CONSPECTUS
Cardiovascular disease (CVD) is a major health problem worldwide. Since adult cardiomyocytes irreversibly withdraw from the cell cycle soon after birth, it is hard for cardiac cells to proliferate and regenerate after myocardial injury, such as that caused myocardial infarction (MI). Live cell-based therapies, which we term as first generation of therapeutic strategies, have been widely used for the treatment of many diseases, including CVD. However, cellular approaches have the problems of poor retention of the transplanted cells and the significant entrapment of the cells in the lungs when delivered intravenously. Another big problem is the low storage/shipping stability of live cells, which limits the manufacturability of living cell products. The field of chemical engineering focuses on designing large-scale processes to convert chemicals, raw materials, living cells, microorganisms, and energy into useful forms and products. By definition, chemical engineers conceive and design processes to produce, transform, and transport materials. This matches the direction that cell therapies are heading toward: “produce”, from live cells to synthetic artificial cells; “transform”, from bare cells to cell/matrix/factor combinations; and “transport”. from simple systemic injections to targeted delivery. Thus, we hereby introduce the “chemical engineering of cell therapies” as a concept.
In this Account, we summarize our recent efforts to develop chemical engineering approaches to repair injured hearts. To address the limitations of poor cellular retention and integration, the first step was the artificial manipulation of stem cells before injections (we term this the second generation of therapeutic strategies). For example, we took advantage of the natural infarct-targeting ability of platelet membranes by fusing them onto the surface of cardiac stromal/stem cells (CSCs). By doing so, we improved the rate at which they were delivered through the vasculature to sites of MI. In addition to modifying natural CSCs, we described a bioengineering approach that involved the encapsulation of CSCs in a polymeric microneedle patch for myocardium regeneration. The painless microneedle patches were used as an in situ delivery device, which directly transported the loaded CSCs to the MI heart. In addition to low cell retention, there are some other barriers that need to be addressed before further clinical application is viable, including the storage/shipping stability of and the evident safety concerns about live cells. Therefore, we developed the third generation of therapeutic strategies, which utilize cell-free approaches for cardiac cell therapies. Numerous studies have indicated that paracrine mechanisms reasonably explain stem cell based heart repair. By imitating or adapting natural stem cells, as well as their secretions, and using them in conjunction with biocompatible materials, we can simulate the function of natural stem cells while avoiding the complications association with the first and second generation therapeutic options. Additionally, we can develop approaches to capture endogenous stem cells and directly transport them to the infarct site. Using these third generation therapeutic strategies, we can provide unprecedented opportunities for cardiac cell therapies. We hope that our designs will promote the use of chemical engineering approaches to transform, transport, and fabricate cell-free systems as novel cardiac cell therapeutic agents for clinical applications.
Graphical Abstract
1. INTRODUCTION
According to the Centers for Disease Control and Prevention, CVD is the primary cause of death in the United States.1 Each year, 735 000 Americans have a heart attack and 600 000 die from one. Ischemic heart disease (IHD), including myocardial infarction (MI), is an especially devastating type of CVD.2 Myocardial ischemia leads to the rapid death of cardiomyocytes, generating an infarcted area and causing defects in contractility. Fibrotic scar will eventually build up in the heart to compensate for the loss of large portions of functional tissue, ultimately affecting heart performance.3 The critical step in preserving heart function post-MI lies in repairing the damaged tissue with healthy myocytes.4 Although human adult cardiomyocytes are able to proliferate, the frequencies are very low (<1%) owing to their postmitotic state.5,6 This low level of cardiomyocyte turnover is not sufficient to compensate for the loss of myocardial mass. Understanding and enhancing cardiac cell proliferation after MI are central themes of heart regenerative medicine.
There has been interest in using stem cell therapies to restore cardiac function in ischemic heart disease. We call this paradigm the first generation of therapeutic strategies.7,8 Up to now, a large number of studies have indicated that transplanted stem cells in animal models of post-myocardial-infarction heart failure enhance cardiomyocyte proliferation and improve cardiac function. Moreover, multiple clinical trials have been conducted to investigate the effects of different types of stem cells on cardiac repair and regeneration.9,10 Despite encouraging preclinical results, however, the efficacy of stem cell therapies in cardiac clinic trials has been disappointing and no benefits, or only small clinical improvements, in cardiac function were recorded.11,12 Scientists sought to identify the root causes of the problem. After dedicated investigation, they found that the transplanted cells have a low retention/engraftment rate in the infarct site (less than 10%).13 Even worse, the survival fraction of transplanted cells is very low, further aggravating the low efficacy of cell therapy. Thus, scientists have realized that stem cells alone are not a magic bullet and that even high doses of stem cells may not be sufficient for the improvement of therapeutic efficacy.
The purpose of chemical engineering is to develop large-scale strategies to produce, transform, and transport (deliver) materials, including drugs. In our arena, the “drugs” are living cells and their derivatives. One example of this is the artificial manipulation of stem cells before they are injected to enhance their targeting of infarct sites and improve their retention rates therein. We term this group of manipulations the second generation of therapeutic strategies. Recent studies have adopted the emerging consensus that paracrine effects play an important role in adult stem cell therapy.14 In other words, stem cell secretions of proteins and microvesicles (i.e., exosomes) are thought to enhance myocardial regeneration and inhibit fibrosis.14 Thus, while the development of artificial stem cells that mimic the paracrine process is challenging, it is nonetheless of high importance.15 Inspired by the biological secretion processes of natural stem cells, it is possible and appealing to utilize chemical engineering methods to develop artificial stem cells that mimic key natural stem cell functions in pursuit of novel, more effective cardiac cell therapies (the third generation of therapeutic strategies). As opposed to natural cells, artificial cells can be easily synthesized at much lower costs and have high storage/shipping stability once manufactured. Thus, the development of chemical engineering approaches that help artificial stem cells target the infarct area would help ensure the viability of a stem cell-free solution.
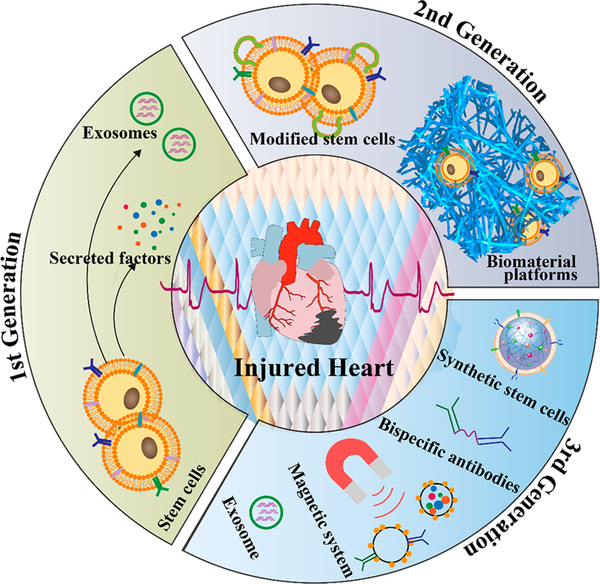
In this Account, we will review these three generations of therapeutic strategies (Figure 1). Our most recent efforts to use chemical engineering tools to develop artificial biomaterials with different formulations as novel cardiac cell therapeutic agents are also discussed. In addition, the challenges and future opportunities of this rapidly growing field are addressed.
Figure 1.
Evolution of translational cardiac regenerative therapies. Chemical engineering strategies were introduced to address the limitations of stem cell therapies for cardiomyocyte regeneration.
2. STEM CELL INJECTIONS: THE FIRST GENERATION OF THERAPEUTIC STRATEGIES
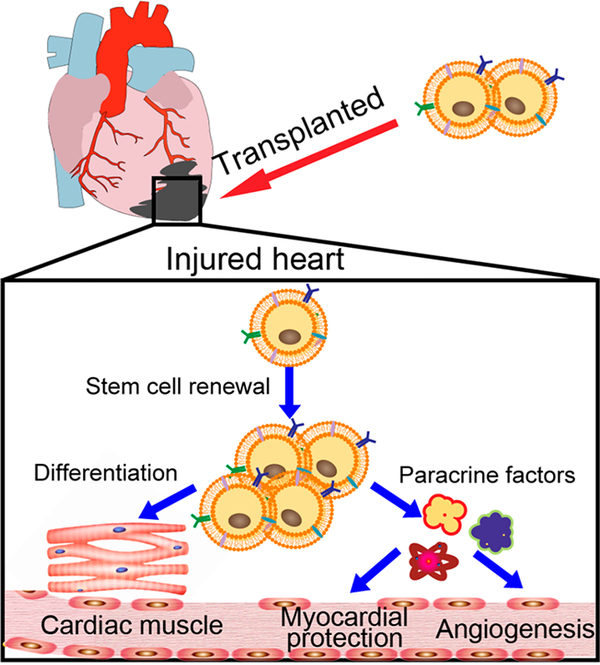
The loss of cardiac cells is irreversible and induces cardiomyocyte injury.16,17 The original intention of stem cell transplantations was for stem cells to differentiate into cardiomyocytes de novo and increase the number of cardiomyocytes in the native tissue. Until now, many cell types have been proven to differentiate into cardiomyocytes in vitro, such as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and endothelial progenitor cells.18 However, the in vivo cardiomyocyte differentiation ability has not been successfully confirmed so far. It is also unclear if the generated cardiomyocytes can electrically couple to the host tissue. Nevertheless, small improvements in cardiac function are observed in most clinical studies.19 The mechanisms of action leading to functional improvements are mainly attributed to the protection of resident cardiomyocytes from apoptosis and enhancements in their proliferation rates (Figure 2). Stem or progenitor cells will release cytokines, chemokines, growth factors, and exosomes as part of their paracrine signaling system to inhibit cardiomyocyte apoptosis and promote resident cardiomyocyte proliferation. Thus, enhancing the number of stem cells in injured hearts and promoting their paracrine effects may be a promising strategy for increasing cardiomyocyte proliferation and repair after injury. This finding has driven us to develop novel methods to enhance the accumulation and retention of stem cells in the injured heart. Chemical engineering, which utilizes chemical modifications and synthesis strategies to transform or transport stem cells, may create new possibilities for stem cell therapies.
Figure 2.
Schematic representation of the mechanisms used by stem cells for heart repair. The transplanted stem cells were expected to repair the injured heart by either the differentiation of cardiomyocytes or the secretion of paracrine factors.
3. TRANSFORM OR TRANSPORT STEM CELLS: THE SECOND GENERATION OF THERAPEUTIC STRATEGIES
As we have mentioned above, although the idea of using stem or progenitor cells for cardiac therapies continues to be at the forefront of research endeavors, after nearly two decades of effort, meta-analytical studies indicate that the benefits of cardiac cell therapies have been none to marginal and that cardiac cell therapy is “far from getting approval”. Researchers seeking to identify the root causes of the problem have found that low retention and engraftment of transplanted cells is among the major hurdles preventing successful clinical translation. For example, intramyocardial injections of MSCs in rat or porcine hearts results in as low as 11% retention after 90 min and 0.6% retention after 24 h.20 In addition, most of the implanted cells are washed off and transported to the lungs and other organs.21 As for the intracoronary and intravenous delivery routes, their cardiac retention rates are much lower than that of intramyocardial injections. Thus, after decades of research, the field of regenerative medicine must come to terms with the very poor retention rate of cells used to target the injured heart, regardless of cell type or delivery route, and primarily due to the strong mechanical flushing forces of the beating myocardium and the inflammatory microenvironment of the MI area. To overcome this limitation, several approaches have been developed, including the enhancement of the cells with infarct-targeting and binding capacity and the provision of material scaffolds to transport the cells and trap them around the infarct area. This section will focus on our recent studies using chemical engineering strategies to transform stem cells or synthesize material scaffolds to deliver cells directly into the injured heart.
3.1. Stem Cell Transformations
As we have indicated, vascular cell delivery routes for heart therapy have poor cell retention rates compared with other delivery routes. However, the intravenous and intracoronary administration of stem cells is much safer and convenient than intramyocardial administration. Therefore, the development of approaches that improve the cell retention rate by vascular routes may be more promising for further clinical applications. To improve cell retention rates, two key points should be considered. One is the need to enhance the targeting of infused cells to the infarct site, and the other is the need to improve the binding capacity of the cells once delivered to the myocardium so that they can overcome the strong flushing forces therein.
Although still nascent, magnetic particle-meditated, targeted drug delivery systems have been widely developed for many biomedical applications.22,23 Superparamagnetic nanoparticles, especially, have been explored to label MSCs and delivery them to local sites by applying an external magnetic field.24 Following the success of magnetic cell delivery, we introduced magnetic nanoparticles to transform the cells with magnetic targeting groups for myocardial repair.25 US Food and Drug Administration (FDA)-approved superparamagnetic nanoparticles were chosen as the magnetic targeting groups for clinical translation because of their favorable regulatory stance. Improved retention was achieved with this magnetic enhancement using human cardiosphere-derived stem cells (hCDCs), which led to improved cardiac function.
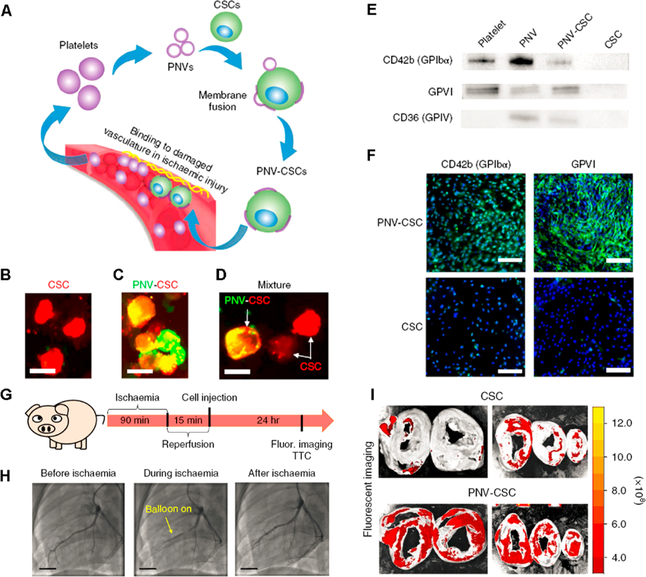
Another strategy that we have experimented with is the use of the natural ability of platelets for homing to MI regions.26 Acute MI induces blood vessel injury, exposing collagen, fibronectin, and von Willebrand factor, which attract platelets. Inspired by this, we artificially modified the natural stem cells with platelet membranes to synthesize infarct-homing stem cells (Figure 3). After confirming the targeting ability of platelet nanovesicle (PNV)-fused stem cells, rat and pig models of myocardial infarction were used to test their cardiac regeneration potential, with positive results.
Figure 3.
(A) Schematic illustration of the fusion of platelet nanovesicles (PNVs) with cardiosphere-derived cardiac stem cells (CSCs) for targeted heart repair. (B−D) Micrographs showing DiI-labeled CSCs (red) decorated with DiO-labeled PNVs (green). Successful fusion is depicted in yellow. Scale bars: 20 μm. (E,F) Western blots and fluorescent micrographs confirming the presence of platelet specific markers in the PNV-CSCs. Scale bars: 200 μm. (G) Protocol design for the pig study. (H) Angiograms confirming the successful pig ischemia/reperfusion model. Scale bars: 15 mm. (I) Ex vivo imaging showing pig hearts 24 h after intracoronary administration of CM-DiI-labeled CSCs or PNV-CSCs. Reprinted with permission from ref 26. Copyright 2018 Nature Publishing Group.
3.2. Chemical Synthesis of Biomaterials to Transport Stem Cells
An alternative to manipulating the stem cells before transplantation is to use biomaterial-based stem cell delivery systems to enhance stem cell homing, engraftment, and survival.27–29 Biomaterials that are retained in the heart can provide a stable microenvironment for transplanted cells and protect them from the mechanical forces of blood flow. In addition, biomaterials protect cells from the regional inflammation and ischemia of the heart injury, which improves their viability. This section will detail the biomaterial approaches that have been developed by our lab to enhance cell retention at the injury site and therapeutic performance, including the use of injectable hydrogels and cell-seeded patches.
Injectable hydrogels are an increasingly promising clinically transferable resource for overcoming low cell retention rates.30 These hydrogels can be delivered into the injured hearts by a minimally invasive injection. Hydrogels protect the cells encapsulated in it from being damaged by the shear forces of the injection itself. To date, various natural materials have been used for the synthesis of injectable hydrogels, including alginate, collagen, fibrin, hyaluronic acid, Matrigel, and decellularized extracellular matrix (ECM). Few of these, however, have been used in clinical trials due to concerns about patient contamination, immunological problems, and batch-to-batch variability. To address these limitations, we pre-encapsulated human cardiac stem cells (hCSCs) into a synthetic polymer-based hydrogel.31 The poly(N-isopropyla-crylamine-co-acrylic acid) (P(NIPAM-AA)) hydrogels have thermosensitive properties and a porous structure that facilitates the exchange of nutrients, oxygen, and cellular secretions between their interior and the surrounding environment. In both mouse and pig MI models, we confirmed that P(NIPAM-AA) hydrogels enhanced cell retention and preserved cardiac function with minimal effect on systemic inflammation or local T cell infiltrations. Although promising, P(NIPAM-AA) hydrogels have a toxic effect on the viability, growth, and biological function of the loaded cells. This is mainly attributed to the physical and chemical properties of the polymer that forms the hydrogel, including its surface charge and hydrophobicity/hydrophilicity. To overcome this hurdle, we synthesized a poly(Nisopropylacrylamide-co-itaconic acid) (P(NIPAM-IA))-based hydrogel, which improved the microenvironment for cardiac stromal cells.32 Compared to P(NIPAM-AA) hydrogels, (P(NIPAM-IA)) hydrogels provide a more hydrophilic and negatively charged microenvironment. In mouse MI models, we verified that P(NIPAM-IA) hydrogels enhanced cell retention and promoted myocardial regeneration through angiogenesis and the protection of cardiomyocytes from apoptosis.
In addition to hydrogels, cardiac patches have been used as scaffolding material for improved cell retention and myocardial regeneration.33 Different three-dimensional (3D) scaffolds made of natural biological materials, have been loaded with cells and used to treat the damage caused by myocardial infarctions. The rationale for their use as stem cell carriers is that cells will enter the injured site from the patch, or release secretions in response to infarct-driven signals. Previous works focused on overcoming the low retention of the cardiac patch method, but few of these reports were concerned with cardiomyogenesis and angiogenesis in the infarct area. Vascularization plays an important role in enhancing cardiomyocyte survival and improves the function of ischemic hearts. We have fabricated a vascularized cardiac patch by combining biomimetic microvessels (BMVs) with cardiac stem cells (CSCs).34 The BMVs were synthesized using human umbilical vein endothelial cells (HUVECs), which create a luminal surface by microfluidic hydrodynamic focusing. After testing in the rat MI model, we found the cardiac patch enhanced angiogenesis and promoted cardiomyocyte proliferation.
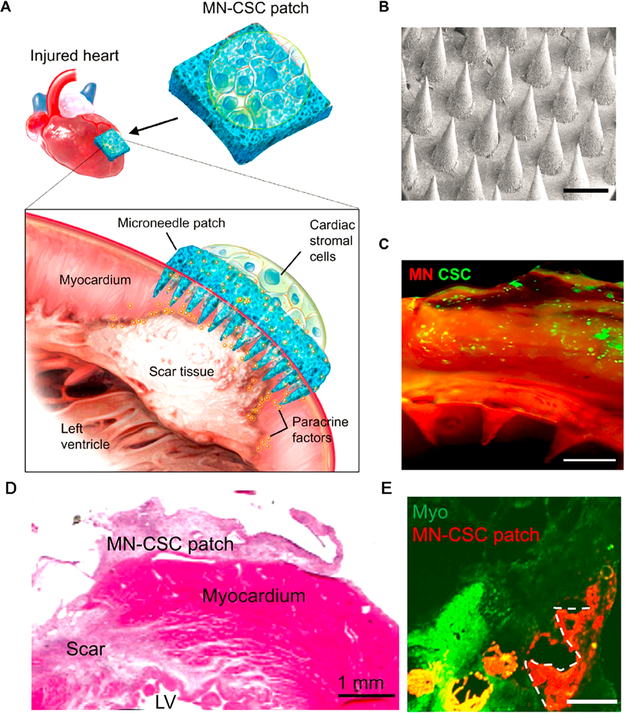
The premise that paracrine signaling is the primary mechanism responsible for stem cell therapy is heavily supported by the scientific literature.14 For this signaling modality to function with the patch treatment paradigm, the secretome released by the cells must be able to reach the host myocardium. In addition, the encapsulated stem cells need to have access to nutrients from the heart environment. Thus, a linker is needed between the cells in the patch and the host myocardium. To achieve this, we developed a cardiac stromal cell-encapsulated microneedle patch (MN-CSCs), ensuring communication between cells and the host myocardium (Figure 4).35 Microneedles provide a new strategy for delivering various therapeutics by the transdermal route. Microneedle-based transdermal delivery has been widely used in the delivery of drugs, biopharmaceuticals, and vaccines. It offers many advantages, such as noninvasive administration, versatile titration of dosage and regimen (as the patch can be on and off), and faster healing at injection site. Our microneedle patch provided channels for the communications of cells with host myocardium and improved cardiac function in both rat and pig MI models.
Figure 4.
(A) Schematic illustration of the use of stromal cell (MN-CSCs)-integrated microneedle patch for myocardium regeneration. (B) SEM image of microneedle. (C) Fluorescent image showing fibrin gels loaded with DiO-labeled cells and combined with MN. Scale bar: 500 mm. (D) Hematoxylin and eosin stain showing the retention of the MN-CSC patch on the infarcted heart. (E) Heart section image showing MN-CSC patch after 7 days of treatment. Scale bar: 500 mm. Reprinted with permission from ref 35. Copyright 2018 American Association for the Advancement of Science.
4. CHEMICAL SYNTHESIS OF CELL-FREE SYSTEMS FOR CARDIAC REPAIR: THE THIRD GENERATION OF THERAPEUTIC STRATEGIES
In addition to low cell retention and off-target delivery issues, there are other issues that need to be addressed before the successful clinical application of stem cells is possible. These include the storage and shipping stability of the live cells and their tumorigenic and immunogenic risks. It is difficult to grow, preserve, and transport stem cells before they are administered to the patient, which creates a big obstacle for manufacturing. The field of chemical engineering aims to design large-scale processes to convert raw materials, including living cells, into useful forms and products. If we adopt chemical engineering principles to develop large-scale manufacturing processes, we can improve manufacturing efficiency, minimize product variability, and reduce costs. In the following section, we will summarize our recent efforts to use chemical strategies to fabricate cell-free systems that can repair injured hearts and successfully overcome the limitations of cellular therapeutic strategies (Table 1).
Table 1.
Summary of Chemical Engineering Approaches to Construct Cell-Free Methods for Myocardial Repair
| chemical engineering | type | principle | refs |
|---|---|---|---|
| produce | synthetic cells | paracrine effect | 41–43 |
| transform | exosomes | 47, 48 | |
| transport | two antibody-modified Fe3O4 | 37 | |
| pretargeting and bioorthogonal chemistry system | capturing endogenous stem cells and targeting to infarct site | 39 | |
| antibody-engineered platelets | 38 |
4.1. Recruit Endogenous Stem Cells
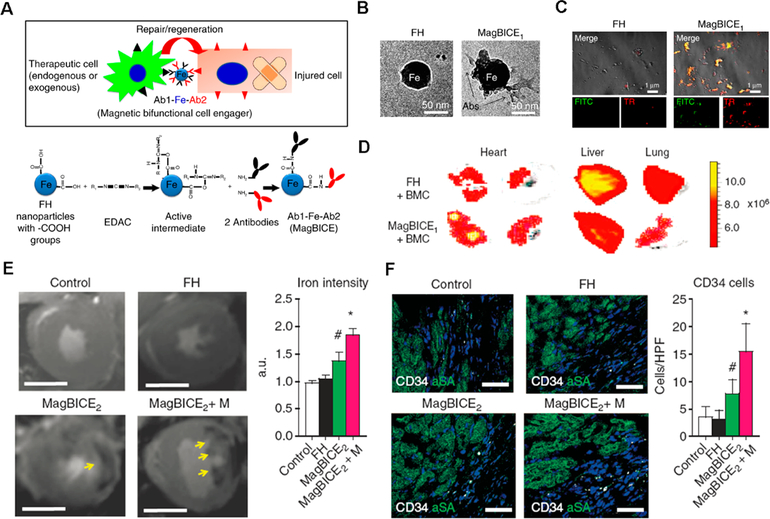
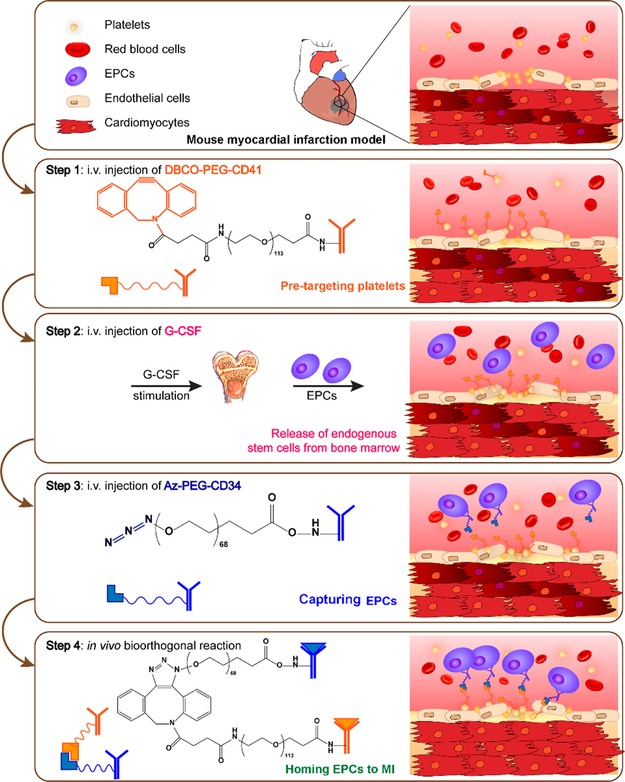
As an alternative to the delivery of exogenous stem cells, the recruitment of endogenous stem cells to the infarct site provides an efficient way to repair the injured heart. The isolation and culture of stem cells is time-consuming, and the transplanted cells may pose safety concerns. Previous reports have indicated that endogenous stem cells are released during MI and migrate to the infarct site.36 However, this inherent recruitment is not capable of inducing significant cardiomyocyte regeneration. To improve the endogenous recruitment of stem cells, we developed iron nanoparticles with two binding regions. The nanoparticle material used was ferumoxytol (trade name: Feraheme (FH)), a clinical contrast agent. We chemically modified the iron nanoparticles with two different antibodies (one bound to CD34-positive cells, and the other targeted to the cardiomyocytes) to capture the endogenous stem cells and transport them more efficiently to the infarct site using an external magnetic field (Figure 5).37 In addition to magnetic targeting, it has been reported that platelets possess the capacity to find cardiac injury sites (relying on the binding motif of their membranes) and can serve as infarct-homing carriers. Thus, we chemically engineered platelets with CD34 antibodies to find CD34-positive cells and transport them to the infarct site.38 While this therapy paradigm still requires the isolation of platelets and their modification in vivo, it is simpler than large-scale cell manufacturing. Alternatively, it is feasible to create bispecific antibodies (BsAb) that possess an anti-CD41 and an anti-CD34 binding arm to capture both endogenous stem cells and platelets in vivo. However, BsAb synthesis is very complicated and time-consuming. More importantly, there is a risk that platelets will conjugate with stem cells and clog the vasculature. To overcome this limitation, we used bioorthogonal click chemistry to recognize circulating CD34-positive cells and direct them to the infarct site for heart repair in a stepwise fashion (Figure 6).39 In our system, anti-CD41 antibodies and CD34 antibodies were not conjugated but used to modify the bioorthogonal chemical groups, Dibenzocyclooctyne (DBCO) and azido, respectively. These were then i.v. administrated separately. The anti-CD41-DBCO group was injected first and given a 48 h pretargeting window to ensure more platelets accumulated in the injured heart. Then, the anti-CD34-azido group was injected to bind to circulating stem cells and then to the infarcted heart via an azido-DBCO reaction.
Figure 5.
(A) Schematic showing the construction of the magnetic bifunctional cell engager (MagBICE) by conjugating two different antibodies. (B) TEM images of nanoparticles before and after antibody modification. (C) Fluorescent images of unconjugated and conjugated magnetic nanoparticles stained with antimouse Texas-Red and antirabbit FITC secondary antibodies. (D) Ex vivo imaging confirming the enhanced accumulation of MagBICE compared to magnetic nanoparticles alone. (E) T2 weighted MRI images were used to confirm the targeting of MagBICE2 to the injured myocardium. (F) Confocal images demonstrating the recruitment of circulating stem cells to the infarct site. Reprinted with permission from ref 37. Copyright 2014 Nature Publishing Group.
Figure 6.
Schematic showing the use of pretargeting and bioorthogonal click chemistry systems to capture circulating CD34-positive cells and transport them to the MI area, in a targeted manner, to increase therapeutic efficiency and efficacy. Reprinted with permission from ref 39. Copyright 2018 American Chemical Society.
4.2. Chemical Synthesis of Artificial Stem Cells to Mimic the Natural Stem Cell Cardiac Repair Function
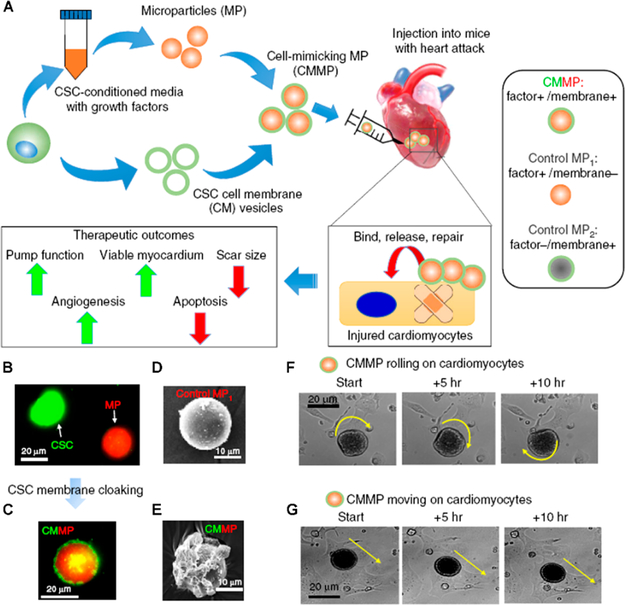
Paracrine secretions, such as vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and exosomes, might be the primary mechanism used by stem cells to impart therapeutic effects. Thus, it is reasonable to develop a therapeutic paradigm to treat MI that delivers just stem cell secretome using biocompatible materials. Biomaterials have been shown to protect protein-based secretome from degradation, possess high loading efficiency, achieve the sustained release of loaded content, and have high cryopreservation stability.40 Poly(lactic-co-glycolic acid) (PLGA) is an FDA-approved biodegradable polymer that has been widely used in drug delivery systems for the treatment of diseases such as cancer and bacterial infections. Thus, PLGA is an ideal candidate for stem cell secretome-loading. We have employed PLGA to load both mesenchymal stem cell (MSC) and cardiosphere-derived stem cell secretome for the repair of injured hearts.41 Furthermore, since stem cell membranes are not null in the repair process, we cloaked the secretome-loaded PLGA capsules with stem cell membranes to construct artificial stem cells that could mimic the therapeutic abilities of natural stem cells (Figure 7).42 We successfully demonstrated that these stem-cell-mimicking microparticles had a higher infarct-targeting capacity and released secretome in a more sustained manner than natural stem cells. Furthermore, biomimetic particles preserved viable myocardium and augmented cardiac function to an extent similar to cardiac stem cell therapy. We also synthesized platelet membrane-coated and secretome-loaded PLGA for myocardium regeneration after ischemia/reperfusion (I/R) injury.43 Moreover, prostaglandin E2 (PGE2), which targets the PGE2 receptors in the pathological cardiac microenvironment resulting after I/R injury, was conjugated to these particles (PINCs) to enhance their targeting ability. The improved cardiac function and reduced cardiac remodeling were confirmed using our PINC delivery system.
Figure 7.
(A) Protocol for the fabrication of cell-mimicking microparticles for myocardial repair. (B,C) Micrographs showing CSC cell membranes (green) coating the microparticles (red). (D,E) SEM images before and after membrane-coating. (F,G) Time-lapse imaging demonstrating the rolling and traveling behavior of CMMPs on attached cardiomyocytes. Reprinted with permission from ref 42. Copyright 2017 Nature Publishing Group.
4.3. Exosomes for Myocardial Regeneration
Exosomes are small microvesicles (~30–100 nm) that are released from late endosomes by most types of cells. They contain various molecular constituents present in their cells of origin, including proteins and/or peptides, microRNAs (miRs), and mRNAs that play an important role in cell−cell communication.44 Recently, exosomes and exosome-based delivery systems have been used in the treatment of disease, including CVD. Exosomes produced by MSCs have been shown to induce myocardial regeneration.45 Cardiosphere-derived stem cells (CDCs) have been applied in clinical trials, including the CADUCEUS and SCIPIO trials.46 In addition, CDC-derived exosomes have been shown to improve cardiac function in murine models of myocardial infarction through intramyocardial injection. Previous research has shown that CDCs are also effective in the treatment of dilated cardiomyopathy (DCM). Thus, we tested the efficacy of intravenously injected CDC-derived exosomes for the treatment of DCM and found that they were capable of improving cardiac function.47 However, it should still be noted that exosomes are mostly distributed in the liver and spleen when administrated by i.v., which may lead to undesired side effects and low therapeutic efficacy. To extend our research, we chemically modified exosomes with a cardiac homing peptide to increase their accumulation in the infarct site while still preserving the i.v. delivery modality.48 Results showed a significant improvement in cardiac function.
5. SUMMARY AND OUTLOOK
Heart disease is the leading cause of death in the United States for both men and women. Each year, 735 000 Americans have a heart attack and 600 000 die from one. Chemical engineering strategies are crucial in the scientific pursuit of improving these statics. The paradigmatic examples discussed in this account mainly highlight the chemical strategies we recently used to modify cellular strategies for the treatment of CVD. While stem cell therapies (first generation) have been on the cutting-edge of researched treatment options for acute and chronic ischemic heart disease for many years, after nearly two decades of effort, meta-analytical research indicates that the benefits of cell therapies have been none to marginal.
Cells are often viewed as “live drugs”. In the past, the field focused on the “live” portion of this moniker, assuming the injected cells were “magic bullets” that could home in on the diseased tissue and start the regenerative process. Unfortunately, the reality is far from what has been expected. Cell therapies have met many bottlenecks on the road to clinical translation, such as low retention and engraftment, lung entrapment, low storage/shipping stability, and safety concerns.49 Switching our focus to the “drug” part of the “live drug” moniker is an essential next step in the development of effective cardiac cell therapies. With the booming of chemical engineering in the biotechnology field, it is possible to chemically manipulate cells to ensure they have better retention and therapeutic efficacy. This can be done through the chemical modification of cell membranes with infarct-targeting molecules or magnetic nanoparticles, and through the chemical synthesis of biomaterials that provide scaffolding for encapsulating cells, among other strategies. These second generation therapeutic strategies help stem cells find the injured site, improve cell retention in the injured heart, and promote therapeutic efficiency.
However, unresolved issues with the use of actual cells, such as storage/shipping stability and safety concerns, paved the way for a third generation of studies aimed at developing cell-free therapeutic methods. Endogenous stem cells are recruited after MI, but at low rates. Improvement of endogenous stem cell accumulation and retention in the infarct site would increase the efficacy of treatment and address the drawbacks related to traditional cell therapy. In addition, given the importance of paracrine signaling in cell therapy, and despite the challenges associated with it, the development of artificial stem cells that mimic the paracrine process is of high importance. Inspired by the biological secretion processes of natural stem cells, it is possible to utilize chemical engineering methods to develop artificial stem cells that mimic key natural stem cell functions in pursuit of novel, more effective cardiac cell therapies. As opposed to natural cells, artificial cells can be easily synthesized at much lower costs and have high storage/shipping stability once manufactured.50 These synthetic cells can be loaded with cell secretome to act as artificial paracrine signal distributors. Exosomes are one of many secretome components that are being studied for the treatment of CVD. They are stable in storage and during shipping, easy to produce, and simple to modify for enhanced permeability and in situ retention.
In the future, exosomes may serve as vehicles to transport therapeutic chemical compounds to the injured heart, in addition to their natural proteomic and genetic cargos, thus enhancing their therapeutic effects. Additionally, the development of exosome isolation strategies for larger-scale production will further accelerate their clinical application. Although the introduction of chemical engineering strategies has contributed to promising advances in cardiac regenerative therapy, many limitations still need to be addressed to accelerate their clinical translation. For example, secretome contains a host of proteins and growth factors. Determining which one or which combination holds the key to cardiomyocyte regeneration remains to be accomplished. Furthermore, since it is difficult to control the ratio of the extracted secretome from one batch to the other, methods to standardize the quantities of secretome-encapsulated particles will need to be developed. The use of exosomes presents a similar standardization problem since they contain various protein factors and miRNAs. Thus, further concentrated research is highly desirable to accelerate clinical translation.
ACKNOWLEDGMENTS
Final grammatical edits were completed by Jhon Cores, Ph.D.
Funding
This work was supported by grants from the National Institutes of Health (R01 HL123920, HL137093, HL144002, and HL146153 to K.C.) and the American Heart Association (18TPA34230092 and 19EIA34660286 to K.C.).
Biographies
Zhenhua Li is an Associate Professor in the College of Chemistry & Environmental Science, Hebei University, China. He received his Ph.D. in Inorganic Chemistry at the Changchun Institute of Applied Chemistry (CIAC), Chinese Academy of Sciences, Jilin, China, 2015. His research interests focus on the synthesis and characterization of functionalized nanomaterials and their applications in the diagnosis and treatment of diseases.
Shiqi Hu received her Ph.D. from the College of Chemical and Biological Engineering, Zhejiang University, China in 2017. Her research focuses on the development of anticancer drug delivery systems and tumor microenvironments. She specializes in polymer synthesis, nanoparticle preparation, and the creation of cancer models for research.
Ke Cheng is a Professor in the Department of Molecular Biomedical Sciences, North Carolina State University, Raleigh, NC. He is also a Professor in the UNC/NCSU Joint Department of Biomedical Engineering. He is an adjunct professor at the UNC Eshelman School of Pharmacy. His formal education began with a B.S. in Pharmaceutical Engineering from Zhejiang University, followed by a Ph.D. in Biological Engineering from the University of Georgia. He received postgraduate training and acquired junior faculty experience at the UCLA School of Medicine and Cedars-Sinai Medical Center.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Shpilsky D; Bambs C; Kip K; Patel S; Aiyer A; Olafiranye O; Reis SE; Erqou S Association between ideal cardiovascular health and markers of subclinical cardiovascular disease. Clin. Cardiol 2018, 41, 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tang J-N; Cores J; Huang K; Cui X-L; Luo L; Zhang J-Y; Li T-S; Qian L; Cheng K Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. Stem Cells Transl. Med 2018, 7, 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Palmquist-Gomes P; Pérez-Pomares JM; Guadix JA Cell-based therapies for the treatment of myocardial infarction: lessons from cardiac regeneration and repair mechanisms in non-human vertebrates. Heart Failure Rev. 2019, 24, 133–142. [DOI] [PubMed] [Google Scholar]
- (4).Amado LC; Saliaris AP; Schuleri KH; St. John M; Xie J-S; Cattaneo S; Durand DJ; Fitton T; Kuang JQ; Stewart G; Lehrke S; Baumgartner WW; Martin BJ; Heldman AW; Hare JM Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 11474–11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zebrowski DC; Becker R; Engel FB Towards regenerating the mammalian heart: challenges in evaluating experimentally induced adult mammalian cardiomyocyte proliferation. Am. J. Physiol. Heart Circ. Physiol 2016, 310, H1045–H1054. [DOI] [PubMed] [Google Scholar]
- (6).Bergmann O; Bhardwaj RD; Bernard S; Zdunek S; Barnabe-´ Heider F; Walsh S; Zupicich J; Alkass K; Buchholz BA; Druid H; Jovinge S; Frisen J Evidence for Cardiomyocyté Renewal in Humans. Science 2009, 324, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Passier R; van Laake LW; Mummery CL Stem-cell-based therapy and lessons from the heart. Nature 2008, 453, 322. [DOI] [PubMed] [Google Scholar]
- (8).Segers VFM; Lee RT Stem-cell therapy for cardiac disease. Nature 2008, 451, 937. [DOI] [PubMed] [Google Scholar]
- (9).Hao M; Wang R; Wang W Cell Therapies in Cardiomyopathy: Current Status of Clinical Trials. Anal. Cell. Pathol 2017, 2017, 9404057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mingliang R; Bo Z; Zhengguo W Stem cells for cardiac repair: status, mechanisms, and new strategies. Stem Cells Int 2011, 2011, 310928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nagpal A; Choy FC; Howell S; Hillier S; Chan F; Hamilton-Bruce MA; Koblar SA Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: a systematic review and meta-analysis. Stem Cell Res. Ther 2017, 8, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Menasché P Cell therapy trials for heart regeneration — lessons learned and future directions. Nat. Rev. Cardiol 2018, 15, 659–671. [DOI] [PubMed] [Google Scholar]
- (13).Lee W-Y; Wei H-J; Lin W-W; Yeh Y-C; Hwang S-M; Wang J-J; Tsai M-S; Chang Y; Sung H-W Enhancement of cell retention and functional benefits in myocardial infarction using human amniotic-fluid stem-cell bodies enriched with endogenous ECM. Biomaterials 2011, 32, 5558–5567. [DOI] [PubMed] [Google Scholar]
- (14).Gartz M; Strande JL Examining the Paracrine Effects of Exosomes in Cardiovascular Disease and Repair. J. Am. Heart Assoc 2018, 7, No. e007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Liang H; Huang K; Su T; Li Z; Hu S; Dinh P-U; Wrona EA; Shao C; Qiao L; Vandergriff AC; Hensley MT; Cores J; Allen T; Zhang H; Zeng Q; Xing J; Freytes DO; Shen D; Yu Z; Cheng K Mesenchymal Stem Cell/Red Blood Cell-Inspired Nanoparticle Therapy in Mice with Carbon Tetrachloride-Induced Acute Liver Failure. ACS Nano 2018, 12, 6536–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Rabinovich-Nikitin I; Dhingra R; Kirshenbaum LA Epigenetic regulation of cardiac cell cycle Re-entry and proliferation. J. Mol. Cell. Cardiol 2018, 121, 297–299. [DOI] [PubMed] [Google Scholar]
- (17).Mohamed TMA; Ang Y-S; Radzinsky E; Zhou P; Huang Y; Elfenbein A; Foley A; Magnitsky S; Srivastava D Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 2018, 173, 104–116.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kehat I; Kenyagin-Karsenti D; Snir M; Segev H; Amit M; Gepstein A; Livne E; Binah O; Itskovitz-Eldor J; Gepstein L Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest 2001, 108, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Trounson A; Thakar RG; Lomax G; Gibbons D Clinical trials for stem cell therapies. BMC Med 2011, 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Follin B; Ghotbi AA; Clemmensen AE; Bentsen S; Juhl M; Søndergaard RH; Lund LD; Haack-Sorensen M; Hasbak P; Cohen S; Ripa RS; Kastrup J; Ekblond A; Kjaer. Retention and Functional Effect of Adipose-Derived Stromal Cells Administered in Alginate Hydrogel in a Rat Model of Acute Myocardial Infarction. Stem Cells Int. 2018, 2018, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dow J; Simkhovich BZ; Kloner RA; Kedes L Washout of transplanted cells from the heart: A potential new hurdle for cell transplantation therapy. Cardiovasc. Res 2005, 67, 301–307. [DOI] [PubMed] [Google Scholar]
- (22).Xie J; Liu G; Eden HS; Ai H; Chen X Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res 2011, 44, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yoo D; Lee J-H; Shin T-H; Cheon J Acc. Chem. Res 2011, 44, 863–874. [DOI] [PubMed] [Google Scholar]
- (24).Wang J; Xiang B; Deng J; Lin H-Y; Zheng D; Freed DH; Arora RC; Tian G Externally Applied Static Magnetic Field Enhances Cardiac Retention and Functional Benefit of Magnetically Iron-Labeled Adipose-Derived Stem Cells in Infarcted Hearts. Stem Cells Transl. Med 2016, 5, 1380–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Vandergriff AC; Hensley TM; Henry ET; Shen D; Anthony S; Zhang J; Cheng K Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014, 35, 8528–8539. [DOI] [PubMed] [Google Scholar]
- (26).Tang J; Su T; Huang K; Dinh P-U; Wang Z; Vandergriff A; Hensley MT; Cores J; Allen T; Li T; Sproul E; Mihalko E; Lobo LJ; Ruterbories L; Lynch A; Brown A; Caranasos TG; Shen D; Stouffer GA; Gu Z; Zhang J; Cheng K Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat. Biomed. Eng 2018, 2, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Chen Q-Z; Harding SE; Ali NN; Lyon AR; Boccaccini AR Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater. Sci. Eng., R 2008, 59, 1–37. [Google Scholar]
- (28).Pascual-Gil S; Garbayo E; Díaz-Herráez P; Prosper F; Blanco-Prieto MJ Heart regeneration after myocardial infarction using synthetic biomaterials. J. Controlled Release 2015, 203, 23–38. [DOI] [PubMed] [Google Scholar]
- (29).O’Neill HS; Gallagher LB; O’Sullivan J; Whyte W; Curley C; Dolan E; Hameed A; O’Dwyer J; Payne C; O’Reilly D; Ruiz-Hernandez E; Roche ET; O’Brien FJ; Cryan SA; Kelly H; Murphy B; Duffy GP Biomaterial-Enhanced Cell and Drug Delivery: Lessons Learned in the Cardiac Field and Future Perspectives. Adv. Mater 2016, 28, 5648–5661. [DOI] [PubMed] [Google Scholar]
- (30).Cai L; Dewi RE; Heilshorn SC Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Adv. Funct. Mater 2015, 25, 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Tang J; Cui X; Caranasos TG; Hensley MT; Vandergriff AC; Hartanto Y; Shen D; Zhang H; Zhang J; Cheng K Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cui X; Tang J; Hartanto Y; Zhang J; Bi J; Dai S; Qiao SZ; Cheng K; Zhang H NIPAM-based Microgel Microenvironment Regulates the Therapeutic Function of Cardiac Stromal Cells. ACS Appl. Mater. Interfaces 2018, 10, 37783–37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dvir T; Timko BP; Brigham MD; Naik SR; Karajanagi SS; Levy O; Jin H; Parker KK; Langer R; Kohane DS Nanowired three-dimensional cardiac patches. Nat. Nanotechnol 2011, 6, 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Su T; Huang K; Daniele MA; Hensley MT; Young AT; Tang J; Allen TA; Vandergriff AC; Erb PD; Ligler FS; Cheng K Cardiac Stem Cell Patch Integrated with Microengineered Blood Vessels Promotes Cardiomyocyte Proliferation and Neovascularization after Acute Myocardial Infarction. ACS Appl. Mater. Interfaces 2018, 10, 33088–33096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Tang J; Wang J; Huang K; Ye Y; Su T; Qiao L; Hensley MT; Caranasos TG; Zhang J; Gu Z; Cheng K Cardiac cell−integrated microneedle patch for treating myocardial infarction. Sci. Adv 2018, 4, No. eaat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Su G; Liu L; Yang L; Mu Y; Guan L Homing of endogenous bone marrow mesenchymal stem cells to rat infarcted myocardium via ultrasound-mediated recombinant SDF-1α adenovirus in microbubbles. Oncotarget 2018, 9, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Cheng K; Shen D; Hensley MT; Middleton R; Sun B; Liu W; De Couto G; Marbán E Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun 2014, 5, 4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Shen D; Li Z; Hu S; Huang K; Su T; Liang H; Liu F; Cheng K Antibody-Armed Platelets for the Regenerative Targeting of Endogenous Stem Cells. Nano Lett. 2019, 19, 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Li Z; Shen D; Hu S; Su T; Huang K; Liu F; Hou L; Cheng K Pretargeting and Bioorthogonal Click Chemistry-Mediated Endogenous Stem Cell Homing for Heart Repair. ACS Nano 2018, 12, 12193–12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Han X; Li Y; Xu Y; Zhao X; Zhang Y; Yang X; Wang Y; Zhao R; Anderson GJ; Zhao Y; Nie G Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun 2018, 9, 3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Luo L; Tang J; Nishi K; Yan C; Dinh P-U; Cores J; Kudo T; Zhang J; Li T-S; Cheng K Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. Circ. Res 2017, 120, 1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tang J; Shen D; Caranasos TG; Wang Z; Vandergriff AC; Allen TA; Hensley MT; Dinh P-U; Cores J; Li T-S; Zhang J; Kan Q; Cheng K Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat. Commun 2017, 8, 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Su T; Huang K; Ma H; Liang H; Dinh P-U; Chen J; Shen D; Allen TA; Qiao L; Li Z; Hu S; Cores J; Frame BN; Young AT; Yin Q; Liu J; Qian L; Caranasos TG; Brudno Y; Ligler FS; Cheng K Platelet-Inspired Nanocells for Targeted Heart Repair After Ischemia/Reperfusion Injury. Adv. Funct. Mater 2019, 29, 1803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Emanueli C; Shearn AIU; Angelini GD; Sahoo S Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vasc. Pharmacol 2015, 71, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Lai RC; Chen TS; Lim SK Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regener. Med 2011, 6, 481–492. [DOI] [PubMed] [Google Scholar]
- (46).Makkar RR; Smith RR; Cheng K; Malliaras K; Thomson LE; Berman D; Czer LS; Marban L; Mendizabal A; Johnston PV; Russell SD; Schuleri KH; Lardo AC; Gerstenblith G; Marban E Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Vandergriff AC; de Andrade JBM; Tang J; Hensley MT; Piedrahita JA; Caranasos TG; Cheng K Intravenous Cardiac Stem Cell-Derived Exosomes Ameliorate Cardiac Dysfunction in Doxorubicin Induced Dilated Cardiomyopathy. Stem Cells Int. 2015, 2015, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Vandergriff A; Huang K; Shen D; Hu S; Hensley MT; Caranasos TG; Qian L; Cheng K Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018, 8, 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Chen Z; Hu Q; Gu Z Leveraging Engineering of Cells for Drug Delivery. Acc. Chem. Res 2018, 51, 668–677. [DOI] [PubMed] [Google Scholar]
- (50).Chen Z; Wang Z; Gu Z Bioinspired and Biomimetic Nanomedicines. Acc. Chem. Res 2019, 52, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]