Abstract
Objectives
Sorghum is one of the most recalcitrant species for transformation. Considering the time and effort required for stable transformation in sorghum, establishing a transient system to screen the efficiency and full functionality of vector constructs is highly desirable.
Results
Here, we report an Agrobacterium-mediated transient transformation assay with intact sorghum leaves using green fluorescent protein as marker. It also provides a good monocot alternative to tobacco and protoplast assays with a direct, native and more reliable system for testing single guide RNA (sgRNA) expression construct efficiency. Given the simplicity and ease of transformation, high reproducibility, and ability to test large constructs, this method can be widely adopted to speed up functional genomic and genome editing studies.
Keywords: Agrobacterium, CRISPR, sgRNA, Sorghum, Transformation, Transient
Introduction
Sorghum is a gluten-free C4 crop, important as both a human dietary staple and animal feed, but more recently also as a potential feedstock for biofuel production [1]. With high collinearity and synteny with other grass genomes, sorghum also provides an ideal template to serve as model for other grasses [2]. However, realizing the full potential of sorghum as feedstock requires bioengineering efforts aimed at tailoring sorghum biomass for biorefining applications [3, 4]. Indeed, while the sorghum genome sequence was completed a decade ago [2], only a handful of genes have been characterized using transgenic approaches.
A major factor in the lack of progress is the low efficiency and time-consuming nature of stable transformation. Indeed, sorghum is one of the most recalcitrant crops to transformation and regeneration. The first sorghum transgenic plants were generated using particle bombardment in 1993 with only 0.28% transformation rate [5]. Subsequently, Zhao and coworkers [6] reported 2.12% transformation rate using Agrobacterium-mediated transformation. Although with recent advancements in technology and optimization of regeneration protocols, several labs have been able to now transform a few limited sorghum cultivars with improved efficiency; reproducibility and consistency still remain major issues [7–9].
When developing engineered plants, due to the time and cost involved, it is highly desirable to test construct functionality in a transient assay. This is particularly true for sorghum. Transient assays in grasses mostly rely on protoplasts [10–12]. However, expression of a gene in protoplasts may not always mimic in planta native state and, also experience inconsistent efficiency due to variability in quality of protoplasts and size of vector transformed [13]. Here, we have established a simplified transient assay with Agrobacterium, also known as agroinfiltration, for transient transformation of sorghum and demonstrated its application by confirming gene editing in sorghum leaves using GFP as a marker. Using our method, researchers can directly test the in planta efficacy of binary constructs that may subsequently be used for stable transformation.
Main text
Methods
Plasmids and bacterial strains
The T-DNA regions of the transformation constructs used in this study are shown in Additional file 1: Fig. S1. Binary vectors C282 and C283 were built based on pTKan-p35S-attR1-GW-attR2 backbone vector [14] using Gateway (Invitrogen, CA, U.S.A.) to introduce codons for GFP (C282) or frame-shifted (fs)GFP (C283) for expression under the CaMV 35S promoter. The fsGFP has a 23 bp positive target control (PTC) sequence inserted after the start codon (5′-gcgcttcaaggtgcacatggagg-3′) [15]. C286 contains GFP driven by maize Ubiquitin 1 promoter, described elsewhere [16, 17]. Binary vectors C475 and C476 were built based on pTKan-pNOS-DsRed-pZmUBQ1-attR1-GW-attR2 backbone vector [16]. The C476 cassette (pTKan-pNOS-DsRed-tNOS-pZmUBQ1-CAS9p-pOsU3-PTC_gRNA-p35S-fsGFP) contains a sgRNA (5′-gcgcttcaaggtgcacatgg-3′) targeting the PTC sequence in fsGFP. CAS9p is a plant codon optimized CAS9 from Streptococcus pyogenes [18]. The C475 cassette (pTKan-pNOS-DsRed-tNOS-pZmUBQ1-CAS9p-pOsU3-nongRNA-p35S-fsGFP) lacking a sgRNA targeting sequence was used as a negative control. Plasmids are available from the JBEI registry: https://registry.jbei.org.
Binary vectors were transformed into Agrobacterium tumefaciens strain GV3101 using electroporation, and grown in Luria Bertani (LB) medium containing 100/30/50 μg/mL rifampicin/gentamicin/spectinomycin at 28 °C. Similarly, A. tumefaciens strain C58C1 containing the P19 suppressor of gene-silencing protein was grown in LB media containing 100/5/50 μg/mL rifampicin/tetracycline/kanamycin.
Leaf infiltration
For agroinfiltration, Agrobacterium was grown in liquid culture (5 mL, 24 h, 30 °C), and cells were pelleted (5000×g, 5 min), and resuspended in infiltration medium containing 50 mM MES, pH 5.6, 2 mM Na3PO4, 0.5% (w/v) dextrose, 200 μM acetosyringone and 0.01% Silwet L-77 with an OD600 of 0.5. The P19 strain was mixed with each of the other strains to ¼ of the final volume. Prior to infiltration, the Agrobacterium suspension was incubated without shaking at 30 °C for about 2 h. The Nicotiana benthamiana plants were grown in a growth chamber under 16/8 h and 26/24 °C day/night cycle, and plants of ~ 4-weeks-old used for infiltration. Sorghum bicolor (L.) Moench inbred line Tx430 plants were grown in a plant growth room under 14/10 h 29/26 °C day/night cycle. Plants at the three-leaf stage (3–4 weeks old), were used for co-infiltration (Fig. 1). The fully expanded sorghum leaves were mechanically wounded with a 40 mm syringe needle of diameter 0.8 mm several times to make the epidermis more conducive to infiltration. No injury was required for tobacco leaf infiltration. The Agrobacterium strains, suspended in infiltration medium, were infiltrated into leaves using a 1 mL syringe without needle. The boundaries of regions infiltrated with Agrobacterium were marked with a permanent marker for later visualization. Typically, each leaf was infiltrated at three different sites on the abaxial surface, with an approximate distance of 2 cm between each site.
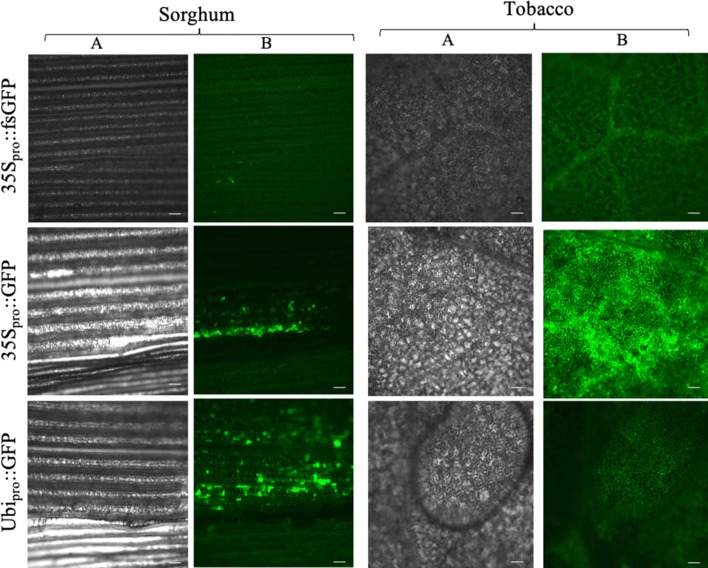
Fig. 1.

Image of sorghum seedling depicting the stage of sorghum plant required for efficient agroinfiltration. Leaves used for syringe-mediated infiltration on abaxial side are marked by white arrows
Microscopy
About 3–4 days after infiltration (DAI), tobacco and sorghum leaves were detached from the plant and observed under a Leica D4000B fluorescence microscope coupled with a Leica DC500 camera using appropriate filters for GFP and DsRed.
Results
Expression of GFP in infiltrated leaves of tobacco and sorghum
We tested binary constructs C282 containing 35Spro::GFP and the modified plasmid C283 with 35Spro::fsGFP (frame-shifted GFP) by agroinfiltration in both tobacco and sorghum leaves. At 3DAI, the GFP signal was examined in detached leaves under a fluorescent microscope. Both sorghum and tobacco leaves infiltrated with C282 showed high and consistent expression of GFP (Fig. 2). However, those infiltrated with C283, containing fsGFP, exhibited no signal. It was noted that the area of detectable GFP expression was much smaller in sorghum as compared to tobacco. This is likely due to the limited infiltration of Agrobacterium suspension in sorghum leaves. The signal could be observed up to 7 DAI, after which the signal declined. The inclusion of P19 helps to both combat siRNA-mediated post transcriptional silencing and enhance the signal in both tobacco and sorghum. Incubation at 30 °C for 2 h was helpful to improve the signal, as well as reproducibility between experiments, likely due to it enhancing active growth of Agrobacteriumm as has been previously demonstrated [19].
Fig. 2.
Results of agroinfiltration with Agrobacterium suspension in sorghum and tobacco leaves. Column A shows bright field images and column B depicts GFP expression detected using fluorescence microscope. Scale bar: 100 μm
Ubiquitin promoter is more effective for sorghum
We compared infiltration of plasmid C282 (35Spro::GFP) with C286 (Ubqpro::GFP) in sorghum. While a higher intensity of GFP signal was observed in tobacco leaves with the 35S promoter compared to sorghum leaves (Fig. 2); GFP expression driven by the maize ubiquitin1 promoter exhibited higher intensity in sorghum leaves.
Demonstration of gene editing in sorghum leaves using GFP as target gene
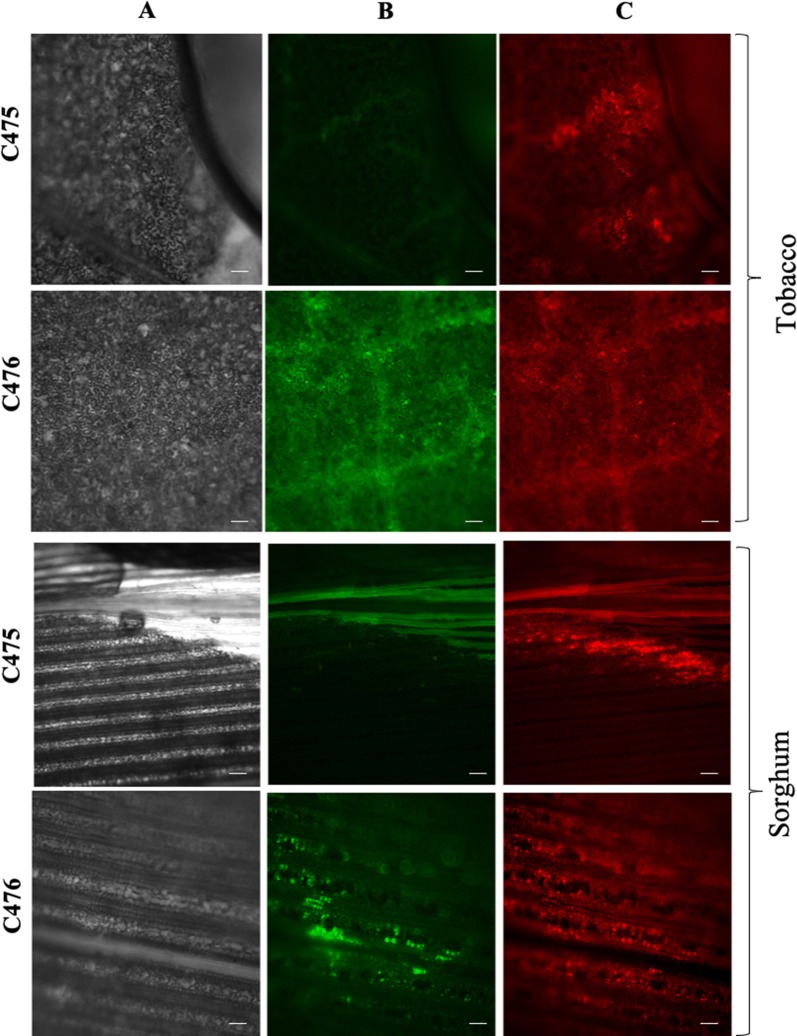
To test whether we can use our transient Agrobacterium-mediated transformation method to determine sgRNA gene editing efficiency in sorghum, we used the binary vectors, C475 and C476 for agroinfiltration. Tobacco leaves were also infiltrated as a comparison. Both C475 and C476 contained constitutively expressed DsRed under the nopaline synthase (NOS) promoter, fsGFP driven by 35S promoter and pUbi-driven CAS9p for CRISPR-mediated genome editing. C476 contained a sgRNA targeting the PTC sequence in fsGFP. As C475 lacked the targeting sgRNA, GFP expression was only expected with C476 vector and only when editing occurs to correct the GFP frame shift.
Following agroinfiltration, DsRed expression could be detected in both sorghum and tobacco leaves with both the constructs, confirming successful infiltration (Fig. 3). However, GFP expression was observed only in the leaves infiltrated with C476 demonstrating successful editing in the intact leaves of both tobacco and sorghum (Fig. 3).
Fig. 3.
Successful editing of GFP in tobacco and sorghum leaves using agroinfiltration. Column A presents bright field images, whereas, columns B and C present expression of GFP and DsRed, respectively. The C476 vector construct contained sgRNA required for editing, while C475 lacked the sgRNA and serves as negative control. Expression of GFP in leaves transformed with C476 demonstrates successful editing. Scale bar: 100 μm
Discussion
Plant transformation is indispensable for elucidating gene function and engineering plant genomes for improved agronomic traits. Several biological, mechanical, chemical and electrical methods of DNA delivery have been developed to facilitate plant transformation over past several decades [20, 21]. Among biological methods, the soil-borne gram-negative bacterium A. tumefaciens is no doubt the most popular and widely used vehicle for DNA delivery in plant cells [22]. Although monocots are outside the host range of this bacterium, Agrobacterium-mediated transformation is now routinely used for transforming monocot genomes as well, though with lower efficiency [23, 24]. Agroinfiltration is also routinely used in several plant species due to rapidity, versatility and convenience [25–31]. However, success of this method in monocot species is very limited primarily due to extensive epidermal cuticular wax, high silica content, and low volume of intercellular space. These morphological features prevent the infiltration of bacterial cells into grasses via the application of simple pressure. Although microprojectile bombardment may be used to introduce expression constructs in cereals, the set-up cost for establishing microprojectile bombardment is high. Moreover, it only targets single cells limiting the scope of screening [32], and often leads to cell damage. Earlier, Andrieu et al. [33] reported Agrobacterium-mediated transient gene expression and silencing in rice leaves by mechanically wounding leaves followed by direct incubation in Agrobacterium suspension. However, we made several attempts to transform sorghum leaves at different stages of development, using their methodology, but could not detect any expression of GFP (data not shown).
Virus-based vectors provide an alternative opportunity for elucidating monocot gene functions. However, instability of the recombinant vector, improper orientation of insert and inconsistency due to inadequate infectivity, inoculation methods, replication/movement of virus in the host, pose serious challenges [34]. Another recent study demonstrated application of nanoparticles in transformation of wheat leaves by combining wounding treatment with syringe infiltration of the nanoparticles [35]. However, the size of plasmid that can be loaded onto nanoparticles is a major constraint due to size exclusion limit of the plant cell wall (~ 20 nm).
To overcome these constraints, we attempted syringe infiltration with recombinant Agrobacterium, containing vectors for in planta GFP expression, at different stages of development in sorghum leaves. As expected, strength of signal in sorghum leaves was higher with the maize ubiquitin promoter as compared to cauliflower mosaic virus 35S promoter, which is reported to perform better in dicots [36]. In our system, although infiltration medium could enter the mature leaves, GFP expression was only detected in the infiltrated younger leaves of 3–4-week-old plants. The expression of GFP seemed to localize to where bacteria were initially infiltrated through mechanical pressure. We did not observe a spread of signal in the adjacent areas, unlike that reported by Andrieu and coworkers [33] for siRNAs in rice. This observation indicated that although bacteria could enter sorghum leaf cells through the wounded regions, they could not passively diffuse to other cells without mechanical pressure in sorghum leaves. We also tried dipping the leaf in Agrobacterium suspension after clipping the leaf from the top, as well as wounding by needle, however Agrobacterium could not detectably enter the sorghum leaves without applied mechanical pressure.
Further, we demonstrated the application of our method to test the efficiency of sgRNA in genome editing constructs. CRISPR-associated Cas9 is a powerful genome editing tool for engineering plants [37]. Although the design of sgRNAs and preparation of constructs is straightforward, the accuracy and efficiency of the method relies on the choice of sgRNAs [38]. Several in silico prediction tools are available to predict the efficiency of sgRNAs based on the sequence features. However, predicted sgRNAs often have vastly different editing efficiencies in planta [17]. Protoplasts have been commonly used to test sgRNA efficiency. However, obtaining high quality protoplasts for genome editing needs extensive standardization, especially for plants such as sorghum. Secondly, additional cloning steps have to be performed to obtain a smaller vector for protoplast transformation. Thirdly and most importantly, the efficiency predicted in protoplasts may not correlate with the efficiency observed in intact plant tissue [38]. Therefore, screening of sgRNAs to achieve high accuracy and efficiency remains a challenge. We adopted our Agrobacterium-mediated transient transformation strategy to test sgRNA-mediated editing efficiency in sorghum leaves. The editing was observed in the transformed tissue within 3 days after infiltration, thereby providing a reliable assay for testing sgRNAs under native conditions.
We used GFP as a reporter in our study as it allows direct visualization in living tissues without being invasive or destructive and does not need any substrate. Gao and workers [39] demonstrated successful use of GFP as marker for stable transformation in sorghum, avoiding use of antibiotics or herbicides. This strategy can be easily applied in our system to quickly assess the full functionality of the vector constructs. For sgRNAs targeting endogenous genes, efficacy can be tested using RT-PCR or sequencing.
Overall, our study demonstrated that in planta Agrobacterium-mediated transient expression of transgenes is achievable in sorghum leaves. High reproducibility, simplicity, rapidity and feasibility to transform large constructs, which can directly be used for stable transformation, are the key advantages of our method. Though this method can be used for subcellular localization studies and physiological assays, the ability to test sgRNA targeting efficiency should be of particular interest.
Limitations
The efficiency of agroinfiltration is much less compared to that observed in tobacco plants and therefore infiltration of more plants may be necessary if significant amount of materials are required for downstream analysis.
Since we were targeting a transgene in our editing assays, editing of an endogenous sorghum gene and confirmation of successful editing by sequencing would be an important step to confirm wide applicability of this method.
Supplementary information
Additional file 1: Figure S1. Schematic presentations of the T-DNA regions of transformation constructs. Elements in each construct are drawn to scale. LB, left border of the T-DNA region; RB, right border of the T-DNA region; PTC, positive target control site for genome editing; fsGFP, frame-shifted GFP with PTC inserted after ATG start codon
Acknowledgements
Not applicable.
Abbreviations
- 35S
Cauliflower mosaic virus 35S promoter
- CRISPR
Clustered regularly interspaced short palindromic repeats
- DAI
Days after infiltration
- fsGFP
Frame-shifted GFP
- GFP
Green fluorescent protein
- LB
Luria Bertani media
- NOS
Nopaline synthase
- P19
Tomato bushy virus P19
- PTC
Positive target control
- sgRNA
Single guide RNA
- Ubi
Zea mays Ubiquitin1 promoter
Authors’ contributions
RS, JCM and HVS conceptualized the study and designed the experiments. RS, YL, VRP and MYL performed the experiments and analyzed the data. RS drafted the manuscript. All authors read and approved the final manuscript.
Funding
R.S. acknowledges IUSSTF-DBT for GETin fellowship. This work was funded by DOE Joint BioEnergy Institute (http://www.jbei.org) supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research through Contract DEAC0205CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rita Sharma and Yan Liang contributed equally to this work
Contributor Information
Rita Sharma, Email: rita.genomics@gmail.com.
Yan Liang, Email: yliang@lbl.gov.
Mi Yeon Lee, Email: miylee@lbl.gov.
Venkataramana R. Pidatala, Email: ramana@lbl.gov
Jenny C. Mortimer, Email: jcmortimer@lbl.gov
Henrik V. Scheller, Email: hscheller@lbl.gov
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13104-020-04968-9.
References
- 1.Mathur S, Umakanth AV, Tonapi VA, Sharma R, Sharma MK. Sweet sorghum as biofuel feedstock: recent advances and available resources. Biotechnol Biofuels. 2017;10:146. doi: 10.1186/s13068-017-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457(7229):551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 3.van der Weijde T, Alvim Kamei CL, Torres AF, Vermerris W, Dolstra O, Visser RG, Trindade LM. The potential of C4 grasses for cellulosic biofuel production. Front Plant Sci. 2013;4:107. doi: 10.3389/fpls.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia R, Gallagher JA, Gomez LD, Bosch M. Genetic engineering of grass cell wall polysaccharides for biorefining. Plant Biotechnol J. 2017;15(9):1071–1092. doi: 10.1111/pbi.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casas AM, Kononowicz AK, Zehr UB, Tomes DT, Axtell JD, Butler LG, Bressan RA, Hasegawa PM. Transgenic sorghum plants via microprojectile bombardment. Proc Natl Acad Sci USA. 1993;90(23):11212–11216. doi: 10.1073/pnas.90.23.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao ZY, Cai T, Tagliani L, Miller M, Wang N, Pang H, Rudert M, Schroeder S, Hondred D, Seltzer J, et al. Agrobacterium-mediated sorghum transformation. Plant Mol Biol. 2000;44(6):789–798. doi: 10.1023/A:1026507517182. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed RI, Ding A, Xie M, Kong Y. Progress in optimization of Agrobacterium-mediated transformation in sorghum (Sorghum bicolor) Int J Mol Sci. 2018;19(10):2983. doi: 10.3390/ijms19102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Che P, Anand A, Wu E, Sander JD, Simon MK, Zhu W, Sigmund AL, Zastrow-Hayes G, Miller M, Liu D, et al. Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application. Plant Biotechnol J. 2018;16(7):1388–1395. doi: 10.1111/pbi.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson-Vasilchik K, Hague J, Mookkan M, Zhang ZJ, Kausch A. Transformation of recalcitrant sorghum varieties facilitated by baby boom and Wuschel2. Curr Protoc Plant Biol. 2018;3(4):e20076. doi: 10.1002/cppb.20076. [DOI] [PubMed] [Google Scholar]
- 10.Burris KP, Dlugosz EM, Collins AG, Stewart CN, Jr, Lenaghan SC. Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.) Plant Cell Rep. 2016;35(3):693–704. doi: 10.1007/s00299-015-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CS, Hsu CT, Yang LH, Lee LY, Fu JY, Cheng QW, Wu FH, Hsiao HC, Zhang Y, Zhang R, et al. Application of protoplast technology to CRISPR/Cas9 mutagenesis: from single-cell mutation detection to mutant plant regeneration. Plant Biotechnol J. 2018;16(7):1295–1310. doi: 10.1111/pbi.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods. 2011;7(1):30. doi: 10.1186/1746-4811-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehman L, Su X, Guo H, Qi X, Cheng H. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol. 2016;16(1):57. doi: 10.1186/s12896-016-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez TL, Liang Y, Nguyen BN, Staskawicz BJ, Loque D, Hammond MC. Tight regulation of plant immune responses by combining promoter and suicide exon elements. Nucleic Acids Res. 2015;43(14):7152–7161. doi: 10.1093/nar/gkv655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013;41:e188. doi: 10.1093/nar/gkt780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Y, Richardson S, Yan J, Benites VT, Cheng-Yue C, Tran T, Mortimer J, Mukhopadhyay A, Keasling JD, Scheller HV, et al. Endoribonuclease-based two-component repressor systems for tight gene expression control in plants. ACS Synth Biol. 2017;6(5):806–816. doi: 10.1021/acssynbio.6b00295. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Eudes A, Yogiswara S, Jing B, Benites VT, Yamanaka R, Cheng-Yue C, Baidoo EE, Mortimer JC, Scheller HV, et al. A screening method to identify efficient sgRNAs in Arabidopsis, used in conjunction with cell-specific lignin reduction. Biotechnol Biofuels. 2019;12:130. doi: 10.1186/s13068-019-1467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant. 2015;8(8):1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Norkunas K, Harding R, Dale J, Dugdale B. Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant Methods. 2018;14:71. doi: 10.1186/s13007-018-0343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altpeter F, Springer NM, Bartley LE, Blechl AE, Brutnell TP, Citovsky V, Conrad LJ, Gelvin SB, Jackson DP, Kausch AP, et al. Advancing crop transformation in the era of genome editing. Plant Cell. 2016;28(7):1510–1520. doi: 10.1105/tpc.16.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barampuram S, Zhang ZJ. Recent advances in plant transformation. Methods Mol Biol. 2011;701:1–35. doi: 10.1007/978-1-61737-957-4_1. [DOI] [PubMed] [Google Scholar]
- 22.Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67(1):16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu E, Zhao ZY. Agrobacterium-mediated sorghum transformation. Methods Mol Biol. 2017;1669:355–364. doi: 10.1007/978-1-4939-7286-9_26. [DOI] [PubMed] [Google Scholar]
- 24.Hiei Y, Ishida Y, Komari T. Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front Plant Sci. 2014;5:628. doi: 10.3389/fpls.2014.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhaskar PB, Venkateshwaran M, Wu L, Ane JM, Jiang J. Agrobacterium-mediated transient gene expression and silencing: a rapid tool for functional gene assay in potato. PLoS ONE. 2009;4(6):e5812. doi: 10.1371/journal.pone.0005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Circelli P, Donini M, Villani ME, Benvenuto E, Marusic C. Efficient Agrobacterium-based transient expression system for the production of biopharmaceuticals in plants. Bioeng Bugs. 2010;1(3):221–224. doi: 10.4161/bbug.1.3.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueiredo JF, Romer P, Lahaye T, Graham JH, White FF, Jones JB. Agrobacterium-mediated transient expression in citrus leaves: a rapid tool for gene expression and functional gene assay. Plant Cell Rep. 2011;30(7):1339–1345. doi: 10.1007/s00299-011-1045-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Baek K, Park CM. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009;28(8):1159–1167. doi: 10.1007/s00299-009-0717-z. [DOI] [PubMed] [Google Scholar]
- 29.Krenek P, Samajova O, Luptovciak I, Doskocilova A, Komis G, Samaj J. Transient plant transformation mediated by Agrobacterium tumefaciens: principles, methods and applications. Biotechnol Adv. 2015;33(6 Pt 2):1024–1042. doi: 10.1016/j.biotechadv.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Li JF, Nebenfuhr A. FAST technique for Agrobacterium-mediated transient gene expression in seedlings of Arabidopsis and other plant species. Cold Spring Harb Protoc. 2010;2010(5):pdb prot5428. doi: 10.1101/pdb.prot5428. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, Liu G, Meng X, Li Y, Wang Y. A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem Genet. 2012;50(9–10):761–769. doi: 10.1007/s10528-012-9518-0. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer P, Pokorny J, Schulze-Lefert P, Dudler R. Technical advance. Double-stranded RNA interferes with gene function at the single-cell level in cereals. Plant J. 2000;24(6):895–903. doi: 10.1046/j.1365-313x.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 33.Andrieu A, Breitler JC, Sire C, Meynard D, Gantet P, Guiderdoni E. An in planta, Agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (Oryza sativa L.) leaves. Rice. 2012;5(1):23. doi: 10.1186/1939-8433-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kant R, Dasgupta I. Gene silencing approaches through virus-based vectors: speeding up functional genomics in monocots. Plant Mol Biol. 2019;100:3–18. doi: 10.1007/s11103-019-00854-6. [DOI] [PubMed] [Google Scholar]
- 35.Demirer GS, Zhang H, Matos JL, Goh NS, Cunningham FJ, Sung Y, Chang R, Aditham AJ, Chio L, Cho MJ, et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat Nanotechnol. 2019;4:456–464. doi: 10.1038/s41565-019-0382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somssich M. A short history of the CaMV 35S promoter. PeerJ PrePr. 2018;6(e27096v2):1–16. [Google Scholar]
- 37.Khatodia S, Bhatotia K, Passricha N, Khurana SM, Tuteja N. The CRISPR/Cas genome-editing tool: application in improvement of crops. Front Plant Sci. 2016;7:506. doi: 10.3389/fpls.2016.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doench JG, Hartenian E, Graham DB, Tothova Z, Hegde M, Smith I, Sullender M, Ebert BL, Xavier RJ, Root DE. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol. 2014;32(12):1262–1267. doi: 10.1038/nbt.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Z, Jayaraj J, Muthukrishnan S, Claflin L, Liang GH. Efficient genetic transformation of sorghum using a visual screening marker. Genome. 2005;48(2):321–333. doi: 10.1139/g04-095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Schematic presentations of the T-DNA regions of transformation constructs. Elements in each construct are drawn to scale. LB, left border of the T-DNA region; RB, right border of the T-DNA region; PTC, positive target control site for genome editing; fsGFP, frame-shifted GFP with PTC inserted after ATG start codon
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.