Short abstract
Objective
The aim of this study was to compare clinical outcomes between patients with carbapenem-resistant Acinetobacter baumannii (CRAB) bacteremia treated with colistin monotherapy and those treated with colistin plus meropenem.
Methods
We retrospectively evaluated data from 71 patients with CRAB bacteremia treated from November 2006 to February 2018. Predictors of 14-day mortality were determined through logistic regression analysis.
Results
Our study cohort included 40 bacteremia patients (44.6 %) treated with colistin monotherapy and 31 (55.4 %) treated with colistin plus meropenem. Overall 14-day mortality tended to be higher with monotherapy rather than combination therapy (47.5% vs 25.8%). The latter also showed a tendency for higher clinical success rate compared with monotherapy (61.3% vs 40.0%). Logistic regression analysis showed that Pitt bacteremia score, pneumonia, and combination therapy were significantly associated with mortality. In patients with higher Pitt bacteremia score (≥4), mortality was significantly higher with monotherapy compared with combination therapy (66.7% vs 27.8%). In patients with lower Pitt bacteremia score (≤3), mortality was similar between the two treatment groups (26.3% vs 23.1%).
Conclusion
Treatment with colistin plus meropenem improves survival in critically-ill patients with CRAB.
Keywords: Bacteremia, Acinetobacter baumannii, colistin, meropenem, outcome, logistic regression
Introduction
Acinetobacter baumannii is a ubiquitous type of bacteria that inhabits water and soil.1 The incidence of A. baumannii bacteremia is increasing, and is associated with a high mortality rate.2–5 Broad-spectrum antibiotics such as carbapenems are frequently used to treat serious infections caused by A. baumannii.6,7 However, carbapenem-resistant A. baumannii (CRAB) infection is increasing in prevalence and is associated with increased mortality, length of hospital stay, and health-care costs.5–10
Although colistin is widely used to treat CRAB infections, its efficacy remains unclear,11–14 as does its use in combination therapy with other antibiotics, especially carbapenems.15 Some in vitro studies have demonstrated a synergistic relationship between colistin and carbapenem against A. baumannii,16,17 and combination therapy has been reported to be superior to monotherapy in some clinical studies.18 Although one meta-analysis showed that combination therapy resulted in better outcomes when high-dose colistin was used,15 combination therapy has not been shown to be superior to monotherapy for a specific type of infection or clinical setting.19 To the best of our knowledge, no study to date has evaluated the combination of colistin and meropenem for treating CRAB bacteremia in Korea. Although CRAB bacteremia can result in detrimental clinical outcomes, there is little evidence of the effectiveness of combination therapy according to disease severity.
In this study, we compared clinical outcomes of patients with CRAB bacteremia treated with colistin monotherapy and colistin plus meropenem. We also analyzed the impact of combination therapy on mortality according to the Pitt bacteremia score, an established tool for assessing disease severity based on mental status, body temperature, blood pressure, mechanical ventilation, and cardiac status20.
Methods
Design, setting, and ethics
We conducted a single-center retrospective cohort study in patients aged ≥18 years with documented cases of CRAB bacteremia between November 2006 and February 2018. Blood culture reports from a university hospital with approximately 750 beds located in Seoul, Korea were reviewed. For patients with multiple episodes of CRAB bacteremia, only the first episode was included in the analysis to avoid multiple entries from a single patient. Cases were sorted into bacteremia treated with colistin monotherapy and bacteremia treated with colistin plus meropenem.
The primary infection source of bacteremia was defined according to the criteria of the Centers for Disease Control and Prevention.21 Co-morbidities were evaluated according to the Charlson Comorbidity Index (CCI)22 using clinical data. The Pitt bacteremia score was evaluated for each patient in the 24-hour period prior to collection of the first positive blood culture.20 The primary outcome was 14-day all-cause mortality, which was also evaluated according to the Pitt bacteremia score. The secondary outcome was clinical success evaluated at 14 days from the onset of infection and defined as improvement or resolution of symptoms including and other signs of infection.23
Steroid/immunosuppressant use was defined as corticosteroid >20 mg/d, prednisone, or the equivalent for >14 days within 4 weeks prior to the onset of bacteremia; or the use of other immunosuppressive medications for organ transplant, malignancy, or autoimmune disorders within the 6 weeks prior to bacteremia. Recent surgery was defined as surgery performed within 4 weeks prior to the onset of bacteremia. This study was approved by the institutional review board at Kangdong Sacred Heart Hospital (reference no. 2018-03-012).
Microbiology
Patients provided 20-mL blood specimens that were divided into 10-mL aliquots in Bactec-plus aerobic/F and anaerobic/F bottles (BD Diagnostic Systems, Franklin Lakes, NJ, USA) and immediately subjected to microbiological testing. The specimens were incubated in a Bactec Fx instrument (BD Diagnostic Systems) and bacterial organisms isolated from positive blood cultures were examined using conventional overnight identification/sensitivity panels with a MicroScan WalkAway system (Siemens, West Sacramento, CA, USA). Tested antibiotic classes included ampicillin/sulbactam, aztreonam, cephalosporins (three third-generation and one fourth-generation), aminoglycosides (gentamicin and tobramycin), quinolones (ciprofloxacin and levofloxacin), carbapenems (imipenem and meropenem), tetracycline, and trimethoprim/sulfamethoxazole. Isolates were considered carbapenem-resistant if they were resistant to imipenem or meropenem.
Data collection
Clinical data from patients with CRAB bacteremia were retrieved from medical records and included initial baseline characteristics such as age, sex, vital signs, underlying diseases, medications, and the primary focus of infection. Clinical data on antimicrobial therapy and mortality were also collected over the entire clinical course.
Statistical analyses
Continuous variables were compared using Student’s t-test, and Pearson’s chi-squared test or Fisher’s exact test were used to compare discrete variables. Kaplan–Meier curves were prepared for the time to mortality, and statistical intergroup differences were examined using the log-rank test.
Logistic regression models were used to identify risk factors for 14-day mortality with baseline clinical characteristics and antibiotic therapy used as variables. Variables with a P-value < 0.20 in the univariate analysis and those considered clinically relevant were eligible for inclusion in the logistic regression model during multivariate analysis. A backward elimination process was used. All significance testing was two-tailed, and values of p < 0.05 were considered statistically significant. All analyses were performed using SPSS for Windows (version 24.0; IBM Corp., Armonk, NY, USA).
Results
Seventy-one cases of CRAB bacteremia were identified from 286 cases of A. baumannii bacteremia during the study period. All cases were nosocomial infections (onset >48 hours after admission), and there were no duplicate cases.
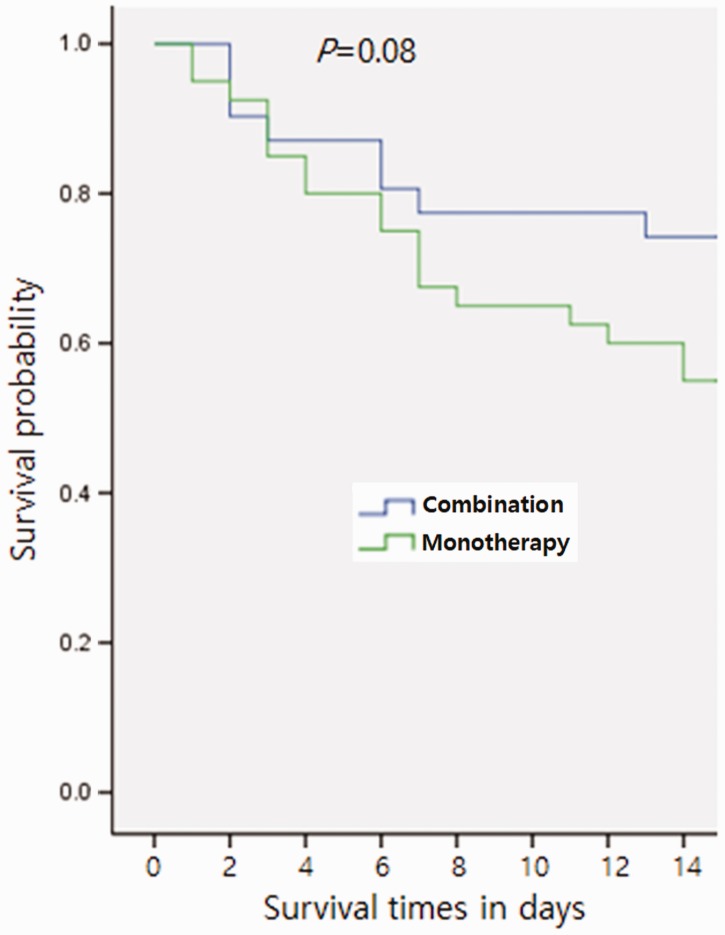
There were 40 cases (56.3 %) of bacteremia treated with colistin monotherapy and 31 cases (43.7%) of bacteremia treated with colistin plus meropenem. Patients received colistin 150 mg every 12 hours with dose adjusted according to renal function. The monotherapy group received colistin for 8.88 ± 4.74 days (mean ± standard deviation, SD). The combination therapy group received additional meropenem 1 g every 8 hours and the dose was also adjusted according to renal function. The combination therapy group was treated for 8.22 ± 4.26 days (mean±SD). Demographic and clinical characteristics are shown in Table 1. There was no significant difference between the groups in any of the evaluated variables, including the Pitt bacteremia score. Steroid and/or immunosuppressant use was reported in 5 of 40 (12.5 %) patients in the colistin monotherapy group and 8 of 31 patients (25.8 %) in the combination therapy group. Sites of recent surgery were central nervous system (11.3%), abdomen (8.5%), bone and joint (1.4%), chest (4.2%), and head and neck (4.2%). Mortality within 14 days of the onset of bacteremia occurred in 19 of 40 (47.5%) patients in the colistin monotherapy group and 8 of 31 (25.8 %) patients in the combination therapy group (Figure 1). The univariate analysis for 14-day mortality is shown in Table 2.
Table 1.
Demographic and clinical characteristics of patients with carbapenem-resistant Acinetobacter baumannii (CRAB) bacteremia treated with colistin vs. colistin plus meropenem.
| Patient characteristics | Colistin (n = 40) | Colistin plus meropenem (n = 31) | p |
|---|---|---|---|
| Age, years | 64.57 ± 16.24 | 70.06 ± 12.01 | 0.12 |
| Sex, male | 22 (55.0%) | 20 (64.5%) | 0.41 |
| Comorbid condition | |||
| Diabetes mellitus | 16 (40.0%) | 12 (38.7%) | 1.00 |
| Cardiovascular disease | 8 (20.0%) | 9 (29.0%) | 0.37 |
| Chronic lung disease | 11 (27.5%) | 9 (29.0%) | 0.88 |
| Renal impairment | 12 (30.0%) | 10 (32.3%) | 0.83 |
| Chronic liver disease | 8 (20.0%) | 3 (9.7%) | 0.32 |
| Cerebrovascular disease | 17 (42.5%) | 13 (41.9%) | 0.96 |
| Malignancy | 6 (15.0%) | 9 (29.0%) | 0.15 |
| Charlson Comorbidity Index | 3.20 ± 1.77 | 3.32 ± 1.44 | 0.75 |
| Pitt bacteremia score | 4.05 ± 2.51 | 3.93 ± 2.30 | 0.84 |
| Steroid/immunosuppressant use | 5 (12.5%) | 8 (25.8%) | 0.15 |
| Recent surgery | 11 (27.5%) | 10 (32.3%) | 0.66 |
| CVC | 36 (90.0%) | 28 (90.3%) | 0.96 |
| Type of infection | |||
| Pneumonia | 15 (37.5) | 16 (51.6) | 0.33 |
| CRI | 17 (42.5%) | 10 (32.3) | 0.46 |
| SSTI | 4 (10.0) | 3 (9.7) | 1.00 |
| IAI | 2 (5.0) | 1 (3.2) | 1.00 |
| UTI | 0 (0) | 1 (3.2) | 0.43 |
| PB | 1 (2.5) | 0 (0) | 1.00 |
| CNS infection | 1(2.5) | 0 (0) | 1.00 |
| ABs before colistin administration | 15 (37.5) | 5 (16.1) | 0.06 |
| ABs during colistin and/or meropenem administration | 8 (20.0) | 3 (9.7) | 0.32 |
Data are presented as mean ± standard deviation, SD for continuous variables and as n (%) of patient s for categorical variables. CVC, central venous catheter; CRI, central line associated infection; UTI, urinary tract infection; SSTI, skin and soft tissue infection; IAI, intra-abdominal infection; PB, primary bacteremia; CNS, central nervous system, ABs, antibiotics other than colistin or meropenem.
Figure 1.
Mortality within 14 days of the onset of bacteremia in the combination therapy and monotherapy groups (25.8% vs 47.5%). Curves are based on Kaplan–Meier estimates.
Table 2.
Univariate analysis of 14-day mortality in patients with CRAB bacteremia.
| Patient characteristics | Survivors (n = 44) | Non-survivors (n = 27) | p |
|---|---|---|---|
| Age, years | 65.34 ± 15.70 | 69.62 ± 12.77 | 0.23 |
| Sex, male | 22 (50%) | 20 (74.1%) | 0.05 |
| Diabetes mellitus | 20 (45.5%) | 8 (29.6%) | 0.21 |
| Cardiovascular disease | 8 (29.6%) | 9 (20.5%) | 0.40 |
| Chronic lung disease | 9 (20.5%) | 11 (40.7%) | 0.10 |
| Renal impairment | 13 (29.5%) | 9 (33.3%) | 0.79 |
| Chronic liver disease | 6 (13.6%) | 5 (18.5%) | 0.73 |
| Cerebrovascular disease | 24 (54.5%) | 6 (22.2%) | 0.01 |
| Malignancy | 9 (20.5%) | 6 (22.2%) | 1.00 |
| Pitt bacteremia score | 3.25 ± 2.22 | 5.22 ± 2.22 | 0.001 |
| Steroid/immunosuppressant use | 9 (20.5%) | 4 (14.8%) | 0.75 |
| Recent surgery | 15 (34.1%) | 6 (22.2%) | 0.42 |
| CVC | 39 (88.6%) | 25 (92.6%) | 0.70 |
| Type of infection | |||
| Pneumonia | 13 (29.5%) | 18 (66.7%) | 0.003 |
| CRI | 19 (43.2%) | 8 (29.6%) | 0.31 |
| SSTI | 6 (13.6%) | 1 (3.7%) | 0.24 |
| IAI | 3 (6.8%) | 0 (0) | 0.28 |
| UTI | 1 (2.3%) | 0 (0) | 1.00 |
| PB | 1 (2.3%) | 0 (0) | 1.00 |
| CNS infection | 1 (2.3%) | 0 (0) | 1.00 |
| Combination therapy | 23 (52.3%) | 8 (29.6%) | 0.08 |
Data are presented as mean ± standard deviation, SD for continuous variables and as n (%) of patients for categorical variables. CVC, central venous catheter; CRI, central line associated infection; UTI, urinary tract infection; SSTI, skin and soft tissue infection; IAI, intra-abdominal infection; PB, primary bacteremia; CNS, central nervous system.
Logistic regression analysis showed that the Pitt bacteremia score, pneumonia, and combination therapy were significantly associated with overall 14-day mortality, with adjusted odds ratios (OR) and 95% confidence intervals (CI) of 1.63 (1.16–2.30), 5.27 (1.11–24.87), and 0.15 (0.03–0.65), respectively (Table 3). In the group with higher Pitt bacteremia score (≥4; n = 39), 14-day mortality was significantly higher for patients with colistin monotherapy (66.7%) compared with those in the colistin plus meropenem group (27.8%) (p = 0.036). In the groups with lower Pitt bacteremia score (≤3; n = 32), 14-day mortality was similar between the two groups (26.3% vs 23.1%) (Figure 2). Clinical success at 14 days after the onset of bacteremia was observed in 16 of 40 (40.0%) patients in the colistin monotherapy group and 19 of 31 (61.3%) patients in the combination therapy group. Univariate analysis revealed that the Pitt bacteremia score was the only significantly different variable, and was lower in the clinical success group than in the failure group (3.31 ± 2.19 vs 4.66 ± 2.44, p = 0.017). Multivariate analysis also showed that the Pitt bacteremia score was the only predictive factor for clinical success (OR 1.29, CI 1.00–1.66).
Table 3.
Risk factors for 14-day mortality in patients with CRAB bacteremia in multivariate analysis.
| Characteristics | Survivors (n = 44) | Non-survivors (n = 27) | Multivariate analysis |
|
|---|---|---|---|---|
| OR (95% CI) | p | |||
| Sex, male | 22 (50%) | 20 (74.1%) | 3.47 (0.79–15.21) | 0.098 |
| Age, years | 65.34 ± 15.70 | 69.62 ± 12.77 | 1.05 (0.99–1.11) | 0.095 |
| Lung disease | 9 (20.5%) | 11 (40.7%) | 1.48 (0.33–6.52) | 0.604 |
| DM | 20 (45.5%) | 7 (25.9%) | 0.25 (0.05–1.19) | 0.082 |
| Cerebrovascular disease | 24 (54.5%) | 6 (22.2%) | 0.37 (0.08–1.76) | 0.215 |
| Pitt bacteremia score | 3.25 ± 2.22 | 5.22 ± 2.22 | 1.63 (1.16–2.30) | 0.005 |
| Type of infection-pneumonia | 13 (29.5%) | 18 (66.7%) | 5.27 (1.11–24.87) | 0.036 |
| Combination therapy | 23 (52.3%) | 8 (29.6%) | 0.15 (0.03–0.65) | 0.011 |
Data are presented as mean±standard deviation, SD for continuous variables and as n (%) of patients for categorical variables. DM, diabetes mellitus.
Figure 2.
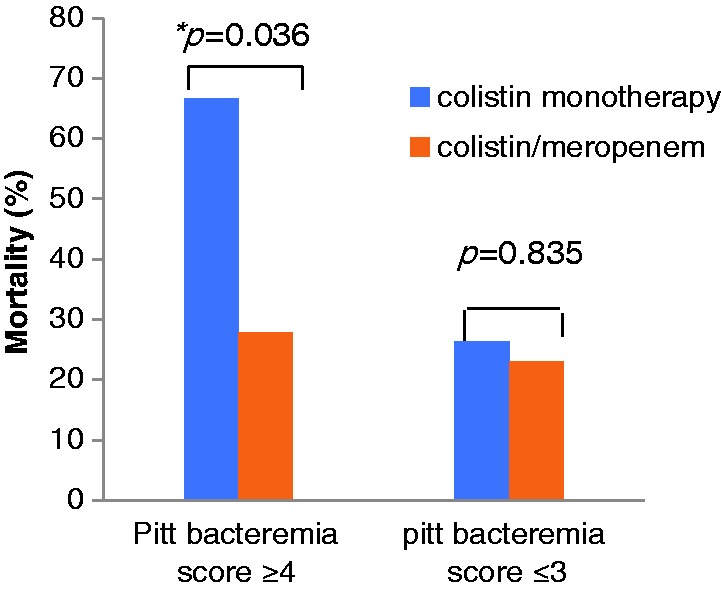
Mortality in the monotherapy and combination therapy groups according to Pitt bacteremia score. *In patients with Pitt bacteremia score ≥4 (n=39), 14-day mortality was significantly higher in patients that received colistin monotherapy (66.7%) compared with those that received colistin plus meropenem (27.8%) (p=0.036).
Discussion
This study compared mortality associated with colistin monotherapy group with that associated with colistin plus meropenem combination therapy in patients with CRAB bacteremia. The notable finding was that mortality was higher in the colistin monotherapy group when the Pitt bacteremia score was high (≥4).
A. baumannii is an important nosocomial pathogens, particularly in the ICU.4,24,25 CRAB bacteremia is increasing and is associated with high mortality.24,26–28 In the present study, all cases were hospital-acquired rather than community-acquired infections. Patients in the monotherapy group had similar demographic and clinical characteristics compared with those in the combination therapy group. Mortality tended to be higher in the monotherapy group, but the difference was not statistically significance (47.5% vs. 25.8%). However, combination therapy was significantly more efficacious in patients with a high Pitt bacteremia score (66.7% vs. 27.8%, p = 0.036). This result is in accordance with findings from previous studies that reported favorable clinical outcomes in patients that received combination therapy, particularly with carbapenem,23,29 and also with a previous meta-analysis of in vitro synergy studies.16 However, a recent randomized controlled trial was unable to show the effectiveness of combination therapy, whereby the addition of meropenem to colistin did not decrease the clinical failure rate in patients with A. baumannii infections.19 The results of another meta-analysis also revealed that combination therapy did not lower mortality in patients with multidrug-resistant Gram-negative infections.15 Our findings showed that the maximal clinical benefit of combination therapy was observed in patients with severe sepsis, which is consistent with data from a previous study of carbapenemase-producing Klebsiella pneumoniae in which combination therapy with carbapenem conferred a survival benefit that was more pronounced when sepsis was more severe or the underlying disease was more likely to be fatal.30 From a different perspective, however, monotherapy may be a better option than combination therapy with respect to cost considerations and the development of resistance in patients with a Pitt bacteremia score <3. Future randomized control studies may therefore be needed to assess the clinical impact of combination therapy according to the severity of infection.
The present study also revealed that mortality may vary depending on the type of infection. In particular, pneumonia as the source of bacteremia was associated with a high mortality rate, consistent with a previous study.3 Further studies examining differences in mortality between pneumonia- and non-pneumonia-related bacteremia are therefore warranted.
This study had several limitations. First, it was a retrospective study that enrolled patients at a single medical center. However, the study was strengthened by the inclusion of a relatively large number of patients with infections of various type and severity. Second, mortality was defined as all-cause mortality and not infection-related mortality. However, infection was considered a major contributing factor for mortality within 14 days of bacteremia.
Conclusion
CRAB bacteremia is associated with significant mortality, and combination treatment with colistin plus meropenem can improve survival in patients with this type of bacteremia, particularly those who are critically ill. Furthermore, high Pitt bacteremia score, pneumonia, and colistin monotherapy may be associated with higher 14-day mortality in this patient population.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research was supported by Hallym University under a grant from the Hallym University Research Fund 2014 (HURF-2014-58).
Notes on contributors
So Yeon Park M.D. is an infectious disease (ID) specialist and assistant professor at Hallym University Medical school. Hye Jin Si M.D. is an ID specialist and clinical instructor at Hallym University Medical School. Joong Sik Eom M.D. is an ID specialist and professor at Gachon University College of Medicine. Jin Seo Lee M.D. is an ID specialist and associate professor at Hallym University Medical School.
References
- 1.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 2008; 46: 1254–1263. [DOI] [PubMed] [Google Scholar]
- 2.Lee HY, Chen CL, Wu SR, et al. Risk factors and outcome analysis of acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med 2014; 42: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 3.Teng SO, Yen MY, Ou TY, et al. Comparison of pneumonia- and non-pneumonia-related Acinetobacter baumannii bacteremia: Impact on empiric therapy and antibiotic resistance. J Microbiol Immunol Infect 2014; 4: 00122–00124. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39: 309–317. [DOI] [PubMed] [Google Scholar]
- 5.Sengstock DM, Thyagarajan R, Apalara J, et al. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis 2010; 50: 1611–1616. DOI: 10.086/652759. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti M, Righi E, Esposito S, et al. Drug treatment for multidrug-resistant Acinetobacter baumannii infections. Future Microbiol 2008; 3: 649–660. DOI: 10.2217/17460913.3.6.649. [DOI] [PubMed] [Google Scholar]
- 7.Jain R, Danziger LH. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann Pharmacother 2004; 38: 1449–1459. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Kim SI, Hong KW, et al. Risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia: impact of appropriate antimicrobial therapy. J Korean Med Sci 2012; 27: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng WH, Liao CH, Lauderdale TL, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis 2010; 14: e764–e769. [DOI] [PubMed] [Google Scholar]
- 10.Wareham DW, Bean DC, Khanna P, et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis 2008; 27: 607–612. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Li W, Feng Y, et al. Efficacy and safety of polymyxins for the treatment of Acinectobacter baumannii infection: a systematic review and meta-analysis. PLoS One 2014; 9: e98091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 2013; 57: 349–358. [DOI] [PubMed] [Google Scholar]
- 13.Gounden R, Bamford C, van Zyl-Smit R, et al. Safety and effectiveness of colistin compared with tobramycin for multi-drug resistant Acinetobacter baumannii infections. BMC Infect Dis 2009; 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalin G, Alp E, Akin Aet al. Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection 2014; 42: 37–42. [DOI] [PubMed] [Google Scholar]
- 15.Vardakas KZ, Mavroudis AD, Georgiou Met al. Intravenous colistin combination antimicrobial treatment vs. monotherapy: a systematic review and meta-analysis. Int J Antimicrob Agents 2018; 51: 535–547. [DOI] [PubMed] [Google Scholar]
- 16.Zusman O, Avni T, Leibovici L, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 2013; 57: 5104–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singkham-In U, Chatsuwan T. In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Diagn Microbiol Infect Dis 2018; 91: 169–174. [DOI] [PubMed] [Google Scholar]
- 18.Poulikakos P, Tansarli GS, Falagas ME. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis 2014; 33: 1675–1685. [DOI] [PubMed] [Google Scholar]
- 19.Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18: 391–400. [DOI] [PubMed] [Google Scholar]
- 20.Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 1991; 115: 585–590. [DOI] [PubMed] [Google Scholar]
- 21.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 23.Shields RK, Clancy CJ, Gillis LM, et al. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PloS one 2012; 7: e52349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis 2012; 55: 209–215. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Choo JW, Kwon SH, et al. Risk factors for mortality in patients with Acinetobacter baumannii bacteremia. Infect Chemother 2013; 45: 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nutman A, Glick R, Temkin E, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect 2014; 20: O1028–O1034. [DOI] [PubMed] [Google Scholar]
- 27.Kwon KT, Oh WS, Song JH, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 2007; 59: 525–530. [DOI] [PubMed] [Google Scholar]
- 28.Punpanich W, Nithitamsakun N, Treeratweeraphong V, et al. Risk factors for carbapenem non-susceptibility and mortality in Acinetobacter baumannii bacteremia in children. Int J Infect Dis 2012; 16: e811–e815. [DOI] [PubMed] [Google Scholar]
- 29.Kuo LC, Lai CC, Liao CH, et al. Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin Microbiol Infect 2007; 13: 196–198. [DOI] [PubMed] [Google Scholar]
- 30.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58: 2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]