Short abstract
Objectives
Bisphenol A diglycidyl ether (BADGE) is an antagonist for PPARγ that reduces bone marrow adiposity and increases bone formation in some animal models of osteoporosis and osteonecrosis. However, the effect of BADGE treatment on glucocorticoid-induced osteoporosis is unknown. This study investigated the preventive effects of BADGE on steroid-induced osteoporosis in mice.
Methods
Thirty-six female C57BL/6J mice were randomly divided into normal (phosphate-buffered saline), model (50 mg/kg dexamethasone sodium phosphate [Dex]), and BADGE (30 mg/kg of BADGE, combined with Dex) groups. All groups received intraperitoneal injections of their treatments, daily for 4 weeks. Protein and mRNA expression levels of gene markers were measured. Micro-computed tomography was used to measure physical parameters of femurs. Bone histomorphology was analyzed by hematoxylin and eosin staining. ELISA was used to measure serum osteocalcin and C-terminal telopeptide of type I collagen (CTX-1).
Results
Glucocorticoid treatment enlarged the marrow fat, concomitant with bone deterioration; BADGE treatment reversed steroid-induced marrow adiposity. Compared with the model group, BADGE treatment improved bone quality and increased bone volume, while increasing osteogenic markers and reducing adipogenic markers at both mRNA and protein levels; moreover, it reduced serum CTX-1 and increased serum osteocalcin.
Conclusion
BADGE treatment ameliorates glucocorticoid-induced osteoporosis by inhibiting PPARγ.
Keywords: BADGE, bone loss, marrow fat, PPARγ, Runx2, glucocorticoid, osteoporosis, mice
Introduction
Steroid-related osteoporosis is a common side effect of steroid therapy.1 Approximately 30% to 50% of patients experience considerable bone loss under long-term glucocorticoid (GC) treatment.2,3 Both the endogenous overproduction of cortisol and chronic glucocorticoid therapy are associated with marked bone marrow adiposity and low bone mineral density, which increase the risk of fracture.4 Fractures in osteoporotic individuals are associated with increased mortality compared with that of healthy individuals; thus, steroid-related osteoporosis are the focus of pharmaceutical therapeutics and regenerative medicine.5
Marrow fat is reportedly increased in patients with glucocorticoid-related osteoporosis.6–8 High concentrations and long durations of steroid treatment can induce bone marrow mesenchymal stem cells (BMSCs) to differentiate into a small number of osteoblasts and a large number of adipocytes.9,10 When BMSCs differentiate into adipocytes, they can no longer be induced to differentiate into osteoblasts. The BMSCs of glucocorticoid-related osteoporotic mice tend to differentiate into adipocytes, but not osteoblasts.11,12 Thus, inhibition of bone marrow adipogenesis presumably could prevent or limit bone loss, based on the close relationship between adipocyte and osteoblast differentiation.
The process of adipocyte differentiation is regulated by many factors. PPARγ, a master regulator of adipocyte differentiation, is closely involved in the adipocyte differentiation of BMSCs. Overexpression of PPARγ increases adipocyte differentiation and decreases osteoblast differentiation, which leads to increased lipid generation and reduced bone formation.13,14 Akune et al.15,16 showed that PPARγ deletion reduced marrow fat and increased bone mass. Bisphenol A diglycidyl ether (BADGE), an antagonist of PPARγ, has been reported to inhibit adipogenesis both in vitro and in vivo.17,18 In mice with age-related osteoporosis, BADGE treatment resulted in reduced marrow adipogenesis, concomitant with increasing levels of osteoblastogenesis and bone formation.19 Moreover, BADGE treatment improved bone formation, concomitant with reduced marrow adiposity in rabbits with steroid-related osteonecrosis.20 However, the effect of BADGE treatment on glucocorticoid-related osteoporosis is unknown. This study was performed to investigate whether BADGE treatment could prevent dexamethasone-induced bone deterioration, and to explore the potential underlying mechanisms of this effect.
Materials and methods
Steroid-induced bone loss in mice
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (no. SY02518072). Thirty-six female C57BL/6J mice (8 weeks old; weight, 21 ± 2.4 g) were housed in a standard animal research environment with consistent temperature and humidity; they had free access to drinking water and food. One week after acclimation to the environment, the mice were randomly divided into three groups—the model group (n = 12) received intraperitoneal injections of 50 mg/kg of dexamethasone sodium phosphate (Dex), daily for 4 weeks;21 the normal group (n = 12) received intraperitoneal injections of phosphate-buffered saline; and the BADGE group (n = 12) received intraperitoneal injections of 30 mg/kg of BADGE, combined with Dex, daily for 4 weeks.22 At the endpoint, animals were anesthetized with pentobarbital sodium and blood samples were obtained; animals were then euthanized by an overdose of pentobarbital sodium. Femurs were dissected from the euthanized mice for analysis in this study.
Micro-computed tomography (µCT) analysis
Left femurs were fixed in 4% paraformaldehyde overnight and scanned with a SCANCO MEDICAL μCT 50 (SCANCO, Brüttisellen, Zurich, Switzerland) to quantify structural parameters. The following parameters were used: X-ray intensity, 100 kVp, 98 μA; slice thickness, 10 μm; rotation between frames, 0.9°; scanning resolution, 1024 × 1024; exposure time, 300 ms; and scanning regions, 7 to 10 mm of the distal femurs. Two-dimensional images of the femurs were used for three-dimensional reconstruction. Measurements were made of the trabecular thickness (Tb.Th), bone mineral density (BMD), trabecular number (Tb.N), and bone volume/total volume (BV/TV).
Histopathology
After left femurs were fixed in 4% paraformaldehyde for 24 hours, they were decalcified in 10% ethylenediaminetetraacetic acid and then embedded in paraffin. The blocks of paraffin were then cut into 4-μm sections and stained with hematoxylin and eosin. The adipocyte number (Ad.N/MV), adipocyte volume (Ad.V/MV), and adipocyte size (Ad.Size/MV) were calculated as in the study by Ko et al.23
PCR analysis
Total RNA from right femurs was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed with the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara Biomedical Technology, Beijing, China). Subsequently, 2 µL cDNA was amplified by using the StepOne™ Real-Time PCR kit (Life Technologies, Beijing, China), in the TC-XP thermocycler (BIOER TECHNOLOGY, Hangzhou, China); primers were specific for the following genes: PPARγ: forward, 5'-TGTCTCACAATGCCATCAGGT-3' and reverse, 5'-TGTGATCTCTTGCACGGCTT-3'; aP2 (FABP4): forward, 5'-TTGGTCACCATCCGGTCAGA-3' and reverse, 5'-CATTCCACCACCAGCTTGTCA-3'; Runx2: forward, 5'-GCGGTGCAAACTTTCTCCAG-3' and reverse, 5'-ACCCAGTCCCTGTTTTAGTTGT-3'; and osteocalcin (OCN): forward, 5'-CCTTGGGTTCTGACTGGGTG-3' and reverse, 5'-GGCCACTTACCCAAGGTAGC-3'. β-actin served as the internal standard. Relative gene expression levels were quantified using the ΔΔCt method.
Western blot
Total protein from right femurs was crushed under liquid nitrogen conditions and prepared in ice-cold RIPA lysis buffer. Protein extracts were resolved by using 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 10% non-fat milk, then incubated with primary antibodies overnight at 4°C; this was followed by incubation at room temperature with horseradish peroxidase-labelled goat anti-rabbit IgG (1:10,000 dilution in 5% skim milk; Cat. No. AS1058; Aspen, Wuhan, China). Protein blots were analyzed using an image analysis system (AlphaEaseFC; Alpha Innotech Corporation, San Leandro, CA, USA). An anti-β-actin antibody was used as a loading control (ab37168, 1:10,000 dilution in 5% skim milk; Abcam, Cambridge, MA, USA). The primary antibodies used were anti-PPARγ (ab59256, 1:500 dilution in 5% skim milk), anti-OCN (ab23981, 1:1000 dilution in 5% skim milk), and anti-RUNX2 (ab93876, 1:500 dilution in 5% skim milk) (Abcam).
ELISA
To evaluate the levels of bone resorption and formation markers in all mice, serum levels of mouse OCN and C-terminal telopeptides of type I collagen (CTX-1) were measured with ELISA kits (Bio-Swamp, Wuhan, China), in accordance with the manufacturer’s instructions.
Statistical analysis
For comparison, two-tailed t-test or one-way analysis of variance were conducted using Prism 6.01 (GraphPad Software Inc., La Jolla, CA, USA). The Student–Newman–Keuls method was used for multiple comparisons among groups. Data are presented as the mean ± standard deviation. Differences with P < 0.05 were considered statistically significant.
Results
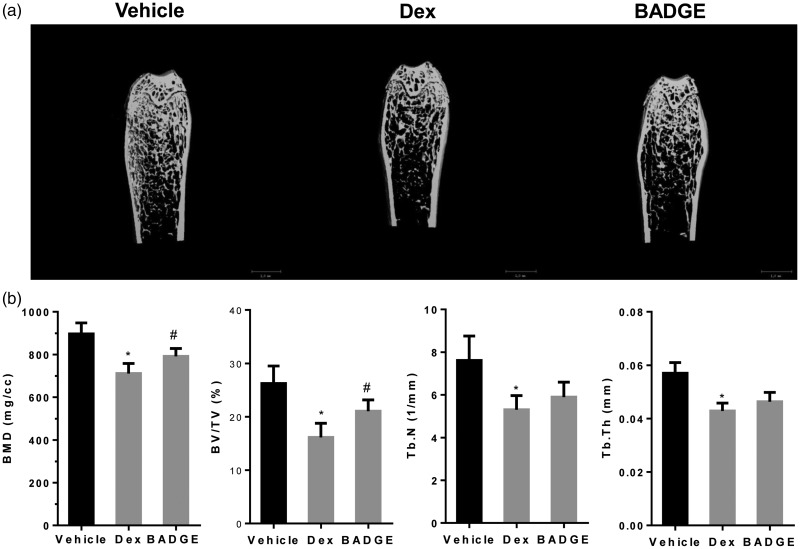
BADGE treatment ameliorated glucocorticoid-induced bone mass loss
μCT analyses showed that Dex treatment led to significantly reduced trabecular BV/TV and BMD, as well as reduced Tb.N and Tb.th (Figure 1a,b). Thus, BADGE treatment improved BMD and trabecular BV/TV, while ameliorating the cortical bone reduction and bone mass loss, compared with those parameters in the model group (P < 0.05 for all comparisons, Figure 1a,b).
Figure 1.
BADGE ameliorates glucocorticoid induced bone mass loss. (a) Representative two-dimensional reconstruction of the femur. (b) Micro-computed tomography revealed that, compared with the normal group, dexamethasone treatment reduced Tb.Th, BV/TV, BMD, and Tb.N. Compared with the model group, BADGE treatment significantly increased trabecular BV/TV and BMD (n = 10). #P < 0.05 vs. model group and *P < 0.05 vs. normal group. BADGE, bisphenol A diglycidyl ether; Tb.Th, trabecular thickness; BV/TV, bone volume/total volume; BMD, bone mineral density; Tb.N, trabecular number.
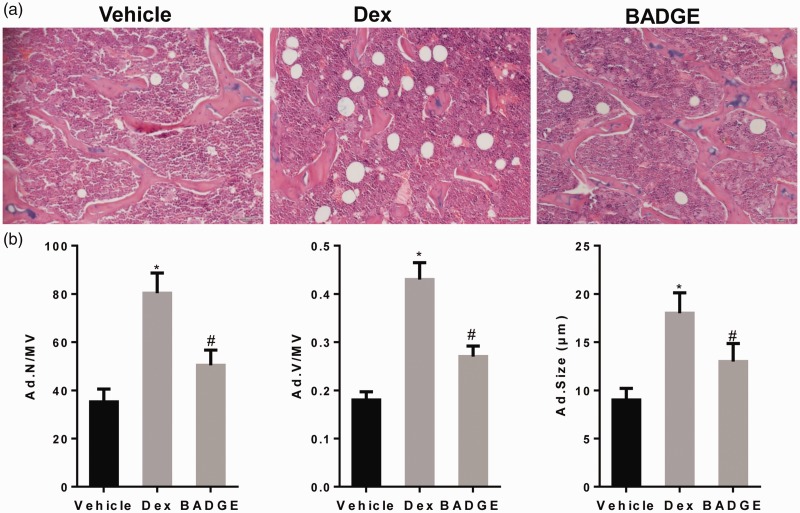
BADGE treatment reduced glucocorticoid-induced bone marrow fat
As shown in Figure 2a and b, more fat cells accumulated in the bone marrow in the steroid-treated group than in the normal group. Thus, BADGE treatment ameliorated glucocorticoid-induced marrow adiposity.
Figure 2.
BADGE treatment reduces glucocorticoid-induced bone marrow fat. (a) Images captured at original magnifications of ×20. In the model group, many fat cells accumulated in the bone marrow, whereas BADGE treatment significantly reduced the volume of marrow fat. (b) BADGE treatment reduced Ad.Size/MV, Ad.V/MV, and Ad.N/MV, which were promoted by dexamethasone in bone tissue. #P < 0.05 vs. model group and *P < 0.05 vs. normal group. BADGE, bisphenol A diglycidyl ether; Ad.Size/MV, adipocyte size; Ad.V/MV, adipocyte volume; Ad.N/MV, adipocyte number.
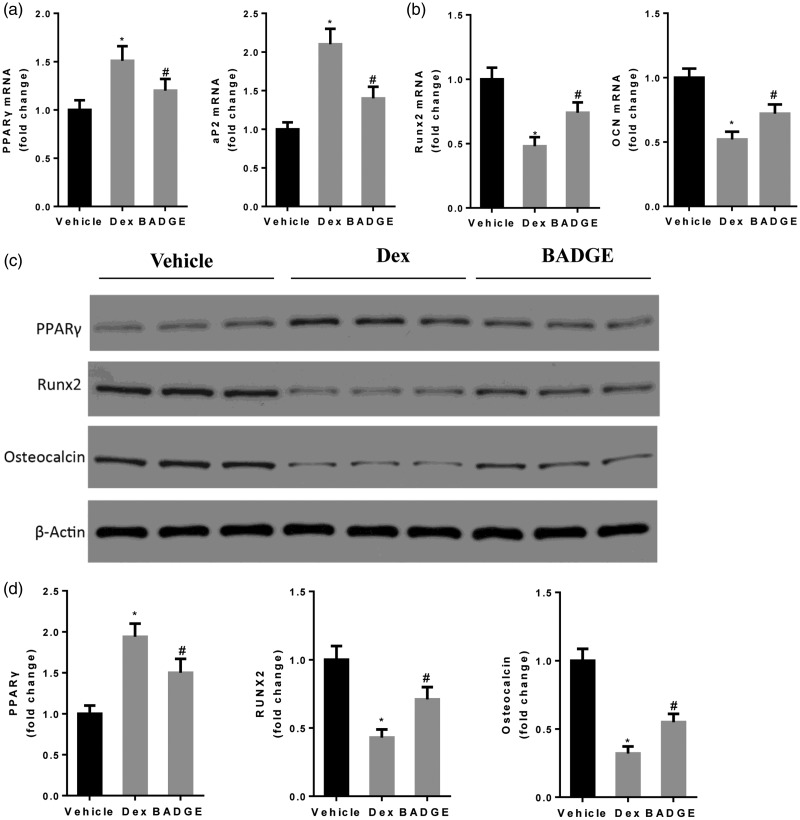
BADGE treatment increased expression levels of osteogenic markers, concomitant with reduced expression levels of adipogenic markers
As shown in Figure 3, compared with the normal group, Dex treatment increased the expression levels of adipogenic markers PPARγ and aP2, but reduced the expression levels of osteogenic markers Runx2 and osteocalcin. The BADGE group exhibited increased expression levels of Runx2 and OCN, as well as reduced expression levels of PPARγ at both the protein and mRNA levels, compared with the model group (P < 0.05 for all comparisons). The mRNA expression level of aP2 was also significantly reduced in the BADGE group, compared with the model group (P < 0.05).
Figure 3.
BADGE treatment increases the expression levels of osteogenic markers and reduces the expression levels of adipogenic markers. (a) BADGE treatment significantly reduced mRNA expression levels of PPARγ and aP2 (n = 12). (b) BADGE treatment increased mRNA expression levels of OCN and Runx2 (n = 12). (c, d) Compared with the model group, BADGE treatment reduced the protein expression level of PPARγ and increased the protein expression levels of OCN and Runx2. *P < 0.05 vs. normal group and #P < 0.05 vs. model group. BADGE, bisphenol A diglycidyl ether; OCN, osteocalcin.
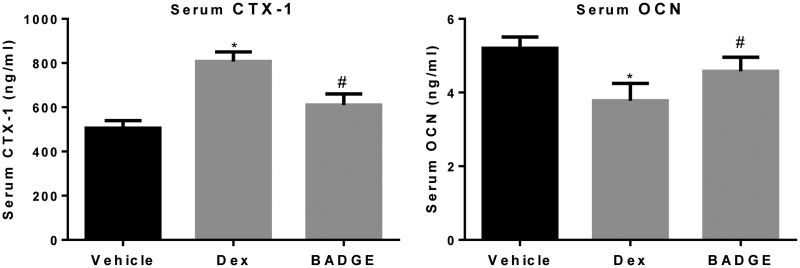
BADGE treatment reduced the level of bone resorption marker concomitant with increased level of bone formation marker
We analyzed the effects of BADGE on the serum levels of a total bone resorption marker, CTX-1, and a bone formation marker, OCN. As shown in Figure 4, compared with the normal group, Dex treatment increased the serum level of CTX-1 and reduced the serum level of OCN. BADGE treatment significantly reduced the serum level of CTX-1 and increased the serum level of OCN, compared with the model group (P < 0.05). The increased bone mass with BADGE treatment was demonstrated to partly result from increased bone formation, concomitant with inhibition of bone resorption.
Figure 4.
BADGE treatment reduces the serum level of CTX-1 while increasing the serum level of OCN. Compared with the normal group, the serum level of CTX-1 was higher and the serum level of OCN was lower in the model group (P < 0.05). BADGE treatment reduced the serum level of CTX-1 and increased the serum level of OCN, compared with the model group (n = 12 for all analyses). #P < 0.05 vs. model group and *P < 0.05 vs. normal group. BADGE, bisphenol A diglycidyl ether; OCN, osteocalcin; CTX-1, C-terminal telopeptide of type I collagen
Discussion
In the current study, BADGE, a PPARγ inhibitor, was found to ameliorate steroid-induced osteoporosis in a mouse model. BADGE treatment increased bone mass, concomitant with reduced infiltration of marrow fat. Expression levels of osteogenic markers were increased, whereas expression levels of adipogenic markers were reduced. Moreover, BADGE treatment reduced the serum level of CTX-1 and increased the serum level of OCN.
Zhang et al.24 demonstrated that BMSCs derived from osteoporotic mice had significantly lower osteogenesis potential and higher adipogenesis potential, compared with those parameters in normal BMSCs; moreover, human mesenchymal stem cell (hMSC)-derived osteoblasts co-cultured with hMSC-derived adipocytes expressed typical amounts of adipogenic genes, but low amounts of osteogenic markers. Martin et al.25 showed that human MSC-derived adipocytes secrete extracellular vesicles that can be transferred to osteoblasts; these vesicles contain anti-osteogenic miRNAs and adipocyte-specific mRNAs.
PPARγ stimulates adipogenesis at the expense of osteoblastogenesis.26 PPARγ deletion induces high bone mass and low marrow fat,15 while activation of PPARγ can induce bone loss and stimulate bone marrow adiposity.27,28 Steroid treatment reportedly increases the gene expression of PPARγ, both in vivo and in vitro.10 BADGE treatment inhibits adipogenesis and reduces fatty marrow;17,18 notably, BADGE treatment has been shown to increase osteoblastogenesis in mouse bone marrow stromal cells in vitro. Gustavo et al.17 indicated that BADGE treatment increased bone mass and osteoblastogenesis in C57BL/6 mice. However, Li et al.29 reported that early BADGE treatment could not increase bone formation in ovariectomized rats, although it could reduce marrow adiposity. Recently, Yuan et al.20 reported that BADGE treatment improved bone formation, concomitant with reduced marrow adiposity, in a rabbit model of steroid-related osteonecrosis. In the current study of mice with glucocorticoid-induced osteoporosis, we found that BADGE treatment increased bone mass and reduced marrow fat infiltration. Importantly, BADGE treatment increased the expression levels of Runx2 and OCN, while reducing the expression levels of PPARγ and aP2.
Glucocorticoid treatment increased the serum level of CTX-1 and reduced the level of OCN, a bone formation marker.4 Zhu et al.30 reported that adipocytes differentiated from BMSCs significantly promoted osteoclast formation. Wan and colleagues reported that PPARγ regulates osteoclastogenesis in mice, and that PPARγ deletion reduced osteoclast differentiation. Treatment with antagonists of PPARγ, such as GW9662 and T0070907, reduced osteoclast gene expression. In contrast, osteoclast differentiation was increased by treatment with rosiglitazone, an agonist of PPAR-γ.31 However, Zhao et al.32 reported that both rosiglitazone and pioglitazone treatment inhibited osteoclastogenesis in a dose-dependent manner. In the current study, we also analyzed the effects of BADGE treatment on serum levels of CTX-1 and OCN. We found that dexamethasone treatment reduced the serum level of OCN and increased the serum level of CTX-1. Compared with the model group, BADGE treatment reduced the serum level of CTX-1 and increased the serum level of OCN. However, we could not distinguish whether BADGE reduced bone resorption markers through effective suppression of fatty marrow or by directly affecting osteoclast differentiation in bone. Further studies are needed to identify the direct effects of BADGE treatment on osteoclast differentiation.
A limitation of our study was that it assessed the preventive effect of BADGE treatment on glucocorticoid-induced osteoporosis, but did not assess its treatment effect. Further studies are thus needed to determine the treatment effect of BADGE on glucocorticoid-induced osteoporosis. BADGE may be effective for clinical prevention or treatment of glucocorticoid-induced osteoporosis in the near future.
Taken together, our data showed that the inhibition of PPARγ by BADGE ameliorated glucocorticoid-induced osteoporosis. BADGE treatment increased bone mass, concomitant with reduced infiltration of marrow fat. Treatment with BADGE increased the transcription of osteogenic genes and reduced the transcription of adipogenic genes. Moreover, BADGE administration reduced the serum level of the bone resorption marker, CTX-1, and increased the serum level of the bone formation marker, OCN, which may have contributed to the increase in bone mass.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The present study was supported by the Technology Innovation Seed Foundation of Zhongnan Hospital of Wuhan University, China (grant no. WJ2018H0002).
References
- 1.Frenkel B, White W, Tuckermann J. Glucocorticoid-induced osteoporosis. Adv Exp Med Biol 2015; 872: 179–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melton LJ., 3rd. How many women have osteoporosis now? J Bone Miner Res 1995; 10: 175–177. [DOI] [PubMed] [Google Scholar]
- 3.Guanabens N, Gifre L, Peris P. The role of Wnt signaling and sclerostin in the pathogenesis of glucocorticoid-induced osteoporosis. Curr Osteoporos Rep 2014; 12: 90–97. [DOI] [PubMed] [Google Scholar]
- 4.Wang FS, Lian WS, Weng WT, et al. Neuropeptide Y mediates glucocorticoid-induced osteoporosis and marrow adiposity in mice. Osteoporos Int 2016; 27: 2777–2789. [DOI] [PubMed] [Google Scholar]
- 5.Svejme O, Ahlborg HG, Nilsson JÅ, et al. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG 2012; 119: 810–816. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 2012; 41: 595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao W, Cheng Z, Busse C, et al. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum 2008; 58: 1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13: 777–787. [DOI] [PubMed] [Google Scholar]
- 9.Gu C, Xu Y, Zhang S, et al. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARgamma and GREM1. Sci Rep 2016; 6: 38491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Li Y, Wang Y, et al. Preventive effects of siRNA targeting PPARgamma gene on steroid-induced osteonecrosis in rabbits. Connect Tissue Res 2014; 55: 322–330. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone 2012; 50: 540–545. [DOI] [PubMed] [Google Scholar]
- 12.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 2009; 66: 236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994; 79: 1147–1156. [DOI] [PubMed] [Google Scholar]
- 15.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 2004; 113: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cock TA, Back J, Elefteriou F, et al. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep 2004; 5: 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotolin S, McCabe LR. Inhibition of PPAR gamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 2006; 209: 967–976. [DOI] [PubMed] [Google Scholar]
- 18.Wright HM, Clish CB, Mikami T, et al. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem 2000; 275: 1873–1877. [DOI] [PubMed] [Google Scholar]
- 19.Duque G, Li W, Vidal C, et al. Pharmacological inhibition of PPARgamma increases osteoblastogenesis and bone mass in male C57BL/6 mice. J Bone Miner Res 2013; 28: 639–648. [DOI] [PubMed] [Google Scholar]
- 20.Yuan N, Li J, Li M, et al. BADGE, a synthetic antagonist for PPARgamma, prevents steroid-related osteonecrosis in a rabbit model. BMC Musculoskelet Disord 2018; 19: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Zhang N, Huang X, et al. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis 2013; 4: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009; 460: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko JY, Chuang PC, Ke HJ, et al. MicroRNA-29a mitigates glucocorticoid induction of bone loss and fatty marrow by rescuing Runx2 acetylation. Bone 2015; 81: 80–88. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Ma C, Liu X, et al. Epigenetic landscape in PPARgamma2 in the enhancement of adipogenesis of mouse osteoporotic bone marrow stromal cell. Biochim Biophys Acta 2015; 1852: 2504–2516. [DOI] [PubMed] [Google Scholar]
- 25.Martin PJ, Haren N, Ghali O, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol 2015; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Ko J. A novel PPARgamma2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation. Cell Death Differ 2014; 21: 1642–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picard F, Auwerx J. PPAR gamma and glucose homeostasis. Annu Rev Nutr 2002; 22: 167–197. [DOI] [PubMed] [Google Scholar]
- 28.Ali AA, Weinstein RS, Stewart SA, et al. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 2005; 146: 1226–1235. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Xu Z, Hou L, et al. Differential effects of bisphenol A diglicydyl ether on bone quality and marrow adiposity in ovary-intact and ovariectomized rats. Am J Physiol Endocrinol Metab 2016; 311: E922–E927. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Liu YL, Chen JD, et al. [Effect of osteogenically and adipogenically differentiated bone mesenchymal stem cells from mouse on osteoclast formation]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2012; 20: 1187–1190. [PubMed] [Google Scholar]
- 31.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med 2007; 13: 1496–1503. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Shi Z, Warriner AH, et al. Molecular mechanism of thiazolidinedione-mediated inhibitory effects on osteoclastogenesis. PLoS One 2014; 9: e102706. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]