Short abstract
Objectives
To evaluate and categorize the survival benefit of tricyclic antidepressants (TCAs) in lung cancer patients based on systematic computational drug repositioning data.
Methods
Data were retrospectively extracted from the medical records of non-small cell lung cancer (NSCLC) patients from the University of Cincinnati Cancer Medical Center database. Patients receiving antidepressants during their course of anti-cancer treatment were compared with those without antidepressants. Data were analyzed using Kaplan–Meier survival curves with the log-rank test, and overall survival (OS) was calculated from the date of diagnosis until last follow-up or death.
Results
The median OS at 2 and 5 years for patients on antidepressants was 20.3 months (54.7% and 42%) vs 44.3 months (47.6% and 43.2%), which was not significant. The median OS for patients receiving TCAs, selective serotonin reuptake inhibitors, and other antidepressants was 3.17 months, 31.33 months, and 18.50 months, respectively.
Conclusion
We found no significant survival benefit for TCA use in combination with anti-cancer agents in NSCLC patients.
Keywords: Lung cancer, tricyclic antidepressants, SSRIs, drug repositioning, apoptosis, overall survival
Introduction
Tricyclic antidepressants (TCAs) are one of the earliest medications used for depression, and function by blocking norepinephrine and serotonin reuptake. Through drug repositioning and validation in preclinical models, TCAs were identified as potent inducers of cell death in small cell lung cancer (SCLC) cells by activating stress pathways. Specifically, they induced apoptosis in both chemo-naïve and chemo-resistant human SCLC tumor cells by blocking G-protein-coupled receptors, which inhibited protein kinase A. The TCA amitriptyline has both pro-apoptotic and anti-myeloma activities and is non-toxic to healthy blood cells.1 New antidepressants such as selective serotonin reuptake inhibitors (SSRIs) have a more favorable toxicity profile compared with TCAs, so TCAs are no longer the first choice of initial treatment for depression although they were shown to be safe for lung cancer patients in particular.2 SSRIs also possess anti-cancerous properties by inhibiting cancer cell proliferation and inducing apoptosis.3 However, for SCLC and other neuroendocrine cancers, TCAs had significant anti-tumor effects in preclinical studies that were comparable to existing chemotherapy regimens. The exact mechanism of these effects is unclear.4
Today, lung cancer is the leading cause of cancer deaths in the United States5 and the main cause of cancer deaths in both men and women in developed countries.6 Non-small cell lung cancer (NSCLC) is the predominant lung cancer disease, but the effect of TCAs on NSCLC tumor cells is poorly studied.7 SCLC is the more aggressive form with frequent early metastases, and the disease is usually asymptomatic until an advanced stage.4 Although SCLC is a chemo-and radio-sensitive disease that usually responds to initial treatment, recurrence is very common, with only 6% of patients surviving 5 years from their initial diagnosis. Cisplatin or carboplatin with etoposide is the first-line chemotherapeutic regimen for both limited and extensive SCLC; this has not changed over the past four decades despite advances in cancer treatment. This combination therapy yields optimal tumor response rates as high as 60% to 80%, but a cure is only achieved in 20% of patients with limited-stage disease.8 Therefore, a conscious effort to find accessible, effective agents to improve patient care is warranted.
Recent advances in computational capacity have enabled more sophisticated computer-assisted drugs to be designed. For example, molecular docking is a computer simulation procedure that predicts the conformation of a receptor–ligand complex, while protein structures determined experimentally by X-ray crystallography or nuclear magnetic resonance have been publicly available for some time. Additionally, many algorithms are available to assess and rationalize ligand–protein or protein–protein interactions. The success of the algorithm in predicting ligand binding is usually measured by the root-mean-squared deviation between experimentally-observed heavy atom positions of the ligands and what was predicted by the algorithm.9 With the continuing use of this novel approach, algorithms have become faster and more accurate.
Through the development of in silico analysis, drug repositioning is finding new indications for existing drugs.10 Not only is this beneficial for known safety, dosage, and toxicity, but it also saves time and money with regard to development.11–14 This can be especially beneficial in patients expressing markers for enhanced metastatic potential.15 Drug repositioning studies have shown promise in complex diseases needing improved therapeutic interventions as well as in diseases lacking effective treatments.16,17 In this study, we retrospectively explored the effect of using TCAs in combination with anti-cancer agents in NSCLC patients treated in our hospital, thus identifying the benefit of this combination therapy.
Materials and methods
Patients and data collection
We retrospectively reviewed the University of Cincinnati Medical Center database and Epic patient medical records, and enrolled patients with NSCLC who underwent evaluation and treatment between January 2004 and December 2014. We compared patients who received antidepressants in the form of TCAs, SSRIs, and other drugs with those who received no antidepressants (Control group). We excluded patients treated at other hospitals. Patient demographics and clinical and pathological data were retrieved from the medical records. Analyzed variables included age, sex, race, histology, tumor–node–metastasis stage, type of treatment, and survival data.18,19 All cases received platinum-based regimes. This study was approved by the review board and ethical committee of the University of Cincinnati Hospital.
Statistical methods
The primary outcome was overall survival (OS) which was analyzed using Kaplan–Meier survival curves with the log-rank test and was calculated from the date of diagnosis until the last follow-up or death. The Cox regression model was used to estimate the hazard ratio (HR) and 95% confidence intervals (95% CI) for long-term mortality in an unadjusted model and after adjustment based on age, sex, and stage. P < 0.05 was considered significant.
Secondary outcomes were: 1) predictors of antidepressant use using Chi-squared and Mann–Whitney U tests, 2) OS among the whole cohort, and 3) OS among patients receiving different antidepressants (TCAs, SSRIs, and others) vs the non-antidepressant cohort.
Categorical variables were reported as absolute numbers (frequency percentages) and compared using the Chi-squared test, while continuous variables were presented as medians and interquartile ranges (IQR) and compared using the Mann–Whitney U test.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA) and IBM SPSS Statistics-Essentials for R 22.0.
Results
Demographics
Data for 174 NSCLC patients were retrieved from the University of Cincinnati database. Thirty-four patients received antidepressants during their treatment course. The median age at diagnosis was 61 years (IQR: 55–69 years) and the majority of patients were men (55.7%). Adenocarcinoma patients were the most frequent subgroup followed by squamous cell lung cancer.
More female patients received antidepressants during their active treatment than male patients (female [f] = 28.6%, male [m] = 12.4%), and SSRIs were the most commonly used antidepressant in both sexes (f = 16.9%, m = 6.2%), followed by other antidepressants (f = 9.1%, m = 5.2%), with TCAs the least common (f = 2.6%, m = 1%; Tables 1–3).
Table 1.
Patient demographics.
| Sex | |
| Male | 97 (55.7%) |
| Female | 77 (44.3%) |
| Age (years) | |
| Median (IQR) | 61 (55-69) |
| Histopathological subtypes | |
| NSCLC, A | 103 (59.2%) |
| NSCLC, SCC | 59 (33.7%) |
| NSCLC, Mixed Adeno/Squamous | 3 (1.7%) |
| NSCLC undifferentiated | 9 (5.2%) |
| Median overall survival | 38.4 months |
IQR, interquartile range; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma.
Table 2.
Antidepressant prescription according to variables.
| Variables | Control (n = 140) | Antidepressants (n = 34) | Total (n = 174) | P value¶ |
|---|---|---|---|---|
| Age, years (median, IQR) | 62 (56–70) | 59 (5075–65.50) | 61 (55–69) | 0.046† |
| Age, years | ||||
| <40 | 1 (50.0%) | 1 (50.0%) | 2 (100.0%) | 0.172 |
| 40–59 | 43 (72.9%) | 16 (27.1%) | 59 (100.0%) | |
| 60–79 | 76 (82.6%) | 16 (17.4%) | 92 (100.0%) | |
| ≥80 | 14 (93.3%) | 1 (6.7%) | 15 (100.0%) | |
| Sex | ||||
| Male | 85 (87.6%) | 12 (12.4%) | 97 (100.0%) | 0.007 |
| Female | 55 (71.4%) | 22 (28.6%) | 77 (100.0%) | |
| Race | ||||
| White | 63 (76.8%) | 19 (23.2%) | 31 (100.0%) | 0.545 |
| Black | 26 (83.9%) | 5 (16.1%) | 82 (100.0%) | |
| Asian | 2 (100.0%) | 0 (0.0%) | 2 (100.0%) | |
| Tumor stage | ||||
| Stage I | 10 (100%) | 0 (0%) | 10 (100.0%) | 0.208 |
| Stage II | 13 (68.4%) | 6 (31.6%) | 19 (100.0%) | |
| Stage III | 33 (76.7%) | 10 (23.3%) | 43 (100.0%) | |
| Stage IV | 76 (81.7%) | 17 (18.3%) | 93 (100.0%) | |
| Statin | ||||
| No | 111 (82.2%) | 24 (17.8%) | 135 (100.0%) | 0.275 |
| Yes | 29 (74.4%) | 10 (25.6%) | 39 (100.0%) | |
| Follow-up, months (Median; 95% CI) | 40 (33.82–46.18) | 36.67 (29.03–44.30) | 40 (35.70–44.30) | |
| Overall survival, median (2 and 5 years) | 44.3 months (47.6% and 43.2%) | 20.3 months (54.7% and 42%) | 38.4 months | 0.466 |
IQR, interquartile range; CI, confidence interval; ¶Chi squared test, †Mann–Whitney U test.
Table 3.
Antidepressant prescription by class according to age and sex.
| Variable | Control group | TCA | SSRI | Other | P value¶ |
|---|---|---|---|---|---|
| Age, years | |||||
| <40 | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 0.309 |
| 40–59 | 43 (72.9%) | 1 (1.7%) | 10 (16.9%) | 5 (8.5%) | |
| 60–79 | 76 (82.6%) | 2 (2.2%) | 8 (8.7%) | 6 (6.5%) | |
| ≥80 | 14 (93.3%) | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) | |
| Sex | |||||
| Male | 85 (87.6%) | 1(1.0%) | 6 (6.2%) | 5 (5.2%) | 0.058 |
| Female | 55 (71.4%) | 2(2.6%) | 13 (16.9%) | 7 (9.1%) |
TCA, tricyclic antidepressants; SSRI, selective serotonin reuptake inhibitors.
The relationship between age and antidepressant intake was inversely proportional (P = 0.046), with patients younger than 40 years having the highest rate of antidepressant prescription during their treatment (50%) compared with 27.1%, 17.4%, and 6.7% for patients aged >40, >60, and >80 years, respectively (Table 2). More detailed demographic age analysis according to antidepressant class is presented in Table 3.
Antidepressant usage was highest among patients with white ethnicity, but the difference was not statistically significant (white = 23.2%, black = 16.1%, Asian = 0%). Similarly, there was no significant difference in antidepressant usage between patients at different stages of disease (0%, 31.6%, 23.3%, and 18.3% for stages I, II, III, and IV, respectively; Table 2).
Survival analyses
The median overall follow-up time was 40 months (95% CI 35.70–44.30); follow-up times for antidepressant and non-antidepressant groups were 36.67 months (95% CI 29.03–44.30) and 40 months (95% CI 33.82–46.18), respectively.
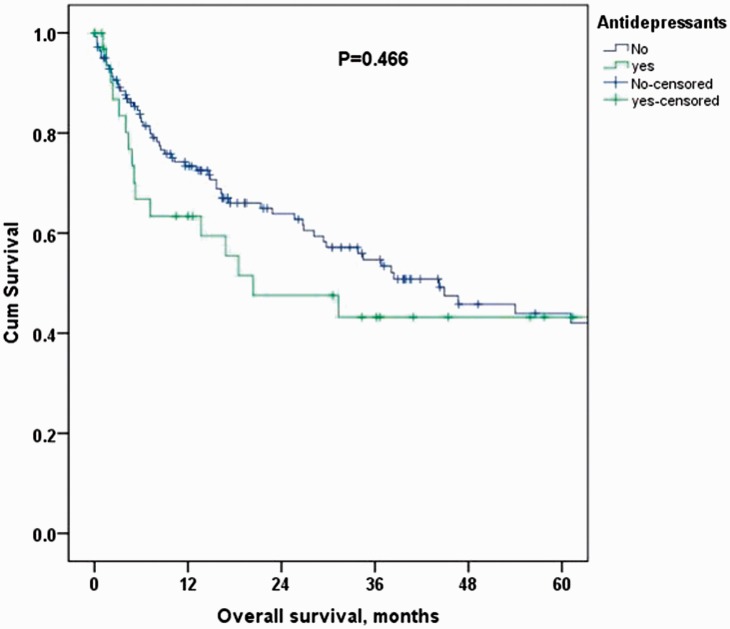
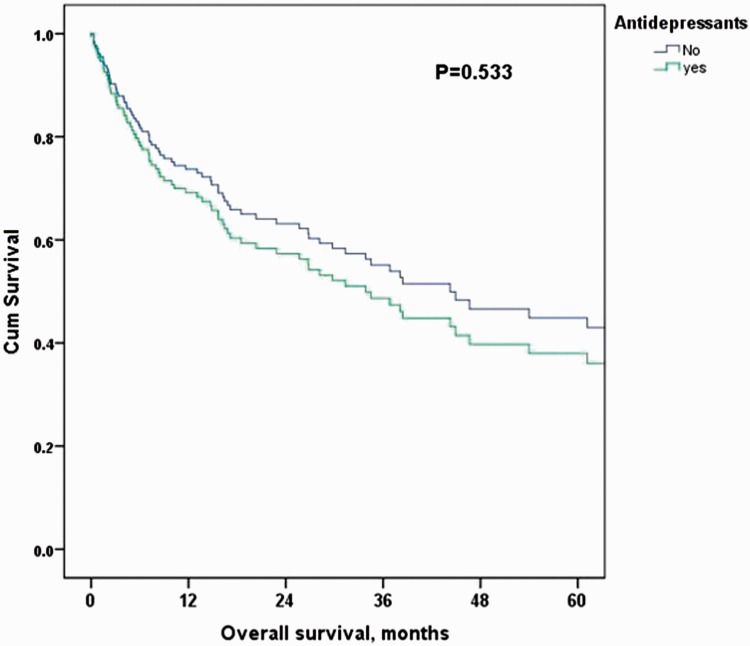
The median OS for the entire cohort was 38.4 months. The median OS at 2 and 5 years for patients receiving antidepressants was 20.3 months (54.7% and 42%, respectively) vs 44.3 months (47.6% and 43.2%, respectively) in the control group. Figure 1 shows the unadjusted Cox regression estimated HR for long-term mortality in the antidepressant group compared with the control group (HR: 1.23 [95% CI 0.71–2.12]), which was further confirmed after adjustment for age, sex, and stage (HR: 1.21 [95% CI 0.67–2.20]; Tables 4 and 5, Figure 2).
Figure 1.
Unadjusted Kaplan–Meier overall survival curves for antidepressant vs control groups.
Table 4.
Cox regression-estimated hazard ratio for overall survival in antidepressant patients compared with controls.
| Variable | HR | 95% confidence interval | P value¶ |
|---|---|---|---|
| Unadjusted model | 1.23 | 0.71–2.12 | 0.467 |
| Adjusted model (age, sex, stage) | 1.21 | 0.67–2.20 | 0.533 |
HR, hazard ratio; OS, overall survival.
Table 5.
Median overall survival for each study arm.
| Drug | N | Deaths | Censored | Median OS | P value¶ | Hazard ratio (95% CI)† |
|---|---|---|---|---|---|---|
| Antidepressant group | 34 | 16 | 18 | 44.3 months | – | Reference |
| Control group | 140 | 68 | 72 | 20.3 months | 0.467 | 1.23 (0.71–2.12) |
OS, overall survival; CI, confidence interval; ¶Log rank test, †Unadjusted HR.
Figure 2.
Cox regression-adjusted Kaplan–Meier overall survival curves for antidepressant vs control groups. Adjustment was for age, sex, and stage.
The overall longest survival time at which at least 75% of patients survived was 8.33 months. In the antidepressant group, this was 4.83 months (3.17, 7.17, and 4.33 months for TCA, SSRI, and other subgroups, respectively) compared with 10.25 months in the control group (Table 6).
Table 6.
Overall survival for antidepressant groups compared with the control arm and the risk of dying.
| Drug | N | Deaths | Censored | OS in study arm (months) | OS in control arm (months) | P value¶ | Hazard ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| TCA | 3 | 2 | 1 | 3.17 | 44.25 | 0.002 | 6.72 (1.59–28.48) |
| SSRI | 19 | 8 | 11 | 31.33 | 44.25 | 0.965 | 0.981 (0.47–2.05) |
| other | 12 | 6 | 6 | 18.50 | 44.25 | 0.522 | 1.31 (0.57–3.02) |
OS, overall survival; CI, confidence interval; ¶Log rank test (pairwise over strata) with overall P value of 0.026
The median OS for patients receiving TCAs, SSRIs, and other antidepressants was 3.17 months (P = 0.002), 31.33 months, and 18.50 months, respectively. The Cox model showed that the impact of SSRIs (HR 0.98 [95% CI 0.47–2.05]) and other antidepressant drugs (HR 1.308 [95% CI 0.57–3.02]) had no difference on survival compared with not taking antidepressants at all. TCAs were associated with an increased risk of death, but the small sample size (n = 3) and very wide confidence interval (HR 6.72, 95% CI 1.59–28.48) argued against this (Table 6).
Discussion
Trends in alternative indications for Food and Drug Administration (FDA)-approved drugs have been the focus of numerous studies.12,16,20,21 Recent technological advancements have helped establish novel relationships between many agents and unconventional diseases. For example, FDA-approved medications such as TCAs, SSRIs, and other antidepressants were shown to have possible anticancer effects on advanced lung cancers. In 2013, Jahchan et al. used in silico analysis to identify the TCA imipramine as a possible treatment for SCLC and other neuroendocrine tumors.10 Experimentally, they established a relationship between imipramine and the induction of apoptosis in both chemo-naïve and chemo-resistant SCLC cell lines. Similar outcomes were obtained in mouse models and xenografts. However, the same results were not obtained in either human or mouse NSCLC cell line cultures. Moreover, these results were not supported by clinical data because they failed to obtain a significant number of patients who received TCA during their cancer treatment by retrospective analysis of Stanford medical records.10 In 2017, assessment of the survival association between lung cancer patients and antidepressants found that those who received TCAs had improved survival compared with patients receiving other antidepressants, even with dose adjustments.12
A study by Amit et al. suggested that SSRIs possess anti-cancerous properties in vitro by exposing malignant T cells to sertraline and paroxetine, resulting in the inhibition of cell proliferation and induction of apoptosis; additionally, sertraline enhances the effects of vincristine and doxorubicin.3 These findings can be explained by the results reported by Gil-Ad et al. who found that paroxetine and sertraline are more effective agents in inhibiting cell viability and proliferation in vitro than other cytotoxic agents including vincristine and doxorubicin.19,22 Their work also revealed possible mechanisms for cancer cell apoptosis, although these were not supported by clinical evidence. In another study of the associated risk of lung cancer with long-term antidepressant use, SSRIs were shown to have a protective effect while a slightly increased risk of lung cancer was observed for TCAs.
Other antidepressants such as duloxetine, trazodone, and mirtazapine also showed a trend towards improved OS, with 805 days compared with 531 in the control group.23 However, no specific trials or reviews have analyzed the potential antineoplastic effects of these agents.
Recent trends in studying alternative indications for FDA-approved drugs have been the focus of numerous studies,16,20,21 and new technological advancements have enabled novel relationships between agents and unconventional diseases to be established. Such indications involving TCAs, SSRIs, and other antidepressants were shown to have possible anticancer effects on advanced lung cancers.
In the present study, we investigated anti-tumor effects that might be attributed to the use of anti-depressants in NSCLC patients; however, we found no clear benefits in this subset of patients. We observed a median OS of 3.17 months among TCA patients, which was inferior to all other groups. We also found that TCA use was associated with a higher risk of death, although this finding was based on a very small population (n = 3). In 2013, Jahchan et al. used in silico analysis to investigate the TCA imipramine as a possible treatment for SCLC and other neuroendocrine tumors.10 Experimentally, they established a relationship between imipramine and the induction of apoptosis in both chemo-naïve and chemo-resistant SCLC cell lines. Similar outcomes were obtained in mouse models and xenografts. However, they did not obtain the same results in either human or mouse NSCLC cell line cultures. Additionally, these results were not supported by clinical data because they failed to identify a significant number of patients treated with TCA by retrospective analysis of Stanford Medical records (fewer than five cases, none of whom received long-term drug treatment).10
Numerous studies have reported improved survival with anti-depressant use. In 2017, the survival association between lung cancer patients and antidepressants was examined.7 Patients who received TCAs 3 months prior to cancer diagnosis showed improved survival compared with those receiving other antidepressants, and this association was still observed with dose adjustments. Another study observed the inhibition of proliferation and induction of apoptosis in malignant T cells following exposure to sertraline and paroxetine, in addition to sertraline-enhanced vincristine and doxorubicin effects, suggesting that SSRIs possess anti-cancerous properties in vitro.3 These findings can be explained by the results reported by Gil-Ad et al. who found that paroxetine and sertraline are more effective at inhibiting cell viability and proliferation in vitro compared with other cytotoxic agents such as vincristine and doxorubicin (Gariboldi et al., 2003).22,23 Their work also suggested possible mechanisms for cancer cell apoptosis, but these were not supported by clinical evidence. Another study of the associated risk of lung cancer with long term use of SSRIs and TCAs documented a protective effect for SSRIs and a slightly increased risk with TCAs.24
Other antidepressants such as duloxetine, trazodone, and mirtazapine also showed a trend towards improved OS.25 However, no specific trials or reviews have analyzed the potential antineoplastic effects of these agents. This is of particular concern because it means that clinical prescription of anti-depressants for anti-neoplastic purposes in cancer patients will be based on a subjective opinion. Despite the numerous reports that support the existence of a benefit from anti-depressants in cancer patients, our results suggest otherwise. We recommend that because depression is such a common co-morbidity in cancer, special attention should be paid to the anti-tumor effects of anti-depressants in larger population studies.
Conclusion
Most previously reported studies used in silico analysis to identify FDA-approved drugs that could be used to treat cancer, then experimentally tested these findings. Preclinical models found that TCAs and SSRIs have apoptotic effects on cancer cells, with TCAs having an advantage in inducing apoptosis in SCLC cells. Our study did not reveal any significantly positive impact of antidepressants on the OS of this cohort of patients. However, we found that the impact of the chemotherapy regimen combined with antidepressants was superior to no antidepressants. None of these findings demonstrated statistical significance, which may be attributed to the small sample size and the fact that the non-antidepressant arm represented more than 50% of our sample. Therefore, future studies with a larger population using propensity scoring or randomized controlled trials will help explore the effect of these non-chemotherapy drugs on OS in advanced lung cancer.
Acknowledgement
We would like to thank our colleague Ola Gaber who helped with the submission process.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics and consent
During the process of data collection, all patients’ data were properly anonymized and written informed consent was obtained at the time of original data collection.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mao X, Hou T, Cao B, et al. The tricyclic antidepressant amitriptyline inhibits D-cyclin transactivation and induces myeloma cell apoptosis by inhibiting histone deacetylases: in vitro and in silico evidence. Mol Pharmacol 2011; 79: 672–680. [DOI] [PubMed] [Google Scholar]
- 2.Ashton JC. The role of amitriptyline in the management of non-small cell lung cancer. Curr Cancer Ther Rev 2015; 10: 284–288. [Google Scholar]
- 3.Amit BH, Gil-Ad I, Taler M, et al. Proapoptotic and chemosensitizing effects of selective serotonin reuptake inhibitors on T cell lymphoma/leukemia (Jurkat) in vitro. Eur Neuropsychopharmacol 2009; 19: 726–734. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Byers LA. Teaching an old dog new tricks: drug repositioning in small cell lung cancer. Cancer Discov 2013; 3: 1333–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 7.Zingone A, Brown D, Bowman ED, et al. Relationship between anti-depressant use and lung cancer survival. Cancer Treat Res Commun 2017; 10: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015; 21: 2244–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernndez-Santoyo A, Tenorio-Barajas A, Altuzar V, et al. Protein-protein and protein-ligand docking. in: Protein Engineering - Technology and Application (InTech, 2013). DOI:10.5772/56376.
- 10.Jahchan NS, Dudley JT, Mazur PK, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov 2013; 3: 1364–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sage J. Development of antidepressants as novel agents to treat small cell lung cancer, https://pdfs.semanticscholar.org/dd46/dbae87ddb3ee795d9c8bb5932a375211d5a8.pdf (2014, accessed 4 July 2019).
- 12.Karim N, Bahassi EM, Gaber O, et al. A phase I study of the non-receptor tyrosine kinase inhibitor (NKI) bosutinib in combination with pemetrexed in patients with advanced solid tumors. J Thorac Oncol 2017; 12: S904. [Google Scholar]
- 13.Eldessouki IA, Kandeel E, Adnan S, et al. Tapering treatment according minimal residual disease. Blood 2015; 126: 4960LP. [Google Scholar]
- 14.Eldessouki I, Gaber O, Namad T, et al. Small or non-small cell lung cancer based therapy for treatment of large cell neuroendocrine cancer of the lung? University of Cincinnati experience. J Oncol 2018; 2018: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karim NA, Eldessouki I, Yellu M, et al. A case study in advanced lung cancer patients with vimentin over expression. Clin Lab 2017; 63: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 16.Hassan R. Impact of low molecular weight heparin on overall survival in patients with advanced lung cancer: a retrospective study. Am J Clin Exp Med 2017; 5: 173. [Google Scholar]
- 17.Shameer K, Readhead B, Dudley JT. Computational and experimental advances in drug repositioning for accelerated therapeutic stratification. Curr Top Med Chem 2015; 15: 5–20. [DOI] [PubMed] [Google Scholar]
- 18.Bunn PAJ. Early-stage non-small-cell lung cancer: current perspectives in combined-modality therapy. Clin Lung Cancer 2004; 6: 85–98. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien ME, Gaafar RM, Popat S, et al. Phase II study of first-line bortezomib and cisplatin in malignant pleural mesothelioma and prospective validation of progression free survival rate as a primary end-point for mesothelioma clinical trials (European Organisation for Research and Treatment of Cancer 08052). Eur J Cancer 2013; 49: 2815–2822. [DOI] [PubMed] [Google Scholar]
- 20.Sotelo J, Briceño E, López-González MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2006; 144: 337–343. [DOI] [PubMed] [Google Scholar]
- 21.Abdel Karim N, Eldessouki I, Taftaf A, et al. Case report GNQ-209P mutation in metastatic uveal melanoma and treatment outcome. Case Rep Oncol Med 2018; 2018: 4256365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gariboldi MB, Ravizza R, Riganti L, et al. Molecular determinants of intrinsic resistance to doxorubicin in human cancer cell lines. Int J Oncol 2003; 22: 1057–1064. [PubMed] [Google Scholar]
- 23.Gil-Ad I, Zolokov A, Lomnitski L, et al. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int J Oncol 2008; 33: 277–286. [PubMed] [Google Scholar]
- 24.Zingone A, Brown D, Bowman E. Relationship between anti-depressant use and lung cancer survival. Cancer Treat Res Commun 2017; 10: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toh S, Rodríguez LA, Hernández-Díaz S. Use of antidepressants and risk of lung cancer. Cancer Causes Control 2007; 18: 1055–1064. [DOI] [PubMed] [Google Scholar]