughton-Pipkin F. New York: Churchill Livingstone; 1984. Medical Statistics Made Easy Edinburgh. [Google Scholar]
- 29.Kawabata A, Kubo S, Ishiki T, Kawao N, Sekiguchi F, Kuroda R, et al. Proteinase-activated receptor-2-mediated relaxation in mouse tracheal and bronchial smooth muscle: Signal transduction mechanisms and distinct agonist sensitivity. J Pharmacol Exp Ther. 2004;311:402–10. doi: 10.1124/jpet.104.068387. [DOI] [PubMed] [Google Scholar]
- 30.Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol. 2003;66:519–25. doi: 10.1016/s0006-2952(03)00292-2. [DOI] [PubMed] [Google Scholar]
- 31.Chambers LS, Black JL, Poronnik P, Johnson PR. Functional effects of protease-activated receptor-2 stimulation on human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1369–78. doi: 10.1152/ajplung.2001.281.6.L1369. [DOI] [PubMed] [Google Scholar]
- 32.Schmidlin F, Amadesi S, Vidil R, Trevisani M, Martinet N, Caughey G, et al. Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle. Am J Respir Crit Care Med. 2001;164:1276–81. doi: 10.1164/ajrccm.164.7.2101157. [DOI] [PubMed] [Google Scholar]
- 33.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–21. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 34.De Campo BA, Henry PJ. Stimulation of protease-activated receptor-2 inhibits airway eosinophilia, hyperresponsiveness and bronchoconstriction in a murine model of allergic inflammation. Br J Pharmacol. 2005;144:1100–8. doi: 10.1038/sj.bjp.0706150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emilsson K, Wahlestedt C, Sun MK, Nystedt S, Owman C, Sundelin J, et al. Vascular effects of proteinase-activated receptor 2 agonist peptide. J Vasc Res. 1997;34:267–72. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- 36.Moffatt JD, Cocks TM. Endothelium-dependent and -independent responses to protease-activated receptor-2 (PAR-2) activation in mouse isolated renal arteries. Br J Pharmacol. 1998;125:591–4. doi: 10.1038/sj.bjp.0702157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito Y, Tajima K. Spontaneous activity in the trachea of dogs treated with indomethacin: An experimental model for aspirin. related asthma. Br J Pharmacol. 1981;73:563–71. doi: 10.1111/j.1476-5381.1981.tb10456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell HW, Adcock J. Vagal mechanisms and the effect of indomethacin on bronchoconstrictor stimuli in the guinea-pig. Br J Pharmacol. 1988;94:522–7. doi: 10.1111/j.1476-5381.1988.tb11556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavord ID, Tattersfield AE. Bronchoprotective role for endogenous prostaglandin E2. Lancet. 1995;345:436–8. doi: 10.1016/s0140-6736(95)90409-3. [DOI] [PubMed] [Google Scholar]
- 40.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 41.Claar D, Hartert TV, Peebles RS., Jr The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 2015;9:55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seymour ML, Binion DG, Compton SJ, Hollenberg MD, MacNaughton WK. Expression of proteinase-activated receptor 2 on human primary gastrointestinal myofibroblasts and stimulation of prostaglandin synthesis. Can J Physiol Pharmacol. 2005;83:605–16. doi: 10.1139/y05-046. [DOI] [PubMed] [Google Scholar]
- 43.Kawao N, Nagataki M, Nagasawa K, Kubo S, Cushing K, Wada T, et al. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin E2 formation in human lung epithelial cells. J Pharmacol Exp Ther. 2005;315:576–89. doi: 10.1124/jpet.105.089490. [DOI] [PubMed] [Google Scholar]
- 44.Chow JM, Moffatt JD, Cocks TM. Effect of protease-activated receptor (PAR)-1, -2 and -4-activating peptides, thrombin and trypsin in rat isolated airways. Br J Pharmacol. 2000;131:1584–91. doi: 10.1038/sj.bjp.0703738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cicala C, Morello S, Vellecco V, Severino B, Sorrentino L, Cirino G, et al. Basal nitric oxide modulates vascular effects of a peptide activating protease-activated receptor 2. Cardiovasc Res. 2003;60:431–7. doi: 10.1016/s0008-6363(03)00565-0. [DOI] [PubMed] [Google Scholar]
- 46.Robin J, Kharbanda R, Mclean P, Campbell R, Vallance P. Protease-activated receptor 2-mediated vasodilatation in humans in vivo: Role of nitric oxide and prostanoids. Circulation. 2003;107:954–9. doi: 10.1161/01.cir.0000050620.37260.75. [DOI] [PubMed] [Google Scholar]
- 47.Risse PA, Naline E, Faisy C, Huchon G, Chung KF, Kleinmann P, et al. Protease-activated receptor 2 in regulation of bronchomotor tone: Effect of tobacco smoking. Life Sci. 2004;75:991–1002. doi: 10.1016/j.lfs.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Black JL, Johnson PR, McKay KO, Carey D, Armour CL. Levcromakalim- and isoprenaline-induced relaxation of human isolated airways – Role of the epithelium and of K+channel activation. Pulm Pharmacol. 1994;7:195–203. doi: 10.1006/pulp.1994.1023. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Tamura G, Iijima H, Shirato K. Effects of an ATP-sensitive K+channel activator, JTV-506, on antigen-induced early and late asthmatic responses in sensitized guinea pigs. Arerugi. 1999;48:1212–6. [PubMed] [Google Scholar]
- 50.McGuire JJ, Hollenberg MD, Andrade-Gordon P, Triggle CR. Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br J Pharmacol. 2002;135:155–69. doi: 10.1038/sj.bjp.0704469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGuire JJ, Hollenberg MD, Bennett BM, Triggle CR. Hyperpolarization of murine small caliber mesenteric arteries by activation of endothelial proteinase-activated receptor 2. Can J Physiol Pharmacol. 2004;82:1103–12. doi: 10.1139/y04-121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of SLIGRL on airway resistance in anesthetized guinea pigs. The response to histamine was evaluated 1, 5, and 10 min after SLIGRL administration


