Short abstract
Objective
This study investigated the effect of recombinant human connective tissue growth factor (hCTGF) on rat adipose-derived stem cells (ADSCs) and explored the feasibility of using ADSCs to treat pelvic organ prolapse.
Methods
ADSCs were isolated from rat inguinal adipose tissue and characterized by flow cytometry and for osteogenic and adipogenic differentiation. ADSCs were treated with recombinant hCTGF and qRT-PCR was performed to detect collagen I and III expression on post-treatment days 7, 14, and 28. Osteogenic and adipogenic differentiation of ADSCs was performed to evaluate the effect of hCTGF. ADSCs were seeded in biological grafting materials, acellular porcine pericardium (APP) and acellular bovine pericardium (ABP), then implanted in the rat vagina. Histology was performed to observe inflammation among different groups.
Results
Collagen I and III expression in ADSCs was significantly increased, and the ability to differentiate into osteogenic and adipogenic lineages was diminished after hCTGF treatment. APP and ABP seeded with ADSCs significantly decreased inflammation and protected from degradation in vivo compared with APP and ABP only; ABP seeded with ADSCs had the lowest inflammation.
Conclusion
hCTGF regulates collagen I and III expression and induces ADSC differentiation in vitro. ADSCs decrease inflammation associated with APP and ABP in vivo.
Keywords: Adipose-derived stem cells (ADSCs), inflammation, acellular pericardium, hCTGF, collagen, pelvic organ prolapse, tissue grafts
Introduction
Pelvic organ prolapse (POP), also called urogenital prolapse, is the leading symptom for hysterectomy among postmenopausal women and the cause of 15% to 18% of procedures in women of all ages.1 POP refers to a descending of many organs, including the uterus, vagina, bladder, and gastrointestinal tract and considerably compromises a woman’s daily activities and quality of life. Many risk factors are included in the cause of POP, such as genetic predisposition, aging, obesity, pregnancy, and vaginal childbirth.2 However, the exact underlying pathophysiology of POP remains unknown. In 1996, Jackson et al.3,4 reported that collagen metabolism is an important etiologic factor in POP, and this conclusion has been subsequently supported. Collagen is a fibrous protein and the primary component of pelvic floor connective tissue, where it acts to maintain the tension and flexibility of pelvic floor connective tissue. Among the different types of collagen, types I and III are the principal determinants that provide strength to soft tissues. Collagen I is universally expressed and confers great resistance to tension, while collagen III is predominant in more flexible tissues, like the vagina.
For women suffering from POP, surgery is the primary treatment option and recurrence is common with ungrafted methods.5 Several complications such as foreign body reactions, excessive inflammatory responses, and vaginal erosion affect the use of synthetic mesh or biological grafts in clinical practice.6 Therefore, multiple treatment methods using stem cells for POP are rapidly emerging as potential strategies to rescue function. Until now, many studies have reported differences between various types of stem cells, including muscle-derived stem cells (MDSCs), endometrial mesenchymal stem cells (EMSCs), and adipose-derived stem cells (ADSCs), which showed promising safety and efficacy for POP in vitro and in vivo.7 ADSCs are multipotent stem cells found in adipose tissue. With advantages such as abundance and easy access compared with other stem cells, ADSCs have attracted interest as a potential treatment option for tissue engineering. Recently, with the progress of tissue engineering and intensive investigations into ADSCs, they have been used to treat various diseases, such as skin damage, cardiovascular disease, and stress-induced urinary incontinence.8–10 Wu et al.11 reported that human ADSCs reseeded on acellular bovine pericardium (ABP) can further enhance the properties of ABP and promote tissue regeneration at the recipient site, indicating that this is a promising treatment method for POP.
Cotreatment with additional trophic factors to cell-based tissue engineering therapy improves surgery results by enhancing regenerative processes. For example, estrogens maintain vaginal and pelvic floor supportive tissue by affecting collagen metabolism.12 Connective tissue growth factor (CTGF) is a member of the CCN family proteins, which are thought to regulate angiogenesis, cell proliferation, apoptosis, and fibrosis and contribute to a variety of protein depositions in the extracellular matrix (ECM).13 However, whether CTGF regulates collagen expression in ADSCs and plays a role in POP treatment with ADSCs remains unknown.
To clarify the effect of CTGF on ADSCs, we treated cultured rat ADSCs with recombinant human CTGF (hCTGF) and investigated its effect on collagen gene expression and the adipogenic and osteogenic differentiation ability of ADSCs. Additionally, previous studies have shown that both acellular porcine pericardium (APP) and ABP are good natural biomaterials for providing a scaffold that favors cell adherence. However, differences in the in vivo efficacy of APP and ABP with or without ADSCs remain unclear. Therefore, we reseeded cultured ADSCs on APP and ABP, then implanted rats with the different materials, including APP, APP with ADSCs, ABP, and ABP with ADSCs. Then, we analyzed inflammation levels at the implant site among all groups to investigate if ADSC–scaffold combinations were more effective for POP treatment.
Materials and methods
Animals
All protocols were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Guangzhou Medical University. Adult female Sprague-Dawley (SD) rats were purchased from the Laboratory Animal Center of Sun Yat-Sen University and housed in groups of two to three animals under specific pathogen-free conditions at 22°C with food and water ad libitum.
Isolation and culture of rat ADSCs
SD rats (12 weeks old) were sacrificed with 3% sodium pentobarbital. The bilateral inguinal fat pads were harvested and digested at 37°C with mild agitation for 1 hour using 0.1% collagenase type I (Sigma-Aldrich, St. Louis, MO, USA). Digestion was terminated by adding an equal volume of Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; GIBCO® Cell Culture, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; GIBCO® Cell Culture). Then, the suspension was centrifuged at 500 ×g for 5 minutes. Next, the cell pellet was resuspended and cultured in DMEM/F12 + 10% FBS + 1% penicillin/streptomycin (GIBCO® Cell Culture). Cultures were maintained at 37°C in a humidified incubator containing 5% CO2.
Flow cytometry analysis
ADSCs of passage 3 were harvested by incubation with 0.25% trypsin-EDTA (GIBCO® Cell Culture), washed twice with phosphate-buffered saline solution (PBS), and stained with phycoerythrin (PE)-conjugated mouse anti-rat CD34, CD44, CD45, CD105, and CD106 (BD Biosciences, San Jose, CA, USA) and fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat CD90 (BD Biosciences) for 30 minutes at room temperature. After washing twice with PBS, the cells were analyzed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
Differentiation
ADSCs of passage 3 were cultured in specific differentiation inducers for adipogenic and osteogenic differentiation. The culture medium was supplemented with dexamethasone (1 μM), insulin (10 μM), 1-methyl-3-isopropyl xanthine (0.5 mM), and indometacin (200 μM) for adipogenic differentiation, or dexamethasone (0.1 μM), β-glycerophosphate (10 mM), and vitamin C (50 mg/L) for osteogenic differentiation (Sigma-Aldrich). After a 14-day induction, verification of adipogenic and osteogenic differentiation were performed by Oil Red O, and Alizarin Red staining (Sigma-Aldrich), respectively.
Oil Red O Staining
Oil Red O staining was used to visualize lipid droplet in ADSCs that underwent adipogenic differentiation. Cells were fixed with 4% neutral buffered formalin for 3 hours at room temperature, washed with 60% isopropanol for 10 minutes, then completely air-dried. Cells were stained with a fresh 60% Oil Red O working solution for 30 minutes, washed four times with distilled water, and images were acquired under a light microscope for analysis.
Alizarin Red staining
Alizarin Red staining was performed to estimate mineralization in ADSCs that underwent osteogenic differentiation. Cells were fixed with 4% neutral buffered formalin for 30 minutes at room temperature, then washed with PBS. After washing, cells were incubated with Alizarin Red S for 15 minutes, washed with PBS, and observed under a light microscope.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from ADSCs using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocol. cDNA was reverse transcribed from 1 µg of RNA using the RevertAid First Strand cDNA Synthesis Kit (Fermentas Life Sciences, Glen Burnie, MD, USA). qRT-PCR was performed using the Fast SYBR Green Master Mix (Thermo Fisher Scientific) and specific primers on the Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocols. The primers used were as follows: β-actin: 5′-AGCCATGTACGTAGCCATCC-3′ (forward) and 5′-ACCCTCATAGATGGGCACAG-3′ (reverse); collagen type I: 5′-GGAGAGTACTGGATCGACCCTA-3′ (forward) and 5′-CTGACCTGTCTCCATGTTGCA-3′ (reverse); and collagen type III: 5′-AGGGAACAACTGATGGTGCTA-3′ (forward) and 5′-GGACTGCTGTGCCAAAATAAG-3′ (reverse). Relative expression values were calculated by the −ΔΔCt method using β-actin as the control.
Culturing ADSCs on APP and ABP
APP and ABP were generously provided by the Guanhao Biotech Corporation (Guangzhou, China). The materials were derived from normal porcine and bovine pericardial tissues. APP and ABP were cut into circular shapes with an area of 0.32 cm2 and washed with 25% alcohol for 5 minutes. After washing with PBS, APP and ABP were placed in 96-well plates with the serosal layer facing up. Then, ADSCs were added onto the APP and ABP at 1 × 106 cells/cm2. Cells were adhered for 48 hours at 37°C in a humidified incubator containing 5% CO2, and then unattached cells were removed with PBS. Using standard preparation methods, APP and ABP tissues seeded with ADSCs for 1 and 7 days were processed for scanning electron microscope (SEM) analysis (Hitachi S3400N, Tokyo, Japan).
Ectopic implantation of APP and ABP
Female adult SD rats were randomly separated into the following five groups (n = 4 per group): 1) “control group”, untreated; 2) “APP group”, treated with APP implantation; 3) “APP + ADSCs group”, treated with APP pre-seeded with ADSC implantation; 4) “ABP group”, treated with ABP implantation; and 5) “ABP + ADSCs group”, treated with ABP pre-seeded with ADSC implantation. All SD rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g body weight) and atropine (0.005 mg/100 g body weight). The bilateral ovaries were removed at the time of grafting, and APP or ABP were implanted in the vaginal mucosa with the serosal surface towards the dorsal side. Then, the engraftment was fixed with 4-0 silk sutures and the incisions were closed using 5-0 absorbable sutures.
Histological analysis
Thirty days after implantation, SD rats were sacrificed, and tissue samples of the vagina were harvested for histological analysis. Briefly, tissues were fixed in 10% formalin, embedded in paraffin, cut into 5-µm sections, which were processed by standard hematoxylin and eosin (H&E) staining. Finally, the number of inflammatory cells was counted.
Statistical analysis
All cell biology experiments were repeated at least three times independently, and data are shown as mean ± standard deviation. One-way and two-way analysis of variance (ANOVA) were used for multiple comparisons, followed by post-hoc comparisons with the Newman–Keuls test. All analyses were performed in SPSS 18.0 software (IBM Corp., Armonk, NY, USA). Differences were considered significant at p ≤ 0.05.
Results
Characterization of ADSCs
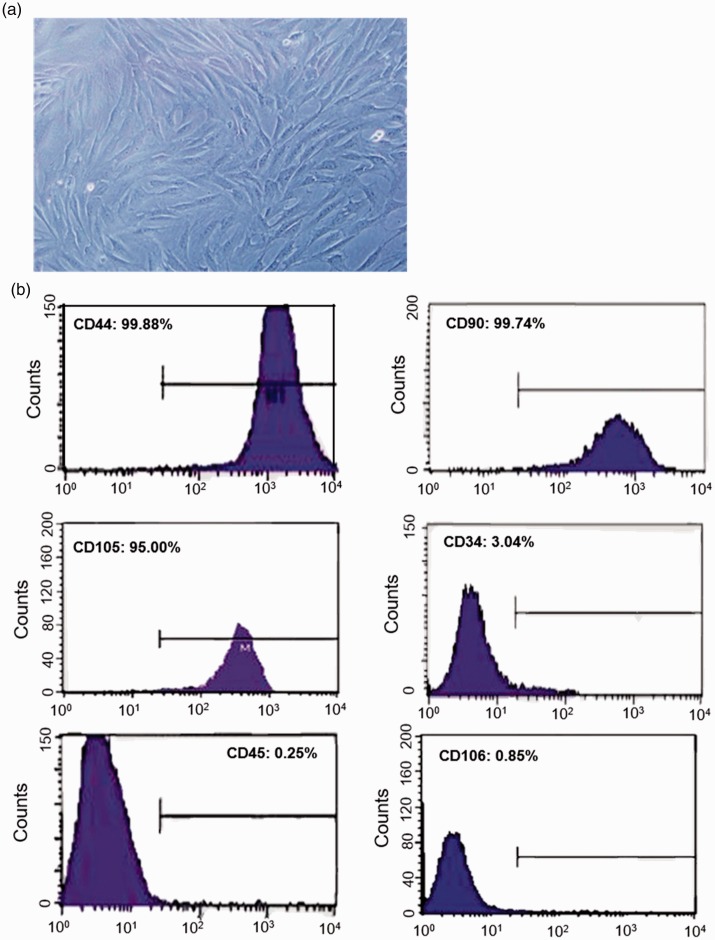
The isolated ADSCs were passaged twice, then cells at passage 3 were used for this study. Light microscopy of ADSCs demonstrated an elongated fibroblast-like morphology (Figure 1a). To characterize the cell populations, flow cytometry was performed to detect the representative surface marker expression profiles. The results showed that ADSCs were positive for the mesenchymal associated markers CD44, CD49d, CD90, and CD105; however, they were negative for CD34, CD45, and CD106 (Figure 1b).
Figure 1.
Identification of adipose-derived stem cells (ADSCs) by flow cytometry. (a) Representative light microscopy of ADSCs at passage 3. The cells show an elongated fibroblast-like morphology. (b) Flow cytometry analysis of ADSCs: as indicated, the cells were stained for the cell surface markers CD44, CD49d, CD90, CD105, CD34, CD45, and CD106.
Adipogenic and osteogenic differentiation of ADSCs
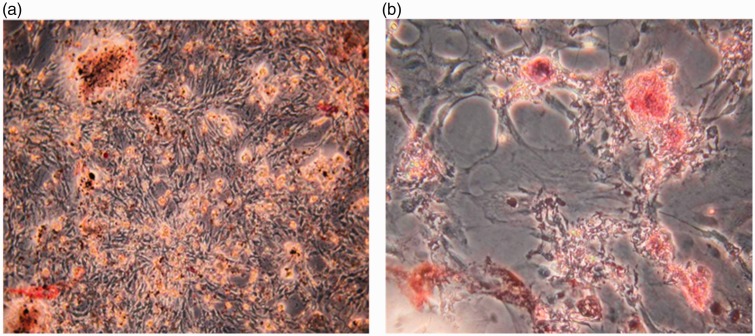
To determine the differentiation potential of isolated rat ADSCs at passage 3, the cells were induced towards adipogenic and osteogenic differentiation using specific methods. After a 14-day culture in adipogenic induction medium, many intracellular lipid-filled droplets were found in most of the ADSCs (Figure 2a). Additionally, Alizarin Red-S stained calcium deposits were found in ADSCs treated with osteogenic medium for 14 days (Figure 2b). These results demonstrated that the isolated cells could undergo adipogenic and osteogenic differentiation in vitro; thus, they were ADSCs according to the characterization results.
Figure 2.
Adipogenic and osteogenic differentiation of adipose-derived stem cells (ADSCs) in specific media. (a) Oil red O staining of ADSCs at passage 3 after incubation with adipogenic medium for 14 days. The accumulation of triglycerides stained by Oil Red O shows the adipogenic differentiation potential of ADSCs. (b) Alizarin Red S staining of ADSCs at passage 3 following treatment with osteogenic medium for 14 days. The calcium deposits indicated by Alizarin Red S staining show the osteogenic differentiation potential of ADSCs.
Effect of hCTGF on collagen gene expression and the differentiation capacity of ADSCs
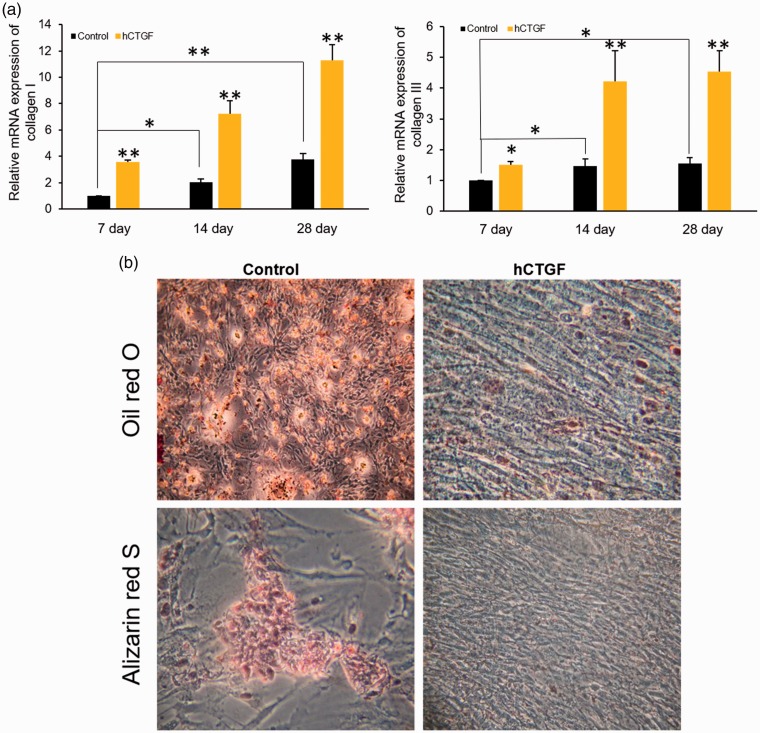
To clarify the effect of CTGF on ADSCs, the cells were treated with recombinant hCTGF at a dose of 100 ng/mL for 7, 14, and 28 days. Collagen I and III mRNA were measured to study the effect of hCTGF on collagen synthesis. Both collagen I and III gene expression levels were increased from day 7 to day 28, and hCTGF induced a further significant increase in collagen I and III mRNA expression after 7, 14, and 28 days compared with the control group (Figure 3a). Additionally, the ADSCs were induced towards adipogenic and osteogenic differentiation following treatment with or without hCTGF for 14 days. The results showed that hCTGF blocked the ability of ADSCs to undergo adipogenic and osteogenic differentiation (Figure 3b). Together, these results suggest that hCTGF can affect collagen synthesis and may induce ADSC differentiation in vitro.
Figure 3.
Human connective tissue growth factor (hCTGF) regulated the mRNA expression of collagen I and III and induced adipose-derived stem cell (ADSC) differentiation. (a) qRT-PCR analysis showed that collagen I and III mRNA levels were upregulated by hCTGF at 7, 14, and 28 days post-treatment (*P < 0.05, **P < 0.01, ANOVA). (b) Representative images of Oil red O staining and Alizarin Red S staining of ADSCs treated with or without hCTGF.
Effect of ADSCs on the biocompatibility of acellular pericardium materials
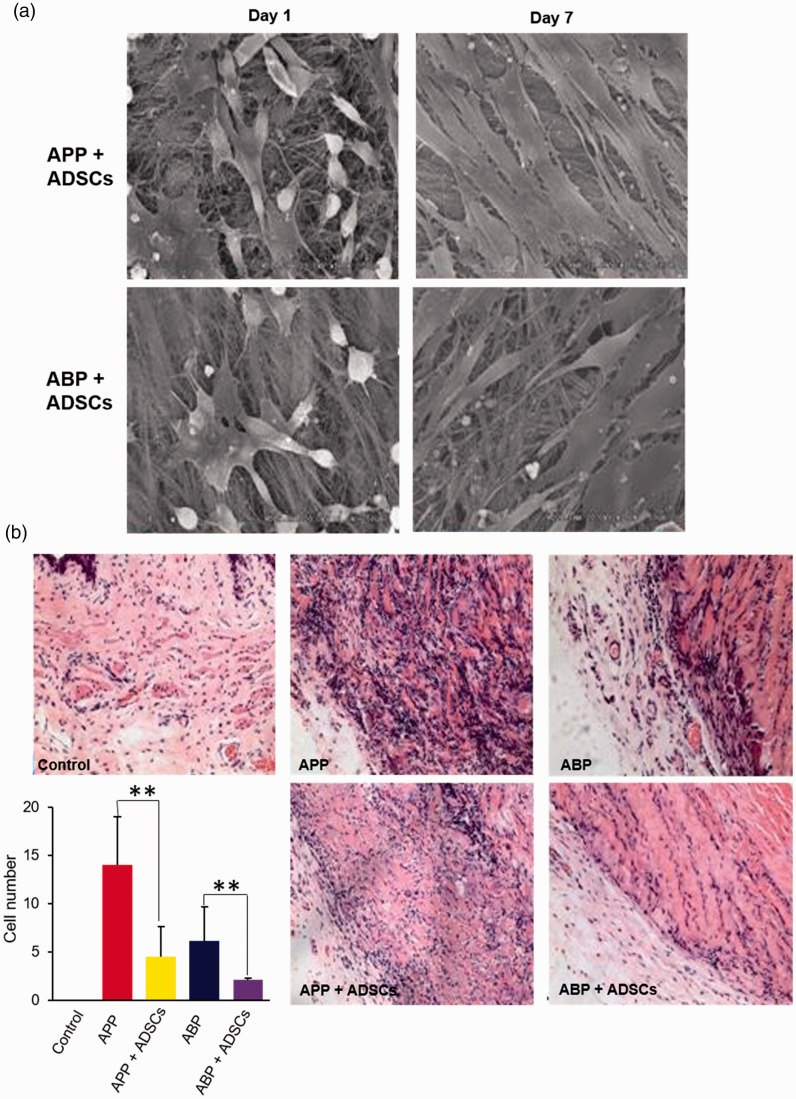
To observe the capacity of ADSCs seeded on APP and ABP in vitro, their morphology was analyzed using scanning electron microscopy 1 and 7 days post-culture. The results showed spindle-shaped adherent cells on APP and ABP 1 day after culture, and it was found that the ADSCs could survive and proliferate such that many ADSCs were found 7 days post in vitro culture. Additionally, cell morphology and viability were similar between the two groups (Figure 4a). To compare the in vivo biocompatibility of the two acellular pericardium models seeded with or without ADSCs, the gross morphology of implanted tissues was observed, and the number of inflammatory cells was counted. Gross examination of the explanted tissues showed that the level of APP degradation was higher than ABP; however, seeding ADSCs dramatically decreased the level of degradation in both pericardium materials. Additionally, a majority of inflammatory cells were found in vaginal tissues around the implanted pericardium. There were no inflammatory cells in the control group, and the number of inflammatory cells was highest in the APP group. Seeding ADSCs significantly decreased the number of inflammatory cells compared with the APP and ABP only group (APP vs. APP + ADSCs: p = 0.006; ABP vs. ABP + ADSCs: p = 0.009.). These data indicate that ADSCs can improve the biocompatibility of acellular pericardium materials by decreasing the infiltration of inflammatory cells.
Figure 4.
Adipose-derived stem cells (ADSCs) decreased the inflammation induced by APP and ABP implantation. (a) The growth of ADSCs on acellular porcine pericardium (APP) and acellular bovine pericardium (ABP) were analyzed by scanning electron microscopy. (b) The level of inflammation upon implantation was assessed by hematoxylin and eosin staining and counting inflammatory cells (**P < 0.01, ANOVA).
Discussion
The fibrillar collagen I and III are primary components of the vaginal and pelvic floor supportive tissues. Collagen I forms thick collagen fibers and provides continuous tension to the pelvic floor tissue. Collagen III mainly affects the flexibility and distension of tissue to overcome periodic stress.4 Recent studies have revealed that ADSCs differentiate into fibroblasts and promote collagen secretion of fibroblasts. Park et al.14 reported that the culture medium of ADSCs significantly increases collagen expression in skin tissue in vitro and that autologous ADSC implantation improves the patient’s skin quality and reduce wrinkles in vivo. Additionally, ADSCs develop into skin substitute material, which is not significantly different from the dermal layer, indicating that ADSCs are the fibroblasts needed to repair skin tissue.15 Among female pelvic floor dysfunctions, many clinical trials have started to repair urethra sphincter damage using periurethral injection of various types of stem cells, such as bone marrow-derived stem cells, muscle-derived stem cells, and ADSCs.8 It has been reported that vascular endothelial growth factor (VEGF) produced by ADSCs protects murine skin fibroblasts from ultraviolet radiation and improves the recovery of UV radiation-induced wounds.16 Compared with other stem cells, ADSCs have many advantages, including ease of collection, high abundance, and low immunogenicity. It has been reported that periurethral injection of ADSCs is a safe and effective treatment for stress-induced urinary incontinence.17 However, the effect of ADSCs on the repair of pelvic floor defects has not been reported.
CTGF is universally expressed in many tissues and organs and has a number of biological functions, including cell proliferation, apoptosis, adhesion, and chemotaxis. hCTGF induces the differentiation of bone marrow mesenchymal stem cells into fibroblasts and increases the mRNA expression of collagen I and III.18 Additionally, another group has reported that recombinant hCTGF induces the differentiation of MSCs and promotes the synthesis of collagen I and III in vitro.19 Similar to their results, our data demonstrated that recombinant hCTGF significantly increased mRNA expression of collagen I and III in ADSCs. Furthermore, recombinant hCTGF also induced the differentiation of ADSCs in vitro because it diminished the capacity of ADSCs for adipogenic and osteogenic differentiation. However, whether recombinant hCTGF induces ADSCs into fibroblasts should be further investigated. Lee et al.18 reported that mesenchymal stem cells express fibroblastic markers such as collagen I and III in the presence of CTGF. Meanwhile, it has also been reported that CTGF-induced fibroblastic differentiation of ADSCs is modulated by simulated microgravity.20
Surgical repair of prolapse is the primary treatment method for severe POP patients and refers to the ‘Three Rs principle,’ which means Retain, Repair, and Replace. Reconstructive surgical materials consist of biologic and synthetic graft materials. Compared with synthetic graft materials, biomaterials have a higher likelihood of biocompatibility. It has been reported that biomaterials undergo long-term degradation by the foreign body response, leading to compromised biomechanical properties and unacceptable surgical failure rates.21 Previous studies have shown that a biodegradable scaffold made from Poly-L-lactic acid showed good acute integration into host tissues; however, an acute macrophage response was found in the ADSC-containing and cell-free scaffolds.22 Evidence of a foreign body reaction was also found in patients who underwent suburethral sling surgery using cross-linked porcine dermis grafts, and the implants appeared to be completely replaced by dense fibroconnective tissue, indicating that the high rate of surgical failure is related to graft degradation.23 In this study, the biocompatibility of ABP was better than that of APP in the rat vagina. Additionally, we also found that ADSCs could adhere and grow in both APP and ABP and that there was no significant difference between these two materials, suggesting that both could be used as a scaffold for ADSCs. Recent studies have shown that embryonic stem cells and mesenchymal stem cells have immunosuppressive effects.24 After seeding ADSCs in APP and ABP, inflammation levels in the rat vagina were significantly decreased compared with the APP and ABP groups. These results revealed that ADSCs could inhibit the immune reaction near the implantation site. However, the immunosuppressive mechanism induced by ADSCs will require further study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xiaoyu Wang https://orcid.org/0000-0003-0335-3322
References
- 1.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet 2007; 369: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 2.Schaffer JI, Wai CY, Boreham MK. Etiology of pelvic organ prolapse. Clin Obstet Gynecol 2005; 48: 639–647. [DOI] [PubMed] [Google Scholar]
- 3.Karin L, Lince SL, Spath MA, et al. Pelvic organ prolapse and collagen-associated disorders. Int Urogynecol J 2012; 23: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerkhof MH, Hendriks L, Brölmann HA. Changes in connective tissue in patients with pelvic organ prolapse–a review of the current literature. Int Urogynecol J 2009; 20: 461–474. [DOI] [PubMed] [Google Scholar]
- 5.Aponte MM, Rosenblum N. Repair of pelvic organ prolapse: what is the goal? Curr Urol Rep 2014; 15: 385. [DOI] [PubMed] [Google Scholar]
- 6.Dällenbach P. To mesh or not to mesh: a review of pelvic organ reconstructive surgery. Int J Womens Health 2015; 7: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emmerson SJ, Gargett CE. Endometrial mesenchymal stem cells as a cell based therapy for pelvic organ prolapse. World J Stem Cells 2016; 8: 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staack A, Rodríguez LV. Stem cells for the treatment of urinary incontinence. Curr Urol Rep 2011; 12: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg 2009; 62: 317–321. [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Ruiz R, Santos MEF, Muñoa MD, et al. Adipose tissue-derived stem cells: the friendly side of a classic cardiovascular foe. J Cardiovasc Transl Res 2008; 1: 55–63. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Dai M, Xu P, et al. In vivo effects of human adipose-derived stem cells reseeding on acellular bovine pericardium in nude mice. Exp Biol Med (Maywood) 2016; 241: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moalli PA, Debes KM, Meyn LA, et al. Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. Am J Obstet Gynecol 2008; 199: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heng ECK, Huang Y, Black SA, Jr, et al. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and α6- and β1 integrins. J Cell Biochem 2006; 98: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park B, Jang KJ, Park J, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 2010; 34: 1323–1326. [DOI] [PubMed] [Google Scholar]
- 15.Trottier V, Marceau-Fortier G, Germain L, et al. IFATS collection: using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2010; 26: 2713–2723. [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Wang Y, Xia Y, et al. Overexpressed Vascular Endothelial Growth Factor (VEGF) in Adipose Derived Stem Cells (ADSCs) attenuates fibroblasts and skin injuries by ultraviolet radiation. Biosci Rep 2019; 39: pii: BSR20190433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokunori Y, Momokazu G, Ryohei H, et al. Periurethral injection of autologous adipose-derived stem cells for the treatment of stress urinary incontinence in patients undergoing radical prostatectomy: report of two initial cases. J Urol 2010; 183: 75–82. [DOI] [PubMed] [Google Scholar]
- 18.Chang HL, Bhranti S, Moioli EK, et al. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 2010; 120: 3340–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong Z, Sant S, Khademhosseini A, et al. Controlling the fibroblastic differentiation of mesenchymal stem cells via the combination of fibrous scaffolds and connective tissue growth factor. Tissue Eng Part A 2011; 17: 2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebnerasuly F, Hajebrahimi Z, Tabaie SM, et al. Effect of simulated microgravity conditions on differentiation of adipose derived stem cells towards fibroblasts using connective tissue growth factor. Iran J Biotechnol 2017; 15: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce LM, Grunlan MA, Hou Y, et al. Biomechanical properties of synthetic and biologic graft materials following long-term implantation in the rabbit abdomen and vagina. Am J Obstet Gynecol 2009; 200: 541–549. [DOI] [PubMed] [Google Scholar]
- 22.Roman Regueros S, Albersen M, Manodoro S, et al. Acute in vivo response to an alternative implant for urogynecology. Biomed Res Int 2014; 2014: 853610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi S, Kubba LM, Abramov Y, et al. Histopathologic changes of porcine dermis xenografts for transvaginal suburethral slings. Am J Obstet Gynecol 2005; 192: 1643–1648. [DOI] [PubMed] [Google Scholar]
- 24.Han KH, Kang HG, Gil HJ, et al. The immunosuppressive effect of embryonic stem cells and mesenchymal stem cells on both primary and secondary alloimmune responses. Transpl Immunol 2010; 23: 141–146. [DOI] [PubMed] [Google Scholar]