Short abstract
Background
The factors that influence cognitive function in patients with atrial fibrillation (AF) remain unclear.
Methods
This study involved an AF group and control group (normal sinus rhythm) of 150 patients each. Cognitive function was assessed with the adjusted Mini-Mental State Examination (MMSEadj) score and Memory and Executive Screening (MES) score. The relationship between cognitive function and the CHA2DS2VASc score was analyzed. Subgroup analysis was performed according to stroke history. Clinical factors affecting the MMSE score were screened by logistic regression analysis.
Results
Baseline data were similar between the two groups. The MMSEadj and MES scores were significantly lower in the AF than control group; the mean MMSEadj score in the AF non-stroke subgroup and control non-stroke subgroup was 26.2 ± 2.7 and 27.9 ± 2.0, respectively. In non-stroke patients with AF, the MMSEadj and MES scores were negatively correlated with the CHA2DS2VASc score. Factors significantly influencing the MMSE score in these patients were age, education, smoking history, NT-proB-type natriuretic peptide, hemoglobin, and anticoagulation.
Conclusion
AF is associated with cognitive dysfunction regardless of stroke history. High CHA2DS2VASc scores are associated with impaired cognitive function. Factors influencing cognitive function in non-stroke patients with AF are age, education, smoking history, NT-proB-type natriuretic peptide, hemoglobin, and anticoagulation.
Keywords: Atrial fibrillation, cognitive function, CHA2DS2VASc score, anticoagulation, Mini-Mental State Examination, Memory and Executive Screening
Background
Cognitive impairment, also termed cognitive dysfunction, refers to an abnormality of the intellectual process by which one becomes aware of, perceives, or comprehends ideas. The incidence of atrial fibrillation (AF) is increasing annually, and the incidence of cognitive dysfunction in patients with AF is simultaneously rising. A few recent studies have focused on the relationship between AF and cognitive function, drawing the conclusion that AF is a risk factor for cognitive dysfunction.1–4 However, both AF and cognitive dysfunction are related to aging, and a causal relationship between these two factors cannot necessarily be established. Additionally, whether AF or subsequent stroke plays a more important role in the development of cognitive dysfunction is unclear. Furthermore, the risk factors associated with cognitive dysfunction in patients with AF have not been clarified.
The present study was performed to further explore the relationship between AF and cognitive dysfunction and examine the factors influencing cognitive function in patients with AF to facilitate early recognition of patients with AF who are at high risk of cognitive decline.
Methods
Study population
From January 2014 to December 2015 in Huashan Hospital Fudan University, patients with AF were consecutively enrolled as the AF group. The control group comprised randomly selected patients with sinus rhythm (matched 1:1 for age, sex, and stroke history) who visited the institute during the same period. The study was approved by the institutional ethics review committee at our institution, and all patients provided written informed consent.
The inclusion criteria for the AF group were an age of ≥18 years and at least one 12-lead electrocardiogram (ECG) or one 24-hour ECG that revealed AF as confirmed by a cardiologist with signature. The exclusion criteria were the presence of acute and unstable diseases such as stroke or myocardial infarction within 3 months; definite brain trauma, encephalopathy, or epilepsy; severe structural heart disease such as ischemic/nonischemic cardiomyopathy; decompensated chronic heart failure (New York Heart Association grade IV); psychiatric diseases (e.g., schizophrenia, depression); and refusal to provide written informed consent.
The inclusion criteria for the control group were an age of ≥18 years and at least one 12-lead ECG or one 24-hour ECG that revealed normal sinus rhythm as confirmed by a cardiologist with signature. The exclusion criteria were any recorded or suspected onset of AF, an arrhythmia such as frequent atrial or ventricular premature contractions, Wolff–Parkinson–White syndrome, high-degree atrioventricular block, history of cardiac electronic device implantation (pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization therapy devices), and all of the above-mentioned exclusion criteria for the AF group.
The patients’ clinical baseline information, comorbidities, and present treatments were carefully recorded. The CHA2DS2VASc score [congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, stroke/transient ischemic attack/thromboembolism (doubled), vascular disease, sex (female)] was evaluated for each patient with AF. Stroke was defined as ischemic/hemorrhagic damage of the central nervous system caused by cerebrovascular diseases. Stroke was diagnosed by a neurologist based on a detailed history, symptoms, and brain magnetic resonance imaging or other head imaging findings. Patients with no history of stroke, symptoms of stroke, or evidence of stroke on cerebral imaging were assigned to the non-stroke group.
Laboratory tests
The following laboratory data were recorded for all patients with AF: complete blood cell count and levels of lipoprotein, troponin T, NT-proB-type natriuretic peptide (pro-BNP), urine protein, serum creatinine, and uric acid. The neutrophil-to-lymphocyte ratio was calculated according to the complete blood cell count.
Cognitive function evaluation
The Mini-Mental State Examination (MMSE) and Memory and Executive Screening (MES) scores were used to evaluate cognitive function. The MMSE is a 30-point test including time and space orientation, temporary and delayed memory, calculation ability, language ability, and visual space ability.5 The MMSE scores were corrected by Mungas adjustment to obtain the adjusted MMSE (MMSEadj) scores.6 The MES test is a further refined neuropsychological assessment based on the MMSE and includes memory and executive function tests totaling 100 points. The MES test has high specificity and sensitivity as well as good reliability and validity without a significant ceiling or floor effect.7 All patients underwent the MMSE. The MES test, which requires higher capability of understanding, was administered only to non-stroke patients who were able to finish it.
First, all baseline data and MMSEadj scores were compared between the AF and control groups. The MES scores of the non-stroke patients who could complete the test in the AF group were also compared with their corresponding patient in the control group. Next, for further analysis, each group (AF and control) was divided into two subgroups according to stroke history: the non-stroke subgroup and the stroke subgroup. Scatter diagrams were used to describe the MMSEadj/MES score and CHA2DS2VASc score of each patient with AF. The relationship between cognitive function and the CHA2DS2VASc score was measured by Spearman’s rank correlation coefficient. Finally, a multivariate regression analysis was performed to screen clinical factors affecting the MMSE score.
Statistical analysis
All data were inputted into Microsoft Excel (Microsoft Corp., Redmond, WA, USA). SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for data processing and analyzing. Normally distributed data are expressed as mean ± standard deviation. The independent-sample t test was used for normally distributed data, and the rank sum test was used for data with a non-normal or approximately normal distribution. Categorical variables are presented as rates. The chi squared test was applied for comparison between groups, and Spearman’s rank test was used to verify the correlation of ranked data. Multivariate regression analysis was performed by logistic regression.
Results
Baseline comparison of cognitive function in AF and control groups
Patients in the AF group (n = 150) and control group (n = 150) underwent 1:1 pair-wise matching by age, sex, and stroke history (Table 1). There was no significant difference in the education level, alcohol or smoking history, coronary heart disease, hypertension, thyroid dysfunction, heart rate, or blood pressure between the two groups.
Table 1.
Comparison of baseline data between AF group and control group.
| AF group(n = 150) | Control group(n = 150) | P value | |
|---|---|---|---|
| Age, years | 71.2 ± 11.2 | 71.2 ± 11.2 | 0.935 |
| Education, years | 8.8 ± 4.5 | 8.0 ± 3.9 | 0.053 |
| Male sex | 92 (0.61) | 92 (0.61) | 1.0 |
| Stroke | 20 (0.13) | 20 (0.13) | 1.0 |
| Smoking history | 48 (0.32) | 53 (0.35) | 0.625 |
| Alcohol abuse | 39 (0.26) | 32 (0.21) | 0.342 |
| Coronary heart disease | 48 (0.32) | 52 (0.35) | 0.713 |
| Diabetes | 44 (0.29) | 45 (0.30) | 0.989 |
| Hypertension | 103 (0.68) | 101 (0.67) | 0.902 |
| Thyroid disease | 15 (0.10) | 10 (0.07) | 0.404 |
| Heart rate, bpm | 81.8 ± 19.1 | 76.1 ± 9.4 | 0.051 |
| Systolic blood pressure, mmHg | 133.5 ± 18.7 | 133.9 ± 19.7 | 0.919 |
| Diastolic blood pressure, mmHg | 81.3 ± 8.8 | 79.3 ± 10.8 | 0.066 |
| MMSE score | 24.2 ± 4.0 | 25.6 ± 2.3 | 0.032* |
| MMSEadj score | 25.9 ± 2.9 | 27.6 ± 2.2 | 0.013* |
Data are presented as mean ± standard deviation or n (%).
*P<0.05.
AF, atrial fibrillation; MMSE, Mini-Mental State Examination; MMSEadj score, MMSE score corrected by Mungas adjustment.
The MMSEadj score was significantly lower in the AF than control group (P = 0.013). Forty-two patients in the AF group without a history of stroke were capable of completing the MES test. The MES scores of these 42 patients were also significantly lower than those of the patients in the control group (n = 42, P = 0.01) (Table 2).
Table 2.
Comparison of MES scores between non-stroke AF group and control group.
| Non-stroke AF groupn = 42 | Control groupn = 42 | P value | |
|---|---|---|---|
| Age, years | 62.2 ± 7.7 | 62.2 ± 7.7 | 1.0 |
| Education, years | 10.9 ± 2.9 | 10.6 ± 3.5 | 0.623 |
| Male sex | 24 (0.57) | 24 (0.57) | 1.0 |
| Smoking history | 14 (0.33) | 12 (0.29) | 0.259 |
| Alcohol abuse | 8 (0.19) | 7 (0.17) | 0.187 |
| Coronary heart disease | 10 (0.24) | 9 (0.21) | 0.786 |
| Diabetes | 5 (0.12) | 6 (0.14) | 0.118 |
| Hypertension | 19 (0.45) | 15 (0.36) | 0.320 |
| Thyroid disease | 5 (0.12) | 3 (0.07) | 0.577 |
| MMSE score | 26.7 ± 1.8 | 27.9 ± 1.2 | 0.006* |
| MMSEadj score | 25.9 ± 2.1 | 27.4 ± 2.2 | 0.001* |
| MES score | 78.8 ± 8.1 | 82.9 ± 5.0 | 0.01* |
| MES-memory score | 36.4 ± 6.5 | 40.1 ± 3.9 | 0.007* |
| MES-excuse score | 42.2 ± 3.8 | 42.9 ± 2.5 | 0.398 |
Data are presented as mean ± standard deviation or n (%).
*P<0.05.
AF, atrial fibrillation; MMSE, Mini-Mental State Examination; MMSEadj score, MMSE score corrected by Mungas adjustment; MES, Memory and Executive Screening.
Subgroup analysis of MMSEadj scores in AF and control groups
For further analysis, all patients were divided into four subgroups: the AF non-stroke group (n = 130), AF stroke group (n = 20), control non-stroke group (n = 130), and control stroke group (n = 20). In the AF stroke group, according to the TOAST classification of ischemic stroke, 10 of 20 patients with stroke had cardiogenic embolism and 5 had atherosclerotic stroke; the remaining 5 were difficult to classify because of the lack of information for further diagnosis. The mean MMSEadj score of the AF patients with and without stroke was 24.1 ± 3.4 and 26.2 ± 2.7, respectively. The subgroup analysis in the AF and control groups showed no significant difference in sex, age, education level, alcohol or smoking history, diabetes, hypertension, coronary heart disease, hyperthyroidism or other diseases, blood pressure, or heart rate. However, the MMSEadj scores of patients with AF were significantly lower than those of patients in the control group regardless of stroke history (patients with stroke, P = 0.031; patients without stroke, P = 0.002) (Table 3).
Table 3.
Comparison of MMSE scores among stroke history subgroups.
| AF non-strokesubgroupn = 130 | Control non-stroke subgroupn = 130 | P value | AF stroke subgroupn = 20 | Control stroke subgroupn = 20 | P value | |
|---|---|---|---|---|---|---|
| Age, years | 70.4 ± 11.5 | 70.4 ± 11.5 | 1.0 | 76.2 ± 7.2 | 76.2 ± 9.0 | 0.985 |
| Education, years | 9.0 ± 4.3 | 8.3 ± 4.0 | 0.53 | 7.9 ± 5.2 | 6.4 ± 4.0 | 0.139 |
| Male sex | 84 (0.65) | 84 (0.65) | 1.0 | 8 (0.40) | 8 (0.40) | 1.0 |
| Smoking history | 41 (0.31) | 44 (0.34) | 0.72 | 7 (0.35) | 9 (0.45) | 0.519 |
| Alcohol abuse | 33 (0.25) | 27 (0.21) | 0.48 | 6 (0.30) | 6 (0.30) | 1.0 |
| Coronary heart disease | 38 (0.29) | 43 (0.33) | 0.592 | 10 (0.50) | 9 (0.45) | 0.752 |
| Diabetes | 35 (0.27) | 39 (0.30) | 0.406 | 9 (0.45) | 6 (0.30) | 0.514 |
| Hypertension | 87 (0.67) | 85 (0.65) | 0.896 | 16 (0.80) | 16 (0.80) | 1.0 |
| Thyroid disease | 11 (0.08) | 8 (0.06) | 0.635 | 4 (0.2) | 2 (0.1) | 0.832 |
| Heart rate, bpm | 82.6 ± 19.1 | 77.3 ± 9.6 | 0.079 | 78.9 ± 20.2 | 74.6 ± 8.2 | 0389 |
| Systolic blood pressure, mmHg | 131.6 ± 17.1 | 132.9 ± 19.8 | 0.752 | 146.6 ± 23.7 | 145.5 ± 15.8 | 0.857 |
| Diastolic blood pressure, mmHg | 81.2 ± 9.7 | 79.2 ± 10.9 | 0.071 | 81.7 ± 9.8 | 76.9 ± 9.5 | 0.117 |
| MMSE score | 24.7 ± 3.8 | 26.0 ± 2.0 | 0.025* | 21.7 ± 4.8 | 22.7 ± 2.4 | 0.049* |
| MMSEadj score | 26.2 ± 2.7 | 27.9 ± 2.0 | 0.031* | 24.1 ± 3.4 | 26.2 ± 2.4 | 0.002* |
Data are presented as mean ± standard deviation or n (%).
*P<0.05
AF, atrial fibrillation; MMSE, Mini-Mental State Examination; MMSEadj score, MMSE score corrected by Mungas adjustment.
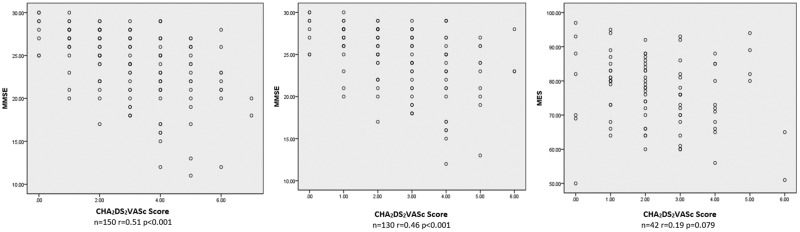
Correlation between cognitive function and CHA2DS2VASc score in patients with AF
The plot diagrams in Figure 1 demonstrate the relationship between cognitive function and the CHA2DS2VASc score. The MMSE score was negatively correlated with the CHA2DS2VASc score in patients with AF (P < 0.001) despite the history of stroke. In non-stroke patients, the MES score also appeared to be inversely associated with the CHA2DS2VASc score, but not significantly.
Figure 1.
Relationship between CHA2DS2VASc score and cognitive function. Left panel and middle panel: plot diagram showing the relationship between the MMSE score and CHA2DS2VASc score in all patients with AF (left) and non-stroke patients with AF (middle). Right panel: relationship between MES score and CHA2DS2VASc score in non-stroke patients with AF. MMSE, Mini-Mental State Examination; AF, atrial fibrillation; MES, Memory and Executive Screening.
Factors influencing cognitive function in non-stroke patients with AF
The median MMSE score in our study was 26. In the single-factor analysis, a higher age, lower education level, stroke, AF, coronary heart disease, hypertension, lower hemoglobin, higher urine protein, and higher pro-BNP were associated with a lower MMSE score (MMSE score of < 26) (Table 4). Among these factors, AF, education, stroke, and age were those that significantly influenced the MMSE score in the multivariate regression (all P < 0.001) (Table 5). In non-stroke patients with AF, a higher MMSE score (MMSE score of ≥26) was significantly associated with a lower age (P < 0.001), higher education level (P < 0.001), lower smoking rate (P = 0.045), lower diastolic blood pressure (P = 0.021), higher rate of receiving anticoagulation therapy (P = 0.022), lower pro-BNP level (P = 0.016), and higher hemoglobin level (P = 0.044) (Table 6). The factors age, sex, education level, anticoagulation, smoking history, diastolic blood pressure, pro-BNP, and hemoglobin were taken into the logistic regression analysis, which showed that the following factors were significantly associated with a higher MMSE score: age (P < 0.001), education level (P < 0.001), smoking history (P = 0.003), anticoagulation (P = 0.03), pro-BNP (P = 0.003), and hemoglobin (P = 0.043). Age, smoking history, and pro-BNP had a negative correlation while education level, anticoagulation, and hemoglobin had a positive correlation with a higher MMSE score (Table 7).
Table 4.
Comparison of features between all patients with higher and lower MMSE scores.
| MMSE score of ≥26n = 159 | MMSE score of < 26n = 141 | P value | |
|---|---|---|---|
| Age, years | 66.4 ± 10.8 | 76.9 ± 9.0 | < 0.001* |
| Education, years | 10.3 ± 3.7 | 6.4 ± 2.9 | < 0.001* |
| Male sex | 101 (0.63) | 83 (0.59) | 0.476 |
| Smoking history | 47 (0.30) | 54 (0.38) | 0.114 |
| Alcohol abuse | 32 (0.20) | 39 (0.28) | 0.136 |
| Heart rate, bpm | 79.1 ± 14.6 | 78.9 ± 16.1 | 0.952 |
| Systolic blood pressure, mmHg | 132.1 ± 17.4 | 135.6 ± 21 | 0.114 |
| Diastolic blood pressure, mmHg | 81.2 ± 9.6 | 79.1 ± 10.9 | 0.07 |
| Stroke | 7 (0.04) | 33 (0.23) | < 0.001* |
| Atrial fibrillation | 70 (0.44) | 80 (0.56) | 0.03* |
| Coronary heart disease | 42 (0.26) | 58 (0.41) | 0.01* |
| Diabetes | 44 (0.28) | 45 (0.31) | 0.448 |
| Hypertension | 98 (0.61) | 106 (0.75) | 0.01* |
| Thyroid disease | 16 (0.10) | 9 (0.06) | 0.128 |
| Antiplatelet therapy | 58 (0.36) | 63 (0.45) | 0.159 |
| Anticoagulation | 36 (0.23) | 23 (0.16) | 0.191 |
| ACEI/ARB | 51 (0.32) | 55 (0.39) | 0.228 |
| Statins | 45 (0.28) | 53 (0.38) | 0.108 |
| β-blocker | 49 (0.31) | 49 (0.35) | 0.538 |
| Hemoglobin, g/L | 128.7 ± 24.9 | 121 ± 21.4 | 0.006* |
| Platelets, 1012/L | 192.1 ± 63.6 | 188.7 ± 72.8 | 0.401 |
| White blood cells, 109/L | 6.1 ± 2.1 | 6.3 ± 2.4 | 0.394 |
| Neutrophil-to-lymphocyte ratio, % | 3.2 ± 2.6 | 4.0 ± 4.1 | 0.08 |
| Troponin T, ng/mL | 0.03 ± 0.09 | 0.04 ± 0.11 | 0.523 |
| Albumin, g/L | 37.8 ± 6.2 | 37.3 ± 6.3 | 0.506 |
| Urine protein | 31 (0.19) | 44 (0.31) | 0.023* |
| NT-proB-type natriuretic peptide, pg/mL | 559.2 ± 1058.2 | 1338.5 ± 3322.6 | 0.008* |
| Low-density lipoprotein, mmol/L | 2.5 ± 1.1 | 2.4 ± 1 | 0.363 |
| Serum creatinine, µmol/L | 78.6 ± 42.7 | 86.5 ± 55.8 | 0.161 |
| Uric acid, µmol/L | 352.9 ± 117.1 | 358.9 ± 106.9 | 0.654 |
Data are presented as mean ± standard deviation or n (%).
*P < 0.05.
MMSE, Mini-Mental State Examination; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 5.
Multivariate logistic regression analysis of risk factors for poor cognitive function in all patients.
| Risk factors | B value | P value | OR | 95% CI |
|---|---|---|---|---|
| Age | −0.090 | <0.001* | 0.914 | 0.881–0.948 |
| Education | 0.280 | <0.001* | 1.323 | 1.201–1.457 |
| Stroke | −2.421 | <0.001* | 0.089 | 0.027–0.293 |
| Atrial fibrillation | −1.799 | <0.001* | 0.165 | 0.071–0.287 |
| Coronary heart disease | −0.410 | 0.257 | 0.663 | 0.326–1.349 |
| Hypertension | −0.538 | 0.138 | 0.584 | 0.287–1.189 |
| NT-proB-type natriuretic peptide | −0.356 | 0.137 | 0.701 | 0.438–1.120 |
| Hemoglobin | 0.012 | 0.136 | 1.012 | 0.996–1.028 |
| Urine protein | −0.512 | 0.221 | 0.600 | 0.264–1.361 |
*P<0.05.
OR, odds ratio; CI, confidence interval.
Table 6.
Comparison of features between non-stroke AF patients with higher and lower MMSE scores.
| MMSE score of ≥26n = 67 | MMSE score of <26n = 63 | P value | |
|---|---|---|---|
| Age, years | 64.9 ± 10.6 | 76.3 ± 9.5 | <0.001* |
| Education, years | 10.7 ± 4.0 | 7.0 ± 4.0 | <0.001* |
| Male sex | 45 (0.67) | 39 (0.62) | 0.584 |
| Smoking history | 17 (0.25) | 27 (0.43) | 0.045* |
| Alcohol abuse | 12 (0.18) | 20 (0.32) | 0.102 |
| Heart rate, bpm | 81.7 ± 18.1 | 82.8 ± 19.9 | 0.723 |
| Systolic blood pressure, mmHg | 131.3 ± 16.7 | 131.8 ± 17.7 | 0.854 |
| Diastolic blood pressure, mmHg | 83.0 ± 9.5 | 79.4 ± 7.6 | 0.021* |
| Coronary heart disease | 15 (0.22) | 22 (0.34) | 0.124 |
| Diabetes | 16 (0.24) | 19 (0.30) | 0.271 |
| Hypertension | 40 (0.60) | 47 (0.74) | 0.093 |
| Thyroid disease | 8 (0.13) | 11 (0.17) | 0.261 |
| Radiofrequency ablation | 13 (0.19) | 5 (0.08) | 0.076 |
| Antiplatelet therapy | 27 (0.40) | 31 (0.49) | 0.378 |
| Anticoagulation | 36 (0.54) | 21 (0.33) | 0.022* |
| ACEI/ARB | 30 (0.45) | 32 (0.51) | 0.598 |
| Statins | 20 (0.30) | 23 (0.37) | 0.495 |
| β-blocker | 30 (0.45) | 28 (0.44) | 0.970 |
| Hemoglobin g/L | 130.8 ± 19.5 | 123.5 ± 21.6 | 0.044* |
| Platelets, 1012/L | 191.1 ± 63.6 | 187.3 ± 63.9 | 0.739 |
| White blood cells, 109/L | 6.5 ± 2.5 | 6.9 ± 2.5 | 0.389 |
| Troponin T, ng/mL | 0.02 ± 0.03 | 0.04 ± 0.11 | 0.548 |
| Albumin, g/L | 37.7 ± 4.6 | 37.2 ± 5.0 | 0.854 |
| Urine protein | 11 (0.16) | 15 (0.24) | 0.518 |
| NT-proB-type natriuretic peptide, pg/mL | 992.3 ± 1517.2 | 2292.9 ± 3330.8 | 0.016* |
| Low-density lipoprotein, mmol/L | 2.0 ± 1.2 | 2.1 ± 1.2 | 0.366 |
| Serum creatinine, µmol/L | 86.5 ± 56.5 | 88.3 ± 37.0 | 0.839 |
| Uric acid, µmol/L | 336.6 ± 113.8 | 350.7 ± 121.8 | 0.494 |
| Neutrophil-to-lymphocyte ratio, % | 2.9 ± 1.8 | 4.0 ± 4.1 | 0.265 |
Data are presented as mean ± standard deviation or n (%).
*P<0.05
MMSE, Mini-Mental State Examination; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 7.
Multivariate logistic regression analysis of risk factors for poor cognitive function in non-stroke AF patients.
| Risk factors | B value | P value | OR | 95% CI |
|---|---|---|---|---|
| Age | −0.156 | <0.001* | 0.645 | 0.802–0.912 |
| Sex | −0.194 | 0.764 | 0.855 | 0.233–2.917 |
| Education | 0.334 | <0.001* | 1.397 | 1.190–1.640 |
| Anti-coagulation | 1.189 | 0.03* | 3.285 | 1.121–9.625 |
| Smoking history | −2.152 | 0.003* | 0.116 | 0.027–0.492 |
| Diastolic blood pressure | 0.063 | 0.08 | 1.065 | 0.993–1.142 |
| NT-proB-type natriuretic peptide | −0.443 | 0.003* | 0.642 | 0.479–0.862 |
| Hemoglobin | 0.031 | 0.043* | 1.031 | 1.001–1.062 |
*P<0.05.
OR, odds ratio; CI, confidence interval.
Discussion
The treatment of AF requires the joint participation and cooperation of the patients and their doctors.8 However, cognitive dysfunction may lead to the reduction or loss of self-management capability, which has negative effects on both the treatment and prognosis. AF is currently regarded as an independent risk factor for cognitive dysfunction.1–4 Two multicenter randomized controlled trials, ONTARGET and TRANSCEND, drew the similar conclusion that AF was independently associated with an increased risk of dementia.1 Therefore, AF and cognitive decline could together form a vicious circle resulting in a poor prognosis.
China has the largest population of patients with stroke and AF worldwide. Cognitive dysfunction is common, but studies of its relationship with AF are still insufficient in China. Compared with the other studies mentioned above, our study enrolled 1:1 matched Chinese patients to avoid the interference of confounding factors. Two scores (MMSE and MES) were used to evaluate cognitive function. The relationship between the CHA2DS2VASc score and cognitive function was also explored.
Stroke is an important factor affecting cognitive function.9 To avoid confusion, our patients were divided into stroke and non-stroke subgroups. In patients with stroke, the MMSEadj scores were still significantly lower than those in patients with sinus rhythm, which corresponds with the theory that AF may increase the risk of dementia in patients with stroke. Most cases of stroke in our study were associated with cardiogenic embolism, and the MMSEadj scores were lower. This is probably because cardiogenic embolism usually causes a larger area of cerebral infarction than cerebrovascular thrombus in situ, and the cerebral infarction area is associated with the decline in cognitive function.10
Both the MMSEadj and MES scores were lower in the non-stroke AF subgroup than in the control subgroup, and no significant difference was found between the two subgroups. The MES test was introduced especially for the evaluation of memory function and executive function. The MES scores in this study demonstrated that the memory function was worse in the AF than control group, while there was no significant difference in the executive function between these two groups. The memory impairment was more obvious than the decline in execution function among patients with AF.
Several authors have stated that AF damages cognitive function through the irregular rhythm and abnormal ventricular rate (>90 or < 50 bpm), which leads to an abnormal distribution of cerebral blood flow.11 However, the present study showed no difference in heart rate between patients with high and low MMSE scores in the non-stroke AF group. Instead, the MMSE score was significantly correlated with the CHA2DS2VASc score. Higher CHA2DS2VASc scores are associated with a higher prevalence of cardiogenic embolism. AF can sometimes cause silent cerebral ischemia, which has been found to be associated with poor cognitive function in previous studies.12,13 Based on our study, the correlation between the MMSE/MES scores and CHA2DS2VASc score might be explained by increased subclinical cerebral ischemia in non-stroke AF patients, suggesting that anticoagulation might be beneficial in those patients.
The multivariate analysis showed that age, education level, stroke, and AF were the factors that influenced the MMSE score in all patients in this study. Stroke is associated with cognitive dysfunction, and the association between AF and cognitive impairment has been proven. Therefore, we also identified factors influencing cognitive function in patients with AF without a history of stroke. Age and educational attainment remained associated with the MMSE score in this population, while smoking, anticoagulation therapy, and the levels of hemoglobin and pro-BNP were found to be risk factors. Our study showed that the patients with lower MMSE scores had a higher incidence of coronary heart disease, diabetes, and thyroid disease and a higher rate of alcohol abuse than those with higher scores; however, the differences were not statistically significant. Whether these factors have an effect on cognitive function remains controversial.
A lower hemoglobin level is associated with worse cognitive function as induced by chronic brain hypoxia and demonstrated by white matter hyperintensity on imaging examinations.14,15 Several causes of anemia (e.g., iron deficiency, lack of folate or vitamin B12) may also directly damage cognitive function.16
As reported in several previous studies, pro-BNP is a marker of cardiac dysfunction that contributes to cognitive decline, thus influencing self-care management.17–19 A higher level of pro-BNP was a risk factor contributing to cognitive impairment in the present study. A higher pro-BNP level may also be associated with increased intracranial pressure, further influencing cognitive function.19 Some authors have stated that a high pro-BNP level was associated with cerebral atherosclerotic disease.17 Anticoagulation therapy was found to be a protective factor for the cognitive function of patients with AF. According to a previous study, patients with both AF and dementia could benefit from anticoagulant therapy.20 Another study of 2605 patients with AF showed that the time within the therapeutic range was significantly associated with dementia in patients with AF. This shorter time may be associated with a higher risk of dementia.21 This can be explained by chronic cerebral damage (such as micro-infarction lesions), which may cause cognitive dysfunction in patients with AF.22 Our study suggests that anticoagulation may be a protective factor in patients with AF. Conversely, patients with AF who have better cognitive function are more likely to receive anticoagulation therapy because of their better self-management.
Study limitations
This study has several limitations. The study had a cross-sectional design. Assessment of cognitive function using the neuropsychological scales was highly dependent upon the patients’ cooperation. Some patients with AF were unable to complete the assessment because of their diseases. Finally, neural imaging examinations were not performed in most patients without stroke.
Conclusions
This cross-sectional clinical study indicates that patients with AF, independent of stroke, have worse cognitive function than those with sinus rhythm. Even in patients without stroke, a high CHA2DS2VASc score is associated with impaired cognitive function. Our data show that the cognitive impairment of patients without stroke was partially due to the lack of intervention for patients with a high CHA2DS2VASc score. In non-stroke patients with AF, anticoagulation and education level are protective factors while age, anemia, a high pro-BNP level, and cigarette smoking are risk factors for poor cognitive dysfunction.
Acknowledgement
We highly appreciate Dr. Qihao Guo, who serves as a neurologist in Huashan Hospital Fudan University. He helped us with the diagnosis, classification, and cognitive function evaluation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The work was supported by funding from Huashan Hospital Fudan University [Grant number: Hospital 716].
References
- 1.Marzona I, O'Donnell M, Teo K, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. Can Med Assoc J 2012; 184: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myserlis PG, Malli A, Kalaitzoglou DK, et al. Atrial fibrillation and cognitive function in patients with heart failure: a systematic review and meta-analysis. Heart Fail Rev 2017; 22: 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Singh-Manoux A, Fayosse A, Sabia S, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J 2017; 38: 2612–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Requena Calleja MA, Arenas Miquélez A, Díez-Manglano J, et al. Sarcopenia, frailty, cognitive impairment and mortality in elderly patients with non-valvular atrial fibrillation. Rev Clin Esp 2019; 219: 424–432. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ. . A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009; 43: 411–431. [DOI] [PubMed] [Google Scholar]
- 6.Mungas D, Marshall SC, Weldon M, et al. Age and education correction of Mini-Mental State Examination for English- and Spanish-speaking elderly. Neurology 1996; 46: 700–706. [DOI] [PubMed] [Google Scholar]
- 7.Guo QH, Zhou B, Zhao QH, et al. Memory and Executive Screening (MES): a brief cognitive test for detecting mild cognitive impairment. BMC Neurol 2012; 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 9.Kaffashian S, Dugravot A, Brunner EJ. Midlife stroke risk and cognitive decline: a 10-year follow-up of the Whitehall II cohort study. Alzheimers Dement 2013; 9: 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996; 27: 1760–1764. [DOI] [PubMed] [Google Scholar]
- 11.Cacciatore F, Testa G, Langellotto A, et al. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: a 10-year study. Dement Geriatr Cogn 2012; 34: 143–148. [DOI] [PubMed] [Google Scholar]
- 12.Conen D, Rodondi N, Muller A, et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol 2019; 73: 989–999. [DOI] [PubMed] [Google Scholar]
- 13.Graffradford J, Madhavan M, Vemuri P, et al. Atrial fibrillation, cognitive impairment, and neuroimaging. Alzheimers Dement 2015; 12: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Le W. Pathological role of hypoxia in Alzheimer's disease. Exp Neurol 2010; 223: 299–303. [DOI] [PubMed] [Google Scholar]
- 15.Inzitari M, Studenski S, Rosano C, et al. Anemia is associated with the progression of white matter disease in older adults with high blood pressure: the cardiovascular health study. J Am Geriatr Soc 2008; 56: 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris MC, Schneider JA, Tangney CC. Thoughts on B-vitamins and dementia. J Alzheimers Dis 2006; 9: 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman M, Callas PW, Mcclure LA, et al. N-terminal pro-B-type natriuretic peptide and risk of future cognitive impairment in the REGARDS cohort. J Alzheimers Dis 2016; 54: 497–503. [DOI] [PubMed] [Google Scholar]
- 18.Hilal S, Chai YL, Ikram MK, et al. Markers of cardiac dysfunction in cognitive impairment and dementia. Medicine 2015; 94: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito J, Naka Y, Watanabe H. Clinical impression of brain natriuretic peptide levels in demented patients without cardiovascular disease. Geriatr Gerontol Int 2009; 9: 242–254. [DOI] [PubMed] [Google Scholar]
- 20.Madhavan M, Holmes DN, Piccini JP, et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J 2019; 211: 77–89. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs V, Woller SC, Stevens S, et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm 2014; 11: 2206–2213. [DOI] [PubMed] [Google Scholar]
- 22.Knecht S, Oelschläger C, Duning T, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J 2008; 29: 2125–2132. [DOI] [PubMed] [Google Scholar]