Short abstract
Objectives
Di(2-ethylhexyl) phthalate (DEHP) is a common endocrine-disrupting chemical, which has potential reproductive toxicity. This study aimed to explore the effects of DEHP exposure in women with polycystic ovary syndrome (PCOS) undergoing in vitro fertilization.
Methods
In this case-control study, DEHP levels in follicular fluid (FF) of women with PCOS (n = 56) and controls (n = 51) were measured. The in vitro effects of DEHP exposure on primary-cultured human granulosa cells (GCs) and a steroidogenic human granulosa-like tumor cell line (KGN cells) were analyzed.
Results
Concentrations of DEHP in FF were significantly higher in women with PCOS than in controls. The clinical pregnancy rate was significantly lower in women with PCOS with high levels of DEHP than in controls. The levels of androgens produced by human GCs were significantly increased following DEHP exposure. Compared with controls, DEHP-treated human GCs and KGN cells showed significantly lower viability, cell cycle arrest, higher apoptosis, and altered expression of apoptosis-related genes.
Conclusion
Women with PCOS are exposed to increased levels of DEHP in follicles, which may be associated with pregnancy loss following in vitro fertilization. DEHP may disrupt steroid production, balance in cellular proliferation, and apoptosis in human granulosa cells.
Keywords: Polycystic ovary syndrome (PCOS), di(2-ethylhexyl) phthalate (DEHP), case-control study, in vitro study, steroid production, apoptosis
Introduction
Polycystic ovary syndrome (PCOS), which affects 5% to 12% of reproductive-aged women,1,2 is the leading cause of sub-fecundity and anovulatory infertility, with characteristics of hyperandrogenism, irregular menstruation, and ovulatory dysfunction.3,4 Women with PCOS who have not responded to first- or second-line ovulation induction therapies or who have additional fertility factors can turn to in vitro fertilization (IVF).5 However, pregnancy outcomes following IVF are not satisfactory because of failed fertilization6 and the risk of ovarian hyperstimulation syndrome.7 Heterogeneous etiology, with genetic, environmental, clinical, and biochemical factors, may be involved in PCOS phenotypes and compromised pregnancy outcomes.8 Remarkably, PCOS patients are thought to represent a subpopulation sensitive to compounds that interfere with the endocrine system.9 For instance, plasticizers such as bisphenol A (BPA) or phthalates, which are endocrine-disrupting chemicals (EDCs), are possible environmental contributors to PCOS pathogenesis.10,11 Serum BPA may be involved in insulin resistance and hyperandrogenism of PCOS.12 The previously reported association of EDCs with menstrual disturbance,13 reduced fecundability,14 and adverse IVF outcomes15 may have been affected by the inclusion of women with PCOS in these studies.
Di(2-ethylhexyl) phthalate (DEHP), is one of the most common EDCs and the most widely used plasticizer indoors, and it can be found in cosmetics, toys, construction materials, and cleaning solutions.16 Concerns have been raised that DEHP is continuously released and exposed in the environment during the use of plastic products.17 DEHP can be absorbed by food and water from contact materials18 and is contained in many medical devices.19 Thus, humans can be exposed to DEHP through oral ingestion, inhalation, and dermal exposure. Furthermore, DEHP is able to cross the placenta, as DEHP and its metabolites have been detected in amniotic fluid, which may result in exposure risk to the developing fetus.20 With the potential to cause reproductive and developmental toxicity, DEHP has shown adverse effects on sexual maturation, fertility, pregnancy, and the female reproductive tract.21 In vitro and in vivo studies have shown that exposure to DEHP impairs folliculogenesis and oocyte maturation in rodents and induces epigenetic changes in germ cells that can be transmitted to subsequent generations.22,23
A few studies have explored the relationship between PCOS and exposure to EDCs (mainly BPA). Considering the high prevalence of PCOS and the potential reproductive toxicity caused by DEHP, we hypothesized that exposure to DEHP may be involved in PCOS and might relate to some of the negative effects on reproduction. The aim of the study was to measure DEHP levels in follicular fluid (FF) of women with and without PCOS and to explore the association of DEHP level with pregnancy outcome in women with PCOS undergoing IVF. Furthermore, as this study aimed to explore the mechanisms underlying the action of DEHP, we also investigated the in vitro effects of DEHP exposure on human granulosa cells (GCs) and the steroidogenic human granulosa-like tumor cell line KGN.
Materials and methods
Participants
The protocol was approved by the Institutional Review Board of School of Medicine, Zhejiang University (Ethics Committee reference number 20170047), and informed consent was obtained from all study participants. Fifty-six infertile women with PCOS and 51 infertile women with tubal blockage (who served as controls) were recruited into this case-control pilot study. Human GCs were collected from an additional 22 infertile women with tubal blockage for the in vitro study. The women with PCOS were diagnosed according to the revised 2003 Rotterdam criteria, indicating PCOS to be present if at least two of the following three criteria are met: oligo- or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries viewed on an ultrasound.24 Inclusion criteria were 20 to 45 years of age, a body mass index (BMI) ≤35, and undergoing IVF. Women with possible ovarian tumors, congenital adrenal hyperplasia, Cushing’s syndrome, diabetes, or cardiovascular disease were excluded. The control subjects had regular menstrual cycles and normal sex hormone levels. No structural abnormalities of the uterus and ovaries were found by vaginal ultrasound or laparoscopy in any of the women. All partners of the women had normal spermiograms and sperm morphology.
All of the women were referred to our department for IVF. They received the long agonist protocol for controlled ovarian hyperstimulation, with administration of recombinant follicle-stimulating hormone (rFSH, Gonal-F; Serono International S.A., Geneva, Switzerland) after being downregulated with triptorelin (Serono International S.A.). The dosages of gonadotropins were individualized according to serum estradiol levels and transvaginal ultrasound measurements of the follicles. When at least one of the follicles reached a diameter of 18 mm, 10,000 IU of human chorionic gonadotropin (Libao Biochemistry Co., Zhuhai, China) was administered 36 hours before ultrasound-guided follicle aspiration.
Sample collection
Peripheral blood was collected on day 3 of a natural menstrual cycle or during withdrawal bleeding. The serum was separated by centrifugation at 3000 × g for 10 minutes and stored at −80°C until measurement of FSH, luteinizing hormone (LH), total testosterone (TT), and estradiol (E2). Follicular fluid and ovarian GCs were collected from women on retrieval day. The FF was sampled by a transvaginal ultrasound-guided puncture and aspiration of 16- to 18-mm-diameter follicles. Fluid collected from a single dominant follicle of the first aspirated follicle of each ovary that did not contain any visible blood contamination was used in this study. The FF samples were centrifuged for 10 minutes at 1300 × g to isolate GCs. The supernatants were stored at −80°C before DEHP concentrations were measured. The human GCs contained in the pellet were purified using Percoll gradient centrifugation to remove red blood cells and cellular debris. If red blood cells were not clearly removed, red blood cell lysis buffer (Biosharp, Hefei, China) was added at a 4:1 ratio for 3 to 5 minutes at 4°C. The collected GCs were used for the in vitro DEHP exposure study.
Measuring DEHP in FF
FF samples used for the detection of DEHP were prepared by adding 200 µL of 0.5 mol/L (pH 5.5) sodium acetate solution to 200 µL of FF sample. After the samples were vortexed for 1 hour at room temperature, 800 µL of ethyl acetate:hexane (60:40, v:v) was added, and the samples were mixed for 2 minutes. To perform liquid-liquid extraction, the samples were centrifuged at 5000 × g for 10 minutes at room temperature; then, the upper layer was removed (640 µL) and added to a 1.5-mL centrifuge tube. Next, 800 µL of ethyl acetate:hexane (60:40, v:v) was added to the lower layer and mixed for 3 minutes, centrifuged at 4000 × g for 15 minutes at room temperature and, again, the upper layer was removed (720 µL). The two extraction solutions were combined, and the solution was separated into two tubes, 680 µL/tube, and vacuum dried. Then, an acetonitrile:water solution (50:50, v:v) was added, the samples were vortexed, centrifuged at 15,000 × g for 10 minutes at 4°C, and the supernatant was subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Eight microliters of sample was injected. A quality control sample was prepared in parallel and tested at the beginning, in the middle, and at the end of the experiment. The LC-MS/MS analysis was carried out using an LC-ESI (QSTAR Elite; Applied Biosystems, Foster City, CA, USA) mass spectrometer.25 Data analysis was performed using Analyst QS Software 2.0 (Applied Biosystems/MDS Sciex) to extract the peak area of ion pair 228.27.
Cell culture and exposure to DEHP
Primary-cultured human GCs were isolated from the FF of 22 women with tubal blockage who were undergoing IVF. The human GCs were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA), supplemented with 10% inactivated fetal bovine serum (FBS; Gibco), and 100 U/mL of penicillin/streptomycin (Gibco) at 37°C in a humidified atmosphere with 5% CO2. The KGN cells were kindly provided by the laboratory of Professor Fan Jin (Women’s Hospital, School of Medicine, Zhejiang University). The KGN cells have similar properties to human GCs, including the expression of functional follicle-stimulating hormone (FSH) receptor and relatively high aromatase activity.26 KGN cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% inactivated FBS and 100 U/mL of penicillin/streptomycin at 37°C in a humidified atmosphere, with 5% CO2. Human GCs and KGN cells were seeded respectively at 2 × 105 cells per well in six-well plates and cultured for 24 hours before DEHP (Sigma-Aldrich Chemical Co., St. Louis, MO) exposure. The medium containing 0 (as control) or 10 nM DEHP was added separately to the cells for 24, 48, or 72 hours.
Measurement of steroid hormone production
After DEHP exposure for 24 hours, the supernatant of human GCs culture medium was collected. The levels of testosterone, androstenedione, estradiol, and estrone in the supernatant were detected by LC-MS/MS.
Western blotting analysis
Primary-cultured human GCs and KGN cells were lysed in sodium dodecyl sulfate buffer after exposure to DEHP for 24 hours. A bicinchoninic acid assay kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the protein concentration. Protein lysates were loaded, separated by 10% SDS-PAGE, and electro-transferred onto a polyvinyl difluoride membrane (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membrane was blocked for 2 hours at room temperature with 5% skimmed milk in Tris buffered saline-Tween (10 mM Tris, pH 7.6, 150 mM NaCl, and 0.05% Tween-20), and then probed with primary antibodies against CYP19A1 (Santa Cruz Biotechnology, 1:2000), CYP17A1 (Santa Cruz Biotechnology, 1:1500), and an internal control, GAPDH (Santa Cruz Biotechnology, 1:3000). After incubation with their corresponding secondary antibody, the signals were visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA). The densitometric intensity was measured using a GS-800 densitometer (Bio-Rad Laboratories, Hercules, CA, USA). CYP17A1 catalyzes two oxidase reactions and leads to the conversion of pregnenolone and progesterone into C19 steroids, such as androstenedione, whereas CYP19A1 can catalyze either androstenedione or testosterone, yielding estrone or estradiol, respectively.27
Cell viability assay
For cell viability analysis, 100 µL of primary-cultured human GCs and KGN cells were seeded in 96-well plates at 7000 cells per well and incubated overnight. For a better observation of cell viability over time, cells were under 0 (control) and 10 nM of DEHP treatment for 24, 48 and 72 hours, respectively, and then cell viability was analyzed using an MTT assay kit (cell proliferation) (Abcam, Cambridge, UK) according to the manufacturer’s protocol. Briefly, after the cell cultures were exposed to DEHP, 10 µL of 5 mg/mL MTT solution was added to the culture of each well. The cells were subsequently incubated at 37°C for 4 hours, and absorbance was measured using a microplate reader at a wavelength of 492 nm (A492). Cell viability rate (%) was calculated by the ratio of A492 in the DEHP exposure group to A492 in the untreated, control group.
Cell cycle assay
For cell cycle analysis, primary-cultured human GCs and KGN cells treated with DEHP were plated in 6-well plates at 2 × 105 cells per well and incubated at 37°C for 48 hours. The cells were then collected, washed twice with phosphate-buffered saline (PBS), and fixed with 70% cold ethanol at 4°C overnight. Then, 50 µg/mL propidium iodide (PI; Sigma-Aldrich Chemical Co.) with 100 µg/mL RNaseA was added to the cells at 4°C in the dark for 30 minutes. The cell cycle distribution was analyzed using flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ, USA).
Apoptosis analysis
To assess whether DEHP had an apoptotic effect, apoptotic cells were determined by annexin V and propidium iodide (PI) staining, and the rate of apoptosis was assessed using flow cytometry analysis. After exposure to DEHP for 48 hours, primary-cultured human GCs and KGN cells were collected and washed with PBS, incubated with annexin V-fluorescein isothiocyanate for 15 minutes and counterstained with PI for 10 minutes at room temperature in the dark. Apoptotic cells were analyzed by flow cytometry (FACSCalibur, Becton Dickinson).
Real-time quantitative PCR analysis
Expression levels of caspase-3, caspase-7, caspase-8, caspase-9, and Bcl-2 mRNA in both primary-cultured human GCs and KGN cells were analyzed with real-time quantitative PCR (RT-qPCR). After DEHP exposure for 24 hours, total RNA was extracted from human GCs and KGN cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to a standard protocol. The concentration and quality of the RNA samples were evaluated using a NanoDrop ND-100 spectrophotometer (Thermo Fisher Scientific). Reverse transcription was performed with the PrimeScript RT Reagent Kit (Perfect Real Time, TaKaRa, Dalian, China) following standard protocols. Quantitative PCR was performed using the Applied Biosystems 7500 System (Thermo Fisher Scientific). The primers specific for caspase-3, caspase-7, caspase-8, caspase-9, Bcl-2, and GAPDH genes for humans were provided by Sangon Biological Engineering Technology (Shanghai, China). PCR reactions were performed using 2 µL of cDNA, 10 µM each primer, 50× ROX reference dye II, and 2× SYBR Premix Ex Taq (TaKaRa) in 20-µL reactions. The thermal cycler protocol was as follows: 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 34 seconds. The experiment was repeated three times. The cycle threshold (Ct) value for GAPDH mRNA was subtracted from that of the target gene, and the relative mRNA expression levels of target genes to GAPDH were expressed as 2−ΔΔCt.
Statistical analysis
Data are presented as mean ± standard deviation (SD), median (quartiles), or n (%). A Shapiro–Wilk test was conducted to determine the normal distribution of raw or logarithmically transformed data. Differences between the two groups were examined using Student’s t-test for normally or lognormally distributed data or Pearson’s Chi-squared (χ2) test for categorical data. The Wilcoxon rank sum test was performed for the non-normally distributed data. Comparisons of multiple groups were conducted with analysis of variance (ANOVA) and Tukey’s post hoc tests. A two-tailed P-value < 0.05 was considered statistically significant. Statistical analyses were carried out with SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) and GraphPad Prism 7.0 (GraphPad Inc., San Diego, CA, USA).
Results
DEHP levels in human follicular fluid
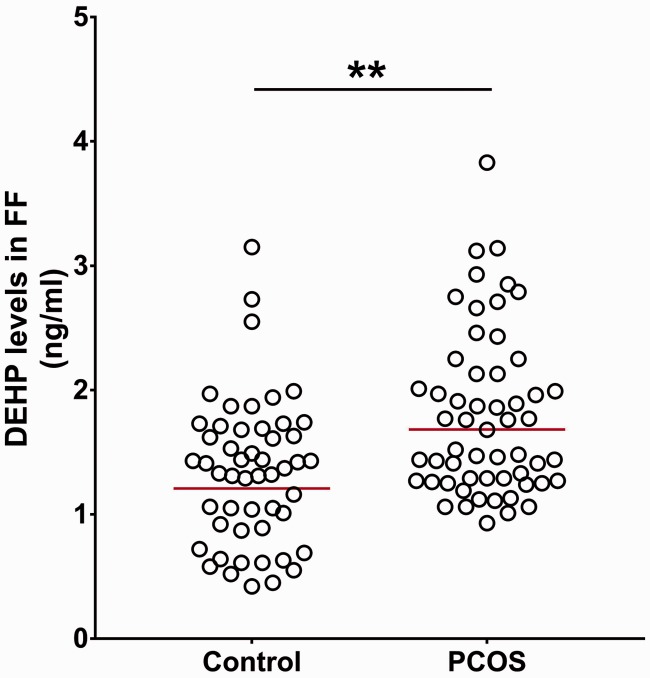
The FF from women with PCOS had significantly higher levels of DEHP (n = 56; geometric mean: 1.68 ng/mL, 95% confidence interval: 1.27–2.13) than that of women in the control group (n = 51; geometric mean: 1.21 ng/mL, 95% confidence interval: 1.06–1.38) (P < 0.01; Figure 1). The women with PCOS whose FF DEHP levels were greater than the geometric mean were defined as the H-DEHP PCOS group (n = 27); those with levels lower than the geometric mean were included in the N-H-DEHP PCOS group (n = 29).
Figure 1.
Concentrations of DEHP in FF of women with PCOS and controls. The data are shown as mean ± SD. The red horizontal line represents the geometric mean. **P < 0.01 versus the control by Student’s t-test. DEHP, di(2-ethylhexyl) phthalate; FF, follicular fluid; PCOS, polycystic ovary syndrome.
Clinical characteristics of participants
There were no significant differences among the control, H-DEHP PCOS, and N-H-DEHP PCOS women for body mass index, duration of infertility, estradiol levels, dosage of rFSH administered, or induction length. No significant differences were found between women in the H-DEHP PCOS and N-H-DEHP PCOS groups in the abovementioned clinical characteristics. However, women in H-DEHP PCOS and N-H-DEHP PCOS groups did differ in LH/FSH ratio, TT levels on day 3 of spontaneous menstrual cycle, and the number of oocytes compared with women in the control group (all P < 0.05; Table 1).
Table 1.
Clinical characteristics of women with PCOS and high or low levels of DEHP in follicular fluid (H-DEHP PCOS, N-H-DEHP PCOS) and controls (women without PCOS).
| Items | Control (n = 51) | N-H-DEHP PCOS (n = 29) | H-DEHP PCOS (n = 27) |
|---|---|---|---|
| Age (years) | 31.59 ± 4.44 | 29.0 ± 3.22* | 29.93 ± 3.56 |
| BMI (kg/m2) | 22.44 ± 3.08 | 23.23 ± 3.32 | 22.41 ± 3.12 |
| Duration of infertility (years) | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 3.0 (2.0–6.0) |
| LH/FSH | 0.66 (0.54–0.97) | 0.89 (0.71–1.80)* | 1.40 (0.70–3.36)* |
| Day 3 TT (nmol/L) | 1.01 ± 0.48 | 1.56 ± 0.76* | 1.34 ± 0.68* |
| Day 3 estradiol (pmol/L) | 107.24 (70.84–169.50) | 83.92 (55.27–126.16) | 98.68 (62.58–146.36) |
| rFSH administered (IU) | 2175 (1650–3300) | 2050 (1650–2625) | 2100 (1800–2700) |
| Induction length (days) | 10 (9–11) | 11 (9–12) | 10 (9–12) |
| Number of oocytes | 10.27 ± 3.56 | 12.9 ± 4.04* | 12.37 ± 3.46* |
| Clinical pregnancy ratea | 24 (47.06) | 10 (34.48) | 6 (22.22)* |
Note: aClinical pregnancy was defined as the presence of a gestational sac with the heartbeat visualized by ultrasound 4–6 weeks after embryo transfer. Data are shown as mean ± SD, median (quartiles) or n (%). *P < 0.05 versus the control by Student’s t-test, Wilcoxon rank sum test, or χ2 test. DEHP, di(2-ethylhexyl) phthalate; PCOS, polycystic ovary syndrome; FSH, follicle-stimulating hormone; LH, luteinizing hormone; Day 3, third day of spontaneous menstrual cycle; rFSH, recombinant FSH; TT, total testosterone.
The clinical pregnancy rates of the control, N-H-DEHP PCOS, and H-DEHP PCOS women were 47.06%, 34.48%, and 22.22%, respectively. The pregnancy rate was significantly lower in H-DEHP PCOS women than in controls (P = 0.032; Table 1).
Effects of DEHP on steroid production of primary-cultured human GCs and KGN cells
The levels of testosterone and androsterone in the supernatant of primary-culture medium with DEHP exposure were significantly higher than those of the control (both P < 0.05). No significant differences existed in the levels of estrone or estradiol between primary-culture medium with DEHP exposure and the control (Table 2).
Table 2.
The levels of steroid hormones in primary-culture medium detected by LC-MS/MS.
| Steroid hormone | Primary-culture control | Primary-culture DEHP |
|---|---|---|
| Testosterone (pg/mL) | 134.14 ± 15.75 | 232.08 ± 22.81* |
| Estrone (pg/mL) | 15.82 ± 3.27 | 17.61 ± 2.55 |
| Estradiol (pg/mL) | 3.00 ± 0.45 | 3.23 ± 0.41 |
| Androsterone (pg/mL) | 14.40 ± 0.62 | 22.04 ± 0.94* |
Note: Data are shown as mean ± SD. *P < 0.05 versus control, by Student’s t-test. LC-MS/MS, liquid chromatography tandem mass spectrometry; DEHP, di(2-ethylhexyl) phthalate.
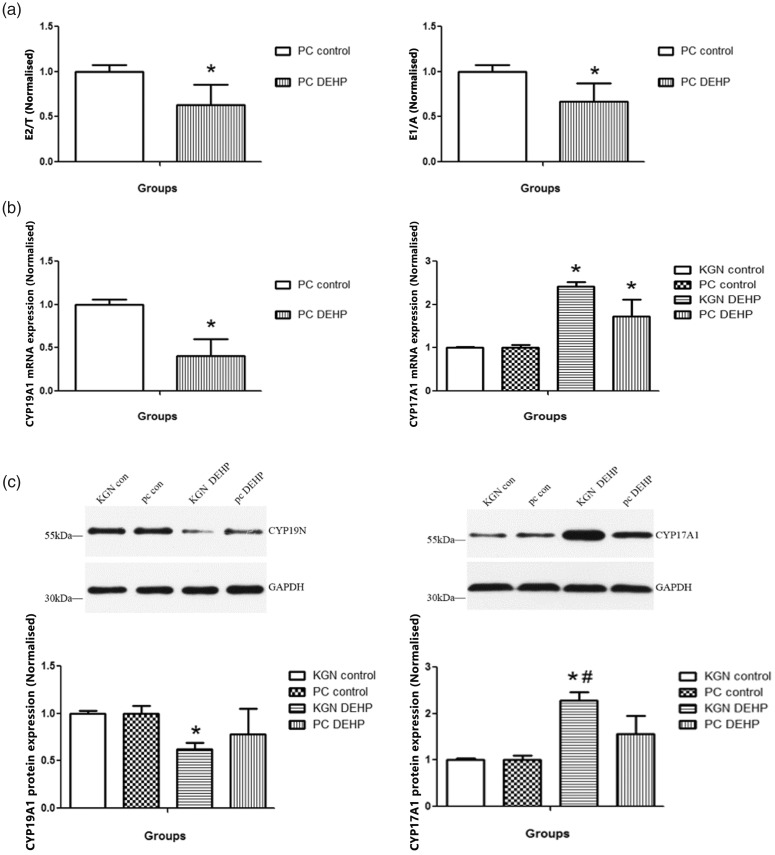
The ratios of estradiol to testosterone and estrone to androsterone were significantly lower in the primary-culture medium exposed to DEHP compared with the control (both P < 0.05; Figure 2a). The mRNA expression level of CYP19A1 in human GCs following DEHP exposure was significantly lower than in control cells (P < 0.05; Figure 2b). The protein expression of CYP19A1 was significantly lower in both primary-cultured human GCs and KGN cells after exposure to DEHP compared with the controls (both P < 0.05; Figure 2c). The mRNA and protein expression of CYP17A1 was significantly higher in both primary-cultured human GCs and KGN cells after exposure to DEHP compared with controls (all P < 0.05; Figure 2b and 2c).
Figure 2.
Effects of DEHP on steroid production of primary-cultured human GCs and KGN cells. (a) The ratios of E2 to T and E1 to A in the supernatant of primary-culture medium. (b) mRNA expression levels of CYP19A1 and CYP17A1 in primary-cultured human GCs or KGN cells. (c) Protein expression levels of CYP19A1 and CYP17A1 in primary-cultured human GCs and KGN cells. Data are shown as mean ± SD. *P < 0.05 versus control by ANOVA, #P < 0.05, KGN DEHP versus PC DEHP by ANOVA. PC, primary-cultured human GCs; KGN, steroidogenic human granulosa-like tumor cell line; DEHP, di(2-ethylhexyl) phthalate; T, testosterone; E1, estrone; E2, estradiol; A, androsterone.
Effect of DEHP on cell viability, cell cycle, and cell apoptosis
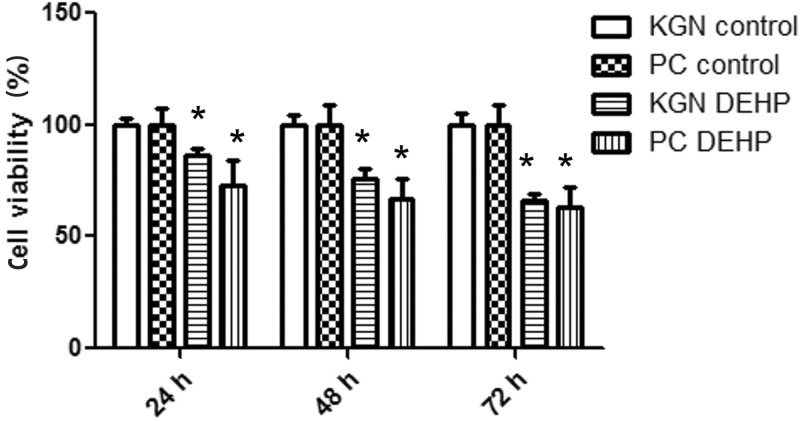
Cell viability analysis showed that exposure of primary-cultured human GCs and KGN cells to DEHP for 24, 48, and 72 hours led to a significant decrease in viability compared with controls (all P < 0.05; Figure 3).
Figure 3.
Effect of DEHP on viability of primary-cultured human GCs and KGN cells measured by MTT assay. Data are shown as mean ± SD. *P < 0.05 versus control by Student’s t-test. PC, primary-cultured human GCs; KGN, steroidogenic human granulosa-like tumor cell line; DEHP, di(2-ethylhexyl) phthalate.
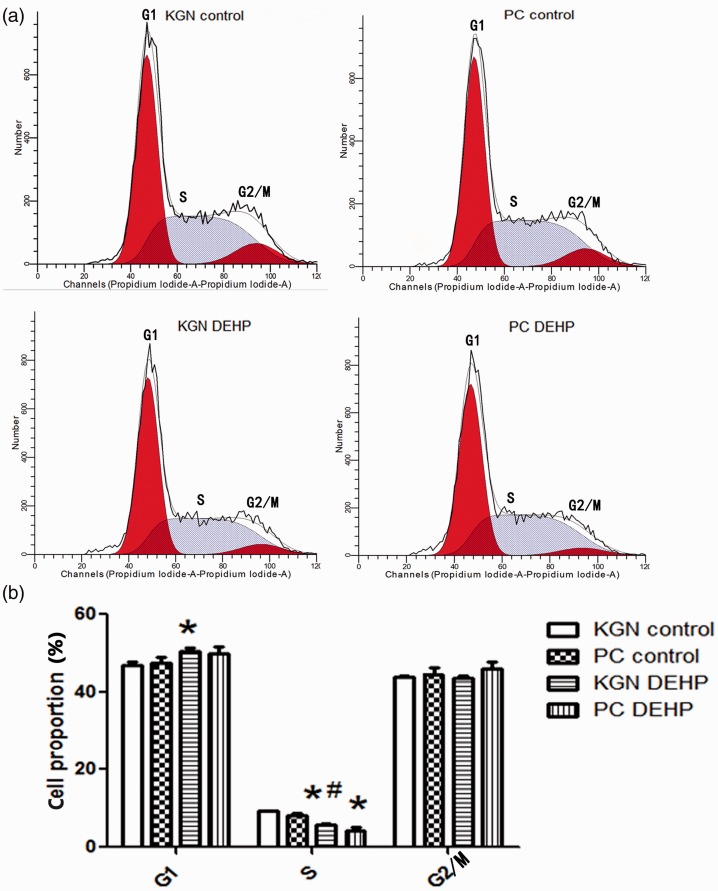
Cell cycle analysis showed that the proportion of KGN cells in G1 phase was significantly higher after exposure to DEHP compared with the KGN control (P < 0.05), whereas the proportions of primary-cultured human GCs and KGN cells in S phase were lower after DEHP exposure compared with the controls (both P < 0.05). The G2/M phase was not influenced by DEHP exposure. These results showed that in vitro DEHP treatment arrested primary-cultured human GCs and KGN cells in the G1 phase and stopped them from entering the S phase (Figure 4a and 4b).
Figure 4.
Effect of DEHP on cell cycle of primary-cultured human GCs and KGN cells analyzed by flow cytometry. (a) Flow cytometry results. The vertical and horizontal axis indicate cell numbers and cell division cycle, respectively. (b) Histogram. Data are shown as mean ± SD. *P < 0.05 versus control by ANOVA, #P < 0.05, KGN DEHP versus PC DEHP group by ANOVA. PC, primary-cultured human GCs; KGN, steroidogenic human granulosa-like tumor cell line; DEHP, di(2-ethylhexyl) phthalate.
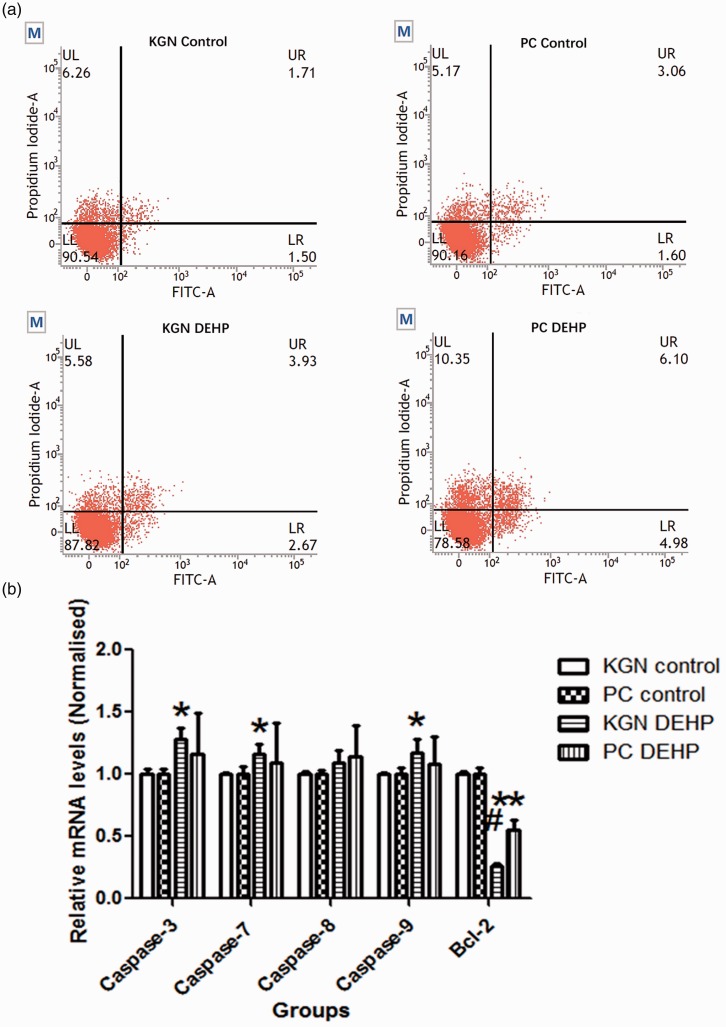
Cell apoptosis analysis showed that the rates of late, early, and total (late + early) apoptosis of primary-cultured human GCs and KGN cells were significantly increased after exposure to DEHP compared with controls (all P < 0.05, Figure 5a). The mRNA expression levels of caspase-3, caspase-7, and caspase-9 were significantly higher in KGN cells after exposure to DEHP compared with controls (all P < 0.05), whereas the mRNA expression of Bcl-2 was significantly lower in primary-cultured human GCs and KGN cells after exposure to DEHP compared with controls (both P < 0.05) (Figure 5b).
Figure 5.
Effect of DEHP on cell of primary-cultured human GCs and KGN cells. (a) Annexin V flow cytometry assay was performed to analyze cell apoptosis. The vertical and horizontal axes indicate PI- and FITC-positive areas, respectively. Identified by flow cytometry, cells were divided into four sections: UL (annexin V-FITC− PI+) was representative of mechanical error; UR (annexin V-FITC+ PI+) was representative of late apoptosis or necrosis cells; LL (annexin V-FITC− PI−) was representative of living cells; LR (annexin V-FITC+ PI−) was representative of early apoptosis cells. (b) mRNA expression levels of caspase-3, caspase-7, caspase-8, caspase-9, and Bcl-2 in primary-cultured human GCs and KGN cells measured using real-time quantitative PCR. Data are shown as mean ± SD. *P < 0.05 versus control by ANOVA, #P < 0.05, KGN DEHP versus PC DEHP group by ANOVA. PI, propidium iodide; FITC, fluorescein isothiocyanate, PC, primary-cultured human GCs; KGN, steroidogenic human granulosa-like tumor cell line; DEHP, di(2-ethylhexyl) phthalate.
Discussion
The current study provided population-based evidence regarding the role of organic pollutants in female reproductive health; we found increased levels of DEHP in the FF of PCOS women, which may be associated with the reduced clinical pregnancy rate when women with PCOS undergo IVF. This study also demonstrated that in vitro DEHP treatment altered steroid production, decreased viability and proliferation, and increased apoptosis in primary-cultured human GCs and KGN cells.
The profile of PCOS, including alterations in both endocrine and reproductive aspects, may indicate that women with PCOS represent a sensitive subpopulation for EDC exposure.9 EDCs represent a group of widespread pollutants that may act as possible environmental contributors to the pathogenesis of PCOS.10,28 Previous studies have shown that DEHP exposure is associated with PCOS characteristics such as altered hormone concentrations,29 cessation of ovulation,30 and polycystic ovarian morphology.29 Prenatal exposure to DEHP that reduces female fertility in a transgenerational manner31 may contribute to altered fetal programming and be involved in the pathogenesis of PCOS.32 Although the mechanism has not been fully elucidated, animal studies have shown that DEHP exposure reduces reproductive functions accompanying changes in sex hormone concentrations and granulosa cell apoptosis,33 disrupts primordial folliculogenesis by inducing autophagy in perinatal ovaries,34 and leads to dysfunction of the hypothalamus-pituitary-ovarian axis35 and altered pulsatile LH secretion.36
According to the traditional two-cell theory of human ovarian steroidogenesis, CYP17A1 expression in the ovary is limited to the theca cells, which are the site of androgen production. Granulosa cells do not express CYP17A1 but express CYP19A1 for estradiol production;37 however, studies suggest that human luteinized granulosa cells in culture do express CYP17A1.38–40 It was reported that C-fos, the AP-1 transcription factor, may inhibit the expression of CYP17A1 and hence suppress the production of androstenedione.41 The steroidogenic pathway and the expression of CYP17A1 in the cultured granulosa cells may be affected by several factors, such as the stage of follicular development, the state of the preovulatory granulosa cell in vivo,42 and the in vitro cell culture system.39 In this study, the results of the in vitro experiment showed that human GCs had altered steroid hormone production after exposure to DEHP. The decreased ratios of estradiol to testosterone and of estrone to androsterone, as well as the increased androstenedione level after DEHP exposure, indicated reduced CYP19A1 activity and enhanced CYP17A1 activity.43 These findings were further supported by DEHP’s inhibition of CYP19A1 expression and upregulation of CYP17A1 expression in both primary human GCs and KGN cells. Thus, DEHP affects sex steroid hormone production by altering expression of steroid hormone–associated synthases, which supports the previously reported effects of DEHP and its metabolite on steroid production, which resulted from suppressed transcript levels of aromatase in animal and human GCs.44,45
DEHP causes an imbalance in cellular proliferation and apoptosis that is associated with the inhibition of the cell cycle and alteration of apoptosis-associated gene expression, which may drive the development of abnormal reproductive functions of PCOS.46 The caspase family of proteins, including caspase-3, caspase-7, and caspase-9, which are involved in the caspase cascade responsible for executing cell death,47 were found to have an upregulated transcript level in KGN cells after DEHP exposure, whereas Bcl-2, an anti-apoptosis factor and an important regulator of programmed cell death,48 was downregulated in both primary human GCs and KGN cells. Similarly, previous studies reported that DEHP treatment arrested mouse GCs at the G0/G1 phase33 and resulted in the increased cell apoptosis,33,45 and that exposure to DEHP or its metabolite induced a decrease in cell viability and promoted apoptosis of rat GCs through increasing caspase-3 activity and Bax/Bcl-2 ratio.49,50
To our knowledge, this is the first study to measure the concentration of DEHP in follicular fluid of women with PCOS. Follicular fluid serves as a medium for communication between oocytes and follicular cells, and it provides the microenvironment for developing oocytes.51 Thus, increased DEHP levels in follicular fluids of women with PCOS found in this study may reflect changes in the ovarian follicular microenvironment and may be associated with the impaired reproductive functions in PCOS. A strength of the study lies in the LC-MS/MS method used to measure the DEHP level in follicular fluids and steroid hormones in cell culture supernatant. With high selectivity and sensitivity, LC-MS/MS is becoming established as the instrument of choice for determining metabolites of phthalates in biological samples52,53 and of steroid hormones.54 Conversely, the study was limited by the small sample size and an insufficient follow-up period. The potential mechanism of DEHP action in the pathogenesis of PCOS needs further investigation. The effects of DEHP on aromatase activity need to be further explored by adding testosterone or androsterone as substrate.
In summary, the clinical data in this study support the hypothesis that exposure to DEHP is involved in PCOS pathogenesis and in the adverse pregnancy outcomes of women with PCOS who undergo IVF. Our in vitro findings add to our understanding of how DEHP affects reproductive functions in women with PCOS. To fully assess the hazard of DEHP to humans, further studies are needed to confirm the role of DEHP exposure in the pathogenesis of PCOS and explore the underlying mechanism. If confirmed, protective strategies and strong recommendations should be considered to reduce human exposure to protect female reproduction from adverse health effects.11
Conclusions
Women with PCOS are exposed to increased levels of DEHP in follicles, which may be involved in pregnancy loss when undergoing IVF. The mechanisms underlying DEHP action may be related to disturbance of steroid production, balance of cellular proliferation, and apoptosis in GCs.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No.LR16H040001) and the National Natural Science Foundation of China (No. 81874480 and 81873837).
References
- 1.Skiba MA, Islam RM, Bell RJ, et al. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2018; 24: 694–709. DOI: 10.1093/humupd/dmy022. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Zhang Q, Yang D, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod 2013; 28: 2562–2569. DOI: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- 3.Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016; 31: 2841–2855. DOI: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 4.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010; 8: 41. DOI: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 2016; 22: 687–708. DOI: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 6.Heijnen EM, Eijkemans MJ, Hughes EG, et al. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update 2006; 12: 13–21. DOI: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 7.Fischer D, Reisenbuchler C, Rosner S, et al. Avoiding OHSS: controlled ovarian low-dose stimulation in women with PCOS. Geburtshilfe Frauenheilkd 2016; 76: 718–726. DOI: 10.1055/s-0042-100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palomba S, de Wilde MA, Falbo A, et al. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015; 21: 575–592. DOI: 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- 9.Heffernan AL, Cunningham TK, Drage DS, et al. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int J Hyg Environ Health 2018; 221: 1068–1075. DOI: 10.1016/j.ijheh.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Palioura E, Diamanti-Kandarakis E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord 2015; 16: 365–371. DOI: 10.1007/s11154-016-9326-7. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowska AZ, Diamanti-Kandarakis E. Polycystic ovary syndrome and environmental toxins. Fertil Steril 2016; 106: 948–958. DOI: 10.1016/j.fertnstert.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Wen S, Yuan D, et al. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol 2018; 34: 370–377. DOI: 10.1080/09513590.2017.1405931. [DOI] [PubMed] [Google Scholar]
- 13.Lyngso J, Ramlau-Hansen CH, Hoyer BB, et al. Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: a cross-sectional study. Hum Reprod 2014; 29: 359–367. DOI: 10.1093/humrep/det390. [DOI] [PubMed] [Google Scholar]
- 14.Velez MP, Arbuckle TE, Fraser WD. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum Reprod 2015; 30: 701–709. DOI: 10.1093/humrep/deu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser R, Gaskins AJ, Souter I, et al. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect 2016; 124: 831–839. DOI: 10.1289/ehp.1509760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schettler T. Human exposure to phthalates via consumer products. Int J Androl 2006; 29: 134–139; discussion 181–135. DOI: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 17.Erythropel HC, Maric M, Nicell JA, et al. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol 2014; 98: 9967–9981. DOI: 10.1007/s00253-014-6183-8. [DOI] [PubMed] [Google Scholar]
- 18.Sui HX, Zhang L, Wu PG, et al. Concentration of di(2-ethylhexyl) phthalate (DEHP) in foods and its dietary exposure in China. Int J Hyg Environ Health 2014; 217: 695–701. DOI: 10.1016/j.ijheh.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Cirillo T, Fasano E, Esposito F, et al. Di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DBP) exposure through diet in hospital patients. Food Chem Toxicol 2013; 51: 434–438. DOI: 10.1016/j.fct.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015; 6: 8. DOI: 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 2013; 43: 200–219. DOI: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Shen W, De Felici M, et al. Di(2-ethylhexyl)phthalate: adverse effects on folliculogenesis that cannot be neglected. Environ Mol Mutagen 2016; 57: 579–588. DOI: 10.1002/em.22037. [DOI] [PubMed] [Google Scholar]
- 23.Mu X, Liao X, Chen X, et al. DEHP exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms. J Hazard Mater 2015; 298: 232–240. DOI: 10.1016/j.jhazmat.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19–25. [DOI] [PubMed] [Google Scholar]
- 25.Lewandowska AE, Macur K, Czaplewska P, et al. Qualitative and quantitative analysis of proteome and peptidome of human follicular fluid using multiple samples from single donor with LC-MS and SWATH methodology. J Proteome Res 2017; 16: 3053–3067. DOI: 10.1021/acs.jproteome.7b00366. [DOI] [PubMed] [Google Scholar]
- 26.Nishi Y, Yanase T, Mu Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001; 142: 437–445. DOI: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 27.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25: 947–970. DOI: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 28.Barrett ES, Sobolewski M. Polycystic ovary syndrome: do endocrine-disrupting chemicals play a role? Semin Reprod Med 2014; 32: 166–176. DOI: 10.1055/s-0034-1371088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol 1994; 128: 216–223. DOI: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- 30.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 2003; 111: 139–145. DOI: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rattan S, Brehm E, Gao L, et al. Di(2-ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol Sci 2018; 163: 420–429. DOI: 10.1093/toxsci/kfy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewlett M, Chow E, Aschengrau A, et al. Prenatal exposure to endocrine disruptors: a developmental etiology for polycystic ovary syndrome. Reprod Sci 2017; 24: 19–27. DOI: 10.1177/1933719116654992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, Liu T, Zhou L, et al. Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol 2012; 34: 869–875. DOI: 10.1016/j.etap.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Mu X, Gao R, et al. Foetal-neonatal exposure of Di (2-ethylhexyl) phthalate disrupts ovarian development in mice by inducing autophagy. J Hazard Mater 2018; 358: 101–112. DOI: 10.1016/j.jhazmat.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Li N, Zhu J, et al. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reprod Toxicol 2014; 46: 141–147. DOI: 10.1016/j.reprotox.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Herreros MA, Encinas T, Torres-Rovira L, et al. Exposure to the endocrine disruptor di(2-ethylhexyl)phthalate affects female reproductive features by altering pulsatile LH secretion. Environ Toxicol Pharmacol 2013; 36: 1141–1149. DOI: 10.1016/j.etap.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Patel SS, Beshay VE, Escobar JC, et al. 17alpha-Hydroxylase (CYP17) expression and subsequent androstenedione production in the human ovary. Reprod Sci 2010; 17: 978–986. DOI: 10.1177/1933719110379055. [DOI] [PubMed] [Google Scholar]
- 38.Park M, Shin E, Won M, et al. FOXL2 interacts with steroidogenic factor-1 (SF-1) and represses SF-1-induced CYP17 transcription in granulosa cells. Mol Endocrinol 2010; 24: 1024–1036. DOI: 10.1210/me.2009-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vireque AA, Campos JR, Dentillo DB, et al. Driving human granulosa-luteal cells recovered from in vitro fertilization cycles toward the follicular phase phenotype. Reprod Sci 2015; 22: 1015–1027. DOI: 10.1177/1933719115570909. [DOI] [PubMed] [Google Scholar]
- 40.Moran FM, VandeVoort CA, Overstreet JW, et al. Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin: inhibition of estradiol secretion due to decreased 17alpha-hydroxylase/17,20-lyase cytochrome P450 expression. Endocrinology 2003; 144: 467–473. DOI: 10.1210/en.2002-220813. [DOI] [PubMed] [Google Scholar]
- 41.Patel SS, Beshay VE, Escobar JC, et al. Molecular mechanism for repression of 17alpha-hydroxylase expression and androstenedione production in granulosa cells. J Clin Endocrinol Metab 2009; 94: 5163–5168. DOI: 10.1210/jc.2009-1341. [DOI] [PubMed] [Google Scholar]
- 42.Voutilainen R, Tapanainen J, Chung BC, et al. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab 1986; 63: 202–207. DOI: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- 43.Alexander SP, Fabbro D, Kelly E, et al. The concise guide to PHARMACOLOGY 2017/18: enzymes. Br J Pharmacol 2017; 174: S272–S359. DOI: 10.1111/bph.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol 2001; 172: 217–224. DOI: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- 45.Xu C, Chen JA, Qiu Z, et al. Ovotoxicity and PPAR-mediated aromatase downregulation in female Sprague-Dawley rats following combined oral exposure to benzo[a]pyrene and di-(2-ethylhexyl) phthalate. Toxicol Lett 2010; 199: 323–332. DOI: 10.1016/j.toxlet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Zheng Q, Li Y, Zhang D, et al. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis 2017; 8: e3145. DOI: 10.1038/cddis.2017.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brentnall M, Rodriguez-Menocal L, De Guevara RL, et al. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 2013; 14: 32. DOI: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 2004; 1644: 83–94. DOI: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Li N, Liu K, Yuan H, et al. The effect of mono-(2-ethylhexyl) phthalate on apoptosis of rat ovarian granulosa cells in vitro. Environ Toxicol Pharmacol 2015; 39: 643–650. DOI: 10.1016/j.etap.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Wang W, Zhu J, et al. Di(2-ethylhexyl) phthalate (DEHP) influences follicular development in mice between the weaning period and maturity by interfering with ovarian development factors and microRNAs. Environ Toxicol 2018; 33: 535–544. DOI: 10.1002/tox.22540. [DOI] [PubMed] [Google Scholar]
- 51.Ambekar AS, Kelkar DS, Pinto SM, et al. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab 2015; 100: 744–753. DOI: 10.1210/jc.2014-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen M, Tao L, Collins EM, et al. Simultaneous determination of multiple phthalate metabolites and bisphenol-A in human urine by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 904: 73–80. DOI: 10.1016/j.jchromb.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsochatzis ED, Tzimou-Tsitouridou R, Gika HG. Analytical methodologies for the assessment of phthalate exposure in humans. Crit Rev Anal Chem 2017; 47: 279–297. DOI: 10.1080/10408347.2016.1273754. [DOI] [PubMed] [Google Scholar]
- 54.Dhayat NA, Marti N, Kollmann Z, et al. Urinary steroid profiling in women hints at a diagnostic signature of the polycystic ovary syndrome: a pilot study considering neglected steroid metabolites. PLoS One 2018; 13: e0203903. DOI: 10.1371/journal.pone.0203903. [DOI] [PMC free article] [PubMed] [Google Scholar]