Short abstract
Solitary fibrous tumors/hemangiopericytomas (SFTs/HPCs) are rare in the central nervous system and intraventricular SFTs/HPCs are even rarer. We present a clinical case of SFT/HPC that was located in the fourth ventricle and performed a literature review of radiological findings of SFT/HPC. The patient was a 52-year-old man who presented to our hospital with dizziness and progressive weakness in his left extremities. Computed tomography and magnetic resonance imaging showed an irregular-shaped mass in the fourth ventricle. The upper anterior and lower posterior parts of the mass showed different densities and signal intensities, which formed a black and white sign. The upper anterior and lower posterior parts of the mass were enhanced to different degrees, and showed heterogeneous reversed enhancement. The patient underwent surgical resection and the mass was histologically confirmed to be an SFT/HPC. SFTs/HPCs of the fourth ventricle are rare, but show characteristic radiological presentations. In the present case, we observed a solid mass with flow voids, black and white sign, and heterogeneously reversed enhancement in the fourth ventricle. Collectively, these radiological features suggested the diagnosis of SFT/HPC.
Keywords: Solitary fibrous tumor/hemangiopericytoma, computed tomography, magnetic resonance imaging, fourth ventricle, flow void, neoplasm, black and white sign
Introduction
Solitary fibrous tumors/hemangiopericytomas (SFTs/HPCs) are an uncommon spindle cell neoplasm of mesenchymal origin. SFTs/HPCs were first described in the pleura and have since been reported in soft tissues and many other organs.1–6 SFTs/HPCs in the central nervous system are rare and intraventricular SFTs/HPCs are even rarer. Most SFTs/HPCs have benign clinical presentations, although some SFTs/HPCs are malignant. We report a new case of SFT/HPC that was located in the fourth ventricle, and present the clinical, radiological, and histopathological characteristics of the case. We also performed a literature review of radiological findings of SFT/HPC. The findings in this case report should facilitate the diagnosis and differential diagnosis of SFTs/HPCs.
Case report
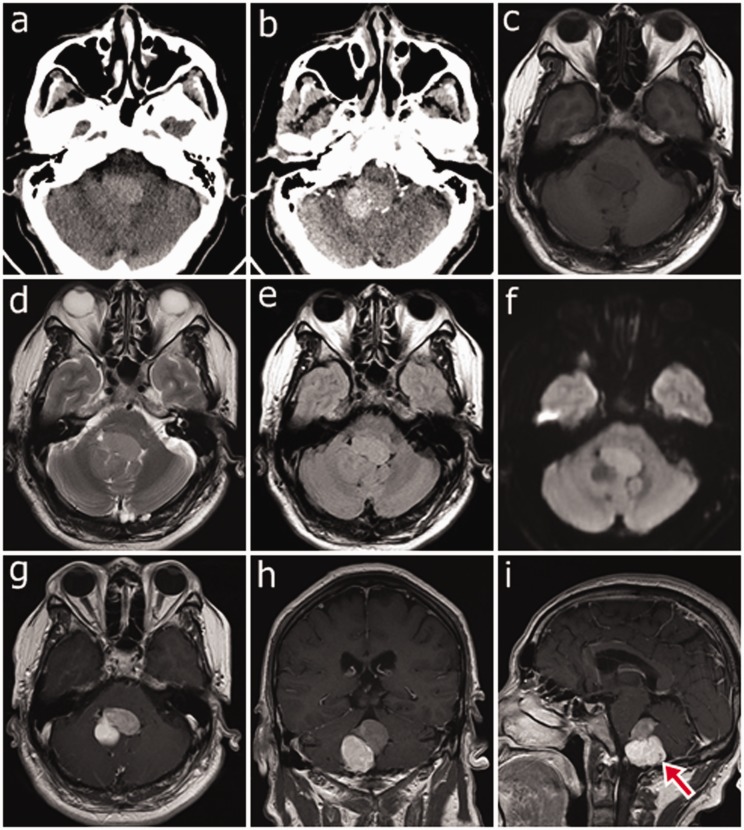
A 52-year-old man presented to our hospital with a 1-month history of dizziness without obvious cause and he had progressive weakness in his left extremities over the past 10 days. The patient had no movement disorder. Unenhanced axial computed tomography (CT) showed an irregular mass in the fourth ventricle. The upper anterior part of the mass was slightly hyperdense and the lower posterior part was isodense (Figure 1a). However, axial contrast-enhanced CT showed that the lower posterior part of the mass had more enhancement than did the upper anterior part (Figure 1b). Axial magnetic resonance imaging (MRI) T1-weighted imaging (T1WI), and fluid-attenuated inversion recovery showed an isointense mass in the fourth ventricle (Figure 1c, e). Axial MRI T2WI and diffusion-weighted imaging (DWI) showed slight hyperintensity in the upper anterior part and slight hypointensity in the lower posterior part of the mass, which formed a black and white sign (Figure 1d, f). After administration of gadolinium, the lower posterior part of the mass had more enhancement than did the upper anterior part, and formed heterogeneous reversed enhancement (Figure 1g–i). The mass was 2.1 × 3.3 × 3.1 cm. A flow void effect was detected at the lower posterior part of the mass in sagittal T1WI-enhanced MRI (Figure 1i), without any signs of necrosis, cystic degeneration, hemorrhage, or calcification. There was a clear dividing line between the mass and normal brain tissue. The brainstem and right cerebellar hemisphere were compressed, but no hydrocephalus was detected.
Figure 1.
(a) Unenhanced axial computed tomography showing an irregular mass in the fourth ventricle. The upper anterior part of the tumor shows slight hyperdensity, while the lower posterior part shows isodensity. (b) Contrast-enhanced axial computed tomography showing that the lower posterior part of the tumor has more pronounced enhancement than the upper anterior part. (c) Axial T1-weighted image showing an isointense mass in the fourth ventricle. (d) and (f) Axial T2-weighted image and diffusion-weighted image showing that the upper anterior part of the mass is hyperintense while the lower posterior part is hypointense, forming a black and white sign. (e) The mass is isointense on fluid-attenuated inversion recovery. (g)–(i) After gadolinium administration, the lower posterior part of the tumor has more enhancement than does the upper anterior part, forming reversed enhancement. (i) Contrast-enhanced T1-weighted sagittal image showing a flow void effect (red arrow) in the lower posterior part of the mass.
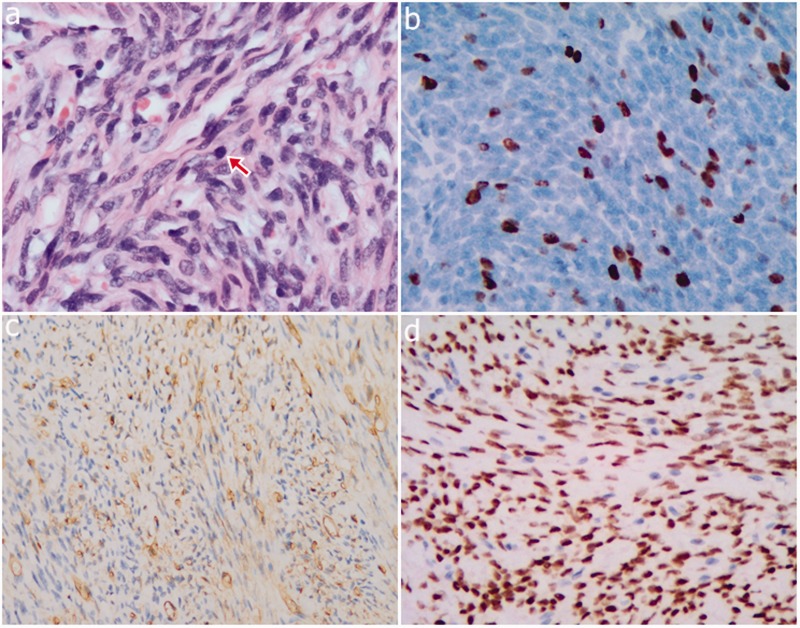
An operation was performed, and a firm, well-circumscribed fourth ventricular tumor was found with brainstem compression. The entire tumor was successfully removed. Part of the tumor appeared as a grayish-white firm mass with an abundant blood supply. Another part of the tumor had a dark red, jelly-like appearance, with a tender texture. A histopathological examination showed that the tumor was composed of spindle cells, collagen matrix, and dilated blood vessels (Figure 2a). Additionally, hypercellular areas alternating with hypocellular areas were detected. No obvious tumor necrosis was observed. Mitotic figures were observed (>5 mitoses/10 HPF) and the molecular immunology borstel-1 proliferation index was 15% (Figure 2b). Neoplastic cells were strongly positive for CD34 (Figure 2c), vimentin, and nuclear STAT6 (Figure 2d). No immunoreactivity with Bcl-2, p53, or epithelial membrane antigen was detected. These pathological features met the diagnosis for malignant SFT/HPC (World Health Organization [WHO] G3) with reference to WHO 2016.7 This patient was well postoperatively. He was discharged and discharged home in a good condition on postoperative week 4. He had received routine radiotherapy.
Figure 2.
Photomicrographs. (a) The tumor is composed of atypical spindle cells and a collagenized stroma, and mitotic figures can be seen (magnification = × 400, red arrow). (b) The molecular immunology borstel-1 proliferation index was 15% (magnification = × 400). (c) Neoplastic cells are strongly immunoreactive to CD34 (magnification = × 200). (d) Neoplastic cells are strongly immunoreactive to nuclear STAT6 (magnification = × 200).
This was a retrospective case report. Therefore, the need for approval of the study protocol was waived. We obtained verbal informed consent for publication from the patient.
Discussion
SFT/HPC is a rare spindle cell soft tissue neoplasm, and was not described pathologically until 1931.1 Since the first report of an SFT/HPC of the meninges in 1996 by Carneiro et al.,2 SFTs/HPCs of the central nervous system continue to be reported.3 SFTs/HPCs originate from CD34-positive dendritic interstitial cells and have the ability to differentiate into fibroblast and myofibroblast cells.3 SFTs/HPCs have a varying degree of differentiation and most SFTs/HPCs are benign histologically. However, predicting the malignant potential of SFTs/HPCs remains challenging. SFTs/HPCs with malignant features, such as local recurrence, invasive growth, or distal metastasis, have been reported in recent years.4,5,8,9
Intraventricular SFTs/HPCs are rare. Only seven cases of intraventricular SFTs/HPCs have been reported in the fourth ventricle3,10–16 and their radiological features varied (Table 1). To date, malignant SFTs/HPCs in the fourth ventricle have not been reported. The pathological and morphological features of SFTs/HPCs are not necessarily correlated with malignant biological behavior. However, according to WHO soft tissue tumor classification criteria, several histomorphological features are useful for identifying malignant SFTs/HPCs. These features include hypercellularity, cytological atypia, necrosis, high mitotic figures, and/or infiltrative margins.17 The present case showed a typical appearance of alternating hypocellular and hypercellular areas. The hypercellular areas had a large amount of blood vessels, while the hypocellular areas had bands of collagen. Additionally, neoplastic cells showed a high proliferation rate with considerable cytological atypia. These features met the diagnostic criteria for malignant SFTs/HPCs.
Table 1.
Summary of clinical and radiological features of solitary fibrous tumors/hemangiopericytomas reported in the literature.
| Reference | Age(years)/sex | Location | Size (greatest dimension, cm) | MRI findings | Peritumoral infiltration | Degree of resection | Adjuvant therapy | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Clarencon et al.11(2006) | 32/F | Fourth ventricle | 2.5 | Hypointense, T1; heterogeneous-hypointense, T2; post-contrast enhancement | NA | NA | NA | NA |
| Gessi et al.12(2006) | 63/F | Fourth ventricle | 2.0 | Isointense, T1; partial post-contrast enhancement | Without infiltration | NA | NA | NA |
| Kim et al.13(2004) | 49/F | Fourth ventricle | NA | Dense post-contrast enhancement | NA | Subtotally | NA | No recurrence after 1 year |
| Montano et al.14(2010) | 61/M | Fourth ventricle | NA | Hypointense, T1; iso-hypointense, T2; marked post-contrast enhancement | Without infiltration of ventricular walls | Totally | NA | No recurrence after 2 years |
| Sawauchi et al.15(2003) | 57/M | Fourth ventricle | NA | Low intensity, T1; homogeneous post-contrast enhancement | NA | NA | NA | NA |
| Cummings et al.16(2001) | 52/M | Fourth ventricle | NA | Homogeneous post-contrast enhancement | NA | NA | NA | NA |
| Wang et al.17(2012) | 52/M | Fourth ventricle | 4.5 | Avid homogeneous post-contrast enhancement | NA | Totally | NA | No recurrence after 1 year |
F = female; M = male; MRI = magnetic resonance imaging; NA = not available
In the present case, the upper anterior part of the tumor was slightly hyperdense on unenhanced CT and slightly hyperintense on T2WI and DWI. However, the lower posterior part of the tumor was isodense on unenhanced CT and slightly hypointense on T2WI and DWI. This radiological feature is known as black and white sign, which may be explained by different tissue composition within the tumor. Hypercellular areas of the tumor showed slight hyperdensity on unenhanced CT and slight hyperintensity on T2WI. Additionally, because of hypercellularity, diffusion of water molecules was restricted, which may lead to hyperintensity on DWI. In contrast, hypocellular areas with abundant collagenous stroma showed isodensity on unenhanced CT and slight hypointensity on T2WI. Collagenous fibers are distributed in compartments, with lower water content, resulting in unrestricted diffusion of water molecules. This may collectively lead to slight hypointensity on DWI. As a result, SFT/HPC shows the characteristic black and white sign on unenhanced CT, and on MRI T2WI and DWI.3,12 In our case, after contrast administration, the lower posterior part of the tumor had marked enhancement compared with the upper anterior part on CT, as well as on MRI. Therefore, reversed enhancement was observed. This phenomenon might be explained by progressive enhancement of collagen fibers.6 Another possible explanation is that the degree of destruction of the blood–brain barrier was inconsistent in the tumor because of intratumoral heterogeneity.
Intraventricular SFT/HPC needs to be differentiated from other tumors. An example of this problem is that intraventricular fibrous meningioma, which mainly consists of fibroblast-like cells and collagen fibers with low free water content, has similar radiological features as SFTs/HPCs. However, intraventricular fibrous meningioma is usually found in the lateral ventricle and is isointense on T2WI.11 Additionally, dural tail sign is common in intraventricular fibrous meningioma, but it does not show black and white sign or reversed enhancement. Ependymoma, which arises from the ependyma, is usually located in the third and fourth ventricles. Ependymoma usually has a heterogeneous texture due to calcification, hemorrhage, and cystic degeneration. This feature can be used to differentiate ependymoma from SFT/HPC. Medulloblastoma is a common brain tumor in children, and is usually located in the cerebellar vermis and grows forward to the fourth ventricle. Cystic degeneration, calcification, and hemorrhage are uncommon in medulloblastoma. Additionally, medulloblastoma can spread through the cerebrospinal fluid and metastasize to different locations.
Surgical resection is the preferred treatment strategy for SFT/HPC and it should have a good prognosis when gross total resections are performed. The curative effect of routine radiotherapy is not definitive. Regular follow-ups are mandatory for all cases of SFT/HPC.
Conclusion
We report a rare case of SFT/HPC in an unusual location. Although this condition is rare, a diagnosis of SFT/HPC should be considered with the following radiological features: a solid mass with flow voids, black and white sign on unenhanced images, reversed enhancement after contrast administration, and the absence of hemorrhage, calcification, or cystic degeneration. In conclusion, SFT/HPC should be included in the differential diagnosis of fourth ventricular tumors in adults.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006; 13: 264–269. DOI: 10.1177/107327480601300403. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro SS, Scheithauer BW, Nascimento AG, et al. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol 1996; 106: 217–224. [DOI] [PubMed] [Google Scholar]
- 3.Clarencon F, Bonneville F, Rousseau A, et al. Intracranial solitary fibrous tumor: imaging findings. Eur J Radiol 2011; 80: 387–394. DOI: 10.1016/j.ejrad.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Cheung F, Talanki VR, Liu J, et al. Metachronous malignant solitary fibrous tumor of kidney: case report and review of literature. Urol Case Rep 2016; 4: 45–47. DOI: 10.1016/j.eucr.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakob M, Schneider M, Hoeller I, et al. Malignant solitary fibrous tumor involving the liver. World J Gastroenterol 2013; 19: 3354–3357. DOI: 10.3748/wjg.v19.i21.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang WD, Chen JY, Cao Y, et al. Computed tomography and magnetic resonance imaging findings of solitary fibrous tumors in the pelvis: correlation with histopathological findings. Eur J Radiol 2011; 78: 65–70. DOI: 10.1016/j.ejrad.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 7. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. DOI: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 8.Law MK, Tung YW, Jinc JS. Malignant transformation in solitary fibrous tumor of the pleura. Asian Cardiovasc Thorac Ann 2014; 22: 981–983. DOI: 10.1177/0218492313498090. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki H, Kurihara T, Katsuoka Y, et al. Distant metastasis from benign solitary fibrous tumor of the kidney. Case Rep Nephrol Urol 2013; 3: 1–8. DOI: 10.1159/000346850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarencon F, Bonneville F, Sichez JP, et al. Atypical location of a solitary fibrous tumor in the fourth ventricle. J Neuroradiol 2006; 33: 279–280. [DOI] [PubMed] [Google Scholar]
- 11.Gessi M, Lauretti L, Fernandez E, et al. Intraventricular solitary fibrous tumor: a rare location for a rare tumor. J Neurooncol 2006; 80: 109–110. DOI: 10.1007/s11060-006-9154-9. [DOI] [PubMed] [Google Scholar]
- 12.Kim KA, Gonzalez I, McComb JG, et al. Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 2004; 54: 1004–1009; discussion 1009. [DOI] [PubMed] [Google Scholar]
- 13.Montano N, Doglietto F, Lauriola L, et al. Solitary fibrous tumour of the IV ventricle. Br J Neurosurg 2010; 24: 495–496. DOI: 10.3109/02688691003675226. [DOI] [PubMed] [Google Scholar]
- 14.Sawauchi S, Arakawa H, Taya K, et al. Solitary fibrous tumor of the fourth ventricle: case report. No Shinkei Geka 2003; 31: 551–555. [PubMed] [Google Scholar]
- 15.Cummings TJ, Burchette JL, McLendon RE. CD34 and dural fibroblasts: the relationship to solitary fibrous tumor and meningioma. Acta Neuropathol 2001; 102: 349–354. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Manucha V, Faro S, et al. Fourth ventricular solitary fibrous tumor: a case report and review of the literature. J Med Case Rep 2012; 6: 205. DOI: 10.1186/1752-1947-6-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology 2014; 64: 2–11. DOI: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]