Abstract
Stem cell therapies have made strides toward the efficacious treatment of injured endometrium and the prevention of intrauterine adhesions, or Asherman’s syndrome (AS). Despite this progress, they are limited by their risk of tumor formation, low engraftment rates, as well as storage and transportation logistics. While attempts have been made to curb these issues, there remains a need for simple and effective solutions. A growing body of evidence supports the theory that delivering media, conditioned with mesenchymal stem cells, might be a promising alternative to live cell therapy. Mesenchymal stem cell-secretome (MSC-Sec) has a superior safety profile and can be stored without losing its regenerative properties. It is versatile enough to be added to a number of delivery vehicles that improve engraftment and control the release of the therapeutic. Thus, it holds great potential for the treatment of AS. Here, a new strategy for loading crosslinked hyaluronic acid gel (HA gel) with MSC-Sec is reported. The HA gel/MSC-Sec treatment paradigm creates a sustained release system that repairs endometrial injury in rats and promotes viable pregnancy.
Keywords: Asherman’s syndrome, endometrial injury, hyaluronic acid, mesenchymal stem cells, secretome
1. Introduction
Asherman’s Syndrome (AS), also commonly known as intrauterine adhesions (IUAs), is a complex gynecological abnormality that leads to scar tissue formation in the uterus. Dilation and curettage (D&C), a common uterine surgery, is the leading cause of IUAs. As reported by an international meta-analysis, about 20% of women treated with D&C developed IUAs within 12 months after surgery.[1] It is mainly caused by operations that impart damage to the endometrium during planned pregnancy terminations, miscarriage, and preterm/term delivery, including operations for the removal of retained products of conception.[2] IUAs can be characterized as fibrous, connective tissue bands, with or without glandular tissue, ranging from filmy to dense. Symptoms caused by IUAs include recurrent pregnancy loss, hypomenorrhea, amenorrhea, cyclic pelvic pain, and infertility.[2]
The goal for treating AS is removing the adhesions and keeping the normal morphology and function of the uteri. Hysteroscopic adhesiolysis is commonly used to manually remove intrauterine adhesions and reconstruct the uterine cavity. Additionally, hormone therapy is needed to regain the uterine lining. However, there is a chance that the adhesions will be recurrent, even after adhesiolysis, depending on the severity of the disease.[3] What’s more, there are complications that can still affect a patient even after a successful pregnancy, such as abnormal placentation, preterm delivery, intrauterine growth restrictions, and higher rates of caesarean sections.[4] Currently, antiadhesion materials, such as hyaluronic acid (HA) gel and intrauterine balloons, are used after adhesiolysis to prevent the recurrence of adhesions.[5]
Stem cells, which have the ability of self-renewal and multilineage differentiation, have been broadly applied in the field of regenerative medicine, for example, acute myocardial infarction, acute liver fibrosis, and idiopathic pulmonary fibrosis.[6] In general, interest for cell-based therapies has skyrocketed, a growing number of scholars have been fixing their sights on using mesenchymal stem cells (MSCs) to treat AS.[7] The first successful application of MSCs in a severe AS patient was reported in India, in 2011.[8] The patient was injected with autologous bone marrow MSCs transvaginally. The procedure increased the thickness of the endometrium and resulted in a successful pregnancy. A number of improvements and additions, based on previous cell delivery strategies, demonstrate better stem cell retention and promotion of endometrial repair after trauma.[9] However, low retention and the propensity for tumorigenicity after administration still remain major obstacles for stem cell–based therapy.[10]
The theory that MSC-based therapy works primarily through paracrine secretions has become more and more accepted.[11] Secretome from MSCs (MSC-Sec) includes a great variety of cytokines and chemokines, which have been shown to play important roles in tissue repair and regeneration.[12] Therapies with the application of MSC-Sec have a superior safety profile compared to live cell administration.[13] Additionally, secretome can be safely stored for long periods of time without risking functional loss. Furthermore, its long shelf-life, and the ability to combine it with biomaterials makes Sec therapy a more clinically translational strategy.
Here, we report a brand-new strategy for protecting the endometrium from trauma after intrauterine surgery and preventing the recurrence of AS. It involves the application of an MSC-Sec-loaded HA gel to the uterine endometrium that can improve endometrial proliferation and function. Our previous studies have shown that MSC-Sec is safe and effective in the treatment of tissue injury and that the repair is mediated primarily through paracrine signaling molecules.[14] Inspired by these studies, we demonstrated an intrauterine drug delivery system aimed at maximizing curative effects by using crosslinked hyaluronic acid as an agent for the sustained release of MSC-Sec. First, we conducted in vitro studies, which verified that MSC-Sec can improve endometrial proliferation and promote angiogenesis. Then, we delivered MSC-Secloaded, crosslinked HA to the injured uteri of rats and showed that the treatment group presents thicker endometrium, more glands, and higher successful pregnancy rates than the control group.
2. Results and Discussion
2.1. Collection and Characterization of MSC-Sec
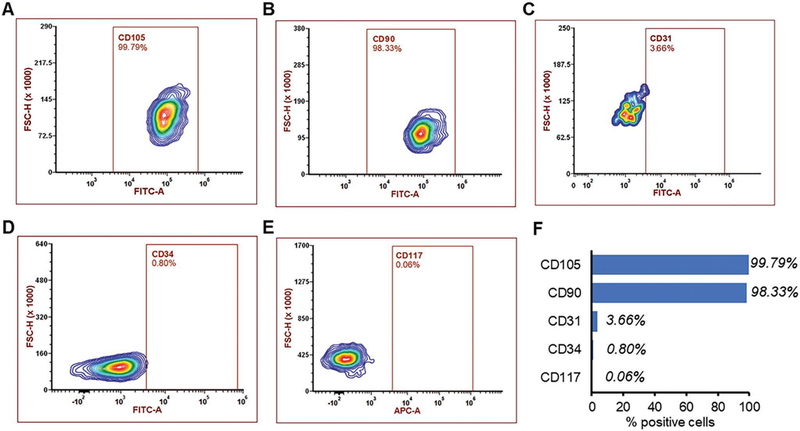
Numerous studies have indicated that stem cells exert their therapeutic benefits mainly through paracrine signaling.[11] Secretome has shown immense potential to treat injured tissues, including injured endometrium.[7a,9b,12a,15,29] Thus, MSC-Sec could be an ideal candidate to treat endometrial injury. To that end, MSC markers were identified via flow cytometry. As shown in Figure 1, the presence of major MSC markers (e.g., CD105 and CD90), but no CD34, CD31, or CD117 expression (Figure 1A–F), was consistent with findings in previous studies.[16] Then, the secretome from MSCs was collected and characterized.
Figure 1.
Characterization of MSCs. Flow cytometry analysis of common MSC markers such as A) CD105 and B) CD90. MSCs are negative for C) CD31, D) CD34, and E) CD117. F) Summary of positive MSC cells with different markers.
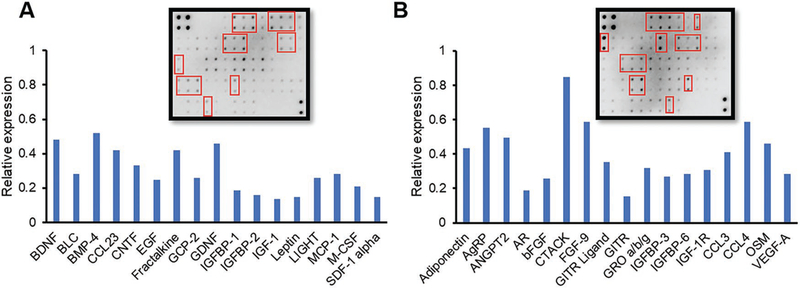
Our previous work reported on an MSC/red blood cell (RBC)-inspired nanoparticle (MRIN), as an effective alternative to the treatment of acute liver failure. This nanoparticle had an MSC-Sec-loaded core and an RBC membrane shell.[14] In this study, the factors that might contribute to the proliferation and migration of endometrial and endothelial cells, as a result of MSC-Sec, were analyzed via cytokine array (Figure 2).[17] There are a number of cytokines associated with tissue regeneration in MSC-Sec, including epidermal growth factor (EGF), insulin-like growth factor binding protein (IGFBP), insulin-like growth factor-1 (IGF), and fibroblast growth factor (FGF).[18] These cytokines have been shown to contribute to endometrial proliferation and glandular reformation after trauma.[19] For example, FGF-9 has been proven to play an important role in endometrial stroma proliferation.[20] EGF has been established as a major effector of angiogenesis in human endometrium.[21] It has also been reported that IGF and IGFBP can promote the proliferation of epithelial cells in the endometrium.[22] Epithelial and stromal cells are major components of the endometrium. Pathological alteration of AS involves injured endometrium and decreased glandular formation. Our assay results (Figure 2A,B) explained the rationale of applying MSC-Sec to treat endometrial injury.
Figure 2.
Human cytokine arrays. A,B) Human cytokine array analysis (AAH-CYT-6 and AAH-CYT-7) of MSC-Sec and densitometric analysis of proteins associated with tissue regeneration. Graph shows the mean value. The proteins in graph are pointed out in red.
2.2. Preparation and Characterization of MSC-Sec Gel
Current treatments of AS disease include hysteroscopic adhesiolysis, the application of antiadhesives, and hormone therapy. Antiadhesives, in combination with local treatments, are optimal for repairing the injured endometrium and preventing adhesion reformation. The intrauterine administration of antiadhesives through the vagina is a suitable and noninvasive approach. Hyaluronic acid gel is being researched as a possible antiadhesive material that could be used after obstetric and gynecological operations.[23] One of the issues with HA gel is that it has a natural half-life of just 1–2 days.[24] This half-life could be even shorter in the uterus due to aqueous dilution. In preliminary experiments, when MSC-Sec-loaded commercial HA gel (non-crosslinked) was placed into saline, the shape of the gel disintegrated immediately with gentle shaking. As a result, the loaded MSC-Sec was released to surrounding environment completely, and rapidly. To achieve a more sustained release of MSC-Sec over a longer period of time, crosslinked HA was used instead.
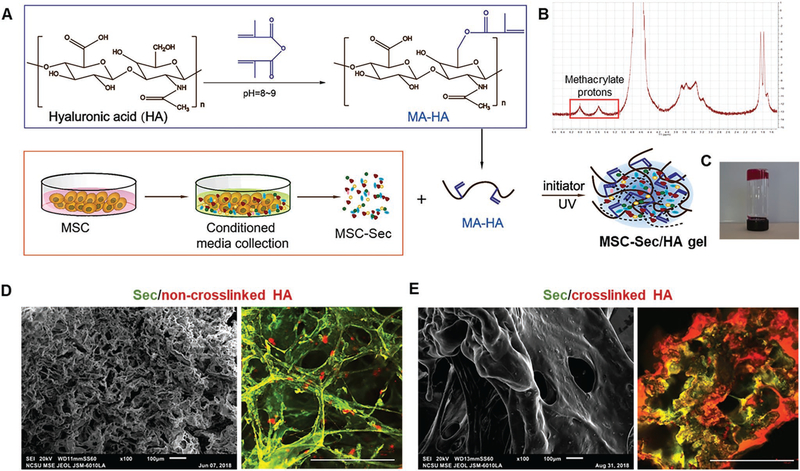
In the procedure shown in Figure 3A, MSC-Sec-loaded, crosslinked HA gel was synthesized as an effective intrauterine sustained-release drug delivery carrier. The proton peaks (methacrylate (MA) protons) at 5.74 and 6.17 ppm represent the successful synthesis of methacrylate hyaluronic acid (MA-HA) (Figure 3B). To create the gel delivery platform, MSC-Sec solution was used to dissolve MA-HA powder and form MSC-Sec/crosslinked HA gel under UV irradiation (MSC-Sec protein concentration: 10 mg mL−1; HA protein concentration: 1 mg mL−1). As shown in Figure 3C, after UV exposure, the gel exhibited the desired stickiness and stiffness. To further observe the detailed morphological structure of the crosslinked HA, scanning electron microscope (SEM) images and confocal images were taken. We loaded MSC-Sec into non-crosslinked, commercial HA and our crosslinked MA-HA. Compared to the non-crosslinked HA (Figure 3D, left panel), which has been approved for postoperative antiadhesive use after uterine surgeries, the crosslinked HA shows more compact construction, with smaller and fewer pores (Figure 3E, left panel). The morphology of the crosslinked HA makes it a more stable carrier than the commercial HA gel and allows it to achieve the sustained and long-term release of MSC-Sec. Corresponding confocal microscopic images (Figure 3D,E, right panel) confirmed the thorough mixture of the MSC-Sec (green) within the HA gel (red). The proper, evenly dispersed integration of the components guaranteed the constant releasing of MSC-Sec along with the degradation of HA. These morphological and physical traits make crosslinked-HA gel a more suitable sustained-release platform for the intrauterine administration of MSC-Sec than commercial HA.
Figure 3.
Fabrication and characterization of MSC-Sec-loaded, crosslinked HA gel. A) Schematic showing the synthesis of MSC-Sec-loaded, crosslinked HA gel. B) MA-HA: 1H NMR (D2O, 300 MHz, δ ppm): 1.85–1.96 (m, 3H, CH2 = C(CH3)CO), 1.99 (s, 3H, NHCOCH3), 5.74 (s, 1H, CH1H2 = C(CH3) CO), 6.17 (s, 1H, CH1H2 = C(CH3)CO). C) State of HA gel at room temperature when the bottle is upside down (gelation). D) Representative SEM (scale bar: 100 μm) and color-depth projection confocal images of MSC-Sec/non-crosslinked HA; green represents MSC-Sec, red represents HA. Scale bar in confocal image: 100 μm. E) Representative SEM (scale bar: 100 μm) and color-depth projection confocal images of MSC-Sec/crosslinked HA; green represents MSC-Sec, red represents HA. Scale bar in confocal image: 100 μm.
2.3. In Vitro Studies of MSC-Sec
MSCs possess qualities that make them favorable candidates for AS therapy.[25,29] Among them are their reported pro-angiogenic effects and their ability to accelerate the proliferation of epithelial and endothelial cells.[26] Herein, a series of in vitro cell assays were conducted to test the bioactive effects of MSC-derived secretome on cultured endothelial, epithelial, and stromal cells.
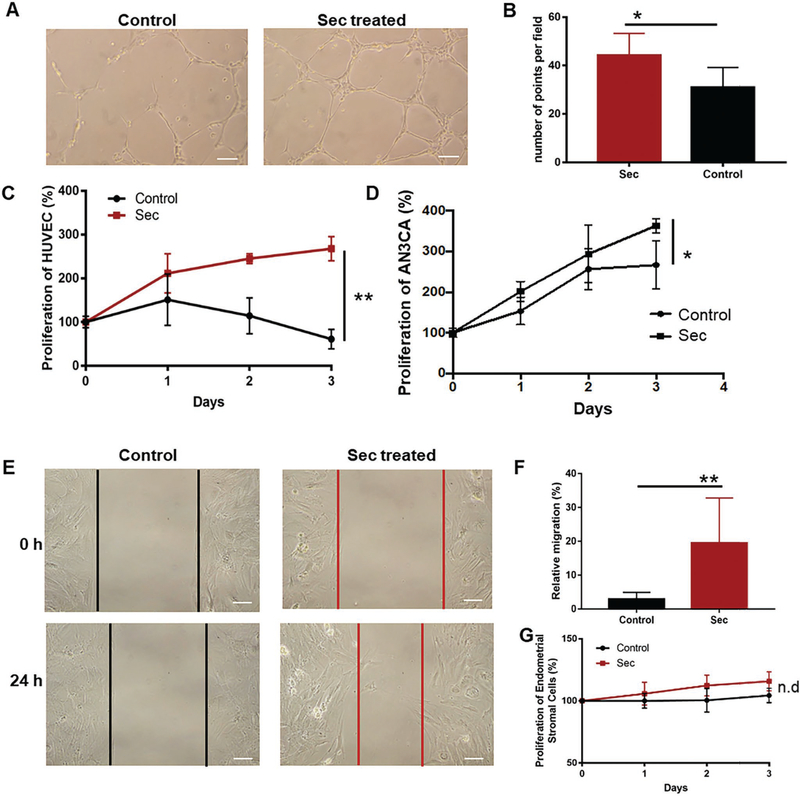
Figure 4A,B shows that the tube-formation ability of human umbilical vein endothelial cells (HUVECs) is enhanced in the MSC-Sec-treated group when compared to the Iscove’s Modified Dulbecco’s Medium (IMDM) group. Additionally, the proliferation rate of HUVECs treated with MSC-Sec was significantly enhanced when compared to the control group (Figure 4C). MSC-Sec not only benefited the proliferation of endothelial cells, but also promoted the growth of human endometrial epithelial cell line, AN3CA (Figure 4D). In addition, to increase the relevance of the cells tested, we isolated endometrial epithelial cells and endometrial stromal cells from rat uteri. As shown in Figure 4E, compared to those incubated with basal medium-IMDM, the rat epithelial cells incubated with MSC-Sec showed an enhanced migratory ability. After quantification, we found that the migration rate of the MSC-Sec group was significantly higher than the basal medium group (Figure 4F).
Figure 4.
In vitro cell-based assays. A) HUVEC tubing images of MSC-Sec-treated group and control group. Scale bar: 20 μm. B) Graph comparing the number of tube points between MSC-Sec-treated group and control group, n = 3. C) Proliferation of HUVEC with IMDM (control group) or MSC-Sec group. D) Proliferation of AN3CA treated with IMDM or MSC-Sec. * indicates P < 0.05 when compared to the control group. E) The image of cell migration of rat endometrial epithelial cells (EEC) cultured with IMDM (control group) or MSC-Sec. Scale bar: 20 μm. F) Analysis of the migration rate of EEC of the control group and MSC-Sec group. G) Proliferation of rat endometrial stromal cells cultured with IMDM or MSC-Sec. n = 6 for each group if no further description, * indicates P < 0.05, ** indicates P < 0.01.
It has been reported that patients with intrauterine adhesions have impaired myometrial and endometrial vascularity. Thus, the prognostic factors of AS include vascularization of endometrium.[7d,27,29] The improvement of tube-formation ability and proliferation of endothelial cells contribute to endometrium recovery. Epithelial cells are the primary components of endometrial glands, which synthesize and secrete substances essential for the survival and development of embryos. Herein, patients who suffer from AS with injured epithelial cells often experience infertility. Thus, the benefits imparted by MSC-Sec on epithelial and endothelial cells support the rationale of applying MSC-Sec to endometrial injuries. In addition, there is no significant difference in the proliferation rates of rat endometrial stromal cells between the MSC-Sec-treated group and IMDM-treated group (Figure 4G). One possible explanation is that epithelial cells and endothelial cells cannot grow well in pure IMDM solution as shown in Figure 4C,D, while stromal cells survived well in basic media and displayed no further promoting effect with the addition of MSC-Sec. In that way, MSC-Sec would not lead to the over-proliferation of endometrial stromal cells. This result indeed benefits the repair of endometrial injury because the over-proliferation of stromal cells is associated with endometriosis, a disease which is characterized by endometrium outside the uterine cavity.[7a,28]
2.4. Ex Vivo Release Study
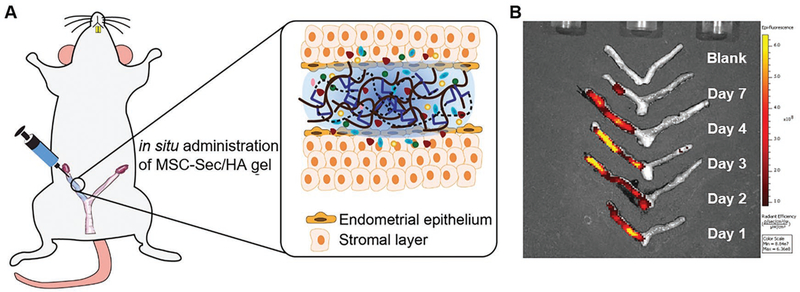
The optimization of therapeutic efficacy requires the maximization of MSC-Sec retention inside the uterus. Before performing in vivo experiments, we studied the kinetics of the MSC-Sec being released from the crosslinked HA in rat uterine cavities. MSC-Sec was labeled with Alexa Fluor 488 for ex vivo IVIS imaging. Then, MSC-Sec gel was administered in vivo into the uteri of rats. The rats were sacrificed on days 1, 2, 3, 4, and 7 and their uteri were collected. Figure 5A illustrates the administration of the crosslinked HA in the uterus. Once in place, MSC-Sec would be released from the gel into the surrounding endometrium over a 7-day period (Figure 5B). It is known that the mean length of the estrous cycle in sexually matured rats is 4 days. The crosslinked HA synthesized in this study can stay in the uterine cavity for roughly two estrous cycles.
Figure 5.
MSC-Sec/HA gel injection and rodent model of endometrium injury. A) Schematic showing intrauterine injection of MSC-Sec/HA gel. B) Ex vivo fluorescent imaging of rat uteri at days 1, 2, 3, 4, and 7 after injection.
2.5. MSC-Sec Gel Treatment Improved Pregnancy
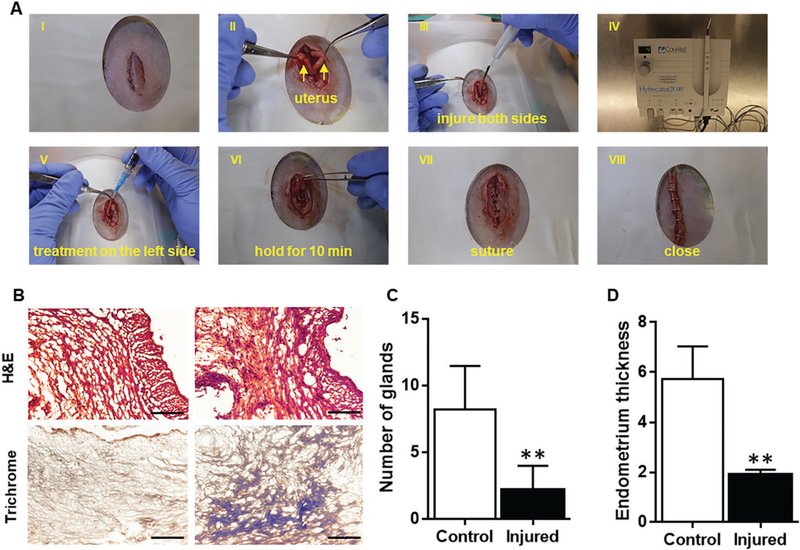
To test the effect of different treatments for the injured uterus, an injured rat uterus model was induced by using electrocoagulation (Figure 6A).[29] Electrocoagulation injures the endometrium of the uterus and reduces angiogenesis therein, simulating the pathological changes that lead to AS.[15,30] Figure 6B shows the hematoxylin and eosin (H&E) and trichrome stained micrographs of the uteri before and after injury. The pathological changes are indicative of the disorganized morphologic structure and enhanced collagen deposition of injured endometrium and myometrium. In addition, the endometrial thickness and the quantity of glands therein was quantified in Figure 6C,D. The subsequent decrease in endometrial thickness and gland quantity indicates the successful establishment of the AS animal model.
Figure 6.
The electrocoagulation-induced animal model of AS. A) Pictures showing the procedure of inducing injury and delivering the treatment. I: abdominal skin incision. II: incision of rectus abdominis and uterus exposure. III: electrocoagulation injury in both sides of uterus. IV: electrocoagulation machine. V: intrauterine injection. Right side is treatment side. Left side is control side. VI: closure of uterus incision. VII: closure of abdominis. VIII: closure of abdominal skin incision. B) Representative H&E and Masson’s trichrome-stained uterus section 14 days after injury (Scale bar: 70 μm). C) Quantitative data comparing the number of glands of endometrium of injured group and control group. D) Quantitative data comparing the thickness of endometrium of injured group and control group. n = 5, ** indicates P < 0.01 when compared to the other group.
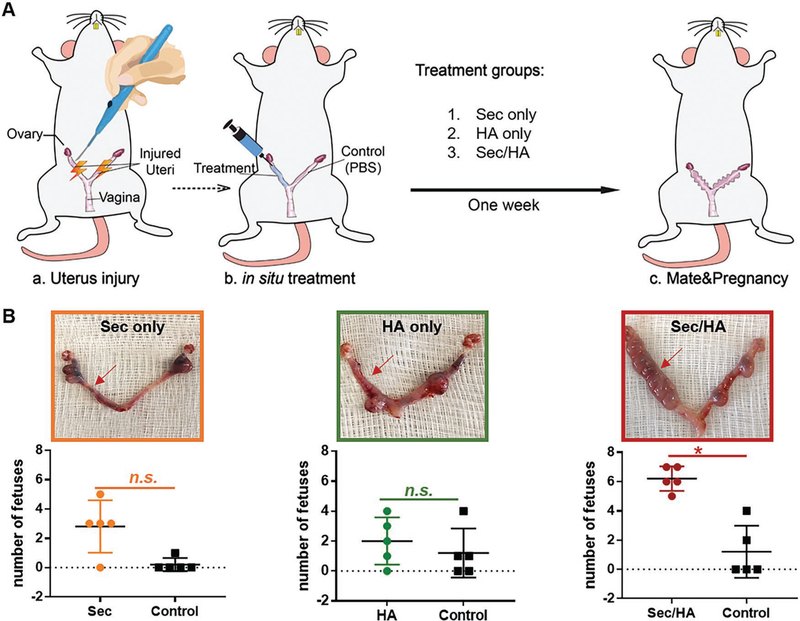
To evaluate the therapeutic effect of MSC-Sec/gel, rats were randomized into three groups to receive crosslinked HA gel only, MSC-Sec only, and MSC-Sec/HA gel, respectively. A schematic image detailing the animal experiment was presented in Figure 7A. In this novel experimental approach, we establish a self-comparison control, to reduce experimental error and interference, by administering PBS into the left injured uterus and one of the three treatments into the right injured uterus. The following treatments were administered: MSC-Sec-loaded, crosslinked HA gel for the MSC-Sec/HA gel group, only crosslinked HA for the HA group, and only MSC-Sec (protein concentration: 10 mg mL−1) for the MSC-Sec group (n = 10). The crosslinked HA gel ensured that the release of MSC-Sec was spread out over a 7-day period in the uterus. After one week, half of these female rats were mated with male rats and half were sacrificed for histology.
Figure 7.
The effects of MSC-Sec HA gel on fetal development. A) Schematic showing the animal study design. Both sides of the uterus are injured. One side gets treatment while left side gets PBS as control. And there are three treatment groups: MSC-Sec, crosslinked HA gel group; crosslinked HA group, and MSC-Sec group, n = 5. B) Representative uterus images and quantitative data comparing the numbers of fetuses on both sides (red arrow indicates treated side). * indicates P < 0.05 when compared to the other side.
As shown in Figure 7B, only ≈1–2 fetuses developed on the PBS control side (left), confirming the successful establishment of the model. As discussed earlier, infertility is a common consequence of AS. After treatment, the right side of the MSC-Sec/HA gel group (red arrow) developed a significantly higher number of fetuses, while in the groups with HA or MSC-Sec only, there was no significant difference between both sides. MSC-Sec itself cannot stay for a long time in the uterine cavity without crosslinked HA gel as the carrier, which leads to its failure to treat. Additionally, crosslinked HA gel alone cannot restore fertility without the infusion of MSC-Sec. Based on significant improvement of the number of fetuses of MSC-Sec/HA gel group, we can conclude that the therapy of MSC-Sec-loaded, crosslinked HA gel makes contribution to the fertility restoration of AS rats.
2.6. Histology Analysis
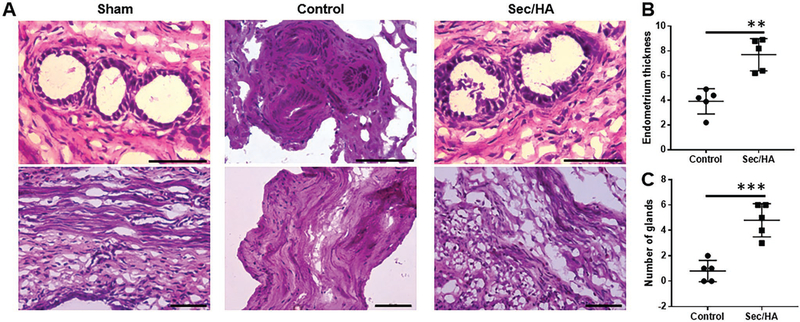
To reveal the pathological changes in the uteri as a result of the treatment, we performed H&E staining on the control sides and the treated sides. Figure 8A showed H&E-stained images of the treated and control sides of the uteri from the MSC-Sec/ HA gel group, endometrium thickness and number of glands were quantified (Figure 8B,C). The morphology of the myometrium on the control side was disorganized because of the trauma, and the boundary between the endometrium and myometrium was not clear. On the MSC-Sec HA gel treated side, we can clearly identify the endometrium, myometrium, and perimetrium. The treated side presented a thicker endometrium and more glands compared to the control side, demonstrating the restoration of endometrial health. Future studies will explore the mechanisms and related pathways involved in MSC-Sec/HA gel treatment.
Figure 8.
MSC-Sec/HA treatment increases gland numbers and endometrium thickness. A) Representative H&E images of sham, the control side of MSC-Sec/HA group, and the treated side of MSC-Sec/HA group. Scale bar: 70 μm. B) Comparison of endometrium thickness between the control side and the treated side of MSC-Sec/HA group. C) Comparison of number of glands between the control side and the treated side of MSC-Sec/HA group. n = 5, ** indicates P < 0.01, *** indicates P < 0.001 when compared to the other group.
3. Conclusion
MSCs, along with other stem cell types, have been used to treat and prevent the formation of recurrent AS.[7a,31] MSC-Sec has shown increasing potential in uterine diseases in recent years.[32] In this study, MSC-Sec-loaded, crosslinked HA gel was developed as a therapeutic that restores injured endometrial morphology and fertility in a rat model of AS. First, we proved that the crosslinked HA gel itself is a good candidate to be as the carrier of intrauterine control released drug delivery system because of its stability and biodegradability. The promising effect of MSC-Sec on endometrial and endothelial cells was verified, and then the therapeutic effect of MSC-Sec HA gel was proved in a murine uterus injury model. The results of this study, both in terms of the morphology and function of the endometrium, highlight the potential benefits of developing this treatment platform in future large-scale animal studies and, potentially, in clinical trials. Inspired by these pilot studies, the functional restoration of endometrium and related mechanisms will be deeply investigated in following studies. Importantly, unlike the laparotomic administration used in this animal study, any subsequent clinical trials stemming from this work will use transvaginal administration to deliver MSC-Sec gel.
To the best of our knowledge, this is the first attempt to combine biomaterials with MSC-Sec in pursuit of uterine restoration/regeneration. By using MSC-Sec, we were able to bypass the tumorigenic risks associated with stem cell therapies. HA, a powerful antiadhesive biomaterial, serves as an optimal carrier for intrauterine administration. It provides protection from recurrent intrauterine adhesions and releases MSC-Sec to accelerate the restoration process. In addition, MSC-Sec/HA gel is clinically translational, given the ease with which it can be prepared and stored, compared to traditional stem cell therapy.
In conclusion, we successfully fabricated a control-released, intrauterine-administered, MSC-Sec-loaded, crosslinked HA gel to restore endometrial morphology and function after electrocoagulation injury in rats. This therapeutic platform utilizes the paracrine signaling proteins similar with cell therapy efficacy, while tackling the challenges of live stem cells.
4. Experimental Section
Culture of MSC and Derivation of MSC-Sec
Human bone marrow–derived MSCs were ordered from the American Type Culture Collection (ATCC; VA, USA). They were cultured in serum-free Iscove’s Modified Dulbecco’s Medium (Thermo Fisher Scientific, MA, USA). After 72 h, the conditioned media or secretome was collected. The Sec was first centrifuged at 0.4 rcf for 5 min and then filtered through a 0.22 μm filter. Sterilized Sec was stored in a −80 °C freezer for at least 24 h, and then lyophilized.
Characterization of MSC and MSC-Sec
The MSC phenotypes were characterized by flow cytometry (Beckman Coulter, Brea, CA). The following primary antibodies were purchased from Becton Dickinson (NJ, USA): CD31 (BD555445), CD34 (BD555821), CD45 (BD 555482), and CD90 (BD 555595). The following primary antibody was purchased from R&D Systems (MN, USA): CD105 (FAB 10971P). The conditioned media of MSC was analyzed by cytokine array (RayBiotech, GA, USA).
Alexa Fluor 488 NHS Ester (A20000, Molecular Probes) was used to react with the amino groups of the proteins to label MSC-Sec. Alexa Fluor 488 NHS Easter labeled Sec was prepared by the following procedures: Lyophilized Sec was dissolved in 1 mL of 0.1 m sodium bicarbonate buffer pH 8.3 at the protein concentration of 10 mg mL−1. The Alexa Fluor 488 NHS Easter was dissolved in DMSO at 10 mg mL−1 and then 50 μL solution was added into the protein solution. After incubating the reaction for 1 h at room temperature, a 10 × 300 mm gel filtration column was equilibrated with PBS and finally the Alexa Fluor 488 labeled Sec was obtained.
Preparation of MSC-Sec-Loaded, Crosslinked Hyaluronic Acid Hydrogel
MSC-Sec-loaded, crosslinked HA gel was fabricated as follows: Briefly, 0.2 g of HA (Sigma-Aldrich, MO, USA), in a sodium salt state, was dissolved in 20 mL of ultrapure water and stirred at 200 rpm for 20 min. Then, 0.8 mL of a 5 n sodium hydroxide (Sigma-Aldrich) solution was added and reacted with 1 mL of methacrylic anhydride (Sigma-Aldrich). The resultant solution was stirred at 400 rpm for 2 h. It was then stored at 4 °C for 24 h, after which it was mixed with 95% ethanol to precipitate. Then, the sediments were washed with 95% ethanol, placed in 40 °C for 3 h, dissolved in ultrapure water, frozen thoroughly, and finally lyophilized. The powder was dissolved by ultrapure water and dialyzed, using a 12 kDa cellulose bag (Spectrum Laboratories, Rancho Dominguez, CA) immersed in 1 L ultrapure water, which served as buffer medium. After 1 week, the resultant MA-HA was lyophilized. The lyophilized Sec was combined with the MA-HA solution and exposed to UV light at a distance of 1.5 cm, using a wavelength of 365 nm (BlueWave 75 UV Curing Spot Lamp), at a power of 4.5 mW cm−2, for 10 s. The final product was the MSC-Sec-loaded, crosslinked hyaluronic acid gel. MA-HA was reacted with cysteamine first to produce amino groups, and then the Alexa Fluor 594 NHS Ester (A20004, Molecular Probes) was used to react with amino groups to fluorescently label MA-HA.
Cell Lines
Immortalized endometrial epithelial cells were obtained from ATCC (AN3CA) and cultured in Eagle’s Minimum Essential Medium (EMEM; Sigma) infused with fetal bovine serum at a concentration of 10% by volume. Human umbilical vein endothelial cells were also obtained from ATCC (CRL1730) and cultured in vascular cell basal medium (ATCC; PCS 100–030) infused with endothelial cell growth kit- VEGF (ATCC; PCS 100–041). Endometrial epithelial cells and stromal cells were separated from 3 eight-week-old rats according to a video protocol.[33] The rat endometrial stromal cells were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12 (DMEM/F12) with l-glutamine, phenol red, 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid, 100 μg mL−1 gentamicin, 0.5 μg mL−1 amphotericin B, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), and fetal bovine serum (FBS; 10% by volume). The rat endometrial epithelial cells were cultured in DMEM containing FBS (10% by volume), 100 μg mL−1 gentamicin, 0.5 μg mL−1 amphotericin B, MCDB-105 medium (25% by volume), and 5 μg mL−1 insulin.
Cell Proliferation Assay
Proliferation was tested on three types of cells: 1) Immortalized human endometrial cells (AN3CA), 2) HUVEC, and 3) rat endometrial stromal cells. The cells were seeded in 96-well plates, at a concentration of 5000 cells per well, in MSC-Sec and plain IMDM. The protein concentration of Sec was 10 mg mL−1 which was measured by BCA protein assay (Thermo Scientific Pierce). CCK8 (Dojindo Molecular Technologies, MD, USA), a proliferation and cytotoxicity assay, was added to each well and analyzed after 4 h in the following 3 days.
Wound Healing Assay
Culture insert 2-well kits (Ibidi; Planegg, Germany) were used to perform the wound healing assay on rat endometrial epithelial cells. 70 μL cell suspension was seeded to both sides of the kit, at a density of 5 × 105 cells mL−1. After cell adherence, the kits were removed gently to create a cell-free gap. After washing with PBS, plain IMDM was added to the control group and MSC-Sec (protein concentration: 10 mg mL−1) was added to the Sec-treated group. The cells were imaged at time points 0 and 24 h.
Tube Formation Assay
Reduced growth factor Matrigel (250 μL) was added into each well of a 24-well plate and allowed to solidify in an incubator, at 37 °C for 30 min. 300 μL of MSC-Sec (protein concentration was 10 mg mL−1), mixed with 10 μL of resuspended HUVEC cells (≈75 000 cells), were added on the Matrigel. Plain IMDM was used as a control. After 4 h, the quantity and the length of tubal connections of HUVECs were compared.
MSC-Sec In Vivo Release Study
Cohorts of rats with endometrial injury were sacrificed 24, 48, 72, 96, and 168 h after receiving intrauterine MSC-Sec HA injections. The HA was previously labeled with Alexa Fluor 594. Uteri were collected for in vivo retention studies using ex vivo fluorescent imaging (IVIS, Caliper Lifesciences, Waltham, MA).
Rat Model of Endometrial Injury
All animal experiment procedures were approved by the IACUC at North Carolina State University. The injury model was induced according to a previous publication.[29] Briefly, 8–10 week-old SD rats who had reached sexual maturity were anesthetized via isoflurane inhalation. A vertical incision was then made on the middle caudal abdomen of each rat to expose their uteri. Next, a small incision was made on both sides of the uteri to insert an electrocoagulation tip. While moving the tip from the uterine horn to the cervix, an electric current (3 V) was delivered over a period of 5 s using an electrocoagulation machine (Hyfrecator 2000 Electrosurgical System). After injury, each study group (n = 10) received a different treatment with syringe directly injecting into the uterine cavity through the previous incision: 1) HA + MSC-Sec group: the right side of uteri received MSC-Sec-loaded, crosslinked HA gel, while the left side received PBS as a control. 2) HA group: right side of the uterus received the blank crosslinked HA gel, while the left side received PBS. 3) MSC-Sec group: the right side of the uterus received only MSC-Sec solution (protein concentration: 10 mg mL−1), while the left side received PBS. After the treatment, sutures were used to close the incisions on the uterus and the abdomen.
Morphological and Functional Test of the Uteri
7 days after the surgery and the treatment injections, half of the rats (n = 5) from each group were sacrificed for uterus removal and histological sectioning/analysis. The remaining rats (n = 5) were relocated to a different cage, where each of them was allowed to mate with a male rat for 96 h. 10 days after mating, the female rats were sacrificed to count the number of fetuses in both sides of their uteri.
Histology
Uterine tissues were embedded in paraffin after fixing in 10% formalin. Sections were cut at 5 μm thicknesses and stained with hematoxylin and eosin as well as Masson’s trichrome. An AZ-100 Nikon multipurpose zoom microscope was used to capture images.
Statistical Analysis
All experiments were repeated independently at least three times and the results were shown as mean ± SD. A two-tailed, paired or unpaired, Student’s t-test was used to analyze data between two groups, depending on the experimental design. Single, double, and triple asterisks represent P < 0.05, 0.01, and 0.001, respectively. P value less than 0.05 means significant difference. Graphpad Prism 7.0 was used for statistical analysis.
Acknowledgements
F.L., S.H., and H.Y. contributed equally to this work. This work was supported by grants from the National Institutes of Health (HL123920, HL137093, HL144002, and HL146153 to K.C.) and the American Heart Association (18TPA34230092 and 19EIA34660 286 to K.C.).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adhm.201900411.
Contributor Information
Feiran Liu, Department of Gynecology and Obstetrics, Beijing Hospital, National Center of Gerontology, Beijing 100730, China; Department of Molecular Biomedical Sciences and Comparative, Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, NC 27695, USA.
Shiqi Hu, Department of Molecular Biomedical Sciences and Comparative, Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, NC 27695, USA.
Hua Yang, Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, NC 27695, USA; Department of Gynecology and Obstetrics, Beijing Friendship Hospital, Capital Medical University, 95 Yong’an Road, Xicheng District, Beijing 100050, China.
Zhenhua Li, Department of Molecular Biomedical Sciences and Comparative, Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, NC 27695, USA.
Ke Huang, Department of Molecular Biomedical Sciences and Comparative, Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Teng Su, Department of Molecular Biomedical Sciences and Comparative, Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, NC 27695, USA.
Shaowei Wang, Department of Gynecology and Obstetrics, Beijing Hospital, National Center of Gerontology, Beijing 100730, China.
Ke Cheng, Department of Molecular Biomedical Sciences and Comparative, Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Joint Department of Biomedical Engineering, North Carolina State University, Raleigh, NC 27695, USA.
References
- [1].Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, Mol BW, Huirne JA, Hum. Reprod. Update 2014, 20, 262. [DOI] [PubMed] [Google Scholar]
- [2].Berman JM, Semin. Reprod. Med. 2008, 26, 349. [DOI] [PubMed] [Google Scholar]
- [3].Chen L, Zhang H, Wang Q, Xie F, Gao S, Song Y, Dong J, Feng H, Xie K, Sui L, J. Minimally Invasive Gynecol. 2017, 24, 299. [DOI] [PubMed] [Google Scholar]
- [4] a).Capella-Allouc S, Morsad F, Rongieres-Bertrand C, Taylor S, Fernandez H, Hum. Reprod 1999, 14, 1230; [DOI] [PubMed] [Google Scholar]; b) Roy KK, Baruah J, Sharma JB, Kumar S, Kachawa G, Singh N, Arch. Gynecol. Obstet 2010, 281, 355. [DOI] [PubMed] [Google Scholar]
- [5] a).Huberlant S, Fernandez H, Vieille P, Khrouf M, Ulrich D, deTayrac R, Letouzey V, PLoS One 2015, 10, e0125610; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mais V, Cirronis MG, Peiretti M, Ferrucci G, Cossu E, Melis GB, Eur. J. Obstet. Gynecol. Reprod. Biol 2012, 160, 1. [DOI] [PubMed] [Google Scholar]
- [6] a).Akram KM, Patel N, Spiteri MA, Forsyth NR, Int. J. Mol. Sci 2016, 17, 128; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ayala P, Caves J, Dai E, Siraj L, Liu L, Chaudhuri O, Haller CA, Mooney DJ, Chaikof EL, Acta Biomater. 2015, 26, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Eom YW, Shim KY, Baik SK, Korean J Intern. Med 2015, 30, 580; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Huang K, Hu S, Cheng K, Adv. Healthcare Mater 2019, 8, 1801011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7] a).Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M, Biomed. Pharmacother 2018, 102, 333; [DOI] [PubMed] [Google Scholar]; b) Hellstrom M, Moreno-Moya JM, Bandstein S, Bom E, Akouri RR, Miyazaki K, Maruyama T, Brannstrom M, Fertil. Steril 2016, 106, 487; [DOI] [PubMed] [Google Scholar]; c) Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS, J. Cell. Mol. Med 2018, 22, 67; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L, Hum. Reprod 2016, 31, 2723. [DOI] [PubMed] [Google Scholar]
- [8].Nagori CB, Panchal SY, Patel H, J. Hum. Reprod. Sci 2011, 4, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9] a).Zhao G, Cao Y, Zhu X, Tang X, Ding L, Sun H, Li J, Li X, Dai C, Ru T, Zhu H, Lu J, Lin C, Wang J, Yan G, Wang H, Wang L, Dai Y, Wang B, Li R, Dai J, Zhou Y, Hu Y, Sci. China: Life Sci 2017, 60, 404; [DOI] [PubMed] [Google Scholar]; b) Li X, Sun H, Lin N, Hou X, Wang J, Zhou B, Xu P, Xiao Z, Chen B, Dai J, Hu Y, Biomaterials 2011, 32, 8172; [DOI] [PubMed] [Google Scholar]; c) Campo H, Cervello I, Simon C, Ann. Biomed. Eng 2017, 45, 1710. [DOI] [PubMed] [Google Scholar]
- [10].Hu S, Ogle BM, Cheng K, Curr. Opin. Cell Biol 2018, 54, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11] a).Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H, Chem. Rev 2018, 118, 1917; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Spees JL, Lee RH, Gregory CA, Stem Cell Res. Ther 2016, 7, 125; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Trounson A, McDonald C, Cell Stem Cell 2015, 17, 11. [DOI] [PubMed] [Google Scholar]
- [12] a).Liang X, Ding Y, Zhang Y, Tse HF, Lian Q, Cell Transplant. 2014, 23, 1045; [DOI] [PubMed] [Google Scholar]; b) Madrigal M, Rao KS, Riordan NH, J. Transl. Med 2014, 12, 260; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Su T, Huang K, Ma H, Liang H, Dinh P-U, Chen J, Shen D, Allen TA, Qiao L, Li Z, Hu S, Cores J, Frame BN, Young AT, Yin Q, Liu J, Qian L, Caranasos TG, Brudno Y, Ligler FS, Cheng K, Adv. Funct. Mater 2019, 29, 1803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA, Hensley MT, Dinh P-U, Cores J, Li T-S, Zhang J, Kan Q, Cheng K, Nat. Commun 2017, 8, 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liang H, Huang K, Su T, Li Z, Hu S, Dinh P-U, Wrona EA, Shao C, Qiao L, Vandergriff AC, Hensley MT, Cores J, Allen T, Zhang H, Zeng Q, Xing J, Freytes DO, Shen D, Yu Z, Cheng K, ACS Nano 2018, 12, 6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Islam MR, Yamagami K, Yoshii Y, Yamauchi N, J. Reprod. Dev 2016, 62, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kern S, Eichler H, Stoeve J, Kluter H, Bieback K, Stem Cells 2006, 24, 1294. [DOI] [PubMed] [Google Scholar]
- [17].Borges FT, Convento MB, Schor N, Stem Cells Cloning: Adv. Appl 2018, 11, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haining RE, Cameron IT, van Papendorp C, Davenport AP, Prentice A, Thomas EJ, Smith SK, Hum. Reprod 1991, 6, 1200. [DOI] [PubMed] [Google Scholar]
- [19].Giudice LC, Fertil. Steril 1994, 61, 1. [DOI] [PubMed] [Google Scholar]
- [20].Chen H-M, Chuang P-C, Tsai S-J, Wing L-YC, Wu M-H, Endocrinology 2002, 143, 2715. [DOI] [PubMed] [Google Scholar]
- [21].Möller B, Rasmussen C, Lindblom B, Olovsson M, MHR: Basic Sci. Reprod. Med 2001, 7, 65. [DOI] [PubMed] [Google Scholar]
- [22].Rutanen EM, Gynecol. Endocrinol 1998, 12, 399. [DOI] [PubMed] [Google Scholar]
- [23].Zhu Y, Hu J, Yu T, Ren Y, Hu L, Med. Sci. Monit 2016, 22, 3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carruthers A, Carruthers J, Plast. Reconstr. Surg 2007, 120, 33S. [DOI] [PubMed] [Google Scholar]
- [25].Ebrahim N, Mostafa O, El Dosoky RE, Ahmed IA, Saad AS, Mostafa A, Sabry D, Ibrahim KA, Farid AS, Stem Cell Res. Ther 2018, 9, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26] a).Boomsma RA, Geenen DL, PLoS One 2012, 7, e35685; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D, Biol. Reprod 2009, 80, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johary J, Xue M, Zhu X, Xu D, Velu PP, Minimally Invasive Gynecol J. 2014, 21, 44. [DOI] [PubMed] [Google Scholar]
- [28].Cousins FL, D. F. O, Gargett CE, Best Pract. Res., Clin. Obstet. Gynaecol 2018, 50, 27. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, Zhang S, Reproduction 2016, 152, 389. [DOI] [PubMed] [Google Scholar]
- [30].Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, Sugino N, Fertil. Steril 2009, 91, 998. [DOI] [PubMed] [Google Scholar]
- [31] a).Cervello I, Gil-Sanchis C, Santamaria X, Cabanillas S, Diaz A, Faus A, Pellicer A, Simon C, Fertil. Steril 2015, 104, 1552; [DOI] [PubMed] [Google Scholar]; b) Cervello I, Santamaria X, Miyazaki K, Maruyama T, Simon C, Semin. Reprod. Med 2015, 33, 366. [DOI] [PubMed] [Google Scholar]
- [32].Ho C-H, Lan C-W, Liao C-Y, Hung S-C, Li H-Y, Sung Y-J, J. Chin. Med. Assoc 2018, 81, 268. [DOI] [PubMed] [Google Scholar]
- [33].De Clercq K, Hennes A, Vriens J, J. Visualized Exp 2017, 121, e55168. [DOI] [PMC free article] [PubMed] [Google Scholar]